Potential Role of Curcumin and Its Nanoformulations to Treat Various Types of Cancers
Abstract
1. Introduction
2. Mechanism of Action of Curcumin as an Anticancer Agent
3. Bioavailability of Curcumin
4. Therapeutic Activity of Curcumin Nanoformulations
4.1. Liposomes
4.2. Nanoparticles
4.2.1. Polymeric Nanoparticles
4.2.2. Solid Lipid Nanoparticles
4.2.3. Magnetic Nanoparticles
4.2.4. Albumin
4.2.5. Gold Nanoparticles
4.3. Conjugates
4.4. Cyclodextrins (CD)
4.5. Solid Dispersions
4.6. Micelles
4.7. Nanospheres and Microcapsules
4.8. Nanogels
4.9. Nanodisks
4.10. Metallo-Complexes
5. Anticancer Activity of Curcumin against Various Types of Cancers
5.1. Gastrointestinal Cancers
5.1.1. Oral Cavity and Salivary Gland Cancers
5.1.2. Esophageal Cancer
5.1.3. Stomach Cancer
5.1.4. Intestinal Cancer
5.1.5. Hepatic Cancer
5.1.6. Pancreatic Cancer
5.2. Head and Neck Cancer
5.3. Glioblastoma and Brain Cancer
5.4. Breast Cancer
5.5. Colorectal Cancer
5.6. Prostate Cancer
5.7. Leukemia
5.7.1. Anticancer Activities of Curcumin against Various Types of Leukemia
Acute Lymphoblastic Leukemia
Acute Myeloid Leukemia
Chronic Lymphocytic Leukemia
Chronic Myeloid Leukemia
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA. Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Gupta, A.P.; Pandotra, P.; Sharma, R.; Kushwaha, M.; Gupta, S. Marine resource: A promising future for anticancer drugs. In Studies in Natural Products Chemistry; Elsevier B.V.: Amsterdam, The Netherlands, 2013; Volume 40, pp. 229–325. ISBN 9780444596031. [Google Scholar]
- Umar, A.; Dunn, B.K.; Greenwald, P. Future directions in cancer prevention. Nat. Rev. Cancer 2012, 12, 835–848. [Google Scholar] [CrossRef]
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X. A review of curcumin and its derivatives as anticancer agents. Int. J. Mol. Sci. 2019, 20, 1033. [Google Scholar] [CrossRef] [PubMed]
- Ochwang’i, D.O.; Kimwele, C.N.; Oduma, J.A.; Gathumbi, P.K.; Mbaria, J.M.; Kiama, S.G. Medicinal plants used in treatment and management of cancer in Kakamega County, Kenya. J. Ethnopharmacol. 2014, 151, 1040–1055. [Google Scholar] [CrossRef]
- Freiburghaus, F.; Kaminsky, R.; Nkunya, M.H.H.; Brun, R. Evaluation of African medicinal plants for their in vitro trypanocidal activity. J. Ethnopharmacol. 1996, 55, 1–11. [Google Scholar] [CrossRef]
- Costa-Lotufo, L.V.; Khan, M.T.H.; Ather, A.; Wilke, D.V.; Jimenez, P.C.; Pessoa, C.; De Moraes, M.E.A.; De Moraes, M.O. Studies of the anticancer potential of plants used in Bangladeshi folk medicine. J. Ethnopharmacol. 2005, 99, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.Z.; Sun, M.; Xing, J.; Luo, Q.; Corke, H. Structure-radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Sci. 2006, 78, 2872–2888. [Google Scholar] [CrossRef] [PubMed]
- Fouche, G.; Cragg, G.M.; Pillay, P.; Kolesnikova, N.; Maharaj, V.J.; Senabe, J. In vitro anticancer screening of South African plants. J. Ethnopharmacol. 2008, 119, 455–461. [Google Scholar] [CrossRef]
- Kamatou, G.P.P.; Van Zyl, R.L.; Davids, H.; Van Heerden, F.R.; Lourens, A.C.U.; Viljoen, A.M. Antimalarial and anticancer activities of selected South African Salvia species and isolated compounds from S. radula. South African J. Bot. 2008, 74, 238–243. [Google Scholar] [CrossRef]
- Gupta, S.C.; Patchva, S.; Koh, W.; Aggarwal, B.B. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin. Exp. Pharmacol. Physiol. 2012, 39, 283–299. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. The chemistry of curcumin: From extraction to therapeutic agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef]
- He, Y.; Yue, Y.; Zheng, X.; Zhang, K.; Chen, S.; Du, Z. Curcumin, inflammation, and chronic diseases: How are they linked? Molecules 2015, 20, 9183–9213. [Google Scholar] [CrossRef] [PubMed]
- Hewlings, S.; Kalman, D. Curcumin: A Review of Its’ Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Giordano, A.; Tommonaro, G. Curcumin and cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E. Curcumin combination chemotherapy: The implication and efficacy in cancer. Molecules 2019, 24, 2527. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.S.Y. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.H.; Helfand, S.L. New tricks of an old molecule: Lifespan regulation by p53. Aging Cell 2006, 5, 437–440. [Google Scholar] [CrossRef]
- Tuorkey, M. Curcumin a potent cancer preventive agent: Mechanisms of cancer cell killing. Interv. Med. Appl. Sci. 2014, 6, 139–146. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Eckert, R.L. Curcumin suppresses AP1 transcription factor-dependent differentiation and activates apoptosis in human epidermal keratinocytes. J. Biol. Chem. 2007, 282, 6707–6715. [Google Scholar] [CrossRef]
- Moragoda, L.; Jaszewski, R.; Majumdar, A.P.N. Curcumin induced modulation of cell cycle and apoptosis in gastric and colon cancer cells. Anticancer Res. 2001, 21, 873–878. [Google Scholar]
- Ashour, A.A.; Abdel-Aziz, A.A.H.; Mansour, A.M.; Neslihan Alpay, S.; Huo, L.; Ozpolat, B. Targeting elongation factor-2 kinase (eEF-2K) induces apoptosis in human pancreatic cancer cells. Apoptosis 2014, 19, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.P.; Li, T.M.; Tsao, J.Y.; Fong, Y.C.; Tang, C.H. Curcumin induces cell apoptosis in human chondrosarcoma through extrinsic death receptor pathway. Int. Immunopharmacol. 2012, 13, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Hahn, Y.I.; Kim, S.J.; Choi, B.Y.; Cho, K.C.; Bandu, R.; Kim, K.P.; Kim, D.H.; Kim, W.; Park, J.S.; Han, B.W.; et al. Curcumin interacts directly with the Cysteine 259 residue of STAT3 and induces apoptosis in H-Ras transformed human mammary epithelial cells. Sci. Rep. 2018, 8, 6409. [Google Scholar] [CrossRef]
- Lee, W.H.; Loo, C.Y.; Young, P.M.; Traini, D.; Mason, R.S.; Rohanizadeh, R. Recent advances in curcumin nanoformulation for cancer therapy. Expert Opin. Drug Deliv. 2014, 11, 1183–1201. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, F.A.; Prakasam, G.; Chattopadhyay, S.; Rehman, A.U.; Padder, R.A.; Ansari, M.A.; Irshad, R.; Mangalhara, K.; Bamezai, R.N.K.; Husain, M.; et al. Curcumin decreases Warburg effect in cancer cells by down-regulating pyruvate kinase M2 via mTOR-HIF1α inhibition. Sci. Rep. 2018, 8, 8323. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Lee, Y.M.; Chang, G.C.; Yu, S.L.; Hsieh, W.Y.; Chen, J.J.W.; Chen, H.W.; Yang, P.C. Curcumin induces EGFR degradation in lung adenocarcinoma and modulates p38 activation in intestine: The versatile adjuvant for gefitinib therapy. PLoS ONE 2011, 6, e23756. [Google Scholar] [CrossRef] [PubMed]
- Shishodia, S.; Singh, T.; Chaturvedi, M.M. Modulation of transcription factors by curcumin. Adv. Exp. Med. Biol. 2007, 595, 127–148. [Google Scholar]
- Davie, J.R.; He, S.; Li, L.; Sekhavat, A.; Espino, P.; Drobic, B.; Dunn, K.L.; Sun, J.M.; Chen, H.Y.; Yu, J.; et al. Nuclear organization and chromatin dynamics - Sp1, Sp3 and histone deacetylases. Adv. Enzyme Regul. 2008, 48, 189–208. [Google Scholar] [CrossRef]
- Zhou, D.-Y.; Zhang, K.; Conney, A.H.; Ding, N.; Cui, X.-X.; Wang, H.; Verano, M.; Zhao, S.; Fan, Y.-X.; Zheng, X.; et al. Synthesis and Evaluation of Curcumin-Related Compounds Containing Benzyl Piperidone for Their Effects on Human Cancer Cells. Chem. Pharm. Bull. 2013, 61, 1149–1155. [Google Scholar] [CrossRef]
- Grabacka, M.M.; Gawin, M.; Pierzchalska, M. Phytochemical modulators of mitochondria: The search for chemopreventive agents and supportive therapeutics. Pharmaceuticals 2014, 7, 913–942. [Google Scholar] [CrossRef]
- Zhou, T.; Ye, L.; Bai, Y.; Sun, A.; Cox, B.; Liu, D.; Li, Y.; Liotta, D.; Snyder, J.P.; Fu, H.; et al. Autophagy and Apoptosis in Hepatocellular Carcinoma Induced by EF25-(GSH)2: A Novel Curcumin Analog. PLoS ONE 2014, 9, e107876. [Google Scholar] [CrossRef] [PubMed]
- Aoki, H.; Takada, Y.; Kondo, S.; Sawaya, R.; Aggarwal, B.B.; Kondo, Y. Evidence that curcumin suppresses the growth of malignant gliomas in vitro and in vivo through induction of autophagy: Role of akt and extracellular signal-regulated kinase signaling pathways. Mol. Pharmacol. 2007, 72, 29–39. [Google Scholar] [CrossRef]
- Dhillon, N.; Aggarwal, B.B.; Newman, R.A.; Wolff, R.A.; Kunnumakkara, A.B.; Abbruzzese, J.L.; Ng, C.S.; Badmaev, V.; Kurzrock, R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin. Cancer Res. 2008, 14, 4491–4499. [Google Scholar] [CrossRef] [PubMed]
- Zlotogorski, A.; Dayan, A.; Dayan, D.; Chaushu, G.; Salo, T.; Vered, M. Nutraceuticals as new treatment approaches for oral cancer-I: Curcumin. Oral Oncol. 2013, 49, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Adams, B.K.; Ferstl, E.M.; Davis, M.C.; Herold, M.; Kurtkaya, S.; Camalier, R.F.; Hollingshead, M.G.; Kaur, G.; Sausville, E.A.; Rickles, F.R.; et al. Synthesis and biological evaluation of novel curcumin analogs as anti-cancer and anti-angiogenesis agents. Bioorganic Med. Chem. 2004, 12, 3871–3883. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Farida, F.H.; Syahida, A.; Abas, F.; Shaari, K.; Israf, D.A.; Lajis, N.H. Synthesis and biological evaluation of curcumin-like diarylpentanoid analogues for anti-inflammatory, antioxidant and anti-tyrosinase activities. Eur. J. Med. Chem. 2009, 44, 3195–3200. [Google Scholar] [CrossRef]
- Zhao, C.; Yang, J.; Wang, Y.; Liang, D.; Yang, X.; Li, X.; Wu, J.; Wu, X.; Yang, S.; Li, X.; et al. Synthesis of mono-carbonyl analogues of curcumin and their effects on inhibition of cytokine release in LPS-stimulated RAW 264.7 macrophages. Bioorg. Med. Chem. 2010, 18, 2388–2393. [Google Scholar] [CrossRef] [PubMed]
- Bala, V.; Rao, S.; Boyd, B.J.; Prestidge, C.A. Prodrug and nanomedicine approaches for the delivery of the camptothecin analogue SN38. J. Control. Release 2013, 172, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Teow, H.M.; Zhou, Z.; Najlah, M.; Yusof, S.R.; Abbott, N.J.; D’Emanuele, A. Delivery of paclitaxel across cellular barriers using a dendrimer-based nanocarrier. Int. J. Pharm. 2013, 441, 701–711. [Google Scholar] [CrossRef]
- Qi, X.; Li, N.; Gu, H.; Xu, Y.; Xu, Y.; Jiao, Y.; Xu, Q.; Li, H.; Lu, J. Amphiphilic oligomer-based micelles as cisplatin nanocarriers for cancer therapy. Nanoscale 2013, 5, 8925–8929. [Google Scholar] [CrossRef]
- Das, M.; Singh, R.P.; Datir, S.R.; Jain, S. Intranuclear drug delivery and effective in vivo cancer therapy via estradiol-peg-appended multiwalled carbon nanotubes. Mol. Pharm. 2013, 10, 3404–3416. [Google Scholar] [CrossRef]
- Arzuman, L.; Beale, P.; Chan, C.; Yu, J.Q.; Huq, F. Synergism From Combinations of Tris(benzimidazole) monochloroplatinum(II) Chloride With Capsaicin, Quercetin, Curcumin and Cisplatin in Human Ovarian Cancer Cell Lines. Anticancer Res. 2014, 34, 5453–5464. [Google Scholar] [PubMed]
- Huq, F.; Yu, J.Q.; Beale, P.; Chan, C.; Arzuman, L.; Nessa, M.U.; Mazumder, M.E.H. Combinations of Platinums and Selected Phytochemicals as a Means of Overcoming Resistance in Ovarian Cancer. Anticancer Res. 2014, 34, 541–545. [Google Scholar] [PubMed]
- Nune, M.; Kumaraswamy, P.; Maheswari Krishnan, U.; Sethuraman, S. Self-Assembling Peptide Nanofibrous Scaffolds for Tissue Engineering: Novel Approaches and Strategies for Effective Functional Regeneration. Curr. Protein Pept. Sci. 2013, 14, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, H.; Wang, J.; Wang, S.; Liao, W.; Yang, Y.; Zhang, Y.; Kong, D.; Yang, Z. Supramolecular hydrogels inspired by collagen for tissue engineering. Org. Biomol. Chem. 2010, 8, 3267–3271. [Google Scholar] [CrossRef]
- Wang, H.; Wei, J.; Yang, C.; Zhao, H.; Li, D.; Yin, Z.; Yang, Z. The inhibition of tumor growth and metastasis by self-assembled nanofibers of taxol. Biomaterials 2012, 33, 5848–5853. [Google Scholar] [CrossRef]
- Zhang, P.; Cheetham, A.G.; Lin, Y.A.; Cui, H. Self-assembled tat nanofibers as effective drug carrier and transporter. ACS Nano 2013, 7, 5965–5977. [Google Scholar] [CrossRef]
