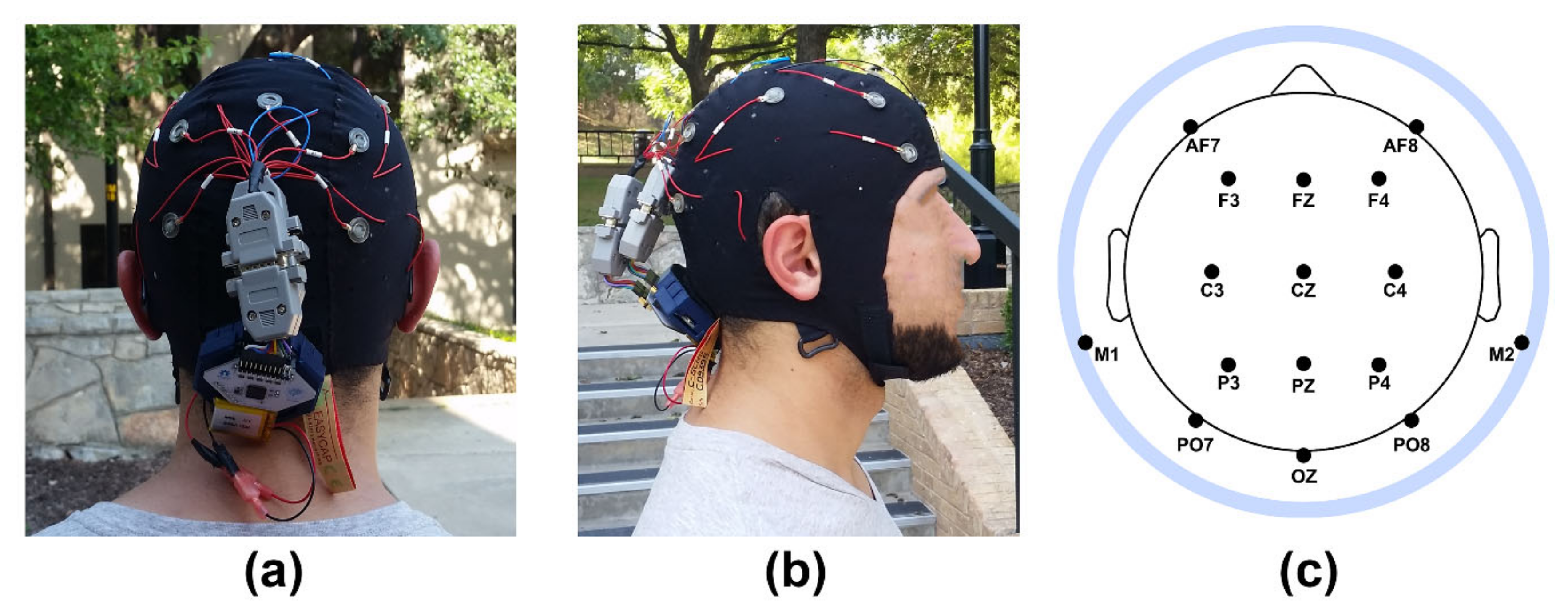
1. Introduction
Quantitative electroencephalography (QEEG) involves the complex numerical analysis of digitally recorded EEG signals that can provide significant insight into the functional relevance of bioelectric brain activity [
1]. Traditionally, these analyses have been applied to EEG data collected within controlled laboratory environments, primarily due to the physical limitations of the recording equipment; traditional EEG systems require large amplifiers and computers that cannot be easily transported. Nevertheless, EEG data collected from controlled recording environments are beneficial for quantitative analysis because such data reflects well-defined experimental manipulations and clean measurement of the EEG signals. This yields strong internal validity among independent and dependent variables and robust performance of numerical analysis algorithms applied to the EEG signals.
