The Root Microbiome of Salicornia ramosissima as a Seedbank for Plant-Growth Promoting Halotolerant Bacteria
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
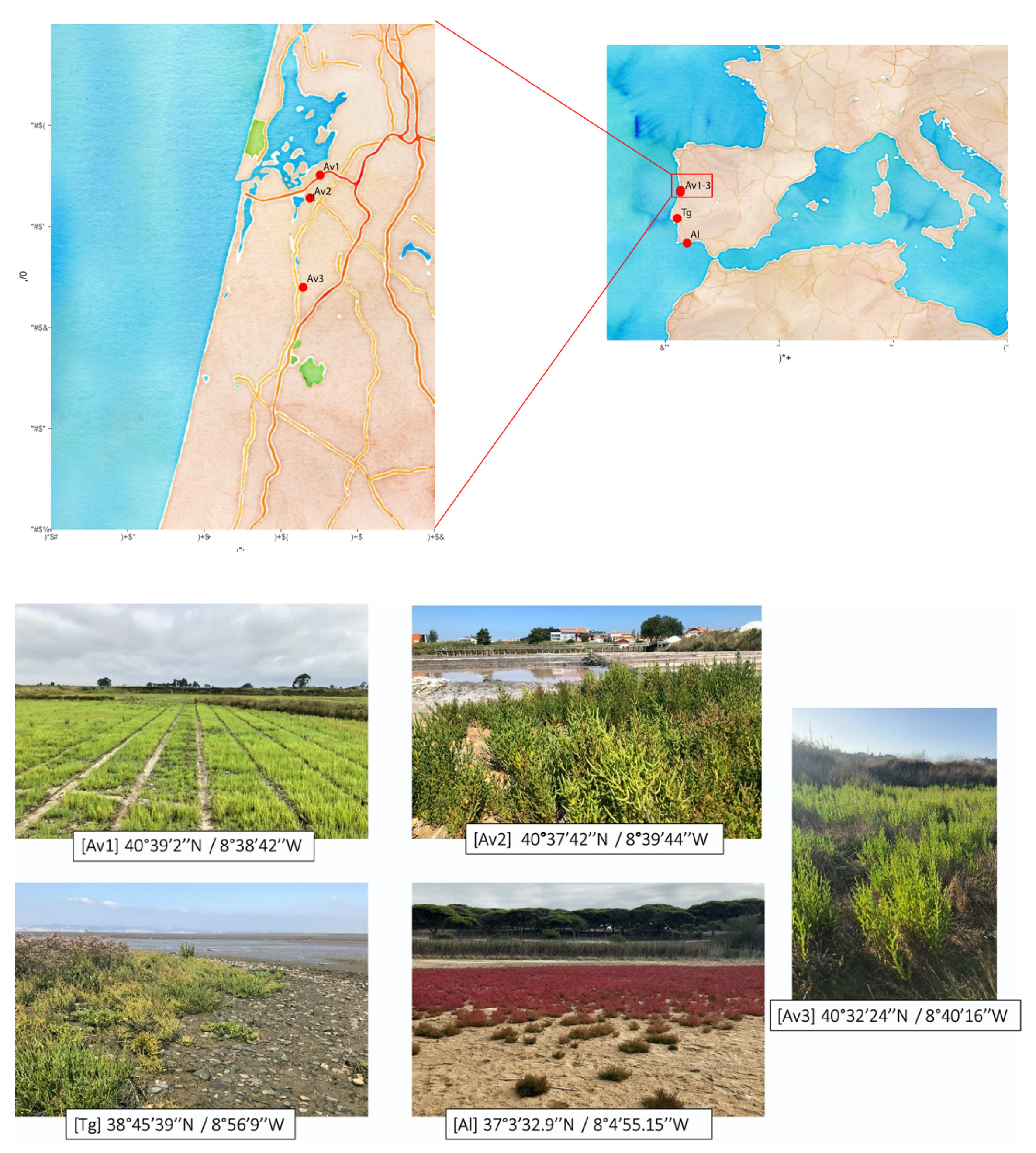
2.1. Sampling Sites
2.2. Physico-Chemical Parameters of Sediments and Pore Water
2.3. Isolation of Halotolerant Rhizosphere and Endophytic Root Bacteria
2.4. Identification of Endophytic and Rhizosphere Bacterial Strains
2.5. Salinity Tolerance
2.6. Screening for Extracellular Enzymatic Activity
2.7. Screening for Other Plant-Growth Promoting Traits
2.8. Statistics
3. Results
3.1. Sediment and Pore Water Characteristics
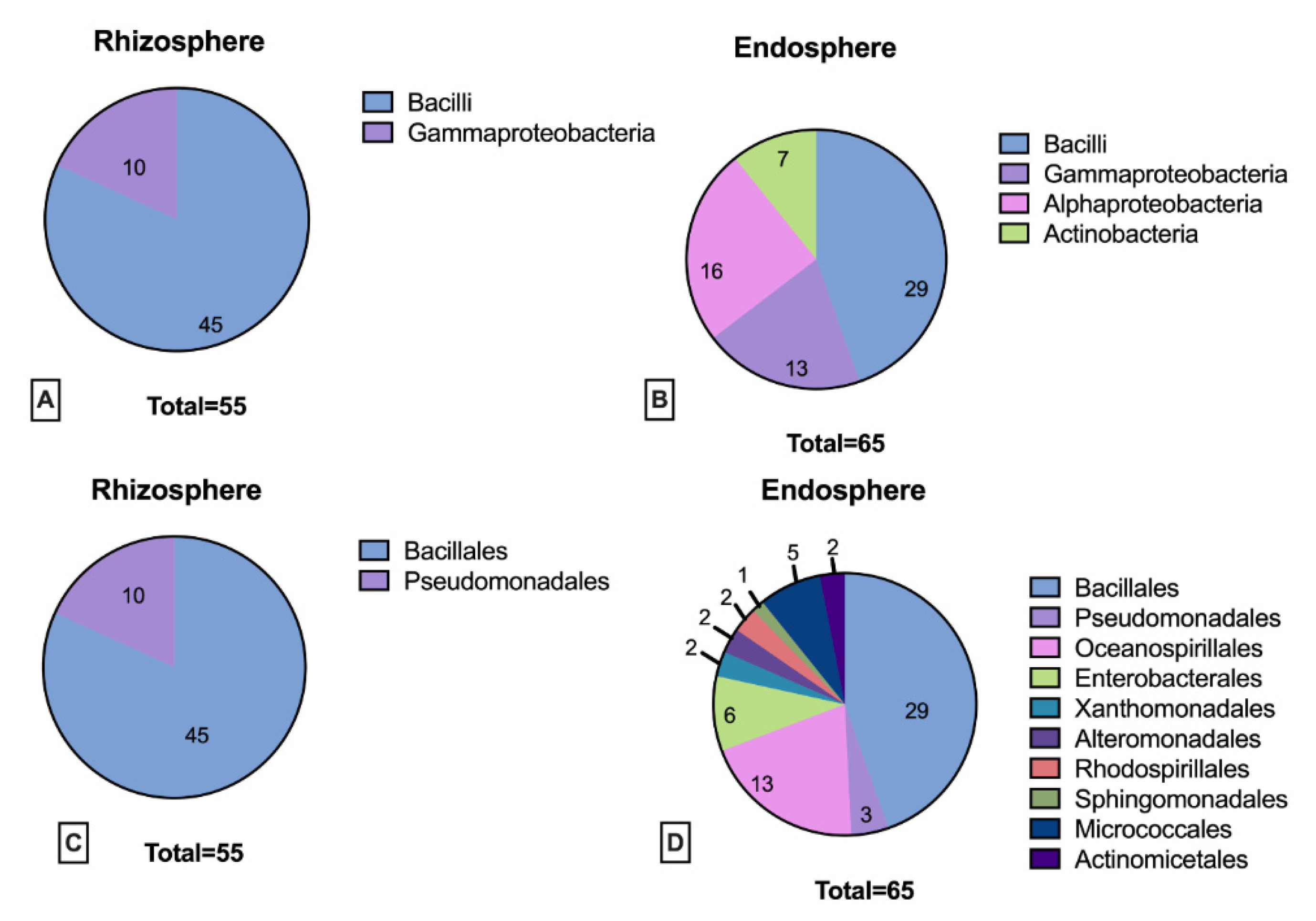
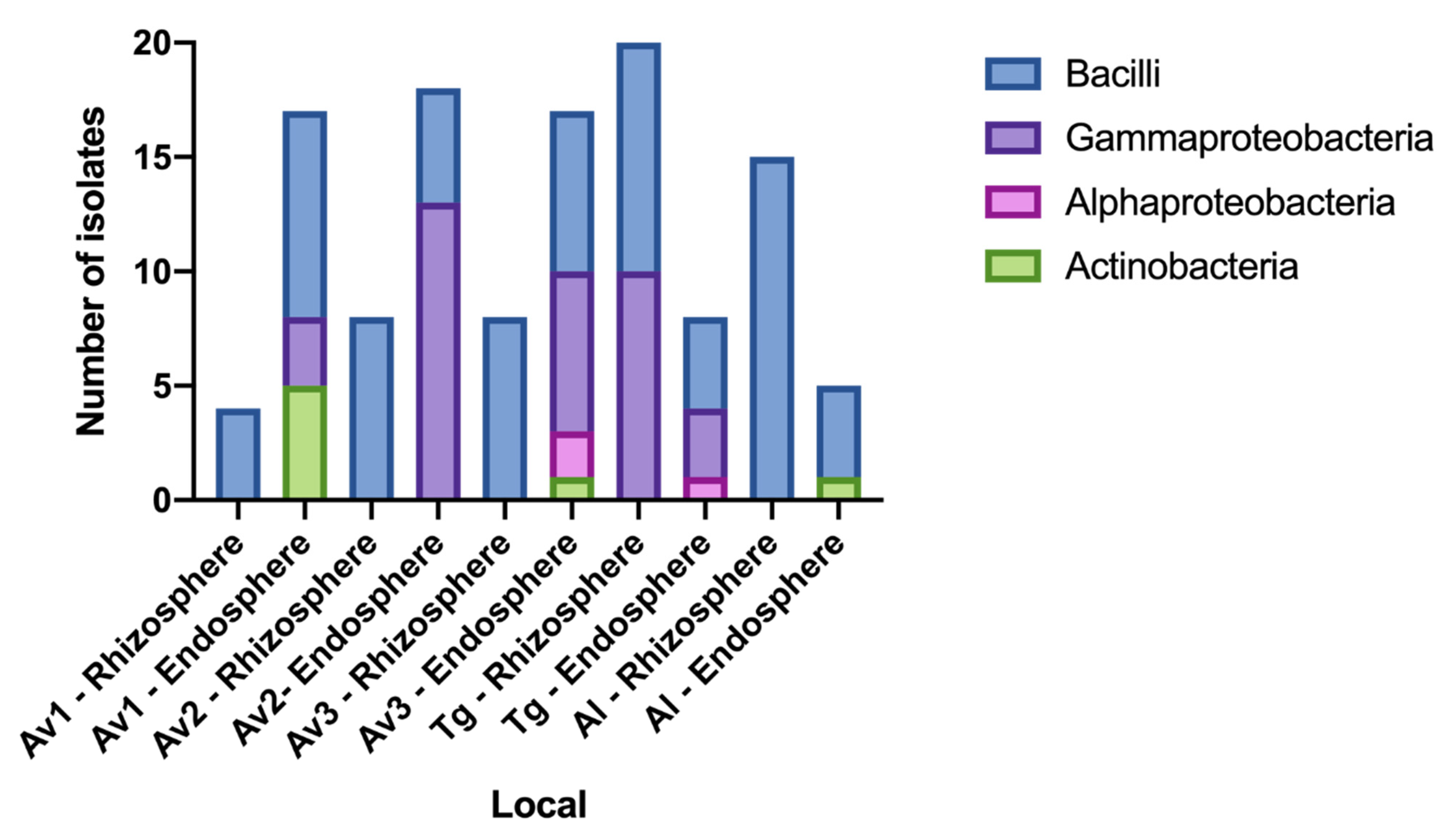
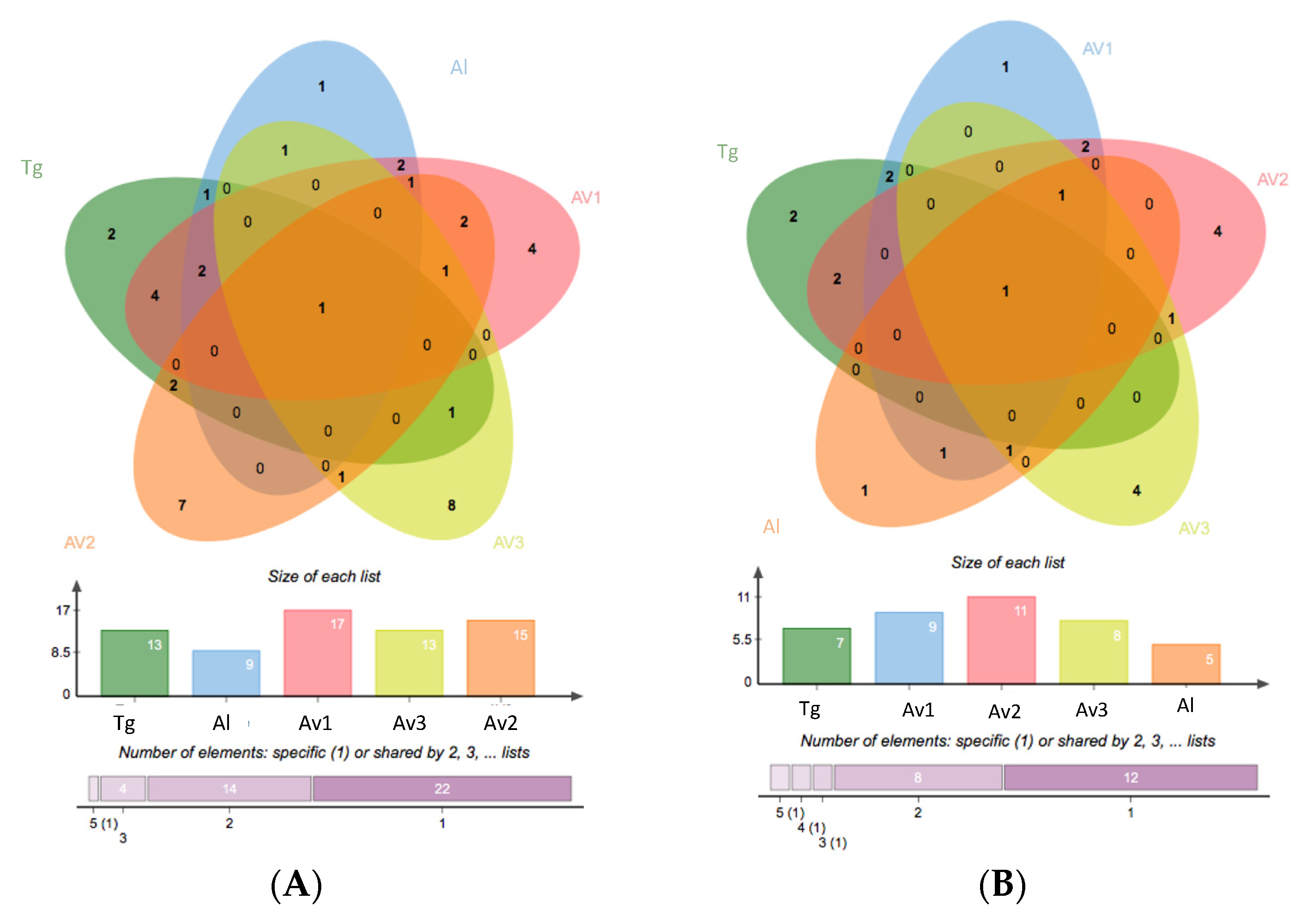
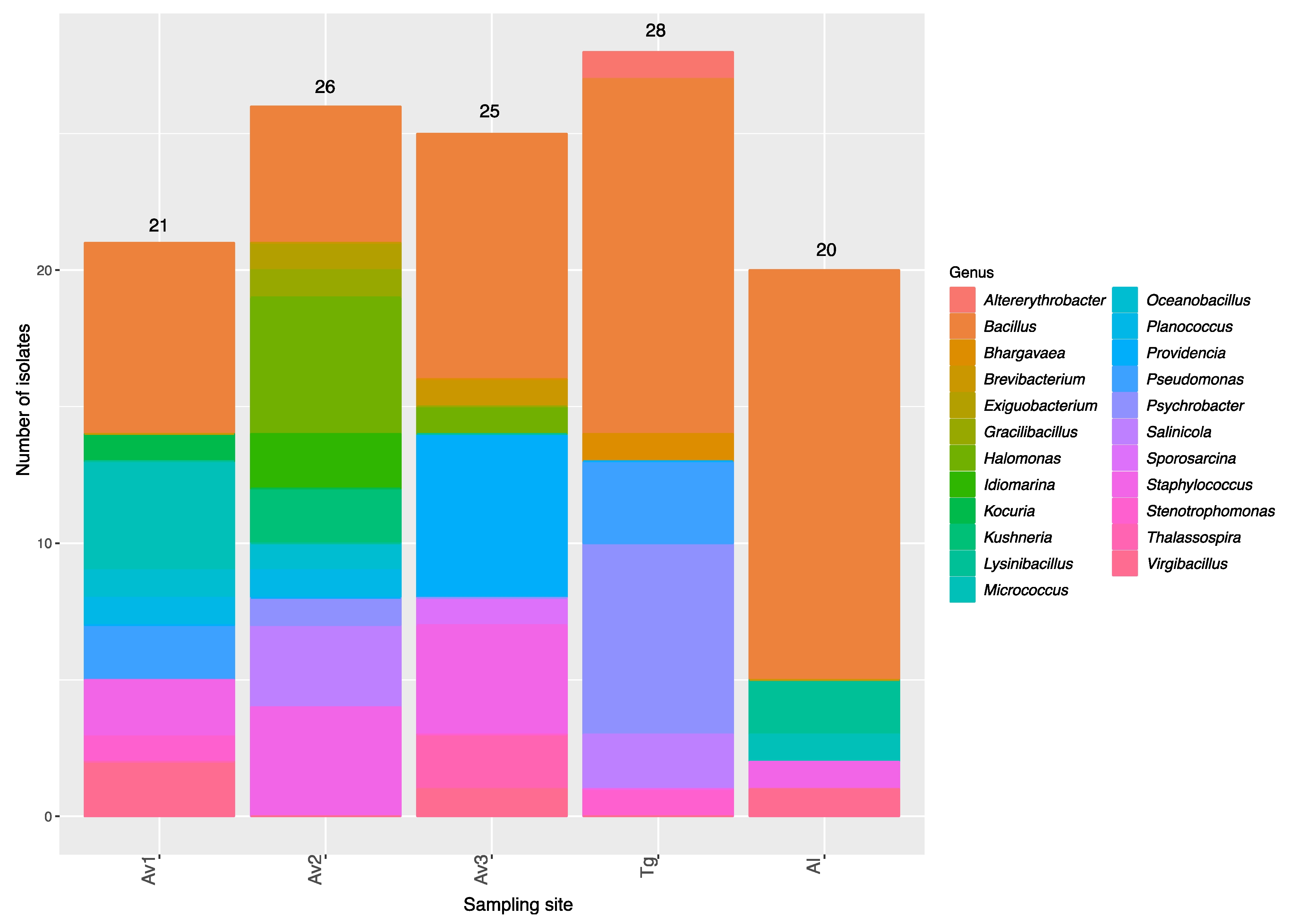
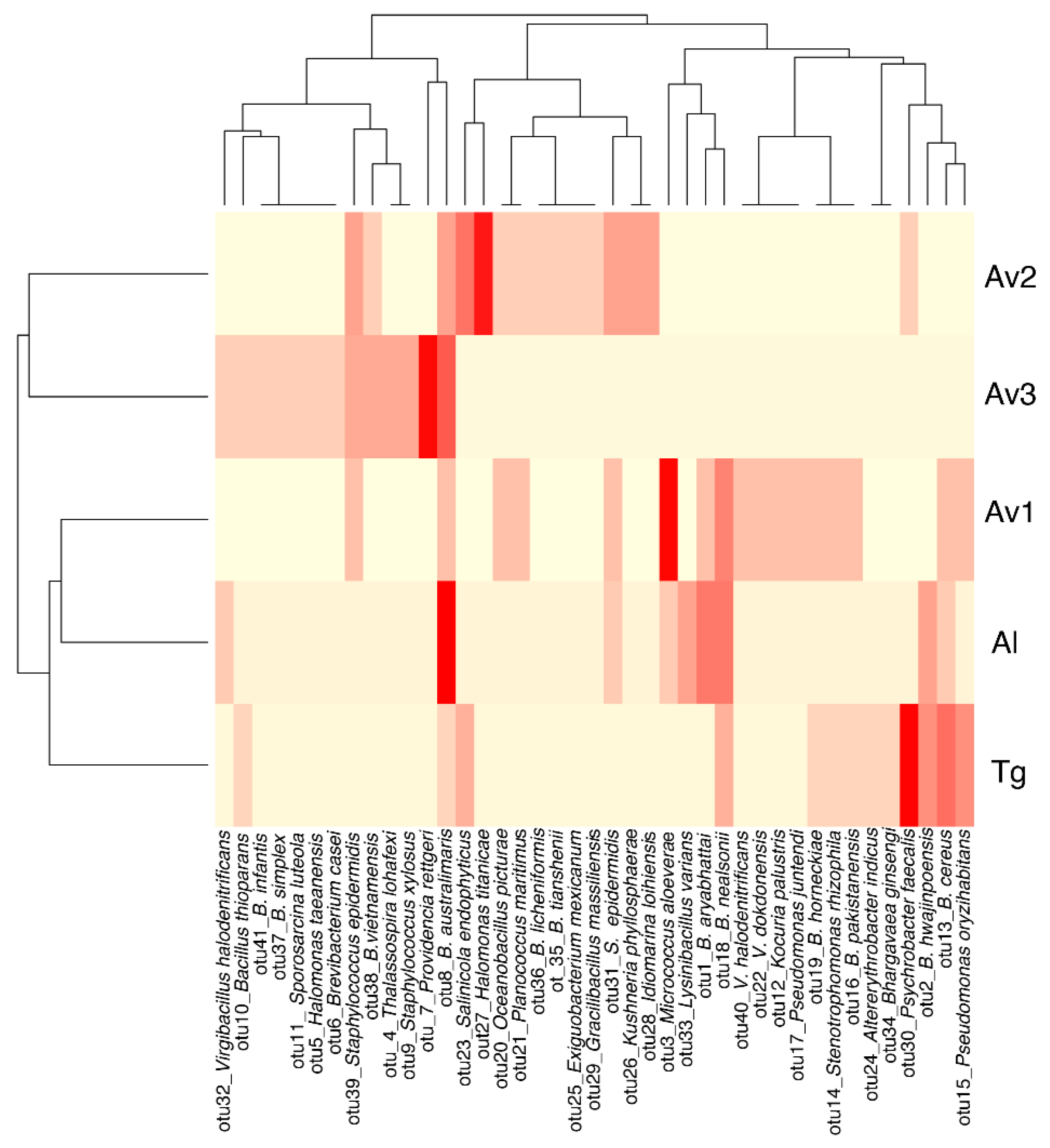
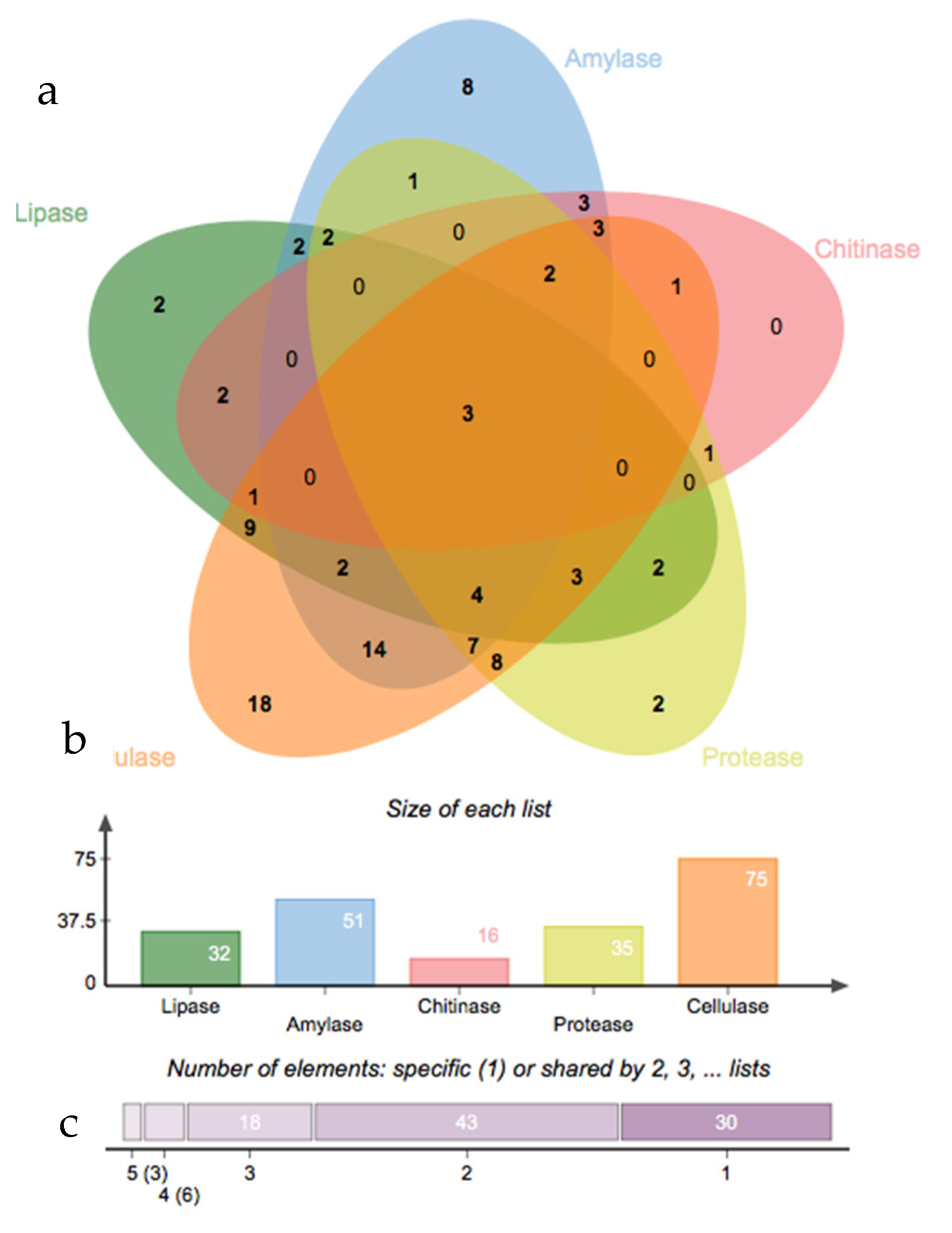
3.2. Rhizosphere and Endosphere Bacterial Isolates
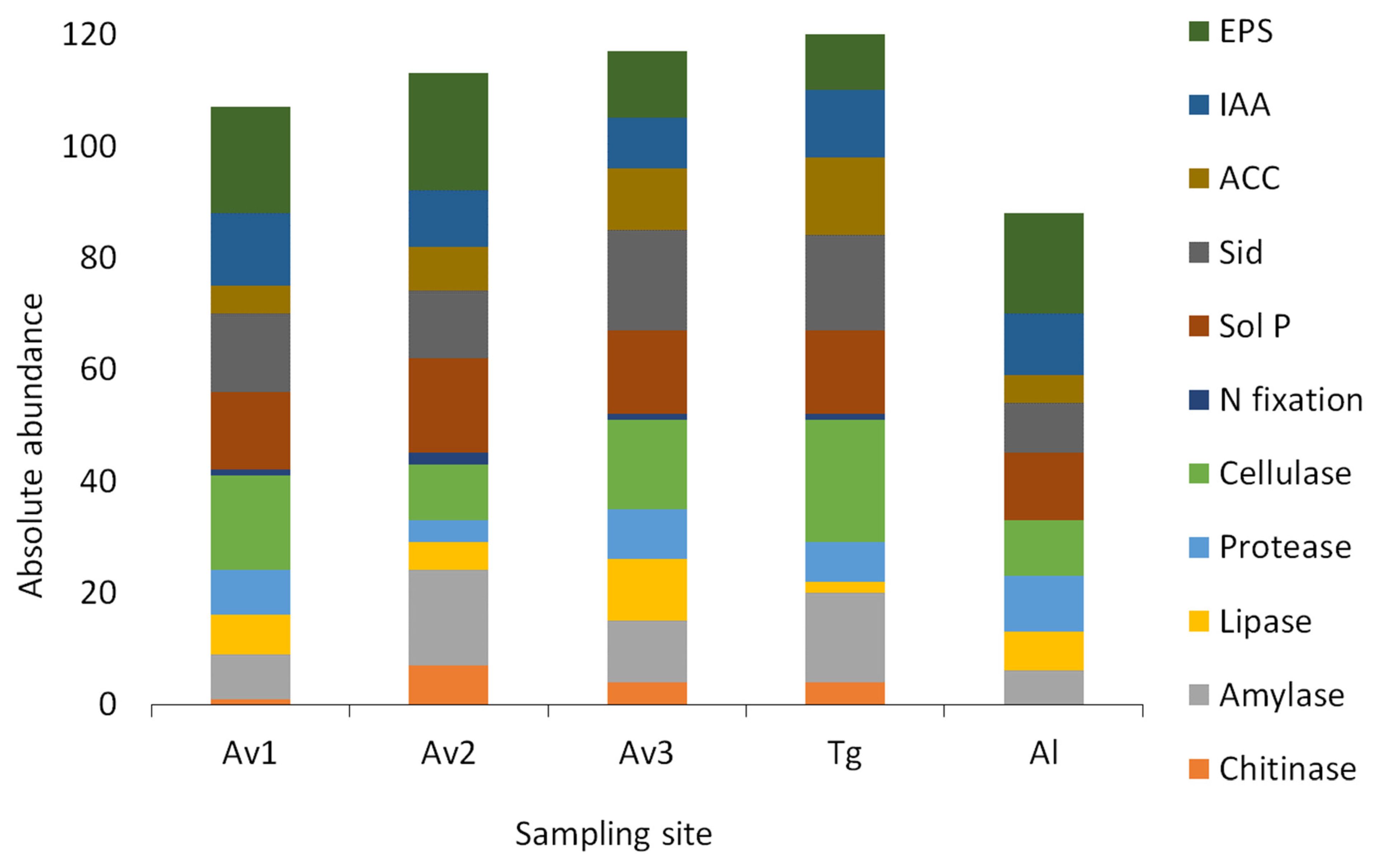
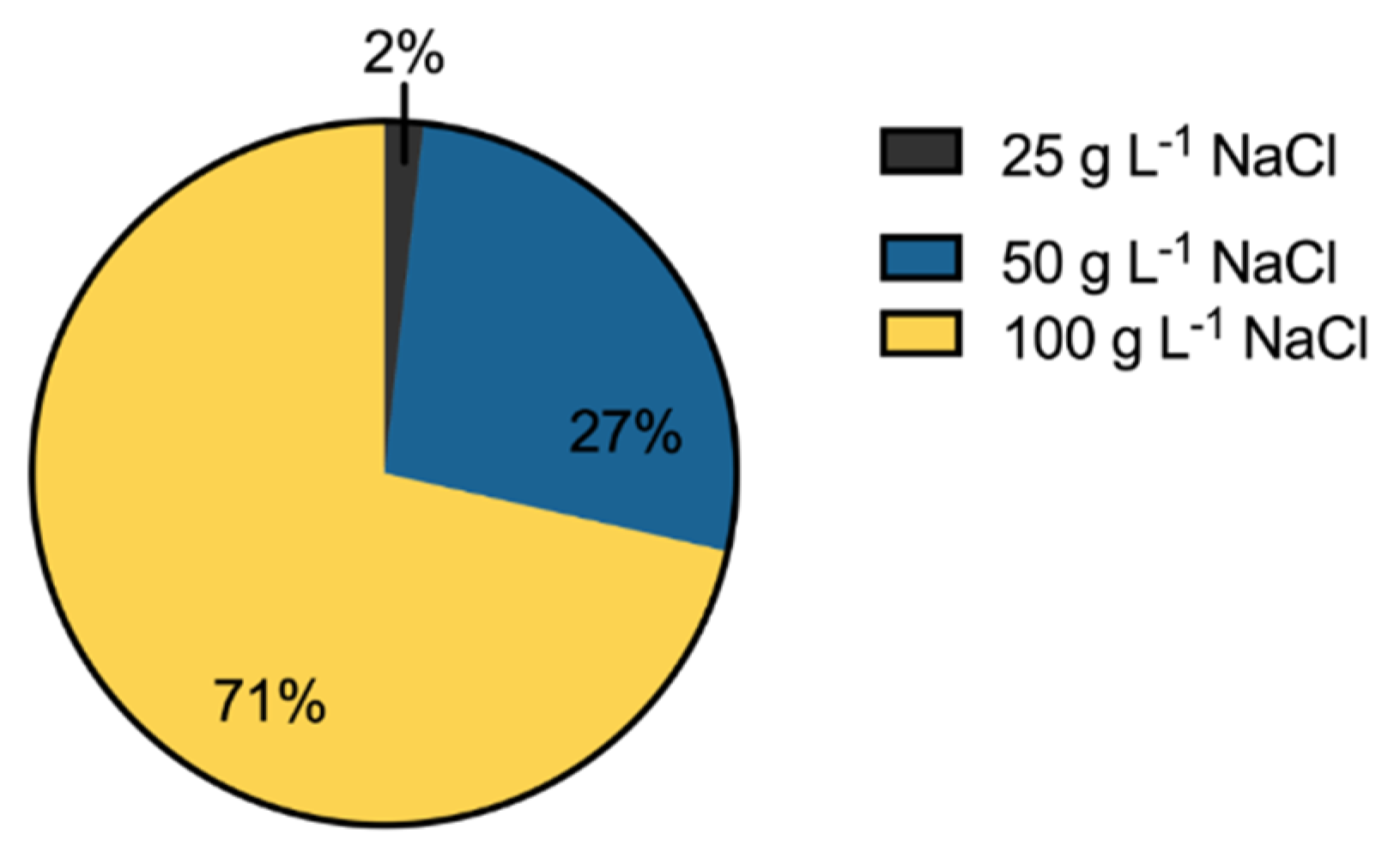
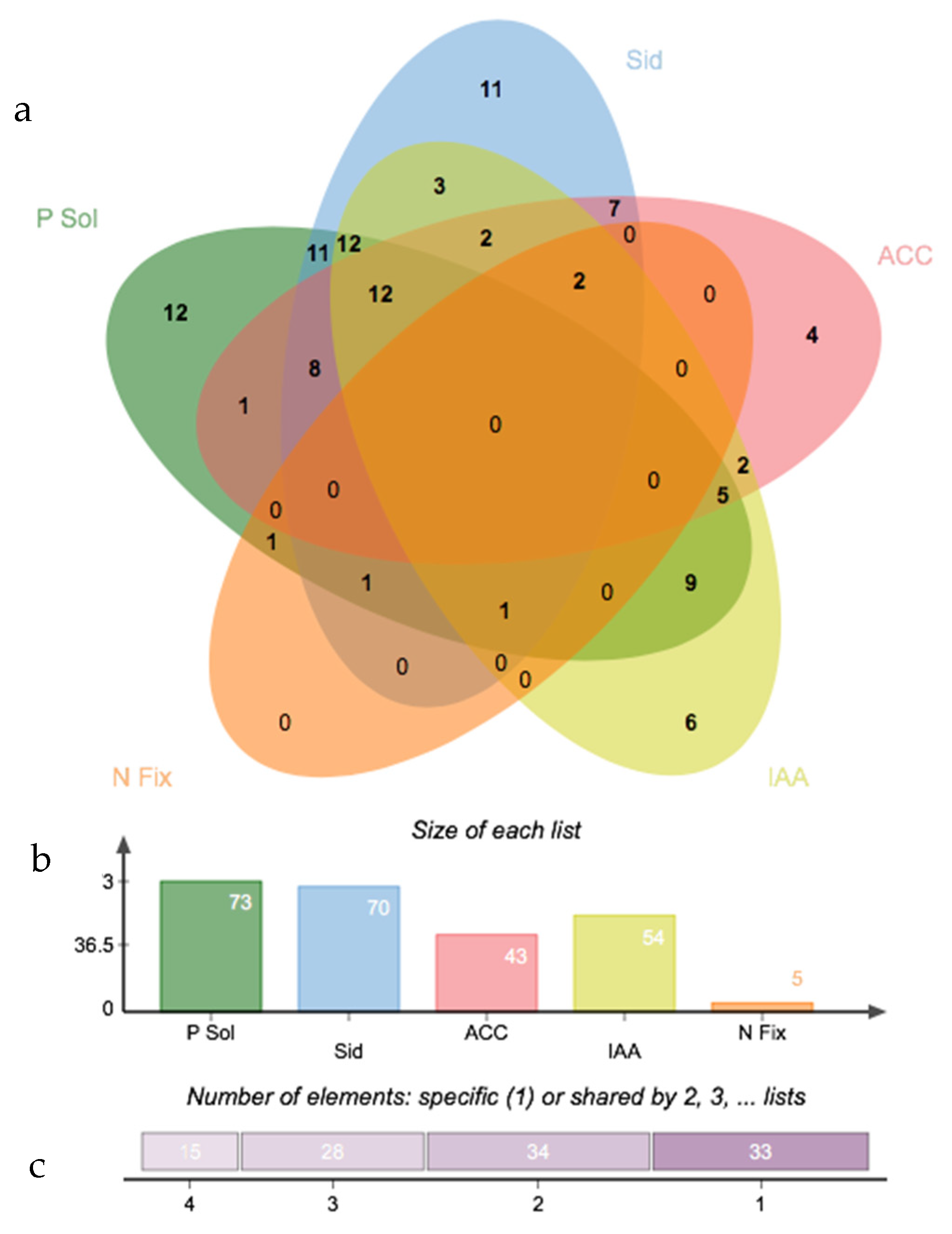
3.3. Salinity Tolerance, Extracellular Enzymatic Activity and Plant-Growth Promoting Traits of Bacterial Isolates
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Isolates | Identification | Limit Salt Tolerance (g L−1) | Extracellular Enzymes | Plant-Growth Promoting Traits | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Code | Site | Source | Phylotype | Candidate Identification a | Q 1 | A 2 | L 3 | P 4 | C 5 | P-Solubilization | Siderophore | ACC Deaminase (nm mg−1 h−1) | IAA (µg mL−1) | N-Fixation | EPS (OD540) | |
| EA1 | Al | E | OTU1 | Bacillus aryabhattai | 100 | − | + | − | + | + | + | − | − | − | − | 0.33 ± 0.01 |
| EA9 | Al | E | 100 | − | − | − | − | − | + | − | − | − | − | 0.46 ± 0.02 | ||
| RA2 | Al | R | 25 | − | + | − | + | + | + | − | − | − | − | 0.20 ± 0.01 | ||
| SP20 | Av1 | R | 50 | + | + | + | + | + | + | + | − | − | − | 0.80 ± 0.01 | ||
| EA4A | Al | E | OTU2 | Bacillus hwajinpoensis | 25 | − | + | − | + | + | − | + | + (38.11 ± 15.67) | − | − | 0.32 ± 0.02 |
| EA4B | Al | E | 100 | − | + | − | − | + | − | − | − | − | − | 0.79 ± 0.02 | ||
| EL9 | Tg | E | 100 | − | + | − | + | + | − | − | − | 9.81 ± 0.46 | − | 0.66 ± 0.01 | ||
| RL11A | Tg | R | 100 | + | + | − | − | + | + | + | + (22.11 ± 5.0) | − | − | 0.33 ± 0.02 | ||
| RL21 | Tg | R | 100 | − | + | − | − | + | − | + | +* (19.57 ± 9.2) | − | − | 0.92 ± 0.03 | ||
| EA7 | Al | E | OTU3 | Micrococcus aloeverae | 100 | − | − | − | + | + | − | + | Doubtful (±) (23.01 ± 15.22) | 18.28 ± 1.96 | − | 0.46 ± 0.02 |
| EH1 | Av1 | E | 100 | − | + | − | + | + | + | − | +* | 11.58 ± 0.53 | − | 0.70 ± 0.01 | ||
| EH14 | Av1 | E | 100 | − | + | + | − | + | + | + | − | − | − | − | ||
| EH21 | Av1 | E | 100 | − | − | + | + | + | − | + | +* | 10.98 ± 1.34 | − | 0.71 ± 0.01 | ||
| EH24 | Av1 | E | 100 | − | − | + | − | + | − | + | Doubtful (±) (12.47 ± 7.29) | − | − | 0.44 ± 0.01 | ||
| EB1 | Av3 | E | OTU4 | Thalassospira lohafexi | 100 | − | + | − | − | − | + | − | − | − | − | |
| EB49 | Av3 | E | 100 | − | + | − | − | + | + | − | − | 21.18 ± 1.5 | − | − | ||
| EB2 | Av3 | E | OTU5 | Halomonas taeanensis | 100 | − | − | − | − | − | − | + | +* (15.98 ± 2.3) | − | − | − |
| EB3 | Av3 | E | OTU6 | Brevibacterium casei | 100 | − | − | − | + | + | − | + | + (17.63 ± 1.35) | 10.87 ± 0.48 | + | 1.24 ± 0.03 |
| EB5 | Av3 | E | OTU7 | Providencia rettgeri | 100 | − | − | − | − | + | + | − | +* | 25.50 ± 2.3 | − | − |
| EB7 | Av3 | E | 50 | − | − | − | − | + | + | + | +* | 27.85 ± 1.37 | − | − | ||
| EB41B | Av3 | E | 100 | − | − | − | + | − | + | + | +* | 15.17 ± 0.96 | − | − | ||
| EB40 | Av3 | E | 100 | − | + | − | − | − | + | + | +* (14.04 ± 4.44) | 17.87 ± 0.54 | − | 2.39 ± 0.1 | ||
| EB41A | Av3 | E | 50 | − | − | − | − | − | + | + | − | − | − | − | ||
| EB42 | Av3 | E | 100 | − | − | − | − | − | + | + | − | − | − | 0.32 ± 0.0 | ||
| EB22 | Av3 | E | OTU8 | Bacillus australimaris | 50 | − | − | + | + | + | + | + | +* | − | − | 0.48 ± 0.01 |
| EB25 | Av3 | E | 50 | − | − | − | − | − | − | + | +* (16.13 ± 2.3) | − | − | − | ||
| EB39 | Av3 | E | 100 | − | + | + | − | − | + | + | +* (14.04 ± 4.68) | 9.47 ± 0.24 | − | 0.35 ± 0.01 | ||
| EH8 | Av1 | E | 50 | − | − | − | + | + | + | − | − | − | − | 2.15 ± 0.13 | ||
| ES13 | Av2 | E | 100 | − | + | − | − | − | − | − | − | 10.53 ± 0.41 | − | − | ||
| ES22 | Av2 | E | 100 | + | + | − | − | − | + | − | − | − | − | 0.54 ± 0.03 | ||
| RA9 | Al | R | 100 | − | − | + | + | − | + | + | +* | 10.28 ± 0.94 | − | 0.63 ± 0.02 | ||
| RA10 | Al | R | 100 | − | − | + | + | − | + | + | + (17.48 ± 3.31) | 10.33 ± 1.36 | − | 0.85 ± 0.03 | ||
| RA14 | Al | R | 50 | − | − | − | + | + | + | + | − | 9.64 ± 1.15 | − | 0.42 ± 0.03 | ||
