Phytoremediation of Heavy Metals in Tropical Soils an Overview
Abstract
1. Introduction
2. Tropical Soils and Heavy Metals
3. Emerging Contaminants and New Nanoagrochemicals as Potential Factors Altering Phytoremediation Processes
4. Plant Defense Mechanisms against Heavy Metals
5. Mobility of Metal and Metalloid Elements
6. Mechanisms Involved in Phytoremediation
7. Plant Strategies for Growing in Contaminated Soils
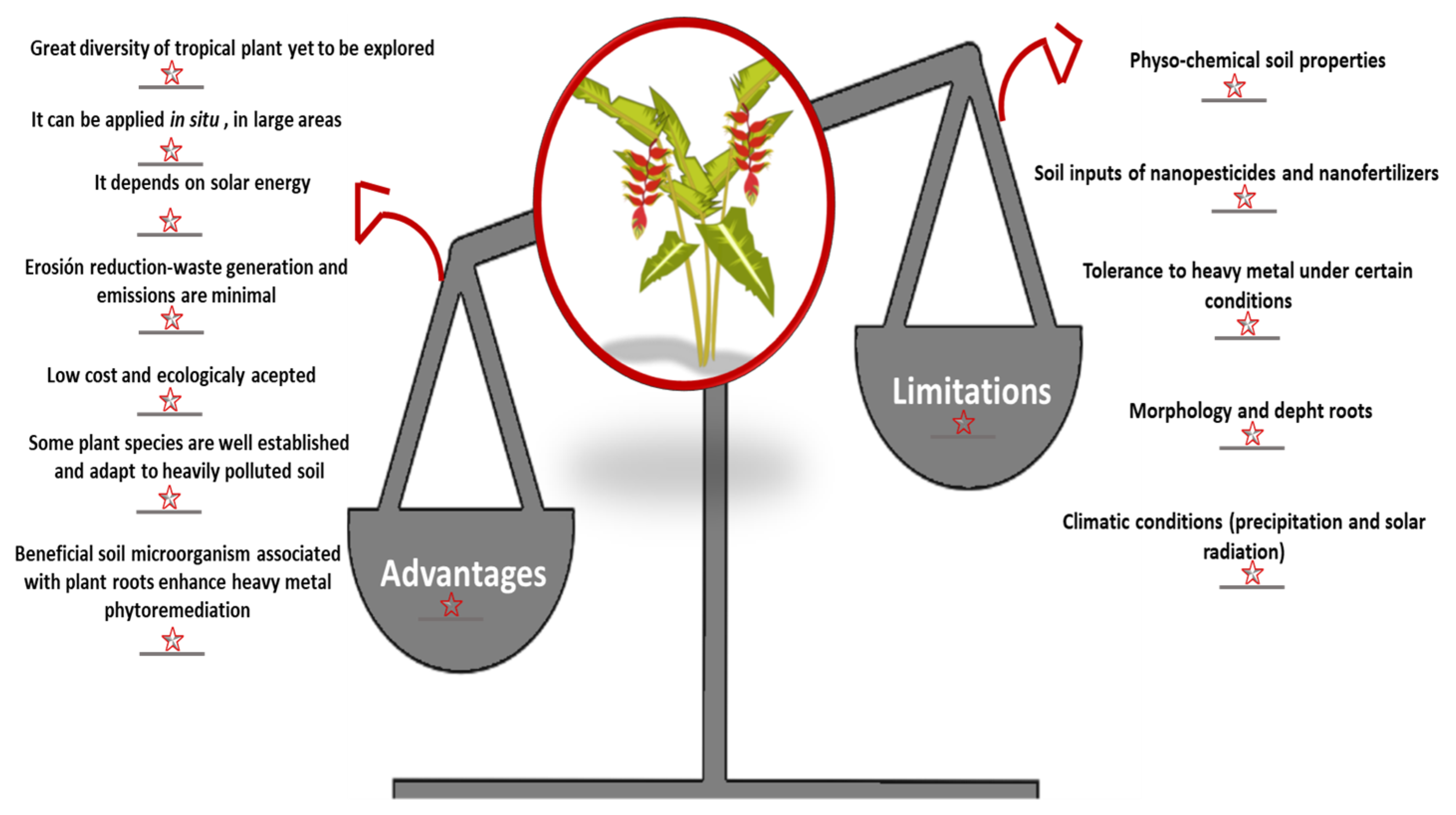
8. Phytoremediation in Tropical Soils—Advantages and Limitations
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Family/Specie | Description | Reference |
|---|---|---|
| Amaranthaceae Amaranthus hybridus L. | The capacity of A. hybridus for the extraction of heavy metals Lead (Pb) and Cadmium (Cd) was evaluated, in symbiosis with arbuscular mycorrhiza. The results showed that the addition of mycorrhizae significantly increased the concentration of Pb and Cd in the roots, stems, and leaves. The concentrations of Pb and Cd increased significantly as the age of the plant increased. | [162] |
| Araceae Xanthosoma undipes | X. undipes was used to evaluate its extraction capacity in soils contaminated with Cadmium (Cd) in areas of oil exploitation in Ecuador. The results showed a Cd removal of up to 79.67% and no sign of oxidative stress. These results make the species a good candidate for phytoremediation of Cd and appropriate for ex situ phytoextraction studies. | [164] |
| Asteraceae Berkheya coddii | B. coddii, a species native to the subtropics of South Africa, has an extraction affinity for metals such as nickel (Ni) and palladium (Pd), making it a candidate for phytoremediation in mining soils affected by high concentrations of these metals. B. coddii in symbiosis with arbuscular mycorrhizae (Glomus intraradices), increases its ability to hyperaccumulate Nickel (Ni). | [167,168] |
| Asteraceae Baccharis trimera | Plant found in areas of southern Brazil. Exhibits spontaneous growth in areas contaminated with various heavy metals, showing greater capacity to accumulate copper (Cu) in its roots. | [56] |
| Asteraceae and Euphorbiaceae Helianthus annuus and Ricinus communis | The tolerance capacity of these two species was evaluated in soils artificially contaminated with Lead (Pb) in a greenhouse. It was found that these species are tolerant to Pb and accumulate it in their roots with little translocation to the shoots, showing evidence of immobilization in the underground parts of these plants. | [169]. |
| Brassicaceae Brassica juncea | Indian mustard (Brassica juncea L. Czern.) tolerates high concentrations of heavy metals and is a promising species for the purpose of phytoextraction of cadmium (Cd) from metal-contaminated soils. | [121] |
| Cyperaceae Cyperus involucratus Cyperus rotundus | This species has demonstrated the ability to hyperaccumulate Cu, Zn, Cd, Ni, Cr, and Pb in various parts of the plant. Cyperus rotundus, may successfully be used as a phytostabiliser for the metals; Zn, Pb, Ni. The grass may also serve as a metal indicator for the Cd. | [174,175] |
| Euphorbiaceae Jatropha curcas | The phytoremediation capability of J. curcas was tested using soils from gold mining areas. This plant species has a good ability to tolerate and accumulate Hg from polluted soils in Colombia. Due to the high translocation and bioaccumulation factors obtained in this study, J. curcas L. can be considered a hyperaccumulating plant of As and Fe. | [158,179] |
| Fabaceae Sesbania drummondii Sesbania rostrata | A shrubby plant 1 to 3 m tall. It is one of the potential tropical plants to remedy soils contaminated with Pb, since it grows naturally in places with this type of contamination and seems to tolerate considerable concentrations. An annual tropical legume, it is a promising candidate species for revegetation at mine tailings. Efficient for hyperaccumulation of cadmium and copper in contaminated soils. | [181,182] |
| Heliconiaceae Heliconia psittacorum | This species was tested in wastewater with leachate from sanitary landfills to phytoremediate heavy metals, finding great potential for phytoextraction of metals such as cadmium (Cd) and lead (Pb). | [14] |
