Improved Heat Stability of Whey Protein Isolate by Glycation with Inulin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Conjugate Preparation
2.3. Visual Observation
2.4. Amadori Compounds and Available Free Amino Groups
2.5. Particle Size and Turbidity
2.6. Zeta Potential
2.7. Differential Scanning Calorimetry (DSC)
2.8. Viscosity Measurement
2.9. Statistical Analysis
3. Results and Discussion
3.1. Visual Observation
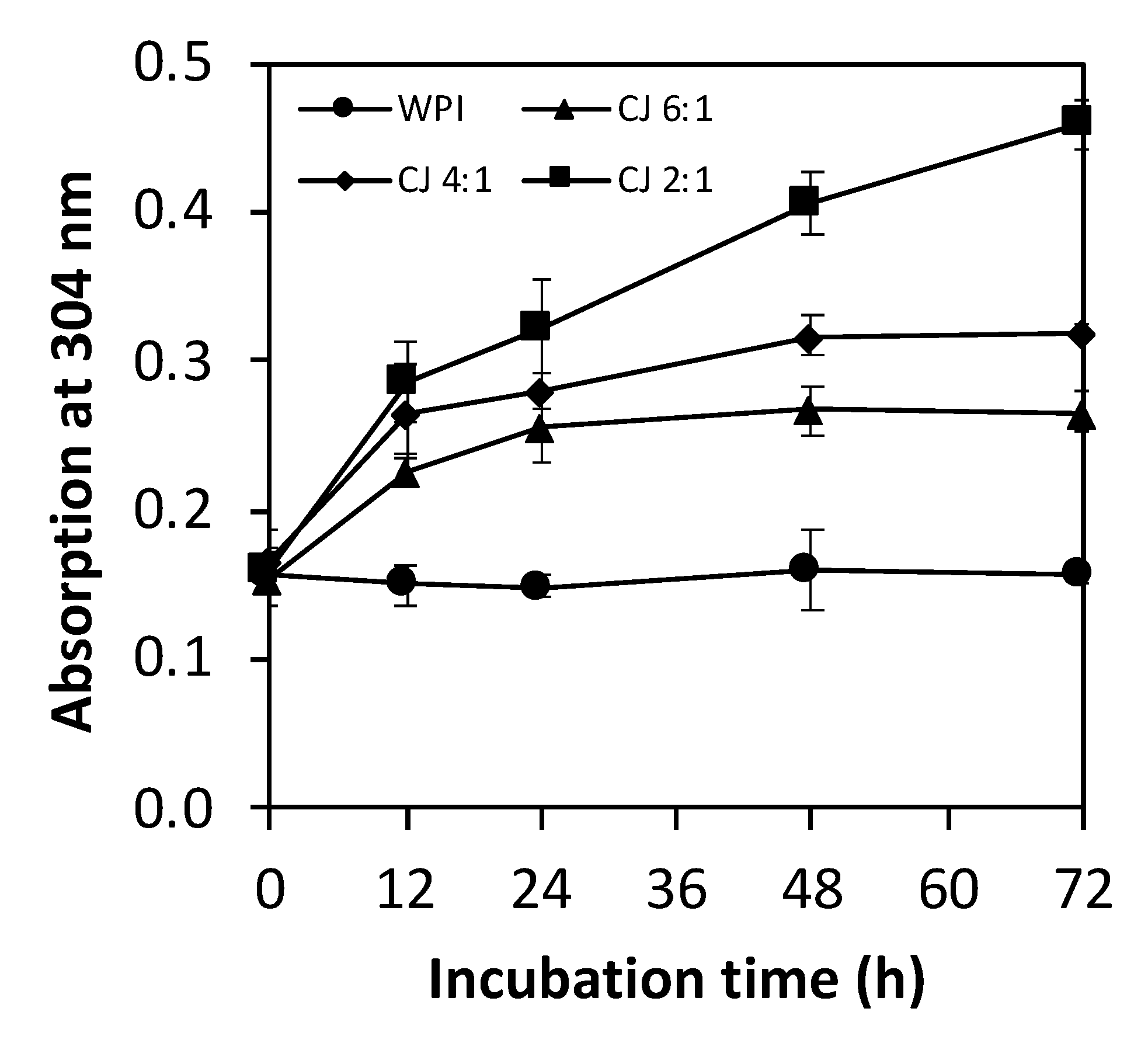
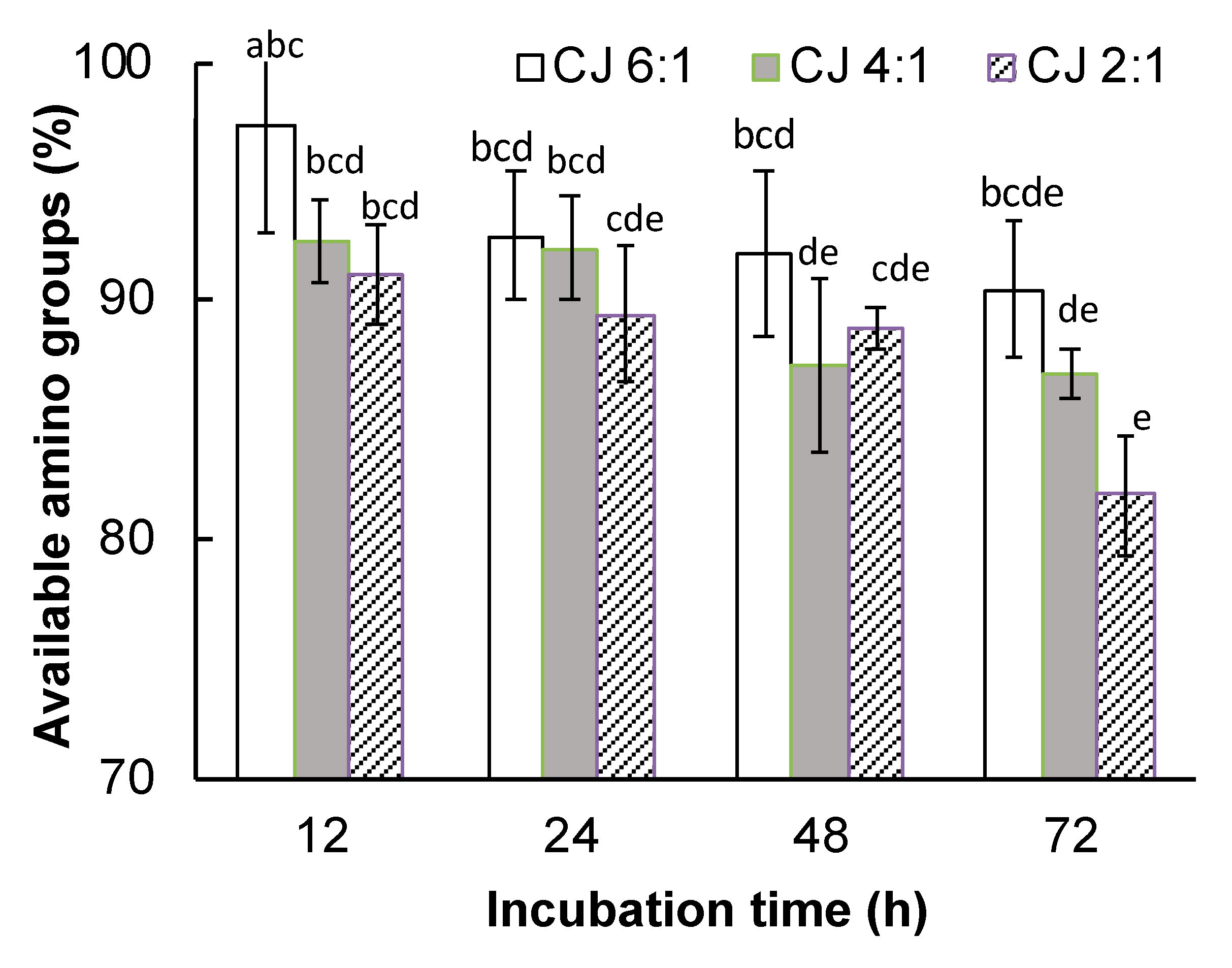
3.2. Degree of Glycation