However, a major drawback to recording EEG in controlled environments is that the external validity of any quantitative results is limited [
2,
3]. There are three main factors limiting the external validity of these results [
4]. First, active behavior of participants in most laboratory studies is highly constrained because the physical effects of gross motor movements often degrades the signal quality of noninvasive brain imaging technologies. Second, most laboratory studies utilize simple static, regularized stimuli that are crude approximations to the dynamic, irregular stimulation found in naturalistic environments. Third, laboratory studies are generally free of environmental background influences (static or dynamic, regular or irregular) that can modify neurocognitive performance. Thus, it remains unclear if the QEEG findings produced by controlled laboratory studies generalize to the case of data collected within uncontrolled environments.
Fortunately, mobile EEG technologies have recently emerged that have opened up new possibilities for the measurement of EEG signals related to active behavior conducted inside or outside of the laboratory [
2]. This technology involves small, battery-powered, wearable EEG amplifiers that can record the brain signals of participants while they naturalistically engage in task performance within a variety of interactive environments [
5,
6,
7,
8,
9,
10,
11]. Research using this technology is still in its early stages, with key methodological, analytical, and interpretational hurdles yet to be resolved [
2,
12,
13,
14,
15,
16]. Nevertheless, mobile EEG technology has reached the point that it can now be used to study the external validity of laboratory QEEG findings, which is a goal of the present study.
In exploring the external validity limits of laboratory-based QEEG findings, it is useful to take an incremental approach in which the main factors affecting these limits (listed earlier) are investigated separately. In the present study, we examined the effect of uncontrolled background influences in the physical environment because manipulating this factor is relatively straightforward and little study has been given to it to date. Previous mobile EEG studies have mainly focused on neurocognitive performance within a single physical or virtual environment (e.g., laboratory, classroom, outdoor, virtual) [
8,
11,
12,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26]. To our knowledge, only two mobile EEG studies to date [
27,
28] have directly compared human neurocognitive performance between different physical environments with different physical characteristics and levels of dynamic irregularity. The first study [
27] examined differences between a controlled indoor laboratory and an uncontrolled outdoor bicycle pathway. However, cognitive task performance in this study (auditory oddball detection) was also paired with a different physical activity in each environment (sitting indoors, bicycling outside). The second study [
28] removed this confound by examining cognition during bicycling activity within a quiet park and a noisy roadway. In the present study, we also recorded mobile EEG from participants performing neurocognitive tasks during the same physical activity within two different physical environments—sitting in a controlled laboratory environment (closed space, minimal noise, static temperature and atmosphere) and a moderately uncontrolled outdoor environment (open space, background noise, weather changes; see
Figure 1).
Another important factor to consider when testing the external validity of laboratory QEEG findings is the particular perceptual, cognitive, and motor functions that an individual performs during mobile EEG recording. Previous mobile EEG studies have focused on several functions (vigilant attention, perceptual novelty and stimulus significance, motor activity) as engaged by a variety of simple and complex tasks [
8,
11,
12,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27]. However, certain tasks might be expected to be more greatly influenced by the background environment than others, particularly tasks engaging executive functioning in complex, flexible combination with other cognitive functions [
29]. In the present study, participants performed the paced auditory serial addition test (PASAT) [
30,
31], a mental task that engages several complex cognitive functions, including number sense, attention, working memory, and executive control [
32,
33,
34]. However, in order to assess QEEG metrics reflecting cognitive engagement during the PASAT, a comparison condition is needed in which cognition is relatively disengaged. Here the control condition was EEG activity recorded while participants performed a simple resting state task [
35,
36,
37,
38], where an individual remains in an unstimulated state of wakeful rest in a manner that engages a default mode of brain activity [
39]. This resting task was also of interest in and of itself as a metric of the effects of background environment on the endogenous neurocognitive processing of the default mode state.