- Soukasene, S.; Toft, D.J.; Moyer, T.J.; Lu, H.; Lee, H.K.; Standley, S.M.; Cryns, V.L.; Stupp, S.I. Antitumor activity of peptide amphiphile nanofiber-encapsulated camptothecin. ACS Nano 2011, 5, 9113–9121. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Indig, G.L.; Weichert, J.; Shin, H.C.; Kwon, G.S. In vivo cancer imaging by poly(ethylene glycol)-b-poly(ε-caprolactone) micelles containing a near-infrared probe. Nanomedi. Nanotechnol. Biol. Med. 2012, 8, 228–236. [Google Scholar] [CrossRef]
- Wagh, A.; Singh, J.; Qian, S.; Law, B. A short circulating peptide nanofiber as a carrier for tumoral delivery. Nanomedi. Nanotechnol. Biol. Med. 2013, 9, 449–457. [Google Scholar] [CrossRef]
- Yadav, B.; Taurin, S.; Rosengren, R.J.; Schumacher, M.; Diederich, M.; Somers-Edgar, T.J.; Larsen, L. Synthesis and cytotoxic potential of heterocyclic cyclohexanone analogues of curcumin. Bioorg. Med. Chem. 2010, 18, 6701–6707. [Google Scholar] [CrossRef]
- Sun, A.; Shoji, M.; Lu, Y.J.; Liotta, D.C.; Snyder, J.P. Synthesis of EF24-tripeptide chloromethyl ketone: A novel curcumin-related anticancer drug delivery system. J. Med. Chem. 2006, 49, 3153–3158. [Google Scholar] [CrossRef]
- Somers-Edgar, T.J.; Taurin, S.; Larsen, L.; Chandramouli, A.; Nelson, M.A.; Rosengren, R.J. Mechanisms for the activity of heterocyclic cyclohexanone curcumin derivatives in estrogen receptor negative human breast cancer cell lines. Invest. New Drugs 2011, 29, 87–97. [Google Scholar] [CrossRef]
- Robinson, T.P.; Ehlers, T.; Hubbard IV, R.B.; Bai, X.; Arbiser, J.L.; Goldsmith, D.J.; Bowen, J.P. Design, synthesis, and biological evaluation of angiogenesis inhibitors: Aromatic enone and dienone analogues of curcumin. Bioorganic Med. Chem. Lett. 2003, 13, 115–117. [Google Scholar] [CrossRef]
- Ohori, H.; Yamakoshi, H.; Tomizawa, M.; Shibuya, M.; Kakudo, Y.; Takahashi, A.; Takahashi, S.; Kato, S.; Suzuki, T.; Ishioka, C.; et al. Synthesis and biolgical analysis of new curcumin analogues bearing an enhanced potential for the medicinal treatment of cancer. Mol. Cancer Ther. 2006, 5, 2563–2571. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, D.; May, R.; Sureban, S.M.; Lee, K.B.; George, R.; Kuppusamy, P.; Ramanujam, R.P.; Hideg, K.; Dieckgraefe, B.K.; Houchen, C.W.; et al. Diphenyl difluoroketone: A curcumin derivative with potent in vivo anticancer activity. Cancer Res. 2008, 68, 1962–1969. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Shao, L.; Wang, Y.; Zhao, C.; Chu, Y.; Xiao, J.; Zhao, Y.; Li, X.; Yang, S. Exploration and synthesis of curcumin analogues with improved structural stability both in vitro and in vivo as cytotoxic agents. Bioorg. Med. Chem. 2009, 17, 2623–2631. [Google Scholar] [CrossRef]
- Karthikeyan, N.S.; Sathiyanarayanan, K.I.; Aravindan, P.G.; Giridharan, P. Synthesis, crystal structure, and anticancer properties of cyclic monocarbonyl analogs of curcumin. Med. Chem. Res. 2011, 20, 81–87. [Google Scholar] [CrossRef]
- Dayton, A.; Selvendiran, K.; Kuppusamy, M.L.; Rivera, B.K.; Meduru, S.; Kálai, T.; Hideg, K.; Kuppusamy, P. Cellular uptake, retention and bioabsorption of HO-3867, a fluorinated curcumin analog with potential antitumor properties. Cancer Biol. Ther. 2010, 10, 1027–1032. [Google Scholar] [CrossRef]
- Lee, H.E.; Choi, E.S.; Jung, J.Y.; You, M.J.; Kim, L.H.; Cho, S.D. Inhibition of specificity protein 1 by dibenzylideneacetone, a curcumin analogue, induces apoptosis in mucoepidermoid carcinomas and tumor xenografts through Bim and truncated Bid. Oral Oncol. 2014, 50, 189–195. [Google Scholar] [CrossRef]
- Novaković, M.; Pešić, M.; Trifunović, S.; Vučković, I.; Todorović, N.; Podolski-Renić, A.; Dinić, J.; Stojković, S.; Tešević, V.; Vajs, V.; et al. Diarylheptanoids from the bark of black alder inhibit the growth of sensitive and multi-drug resistant non-small cell lung carcinoma cells. Phytochemistry 2014, 97, 46–54. [Google Scholar] [CrossRef]
- Zheng, A.; Li, H.; Wang, X.; Feng, Z.; Xu, J.; Cao, K.; Zhou, B.; Wu, J.; Liu, J. Anticancer Effect of a Curcumin Derivative B63: ROS Production and Mitochondrial Dysfunction. Curr. Cancer Drug Targets 2014, 14, 156–166. [Google Scholar] [CrossRef]
- Ucisik, M.H.; Küpcü, S.; Schuster, B.; Sleytr, U.B. Characterization of CurcuEmulsomes: Nanoformulation for enhanced solubility and delivery of curcumin. J. Nanobiotechnol. 2013, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Sahu, A.; Kasoju, N.; Goswami, P.; Bora, U. Encapsulation of curcumin in Pluronic block copolymer micelles for drug delivery applications. J. Biomater. Appl. 2011, 25, 619–639. [Google Scholar] [CrossRef]
- Basniwal, R.K.; Khosla, R.; Jain, N. Improving the anticancer activity of curcumin using nanocurcumin dispersion in water. Nutr. Cancer 2014, 66, 1015–1022. [Google Scholar] [CrossRef]
- Esmaili, M.; Ghaffari, S.M.; Moosavi-Movahedi, Z.; Atri, M.S.; Sharifizadeh, A.; Farhadi, M.; Yousefi, R.; Chobert, J.M.; Haertlé, T.; Moosavi-Movahedi, A.A. Beta casein-micelle as a nano vehicle for solubility enhancement of curcumin; food industry application. LWT-Food Sci. Technol. 2011, 44, 2166–2172. [Google Scholar] [CrossRef]
- Anuchapreeda, S.; Fukumori, Y.; Okonogi, S.; Ichikawa, H. Preparation of Lipid Nanoemulsions Incorporating Curcumin for Cancer Therapy. J. Nanotechnol. 2012, 2012, 270383. [Google Scholar] [CrossRef]
- Basnet, P.; Hussain, H.; Tho, I.; Skalko-Basnet, N. Liposomal delivery system enhances anti-inflammatory properties of curcumin. J. Pharm. Sci. 2012, 101, 598–609. [Google Scholar] [CrossRef]
- Rogers, N.M.; Stephenson, M.D.; Kitching, A.R.; Horowitz, J.D.; Coates, P.T.H. Amelioration of renal ischaemia-reperfusion injury by liposomal delivery of curcumin to renal tubular epithelial and antigen-presenting cells. Br. J. Pharmacol. 2012, 166, 194–209. [Google Scholar] [CrossRef] [PubMed]
- Tefas, L.R.; Sylvester, B.; Tomuta, I.; Sesarman, A.; Licarete, E.; Banciu, M.; Porfire, A. Development of antiproliferative long-circulating liposomes co-encapsulating doxorubicin and curcumin, through the use of a quality-by-design approach. Drug Des. Devel. Ther. 2017, 11, 1605–1621. [Google Scholar] [CrossRef] [PubMed]
- Sesarman, A.; Tefas, L.; Sylvester, B.; Licarete, E.; Rauca, V.; Luput, L.; Patras, L.; Banciu, M.; Porfire, A. Anti-angiogenic and anti-inflammatory effects of long-circulating liposomes co-encapsulating curcumin and doxorubicin on C26 murine colon cancer cells. Pharmacol. Rep. 2018, 70, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Chen, X.F.; Wang, T.; Wang, Z.; Liao, C.; Wang, Z.; Huang, R.; Wang, D.; Li, X.; Wu, L.; et al. Soluble TREM2 induces inflammatory responses and enhances microglial survival. J. Exp. Med. 2017, 214, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Vetha, B.S.S.; Kim, E.M.; Oh, P.S.; Kim, S.H.; Lim, S.T.; Sohn, M.H.; Jeong, H.J. Curcumin Encapsulated Micellar Nanoplatform for Blue Light Emitting Diode Induced Apoptosis as a New Class of Cancer Therapy. Macromol. Res. 2019, 27, 1179–1184. [Google Scholar] [CrossRef]
- Chen, Y.; Du, Q.; Guo, Q.; Huang, J.; Liu, L.; Shen, X.; Peng, J. A W/O emulsion mediated film dispersion method for curcumin encapsulated pH-sensitive liposomes in the colon tumor treatment. Drug Dev. Ind. Pharm. 2019, 45, 282–291. [Google Scholar] [CrossRef]
- Huang, M.; Liang, C.; Tan, C.; Huang, S.; Ying, R.; Wang, Y.; Wang, Z.; Zhang, Y. Liposome co-encapsulation as a strategy for the delivery of curcumin and resveratrol. Food Funct. 2019, 10, 6447–6458. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Zhang, B.; Li, M.; Diao, K.; Zhang, Z.; Li, J.; Xu, Y.; Wang, X.; Chen, H. In situ injectable nano-composite hydrogel composed of curcumin, N,O-carboxymethyl chitosan and oxidized alginate for wound healing application. Int. J. Pharm. 2012, 437, 110–119. [Google Scholar] [CrossRef]
- Anitha, A.; Sreeranganathan, M.; Chennazhi, K.P.; Lakshmanan, V.K.; Jayakumar, R. In vitro combinatorial anticancer effects of 5-fluorouracil and curcumin loaded N,O-carboxymethyl chitosan nanoparticles toward colon cancer and in vivo pharmacokinetic studies. Eur. J. Pharm. Biopharm. 2014, 88, 238–251. [Google Scholar] [CrossRef]
- Chaurasia, S.; Chaubey, P.; Patel, R.R.; Kumar, N.; Mishra, B. Curcumin-polymeric nanoparticles against colon-26 tumor-bearing mice: Cytotoxicity, pharmacokinetic and anticancer efficacy studies. Drug Dev. Ind. Pharm. 2016, 42, 694–700. [Google Scholar] [CrossRef]
- Xie, M.; Fan, D.; Li, Y.; He, X.; Chen, X.; Chen, Y.; Zhu, J.; Xu, G.; Wu, X.; Lan, P. Supercritical carbon dioxide-developed silk fibroin nanoplatform for smart colon cancer therapy. Int. J. Nanomed. 2017, 12, 7751–7761. [Google Scholar] [CrossRef]
- Nasery, M.M.; Abadi, B.; Poormoghadam, D.; Zarrabi, A.; Keyhanvar, P.; Khanbabaei, H.; Ashrafizadeh, M.; Mohammadinejad, R.; Tavakol, S.; Sethi, G. Curcumin delivery mediated by bio-based nanoparticles: A review. Molecules 2020, 25, 689. [Google Scholar] [CrossRef] [PubMed]
- Sanoj Rejinold, N.; Thomas, R.G.; Muthiah, M.; Chennazhi, K.P.; Manzoor, K.; Park, I.K.; Jeong, Y.Y.; Jayakumar, R. Anti-cancer, pharmacokinetics and tumor localization studies of pH-, RF- and thermo-responsive nanoparticles. Int. J. Biol. Macromol. 2015, 74, 249–262. [Google Scholar] [CrossRef]
- Nambiar, S.; Osei, E.; Fleck, A.; Darko, J.; Mutsaers, A.J.; Wettig, S. Synthesis of curcumin-functionalized gold nanoparticles and cytotoxicity studies in human prostate cancer cell line. Appl. Nanosci. 2018, 8, 347–357. [Google Scholar] [CrossRef]
- Elbialy, N.S.; Abdelfatah, E.A.; Khalil, W.A. Antitumor Activity of Curcumin-Green Synthesized Gold Nanoparticles: In Vitro Study. Bionanoscience 2019, 9, 813–820. [Google Scholar] [CrossRef]
- Yallapu, M.M.; Othman, S.F.; Curtis, E.T.; Bauer, N.A.; Chauhan, N.; Kumar, D.; Jaggi, M.; Chauhan, S.C. Curcumin-loaded magnetic nanoparticles for breast cancer therapeutics and imaging applications. Int. J. Nanomed. 2012, 7, 1761–1779. [Google Scholar]
- Bhandari, R.; Gupta, P.; Dziubla, T.; Hilt, J.Z. Single step synthesis, characterization and applications of curcumin functionalized iron oxide magnetic nanoparticles. Mater. Sci. Eng. C 2016, 67, 59–64. [Google Scholar] [CrossRef]
- Saikia, C.; Das, M.K.; Ramteke, A.; Maji, T.K. Controlled release of curcumin from thiolated starch-coated iron oxide magnetic nanoparticles: An in vitro evaluation. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 349–358. [Google Scholar] [CrossRef]
- Aeineh, N.; Salehi, F.; Akrami, M.; Nemati, F.; Alipour, M.; Ghorbani, M.; Nikfar, B.; Salehian, F.; Riyahi Alam, N.; Sadat Ebrahimi, S.E.; et al. Glutathione conjugated polyethylenimine on the surface of Fe 3 O 4 magnetic nanoparticles as a theranostic agent for targeted and controlled curcumin delivery. J. Biomater. Sci. Polym. Ed. 2018, 29, 1109–1125. [Google Scholar] [CrossRef]
- Ayubi, M.; Karimi, M.; Abdpour, S.; Rostamizadeh, K.; Parsa, M.; Zamani, M.; Saedi, A. Magnetic nanoparticles decorated with PEGylated curcumin as dual targeted drug delivery: Synthesis, toxicity and biocompatibility study. Mater. Sci. Eng. C 2019, 104, 109810. [Google Scholar] [CrossRef] [PubMed]
- VR, Y.; S, S.; K, D.; S, Y. Novel formulation of solid lipid microparticles of curcumin for anti-angiogenic and anti-inflammatory activity for optimization of therapy of inflammatory bowel disease. J. Pharm. Pharmacol. 2009, 61, 311–321. [Google Scholar]
- Kakkar, V.; Singh, S.; Singla, D.; Kaur, I.P. Exploring solid lipid nanoparticles to enhance the oral bioavailability of curcumin. Mol. Nutr. Food Res. 2011, 55, 495–503. [Google Scholar] [CrossRef]
- Kakkar, V.; Muppu, S.K.; Chopra, K.; Kaur, I.P. Curcumin loaded solid lipid nanoparticles: An efficient formulation approach for cerebral ischemic reperfusion injury in rats. Eur. J. Pharm. Biopharm. 2013, 85, 339–345. [Google Scholar] [CrossRef]