3.2.1. Amadori Compounds
3.2.2. Available Amino Groups
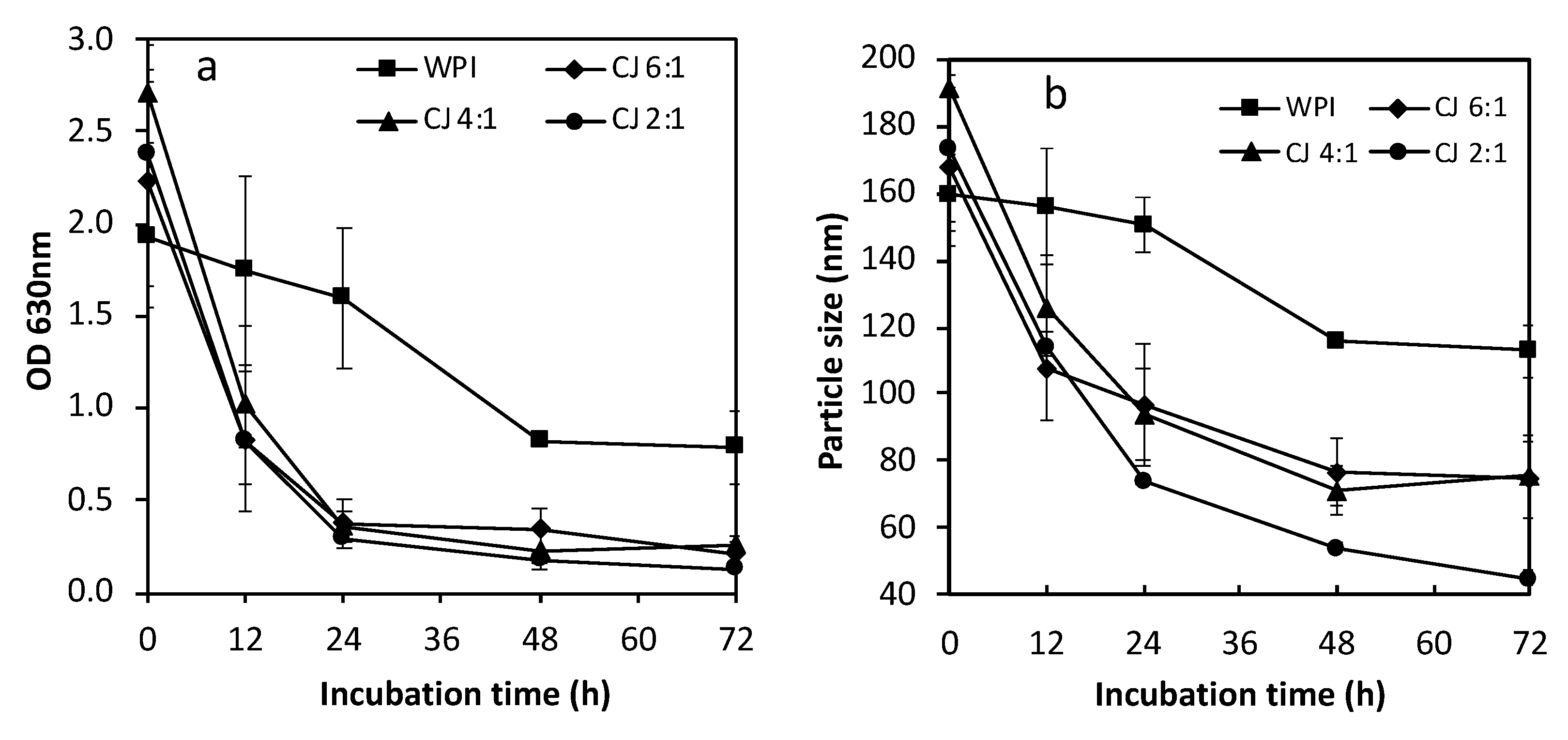
3.3. Heat Stability
3.4. Flow Behavior
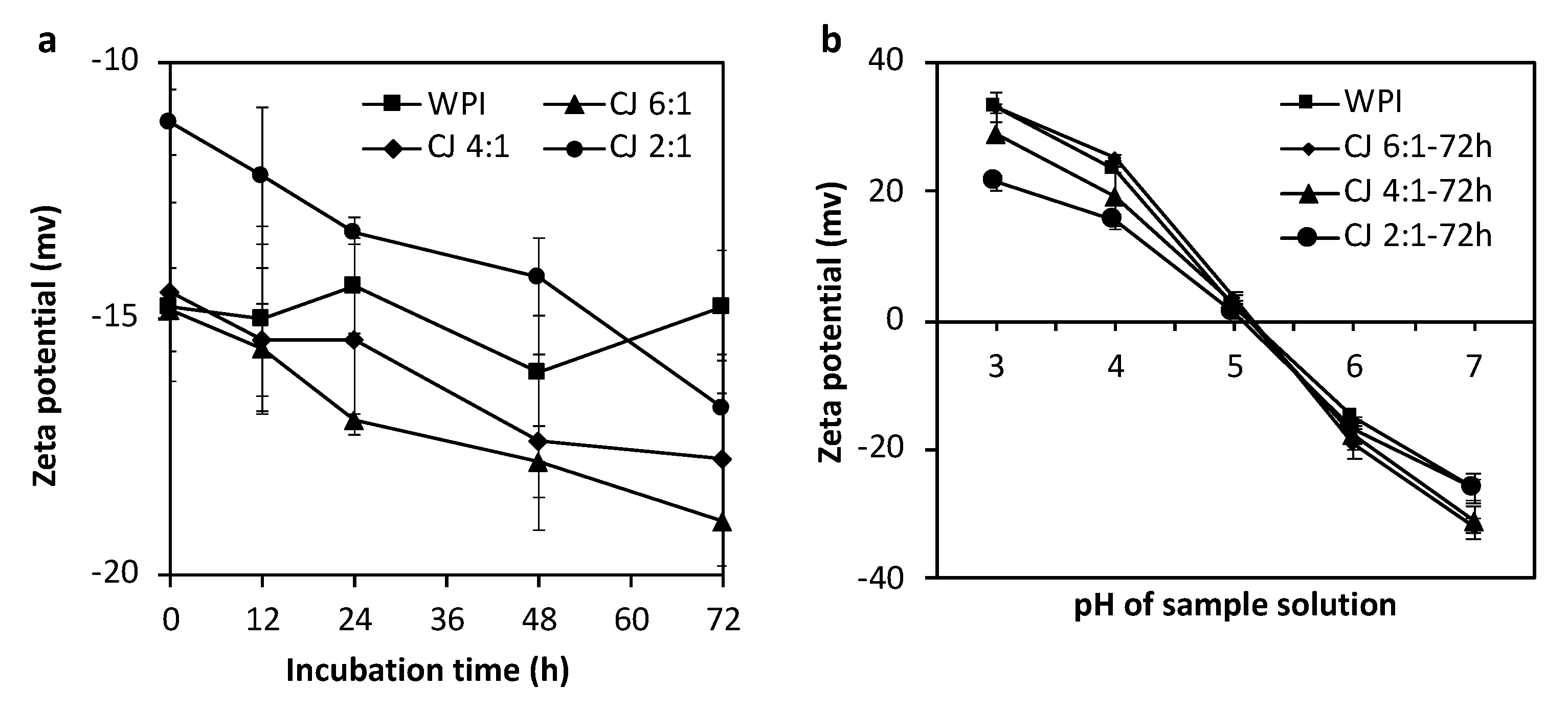
3.5. Zeta Potential
3.6. Differential Scanning Calorimetry
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holtz, S. Beverage Report—Trend: The Stairway to Health. Counter Trend: The Road to Flavor. Available online: https://www.cspdailynews.com/csp-magazine/2014-beverage-report-trend-stairway-health-countertrend-road-flavor (accessed on 31 March 2020).