| RA25 | Al | R | 100 | − | − | + | − | − | + | + | − | 11.04 ± 3.01 | − | 0.54 ± 0.05 | ||
| RA27 | Al | R | 100 | − | − | + | − | + | + | − | − | 13.71 ± 7.15 | − | − | ||
| RA33 | Al | R | 100 | − | − | + | − | + | + | + | +* | 11.91 ± 6.31 | − | 0.53 ± 0.02 | ||
| RL9 | Tg | R | 100 | + | − | + | − | − | + | + | +* | − | − | 0.40 ± 0.02 | ||
| SB4 | Av3 | R | 100 | + | + | + | + | + | − | − | − | − | − | 0.3 ± 0.01 | ||
| EB45 | Av3 | E | OTU9 | Staphylococcus xylosus | 100 | − | − | + | − | + | + | + | +* | − | − | 0.91 ± 0.02 |
| EB46 | Av3 | E | 100 | − | − | + | − | + | + | + | − | − | − | 2.92 ± 0.2 | ||
| EB48 | Av3 | E | OTU10 | Bacillus thioparans | 100 | − | + | − | − | + | − | − | − | − | − | |
| RL1 | Tg | R | 50 | − | + | − | − | + | − | + | − | − | − | − | ||
| EB50 | Av3 | E | OTU11 | Sporosarcina luteola | 50 | − | − | + | + | + | + | + | +* | − | − | − |
| EH2 | Av1 | E | OTU12 | Kocuria palustris | 100 | − | − | − | + | + | + | + | +* | 11.85 ± 0.29 | − | 0.92 ± 0.05 |
| EH6 | Av1 | E | OTU13 | Bacillus cereus | 50 | − | + | + | + | + | + | + | − | 10.65 ± 0.76 | − | 0.42 ± 0.0 |
| EL6 | Tg | E | 50 | − | + | − | − | + | + | − | − | 5.99 ± 1.84 | − | − | ||
| RA5 | Al | R | 50 | − | − | + | − | − | − | − | + (14.19 ± 0.69) | − | − | 0.49 ± 0.07 | ||
| RL11B | Tg | R | 50 | + | + | − | + | + | + | + | − | − | − | − | ||
| RL15 | Tg | R | 50 | + | + | − | + | + | + | + | +* | − | − | 0.33 ± 0.02 | ||
| RL25 | Tg | R | 100 | − | + | − | − | + | + | − | − | − | − | − | ||
| EH7 | Av1 | E | OTU14 | Stenotrophomonas rhizophila | 50 | − | − | − | + | + | + | + | − | 15.02 ± 0.31 | + | 0.31 ± 0.0 |
| EL11 | Tg | E | 50 | − | + | − | + | − | + | − | +* | 14.59 ± 1.05 | − | − | ||
| EH9 | Av1 | E | OTU15 | Pseudomonas oryzihabitans | 50 | − | − | − | − | + | + | − | +* (26.9 ± 14.13) | 17.27 ± 1.62 | − | − |
| RL17 | Tg | R | 100 | − | − | − | − | + | − | − | − | − | − | − | ||
| RL18 | Tg | R | 100 | − | + | − | − | + | + | + | +* (44.09 ± 4.33) | 39.55 ± 1.01 | − | 0.40 ± 0.01 | ||
| RL20 | Tg | R | 50 | − | + | − | − | + | + | − | − | 23.69 ± 1.94 | − | 0.85 ± 0.02 | ||
| EH10 | Av1 | E | OTU16 | Bacillus pakistanensis | 100 | − | − | − | − | − | − | + | − | − | − | 0.34 ± 0.02 |
| RL16 | Tg | R | 100 | − | + | − | − | + | − | + | + (35.57 ± 10.0) | − | − | 0.31 ± 0.02 | ||
| EH11 | Av1 | E | OTU17 | Pseudomonas juntendi | 100 | − | + | − | − | + | + | + | − | 10.90 ± 2.07 | − | 0.97 ± 0.02 |
| EH12 | Av1 | E | OTU18 | Bacillus nealsonii | 50 | − | + | + | − | + | + | − | − | 20.92 ± 0.69 | − | 0.47 ± 0.02 |
| EH13 | Av1 | E | 50 | − | + | − | − | − | + | − | − | 20.99 ± 0.51 | − | 0.60 ± 0.01 | ||
| EL10 | Tg | E | 50 | − | − | − | − | − | + | − | − | 13.12 ± 0.52 | − | 0.33 ± 0.04 | ||
| EL12 | Tg | E | 50 | − | + | − | + | + | − | + | +* | − | − | − | ||
| RA3 | Al | R | 50 | − | + | − | + | − | + | − | − | 9.80 ± 0.51 | − | 0.60 ± 0.03 | ||
| RA30 | Al | R | 100 | − | + | − | + | + | + | − | − | 13.63 ± 0.37 | − | 0.44 ± 0.04 | ||
| RA32 | Al | R | 100 | − | − | − | + | − | + | − | − | − | − | 0.69 ± 0.06 | ||
| EH16 | Av1 | E | OTU19 | Bacillus horneckiae | 100 | − | − | − | − | + | + | + | − | 12.42 ± 0.35 | − | 0.43 ± 0.01 |
| RL5 | Tg | R | 100 | − | − | − | − | − | − | + | +* | − | − | 0.34 ± 0.04 | ||
| EH19 | Av1 | E | OTU20 | Oceanobacillus picturae | 100 | − | + | − | − | + | − | + | Doubtful (±) (20.32 ± 14.05) | 55.33 ± 1.38 | − | 0.41 ± 0.01 |
| ES4 | Av2 | E | 50 | − | − | − | − | − | + | + | +* | 11.97 ± 4.46 | − | 0.37 ± 0.01 | ||
| EH20 | Av1 | E | OTU21 | Planococcus maritimus | 100 | − | − | − | + | + | − | + | − | − | − | 0.44 ± 0.01 |
| SF92 | Av2 | R | 100 | − | − | + | − | + | + | − | + (21.21 ± 1.19) | 14.60 ± 5.76 | − | − | ||
| EH25 | Av1 | E | OTU22 | Virgibacillus dokdonensis | 100 | − | − | − | − | − | − | + | − | − | − | 0.61 ± 0.01 |
| EL13 | Tg | E | OTU23 | Salinicola endophyticus | 100 | − | + | − | − | + | + | + | +* | 71.35 ± 6.86 | − | 0.54 ± 0.01 |
| EL14 | Tg | E | 100 | − | + | − | − | + | + | + | − | 49.18 ± 25.05 | 1.35 ± 0.03 | |||
| ES6 | Av2 | E | 100 | − | + | − | − | + | + | − | +* (16.95 ± 4.12) | − | − | 0.45 ± 0.02 | ||
| ES7 | Av2 | E | 100 | − | + | − | − | + | + | − | − | − | − | 0.33 ± 0.03 | ||
| ES25 | Av2 | E | 100 | − | + | − | − | − | + | + | − | − | − | 0.56 ± 0.04 | ||
| EL16 | Tg | E | OTU24 | Altererythrobacter indicus | 50 | − | − | − | − | + | − | + | +* | 45.66 ± 2.11 | − | − |
| ES1 | Av2 | E | OTU25 | Exiguobacterium mexicanum | 100 | − | + | − | + | + | + | − | − | − | − | 0.40 ± 0.02 |
| ES2 | Av2 | E | OTU26 | Kushneria phyllosphaerae | 100 | − | + | − | − | + | + | + | − | − | + | 0.56 ± 0.02 |