- Abd-Ellatef, G.E.F.; Gazzano, E.; Chirio, D.; Hamed, A.R.; Belisario, D.C.; Zuddas, C.; Peira, E.; Rolando, B.; Kopecka, J.; Marie, M.A.S.; et al. Curcumin-loaded solid lipid nanoparticles bypass p-glycoprotein mediated doxorubicin resistance in triple negative breast cancer cells. Pharmaceutics 2020, 12, 96. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, R.; Xie, Q.; Li, A.; Xiao, Y.; Li, K.; Liu, H.; Cui, D.; Chen, Y.; Wang, S. Enhanced bioavailability and efficiency of curcumin for the treatment of asthma by its formulation in solid lipid nanoparticles. Int. J. Nanomed. 2012, 7, 3667–3677. [Google Scholar] [CrossRef]
- Wang, S.; Ha, Y.; Huang, X.; Chin, B.; Sim, W.; Chen, R. A New Strategy for Intestinal Drug Delivery via pH-Responsive and Membrane-Active Nanogels. ACS Appl. Mater. Interfaces 2018, 10, 36622–36627. [Google Scholar] [CrossRef] [PubMed]
- Manju, S.; Sreenivasan, K. Conjugation of curcumin onto hyaluronic acid enhances its aqueous solubility and stability. J. Colloid Interface Sci. 2011, 359, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Brahmkhatri, V.P.; Sharma, N.; Sunanda, P.; D’Souza, A.; Raghothama, S.; Atreya, H.S. Curcumin nanoconjugate inhibits aggregation of N-terminal region (Aβ-16) of an amyloid beta peptide. New J. Chem. 2018, 42, 19881–19892. [Google Scholar] [CrossRef]
- Siddique, Y.H.; Khan, W.; Singh, B.R.; Naqvi, A.H. Synthesis of Alginate-Curcumin Nanocomposite and Its Protective Role in Transgenic Drosophila Model of Parkinson’s Disease. ISRN Pharmacol. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Yallapu, M.M.; Jaggi, M.; Chauhan, S.C. β-Cyclodextrin-curcumin self-assembly enhances curcumin delivery in prostate cancer cells. Colloids Surf. B Biointerfaces 2010, 79, 113–125. [Google Scholar] [CrossRef]
- Yadav, A.; Lomash, V.; Samim, M.; Flora, S.J.S. Curcumin encapsulated in chitosan nanoparticles: A novel strategy for the treatment of arsenic toxicity. Chem. Biol. Interact. 2012, 199, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Dandawate, P.R.; Vyas, A.; Ahmad, A.; Banerjee, S.; Deshpande, J.; Swamy, K.V.; Jamadar, A.; Dumhe-Klaire, A.C.; Padhye, S.; Sarkar, F.H. Inclusion complex of novel curcumin analogue CDF and β-cyclodextrin (1:2) and its enhanced in vivo anticancer activity against pancreatic cancer. Pharm. Res. 2012, 29, 1775–1786. [Google Scholar] [CrossRef]
- Abruzzo, A.; Zuccheri, G.; Belluti, F.; Provenzano, S.; Verardi, L.; Bigucci, F.; Cerchiara, T.; Luppi, B.; Calonghi, N. Chitosan nanoparticles for lipophilic anticancer drug delivery: Development, characterization and in vitro studies on HT29 cancer cells. Colloids Surf. B Biointerfaces 2016, 145, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Ndong Ntoutoume, G.M.A.; Granet, R.; Mbakidi, J.P.; Brégier, F.; Léger, D.Y.; Fidanzi-Dugas, C.; Lequart, V.; Joly, N.; Liagre, B.; Chaleix, V.; et al. Development of curcumin-cyclodextrin/cellulose nanocrystals complexes: New anticancer drug delivery systems. Bioorganic Med. Chem. Lett. 2016, 26, 941–945. [Google Scholar] [CrossRef] [PubMed]
- Maria, D.N.; Mishra, S.R.; Wang, L.; Abd-Elgawad, A.-E.H.; Soliman, O.A.-E.; El-Dahan, M.S.; Jablonski, M.M. Water-soluble Complex of Curcumin with Cyclodextrins: Enhanced Physical Properties For Ocular Drug Delivery. Curr. Drug Deliv. 2016, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Sun, L.; Wu, Q.; Guo, W.; Li, L.; Chen, Y.; Li, Y.; Gong, C.; Qian, Z.; Wei, Y. Curcumin loaded polymeric micelles inhibit breast tumor growth and spontaneous pulmonary metastasis. Int. J. Pharm. 2013, 443, 175–182. [Google Scholar] [CrossRef]
- Teixeira, C.C.C.; Mendonça, L.M.; Bergamaschi, M.M.; Queiroz, R.H.C.; Souza, G.E.P.; Antunes, L.M.G.; Freitas, L.A.P. Microparticles Containing Curcumin Solid Dispersion: Stability, Bioavailability and Anti-Inflammatory Activity. AAPS Pharm. Sci. Tech. 2016, 17, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Silva de Sá, I.; Peron, A.P.; Leimann, F.V.; Bressan, G.N.; Krum, B.N.; Fachinetto, R.; Pinela, J.; Calhelha, R.C.; Barreiro, M.F.; Ferreira, I.C.F.R.; et al. In vitro and in vivo evaluation of enzymatic and antioxidant activity, cytotoxicity and genotoxicity of curcumin-loaded solid dispersions. Food Chem. Toxicol. 2019, 125, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhuang, B.; Du, G.; Han, G.; Jin, Y. Curcumin solid dispersion-loaded in situ hydrogels for local treatment of injured vaginal bacterial infection and improvement of vaginal wound healing. J. Pharm. Pharmacol. 2019, 71, 1044–1054. [Google Scholar] [CrossRef]
- Adhikary, R.; Carlson, P.J.; Kee, T.W.; Petrich, J.W. Excited-state intramolecular hydrogen atom transfer of curcumin in surfactant micelles. J. Phys. Chem. B 2010, 114, 2997–3004. [Google Scholar] [CrossRef]
- Podaralla, S.; Averineni, R.; Alqahtani, M.; Perumal, O. Synthesis of novel biodegradable methoxy poly(ethylene glycol)-zein micelles for effective delivery of curcumin. Mol. Pharm. 2012, 9, 2778–2786. [Google Scholar] [CrossRef]
- Raveendran, R.; Bhuvaneshwar, G.S.; Sharma, C.P. In vitro cytotoxicity and cellular uptake of curcumin-loaded Pluronic/Polycaprolactone micelles in colorectal adenocarcinoma cells. J. Biomater. Appl. 2013, 27, 811–827. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zou, Y.; Zhang, M.; Zhao, N.; Tian, Q.; Gu, M.; Liu, W.; Shi, R.; Lü, Y.; Yu, W. Mitochondrial Sirt3 Expression is Decreased in APP/PS1 Double Transgenic Mouse Model of Alzheimer’s Disease. Neurochem. Res. 2015, 40, 1576–1582. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.; Trench, D.; Putnam, J.; Stenzel, M.H.; Lord, M.S. Curcumin-Loading-Dependent Stability of PEGMEMA-Based Micelles Affects Endocytosis and Exocytosis in Colon Carcinoma Cells. Mol. Pharm. 2016, 13, 924–932. [Google Scholar] [CrossRef]
- Javadi, S.; Rostamizadeh, K.; Hejazi, J.; Parsa, M.; Fathi, M. Curcumin mediated down-regulation of αVβ3 integrin and up-regulation of pyruvate dehydrogenase kinase 4 (PDK4) in Erlotinib resistant SW480 colon cancer cells. Phyther. Res. 2018, 32, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, Q.; Li, H.; Yang, L.; Yi, J.Z.; Xie, M.; Zhang, L.M. Long-circulating zein-polysulfobetaine conjugate-based nanocarriers for enhancing the stability and pharmacokinetics of curcumin. Mater. Sci. Eng. C 2020, 109, 110636. [Google Scholar] [CrossRef]
- Arunraj, T.R.; Rejinold, N.S.; Mangalathillam, S.; Saroj, S.; Biswas, R.; Jayakumar, R. Synthesis, characterization and biological activities of curcumin nanospheres. J. Biomed. Nanotechnol. 2014, 10, 238–250. [Google Scholar] [CrossRef]
- Liang, H.; Friedman, J.M.; Nacharaju, P. Fabrication of biodegradable PEG–PLA nanospheres for solubility, stabilization, and delivery of curcumin. Artif. Cells Nanomed. Biotechnol. 2017, 45, 297–304. [Google Scholar] [CrossRef]
- Huo, X.; Zhang, Y.; Jin, X.; Li, Y.; Zhang, L. A novel synthesis of selenium nanoparticles encapsulated PLGA nanospheres with curcumin molecules for the inhibition of amyloid β aggregation in Alzheimer’s disease. J. Photochem. Photobiol. B Biol. 2019, 190, 98–102. [Google Scholar] [CrossRef]
- Farkhonde Masoule, S.; Pourhajibagher, M.; Safari, J.; Khoobi, M. Base-free green synthesis of copper(II) oxide nanoparticles using highly cross-linked poly(curcumin) nanospheres: Synergistically improved antimicrobial activity. Res. Chem. Intermed. 2019, 45, 4449–4462. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Lee, Y.-M.; Kim, D.-W.; Min, T.; Lee, S.-J. Nanosphere Loaded With Curcumin Inhibits the Gastrointestinal Cell Death Signaling Pathway Induced by the Foodborne Pathogen Vibrio vulnificus. Cells 2020, 9, 631. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Senanayake, T.H.; Warren, G.; Vinogradov, S. V. Hyaluronic acid-based nanogel-drug conjugates with enhanced anticancer activity designed for the targeting of cd44-positive and drug-resistant tumors. Bioconjug. Chem. 2013, 24, 658–668. [Google Scholar] [CrossRef]
- Miron, V.E.; Boyd, A.; Zhao, J.W.; Yuen, T.J.; Ruckh, J.M.; Shadrach, J.L.; Van Wijngaarden, P.; Wagers, A.J.; Williams, A.; Franklin, R.J.M.; et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat. Neurosci. 2013, 16, 1211–1218. [Google Scholar] [CrossRef]
- Amanlou, N.; Parsa, M.; Rostamizadeh, K.; Sadighian, S.; Moghaddam, F. Enhanced cytotoxic activity of curcumin on cancer cell lines by incorporating into gold/chitosan nanogels. Mater. Chem. Phys. 2019, 226, 151–157. [Google Scholar] [CrossRef]
- Priya, P.; Mohan Raj, R.; Vasanthakumar, V.; Raj, V. Curcumin-loaded layer-by-layer folic acid and casein coated carboxymethyl cellulose/casein nanogels for treatment of skin cancer. Arab. J. Chem. 2020, 13, 694–708. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, R.X.; Zhang, C.; Dai, C.; Ju, X.; He, R. Fabrication of Stable and Self-Assembling Rapeseed Protein Nanogel for Hydrophobic Curcumin Delivery. J. Agric. Food Chem. 2019, 67, 887–894. [Google Scholar] [CrossRef]
- Subramani, P.A.; Panati, K.; Narala, V.R. Curcumin Nanotechnologies and Its Anticancer Activity. Nutr. Cancer 2017, 69, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Singh, A.T.K.; Xu, W.; Sulchek, T.; Gordon, L.I.; Ryan, R.O. Curcumin nanodisks: Formulation and characterization. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 162–167. [Google Scholar] [CrossRef]
- Singh, A.T.K.; Ghosh, M.; Forte, T.M.; Ryan, R.O.; Gordon, L.I. Curcumin nanodisk-induced apoptosis in mantle cell lymphoma. Leuk. Lymphoma 2011, 52, 1537–1543. [Google Scholar] [CrossRef] [PubMed]
- Faraji, A.H.; Wipf, P. Nanoparticles in cellular drug delivery. Bioorg. Med. Chem. 2009, 17, 2950–2962. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Wu, M.; Li, H. Antitumor activities of novel glycyrrhetinic acid-modified curcumin-loaded cationic liposomes in vitro and in H22 tumor-bearing mice. Drug Deliv. 2018, 25, 1984–1995. [Google Scholar] [CrossRef]
- Dhule, S.S.; Penfornis, P.; Frazier, T.; Walker, R.; Feldman, J.; Tan, G.; He, J.; Alb, A.; John, V.; Pochampally, R. Curcumin-loaded γ-cyclodextrin liposomal nanoparticles as delivery vehicles for osteosarcoma. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Wei, Y.; Lee, R.J.; Zhao, L. Liposomal curcumin and its application in cancer. Int. J. Nanomed. 2017, 12, 6027–6044. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ahmed, B.; Mehta, K.; Kurzrock, R. Liposomal curcumin with and without oxaliplatin: Effects on cell growth, apoptosis, and angiogenesis in colorectal cancer. Mol. Cancer Ther. 2007, 6, 1276–1282. [Google Scholar] [CrossRef]
- Biswas, A.K.; Islam, M.R.; Choudhury, Z.S.; Mostafa, A.; Kadir, M.F. Nanotechnology based approaches in cancer therapeutics. Adv. Nat. Sci. Nanosci. Nanotechnol. 2014, 5, 043001. [Google Scholar] [CrossRef]
- Rudramurthy, G.R.; Swamy, M.K.; Sinniah, U.R.; Ghasemzadeh, A. Nanoparticles: Alternatives against drug-resistant pathogenic microbes. Molecules 2016, 21, 836. [Google Scholar] [CrossRef]
- Ferrari, R.; Sponchioni, M.; Morbidelli, M.; Moscatelli, D. Polymer nanoparticles for the intravenous delivery of anticancer drugs: The checkpoints on the road from the synthesis to clinical translation. Nanoscale 2018, 10, 22701–22719. [Google Scholar] [CrossRef]
- Shome, S.; Talukdar, A. Das; Choudhury, M.D.; Bhattacharya, M.K.; Upadhyaya, H. Curcumin as potential therapeutic natural product: A nanobiotechnological perspective. J. Pharm. Pharmacol. 2016, 68, 1481–1500. [Google Scholar] [CrossRef]
- Chang, P.Y.; Peng, S.F.; Lee, C.Y.; Lu, C.C.; Tsai, S.C.; Shieh, T.M.; Wu, T.S.; Tu, M.G.; Chen, M.Y.; Yang, J.S. Curcumin-loaded nanoparticles induce apoptotic cell death through regulation of the function of MDR1 and reactive oxygen species in cisplatin-resistant CAR human oral cancer cells. Int. J. Oncol. 2013, 43, 1141–1150. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.; Haggag, Y.A.; Lane, M.E.; McCarron, P.A.; Tambuwala, M.M. Polymeric Nano-Encapsulation of Curcumin Enhances its Anti-Cancer Activity in Breast (MDA-MB231) and Lung (A549) Cancer Cells Through Reduction in Expression of HIF-1α and Nuclear p65 (Rel A). Curr. Drug Deliv. 2018, 15, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, H.; Rompicharla, S.V.K.; Komanduri, N.; Aashma, S.; Paradkar, S.; Ghosh, B.; Biswas, S. Development of Curcumin-Loaded Solid Lipid Nanoparticles Utilizing Glyceryl Monostearate as Single Lipid Using QbD Approach: Characterization and Evaluation of Anticancer Activity Against Human Breast Cancer Cell Line. Curr. Drug Deliv. 2018, 15, 1271–1283. [Google Scholar] [CrossRef] [PubMed]