- Beverage-Digest. Special Issue: U.S. Beverage Results for 2013. Available online: http://www.beverage-digest.com/pdf/top-10_2014.pdf (accessed on 31 March 2020).
- Park, Y.W.; Haenlein, G.F.W. Milk and Dairy Products in Human Nutrition: Production, Composition and Health; John Wiley & Sons: Hoboken, NJ, USA, 2013; p. 742. [Google Scholar]
- Wang, W.; Zhong, Q. Properties of whey protein–maltodextrin conjugates as impacted by powder acidity during the Maillard reaction. Food Hydrocoll. 2014, 38, 85–94. [Google Scholar] [CrossRef]
- Nastaj, M.; Sołowiej, B.; Terpiłowski, K.; Mleko, S. Effect of erythritol on physicochemical properties of reformulated high protein meringues obtained from whey protein isolate. Int. Dairy J. 2020, 105, 104672. [Google Scholar] [CrossRef]
- Baier, S.K.; McClements, D.J. Influence of Cosolvent Systems on the Gelation Mechanism of Globular Protein: Thermodynamic, Kinetic, and Structural Aspects of Globular Protein Gelation. Compr. Rev. Food Sci. Food Saf. 2005, 4, 43–54. [Google Scholar] [CrossRef] [Green Version]
- Roefs, S.P.; Kruif, K.G.d. A model for the denaturation and aggregation of beta-lactoglobulin. Eur. J. Biochem. 1994, 226, 883–889. [Google Scholar] [CrossRef] [PubMed]
- And, M.A.M.H.; Van Mil, P.J.J.M. Heat-Induced Aggregation of β-Lactoglobulin: Role of the Free Thiol Group and Disulfide Bonds. J. Agric. Food Chem. 1997, 45, 2942–2948. [Google Scholar] [CrossRef]
- Nastaj, M.; Sołowiej, B.G. The effect of various pH values on foaming properties of whey protein preparations. Int. J. Dairy Technol. 2020, 73, 683–694. [Google Scholar] [CrossRef]
- Etzel, M.R. Manufacture and Use of Dairy Protein Fractions. J. Nutr. 2004, 134, 996S–1002S. [Google Scholar] [CrossRef]
- Aoki, T.; Hiidome, Y.; Sugimoto, Y.; Ibrahim, H.R.; Kato, Y. Modification of ovalbumin with oligogalacturonic acids through the Maillard reaction. Food Res. Int. 2001, 34, 127–132. [Google Scholar] [CrossRef]
- Aoki, T.; Hiidome, Y.; Kitahata, K.; Sugimoto, Y.; Ibrahim, H.R.; Kato, Y. Improvement of heat stability and emulsifying activity of ovalbumin by conjugation with glucuronic acid through the Maillard reaction. Food Res. Int. 1999, 32, 129–133. [Google Scholar] [CrossRef]
- Jiménez-Castaño, L.; Villamiel, M.; López-Fandiño, R. Glycosylation of individual whey proteins by Maillard reaction using dextran of different molecular mass. Food Hydrocoll. 2007, 21, 433–443. [Google Scholar] [CrossRef]
- Jimenezcastano, L.; Lopezfandino, R.; Olano, A.; Villamiel, M. Study on β-lactoglobulin glycosylation with dextran: Effect on solubility and heat stability. Food Chem. 2005, 93, 689–695. [Google Scholar] [CrossRef]
- Kato, Y.; Aoki, T.; Kato, N.; Nakamura, R.; Matsuda, T. Modification of Ovalbumin with Glucose 6-Phosphate by Amino-Carbonyl Reaction. Improvement of Protein Heat Stability and Emulsifying Activity. J. Agric. Food Chem. 1995, 43, 301–305. [Google Scholar] [CrossRef]