| ES18 | Av2 | E | 100 | − | + | − | − | + | − | − | +* | − | − | 0.56 ± 0.03 | ||
| ES3 | Av2 | E | OTU27 | Halomonas titanicae | 100 | − | − | − | − | − | + | − | − | 12.22 ± 0.50 | − | − |
| ES10 | Av2 | E | 100 | − | + | − | − | − | + | − | + (24.8 ± 14.51) | − | + | 0.58 ± 0.02 | ||
| ES12 | Av2 | E | 100 | + | + | − | − | − | − | − | + (51.49 ± 4.17) | − | − | 0.63 ± 0.02 | ||
| ES14 | Av2 | E | 100 | + | + | − | − | + | + | +* (45.32 ± 4.5) | 10.45 ± 0.18 | − | 0.61 ± 0.04 | |||
| ES15 | Av2 | E | 50 | − | + | − | − | − | − | − | +* (23.91 ± 3.17) | − | − | 2.65 ± 0.1 | ||
| ES17 | Av2 | E | OTU28 | Idiomarina loihiensis | 100 | − | + | − | − | − | − | − | − | − | − | 0.80 ± 0.05 |
| ES20 | Av2 | E | 100 | − | − | − | − | − | − | − | − | − | − | 1.82 ± 0.07 | ||
| ES19 | Av2 | E | OTU29 | Gracilibacillus massiliensis | 50 | − | + | + | − | − | − | + | − | − | − | 0.50 ± 0.01 |
| ES21 | E | OTU30 | Psychrobacter faecalis | 100 | + | − | + | − | − | + | + | Doubtful (±) (13.14 ± 3.5) | 12.11 ± 0.58 | − | 0.36 ± 0.02 | |
| RL3 | Tg | R | 100 | − | − | + | − | + | − | + | + (25.03 ± 2.85) | 59.64 ± 4.50 | + | 0.32 ± 0.0 | ||
| RL4 | Tg | R | 100 | − | − | − | − | + | − | − | +* (12.09 ± 1.13) | 22.06 ± 0.92 | − | − | ||
| RL6 | Tg | R | 100 | − | − | − | − | − | − | − | − | 31.50 ± 0.58 | − | − | ||
| RL13 | Tg | R | 50 | − | + | − | + | + | + | + | + (23.91 ± 1.27) | − | − | − | ||
| RL14 | Tg | R | 100 | − | − | − | − | − | − | − | 14.02 ± 0.35 | − | − | |||
| RL19 | Tg | R | 100 | − | − | − | − | + | + | + | − | 17.79 ± 1.88 | − | 0.42 ± 0.01 | ||
| RL23 | Tg | R | 100 | − | − | − | − | + | + | + | + (25.03 ± 10.31) | − | − | 0.40 ± 0.01 | ||
| RA13 | Al | R | OTU31 | Staphylococcus epidermidis | 100 | − | − | + | − | + | + | + | + (18.67 ± 2.47) | − | − | 1.10 ± 0.09 |
| SF69 | Av2 | R | 100 | + | + | − | − | + | + | + | − | 15.86 ± 2.02 | − | 0.64 ± 0.02 | ||
| SF68 | Av2 | R | 100 | − | − | − | − | + | + | + | − | 10.67 ± 4.79 | − | 0.71 ± 0.02 | ||
| SP32 | Av1 | R | 50 | + | − | − | − | + | + | + | − | − | − | 0.98 ± 0.03 | ||
| RA21 | Al | R | OTU32 | Virgibacillus halodenitrificans | 100 | − | − | − | − | − | − | − | − | − | − | 0.61 ± 0.07 |
| SB4B | Av3 | R | 100 | + | + | − | − | + | − | + | − | − | − | 0.47 ± 0.01 | ||
| RA24 | Al | R | OTU33 | Lysinibacillus varians | 100 | − | − | − | − | − | − | − | − | 28.08 ± 0.82 | − | 0.34 ± 0.05 |
| RA35 | Al | R | 100 | − | − | − | − | − | − | − | +* (12.39 ± 0.69) | 17.94 ± 0.61 | − | 0.59 ± 0.04 | ||
| RL12 | Tg | R | OTU34 | Bhargavaea ginsengi | 100 | − | − | − | + | + | − | − | − | − | − | − |
| SF27 | Av2 | R | OTU35 | Bacillus tianshenii | 100 | − | + | + | + | − | + | + | + (12.69 ± 1.96) | − | − | 0.46 ± 0.01 |
| SF100 | Av2 | R | OTU36 | Bacillus licheniformis | 100 | − | − | − | − | + | − | + | Doubtful (±) (10.3 ± 2.92) | − | − | − |
| SB115 | Av3 | R | OTU37 | Bacillus simplex | 50 | − | + | + | + | + | − | − | Doubtful (±) (12.02 ± 3.54) | 10.60 ± 3.53 | − | 3.22 ± 0.01 |
| SF59 | Av2 | R | OTU38 | Bacillus vietnamensis | 50 | + | + | + | + | + | − | − | + (22.11 ± 3.14) | − | − | 0.39 ± 0.02 |
| SB113 | Av3 | R | 50 | − | + | + | + | + | − | + | − | − | − | 0.35 ± 0.0 | ||
| SB8 | Av3 | R | 100 | − | + | + | + | − | − | + | − | − | − | − | ||
| SB78 | Av3 | R | OTU39 | Staphylococcus epidermidis | 25 | − | − | − | − | + | + | + | − | − | − | − |
| SF91 | Av2 | R | 25 | − | − | − | − | − | + | + | + (141.86 ± 1.27) | 9.27 ± 2.78 | − | − | ||
| SP103 | Av1 | R | 50 | − | − | − | − | − | + | − | − | 19.12 ± 3.46 | − | 0.39 ± 0.01 | ||
| SF104 | Av2 | R | 100 | + | − | − | + | − | + | + | + (13.81 ± 0.95) | 9.79 ± 3.98 | − | 1.35 ± 0.05 | ||
| SB112 | Av3 | R | 100 | + | − | + | − | + | + | − | − | − | − | 0.40 ± | ||
| SP86 | Av1 | R | OTU40 | Virgibacillus halodenitrificans | 100 | − | − | + | − | + | − | − | − | 11.34 ± 5.3 | − | 1.58 ± 0.06 |
| SB102 | Av3 | R | OTU41 | Bacillus infantis | 100 | + | + | + | + | + | − | + | Doubtful (+−+) (12.39 ± 2.99) | 25.19 ± 5.36 | − | − |
| Control (+) | Pseudomonas aeruginosa | 115.4 | 47.26 ± 0.66 | 0.66 ± 0.02 | ||||||||||||
| Av1 | Av2 | Av3 | Tg | Al | |
|---|---|---|---|---|---|
| Chitinase | 5% | 27% | 16% | 14% | 0% |
| Amylase | 38% | 65% | 44% | 57% | 30% |
| Lipase | 33% | 19% | 44% | 7% | 35% |
| Protease | 38% | 15% | 36% | 25% | 50% |
| Cellulase | 81% | 38% | 64% | 79% | 50% |
| N fixation | 5% | 8% | 4% | 4% | 0% |
| Sol P | 67% | 65% | 60% | 54% | 60% |
| Siderophores | 67% | 46% | 72% | 61% | 45% |
| ACC deaminase | 24% | 42% | 44% | 75% | 35% |
| IAA | 62% | 38% | 36% | 43% | 55% |
| EPS | 90% | 81% | 48% | 54% | 90% |
References