- Nahar, P.P.; Slitt, A.L.; Seeram, N.P. Anti-Inflammatory Effects of Novel Standardized Solid Lipid Curcumin Formulations. J. Med. Food 2015, 18, 786–792. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, Y.; Hu, J. Curcumin Inhibits Imiquimod-Induced Psoriasis-Like Inflammation by Inhibiting IL-1beta and IL-6 Production in Mice. PLoS ONE 2013, 8, e67078. [Google Scholar] [CrossRef]
- Wang, W.; Chen, T.; Xu, H.; Ren, B.; Cheng, X.; Qi, R.; Liu, H.; Wang, Y.; Yan, L.; Chen, S.; et al. Curcumin-loaded solid lipid nanoparticles enhanced anticancer efficiency in breast cancer. Molecules 2018, 23, 1578. [Google Scholar] [CrossRef]
- Roacho-Pérez, J.A.; Ruiz-Hernandez, F.G.; Chapa-Gonzalez, C.; Martínez-Rodríguez, H.G.; Flores-Urquizo, I.A.; Pedroza-Montoya, F.E.; Garza-Treviño, E.N.; Bautista-Villarea, M.; García-Casillas, P.E.; Sánchez-Domínguez, C.N. Magnetite nanoparticles coated with PEG 3350-Tween 80: In vitro characterization using primary cell cultures. Polymers 2020, 12, 300. [Google Scholar] [CrossRef]
- Yallapu, M.M.; Ebeling, M.C.; Khan, S.; Sundram, V.; Chauhan, N.; Gupta, B.K.; Puumala, S.E.; Jaggi, M.; Chauhan, S.C. Novel curcumin-loaded magnetic nanoparticles for pancreatic cancer treatment. Mol. Cancer Ther. 2013, 12, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Jiang, H.H.; Youn, Y.S.; Park, C.W.; Tak, K.K.; Lee, S.; Kim, H.; Jon, S.; Chen, X.; Lee, K.C. Preparation and characterization of water-soluble albumin-bound curcumin nanoparticles with improved antitumor activity. Int. J. Pharm. 2011, 403, 285–291. [Google Scholar] [CrossRef]
- Thadakapally, R.; Aafreen, A.; Aukunuru, J.; Habibuddin, M.; Jogala, S. Preparation and characterization of PEG-albumin-curcumin nanoparticles intended to treat breast cancer. Indian J. Pharm. Sci. 2016, 78, 65–72. [Google Scholar]
- Kim, B.; Lee, C.; Lee, E.S.; Shin, B.S.; Youn, Y.S. Paclitaxel and curcumin co-bound albumin nanoparticles having antitumor potential to pancreatic cancer. Asian J. Pharm. Sci. 2016, 11, 708–714. [Google Scholar] [CrossRef]
- Li, W.; Liu, R.; Pei, Q.; Shou, T.; Zhang, W.; Hu, J. Apoptotic effect of green synthesized gold nanoparticles from curcuma wenyujin extract against human renal cell carcinoma a498 cells. Int. J. Nanomed. 2019, 14, 4091–4103. [Google Scholar] [CrossRef] [PubMed]
- Kondath, S.; Rajaram, R.; Anantanarayanan, R. Curcumin reduced gold nanoparticles synergistically induces ROS mediated apoptosis in MCF-7 cancer cells. Inorg. Nano-Metal Chem. 2021, 1–13. [Google Scholar] [CrossRef]
- Muangnoi, C.; Jithavech, P.; Na Bhuket, P.R.; Supasena, W.; Wichitnithad, W.; Towiwat, P.; Niwattisaiwong, N.; Haworth, I.S.; Rojsitthisak, P. A curcumin-diglutaric acid conjugated prodrug with improved water solubility and antinociceptive properties compared to curcumin. Biosci. Biotechnol. Biochem. 2018, 82, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Meghwal, M.; Goswami, T.K. Piper nigrum and piperine: An update. Phyther. Res. 2013, 27, 1121–1130. [Google Scholar] [CrossRef]
- Srinivasan, K. Black pepper and its pungent principle-piperine: A review of diverse physiological effects. Crit. Rev. Food Sci. Nutr. 2007, 47, 735–748. [Google Scholar] [CrossRef] [PubMed]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S.S.R. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef]
- Tang, H.; Murphy, C.J.; Zhang, B.; Shen, Y.; Van Kirk, E.A.; Murdoch, W.J.; Radosz, M. Curcumin polymers as anticancer conjugates. Biomaterials 2010, 31, 7139–7149. [Google Scholar] [CrossRef]
- Szejtli, J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 1998, 98, 1743–1753. [Google Scholar] [CrossRef]
- Santos, A.C.; Costa, D.; Ferreira, L.; Guerra, C.; Pereira-Silva, M.; Pereira, I.; Peixoto, D.; Ferreira, N.R.; Veiga, F. Cyclodextrin-based delivery systems for in vivo-tested anticancer therapies. Drug Deliv. Transl. Res. 2020, 11, 49–71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Man, S.; Qiu, H.; Liu, Z.; Zhang, M.; Ma, L.; Gao, W. Curcumin-cyclodextrin complexes enhanced the anti-cancer effects of curcumin. Environ. Toxicol. Pharmacol. 2016, 48, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Flora, G.; Gupta, D.; Tiwari, A. Nanocurcumin: A promising therapeutic advancement over native curcumin. Crit. Rev. Ther. Drug Carrier Syst. 2013, 30, 331–368. [Google Scholar] [CrossRef]
- Karthikeyan, A.; Senthil, N.; Min, T. Nanocurcumin: A Promising Candidate for Therapeutic Applications. Front. Pharmacol. 2020, 11, 1. [Google Scholar] [CrossRef]
- Song, I.-S.; Cha, J.-S.; Choi, M.-K. Characterization, in Vivo and in Vitro Evaluation of Solid Dispersion of Curcumin Containing d-α-Tocopheryl Polyethylene Glycol 1000 Succinate and Mannitol. Molecules 2016, 21, 1386. [Google Scholar] [CrossRef]
- Kaushik, D.; Kumar, A.; Kaushik, P.; Rana, A.C. Analgesic and Anti-Inflammatory Activity of Pinus roxburghii Sarg. Adv. Pharmacol. Sci. 2012, 2012, 1–6. [Google Scholar]
- Gong, C.; Deng, S.; Wu, Q.; Xiang, M.; Wei, X.; Li, L.; Gao, X.; Wang, B.; Sun, L.; Chen, Y.; et al. Improving antiangiogenesis and anti-tumor activity of curcumin by biodegradable polymeric micelles. Biomaterials 2013, 34, 1413–1432. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, T.; Xu, L.; Zhang, Z.; Li, L.; Chen, H. Preparation of curcumin micelles and the in vitro and in vivo evaluation for cancer therapy. J. Biomed. Nanotechnol. 2014, 10, 1458–1468. [Google Scholar] [CrossRef] [PubMed]
- Verderio, P.; Pandolfi, L.; Mazzucchelli, S.; Marinozzi, M.R.; Vanna, R.; Gramatica, F.; Corsi, F.; Colombo, M.; Morasso, C.; Prosperi, D. Antiproliferative effect of ASC-J9 delivered by PLGA nanoparticles against estrogen-dependent breast cancer cells. Mol. Pharm. 2014, 11, 2864–2875. [Google Scholar] [CrossRef]
- Mukerjee, A.; Vishwanatha, J.K. Formulation, Characterization and Evaluation of Curcumin-loaded PLGA Nanospheres for Cancer Therapy. Anticancer Res. 2009, 29, 3867–3875. [Google Scholar] [PubMed]
- Kim, S.; Diab, R.; Joubert, O.; Canilho, N.; Pasc, A. Core-shell microcapsules of solid lipid nanoparticles and mesoporous silica for enhanced oral delivery of curcumin. Colloids Surf. B Biointerfaces 2016, 140, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Mai, Z.; Chen, J.; He, T.; Hu, Y.; Dong, X.; Zhang, H.; Huang, W.; Ko, F.; Zhou, W. Electrospray biodegradable microcapsules loaded with curcumin for drug delivery systems with high bioactivity. RSC Adv. 2017, 7, 1724–1734. [Google Scholar] [CrossRef]
- Reeves, A.; Vinogradov, S. V.; Morrissey, P.; Chernin, M.; Ahmed, M.M. Curcumin-encapsulating nanogels as an effective anticancer formulation for intracellular uptake. Mol. Cell. Pharmacol. 2015, 7, 25–40. [Google Scholar]
- Dandekar, P.P.; Jain, R.; Patil, S.; Dhumal, R.; Tiwari, D.; Sharma, S.; Vanage, G.; Patravale, V. Curcumin-loaded hydrogel nanoparticles: Application in anti-malarial therapy and toxicological evaluation. J. Pharm. Sci. 2010, 99, 4992–5010. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Ryan, R.O. ApoE enhances nanodisk-mediated curcumin delivery to glioblastoma multiforme cells. Nanomedicine 2014, 9, 763–771. [Google Scholar] [CrossRef]
- Mangalathillam, S.; Rejinold, N.S.; Nair, A.; Lakshmanan, V.K.; Nair, S.V.; Jayakumar, R. Curcumin loaded chitin nanogels for skin cancer treatment via the transdermal route. Nanoscale 2012, 4, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gu, Z.; Zhang, C.; Li, S.; Zhang, L.; Zhou, G.; Wang, S.; Zhang, J. Synthesis, characterization and ROS-mediated antitumor effects of palladium(II) complexes of curcuminoids. Eur. J. Med. Chem. 2018, 144, 662–671. [Google Scholar] [CrossRef]
- Vellampatti, S.; Chandrasekaran, G.; Mitta, S.B.; Lakshmanan, V.K.; Park, S.H. Metallo-Curcumin-Conjugated DNA Complexes Induces Preferential Prostate Cancer Cells Cytotoxicity and Pause Growth of Bacterial Cells. Sci. Rep. 2018, 8, 14929. [Google Scholar] [CrossRef] [PubMed]
- Bayet-Robert, M.; Kwiatkowski, F.; Leheurteur, M.; Gachon, F.; Planchat, E.; Abrial, C.; Mouret-Reynier, M.A.; Durando, X.; Barthomeuf, C.; Chollet, P. Phase I dose escalation trial of docetaxel plus curcumin in patients with advanced and metastatic breast cancer. Cancer Biol. Ther. 2010, 9, 9. [Google Scholar] [CrossRef]
- Ledda, A.; Belcaro, G.; Dugall, M.; Luzzi, R.; Scoccianti, M.; de Togni, S.; Appendino, G.; Ciammaichella, G. Meriva®, a lecithinized curcumin delivery system, in the control of benign prostatic hyperplasia: A pilot, product evaluation registry study. Panminerva Med. 2012, 54, 17–22. [Google Scholar] [PubMed]
- Plummer, S.M.; Hill, K.A.; Festing, M.F.W.; Steward, W.P.; Gescher, A.J.; Sharma, R.A. Clinical Development of Leukocyte Cyclooxygenase 2 Activity as a Systemic Biomarker for Cancer Chemopreventive Agents. Cancer Epidemiol. Biomarkers Prev. 2001, 10, 1295–1299. [Google Scholar] [PubMed]
- Sharma, R.A.; Euden, S.A.; Platton, S.L.; Cooke, D.N.; Shafayat, A.; Hewitt, H.R.; Marczylo, T.H.; Morgan, B.; Hemingway, D.; Plummer, S.M.; et al. Phase I clinical trial of oral curcumin: Biomarkers of systemic activity and compliance. Clin. Cancer Res. 2004, 10, 6847–6854. [Google Scholar] [CrossRef] [PubMed]
- Garcea, G.; Berry, D.P.; Jones, D.J.L.; Singh, R.; Dennison, A.R.; Farmer, P.B.; Sharma, R.A.; Steward, W.P.; Gescher, A.J. Consumption of the Putative Chemopreventive Agent Curcumin by Cancer Patients: Assessment of Curcumin Levels in the Colorectum and their Pharmacodynamic Consequences. Cancer Epidemiol. Biomarkers Prev. 2005, 14, 120–125. [Google Scholar]
- He, Z.Y.; Shi, C.B.; Wen, H.; Li, F.L.; Wang, B.L.; Wang, J. Upregulation of p53 expression in patients with colorectal cancer by administration of curcumin. Cancer Invest. 2011, 29, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Carroll, R.E.; Benya, R. V.; Turgeon, D.K.; Vareed, S.; Neuman, M.; Rodriguez, L.; Kakarala, M.; Carpenter, P.M.; McLaren, C.; Meyskens, F.L.; et al. Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prev. Res. 2011, 4, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Irving, G.R.B.; Howells, L.M.; Sale, S.; Kralj-Hans, I.; Atkin, W.S.; Clark, S.K.; Britton, R.G.; Jones, D.J.L.; Scott, E.N.; Berry, D.P.; et al. Prolonged biologically active colonic tissue levels of curcumin achieved after oral administration-a clinical pilot study including assessment of patient acceptability. Cancer Prev. Res. 2013, 6, 119–128. [Google Scholar] [CrossRef]
- Ghalaut, V.S.; Sangwan, L.; Dahiya, K.; Ghalaut, P.S.; Dhankhar, R.; Saharan, R. Effect of imatinib therapy with and without turmeric powder on nitric oxide levels in chronic myeloid leukemia. J. Oncol. Pharm. Pract. 2012, 18, 186–190. [Google Scholar] [CrossRef]
- Cruz-Correa, M.; Hylind, L.M.; Marrero, J.H.; Zahurak, M.L.; Murray-Stewart, T.; Casero, R.A.; Montgomery, E.A.; Iacobuzio-Donahue, C.; Brosens, L.A.; Offerhaus, G.J.; et al. Efficacy and Safety of Curcumin in Treatment of Intestinal Adenomas in Patients With Familial Adenomatous Polyposis. Gastroenterology 2018, 155, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Veena, M.S.; Basak, S.K.; Han, E.; Tajima, T.; Gjertson, D.W.; Starr, J.; Eidelman, O.; Pollard, H.B.; Srivastava, M.; et al. Curcumin treatment suppresses IKKβ kinase activity of salivary cells of patients with head and neck cancer: A pilot study. Clin. Cancer Res. 2011, 17, 5953–5961. [Google Scholar] [CrossRef]
- Panahi, Y.; Saadat, A.; Beiraghdar, F.; Sahebkar, A. Adjuvant therapy with bioavailability-boosted curcuminoids suppresses systemic inflammation and improves quality of life in patients with solid tumors: A randomized double-blind placebo-controlled trial. Phyther. Res. 2014, 28, 1461–1467. [Google Scholar] [CrossRef]