| Poaceae Gynerium sagittatum (caña fleche) | The mercury (Hg) accumulation capacity of the plant com-monly called “caña fleche” (Gynerium sagittatum) was evalu-ated in vitro. It was found that this plant is hyperaccumulative of mercury metal, with metal concentrations increasing in its roots and stems over time. | [191] |
| Chrysopogon zizanioides (Vetiver Grass) | Commonly found on flood plains and stream banks, but also appears throughout the tropical and subtropical regions of Africa, Asia, America, Australasia, and Mediterranean Europe. Highly tolerate extreme climatic variation such as prolonged drought, flood, submergence and temperatures, soils high in acidity and alkalinity. Accumulates high concentrations of heavy metals (lead, zinc, arsenic, cadmium, zinc, chromiun, and copper) in its roots and shoots | [192] |
| Pennisetum purpureum (Napier grass) | Commonly known as Napier grass, due to its rapid growth rate and ability to survive in highly contaminated soils, is a candidate plant for phytoremediation processes. In this study, a phytobacterial system was used that uses Pennisetum purpureum together with a lead-resistant bacterium (LRB) for lead uptake. | [193] |
| Urticaceae Cecropia peltata | This species, commonly called “guarumo”, was used as a phytoextractor of mercury (Hg) present in contaminated soils in the south of Bolívar (Colombia). An elevated concentration of mercury was found in its roots and leaves. | [194] |
| Verbenaceae Phyla nodiflora | Species selected among 17 germinated in sites in North Florida contaminated with metals: lead (Pb), copper (Cu) and zinc (Zn). P. nodiflora was the most efficient at accumulating Cu and Zn in its stems and is a native species of the area considered to exhibit phytoremediation potential. | [155] |
References
- Coyne, M.; Rasskin, M. Microbiología del Suelo: Un Enfoque Exploratorio; Paraninfo: Madrid, Spain, 2000. [Google Scholar]
- Chapin, F.; Matson, P.; Money, H. Principles of Terrestrial Ecosystem Ecology, 1st ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Baillie, I.C.; Anderson, J.M.; Ingram, J.S.I. Tropical Soil Biology and Fertility: A Handbook of Methods. J. Ecol. 1990, 78, 547. [Google Scholar] [CrossRef]
- Matson, P.A.; Parton, W.J.; Power, A.G.; Swift, M.J. Agricultural Intensification and Ecosystem Properties. Science 1997, 277, 504–509. [Google Scholar] [CrossRef]
- Balland-Bolou-Bi, C.; Turc, B.; Alphonse, V.; Bousserrhine, N. Impact of microbial communities from tropical soils on the mobilization of trace metals during dissolution of cinnabar ore. J. Environ. Sci. 2017, 56, 122–130. [Google Scholar] [CrossRef]
- Osakwe, S.A.; Okolie, L.P. Physicochemical Characteristics and Heavy Metals Contents in Soils and Cassava Plants from Farmlands along A Major Highway in Delta State, Nigeria. J. Appl. Sci. Environ. Manag. 2016, 19, 695. [Google Scholar] [CrossRef]
- Jakimska, A.; Konieczka, P.; Skora, K.; Namiesnik, J. Bioaccumulation of Metals in Tissues of Marine Animals, Part II: Metal Concentrations in Animal Tissues. Pol. J. Environ. Stud. 2011, 20, 1127–1146. [Google Scholar]
- Broos, K.; Mertens, J.; Smolders, E. Toxicity of heavy metals in soil assessed with various soil microbial and plant growth assays: A comparative study. Environ. Toxicol. Chem. 2005, 24, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Motuzova, G.; Minkina, T.M.; Karpova, E.; Barsova, N.; Mandzhieva, S. Soil contamination with heavy metals as a potential and real risk to the environment. J. Geochem. Explor. 2014, 144, 241–246. [Google Scholar] [CrossRef]
- Huauya, M.; Huamaní, H. Macrofauna edáfica y metales pesados en el cultivo de cacao Theobroma cacao L, (Malvaceae). Biologist 2014, 12, 45–55. [Google Scholar]
- Sauvé, S.; Desrosier, M. A review of what is an emerging contaminant. Hem. Cent. J. 2014, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Kabata-Pendias, A. Trace Elements in Soils and Plants; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Prieto, J.; Gonzalez, C.; Román, A.; Prieto, F. Contaminación y fitotoxicidad en plantas por metals pesados provenientes de suelos y agua. Trop. Subtrop. Agroecosyst. 2009, 10, 29–44. Available online: https://www.redalyc.org/articulo.oa?id=939/93911243003 (accessed on 4 December 2019).