- Liu, G.; Zhong, Q. Thermal aggregation properties of whey protein glycated with various saccharides. Food Hydrocoll. 2013, 32, 87–96. [Google Scholar] [CrossRef]
- Liu, G.; Zhong, Q. Glycation of Whey Protein to Provide Steric Hindrance against Thermal Aggregation. J. Agric. Food Chem. 2012, 60, 9754–9762. [Google Scholar] [CrossRef]
- Wang, Q.; Ismail, B. Effect of Maillard-induced glycosylation on the nutritional quality, solubility, thermal stability and molecular configuration of whey proteinv. Int. Dairy J. 2012, 25, 112–122. [Google Scholar] [CrossRef]
- Xu, C.-H.; Yang, X.-Q.; Yu, S.-J.; Qi, J.-R.; Guo, R.; Sun, W.-W.; Yao, Y.-J.; Zhao, M.-M. The effect of glycosylation with dextran chains of differing lengths on the thermal aggregation of β-conglycinin and glycinin. Food Res. Int. 2010, 43, 2270–2276. [Google Scholar] [CrossRef]
- Oliver, C.M.; Melton, L.D.; Stanley, R.A. Creating Proteins with Novel Functionality via the Maillard Reaction: A Review. Crit. Rev. Food Sci. Nutr. 2006, 46, 337–350. [Google Scholar] [CrossRef]
- Liu, J.; Ru, Q.; Ding, Y. Glycation a promising method for food protein modification: Physicochemical properties and structure, a review. Food Res. Int. 2012, 49, 170–183. [Google Scholar] [CrossRef]
- Jiménez-Castaño, L.; Villamiel, M.; Martin-Álvarez, P.J.; Olano, A.; López-Fandiño, R. Effect of the dry-heating conditions on the glycosylation of β-lactoglobulin with dextran through the Maillard reaction. Food Hydrocoll. 2005, 19, 831–837. [Google Scholar] [CrossRef]
- Malec, L.; Gonzales, A.P.; Naranjo, G.; Vigo, M. Influence of water activity and storage temperature on lysine availability of a milk like system. Food Res. Int. 2002, 35, 849–853. [Google Scholar] [CrossRef]
- Pan, G.G.; Melton, L.D. Nonenzymatic Browning of Lactose and Caseinate during Dry Heating at Different Relative Humidities. J. Agric. Food Chem. 2007, 55, 10036–10042. [Google Scholar] [CrossRef] [PubMed]
- Ames, J.M. The Maillard Reaction. In Biochemistry of Food Proteins; Hudson, B.J.F., Ed.; Springer: Boston, MA, USA, 1992; pp. 99–153. [Google Scholar]
- Barclay, T.; Ginic-Markovic, M.; Cooper, P.; Petrovsky, N. Inulin—A Versatile Polysaccharide with Multiple Pharmaceutical and Food Chemical Uses; J. Excip. Food Chem. 2007, 1, 27–50. [Google Scholar]
- Oliver, C.M.; Melton, L.D.; A Stanley, R. Glycation of caseinate by fructose and fructo-oligosaccharides during controlled heat treatment in the ‘dry’ state. J. Sci. Food Agric. 2006, 86, 722–731. [Google Scholar] [CrossRef]
- Nair, K.K.; Kharb, S.; Thompkinson, D.K. Inulin Dietary Fiber with Functional and Health Attributes—A Review. Food Rev. Int. 2010, 26, 189–203. [Google Scholar] [CrossRef]
- Roberfroid, M.B. Introducing inulin-type fructans. Br. J. Nutr. 2005, 93, S13–S25. [Google Scholar] [CrossRef]
- Ryan, K.N.; Foegeding, E.A. Formation of soluble whey protein aggregates and their stability in beverages. Food Hydrocoll. 2015, 43, 265–274. [Google Scholar] [CrossRef]