- Yuan, Z.; Druzhinina, I.S.; Labbé, J.; Redman, R.; Qin, Y.; Rodriguez, R.; Zhang, C.; Tuskan, G.A.; Lin, F. Specialized microbiome of a halophyte and its role in helping non-host plants to withstand salinity. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef]
- Volkov, V.; Beilby, M.J. Editorial: Salinity tolerance in plants: Mechanisms and regulation of ion transport. Front. Plant Sci. 2017, 8, 1795. [Google Scholar] [CrossRef] [PubMed]
- Sgroy, V.; Cassán, F.; Masciarelli, O.; Del Papa, M.F.; Lagares, A.; Luna, V. Isolation and characterization of endophytic plant growth-promoting (PGPB) or stress homeostasis-regulating (PSHB) bacteria associated to the halophyte Prosopis strombulifera. Appl. Microbiol. Biotechnol. 2009, 85, 371–381. [Google Scholar] [CrossRef]
- Bragina, A.; Oberauner-Wappis, L.; Zachow, C.; Halwachs, B.; Thallinger, G.G.; Müller, H.; Berg, G. The Sphagnum microbiome supports bog ecosystem functioning under extreme conditions. Mol. Ecol. 2014, 23, 4498–4510. [Google Scholar] [CrossRef] [PubMed]
- Trognitz, F.; Hackl, E.; Widhalm, S.; Sessitsch, A. The role of plant-microbiome interactions in weed establishment and control. FEMS Microbiol. Ecol. 2016, 92, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Hartman, K.; van der Heijden, M.G.A.; Roussely-Provent, V.; Walser, J.C.; Schlaeppi, K. Deciphering composition and function of the root microbiome of a legume plant. Microbiome 2017, 5, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egamberdieva, D.; Wirth, S.; Bellingrath-Kimura, S.D.; Mishra, J.; Arora, N.K. Salt-Tolerant Plant Growth Promoting Rhizobacteria for Enhancing Crop Productivity of Saline Soils. Front. Microbiol. 2019, 10, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Dimkpa, C.; Weinand, T.; Asch, F. Plant-rhizobacteria interactions alleviate abiotic stress conditions. Plant Cell Environ. 2009, 32, 1682–1694. [Google Scholar] [CrossRef] [PubMed]
- Jha, B.; Gontia, I.; Hartmann, A. The roots of the halophyte Salicornia brachiata are a source of new halotolerant diazotrophic bacteria with plant growth-promoting potential. Plant Soil 2012, 356, 265–277. [Google Scholar] [CrossRef]
- Ruppel, S.; Franken, P.; Witzel, K. Properties of the halophyte microbiome and their implications for plant salt tolerance. Funct. Plant Biol. 2013, 40, 940–951. [Google Scholar] [CrossRef] [Green Version]
- Castroviejo, S. Salicornia L. Stud. Plant Sci. 1991, 2, 69. [Google Scholar] [CrossRef]
- Silva, H.; Caldeira, G.; Freitas, H. Salicornia ramosissima population dynamics and tolerance of salinity. Ecol. Res. 2007, 22, 125–134. [Google Scholar] [CrossRef] [Green Version]
- Jefferies, R.L.; Gottlieb, L.D. Genetic differentiation of the microspecies Salicornia europaea L. (sensu stricto) and S. ramosissima. J. Woods. New Phytol. 1982. [Google Scholar] [CrossRef]
- Milić, D.; Luković, J.; Dan, M.; Zorić, L.; Obreht, D.; Veselić, S.; Anačkov, G.; Petanidou, T. Identification of salicornia population: Anatomical characterization and RAPD fingerprinting. Arch. Biol. Sci. 2011. [Google Scholar] [CrossRef]
- Ingrouille, M.; Pearson, J. The pattern of morphological variation in the Salicornia europaea L. aggregate (Chenopodiaceae). Watsonia 1987. [Google Scholar]
- Bae, J.Y.; Park, L.Y.; Lee, S.H. Effect of Salicornia herbacea L. powder on the quality characteristics of bread. J. Korean Soc. Food Sci. Nutr. 2008. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Kim, H.J.; Rhim, J.W. Vacuum drying characteristics of Salicornia herbacea L. J. Agric. Sci. Technol. 2012, 14, 587–598. [Google Scholar]
- Elsebaie, E.M.; Elsanat, S.Y.; Gouda, M.S.; Elnemr, K.M. Oil and Fatty Acids Composition in Glasswort (Salicornia Fruticosa) Seeds. IOSR J. Appl. Chem. 2013, 4, 06–09. [Google Scholar] [CrossRef]
- Isca, V.M.S.; Seca, A.M.L.; Pinto, D.C.G.A.; Silva, H.; Silva, A.M.S. Lipophilic profile of the edible halophyte Salicornia ramosissima. Food Chem. 2014, 165, 330–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.W.; Lou, K.; Li, C.; Wang, L.; Zhao, Z.Y.; Zhao, S.; Tian, C.Y. Illumina-based analysis of bacterial diversity related to halophytes Salicornia europaea and Sueada aralocaspica. J. Microbiol. 2015, 53, 678–685. [Google Scholar] [CrossRef]
- Furtado, B.U.; Gołebiewski, M.; Skorupa, M.; Hulisz, P.; Hrynkiewicz, K. Bacterial and fungal endophytic microbiomes of Salicornia europaea. Appl. Environ. Microbiol. 2019, 85, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szymańska, S.; Borruso, L.; Brusetti, L.; Hulisz, P.; Furtado, B.; Hrynkiewicz, K. Bacterial microbiome of root-associated endophytes of Salicornia europaea in correspondence to different levels of salinity. Environ. Sci. Pollut. Res. 2018, 25, 25420–25431. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Zhou, N.; Zhao, Z.Y.; Zhang, K.; Wu, G.H.; Tian, C.Y. Isolation of Endophytic Plant Growth-Promoting Bacteria Associated with the Halophyte Salicornia europaea and Evaluation of their Promoting Activity Under Salt Stress. Curr. Microbiol. 2016, 73, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Razzaghi Komaresofla, B.; Alikhani, H.A.; Etesami, H.; Khoshkholgh-Sima, N.A. Improved growth and salinity tolerance of the halophyte Salicornia sp. by co–inoculation with endophytic and rhizosphere bacteria. Appl. Soil Ecol. 2019, 138, 160–170. [Google Scholar] [CrossRef]