A third factor to consider when testing the external validity of laboratory QEEG findings is the particular QEEG metric utilized to index brain functioning. The majority of previous studies using mobile EEG to study brain function have utilized event-related potential (ERP) measures of averaged stimulus-locked EEG activity [
5,
12,
17,
19,
20,
21,
22,
25,
27,
40], with few studies examining tonic or event-related spectral power [
8,
16,
23,
24,
26]. Other studies have used measures of spectral power or functional connectivity to study the technical performance of mobile EEG equipment rather than brain function per se [
7,
9]. Here, we utilized EEG spectral power as the QEEG metric because previous research has demonstrated correlations between EEG oscillations within three frequency ranges (theta: 4–7 Hz, alpha: 8–13 Hz, low beta: 14–20 Hz) and the neurocognitive states engaged by the PASAT and resting state task [
35,
36,
37,
38,
41,
42,
43,
44,
45,
46,
47,
48,
49,
50].
Finally, we expected that a moderate influence of background environment would likely be small and idiosyncratic. Thus, to ensure sufficient sensitivity to both real and null effects, we employed null hypothesis significance testing (NHST) augmented by Bayesian model selection via use of Bayes factors. We observed EEG power effects typical of the neurocognitive states engaged by the PASAT and resting state task, but did not observe major EEG power differences between the two background recording environments for all three frequency bands. These results support the external validity of the EEG spectral power metric for complex and default neurocognitive states. (Note: This manuscript is based in part on an unpublished Master’s thesis submitted by the first author to Texas State University [
51].)
4. Discussion
In the present study, we explored the external validity limits of laboratory-based QEEG findings by examining the influence of physical environment background on complex and simple neurocognitive states as, respectively, engaged by the PASAT and resting state task. Each participant performed these tasks in a controlled laboratory environment (closed space, minimal noise, static temperature and atmosphere) and a mildly uncontrolled outdoor environment (open space, background noise, weather changes). Brain activity associated with these neurocognitive states was quantitatively indexed via computation of EEG spectral power. We observed a weak between-environment beta band power effect for the resting task, with greater beta power in the outdoor versus laboratory environment, but no other reliable spectral power differences between the two background recording environments in all spectral power bands for either task.
4.1. Resting Task
The resting state task engages a default mental state, characterized by internal cognitive processing (conceptualization, episodic working memory, unconstrained verbally mediated thoughts), endogenously directed attention, and the monitoring of the body, external environment, and emotional states [
39,
75,
76,
77,
78]. These mental states are correlated with the oscillatory activity of several resting state brain networks distributed across multiple brain regions [
76], where such activity is disengaged during active goal-directed behavior [
79]. Importantly, these resting state networks are associated with unique patterns of wideband (0.1–100 Hz) EEG power [
76], although network activity in the theta, alpha, and beta band ranges predominates [
80]. Moreover, the resting EEG power spectrum is modulated according to whether an individual maintains the resting state with eyes open and closed. Most studies consistently find greater theta, alpha, and beta spectral power during eyes closed versus eyes open states [
35,
36,
37,
38,
41,
47,
61,
62], differences that in part reflect a transition from “cortical idling” in the absence of visual or cognitive stimulation to active perceptual and cognitive engagement [
80]. We also observed this eyes closed versus eyes open activity pattern across both environments within all three frequency bands in the present study. The observation of greater eyes closed versus eyes open EEG power for both environments supports the external validity of laboratory observations of the resting state EEG power across all three frequency ranges.
One concern with this observation is the degree to which it reflects the typical transition from one default mode state to another, or if it reflects environmental factors as well. For example, eyes open alpha rhythms can also be can be blocked by changes in visual stimulation via eye movements as participants fixate attention on different visual elements in their environment [
81]. Given that the outdoor environment provided a more complex and dynamic scene than the laboratory (see
Section 2.2 Background Recording Environments), some proportion of the alpha blocking observed in the eyes open resting state may have been due to eye movements rather than a general transition away from an idle cortical state. In addition, resting state EEG power responses in the outdoor environment may also reflect higher arousal levels due to embarrassment at being seen by others during the experiment while wearing an awkward-looking EEG cap. Nevertheless, we did not find statistically meaningful between-environment differences for resting state theta and alpha EEG power or EEG power variability, nor did we find such differences to correlate with the six weather-related variables we indexed in the outdoor environment (see
Section 2.2 Background Recording Environments). This suggests that such visual factor or general arousal differences between the two environments did not contribute much variance to our present EEG power measurements in the theta and alpha frequency bands.