- Zhu, D.; Damodaran, S.; Lucey, J.A. Formation of Whey Protein Isolate (WPI)−Dextran Conjugates in Aqueous Solutions. J. Agric. Food Chem. 2008, 56, 7113–7118. [Google Scholar] [CrossRef]
- Tang, C.-H.; Sun, X.; Foegeding, E.A. Modulation of Physicochemical and Conformational Properties of Kidney Bean Vicilin (Phaseolin) by Glycation with Glucose: Implications for Structure–Function Relationships of Legume Vicilins. J. Agric. Food Chem. 2011, 59, 10114–10123. [Google Scholar] [CrossRef]
- Van Boekel, M.a.J.S. Kinetic aspects of the Maillard reaction: A critical review. Nahrung 2001, 45, 150–159. [Google Scholar] [CrossRef]
- Kroh, L.W.; Jalyschko, W.; Häseler, J. Non-volatile Reaction Products by Heat-induced Degradation of α-Glucans. Part I: Analysis of Oligomeric Maltodextrins and Anhydrosugars. Starch Stärke 1996, 48, 426–433. [Google Scholar] [CrossRef]
- Oliver, C.M.; Melton, L.D.; A Stanley, R. Functional properties of caseinate glycoconjugates prepared by controlled heating in the ‘dry’ state. J. Sci. Food Agric. 2006, 86, 732–740. [Google Scholar] [CrossRef]
- Dan, A.; Ghosh, S.; Moulik, S.P. Physicochemical studies on the biopolymer inulin: A critical evaluation of its self-aggregation, aggregate-morphology, interaction with water, and thermal stability. Biopolymers 2009, 91, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Heinert, D.; Martell, A.E. Pyridoxine and Pyridoxal Analogs. VI. Electronic Absorption Spectra of Schiff Bases. J. Am. Chem. Soc. 1963, 85, 183–188. [Google Scholar] [CrossRef]
- Blonski, C.; De Moissac, D.; Périé, J.; Sygusch, J. Inhibition of rabbit muscle aldolase by phosphorylated aromatic compounds. Biochem. J. 1997, 323, 71–77. [Google Scholar] [CrossRef] [Green Version]
- Joubran, Y.; Mackie, A.; Lesmes, U. Impact of the Maillard reaction on the antioxidant capacity of bovine lactoferrin. Food Chem. 2013, 141, 3796–3802. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Alvarenga, M.; Martinez-Rodriguez, E.; Garcia-Amezquita, L.; Olivas, G.; Zamudio-Flores, P.; Acosta-Muniz, C.; Sepulveda, D. Effect of Maillard reaction conditions on the degree of glycation and functional properties of whey protein isolate—Maltodextrin conjugates. Food Hydrocoll. 2014, 38, 110–118. [Google Scholar] [CrossRef]
- Stevens, C.V.; Meriggi, A.; Booten, K. Chemical Modification of Inulin, a Valuable Renewable Resource, and Its Industrial Applications. Biomacromolecules 2001, 2, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.-J.; Qiu, A.-Y.; Liu, X.-Y.; Hua, Y.-F.; Ma, Y.-H. Microwave improvement of soy protein isolate–saccharide graft reactions. Food Chem. 2006, 97, 577–585. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Intermolecular and Surface Forces; Academic Press: Burlington, MA, USA, 2011. [Google Scholar]