- Figueira, C.; Ferreira, M.J.; Silva, H.; Cunha, A. Improved germination efficiency of Salicornia ramosissima seeds inoculated with Bacillus aryabhattai SP1016-20. Ann. Appl. Biol. 2019, 174, 319–328. [Google Scholar] [CrossRef]
- Mesa-Marín, J.; Pérez-Romero, J.A.; Mateos-Naranjo, E.; Bernabeu-Meana, M.; Pajuelo, E.; Rodríguez-Llorente, I.D.; Redondo-Gómez, S. Effect of plant growth-promoting rhizobacteria on salicornia ramosissima seed germination under salinity, CO2 and temperature stress. Agronomy 2019, 9, 655. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, M.; Torres, M.; Blanco, L.; Béjar, V.; Sampedro, I.; Llamas, I. Plant growth-promoting activity and quorum quenching-mediated biocontrol of bacterial phytopathogens by Pseudomonas segetis strain P6. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Piernik, A.; Hrynkiewicz, K.; Wojciechowska, A.; Szymańska, S.; Lis, M.I.; Muscolo, A. Effect of halotolerant endophytic bacteria isolated from Salicornia europaea L. on the growth of fodder beet (Beta vulgaris L.) under salt stress. Arch. Agron. Soil Sci. 2017, 63, 1404–1418. [Google Scholar] [CrossRef]
- Hrynkiewicz, K.; Patz, S.; Ruppel, S. Salicornia europaea L. as an underutilized saline-tolerant plant inhabited by endophytic diazotrophs. J. Adv. Res. 2019, 19, 49–56. [Google Scholar] [CrossRef]
- Schulte, E.E.; Hopkins, B.G. Estimation of Soil Organic Matter by Weight Loss-On-Ignition. In Soil Organic Matter: Analysis and Interpretation; Magdoff, F., Tabatabai, M.A., Hanlon, E.A., Eds.; Soil Science Society of America: Madison, WI, USA, 1996; Volume 049, pp. 21–31. [Google Scholar]
- Zaman, M.; Shahid, S.A.; Heng, L. Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Springer: Berlin, Germany, 2018; ISBN 9783319961897. [Google Scholar]
- Sonmez, S.; Buyuktas, D.; Okturen, F.; Citak, S. Assessment of different soil to water ratios (1:1, 1:2.5, 1:5) in soil salinity studies. Geoderma 2008. [Google Scholar] [CrossRef]
- Barillot, C.D.C.; Sarde, C.O.; Bert, V.; Tarnaud, E.; Cochet, N. A standardized method for the sampling of rhizosphere and rhizoplan soil bacteria associated to a herbaceous root system. Ann. Microbiol. 2013, 63, 471–476. [Google Scholar] [CrossRef]
- Oren, A. Life at High Salt Concentrations. Prokaryotes 2006, 3, 263–282. [Google Scholar]
- Szymańska, S.; Płociniczak, T.; Piotrowska-Seget, Z.; Hrynkiewicz, K. Endophytic and rhizosphere bacteria associated with the roots of the halophyte Salicornia europaea L.—Community structure and metabolic potential. Microbiol. Res. 2016, 192, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cole, J.R.; Wang, Q.; Fish, J.A.; Chai, B.; McGarrell, D.M.; Sun, Y.; Brown, C.T.; Porras-Alfaro, A.; Kuske, C.R.; Tiedje, J.M. Ribosomal Database Project: Data and tools for high throughput rRNA analysis. Nucleic Acids Res. 2014. [Google Scholar] [CrossRef] [Green Version]
- Agarwala, R.; Barrett, T.; Beck, J.; Benson, D.A.; Bollin, C.; Bolton, E.; Bourexis, D.; Brister, J.R.; Bryant, S.H.; Canese, K.; et al. Database Resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2017. [Google Scholar] [CrossRef] [Green Version]
- Yoon, S.H.; Ha, S.M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 2017. [Google Scholar] [CrossRef]
- Brígido, C.; Singh, S.; Menéndez, E.; Tavares, M.J.; Glick, B.R.; Do Rosário Félix, M.; Oliveira, S.; Carvalho, M. Diversity and functionality of culturable endophytic bacterial communities in chickpea plants. Plants 2019, 8, 42. [Google Scholar] [CrossRef] [Green Version]
- Kouker, G.; Jaeger, K.E. Specific and sensitive plate assay for bacterial lipases. Appl. Environ. Microbiol. 1987, 53, 211–213. [Google Scholar] [CrossRef] [Green Version]