However, the present observation of greater resting state beta-band EEG power for the outdoor versus indoor environments suggests that certain resting state processes can be affected by the environment. In general, the frequency of EEG oscillation reflects the size of the recurrent neural network mediating the oscillation [
82,
83], with higher frequency oscillations reflecting small (local) network activity and low frequency oscillations reflecting large (global) network activity. Thus, the present between-environment beta-band difference may reflect differences in local network processing. Moreover, given that this effect did not differ across the scalp (as indicated by a null Environment × Electrode effect; see
Section 3.1. Resting Task EEG Power, above), it is possible that these beta-band differences are present in local networks spread across the cortex rather than in specific brain regions. Furthermore, given that the present beta-band resting state EEG power effects were observed in the context of greater beta-band EEG power variability in the outdoor environment (see
Section 3.3 Resting Task and PASAT EEG Power Variability Analysis), it is possible that the beta-band EEG power effect reflects more variable activity of these local networks in response to the unstable outdoor environment. However, testing these possibilities requires additional EEG source localization analysis to account for the known volume conduction and spatial dispersion of cortical EEG signals as they travel through the head from the cortex to the scalp [
84,
85]. Such analysis is beyond the scope of the present study and is a goal for future research.
We must note that the significant beta-band effect was weak according to Bayesian evidence criteria (see
Section 3.1. Resting Task EEG Power, above). Given the weakness of the effect, we cannot rule out the alternative possibility that it merely reflects residual EMG contamination of the EEG signal that was not entirely removed by our artifact rejection procedure (see
Section 2.7 EEG Preprocessing and Spectral Power Analysis, above). Muscle activity generates wide-band high frequency gamma (25–100 Hz) EEG signals that overlap with the beta range [
82,
86], and it is possible that participants were less relaxed and exhibited more muscular tension and movement in the outdoor environment relative to the laboratory environment. This interpretation would be consistent with our observation that between-environment differences in resting state beta power did not correlate with the six weather-related variables we indexed in the outdoor environment. Although, the likelihood of this alternative possibility is low given that we focused on the lower beta band (14–20 Hz) that is less susceptible to EMG contamination, future research is needed to replicate the present beta-band power finding.
We also performed an effect size analysis in which we compared the observed sizes of the resting task’s primary, neurocognitively relevant main effect (differences in eyes closed versus open resting states) to the effect sizes obtained from a simple meta-analysis of several laboratory EEG studies that utilized this task. We found the directions of the presently observed resting state primary effects were consistent with the directions of the meta-analysis effects. This was case for the EEG data recorded in the laboratory and outdoor environments, which supports the external validity of laboratory resting state EEG findings. However, we also found the presently observed effects sizes to be smaller than the meta-analytic effect sizes obtained from laboratory-recorded data. Given that smaller effect sizes were observed for the present resting state data recorded in both environments, it is likely that this reflects a performance difference between traditional laboratory and mobile EEG technology. Mobile EEG technology is known to exhibit lower signal-to-noise ratios than traditional EEG systems [
9,
13,
15,
16], and it is possible that the smaller effect sizes observed in the present study reflect this factor. This is a topic for further research.