- Ide, H.; Tokiwa, S.; Sakamaki, K.; Nishio, K.; Isotani, S.; Muto, S.; Hama, T.; Masuda, H.; Horie, S. Combined inhibitory effects of soy isoflavones and curcumin on the production of prostate-specific antigen. Prostate 2010, 70, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Hejazi, J.; Rastmanesh, R.; Taleban, F.A.; Molana, S.H.; Hejazi, E.; Ehtejab, G.; Hara, N. Effect of curcumin supplementation during radiotherapy on oxidative status of patients with prostate cancer: A double blinded, randomized, placebo-controlled study. Nutr. Cancer 2016, 68, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Epelbaum, R.; Schaffer, M.; Vizel, B.; Badmaev, V.; Bar-Sela, G. Curcumin and gemcitabine in patients with advanced pancreatic cancer. Nutr. Cancer 2010, 62, 1137–1141. [Google Scholar] [CrossRef] [PubMed]
- Kanai, M.; Yoshimura, K.; Asada, M.; Imaizumi, A.; Suzuki, C.; Matsumoto, S.; Nishimura, T.; Mori, Y.; Masui, T.; Kawaguchi, Y.; et al. A phase I/II study of gemcitabine-based chemotherapy plus curcumin for patients with gemcitabine-resistant pancreatic cancer. Cancer Chemother. Pharmacol. 2011, 68, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Kanai, M.; Otsuka, Y.; Otsuka, K.; Sato, M.; Nishimura, T.; Mori, Y.; Kawaguchi, M.; Hatano, E.; Kodama, Y.; Matsumoto, S.; et al. A phase i study investigating the safety and pharmacokinetics of highly bioavailable curcumin (Theracurmin®) in cancer patients. Cancer Chemother. Pharmacol. 2013, 71, 1521–1530. [Google Scholar] [CrossRef]
- Krishnaswamy, K.; Goud, V.K.; Sesikeran, B.; Mukundan, M.A.; Krishna, T.P. Retardation of experimental tumorigenesis and reduction in DNA adducts by turmeric and curcumin. Nutr. Cancer 1998, 30, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Chen, X.; Liao, J.; Yang, G.; Wang, S.; Josephson, Y.; Han, C.; Chen, J.; Huang, M.T.; Yang, C.S. Inhibition of 7,12-dimethylbenz[a]anthracene (DMBA)-induced oral carcinogenesis in hamsters by tea and curcumin. Carcinogenesis 2002, 23, 1307–1313. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, S.; Balakrishnan, S.; Menon, V.P.; Alias, L.M.; Reena, A.R. Chemopreventive efficacy of curcumin and piperine during 7,12-dimethylbenz[a]anthracene-induced hamster buccal pouch carcinogenesis. Singapore Med. J. 2009, 50, 139–146. [Google Scholar] [PubMed]
- Rai, B.; Kaur, J.; Jacobs, R.; Singh, J. Possible action mechanism for curcumin in pre-cancerous lesions based on serum and salivary markers of oxidative stress. J. Oral Sci. 2010, 52, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, A.L.; Morse, M.A.; Fields, H.W.; Rothas, D.A.; Pei, P.; Rodrigo, K.A.; Renner, R.J.; Mallery, S.R. Curcumin activates the aryl hydrocarbon receptor yet significantly inhibits (-)-benzo(a)pyrene-7R-trans-7,8-dihydrodiol bioactivation in oral squamous cell carcinoma cells and oral mucosa. Cancer Res. 2002, 62, 5451–5456. [Google Scholar] [PubMed]
- Chakravarti, N.; Kadara, H.; Yoon, D.J.; Shay, J.W.; Myers, J.N.; Lotan, D.; Sonenberg, N.; Lotan, R. Differential inhibition of protein translation machinery by curcumin in normal, immortalized, and malignant oral epithelial cells. Cancer Prev. Res. 2010, 3, 331–338. [Google Scholar] [CrossRef]
- Lee, S.S.; Tsai, C.H.; Ho, Y.C.; Chang, Y.C. The upregulation of heat shock protein 70 expression in areca quid chewing-associated oral squamous cell carcinomas. Oral Oncol. 2008, 44, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.W.; Hung, P.S.; Lin, I.Y.; Hou, C.P.; Chen, L.K.; Tsai, Y.M.; Lin, S.C. Curcumin upregulates insulin-like growth factor binding protein-5 (IGFBP-5) and C/EBPα during oral cancer suppression. Int. J. Cancer 2010, 127, 9–20. [Google Scholar] [CrossRef]
- Shin, H.K.; Kim, J.; Lee, E.J.; Kim, S.H. Inhibitory effect of curcumin on motility of human oral squamous carcinoma YD-10B cells via suppression of ERK and NF-κB activations. Phyther. Res. 2010, 24, 577–582. [Google Scholar] [CrossRef]
- Sharma, C.; Kaur, J.; Shishodia, S.; Aggarwal, B.B.; Ralhan, R. Curcumin down regulates smokeless tobacco-induced NF-κB activation and COX-2 expression in human oral premalignant and cancer cells. Toxicology 2006, 228, 1–15. [Google Scholar] [CrossRef]
- Atsumi, T.; Tonosaki, K.; Fujisawa, S. Induction of early apoptosis and ROS-generation activity in human gingival fibroblasts (HGF) and human submandibular gland carcinoma (HSG) cells treated with curcumin. Arch. Oral Biol. 2006, 51, 913–921. [Google Scholar] [CrossRef]
- Atsumi, T.; Fujisawa, S.; Tonosaki, K. Relationship between intracellular ROS production and membrane mobility in curcumin- and tetrahydrocurcumin-treated human gingival fibroblasts and human submandibular gland carcinoma cells. Oral Dis. 2005, 11, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Atsumi, T.; Murakami, Y.; Shibuya, K.; Tonosaki, K.; Fujisawa, S. Induction of cytotoxicity and apoptosis and inhibition of cyclooxygenase-2 gene expression, by curcumin and its analog, α-diisoeugenol. Anticancer Res. 2005, 25, 4029–4036. [Google Scholar] [PubMed]
- Fujisawa, S.; Atsumi, T.; Ishihara, M.; Kadoma, Y. Cytotoxicity, ROS-generation activity and radical-scavenging activity of curcumin and related compounds. Anticancer Res. 2004, 24, 563–569. [Google Scholar] [PubMed]
- Rajasekaran, S.A. Therapeutic potential of curcumin in gastrointestinal diseases. World J. Gastrointest. Pathophysiol. 2011, 2, 1. [Google Scholar] [CrossRef]
- Hartojo, W.; Silvers, A.L.; Thomas, D.G.; Seder, C.W.; Lin, L.; Rao, H.; Wang, Z.; Greenson, J.K.; Giordano, T.J.; Orringer, M.B.; et al. Curcumin promotes apoptosis, increases chemosensitivity, and inhibits nuclear factor κB in esophageal adenocarcinoma. Transl. Oncol. 2010, 3, 99–108. [Google Scholar] [CrossRef]
- O’Sullivan-Coyne, G.; O’Sullivan, G.C.; O’Donovan, T.R.; Piwocka, K.; McKenna, S.L. Curcumin induces apoptosis-independent death in oesophageal cancer cells. Br. J. Cancer 2009, 101, 1585–1595. [Google Scholar] [CrossRef]
- Yu, L.; Wu, W.K.K.; Li, Z.J.; Wong, H.P.S.; Tai, E.K.K.; Li, H.T.; Wu, Y.C.; Cho, C.H. E series of prostaglandin receptor 2-mediated activation of extracellular signal-regulated kinase/activator protein-1 signaling is required for the mitogenic action of prostaglandin E2 in esophageal squamous-cell carcinoma. J. Pharmacol. Exp. Ther. 2008, 327, 258–267. [Google Scholar] [CrossRef]
- Ushida, J.; Sugie, S.; Kawabata, K.; Pham, Q.V.; Tanaka, T.; Fujii, K.; Takeuchi, H.; Ito, Y.; Mori, H. Chemopreventive effect of curcumin on N-Nitrosomethylbenzylamine-induced esophageal carcinogenesis in rats. Jpn. J. Cancer Res. 2000, 91, 893–898. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Marusawa, H.; Kinoshita, K.; Endo, Y.; Kou, T.; Morisawa, T.; Azuma, T.; Okazaki, I.M.; Honjo, T.; Chiba, T. Helicobacter pylori infection triggers aberrant expression of activation-induced cytidine deaminase in gastric epithelium. Nat. Med. 2007, 13, 470–476. [Google Scholar] [CrossRef]
- Zaidi, S.F.H.; Yamamoto, T.; Refaat, A.; Ahmed, K.; Sakurai, H.; Saiki, I.; Kondo, T.; Usmanghani, K.; Kadowaki, M.; Sugiyama, T. Modulation of activation-induced cytidine deaminase by curcumin in helicobacter pylori-infected gastric epithelial cells. Helicobacter 2009, 14, 588–595. [Google Scholar] [CrossRef]
- De, R.; Kundu, P.; Swarnakar, S.; Ramamurthy, T.; Chowdhury, A.; Nair, G.B.; Mukhopadhyay, A.K. Antimicrobial activity of curcumin against helicobacter pylori isolates from India and during infections in mice. Antimicrob. Agents Chemother. 2009, 53, 1592–1597. [Google Scholar] [CrossRef]
- Mahady, G.B.; Pendland, S.L.; Yun, G.; Lu, Z.Z. Turmeric (Curcuma longa) and curcumin inhibit the growth of Helicobacter pylori, a group 1 carcinogen. Anticancer Res. 2002, 22, 4179–4181. [Google Scholar]
- Münzenmaier, A.; Lange, C.; Glocker, E.; Covacci, A.; Moran, A.; Bereswill, S.; Baeuerle, P.A.; Kist, M.; Pahl, H.L. A secreted/shed product of Helicobacter pylori activates transcription factor nuclear factor-kappa B. J. Immunol. 1997, 159, 159. [Google Scholar]
- Liu, C.A.; Wang, M.J.; Chi, C.W.; Wu, C.W.; Chen, J.Y. Rho/Rhotekin-mediated NF-κB activation confers resistance to apoptosis. Oncogene 2004, 23, 8731–8742. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.Y.; Kim, H.J.; Jung, K.O.; Park, K.Y. Curcumin inhibits the growth of AGS human gastric carcinoma cells in vitro and shows synergism with 5-fluorouracil. J. Med. Food 2004, 7, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.Z.; Wang, J.; Li, X.D.; Wang, G.L.; Liu, F.N.; Cheng, M.S.; Li, F. Curcumin suppresses proliferation and invasion in human gastric cancer cells by downregulation of PAK1 activity and cyclin D1 expression. Cancer Biol. Ther. 2009, 8, 1360–1368. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.Q.; Bi, H.; Feng, J.Q.; Cao, J.G. Effect of curcumin on multidrug resistance in resistant human gastric carcinoma cell line SGC7901/VCR. Acta Pharmacol. Sin. 2005, 26, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.V.; Hu, X.; Srivastava, S.K.; Singh, M.; Xia, H.; Orchard, J.L.; Zaren, H.A. Mechanism of inhibition of benzo[a]pyrene-induced forestomach cancer in mice by dietary curcumin. Carcinogenesis 1998, 19, 1357–1360. [Google Scholar] [CrossRef] [PubMed]
- Azuine, M.A.; Bhide, S.V. Adjuvant chemoprevention of experimental cancer: Catechin and dietary turmeric in forestomach and oral cancer models. J. Ethnopharmacol. 1994, 44, 211–217. [Google Scholar] [CrossRef]
- Deshpande, S.S.; Ingle, A.D.; Maru, G.B. Inhibitory effects of curcumin-free aqueous turmeric extract on benzo[a]pyrene-induced forestomach papillomas in mice. Cancer Lett. 1997, 118, 79–85. [Google Scholar] [CrossRef]
- Huang, M.-T.; Lou, Y.-R.; Ma, W.; Newmark, H.L.; Reuhl, K.R.; Conney, A.H. Inhibitory Effects of Dietary Curcumin on Forestomach, Duodenal, and Colon Carcinogenesis in Mice. Cancer Res. 1994, 54, 5841–5847. [Google Scholar] [PubMed]
- Ikezaki, S.; Nishikawa, A.; Furukawa, F.; Kudo, K.; Nakamura, H.; Tamura, K.; Mori, H. Chemopreventive effects of curcumin on glandular stomach carcinogenesis induced by N-methyl-N’-nitro-N-nitrosoguanidine and sodium chloride in rats - PubMed. Anticancer Res. 2001, 21, 3407–3411. [Google Scholar] [PubMed]
- Azuine, M.A.; Bhide, S.V. Chemopreventive Effect of Turmeric Against Stomach and Skin Tumors Induced by Chemical Carcinogens in Swiss Mice. Nutr. Cancer 1992, 17, 77–83. [Google Scholar] [CrossRef]
- Azuine, M.A.; Kayal, J.J.; Bhide, S.V. Protective role of aqueous turmeric extract against mutagenicity of direct-acting carcinogens as well as Benzo[a]pyrene-induced genotoxicity and carcinogenicity. J. Cancer Res. Clin. Oncol. 1992, 118, 447–452. [Google Scholar] [CrossRef]
- Chen, A.L.; Hsu, C.H.; Lin, J.K.; Hsu, M.M.; Ho, Y.F.; She, T.S.; Ko, J.Y.; Lin, J.T.; Lin, B.R.; Wu, M.S.; et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001, 21, 2895–2900. [Google Scholar]
- Hsu, C.H.; Cheng, A.L. Clinical studies with curcumin. Adv. Exp. Med. Biol. 2007, 595, 471–480. [Google Scholar]
- Johnson, J.J.; Mukhtar, H. Curcumin for chemoprevention of colon cancer. Cancer Lett. 2007, 255, 170–181. [Google Scholar] [CrossRef]
- Sharma, R.A.; McLelland, H.R.; Hill, K.A.; Euden, S.A.; Steward, W.P.; Gescher, A.J.; Ireson, C.R.; Manson, M.M.; Pirmohamed, M.; Marnett, L.J. Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin. Cancer Res. 2001, 7, 1894–1900. [Google Scholar]
- Sharma, R.A.; Steward, W.P.; Gescher, A.J. Pharmacokinetics and pharmacodynamics of curcumin. Adv. Exp. Med. Biol. 2007, 595, 453–470. [Google Scholar] [PubMed]
- Devasena, T.; Rajasekaran, K.N.; Gunasekaran, G.; Viswanathan, P.; Menon, V.P. Anticarcinogenic effect of bis-1,7-(2-hydroxyphenyl)-hepta-1,6-diene-3,5-dione a curcumin analog on DMH-induced colon cancer model. Pharmacol. Res. 2003, 47, 133–140. [Google Scholar] [CrossRef]
- Kawamori, T.; Lubet, R.; Steele, V.E.; Kelloff, G.J.; Kaskey, R.B.; Rao, C.V; Reddy, B.S. Chemopreventive Effect of Curcumin, a Naturally Occurring Anti-Inflammatory Agent, during the Promotion/Progression Stages of Colon Cancer. Cancer Res. 1999, 59, 597–601. [Google Scholar]