- Dunne, C.; Crowley, J.J.; Moënne-Loccoz, Y.; Dowling, D.N.; De Bruijn, F.J.; O’Gara, F. Biological control of Pythium ultimum by Stenotrophomonas maltophilia W81 is mediated by an extracellular proteolytic activity. Microbiology 1997, 143, 3921–3931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordon, S.A.; Weber, R.P. Colorimetric Estimation of Inodoleacetic Acid. Plant Physiol. 1951, 26, 192–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khare, E.; Arora, N. Effect of Indole-3-Acetic Acid (IAA) Produced by Pseudomonas aeruginosa in Suppression of Charcoal Rot Disease of Chickpea. Curr. Microbiol. 2010, 61, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Penrose, D.M.; Glick, B.R. Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol. Plant. 2003, 118, 10–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honma, M.; Smmomura, T. Metabolism of 1-aminocyclopropane-1-carboxylic acid. Agric. Biol. Chem. 1978, 42, 1825–1831. [Google Scholar] [CrossRef]
- Balado, M.; Souto, A.; Vences, A.; Careaga, V.P.; Valderrama, K.; Segade, Y.; Rodríguez, J.; Osorio, C.R.; Jiménez, C.; Lemos, M.L. Two Catechol Siderophores, Acinetobactin and Amonabactin, Are Simultaneously Produced by Aeromonas salmonicida subsp. salmonicida Sharing Part of the Biosynthetic Pathway. ACS Chem. Biol. 2015, 10, 2850–2860. [Google Scholar] [CrossRef]
- Pérez-Miranda, S.; Cabirol, N.; George-Téllez, R.; Zamudio-Rivera, L.S.; Fernández, F.J. O-CAS, a fast and universal method for siderophore detection. J. Microbiol. Methods 2007, 70, 127–131. [Google Scholar] [CrossRef]
- Vazquez, P.; Holguin, G.; Puente, M.E.; Lopez-Cortes, A.; Bashan, Y. Phosphate-solubilizing microorganisms associated with the rhizosphere of mangroves in a semiarid coastal lagoon. Biol. Fertil. Soils 2000, 30, 460–468. [Google Scholar] [CrossRef]
- Paul, D.; Sinha, S.N. Isolation and characterization of phosphate solubilizing bacterium Pseudomonas aeruginosa KUPSB12 with antibacterial potential from river Ganga, India. Ann. Agrar. Sci. 2017, 15, 130–136. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, U.; Roy, S.; Chakraborty, A.; Dey, P.; Chakraborty, B. Plant Growth Promotion and Amelioration of Salinity Stress in Crop Plants by a Salt-Tolerant Bacterium. Rec. Res. Sci. Tech. 2011, 3, 61–70. [Google Scholar]
- Yu, S.S.; Kyaw, E.P.; Lynn, T.; Latt, Z.K.; Aung, A.; Sev, T.M.; Thet, M. The correlation of carbon source and ammonium accumulation in culture broth by nitrogen-fixing bacterial isolates The correlation of carbon source and ammonium accumulation in culture broth by nitrogen-fixing bacterial isolates. J. Sci. Innov. Res. 2017, 6, 63–67. [Google Scholar]
- Stepanović, S.; Vuković, D.; Dakić, I.; Savić, B.; Švabić-Vlahović, M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods 2000, 40, 175–179. [Google Scholar] [CrossRef]
- Myszka, K.; Czaczyk, K. Characterization of adhesive exopolysaccharide (EPS) produced by Pseudomonas aeruginosa under starvation conditions. Curr. Microbiol. 2009, 58, 541–546. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. 2014. Available online: https://www.R-project.org (accessed on 23 February 2021).
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.; Wagner, H. Vegan: Community Ecology Package. R Package Version 2.0-9. Available online: https://CRAN.R-project.org/package=vegan (accessed on 23 February 2021).
- USDA. Soil Survey Manual Agriculture. Handbook 18; USDA Natural Resources Conservation Service: Washington, DC, USA, 2017.
- Rhoades, J.; Kandiah, A.; Mashali, A. The Use of Saline Waters for Crop Production; Food and Agriculture Organization of the United Nations: Rome, Italy, 1992; ISBN 9251032378. [Google Scholar]
- Etesami, H.; Beattie, G.A. Mining halophytes for plant growth-promoting halotolerant bacteria to enhance the salinity tolerance of non-halophytic crops. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szoboszlay, M.; Näther, A.; Liu, B.; Carrillo, A.; Castellanos, T.; Smalla, K.; Jia, Z.; Tebbe, C.C. Contrasting microbial community responses to salinization and straw amendment in a semiarid bare soil and its wheat rhizosphere. Sci. Rep. 2019. [Google Scholar] [CrossRef] [PubMed]
- Garza, D.R.; Dutilh, B.E. From cultured to uncultured genome sequences: Metagenomics and modeling microbial ecosystems. Cell. Mol. Life Sci. 2015, 72, 4287–4308. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, K.; Shiwa, Y.; Ishige, T.; Sakamoto, H.; Tanaka, K.; Uchino, M.; Tanaka, N.; Oguri, S.; Saitoh, H.; Tsushima, S. Bacterial diversity associated with the rhizosphere and endosphere of two halophytes: Glaux maritima and Salicornia europaea. Front. Microbiol. 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Ravikumar, S.; Inbaneson, S.J.; Uthiraselvam, M.; Priya, S.R.; Ramu, A.; Banerjee, M.B. Diversity of Endophytic Actinomycetes from Karangkadu Mangrove Ecosystem and Its Antibacterial Potential Against Bacterial Pathogens. J. Pharm. Res. 2011, 4, 294–296. [Google Scholar]
- De Ridder-Duine, A.S.; Kowalchuk, G.A.; Klein Gunnewiek, P.J.A.; Smant, W.; Van Veen, J.A.; De Boer, W. Rhizosphere bacterial community composition in natural stands of Carex arenaria (sand sedge) is determined by bulk soil community composition. Soil Biol. Biochem. 2005, 37, 349–357. [Google Scholar] [CrossRef]