- Madera-Parra, C.; Peña-Salamanca, E.J.; Pena, M.R.; Rousseau, D.P.L.; Lens, P.N.L. Phytoremediation of Landfill Leachate withColocasia esculenta, Gynerum sagittatumandHeliconia psittacorumin Constructed Wetlands. Int. J. Phytoremediat. 2014, 17, 16–24. [Google Scholar] [CrossRef]
- Paz-Alberto, A.M.; Sigua, G.C. Phytoremediation: A Green Technology to Remove Environmental Pollutants. Am. J. Clim. Chang. 2013, 2, 71–86. [Google Scholar] [CrossRef]
- Bhatnagar, N. Phytoremediation: A Promising Technology of Environmental Pollution Control. Glob. J. Microbiol. Bio Technol. 2016, 4, 39–42. [Google Scholar]
- Rajkumar, M.; Sandhya, S.; Prasad, M.; Freitas, H. Perspectives of plant-associated microbes in heavy metal phytoremediation. Bio Technol. Adv. 2012, 30, 1562–1574. [Google Scholar] [CrossRef] [PubMed]
- González-Chávez, M. Remediation of soils polluted with heavy metals using plants and rhizospheric microorganisms. Terra Organo Cient. Soc. Mex. Cienc. Suelo A.C. 2013, 23, 29–37. [Google Scholar]
- Raven, P.H.; Gereau, R.E.; Phillipson, P.B.; Chatelain, C.; Jenkins, C.N.; Ulloa, C.U. The distribution of biodiversity richness in the tropics. Sci. Adv. 2020, 6, eabc6228. [Google Scholar] [CrossRef]
- Stocking, M.A. Tropical Soils and Food Security: The Next 50 Years. Science 2003, 302, 1356–1359. [Google Scholar] [CrossRef]
- Hartemink, A.E. Tropical soils—properties and management for sustainable agriculture. Geoderma 2004, 123, 373–375. [Google Scholar] [CrossRef]
- Wohl, E.; Barros, A.P.; Brunsell, N.; Chappell, N.A.; Coe, M.T.; Giambelluca, T.W.; Goldsmith, S.T.; Harmon, R.S.; Hendrickx, J.M.H.; Juvik, J.; et al. The hydrology of the humid tropics. Nat. Clim. Chang. 2012, 2, 655–662. [Google Scholar] [CrossRef]
- Oliveira, F.C.C.; Silva, I.R.; Ferreira, G.W.D.; Soares, E.M.; Silva, S.R.; Silva, E.F. Contribution of Eucalyptus Harvest Residues and Nitrogen Fertilization to Carbon Stabilization in Ultisols of Southern Bahia. Rev. Braz. Ciência Solo 2018, 42. [Google Scholar] [CrossRef]
- Fontes, M.P.F.; Carvalho, I.A. Color Attributes and Mineralogical Characteristics, Evaluated by Radiometry, of Highly Weathered Tropical Soils. Soil Sci. Soc. Am. J. 2005, 69, 1162–1172. [Google Scholar] [CrossRef]
- Galvão, L.S.; Formaggio, A.R.; Couto, E.G.; Roberts, D.A. Relationships between the mineralogical and chemical composition of tropical soils and topography from hyperspectral remote sensing data. ISPRS J. Photogramm. Remote Sens. 2008, 63, 259–271. [Google Scholar] [CrossRef]
- Sanchez, P.A.; Shepherd, K.D.; Soule, M.J.; Place, F.M.; Buresh, R.J.; Izac, A.-M.N.; Mokwunye, A.U.; Kwesiga, F.R.; Ndiritu, C.G.; Woomer, P.L. Soil Fertility Replenishment in Africa: An Investment in Natural Resource Capital. In SSSA Special Publications; John Wiley & Sons, Inc: Hoboken, NJ, USA, 2015; pp. 1–46. [Google Scholar]
- Viciedo, D.O.; Hernández, A.; Rodríguez, M.; Lizcano, R.; Calero, A.; Peña, K. Effects of land-use change on Nitisols properties in a tropical climate. Rev. Fac. Nac. Agron. Medellín 2018, 71, 8601–8608. [Google Scholar] [CrossRef]
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal–A review. Biol. Fertil. Soils 2002, 35, 219–230. [Google Scholar] [CrossRef]
- Bogado, G.O.; Reinert, H.O.; Francisca, F.M. Geotechnical properties of residual soils from the North-east of Argentina. Int. J. Geotech. Ing. 2019, 13, 112–121. [Google Scholar] [CrossRef]
- Rigo, M.L.; Pinheiro, R.J.B.; Bressani, L.A.; Bica, A.V.D.; Da Silveira, R.M. The residual shear strength of tropical soils. Can. Geotech. J. 2006, 43, 431–447. [Google Scholar] [CrossRef]
- Laurance, W.F.; Sayer, J.; Cassman, K.G. Agricultural expansion and its impacts on tropical nature. Trends Ecol. Evol. 2014, 29, 107–116. [Google Scholar] [CrossRef]
- Sala, O.E.; Chapin, F.S.; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Leemans, R.; et al. Global biodiversity scenarios for the year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef]
- Gisbert, J.M.; Ibáñez, S.; Moreno, H. Génesis del Suelo; Universidad Politécnica de Valencia Editorial UPV: Valencia, Spain, 2010. [Google Scholar]
- Musinguzi, P.; Tenywa, J.S.; Ebanyat, P.; Tenywa, M.M.; Mubiru, D.N.; Basamba, T.A.; Leip, A. Soil Organic Carbon Thresholds and Nitrogen Management in Tropical Agroecosystems: Concepts and Prospects. J. Sustain. Dev. 2013, 6, 31. [Google Scholar] [CrossRef]
- Vásquez-Polo, J.R.; Vázquez, F.M.; Menjivar-Flores, J.C. Formas de carbono orgánico en suelos con diferentes usos en el departamento del Magdalena (Colombia). Acta Agron. 2011, 60, 369–379. Available online: http://www.scielo.org.co/scielo.php?script=sci_abstract&pid=S0120-28122011000400010 (accessed on 22 May 2020).