- Pereira, M.A.; Grubbs, C.J.; Barnes, L.H.; Li, H.; Olson, G.R.; Eto, I.; Juliana, M.; Whitaker, L.M.; Kelloff, G.J.; Steele, V.E.; et al. Effects of the phytochemicals, curcumin and quercetin, upon azoxymethane-induced colon cancer and 7, 12-dimethylbenz[a]anthracene-induced mammary cancer in rats. Carcinogenesis 1996, 17, 1305–1311. [Google Scholar] [CrossRef]
- Rao, C.V; Rivenson, A.; Simi, B.; Reddy, B.S. Chemoprevention of Colon Carcinogenesis by Dietary Curcumin, a Naturally Occurring Plant Phenolic Compound. Cancer Res. 1995, 55, 259–266. [Google Scholar]
- Volate, S.R.; Davenport, D.M.; Muga, S.J.; Wargovich, M.J. Modulation of aberrant crypt foci and apoptosis by dietary herbal supplements (quercetin, curcumin, silymarin, ginseng and rutin). Carcinogenesis 2005, 26, 1450–1456. [Google Scholar] [CrossRef]
- Shpitz, B.; Giladi, N.; Sagiv, E.; Lev-Ari, S.; Liberman, E.; Kazanov, D.; Arber, N. Celecoxib and curcumin additively inhibit the growth of colorectal cancer in a rat model. Digestion 2007, 74, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Wijnands, M.V.W.; Van Erk, M.J.; Doornbos, R.P.; Krul, C.A.M.; Woutersen, R.A. Do aberrant crypt foci have predictive value for the occurrence of colorectal tumours? Potential of gene expression profiling in tumours. Food Chem. Toxicol. 2004, 42, 1629–1639. [Google Scholar] [CrossRef]
- Milacic, V.; Banerjee, S.; Landis-Piwowar, K.R.; Sarkar, F.H.; Majumdar, A.P.N.; Dou, Q.P. Curcumin inhibits the proteasome activity in human colon cancer cells in vitro and in vivo. Cancer Res. 2008, 68, 7283–7292. [Google Scholar] [CrossRef]
- Cen, L.; Hutzen, B.; Ball, S.; DeAngelis, S.; Chen, C.L.; Fuchs, J.R.; Li, C.; Li, P.K.; Lin, J. New structural analogues of curcumin exhibit potent growth suppressive activity in human colorectal carcinoma cells. BMC Cancer 2009, 9, 99. [Google Scholar] [CrossRef]
- Shibata, H.; Yamakoshi, H.; Sato, A.; Ohori, H.; Kakudo, Y.; Kudo, C.; Takahashi, Y.; Watanabe, M.; Takano, H.; Ishioka, C.; et al. Newly synthesized curcumin analog has improved potential to prevent colorectal carcinogenesis in vivo. Cancer Sci. 2009, 100, 956–960. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.B.; Majumdar, A.P.N. Synergistic role of curcumin with current therapeutics in colorectal cancer: Minireview. Nutr. Cancer 2009, 61, 842–846. [Google Scholar] [CrossRef] [PubMed]
- Nautiyal, J.; Banerjee, S.; Kanwar, S.S.; Yu, Y.; Patel, B.B.; Sarkar, F.H.; Majumdar, A.P.N. Curcumin enhances dasatinib-induced inhibition of growth and transformation of colon cancer cells. Int. J. Cancer 2011, 128, 951–961. [Google Scholar] [CrossRef]
- Majumdar, A.P.N.; Banerjee, S.; Nautiyal, J.; Patel, B.B.; Patel, V.; Du, J.; Yu, Y.; Elliott, A.A.; Levi, E.; Sarkar, F.H. Curcumin synergizes with resveratrol to inhibit colon cancer. Nutr. Cancer 2009, 61, 544–553. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Diagaradjane, P.; Anand, P.; Kuzhuvelil, H.B.; Deorukhkar, A.; Gelovani, J.; Guha, S.; Krishnan, S.; Aggarwal, B.B. Curcumin sensitizes human colorectal cancer to capecitabine by modulation of cyclin D1, COX-2, MMP-9, VEGF and CXCR4 expression in an orthotopic mouse model. Int. J. Cancer 2009, 125, 2187–2197. [Google Scholar] [CrossRef]
- Patel, B.B.; Sengupta, R.; Qazi, S.; Vachhani, H.; Yu, Y.; Rishi, A.K.; Majumdar, A.P.N. Curcumin enhances the effects of 5-fluorouracil and oxaliplatin in mediating growth inhibition of colon cancer cells by modulating EGFR and IGF-1R. Int. J. Cancer 2008, 122, 267–273. [Google Scholar] [CrossRef]
- Howells, L.M.; Mitra, A.; Manson, M.M. Comparison of oxaliplatin- and curcumin-mediated antiproliferative effects in colorectal cell lines. Int. J. Cancer 2007, 121, 175–183. [Google Scholar] [CrossRef]
- Du, B.; Jiang, L.; Xia, Q.; Zhong, L. Synergistic inhibitory effects of curcumin and 5-fluorouracil on the growth of the human colon cancer cell line HT-29. Chemotherapy 2005, 52, 23–28. [Google Scholar] [CrossRef]
- Giladi, N.; Kazanov, D.; Shpitz, B.; Aroch, I.; Kraus, S.; Arber, N. Curcumin potentiates the pro-apoptotic effects of sulindac sulfone in colorectal cancer. Expert Opin. Investig. Drugs 2010, 19, S117–S124. [Google Scholar]
- Binion, D.G.; Otterson, M.F.; Rafiee, P. Curcumin inhibits VEGF-mediated angiogenesis in human intestinal microvascular endothelial cells through COX-2 and MAPK inhibition. Gut 2008, 57, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Diagaradjane, P.; Guha, S.; Deorukhkar, A.; Shentu, S.; Aggarwal, B.B.; Krishnan, S. Curcumin sensitizes human colorectal cancer xenografts in nude mice to γ-radiation by targeting nuclear factor-κB-regulated gene products. Clin. Cancer Res. 2008, 14, 2128–2136. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, P.; Binion, D.G.; Wellner, M.; Behmaram, B.; Floer, M.; Mitton, E.; Nie, L.; Zhang, Z.; Otterson, M.F. Modulatory effect of curcumin on survival of irradiated human intestinal microvascular endothelial cells: Role of Akt/mTOR and NF-κB. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298. [Google Scholar] [CrossRef]
- Sandur, S.K.; Deorukhkar, A.; Pandey, M.K.; Pabón, A.M.; Shentu, S.; Guha, S.; Aggarwal, B.B.; Krishnan, S. Curcumin Modulates the Radiosensitivity of Colorectal Cancer Cells by Suppressing Constitutive and Inducible NF-κB Activity. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Su, C.C.; Chen, G.W.; Lin, J.G.; Wu, L.T.; Chung, J.G. Curcumin inhibits cell migration of human colon cancer colo 205 cells through the inhibition of nuclear factor kappa B /p65 and down-regulates cyclooxygenase-2 and matrix metalloproteinase-2 expressions. Anticancer Res. 2006, 26, 1281–1288. [Google Scholar]
- Shim, J.S.; Lee, J.; Park, H.J.; Park, S.J.; Kwon, H.J. A new curcumin derivative, HBC, interferes with the cell cycle progression of colon cancer cells via antagonization of the Ca2+/calmodulin function. Chem. Biol. 2004, 11, 1455–1463. [Google Scholar] [CrossRef]
- Collett, G.P.; Campbell, F.C. Curcumin induces c-jun N-terminal kinase-dependent apoptosis in HCT116 human colon cancer cells. Carcinogenesis 2004, 25, 2183–2189. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.M.; Gulhati, P.; Arrieta, I.; Wang, X.; Uchida, T.; Gao, T.; Evers, B.M. Curcumin inhibits proliferation of colorectal carcinoma by modulating Akt/mTOR signaling. Anticancer Res. 2009, 29, 3185–3190. [Google Scholar]
- Patel, B.B.; Gupta, D.; Elliott, A.A.; Sengupta, V.; Yu, Y.; Majumdar, A.P.N. Curcumin targets FOLFOX-surviving colon cancer cells via inhibition of EGFRs and IGF-1R. Anticancer Res. 2010, 30, 319–325. [Google Scholar]
- Jaiswal, A.S.; Marlow, B.P.; Gupta, N.; Narayan, S. β-catenin-mediated transactivation and cell - cell adhesion pathways are important in curcumin (diferuylmethane)-induced growth arrest and apoptosis in colon cancer cells. Oncogene 2002, 21, 8414–8427. [Google Scholar] [CrossRef] [PubMed]
- Narayan, S. Curcumin, a multi-functional chemopreventive agent, blocks growth of colon cancer cells by targeting β-catenin-mediated transactivation and cell-cell adhesion pathways. J. Mol. Histol. 2004, 35, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Ryu, M.J.; Cho, M.; Song, J.Y.; Yun, Y.S.; Choi, I.W.; Kim, D.E.; Park, B.S.; Oh, S. Natural derivatives of curcumin attenuate the Wnt/β-catenin pathway through down-regulation of the transcriptional coactivator p300. Biochem. Biophys. Res. Commun. 2008, 377, 1304–1308. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Q.; Ives, K.L.; Evers, B.M. Curcumin inhibits neurotensin-mediated interleukin-8 production and migration of HCT116 human colon cancer cells. Clin. Cancer Res. 2006, 12, 5346–5355. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Correa, M.; Shoskes, D.A.; Sanchez, P.; Zhao, R.; Hylind, L.M.; Wexner, S.D.; Giardiello, F.M. Combination Treatment With Curcumin and Quercetin of Adenomas in Familial Adenomatous Polyposis. Clin. Gastroenterol. Hepatol. 2006, 4, 1035–1038. [Google Scholar] [CrossRef] [PubMed]
- Perkins, S.; Verschoyle, R.D.; Hill, K.; Sharma, R.A.; Williams, M.L.; Steward, W.P.; Gescher, A.J.; Parveen, I.; Threadgill, M.D. Chemopreventive efficacy and pharmacokinetics of curcumin in the min/+ mouse, a model of familial adenomatous polyposis. Cancer Epidemiol. Biomarkers Prev. 2002, 11, 535–540. [Google Scholar]
- Tunstall, R.G.; Sharma, R.A.; Perkins, S.; Sale, S.; Singh, R.; Farmer, P.B.; Steward, W.P.; Gescher, A.J. Cyclooxygenase-2 expression and oxidative DNA adducts in murine intestinal adenomas: Modification by dietary curcumin and implications for clinical trials. Eur. J. Cancer 2006, 42, 415–421. [Google Scholar] [CrossRef]
- Gao, X.Q.; Yang, C.X.; Chen, G.J.; Wang, G.Y.; Chen, B.; Tan, S.K.; Liu, J.; Yuan, Q.L. Ginsenoside Rb1 regulates the expressions of brain-derived neurotrophic factor and caspase-3 and induces neurogenesis in rats with experimental cerebral ischemia. J. Ethnopharmacol. 2010, 132, 393–399. [Google Scholar] [CrossRef]
- Wang, W.Z.; Cheng, J.; Luo, J.; Zhuang, S.M. Abrogation of G2/M arrest sensitizes curcumin-resistant hepatoma cells to apoptosis. FEBS Lett. 2008, 582, 2689–2695. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Jia, L.; Zhou, H.M.; Liu, Y.; Zhong, L.F. Mitochondrial and nuclear DNA damage induced by curcumin in human hepatoma G2 cells. Toxicol. Sci. 2006, 91, 476–483. [Google Scholar] [CrossRef]
- Cao, J.; Liu, Y.; Jia, L.; Zhou, H.M.; Kong, Y.; Yang, G.; Jiang, L.P.; Li, Q.J.; Zhong, L.F. Curcumin induces apoptosis through mitochondrial hyperpolarization and mtDNA damage in human hepatoma G2 cells. Free Radic. Biol. Med. 2007, 43, 968–975. [Google Scholar] [CrossRef]
- Jiang, M.C.; Yang-Yen, H.F.; Lin, J.K.; Yen, J.J.Y. Differential regulation of p53, c-Myc, Bcl-2 and Bax protein expression during apoptosis induced by widely divergent stimuli in human hepatoblastoma cells. Oncogene 1996, 13, 609–616. [Google Scholar]
- Bae, M.K.; Kim, S.H.; Jeong, J.W.; Lee, Y.M.; Kim, H.S.; Kim, S.R.; Yun, I.; Bae, S.K.; Kim, K.W. Curcumin inhibits hypoxia-induced angiogenesis via down-regulation of HIF-1. Oncol. Rep. 2006, 15, 1557–1562. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Wang, H.; Qu, S.; Miao, X.; Zhang, J. CD147 regulates vascular endothelial growth factor - A expression, tumorigenicity, and chemosensitivity to curcumin in hepatocellular carcinoma. IUBMB Life 2008, 60, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Seol, D.W.; Chen, Q.; Zarnegar, R. Transcriptional activation of the Hepatocyte Growth Factor receptor (c-met) gene by its ligand (Hepatocyte Growth Factor) is mediated through AP-1. Oncogene 2000, 19, 1132–1137. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.I.; Ke, Y.F.; Ko, Y.C.; Lin, J.K. Curcumin inhibits SK-Hep-1 hepatocellular carcinoma cell invasion in vitro and suppresses matrix metalloproteinase-9 secretion. Oncology 1998, 55, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Notarbartolo, M.; Poma, P.; Perri, D.; Dusonchet, L.; Cervello, M.; D’Alessandro, N. Antitumor effects of curcumin, alone or in combination with cisplatin or doxorubicin, on human hepatic cancer cells. Analysis of their possible relationship to changes in NF-kB activation levels and in IAP gene expression. Cancer Lett. 2005, 224, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.C.; Lin, S.Y.; Su, C.C.; Lin, S.S.; Ho, C.C.; Hsia, T.C.; Chiu, T.H.; Yu, C.S.; Ip, S.W.; Lin, T.P.; et al. Effects of curcumin on N-bis(2-hydroxypropyl) nitrosamine (DHPN)-induced lung and liver tumorigenesis in BALB/c mice in vivo. In Vivo (Brooklyn) 2008, 22, 781–786. [Google Scholar]
- Chuang, S.E.; Kuo, M.L.; Hsu, C.H.; Chen, C.R.; Lin, J.K.; Lai, G.M.; Hsieh, C.Y.; Cheng, A.L. Curcumin-containing diet inhibits diethylnitrosamine-induced murine hepatocarcinogenesis. Carcinogenesis 2000, 21, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Yoysungnoen, P.; Wirachwong, P.; Bhattarakosol, P.; Niimi, H.; Patumraj, S. Antiangiogenic activity of curcumin in hepatocellular carcinoma cells implanted nude mice. Clin. Hemorheol. Microcirc. 2005, 33, 127–135. [Google Scholar] [PubMed]