4.2. PASAT
The PASAT is an arithmetic task that requires numerical information to be represented, retained, and transformed within the mind. The task engages a complex combination of cognitive functions, including number sense, attention, short- and long-term memory, and executive control [
32,
33,
34]. Similar to the resting task, the mental states of the PASAT are correlated with the oscillatory activity of several brain networks distributed across multiple brain regions [
76,
79,
87]. Unlike the resting task, however, these networks are engaged during active goal-directed behavior, with their activity anti-correlated with the activation of the resting state default mode network [
79]. Relative to a rest or baseline state, EEG spectral power patterns elicited during simple arithmetic tasks typically exhibit increases in frontal theta and/or beta power [
63,
64,
65,
66,
67,
71], decreases in posterior beta power [
66,
69], and decreases in posterior or scalp-wide alpha power [
63,
64,
65,
68,
69,
70]. The frontal theta and beta power changes are thought to reflect executive engagement, active cognition and concentration, and/or functional binding [
48,
66,
67,
88,
89]. The alpha power changes likely reflect a transition from “cortical idling” in the absence of visual or cognitive stimulation to active perceptual and cognitive engagement [
64,
65,
69,
90], similar to the alpha power decrease seen during the resting task when transitioning from eyes closed to eyes open states.
In the present study, we observed similar alpha power decreases during PASAT performance relative to the resting task eyes open control condition (see
Section 3.2 PASAT EEG Power and Behavioral Performance, above). This finding was observed in the context of a null effect of background recording environment for the alpha power bands and an absence of a correlation between the six weather-related variables we indexed in the outdoor environment and the difference between the outdoor and laboratory between-task differences in EEG power. Furthermore, we compared the observed sizes of the PASAT’s primary, neurocognitively relevant main effect (differences in PASAT versus eyes open resting state control) to the sizes of similar effects obtained from a simple meta-analysis of several laboratory EEG studies that utilized this task. This analysis yielded observed alpha-band effect sizes that were negative and large in magnitude, consistent with the meta-analytic alpha-band effects. These results support the external validity of these alpha EEG power effects as observed in the laboratory.
However, we did not observe any meaningful between-task differences in theta-power. It is unclear if the lack of these between-task differences is due to effects of environment or something specific to the PASAT. If environmental influences obscured the presence of a true theta power effect, then this should be detectable in the form of a main ANOVA effect of environment and/or an interaction of environment with other experimental variables. Yet, we did not observe any significant theta-band effects of environment (both from the ANOVAs and the Pearson correlations between EEG power and the six weather-related variables we indexed in the outdoor environment), and the Bayesian evidence for these null effects were mostly well-above chance;
P(
H0|Data) ranged from 0.72 to 0.82, save for an Environment × Task ANOVA interaction,
P(
H0|Data) = 0.48 (see
Section 3.2 PASAT, above). In addition, the present effect size analysis showed that the observed theta-band effects were either positive but near zero in value, or negative valued. This is in contrast to the large positive-valued meta-analytic theta-band effect, but it is consistent with the inferential tests for theta-band EEG power during the PASAT. Regarding the possibility that these discrepant null theta-band task effects are specific to the PASAT, a survey of past EEG research literature yields little insight. Thus, far, surprisingly few EEG studies have focused directly on PASAT performance-elicited electrophysiological responses. Instead, most past studies have focused on using this task in conjunction with EEG to understand longitudinal changes in PASAT performance due to practice or brain injury [
91,
92,
93]. The few studies that have focused on PASAT-elicited EEG responses found no theta band differences when PASAT performance is compared to some control condition (e.g., another active task or default resting states) [
70,
94]. Thus, while the present null theta power effects are in line with previous findings, and thus support a conclusion of external validity for these effects, this conclusion is tentative until the present theta power null finding is replicated by future research.
We also did not observe clear between-task differences in beta-band power. We did find a significant Task × Electrode interaction for beta-band power, but further analysis showed that this interaction reflected a positive difference between posterior versus frontal power that was stronger for the PASAT than the eyes open resting state. However, there were no significant between-task differences at either scalp location (see
Section 3.2. PASAT EEG Power and Behavioral Performance). The effect size analysis showed a different pattern of task-related beta-band EEG power compared to the meta-analytic effect estimate obtained from previous studies. Task-related posterior beta-band EEG power was positive-valued, in contrast to the negative value of the corresponding meta-analytic effect. The effect size analysis also suggested a different pattern of task-related beta-band EEG power across environments. Task-related frontal beta-band power was positive-valued (though near zero) within the laboratory environment, in agreement with the positive value of the corresponding meta-analytic effect, yet was negative-valued within the outdoor environment. Nevertheless, statistical tests (ANOVAs, Pearson correlations) involving the factor of environment were non-significant with above-chance Bayesian evidence for these null effects (
P(
H0|Data) ranged from 0.70 to 0.82). We suggest that this complex pattern of discrepant results for the PASAT beta-band power could be due to two factors: (1) neurocognitive differences in arithmetic versus PASAT performance (the latter of which involves both mathematical and working memory cognition) or (2) a true difference in arithmetical neurocognition across different environments that was too small to be fully detected by our current experimental design or was otherwise obscured by other factors (for example, unmeasured environmental characteristics or a lower-signal to-noise ratio for mobile EEG). Determining which (if any) of these factors is responsible for the present observations is a topic for future research.