- Bodek, I.; Lyman, W.J.; Reehl, W.F. Environmental Inorganic Chemistry: Properties, Processes, and Estimation Methods; Walton, B.T., Conway, R.A., Eds.; SETAC Special Publication Series; Pergamon: New York, NY, USA, 1988. [Google Scholar]
- Kämp, F.N.; Curi, N. Argilominerais em Solos Brazileiros. In Tópicos em Ciência do Solo; Curi, N., Ed.; Sociedade Brazileira de Ciência do Solo: Viçosa, Brazil, 2003; Volume 3, pp. 1–54. [Google Scholar]
- Rieuwerts, J.S. The mobility and bioavailability of trace metals in tropical soils: A review. Chem. Speciat. Bioavailab. 2007, 19, 75–85. [Google Scholar] [CrossRef]
- Fontes, M.P.F.; Gomes, P.C. Simultaneous competitive adsorption of heavy metals by the mineral matrix of tropical soils. Appl. Geochem. 2003, 18, 795–804. [Google Scholar] [CrossRef]
- Schloter, M.; Dilly, O.; Munch, J. Indicators for evaluating soil quality. Agric. Ecosyst. Environ. 2003, 98, 255–262. [Google Scholar] [CrossRef]
- Chang, T.W.; Wang, M.-K.; Lin, C. Adsorption of Copper in the Different Sorbent/Water Ratios of Soil Systems. Water Air Soil Pollut. 2002, 138, 199–209. [Google Scholar] [CrossRef]
- Silveira, M.L.A.; Alleoni, L.R.F.; Camargo, O.A.; Casagrande, J.C. Copper adsorption in oxidic soils after removal of organic matter and iron oxides. Commun. Soil Sci. Plant Anal. 2002, 33, 3581–3592. [Google Scholar] [CrossRef]
- Duffus, J.H. “Heavy metals” a meaningless term? (IUPAC Technical Report). Pure Appl. Chem. 2002, 74, 793–807. [Google Scholar] [CrossRef]
- Alloway, B.J. Sources of Heavy Metals and Metalloids in Soils. In Water Management and the Environment: Case Studies; Springer: Cham, Switzerland, 2013; pp. 11–50. [Google Scholar]
- Lori, V.; Pietrini, F.; Massacci, A.; Zacchini, M. Morphophysiological Responses, Heavy Metal Accumulation and Phy-Toremoval Ability in Four Willow Clones Exposed To Cadmium Under Hydroponics. In Phytoremediation; Springer International Publishing: Cham, Switzerland, 2015; pp. 87–98. [Google Scholar]
- Wuana, R.A.; Okieimen, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. ISRN Ecol. 2011, 2011, 1–20. [Google Scholar] [CrossRef]
- Alyemeni, M.N.; Almohisen, I. Effect of Anthropogenic Activities on Accumulation of Heavy Metals in Legumes Crops, Riyadh, Saudi Arabia. APCBEE Procedia 2014, 10, 275–280. [Google Scholar] [CrossRef][Green Version]
- Aprile, A.; De Bellis, L. Editorial for Special Issue “Heavy Metals Accumulation, Toxicity, and Detoxification in Plants”. Int. J. Mol. Sci. 2020, 21, 4103. [Google Scholar] [CrossRef]
- Alkhader, A.M.F. The impact of phosphorus fertilizers on heavy metals content of soils and vegetables grown on selected farms in Jordan. Agrotechnology 2015, 5, 137. [Google Scholar] [CrossRef]
- Jayasumana, C.; Fonseka, S.; Fernando, A.; Jayalath, K.; Amarasinghe, M.; Siribaddana, S.; Gunatilake, S.; Paranagama, P. Phosphate fertilizer is a main source of arsenic in areas affected with chronic kidney disease of unknown etiology in Sri Lanka. SpringerPlus 2015, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Atafar, Z.; Mesdaghinia, A.; Nouri, J.; Homaee, M.; Yunesian, M.; Ahmadimoghaddam, M.; Mahvi, A.H. Effect of fertilizer application on soil heavy metal concentration. Environ. Monit. Assess. 2008, 160, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Sumner, M.E. Beneficial use of effluents, wastes, and biosolids. Commun. Soil Sci. Plant Anal. 2000, 31, 1701–1715. [Google Scholar] [CrossRef]
- Basta, N.; McGowen, S. Evaluation of chemical immobilization treatments for reducing heavy metal transport in a smelter-contaminated soil. Environ. Pollut. 2004, 127, 73–82. [Google Scholar] [CrossRef]
- Caviedes Rubio, D.I.; Delgado, D.R.; Olaya Amaya, A. Remoción de metales pesados comúnmente generados por la actividad industrial, empleando macrófitas neotropicales. Producción Limpia 2016, 11, 127–128. [Google Scholar] [CrossRef]
- Mapanda, F.; Mangwayana, E.; Nyamangara, J.; Giller, K. The effect of long-term irrigation using wastewater on heavy metal contents of soils under vegetables in Harare, Zimbabwe. Agric. Ecosyst. Environ. 2005, 107, 151–165. [Google Scholar] [CrossRef]
- Boechat, C.L.; Pistóia, V.C.; Gianelo, C.; Camargo, F.A.D.O. Accumulation and translocation of heavy metal by spontaneous plants growing on multi-metal-contaminated site in the Southeast of Rio Grande do Sul state, Brazil. Environ. Sci. Pollut. Res. 2016, 23, 2371–2380. [Google Scholar] [CrossRef]
- Sharma, S.S.; Dietz, K.-J. The relationship between metal toxicity and cellular redox imbalance. Trends Plant Sci. 2009, 14, 43–50. [Google Scholar] [CrossRef]
- Rajewska, I.; Talarek, M.; Bajguz, A. Brassinosteroids and Response of Plants to Heavy Metals Action. Front. Plant Sci. 2016, 7, 629. [Google Scholar] [CrossRef]
- Oliveira, A.P.; Pampulha, M.E. Effects of long-term heavy metal contamination on soil microbial characteristics. J. Biosci. Bioeng. 2006, 102, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Olaniran, A.O.; Balgobind, A.; Pillay, B. Bioavailability of Heavy Metals in Soil: Impact on Microbial Biodegradation of Organic Compounds and Possible Improvement Strategies. Int. J. Mol. Sci. 2013, 14, 10197–10228. [Google Scholar] [CrossRef]
- He, Z.; Shentu, J.; Yang, X.; Baligar, V.C.; Zhang, T.; Stoffella, P.J. Heavy metal contamination of soils: Sources, in-dicators, and assessment. J. Environ. Indic. 2015, 9, 17–18. Available online: www.environmentalindicators.net (accessed on 17 August 2019).