- Yoysungnoen, P.; Wirachwong, P.; Bhattarakosol, P.; Niimi, H.; Patumraj, S. Effects of curcumin on tumor angiogenesis and biomarkers, COX-2 and VEGF, in hepatocellular carcinoma cell-implanted nude mice. Clin. Hemorheol. Microcirc. 2006, 34, 109–115. [Google Scholar] [PubMed]
- Yoysungnoen, P.; Wirachwong, P.; Changtam, C.; Suksamram, A.; Patumraj, S. Anti-cancer and anti-angiogenic effects of curcumin and tetrahydrocurcumin on implanted hepatocellular carcinoma in nude mice. World J. Gastroenterol. 2008, 14, 2003–2009. [Google Scholar] [CrossRef]
- Ohashi, Y.; Tsuchiya, Y.; Koizumi, K.; Sakurai, H.; Saiki, I. Prevention of intrahepatic metastasis by curcumin in an orthotopic implantation model. Oncology 2003, 65, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Piper, J.T.; Singhal, S.S.; Salameh, M.S.; Torman, R.T.; Awasthi, Y.C.; Awasthi, S. Mechanisms of anticarcinogenic properties of curcumin: The effect of curcumin on glutathione linked detoxification enzymes in rat liver. Int. J. Biochem. Cell Biol. 1998, 30, 445–456. [Google Scholar] [CrossRef]
- Sreepriya, M.; Bali, G. Chemopreventive effects of embelin and curcumin against N-nitrosodiethylamine/phenobarbital-induced hepatocarcinogenesis in Wistar rats. Fitoterapia 2005, 76, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Sreepriya, M.; Bali, G. Effects of administration of Embelin and Curcumin on lipid peroxidation, hepatic glutathione antioxidant defense and hematopoietic system during N-nitrosodiethylamine/Phenobarbital-induced hepatocarcinogenesis in Wistar rats. Mol. Cell. Biochem. 2006, 284, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Bisht, S.; Mizuma, M.; Feldmann, G.; Ottenhof, N.A.; Hong, S.M.; Pramanik, D.; Chenna, V.; Karikari, C.; Sharma, R.; Goggins, M.G.; et al. Systemic administration of polymeric nanoparticle-encapsulated curcumin (NanoCurc) blocks tumor growth and metastases in preclinical models of pancreatic cancer. Mol. Cancer Ther. 2010, 9, 2255–2264. [Google Scholar] [CrossRef]
- Swamy, M.V.; Citineni, B.; Patlolla, J.M.R.; Mohammed, A.; Zhang, Y.; Rao, C.V. Prevention and treatment of pancreatic cancer by curcumin in combination with omega-3 fatty acids. Nutr. Cancer 2008, 60, 81–89. [Google Scholar] [CrossRef]
- Lev-Ari, S.; Zinger, H.; Kazanov, D.; Yona, D.; Ben-Yosef, R.; Starr, A.; Figer, A.; Arber, N. Curcumin synergistically potentiates the growth inhibitory and pro-apoptotic effects of celecoxib in pancreatic adenocarcinoma cells. Biomed. Pharmacother. 2005, 59, S276–S280. [Google Scholar] [CrossRef]
- Holcomb, B.; Yip-Schneider, M.T.; Matos, J.M.; Dixon, J.; Kennard, J.; Mahomed, J.; Shanmugam, R.; Sebolt-Leopold, J.; Schmidt, C.M. Pancreatic cancer cell genetics and signaling response to treatment correlate with efficacy of gemcitabine-based molecular targeting strategies. J. Gastrointest. Surg. 2008, 12, 288–296. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Guha, S.; Krishnan, S.; Diagaradjane, P.; Gelovani, J.; Aggarwal, B.B. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-κB-regulated gene products. Cancer Res. 2007, 67, 3853–3861. [Google Scholar] [CrossRef]
- Lev-Ari, S.; Vexler, A.; Starr, A.; Ashkenazy-Voghera, M.; Greif, J.; Aderka, D.; Ben-Yosef, R. Curcumin augments gemcitabine cytotoxic effect on pancreatic adenocarcinoma cell lines. Cancer Invest. 2007, 25, 411–418. [Google Scholar] [CrossRef]
- Ramachandran, C.; Resek, A.P.; Escalon, E.; Aviram, A.; Melnick, S.J. Ramachandran Potentiation of gemcitabine by Turmeric Force in pancreatic cancer cell lines. Oncol. Rep. 2010, 23, 1529–1535. [Google Scholar] [CrossRef][Green Version]
- Mach, C.M.; Mathew, L.; Mosley, S.A.; Kurzrock, R.; Smith, J.A. Determination of minimum effective dose and optimal dosing schedule for liposomal curcumin in a xenograft human pancreatic cancer model. Anticancer Res. 2009, 29, 1895–1899. [Google Scholar] [PubMed]
- Li, L.; Braiteh, F.S.; Kurzrock, R. Liposome-encapsulated curcumin: In vitro and in vivo effects on proliferation, apoptosis, signaling, and angiogenesis. Cancer 2005, 104, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, C.; Acharya, S.; Mohanty, A.K.; Dilnawaz, F.; Sahoo, S.K. Curcumin-encapsulated MePEG/PCL diblock copolymeric micelles: A novel controlled delivery vehicle for cancer therapy. Nanomedicine 2010, 5, 433–449. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Y.; Yang, C.; Wang, P.; Oelschlager, D.K.; Zheng, Y.; Tian, D.A.; Grizzle, W.E.; Buchsbaum, D.J.; Wan, M. Polyethylene glycosylated curcumin conjugate inhibits pancreatic cancer cell growth through inactivation of Jab1. Mol. Pharmacol. 2009, 76, 81–90. [Google Scholar] [CrossRef]
- Jutooru, I.; Chadalapaka, G.; Lei, P.; Safe, S. Inhibition of NFκB and pancreatic cancer cell and tumor growth by curcumin is dependent on specificity protein down-regulation. J. Biol. Chem. 2010, 285, 25332–25344. [Google Scholar] [CrossRef]
- Khanbolooki, S.; Nawrocki, S.T.; Arumugam, T.; Andtbacka, R.; Pino, M.S.; Kurzrock, R.; Logsdon, C.D.; Abbruzzese, J.L.; McConkey, D.J. Nuclear factor-κB maintains TRAIL resistance in human pancreatic cancer cells. Mol. Cancer Ther. 2006, 5, 2251–2260. [Google Scholar] [CrossRef]
- Li, L.; Aggarwal, B.B.; Shishodia, S.; Abbruzzese, J.; Kurzrock, R. Nuclear factor-κB and IκB are constitutively active in human pancreatic cells, and their down-regulation by curcumin (Diferuloylmethane) is associated with the suppression of proliferation and the induction of apoptosis. Cancer 2004, 101, 2351–2362. [Google Scholar] [CrossRef]
- Sun, M.; Estrov, Z.; Ji, Y.; Coombes, K.R.; Harris, D.H.; Kurzrock, R. Curcumin (diferuloylmethane) alters the expression profiles of microRNAs in human pancreatic cancer cells. Mol. Cancer Ther. 2008, 7, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Glienke, W.; Maute, L.; Wicht, J.; Bergmann, L. Wilms’ tumour gene 1 (WT1) as a target in curcumin treatment of pancreatic cancer cells. Eur. J. Cancer 2009, 45, 874–880. [Google Scholar] [CrossRef]
- Sahu, R.P.; Batra, S.; Srivastava, S.K. Activation of ATM/Chk1 by curcumin causes cell cycle arrest and apoptosis in human pancreatic cancer cells. Br. J. Cancer 2009, 100, 1425–1433. [Google Scholar] [CrossRef]
- Glienke, W.; Maute, L.; Wicht, J.; Bergmann, L. Curcumin inhibits constitutive STAT3 phosphorylation in human pancreatic cancer cell lines and downregulation of Survivin/BIRC5 gene expression. Cancer Invest. 2010, 28, 166–171. [Google Scholar] [CrossRef]
- Hidaka, H.; Ishiko, T.; Furuhashi, T.; Kamohara, H.; Suzuki, S.; Miyazaki, M.; Ikeda, O.; Mita, S.; Setoguchi, T.; Ogawa, M. Curcumin inhibits interleukin 8 production and enhances interleukin 8 receptor expression on the cell surface: Impact on human pancreatic carcinoma cell growth by autocrine regulation. Cancer 2002, 95, 1206–1214. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, Y.; Banerjee, S.; Li, Y.; Sarkar, F.H. Notch-1 down-regulation by curcumin is associated with the inhibition of cell growth and the induction of apoptosis in pancreatic cancer cells. Cancer 2006, 106, 2503–2513. [Google Scholar] [CrossRef]
- Lev-Ari, S.; Starr, A.; Vexler, A.; Karaush, V.; Loew, V.; Greif, J.; Fenig, E.; Aderka, D.; Ben-Yosef, R. Inhibition of pancreatic and lung adenocarcinoma cell survival by curcumin is associated with increased apoptosis, down-regulation of COX-2 and EGFR and inhibition of Erk1/2 activity. Anticancer Res. 2006, 26, 4423–4430. [Google Scholar] [PubMed]
- Lin Curcumin analogue GO-Y030 inhibits STAT3 activity and cell growth in breast and pancreatic carcinomas. Int. J. Oncol. 2009, 35, 35.
- Friedman, L.; Lin, L.; Ball, S.; Bekaii-Saab, T.; Fuchs, J.; Li, P.K.; Li, C.; Lin, J. Curcumin analogues exhibit enhanced growth suppressive activity in human pancreatic cancer cells. Anticancer Drugs 2009, 20, 444–449. [Google Scholar] [CrossRef]
- Padhye, S.; Banerjee, S.; Chavan, D.; Pandye, S.; Swamy, K.V.; Ali, S.; Li, J.; Dou, Q.P.; Sarkar, F.H. Fluorocurcumins as cyclooxygenase-2 inhibitor: Molecular docking, pharmacokinetics and tissue distribution in mice. Pharm. Res. 2009, 26, 2438–2445. [Google Scholar] [CrossRef] [PubMed]
- Grossman, S.A.; Batara, J.F. Current management of glioblastoma multiforme. Semin. Oncol. 2004, 31, 635–644. [Google Scholar] [CrossRef]
- Klinger, N. V; Mittal, S. Therapeutic Potential of Curcumin for the Treatment of Brain Tumors. Oxid. Med. Cell. Longev. 2016, 2016, 9324085. [Google Scholar] [CrossRef]
- Chintala, S.K.; Tonn, J.C.; Rao, J.S. Matrix metalloproteinases and their biological function in human gliomas. Int. J. Dev. Neurosci. 1999, 17, 495–502. [Google Scholar] [CrossRef]
- Perry, M.C.; Demeule, M.; Régina, A.; Moumdjian, R.; Béliveau, R. Curcumin inhibits tumor growth and angiogenesis in glioblastoma xenografts. Mol. Nutr. Food Res. 2010, 54, 1192–1201. [Google Scholar] [CrossRef]
- Wu, B.; Yao, H.; Wang, S.; Xu, R. DAPK1 modulates a curcumin-induced G2/M arrest and apoptosis by regulating STAT3, NF-κB, and caspase-3 activation. Biochem. Biophys. Res. Commun. 2013, 434, 75–80. [Google Scholar] [CrossRef]
- Houssami, N.; MacAskill, P.; Marinovich, M.L.; Dixon, J.M.; Irwig, L.; Brennan, M.E.; Solin, L.J. Meta-analysis of the impact of surgical margins on local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy. Eur. J. Cancer 2010, 46, 3219–3232. [Google Scholar] [CrossRef]
- Carrato, A. Adjuvant treatment of colorectal cancer. Gastrointest. Cancer Res. 2008, 2, S42–S46. [Google Scholar]
- Mudduluru, G.; George-William, J.N.; Muppala, S.; Asangani, I.A.; Kumarswamy, R.; Nelson, L.D.; Allgayer, H. Curcumin regulates miR-21 expression and inhibits invasion and metastasis in colorectal cancer. Biosci. Rep. 2011, 31, 185–197. [Google Scholar] [CrossRef]
- Xu, H.; Yu, Y.; Marciniak, D.; Rishi, A.K.; Sarkar, F.H.; Kucuk, O.; Majumdar, A.P.N. Epidermal growth factor receptor (EGFR)-related protein inhibits multiple members of the EGFR family in colon and breast cancer cells. Mol. Cancer Ther. 2005, 4, 435–442. [Google Scholar] [CrossRef]
- Cheng, M.A.; Chou, F.J.; Wang, K.; Yang, R.; Ding, J.; Zhang, Q.; Li, G.; Yeh, S.; Chang, C.; Xu, D. Androgen receptor (AR) degradation enhancer ASC-J9® in an FDA-approved formulated solution suppresses castration resistant prostate cancer cell growth. Cancer Lett. 2018, 417, 182–191. [Google Scholar] [CrossRef]
- Ahmed, A.; Ali, S.; Sarkar, F.H. Advances in androgen receptor targeted therapy for prostate cancer. J. Cell. Physiol. 2014, 229, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Dorai, T.; Cao, Y.C.; Dorai, B.; Buttyan, R.; Katz, A.E. Therapeutic potential of curcumin in human prostate cancer. III. Curcumin inhibits proliferation, induces apoptosis, and inhibits angiogenesis of LNCaP prostate cancer cells in vivo. Prostate 2001, 47, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, A.; Bueso-Ramos, C.; Chatterjee, D.; Pantazis, P.; Aggarwal, B.B. Curcumin downregulates cell survival mechanisms in human prostate cancer cell lines. Oncogene 2001, 20, 7597–7609. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F. Targeting multiple signaling pathways as a strategy for managing prostate cancer: Multifocal signal modulation therapy. Integr. Cancer Ther. 2004, 3, 349–380. [Google Scholar] [CrossRef]
- Sundram, V.; Chauhan, S.C.; Jaggi, M. Emerging roles of protein kinase D1 in cancer. Mol. Cancer Res. 2011, 9, 985–996. [Google Scholar] [CrossRef] [PubMed]
- LaValle, C.R.; George, K.M.; Sharlow, E.R.; Lazo, J.S.; Wipf, P.; Wang, Q.J. Protein kinase D as a potential new target for cancer therapy. Biochim. Biophys. Acta Rev. Cancer 2010, 1806, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Lai, K.P.; Chuang, K.L.; Xu, D.; Miyamoto, H.; Tochigi, T.; Pang, S.T.; Li, L.; Arai, Y.; Kung, H.J.; et al. ASC-J9 suppresses castration-resistant prostate cancer growth through degradation of full-length and splice variant androgen receptors. Neoplasia 2012, 14, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Shih, C.; Lee, K. Novel Anti-Prostate Cancer Curcumin Analogues That Enhance Androgen Receptor Degradation Activity. Anticancer. Agents Med. Chem. 2009, 9, 904–912. [Google Scholar] [CrossRef]