- Chaudhary, D.R.; Saxena, J.; Lorenz, N.; Dick, L.K.; Dick, R.P. Microbial Profiles of Rhizosphere and Bulk Soil Microbial Communities of Biofuel Crops Switchgrass (Panicum virgatum L.) and Jatropha (Jatropha curcas L.). Appl. Environ. Soil Sci. 2012, 2012, 906864. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.-Y.; Yuan, X.-F.; Lin, H.-R.; Yang, Y.-Q.; Li, Z.-Y. Differences in soil properties and bacterial communities between the rhizosphere and bulk soil and among different production areas of the medicinal plant Fritillaria thunbergii. Int. J. Mol. Sci. 2011, 12, 3770–3785. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, N.; Beaupré, S.R.; Steen, A.D.; Pearson, A. Sequential bioavailability of sedimentary organic matter to heterotrophic bacteria. Environ. Microbiol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Fidalgo, C.; Henriques, I.; Rocha, J.; Tacão, M.; Alves, A. Culturable endophytic bacteria from the salt marsh plant Halimione portulacoides: Phylogenetic diversity, functional characterization, and influence of metal(loid) contamination. Environ. Sci. Pollut. Res. 2016, 23, 10200–10214. [Google Scholar] [CrossRef] [PubMed]
- Menendez, E.; Garcia-Fraile, P.; Rivas, R. Biotechnological applications of bacterial cellulases. AIMS Bioeng. 2015, 2, 163–182. [Google Scholar] [CrossRef]
- Olanrewaju, O.S.; Glick, B.R.; Babalola, O.O. Mechanisms of action of plant growth promoting bacteria. World J. Microbiol. Biotechnol. 2012, 1, 197. [Google Scholar] [CrossRef] [Green Version]
- Glick, B.R.; Glick, B.R. Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientifica 2012, 2012, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Kaur, T.; Sharma, D.; Kaur, A.; Manhas, R.K. Antagonistic and plant growth promoting activities of endophytic and soil actinomycetes. Arch. Phytopathol. Plant Prot. 2013, 46, 1756–1768. [Google Scholar] [CrossRef]
- Etesami, H.; Alikhani, H.A. Bacillus species as the most promising bacterial biocontrol agents in rhizosphere and endorhiza of plants grown in rotation with each other. Eur. J. Plant Pathol. 2018. [Google Scholar] [CrossRef]
- Szymańska, S.; Płociniczak, T.; Piotrowska-Seget, Z.; Złoch, M.; Ruppel, S.; Hrynkiewicz, K. Metabolic potential and community structure of endophytic and rhizosphere bacteria associated with the roots of the halophyte Aster tripolium L. Microbiol. Res. 2016, 182, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Siddikee, M.A.; Tipayno, S.C.; Kim, K.; Chung, J.; Sa, T. Influence of varying degree of salinity-sodicity stress on enzyme activities and bacterial populations of coastal soils of yellow sea, South Korea. J. Microbiol. Biotechnol. 2011, 21, 341–346. [Google Scholar] [CrossRef]
- Costa, O.Y.A.; Raaijmakers, J.M.; Kuramae, E.E. Microbial extracellular polymeric substances: Ecological function and impact on soil aggregation. Front. Microbiol. 2018, 9, 1636. [Google Scholar] [CrossRef] [Green Version]
- Upadhyay, S.K.; Singh, J.S.; Singh, D.P. Exopolysaccharide-producing plant growth-promoting rhizobacteria under salinity condition. Pedosphere 2011. [Google Scholar] [CrossRef]
- Bremer, E.; Krämer, R. Responses of microorganisms to osmotic stress. Annu. Rev. Microbiol. 2019, 73, 313–334. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Snehi, S.K.; Singh, V. Role of PGPR in Biofilm Formations and Its Importance in Plant Health. Biofilms Plant Soil Health 2017, 27, 27–40. [Google Scholar]
- Khan, M.Z.; Islam, M.A.; Azom, M.G.; Amin, M.S. Short Term Influence of Salinity on Uptake of Phosphorus by Ipomoea aquatica. Int. J. Plant Soil Sci. 2018, 25, 1–9. [Google Scholar] [CrossRef]
- Qin, Y.; Druzhinina, I.S.; Pan, X.; Yuan, Z. Microbially Mediated Plant Salt Tolerance and Microbiome-based Solutions for Saline Agriculture. Biotechnol. Adv. 2016, 34, 1245–1259. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, H.; Fraga, R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol. Adv. 1999, 17, 319–339. [Google Scholar] [CrossRef]
- Yi, Y.; Huang, W.; Ge, Y. Exopolysaccharide: A novel important factor in the microbial dissolution of tricalcium phosphate. World J. Microbiol. Biotechnol. 2008, 24, 1059–1065. [Google Scholar] [CrossRef]
- Hamdali, H.; Bouizgarne, B.; Hafidi, M.; Lebrihi, A.; Virolle, M.J.; Ouhdouch, Y. Screening for rock phosphate solubilizing Actinomycetes from Moroccan phosphate mines. Appl. Soil Ecol. 2008, 38, 12–19. [Google Scholar] [CrossRef]
- Hussain, R.M. The Effect of Phosphorus in Nitrogen Fixation in Legumes. Agric. Res. Technol. Open Access J. 2017, 5, 12–14. [Google Scholar] [CrossRef]