- Appel, C.; Ma, L. Concentration, pH, and Surface Charge Effects on Cadmium and Lead Sorption in Three Tropical Soils. J. Environ. Qual. 2002, 31, 581–589. [Google Scholar] [CrossRef]
- Bradl, H.B. Adsorption of heavy metal ions on soils and soils constituents. J. Colloid Interface Sci. 2004, 277, 1–18. [Google Scholar] [CrossRef]
- Oseniet Olalekan, A.; Taiwo Abayomi, G.; Ijaola Taiwo, O.; Yenusa Luqman, A. The Effects of pH On the Levels of Some Heavy Metals in Soil Samples of Five Dumpsites in Abeokuta and its Environs. Int. J. Sci. Res. 2016, 5, 1543–1545. [Google Scholar] [CrossRef]
- Bolan, N.S.; Adriano, D.C.; Curtin, D. Soil acidification and liming interactions with nutrient and heavy metal transformation and bioavailability. Adv. Agron. 2003, 78, 5–272. [Google Scholar] [CrossRef]
- Tutic, A.; Novakovic, S.; Lutovac, M.; Biocanin, R.; Ketin, S.; Omerovic, N. The Heavy Metals in Agrosystems and Impact on Health and Quality of Life. Open Access Maced. J. Med. Sci. 2015, 3, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Tóth, G.; Hermann, T.; Da Silva, M.; Montanarella, L. Heavy metals in agricultural soils of the European Union with implications for food safety. Environ. Int. 2016, 88, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, P.E.; Feng, L.G. Risks of Hazardous Wastes; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar] [CrossRef]
- Spear, R. Recognized and Possible Exposure to Pesticides. In Handbook of Pesticide Toxicology, 1st ed.; Hayes, W.J., Jr., Edwards, R.L., Jr., Eds.; Academic Press: New York, NY, USA, 1999; pp. 245–274. [Google Scholar]
- Zaragoza-Bastida, A.; Valladares-Carranza, B.; Ortega-Santana, C.; Zamora-Espinosa, J.; Velázquez- Ordoñez, V.; Aparicio Burgos, J. Implications of the use of organochlorine in the environment, and public health. Abanico Veterinario 2016, 6, 43–55. [Google Scholar]
- Benbrook, C. Trends in glyphosate herbicide use in the United States and globally. Environ. Sci. Eur. 2016, 28, 1–15. [Google Scholar] [CrossRef]
- Blackburn, L.G.; Boutin, C. Subtle effects of herbicide use in the context of genetically modified crops: A case study with glyphosate (Roundup). Ecotoxicology 2003, 12, 271–285. [Google Scholar] [CrossRef]
- Osten, J.R.-V.; Dzul-Caamal, R. Glyphosate Residues in Groundwater, Drinking Water and Urine of Subsistence Farmers from Intensive Agriculture Localities: A Survey in Hopelchén, Campeche, Mexico. Int. J. Environ. Res. Public Health 2017, 14, 595. [Google Scholar] [CrossRef]
- Piola, L.; Fuchs, J.; Oneto, M.L.; Basack, S.; Kesten, E.; Casabé, N. Comparative toxicity of two glyphosate-based formulations to Eisenia andrei under laboratory conditions. Chemosphere 2013, 91, 545–551. [Google Scholar] [CrossRef]
- Helander, M.; Saloniemi, I.; Omacini, M.; Druille, M.; Salminen, J.P.; Saikkonen, K. Glyphosatedecreases mycorrhizal colonization and affects plant-soil feedback. Sci. Total Environ. 2018, 642, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Environmental Protection Agency (EPA) Emerging Contaminants Nanomaterials. 2010 United States. EPA 505-F-10-008. Available online: https://archive.epa.gov/region9/mediacenter/web/pdf/emerging_contaminant_nanomaterials.pdf (accessed on 17 January 2020).
- Wang, P.; Lombi, E.; Zhao, F.-J.; Kopittke, P.M. Nanotechnology: A New Opportunity in Plant Sciences. Trends Plant Sci. 2016, 21, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Pakrashi, S.; Jain, N.; Dalai, S.; Jayakumar, J.; Chandrasekaran, P.T.; Raichur, A.M.; Chandrasekaran, N.; Mukherjee, A. In Vivo Genotoxicity Assessment of Titanium Dioxide Nanoparticles by Allium cepa Root Tip Assay at High Exposure Concentrations. PLoS ONE 2014, 9, e87789. [Google Scholar] [CrossRef] [PubMed]
- Hossain, Z.; Mustafa, G.; Komatsu, S. Plant Responses to Nanoparticle Stress. Int. J. Mol. Sci. 2015, 16, 26644–26653. [Google Scholar] [CrossRef] [PubMed]
- Skiba, E.; Wolf, W.M. Cerium Oxide Nanoparticles Affect Heavy Metals Uptake by Pea in a Divergent Way than Their Ionic and Bulk Counterparts. Water Air Soil Pollut. 2019, 230, 248. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Singh, S.; Singh, S.; Srivastava, P.K.; Singh, V.P.; Singh, S.; Prasad, S.M.; Singh, P.K.; Dubey, N.K.; Pandey, A.C.; et al. Nitric oxide alleviates silver nanoparticles (AgNps)-induced phytotoxicity in Pisum sativum seedlings. Plant Physiol. Biochem. 2017, 110, 167–177. [Google Scholar] [CrossRef]
- Cvjetko, P.; Milošić, A.; Domijan, A.-M.; Vrček, I.V.; Tolić, S.; Štefanić, P.P.; Letofsky-Papst, I.; Tkalec, M.; Balen, B. Toxicity of silver ions and differently coated silver nanoparticles in Allium cepa roots. Ecotoxicol. Environ. Saf. 2017, 137, 18–28. [Google Scholar] [CrossRef]
- Jo, Y.-K.; Kim, B.H.; Jung, G. Antifungal Activity of Silver Ions and Nanoparticles on Phytopathogenic Fungi. Plant Dis. 2009, 93, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Rafique, R.; Arshad, M.; Khokhar, M.F.; Qazi, I.A.; Hamza, A.; Virk, N. Growth response of wheat to titania na-noparticles application. Nust J. Eng. Sci. 2014, 7, 42–46. Available online: http://journals.nust.edu.pk/index.php/njes/article/view/133 (accessed on 22 May 2019).