4.3. Study Limitations
Interpretation of the present observations must be tempered by consideration of the limitations of the present study. One limitation was the small sample size and resulting low statistical power. However, this limitation is somewhat mitigated by the use of Bayes factors, which allow us to estimate the probability of null and alternative effects. This allowed us to interpret non-significant NHST outcomes as evidence for the null hypothesis [
55].
A second limitation of this study is that it has lowered internal validity with respect to the main factor of interest, the effect of background influences in the physical environment. This was in part by design because the objective of our study was to evaluate the external validity of QEEG research in different physical environments. We had no direct control of these influences in the outdoor environment, influences that fluctuated greatly. We had no control over levels of student activity on campus, noise levels, and weather activity. We did implement limited control over the time of day experimental sessions were conducted; the majority of participants were run during mid-afternoon hours when the campus was highly active. However, due to time constraints, not all experimental sessions were conducted at the same hours of the day and thus some sessions were conducted during periods of quiet campus activity. Thus, it remains possible that the present null effects of environment resulted in part from limited distractions in the outdoor environment that were not strong enough to influence task performance.
A third limitation of the present study is that measurement of the various characteristics of the two physical environments was limited. While we did not observe a direct relationship between several key weather variables (see
Section 2.2 Background Recording Environments) and EEG power, there are multiple other characteristics of these environments that we did not assess. Thus, it is unclear to which the present results can be generalized to other environments.
A fourth limitation of the present study is that we only used two basic behavioral tasks that did not involve large-scale active movement to appraise neurocognitive functioning. Thus, we cannot generalize our findings to other tasks engaging similar cognitive processes as studied here; this is a topic for future research.
Finally, a fifth limitation of this study is that our population sample was entirely composed of young, college students. It is likely that their demographic characteristics (e.g., level of education, mathematical experience) influenced PASAT performance and electrophysiological outcomes. Investigating the external validity of the present QEEG metrics for other populations performing these tasks is a topic for future research.
5. Conclusions
In conclusion, the present study probed the validity limits of laboratory QEEG by using a mobile EEG system to record EEG signals from human participants while they performed two neurocognitive tasks (PASAT, resting state task) within a controlled laboratory environment and a moderately uncontrolled outdoor environment. Null hypothesis significance testing (NHST) showed significant EEG spectral power effects typical of the neurocognitive states engaged by these tasks (number sense, attention, memory, executive function), but only a beta-band EEG power difference between the two recording environments for the resting task. Bayesian analysis showed that the remaining null effects of environment were unlikely to reflect measurement insensitivities. The overall pattern of these results supports the external validity of laboratory EEG spectral power findings for the complex and default neurocognitive states engaged in moderately uncontrolled environments. They also serve to bolster the credibility of efforts to use mobile EEG systems to index neurocognitive performance in non-laboratory environments.
Human beings operate in a multitude of environments each day, which range drastically regarding the type and concentration of stimuli that are present, yet differences in environmental influences on cognition have been understudied. The present study used QEEG methods to only evaluate the effects of two particular physical environments using two specific cognitive tasks. Future mobile EEG research should study additional physical environments and tasks in order to further examine the external validity of QEEG and to better understand human neurocognition in real-world environments.