- Zand, A.M.; Imani, S.; Mojtaba, S.; Borna, H.; Ziaei, R.; Honari, H. Effect of Age, Gender and Blood Group on Blood Cancer Types. Kowsar Med. J. 2010, 15, 111–114. [Google Scholar]
- Rafiq, S.; Raza, M.H.; Younas, M.; Naeem, F.; Adeeb, R.; Iqbal, J.; Anwar, P.; Sajid, U.; Manzoor, H.M. Molecular Targets of Curcumin and Future Therapeutic Role in Leukemia. J. Biosci. Med. 2018, 06, 33–50. [Google Scholar] [CrossRef]
- Koohi, F.; Salehiniya, H.; Shamlou, R.; Eslami, S.; Ghojogh, Z.M.; Kor, Y.; Rafiemanesh, H. Leukemia in Iran: Epidemiology and morphology trends. Asian Pacific J. Cancer Prev. 2015, 16, 7759–7763. [Google Scholar] [CrossRef] [PubMed]
- Chaitanya, G.V.; Alexander, J.S.; Babu, P.P. PARP-1 cleavage fragments: Signatures of cell-death proteases in neurodegeneration. Cell Commun. Signal. 2010, 8, 31. [Google Scholar] [CrossRef]
- Green, A.R.; Caracappa, D.; Benhasouna, A.A.; Alshareeda, A.; Nolan, C.C.; Macmillan, R.D.; Madhusudan, S.; Ellis, I.O.; Rakha, E.A. Biological and clinical significance of PARP1 protein expression in breast cancer. Breast Cancer Res. Treat. 2015, 149, 353–362. [Google Scholar] [CrossRef]
- Mishra, D.; Singh, S.; Narayan, G. Curcumin Induces Apoptosis in Pre-B Acute Lymphoblastic Leukemia Cell Lines Via PARP-1 Cleavage. Asian Pacific J. Cancer Prev. 2016, 17, 3865–3869. [Google Scholar]
- Sharma, V.; Jha, A.K.; Kumar, A.; Bhatnagar, A.; Narayan, G.; Kaur, J. Curcumin-mediated reversal of p15 gene promoter methylation: Implication in anti-neoplastic action against acute lymphoid leukaemia cell line. Folia Biol. (Czech Republic) 2015, 61, 80–89. [Google Scholar]
- Druker, B.J.; Sawyers, C.L.; Kantarjian, H.; Resta, D.J.; Reese, S.F.; Ford, J.M.; Capdeville, R.; Talpaz, M. Activity of a Specific Inhibitor of the BCR-ABL Tyrosine Kinase in the Blast Crisis of Chronic Myeloid Leukemia and Acute Lymphoblastic Leukemia with the Philadelphia Chromosome. N. Engl. J. Med. 2001, 344, 1038–1042. [Google Scholar] [CrossRef]
- Ottmann, O.G.; Druker, B.J.; Sawyers, C.L.; Goldman, J.M.; Reiffers, J.; Silver, R.T.; Tura, S.; Fischer, T.; Deininger, M.W.; Schiffer, C.A.; et al. A phase 2 study of imatinib in patients with relapsed or refractory Philadelphia chromosome-positive acute lymphoid leukemias. Blood 2002, 100, 1965–1971. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, Y.; Shan, Q.; He, G.; Lin, J.; Gong, Y. Curcumin potentiates the anti-leukemia effects of imatinib by downregulation of the AKT/mTOR pathway and BCR/ABL gene expression in Ph+ acute lymphoblastic leukemia. Int. J. Biochem. Cell Biol. 2015, 65, 1–11. [Google Scholar] [CrossRef]
- Yu, J.; Peng, Y.; Wu, L.C.; Xie, Z.; Deng, Y.; Hughes, T.; He, S.; Mo, X.K.; Chiu, M.; Wang, Q.E.; et al. Curcumin Down-Regulates DNA Methyltransferase 1 and Plays an Anti-Leukemic Role in Acute Myeloid Leukemia. PLoS ONE 2013, 8, e55934. [Google Scholar] [CrossRef] [PubMed]
- Toyota, M.; Kopecky, K.J.; Toyota, M.O.; Jair, K.W.; Willman, C.L.; Issa, J.P.J. Methylation profiling in acute myeloid leukemia. Blood 2001, 97, 2823–2829. [Google Scholar] [CrossRef] [PubMed]
- Hallek, M. Chronic lymphocytic leukemia: 2013 update on diagnosis, risk stratification and treatment. Am. J. Hematol. 2013, 88, 803–816. [Google Scholar] [CrossRef] [PubMed]
- Stephens, J.M.; Gramegna, P.; Laskin, B.; Botteman, M.F.; Pashos, C.L. Chronic lymphocytic leukemia: Economic burden and quality of life: Literature review. Am. J. Ther. 2005, 12, 460–466. [Google Scholar] [CrossRef]
- Golombick, T.; Diamond, T.H.; Manoharan, A.; Ramakrishna, R. B-Cell Disorders and Curcumin. Integr. Cancer Ther. 2017, 16, 255–257. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Kay, N.E.; Secreto, C.R.; Shanafelt, T.D. Curcumin inhibits prosurvival pathways in chronic lymphocytic leukemia B cells and may overcome their stromal protection in combination with EGCG. Clin. Cancer Res. 2009, 15, 1250–1258. [Google Scholar] [CrossRef]
- Jabbour, E.; Kantarjian, H. Chronic myeloid leukemia: 2016 update on diagnosis, therapy, and monitoring. Am. J. Hematol. 2016, 91, 252–265. [Google Scholar] [CrossRef] [PubMed]
- Nandagopalan, S.R.; Kuila, N.; Biswas, S.; Pattnayak, N.C.; Biswas, G.; Chakraborty, S. Dual transcripts of BCR-ABL & different polymorphisms in chronic myeloid leukaemia patients. Indian J. Med. Res. Suppl. 2016, 143, 136–141. [Google Scholar]
- Williams, L.A.; Gonzalez, A.G.G.; Ault, P.; Mendoza, T.R.; Sailors, M.L.; Williams, J.L.; Huang, F.; Nazha, A.; Kantarjian, H.M.; Cleeland, C.S.; et al. Measuring the symptom burden associated with the treatment of chronic myeloid leukemia. Blood 2013, 122, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.-X.; Xu, J.-H.; Wu, G.-H.; Chen, Y.-Z. Inhibitory effect of curcumin on proliferation of K562 cells involves down-regulation of p210(bcr/abl) initiated Ras signal transduction pathway. Acta Pharmacol. Sin. 2003, 24, 1155–1160. [Google Scholar]
- Mukherjee, A.; Sarkar, R.; Mukherjee, S.; Biswas, J.; Roy, M. Curcumin Boosts up the Efficacy of Imatinib Mesylate in Chronic Myelogenic Leukemia Cell Line K-562 by Modulation of Various Markers. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 240–255. [Google Scholar] [CrossRef]
- Zhang, K.Z.; Xu, J.H.; Huang, X.W.; Wu, L.X.; Su, Y.; Chen, Y.Z. Curcumin synergistically augments bcr/abl phosphorothioate antisense oligonucleotides to inhibit growth of chronic myelogenous leukemia cells. Acta Pharmacol. Sin. 2007, 28, 105–110. [Google Scholar] [CrossRef] [PubMed][Green Version]


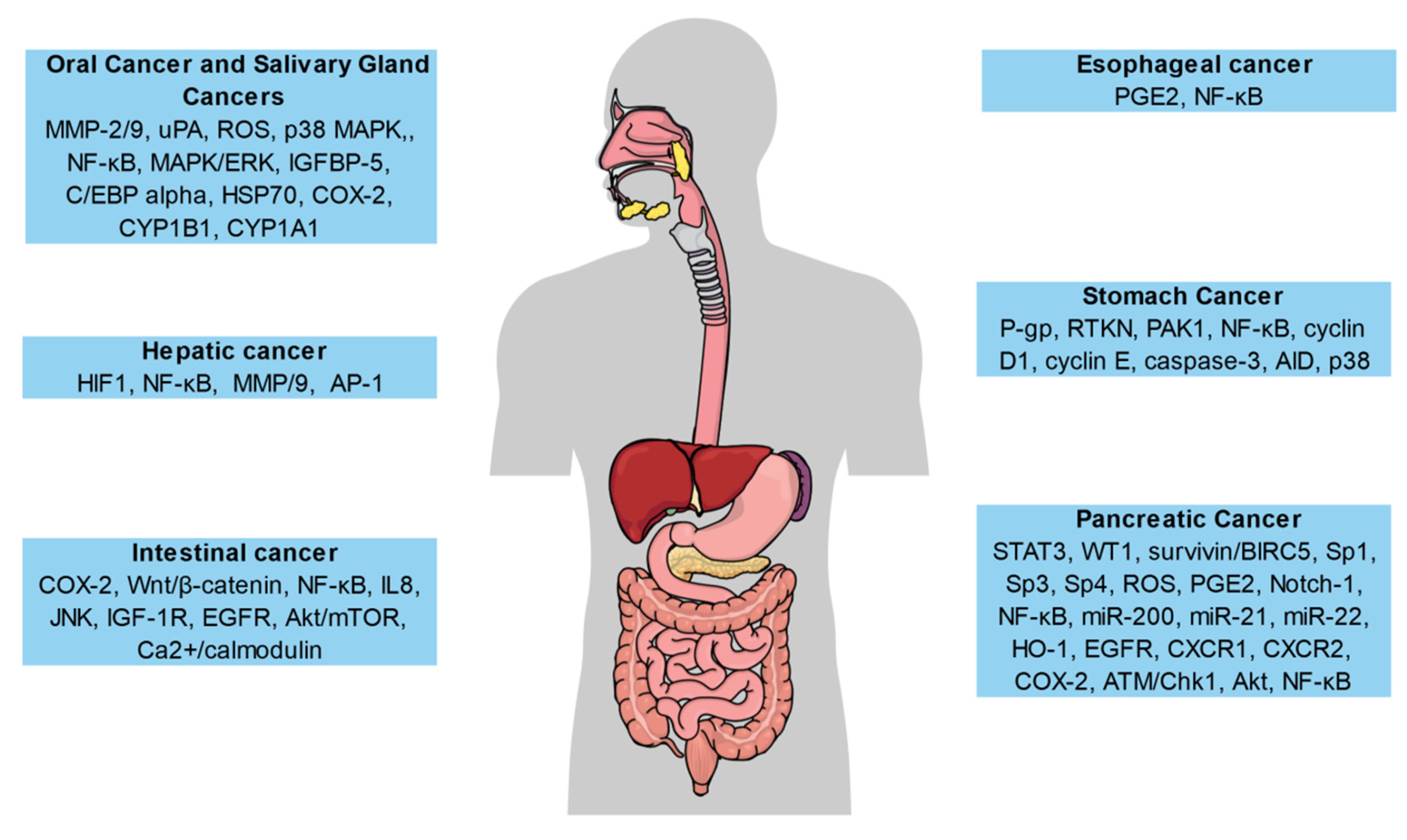
| Nanoformulations | Cancer Models | Major Effects | References |
|---|---|---|---|
| Liposomes | Melanoma, colorectal cancer, and lung cancer | Enhanced bioactivity; antimelanoma effects; increased encapsulation efficiency; increased anticancer effect | [69,70,71,72,73,74,75,76] |
| Polymers | Colorectal cancer | Inhibited tumor growth; increased growth suppression in cancer cells as compared to free curcumin (CUR); enhanced cellular uptake; improved anticancer effect | [77,78,79,80,81] |
| Gold nanoparticles | Prostate and colorectal cancer cells | Ameliorated antioxidant activity; improved stability and solubility; increased biocompatibility and anticancer effect | [82,83,84] |
| Magnetic nanoparticles | Cancer and inflammatory cells | Ameliorated cellular uptake; potent targeting ability of CUR; controlled delivery of CUR; increased biocompatibility and anticancer activity | [85,86,87,88,89] |
| Solid lipid nanoparticles (SLNs) | Breast cancer lines | Prolonged blood circulation, enhanced anti-inflammatory activities; improved anticancer effect | [90,91,92,93,94,95] |
| Conjugates | Breast cancer | Enhanced stability, solubility, and bioavailability; potent anticancer effect | [96,97,98] |
| Cyclodextrins | Lung, breast, pancreatic, colorectal, and prostate cancer cells | Increased solubility, bioavailability, antiproliferation, and anticancer effects | [99,100,101,102,103,104] |
| Solid dispersions | Breast tumor | Prolonged survival, antitumor and anti-metastatic activity; Increased stability, bioavailability and anti-inflammatory effects | [105,106,107,108] |
| Micelles | Lung tumor and colorectal cancer | Improved solubility and bioavailability; extended life, targeted delivery of drug; increased chemical stability; improved anticancer and antitumor activities | [109,110,111,112,113,114,115] |
| Nanospheres | Breast cancer and melanoma cells | Potent antimicrobial and anticancer activities; effective targeted drug delivery | [116,117,118,119,120] |
| Nanogels | Colorectal cancer, pancreatic cancer and skin cancer cells | Controlled and targeted release of drug; prolonged circulation; increased bioavailability; improved anticancer activity | [121,122,123,124,125] |
| Nanodisks | Mantle cell lymphoma | Ameliorated biological action and apoptosis to mantle cell lymphoma and anticancer effect | [126,127,128] |
| Cancer Type | Study Type | Study Duration | Number of Participants | Outcomes | References |
|---|---|---|---|---|---|
| Breast cancer | Phase I clinical trial | 7 days | 14 | Reduced vascular endothelial growth factor levels, decreased harmful effects, no cancer progression, partial response in some individuals | [176] |
| Benign prostatic hypertrophy | Pilot product evaluation study | 24 weeks | 61 | Enhanced quality of life, decreased signs and symptoms, | [177] |
| Colorectal cancer | dose-escalation pilot study | 29 days | 15 | Dose-dependently decreased the prostaglandin E2 (PGE2) levels | [178] |
| Phase I dose-escalation trial | 4 months | 15 | Lower concentrationsof curcumin (CUR) and its metabolites in urine and plasma, dose-dependently decreased the PGE2 levels | [179] | |
| Phase I dose-escalation trial | 7 days | 12 | Biologically active CUR levels in the colorectal tissue | [180] | |
| Phase I clinical trial | 30 days | 126 | Reduced concentrationsof tumor necrosis factor-alpha in serum, elevated p53 expression in colorectal tissue | [181] | |
| Phase II clinical trial | 1 month | 44 | Decreased number of aberrant crypt foci | [182] | |
| Pilot study | 14 days | 26 | Extended levels of biologically active CUR in colon tissue, safe and well-tolerated | [183] | |
| Chronic myeloid leukemia | Randomized controlled trial | 6 weeks | 50 | Decreased levels of nitric oxide | [184] |
| Intestinal Adenoma | Randomized controlled trial | 12 months | 44 | Very few adverse effects, no noticeable clinical response | [185] |
| Head and neck squamous cell carcinoma | Pilot study | - | 21 | Decreased activity of IκB kinase β in the salivary cells | [186] |
| Solid tumors | Randomized controlled trial | 8 weeks | 80 | Enhanced quality of life, decreased inflammatory mediator levels | [187] |
| Prostate cancer | Randomized controlled trial | 6 months | 85 | Reduced prostate-specific antigen levels in individuals with an initial PSA ≥ 10 µg/mL | [188] |
| Randomized controlled trial | 3 months | 40 | The considerable antioxidant effect, decreased levels of PSA | [189] | |
| Pancreatic cancer | Phase II clinical trial | 8 weeks | 25 | No toxicities, biological effect only in 2 individuals, poor oral bioavailability | [34] |
| Phase II clinical trial | 4 weeks | 17 | Increased incidence of side effects | [190] | |
| Phase I/II clinical trial | 14 days | 21 | Safe and well-tolerated | [191] | |
| Phase I clinical trial | 9 months | 16 | Enhanced quality of life, highly bioavailable, safe, no marked alterations in cytokine levels or nuclear factor kappa B activity | [192] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kabir, M.T.; Rahman, M.H.; Akter, R.; Behl, T.; Kaushik, D.; Mittal, V.; Pandey, P.; Akhtar, M.F.; Saleem, A.; Albadrani, G.M.; et al. Potential Role of Curcumin and Its Nanoformulations to Treat Various Types of Cancers. Biomolecules 2021, 11, 392. https://doi.org/10.3390/biom11030392
Kabir MT, Rahman MH, Akter R, Behl T, Kaushik D, Mittal V, Pandey P, Akhtar MF, Saleem A, Albadrani GM, et al. Potential Role of Curcumin and Its Nanoformulations to Treat Various Types of Cancers. Biomolecules. 2021; 11(3):392. https://doi.org/10.3390/biom11030392
Chicago/Turabian StyleKabir, Md. Tanvir, Md. Habibur Rahman, Rokeya Akter, Tapan Behl, Deepak Kaushik, Vineet Mittal, Parijat Pandey, Muhammad Furqan Akhtar, Ammara Saleem, Ghadeer M. Albadrani, and et al. 2021. "Potential Role of Curcumin and Its Nanoformulations to Treat Various Types of Cancers" Biomolecules 11, no. 3: 392. https://doi.org/10.3390/biom11030392
APA StyleKabir, M. T., Rahman, M. H., Akter, R., Behl, T., Kaushik, D., Mittal, V., Pandey, P., Akhtar, M. F., Saleem, A., Albadrani, G. M., Kamel, M., Khalifa, S. A. M., El-Seedi, H. R., & Abdel-Daim, M. M. (2021). Potential Role of Curcumin and Its Nanoformulations to Treat Various Types of Cancers. Biomolecules, 11(3), 392. https://doi.org/10.3390/biom11030392