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Gupta, D.K.; Huang, H.G.; Corpas, F.J. Lead tolerance in plants: Strategies for phytoremediation. Environ. Sci. Pollut. Res. 2013, 20, 2150–2161. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.I.; Shahzad, T.; Shahid, M.; Ismail, I.M.; Shah, G.M.; Almeelbi, T. Zinc oxide nanoparticles affect carbon and nitrogen mineralization of Phoenix dactylifera leaf litter in a sandy soil. J. Hazard. Mater. 2017, 324, 298–305. [Google Scholar] [CrossRef]
- Ben-Moshe, T.; Frenk, S.; Dror, I.; Minz, D.; Berkowitz, B. Effects of metal oxide nanoparticles on soil properties. Chemosphere 2013, 90, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Dinesh, R.; Anandaraj, M.; Srinivasan, V.; Hamza, S. Engineered nanoparticles in the soil and their potential implications to microbial activity. Geoderma 2012, 173, 19–27. [Google Scholar] [CrossRef]
- Rajput, V.D.; Minkina, T.; Sushkova, S.; Tsitsuashvili, V.; Mandzhieva, S.; Gorovtsov, A.; Gromakova, N. Effect of nanoparticles on crops and soil microbial communities. J. Soils Sediments 2018, 18, 2179–2187. [Google Scholar] [CrossRef]
- Feng, Y.; Cui, X.; He, S.; Dong, G.; Chen, M.; Wang, J.; Lin, X. The Role of Metal Nanoparticles in Influencing Arbuscular Mycorrhizal Fungi Effects on Plant Growth. Environ. Sci. Technol. 2013, 47, 9496–9504. [Google Scholar] [CrossRef]
- Handojo, L.; Ikhsan, N.A.; Mukti, R.R.; Indarto, A. Nanomaterials for remediations of agrochemicals. In Agrochemicals Detection, Treatment and Remediation; Elsevier BV: Amsterdam, The Netherlands, 2020; pp. 535–567. [Google Scholar]
- Valko, M.; Jamova, K.; Rhodes, C.J.; Kuča, K.; Musilek, K. Redox- and non-redox-metal-induced formation of free rad-icals and their role in human disease. Arch. Toxicol. 2016, 90, 1–37. [Google Scholar] [CrossRef]
- Rinalducci, S.; Murgiano, L.; Zolla, L. Redox proteomics: Basic principles and future perspectives for the detection of protein oxidation in plants. J. Exp. Bot. 2008, 59, 3781–3801. [Google Scholar] [CrossRef]
- Flora, S.J.; Mittal, M.; Mehta, A. Heavy metal induced oxidative stress & its possible reversal by chelation therapy. Indian J. Med. Res. 2008, 128, 501–523. [Google Scholar]
- Pelaez, M.P.; Casierra-Posada, F.; Torres, G.A.R. Tóxidad de cadmio y plomo en Pasto Tanner Brachiaria arrecta. Rev. Cienc. Agrícolas 2014, 31, 3–13. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2. [Google Scholar] [CrossRef]
- Appenroth, K.J. Definition of “Heavy Metals” and Their Role in Biological Systems. Soil Heavy Metals -19-29; Springer: Berlin/Heidelberg, Germany, 2010; ISBN 978-3-642-02435-1. [Google Scholar]
- Shukla, U.C.; Murthy, R.C.; Kakkar, P. Combined effect of ultraviolet-B radiation and cadmium contamination on nutrient uptake and photosynthetic pigments inBrassica campestrisL. seedlings. Environ. Toxicol. 2008, 23, 712–719. [Google Scholar] [CrossRef]
- Ying, R.R.; Qiu, R.L.; Tang, Y.T.; Hu, P.J.; Qiu, H.; Chen, H.R.; Morel, J.L. Cadmium tolerance of carbon assim-ilation enzymes and chloroplast in Zn/Cd hyperaccumulator Picris divaricata. J. Plant Physiol. 2010, 167, 81–87. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Gechev, T.S.; Van Breusegem, F.; Stone, J.M.; Denev, I.; Laloi, C. Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. BioEssays 2006, 28, 1091–1101. [Google Scholar] [CrossRef]
- Jain, R.; Chandra, A.; Venugopalan, V.K.; Solomon, S. Physiological Changes and Expression of SOD and P5CS Genes in Response to Water Deficit in Sugarcane. Sugar Tech. 2015, 17, 276–282. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Zhou, Y.; Gong, T.; Wang, J.; Ge, Y. Enhanced phytoremediation of mixed heavy metal (mercury)–organic pollutants (trichloroethylene) with transgenic alfalfa co-expressing glutathione S-transferase and human P450 2E1. J. Hazard. Mater. 2013, 260, 1100–1107. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Wang, A.; Zhang, X.; Wu, Y.; Wang, R.; Cui, H.; Huang, R.; Luo, Y. Identification and analysis of glutathione S-transferase gene family in sweet potato reveal divergent GST-mediated networks in aboveground and underground tissues in response to abiotic stresses. BMC Plant Biol. 2017, 17, 1–15. [Google Scholar] [CrossRef]
- Nianiou-Obeidat, I.; Madesis, P.; Kissoudis, C.; Voulgari, G.; Chronopoulou, E.; Tsaftaris, A.; Labrou, N.E. Plant glutathione transferase-mediated stress tolerance: Functions and biotechnological applications. Plant Cell Rep. 2017, 36, 791–805. [Google Scholar] [CrossRef]
- Chronopoulou, E.; Georgakis, N.; Nianiou-Obeidat, I.; Madesis, P.; Perperopoulou, F.; Pouliou, F.; Vasilopoulou, E.; Ioannou, E.; Ataya, F.S.; Labrou, N.E. Plant Glutathione Transferases in Abiotic Stress Response and Herbicide Resistance. In Glutathione in Plant Growth, Development, and Stress Tolerance; Springer Science and Business Media LLC: Berlin, Germany, 2017; pp. 215–233. [Google Scholar]
- Rascio, N.; Navari-Izzo, F. Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting? Plant Sci. 2011, 180, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Kłos, A.; Czora, M.; Rajfur, M.; Wacławek, M. Mechanisms for Translocation of Heavy Metals from Soil to Epigeal Mosses. Water Air Soil Pollut. 2011, 223, 1829–1836. [Google Scholar] [CrossRef]
- Peijnenburg, W.J.; Posthuma, L.; Eijsackers, H.; Allen, H. A Conceptual Framework for Implementation of Bioavailability of Metals for Environmental Management Purposes. Ecotoxicol. Environ. Saf. 1997, 37, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Lanno, R.; Wells, J.; Conder, J.; Bradham, K.; Basta, N. The bioavailability of chemicals in soil for earthworms. Ecotoxicol. Environ. Saf. 2004, 57, 39–47. [Google Scholar] [CrossRef] [PubMed]
- NRC Committee—National Research Council Committee on Bioavailability of Contaminants in Soils and Sediments. Bioavailability of Contaminants in Soils and Sediments: Processes, Tools, and Applications; The National Academic Press: Washington, DC, USA, 2003. [Google Scholar]