- Shu, Y.-W.; Sahara, S.; Nakamura, S.; Kato, A. Effects of the Length of Polysaccharide Chains on the Functional Properties of the Maillard-Type Lysozyme−Polysaccharide Conjugate. J. Agric. Food Chem. 1996, 44, 2544–2548. [Google Scholar] [CrossRef]
- Wang, Q.; He, L.; Labuza, T.P.; Ismail, B. Structural characterisation of partially glycosylated whey protein as influenced by pH and heat using surface-enhanced Raman spectroscopy. Food Chem. 2013, 139, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Baniel, A.; Caer, D.; Colas, B.; Gueguen, J. Functional properties of glycosylated derivatives of the 11S storage protein from pea (Pisum sativum L.). J. Agric. Food Chem. 1992, 40, 200–205. [Google Scholar] [CrossRef]
- Corzo-Martínez, M.; Moreno, F.J.; Villamiel, M.; Harte, F.M. Characterization and improvement of rheological properties of sodium caseinate glycated with galactose, lactose and dextran. Food Hydrocoll. 2010, 24, 88–97. [Google Scholar] [CrossRef] [Green Version]
- O’Regan, J.; Mulvihill, D.M. Preparation, characterisation and selected functional properties of sodium caseinate–maltodextrin conjugates. Food Chem. 2009, 115, 1257–1267. [Google Scholar] [CrossRef]
- Paraman, I.; Hettiarachchy, N.S.; Schaefer, C. Glycosylation and Deamidation of Rice Endosperm Protein for Improved Solubility and Emulsifying Properties. Cereal Chem. J. 2007, 84, 593–599. [Google Scholar] [CrossRef]
- McGuffey, M.K.; Epting, K.L.; Kelly, R.M.; Foegeding, E.A. Denaturation and Aggregation of Three α-Lactalbumin Preparations at Neutral pH. J. Agric. Food Chem. 2005, 53, 3182–3190. [Google Scholar] [CrossRef]
- Xing, L.; Lin, K.; Zhou, X.; Liu, S.; Luo, Y. Multistate Mechanism of Lysozyme Denaturation through Synchronous Analysis of Raman Spectra. J. Phys. Chem. B 2016, 120, 10660–10667. [Google Scholar] [CrossRef] [PubMed]
- Medrano, A.; Abirached, C.; Panizzolo, L.; Moyna, P.; Añón, M. The effect of glycation on foam and structural properties of β-lactoglobulin. Food Chem. 2009, 113, 127–133. [Google Scholar] [CrossRef]
- Broersen, K.; Voragen, A.G.J.; Hamer, R.J.; De Jongh, H.H.J. Glycoforms of β-lactoglobulin with improved thermostability and preserved structural packing. Biotechnol. Bioeng. 2004, 86, 78–87. [Google Scholar] [CrossRef] [PubMed]

| Sample | Td (°C) | ΔH (kJ/g) |
|---|---|---|
| WPI | 71.21 ± 0.04 a | 6.90 ± 0.54 a |
| CJ 6:1-72 h | 71.82 ± 0.03 b | 3.60 ± 0.54 b |
| CJ 2:1-72 h | 73.60 ± 0.08 c | 2.85 ± 0.86 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Vardhanabhuti, B. Improved Heat Stability of Whey Protein Isolate by Glycation with Inulin. Dairy 2021, 2, 135-147. https://doi.org/10.3390/dairy2010013
He Y, Vardhanabhuti B. Improved Heat Stability of Whey Protein Isolate by Glycation with Inulin. Dairy. 2021; 2(1):135-147. https://doi.org/10.3390/dairy2010013
Chicago/Turabian StyleHe, Yue, and Bongkosh Vardhanabhuti. 2021. "Improved Heat Stability of Whey Protein Isolate by Glycation with Inulin" Dairy 2, no. 1: 135-147. https://doi.org/10.3390/dairy2010013