- Rotaru, V.; Sinclair, T.R. Interactive influence of phosphorus and iron on nitrogen fixation by soybean. Environ. Exp. Bot. 2009, 66, 94–99. [Google Scholar] [CrossRef]
- Mapelli, F.; Marasco, R.; Rolli, E.; Barbato, M.; Cherif, H.; Guesmi, A.; Ouzari, I.; Daffonchio, D.; Borin, S. Potential for plant growth promotion of rhizobacteria associated with Salicornia growing in Tunisian hypersaline soils. BioMed Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [Green Version]
- Navarro-Noya, Y.E.; Luna-Guido, M.; Dendooven, L. Cultivable Nitrogen Fixing Bacteria from Extremely Alkaline-Saline Soils. Adv. Microbiol. 2016, 6, 412–423. [Google Scholar] [CrossRef] [Green Version]
- Kayasth, M.; Gera, R.; Dudeja, S.S.; Sharma, P.K.; Kumar, V. Studies on salinization in Haryana soils on free-living nitrogen-fixing bacterial populations and their activity. J. Basic Microbiol. 2014, 54, 170–179. [Google Scholar] [CrossRef]
- Jones, K. Nitrogen Fixation in a Salt Marsh. J. Ecol. 1974, 62, 553–565. [Google Scholar] [CrossRef]
- Elmerich, C.; Newton, W.E. Associative and Endophytic Nitrogen-fixing Bacteria and Cyanobacterial Associations. Assoc. Endophytic Nitrogen-Fixing Bact. Cyanobacterial Assoc. 2007. [Google Scholar] [CrossRef]
- Currin, C.A.; Paerl, H.W. Environmental and physiological controls on diel patterns of N2 fixation in epiphytic cyanobacterial communities. Microb. Ecol. 1998, 35, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H.; Alikhani, H.A.; Hosseini, H.M. Indole-3-acetic acid (IAA) production trait, a useful screening to select endophytic and rhizosphere competent bacteria for rice growth promoting agents. MethodsX 2015, 2, 72–78. [Google Scholar] [CrossRef]
- Leontidou, K.; Genitsaris, S.; Papadopoulou, A.; Kamou, N.; Bosmali, I.; Matsi, T.; Madesis, P.; Vokou, D.; Karamanoli, K.; Mellidou, I. Plant growth promoting rhizobacteria isolated from halophytes and drought-tolerant plants: Genomic characterisation and exploration of phyto-beneficial traits. Sci. Rep. 2020, 10, 14857. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R.; Penrose, D.M.; Li, J. A model for the lowering of plant ethylene concentrations by plant growth-promoting bacteria. J. Theor. Biol. 1998, 190, 63–68. [Google Scholar] [CrossRef]
- Oliveira, A.L.M.; Canuto, E.L.; Silva, E.E.; Reis, V.M.; Baldani, J.I. Survival of endophytic diazotrophic bacteria in soil under different moisture levels. Braz. J. Microbiol. 2004. [Google Scholar] [CrossRef] [Green Version]
- Han, L.L.; Wang, Q.; Shen, J.P.; Di, H.J.; Wang, J.T.; Wei, W.X.; Fang, Y.T.; Zhang, L.M.; He, J.Z. Multiple factors drive the abundance and diversity of the diazotrophic community in typical farmland soils of China. FEMS Microbiol. Ecol. 2019. [Google Scholar] [CrossRef] [PubMed]
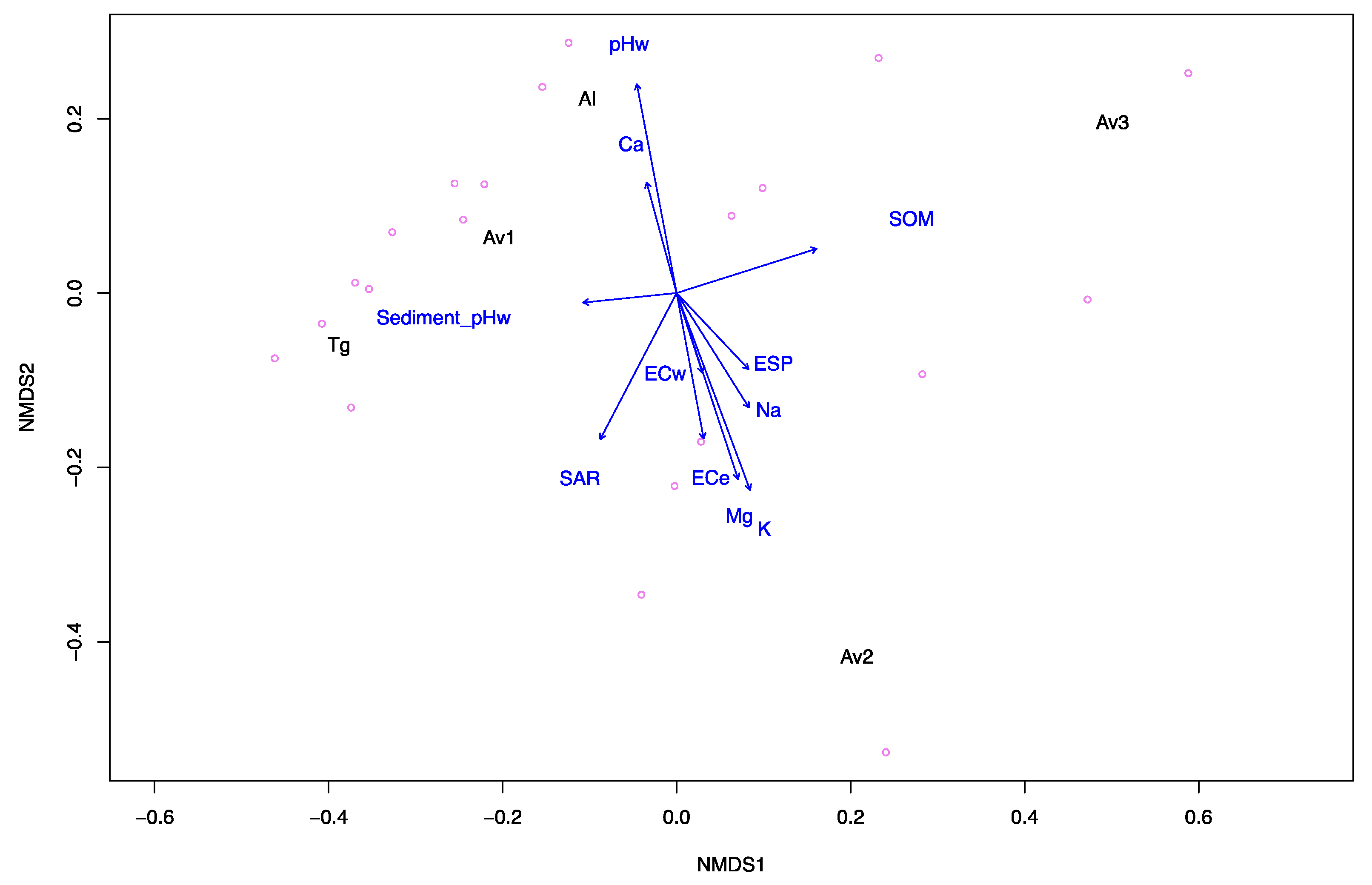
| Sites | Sediment | Pore Water | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pHw a | pHca b | ECe (dS m−1) c | SOM (% w/w) d | Na (mg kg−1) | Mg (mg kg−1) | K (mg kg−1) | Ca (mg kg−1) | SAR e | ESP f | pHw g | ECw (dS m−1) h | |
| Av1 | 7.3 | 7.2 | 129 | 6.08 | 972 | 139 | 47 | 176 | 13.3 | 17 | 7.3 | 113.1 |
| Av2 | 7.5 | 7.4 | 177 | 4.26 | 994 | 355 | 84 | 90 | 10.5 | 14 | 7 | 128.5 |
| Av3 | 6.7 | 6.7 | 56 | 7.22 | 711 | 109 | 43 | 84 | 12 | 16 | 7.6 | 68.5 |
| Tg | 7.2 | 7.2 | 39 | 2.55 | 385 | 70 | 31 | 89 | 7.4 | 10 | 6.8 | 47.1 |
| Al | 8.1 | 8.0 | 89 | 2.21 | 476 | 132 | 19 | 3016 | 2.3 | 2 | 7.2 | 108.1 |
| Sites | Number of Isolates | Average 16SrRNA Sequence Length (bp) | Number of OTUs a | Average Shannon’s H’ | Average Pielou’s Evenness |
|---|---|---|---|---|---|
| Av1 | 21 | 889 | 17 | 2.71 | 0.95 |
| Av2 | 26 | 884 | 15 | 2.55 | 0.94 |
| Av3 | 25 | 905 | 13 | 2.34 | 0.91 |
| Tg | 28 | 929 | 13 | 2.31 | 0.90 |
| Al | 20 | 940 | 9 | 1.98 | 0.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, M.J.; Cunha, A.; Figueiredo, S.; Faustino, P.; Patinha, C.; Silva, H.; Sierra-Garcia, I.N. The Root Microbiome of Salicornia ramosissima as a Seedbank for Plant-Growth Promoting Halotolerant Bacteria. Appl. Sci. 2021, 11, 2233. https://doi.org/10.3390/app11052233
Ferreira MJ, Cunha A, Figueiredo S, Faustino P, Patinha C, Silva H, Sierra-Garcia IN. The Root Microbiome of Salicornia ramosissima as a Seedbank for Plant-Growth Promoting Halotolerant Bacteria. Applied Sciences. 2021; 11(5):2233. https://doi.org/10.3390/app11052233
Chicago/Turabian StyleFerreira, Maria J., Angela Cunha, Sandro Figueiredo, Pedro Faustino, Carla Patinha, Helena Silva, and Isabel N. Sierra-Garcia. 2021. "The Root Microbiome of Salicornia ramosissima as a Seedbank for Plant-Growth Promoting Halotolerant Bacteria" Applied Sciences 11, no. 5: 2233. https://doi.org/10.3390/app11052233