- Violante, A.; Cozzolino, V.; Perelomov, L.V.; Caporale, A.; Pigna, M. Mobility and bioavailability of heavy metals and metalloids in soil environments. J. Soil Sci. Plant Nutr. 2010, 10, 268–292. [Google Scholar] [CrossRef]
- Senila, M. Real and simulated bioavailability of lead in contaminated and uncontaminated soils. J. Environ. Health Sci. Eng. 2014, 12, 108. [Google Scholar] [CrossRef]
- Zhang, H.; Davison, W. Use of diffusive gradients in thin-films for studies of chemical speciation and bioavailability. Environ. Chem. 2015, 12, 85–101. [Google Scholar] [CrossRef]
- Senila, M.; Levei, E.A.; Senila, L. Assessment of metals bioavailability to vegetables under field conditions using DGT, single extractions and multivariate statistics. Chem. Cent. J. 2012, 6, 119. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bai, L.; Zeng, X.; Su, S.; Wang, Y.; Wu, C. Assessment of arsenic availability in soilsusing the diffusive gra-dients in thin films (DGT) technique—a comparison study of DGT and classic extraction methods. Environ. Sci. Process. Impacts 2014, 16, 2355–2361. [Google Scholar] [CrossRef] [PubMed]
- Atangana, A.; Khasa, D.; Chang, S.; Degrande, A. Tropical Agroforestry. In Tropical Agroforestry; Springer Nature: Cham, Switzerland, 2014. [Google Scholar]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Butcher, D.J. Phytoremediation of Lead in Soil: Recent Applications and Future Prospects. Appl. Spectrosc. Rev. 2009, 44, 123–139. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Castagna, A.; Ranieri, A.; Di Toppi, L.S. Cadmium tolerance in Brassica juncea roots and shoots is affected by antioxidant status and phytochelatin biosynthesis. Plant Physiol. Biochem. 2012, 57, 15–22. [Google Scholar] [CrossRef]
- Lu, X.Y.; He, C.Q. Tolerance uptake and accumulation of cadmium by Ricinus communis L. J. Agro-Environ. Sci. 2005, 24, 674–677. [Google Scholar]
- King, D.J.; Doronila, A.I.; Feenstra, C.; Baker, A.J.; Woodrow, I.E. Phytostabilisation of arsenical gold mine tailings using four Eucalyptus species: Growth, arsenic uptake and availability after five years. Sci. Total Environ. 2008, 406, 35–42. [Google Scholar] [CrossRef]
- Fujii, T.; Murakami, K.; Endo, T.; Fujimoto, S.; Minowa, T.; Matsushika, A.; Yano, S.; Sawayama, S. Bench-scale bioethanol production from eucalyptus by high solid saccharification and glucose/xylose fermentation method. Bioprocess Biosyst. Eng. 2013, 37, 749–754. [Google Scholar] [CrossRef]
- Antonkiewicz, J.; Kołodziej, B.; Bielińska, E.J. The use of reed canary grass and giant miscanthus in the phytoremediation of municipal sewage sludge. Environ. Sci. Pollut. Res. 2016, 23, 9505–9517. [Google Scholar] [CrossRef]
- Radziemska, M.; Koda, E.; Bilgin, A.; Vaverková, M.D. Concept of Aided Phytostabilization of Contaminated Soils in Postindustrial Areas. Int. J. Environ. Res. Public Health 2017, 15, 24. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Saxena, S. Arbuscular Mycorrhizal Fungi (AMF) from Heavy Metal-Contaminated Soils: Molecular Ap-proach and Application in Phytoremediation. In Biofertilizers for SustainableAgriculture and Environment; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Cheng, L.; Zhou, Q.; Yu, B. Responses and roles of roots, microbes, and degrading genes in rhizosphere during phy-toremediation of petroleum hydrocarbons contaminated soil. Int. J. Phytoremediat. 2019, 21, 1161–1169. [Google Scholar] [CrossRef]
- Mahar, A.; Wang, P.; Ali, A.; Awasthi, M.K.; Lahori, A.H.; Wang, Q.; Li, R.; Zhang, Z. Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: A review. Ecotoxicol. Environ. Saf. 2016, 126, 111–121. [Google Scholar] [CrossRef]
- Suman, J.; Uhlik, O.; Viktorova, J.; Macek, T. Phytoextraction of Heavy Metals: A Promising Tool for Clean-Up of Polluted Environment? Front. Plant Sci. 2018, 9, 1476. [Google Scholar] [CrossRef]
- Baker, A.J.; Walker, P.L. Ecophysiology of Metal Uptake by Tolerant Plants. In Heavy Metal Tolerance in Plants: Evolutionary Aspects; Shaw, A.J., Ed.; CRC Press Inc.: Boca Raton, FL, USA, 1990; pp. 155–165. [Google Scholar]
- Mishra, S.; Dubey, R.S. Heavy Metal Uptake and Detoxification Mechanisms in Plants. Int. J. Agric. Res. 2006, 1, 122–141. [Google Scholar] [CrossRef]
- Benzarti, S.; Mohri, S.; Ono, Y. Plant response to heavy metal toxicity: Comparative study between the hyperaccumulatorThlaspi caerulescens(ecotype Ganges) and nonaccumulator plants: Lettuce, radish, and alfalfa. Environ. Toxicol. 2008, 23, 607–616. [Google Scholar] [CrossRef]
- Singh, R.; Gautam, N.; Mishra, A.; Gupta, R. Heavy metals and living systems: An overview. Indian J. Pharmacol. 2011, 43, 246–253. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Xie, Y. Heavy Metal Stress and Some Mechanisms of Plant Defense Response. Sci. World J. 2015, 2015, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Maksymiec, W.; Wójcik, M.; Krupa, Z. Variation in oxidative stress and photochemical activity in Arabidopsis thaliana leaves subjected to cadmium and excess copper in the presence or absence of jasmonate and ascorbate. Chemosphere 2007, 66, 421–427. [Google Scholar] [CrossRef]
- Assuncao, A.G.L.; Schat, H.; Aarts, M.G.M. Thlaspi caerulescens, an attractive model species to study heavy metal hyperaccumulation in plants. New Phytol. 2003, 159, 351–360. [Google Scholar] [CrossRef]
- Yang, X.; Long, X.; Ye, H.; He, Z.; Calvert, D.; Stoffella, P. Cadmium tolerance and hyperaccumulation in a new Zn-hyperaccumulating plant species (Sedum alfredii Hance). Plant Soil 2004, 259, 181–189. [Google Scholar] [CrossRef]
- Ali, H.; Naseer, M.; Sajad, M.A. Fitorremediación de metales pesados por Trifolium alexandrinum. Int. J. Environ. Sci. 2012, 2, 1459–1469. [Google Scholar]
- Baker, A.J.; Brooks, R. Terrestrial higher plants which hyperaccumulate metallic elements–A review of their distribution, ecology and phytochemistry. Biorecovery 1989, 1, 81–126. [Google Scholar]
- Baker, A.J.; McGrath, S.P.; Reeves, R.D.; Smith, J.A. Metal Hyperaccumulator Plants: A Review of The Ecology and Physiology of a Biological Resource For Phytoremediation Of Met-Al-Polluted Soils. In Phytoremediation of Contaminated Soil and Water; Terry, N., Bañuelos, G., Eds.; Lewis Publishers: Boca Ratón, FL, USA, 2000; pp. 85–107. [Google Scholar]
- Van Der Ent, A.; Baker, A.J.M.; Reeves, R.D.; Pollard, A.J.; Schat, H. Hyperaccumulators of metal and metalloid trace elements: Facts and fiction. Plant Soil 2013, 362, 319–334. [Google Scholar] [CrossRef]
- Memon, A.R.; Schröder, P. Implications of metal accumulation mechanisms to phytoremediation. Environ. Sci. Pollut. Res. 2009, 16, 162–175. [Google Scholar] [CrossRef]
- Sheoran, V.; Sheoran, A.S.; Poonia, P. Role of Hyperaccumulators in Phytoextraction of Metals from Contaminated Mining Sites: A Review. Crit. Rev. Environ. Sci. Technol. 2010, 41, 168–214. [Google Scholar] [CrossRef]
- Alford, É.R.; Pilon-Smits, E.A.H.; Paschke, M.W. Metallophytes—a view from the rhizosphere. Plant Soil 2010, 337, 33–50. [Google Scholar] [CrossRef]
- Corso, M.; De La Torre, V.S.G. Biomolecular approaches to understanding metal tolerance and hyperaccumulation in plants. Metallomics 2020, 12, 840–859. [Google Scholar] [CrossRef] [PubMed]
- Whiting, S.N.; Neumann, P.M.; Baker, A.J.M. Nickel and zinc hyperaccumulation by Alyssum murale and Thlaspi caerulescens (Brassicaceae) do not enhance survival and whole-plant growth under drought stress. Plant Cell Environ. 2003, 26, 351–360. [Google Scholar] [CrossRef]
- Whiting, S.N.; Leake, J.R.; McGrath, S.P.; Baker, A.J.M. Hyperaccumulation of Zn byThlaspi caerulescensCan Ameliorate Zn Toxicity in the Rhizosphere of CocroppedThlaspi arvense. Environ. Sci. Technol. 2001, 35, 3237–3241. [Google Scholar] [CrossRef] [PubMed]
- Pollard, A.J.; Baker, A.J.M. Deterrence of herbivory by zinc hyperaccumulation in Thlaspi caerulescens (Brassicaceae). New Phytol. 1997, 135, 655–658. Available online: http://www.jstor.org/stable/2558997 (accessed on 22 May 2020). [CrossRef]
- Behmer, S.T.; Lloyd, C.M.; Raubenheimer, D.; Stewart-Clark, J.; Knight, J.; Leighton, R.S.; Smith, J.A.C. Metal hyperaccumulation in plants: Mechanisms of defense against insect herbivores. Funct. Ecol. 2005, 19, 55–66. [Google Scholar] [CrossRef]
- Boyd, R.S. Plant defense using toxic inorganic ions: Conceptual models of the defensive enhancement and joint effects hypotheses. Plant Sci. 2012, 195, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Pittarello, M.; Busato, J.G.; Carletti, P.; Dobbss, L. Possible developments for ex situ phytoremediation of contaminated sediments, in tropical and subtropical regions—Review. Chemosphere 2017, 182, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, G.C.G.; Rodella, A.A.; De Abreu, C.A.; Coscione, A.R. Vegetable species for phytoextraction of boron, copper, lead, manganese and zinc from contaminated soil. Sci. Agric. 2010, 67, 713–719. [Google Scholar] [CrossRef]
- Gao, G.; Zeng, X.; Li, Z.; Chen, A.; Yang, Z. Variations in several morphological characteristics and Cd/Pb accumulation capacities among different ecotypes of torpedograss responding to Cd-Pb stresses. Int. J. Phytoremediat. 2017, 19, 844–861. [Google Scholar] [CrossRef]
- Yoon, J.; Cao, X.; Zhou, Q.; Ma, L.Q. Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci. Total Environ. 2006, 368, 456–464. [Google Scholar] [CrossRef]
- Alves, M.; Araújo, A.C.; Prata, A.P.; Vitta, F.; Hefler, S.; Trevisan, R.; Giol, A.d.S.B.; Martins, S.; Thomas, W. Diversidade de Cyperaceae no Brazil. Rodriguésia 2009, 60, 771–782. [Google Scholar] [CrossRef]
- Barceló, J.; Poschenrieder, C. Phytoremediation: Principles and perspectives. Contrib. Sci. 2003, 2, 333–344. [Google Scholar]
- Marrugo-Negrete, J.L.; Marrugo-Madrid, S.; Pinedo-Hernández, J.; Durango-Hernández, J.; Díez, S. Screening of native plant species for phytoremediation potential at a Hg-contaminated mining site. Sci. Total Environ. 2016, 542, 809–816. [Google Scholar] [CrossRef]
- Vara Prasad, M.N.; de Oliveira Freitas, H.M. Metal hyperaccumulation in plants: Biodiversity prospecting for phy-toremediation technology. Electron. J. Biotechnol. 2003, 6, 285–321. [Google Scholar] [CrossRef]
- Christenhusz, M.J.; Byng, J.W. The number of known plants species in the world and its annual increase. Phytotaxa 2016, 261, 201–217. [Google Scholar] [CrossRef]
- Costea, M.; Sanders, A.; Waines, G. Preliminary results towards a revision of the Amaranthus hybridus complex (Amaranthaceae). Sida Contrib. Bot. 2001, 19, 931–974. [Google Scholar]
- Ortiz-Cano, H.G.; Trejo-Calzada, R.; Valdez-Cepeda, R.D.; Arreola-Avila, J.G.; Flores-Hernandez, A.; Lopez-Ariza, B. Phytoextraction of lead and cadmium in contaminated soils using pigweed (Amaranthus hybridus L.) and mycorrhiza. Revista Chapingo. Ser. Hortic. 2009, 15, 161–168. Available online: http://www.scielo.org.mx/pdf/rcsh/v15n2/v15n2a9.pdf (accessed on 4 December 2019).
- Croat, T.B.; Delannay, X.; Ortiz, O.O. A revision of Xanthosoma (Araceae). Part 2: Central America. J. Int. Aroid Soc. 2017, 40, 504–581. [Google Scholar]
- Muso Cachumba, J. Determinación de la capacidad fitorremediadora de cadmio del Camacho (Xanthosoma undipies koch) especie vegetal nativa en el área de influencia de Ep Ecuador en el distrito amazónico. Sangolqui, Ecuador: Escuela politécnica del ejército. 2012. Available online: http://repositorio.espe.edu.ec/handle/21000/6255 (accessed on 4 December 2019).
- Funk, V.A.; Bayer, R.J.; Keeley, S.; Chan, R.; Watson, L.; Gemeinholzer, B.; Schilling, E.E.; Panero, J.L.; Baldwin, B.G.; Garcia-Jacas, N.T.; et al. Everywhere but Antarctica: Using a supertree to understand the diversity and distribution of the Compositae. Biol. Skr. 2005, 55, 343–373. [Google Scholar]
- Appezzato da Glória, B. Morfologia de Sistemas Subterrâneos de Plantas; 3i Editora: Belo Horizonte, Brazil, 2015; p. 160. [Google Scholar]
- Robinson, B.; Clothier, B.; Brooks, R.R. Soil Amendments Affecting Nickel and Cobalt Uptake by Berkheya coddii: Potential Use for Phytomining and Phytoremediation. Ann. Bot. 1999, 84, 689–694. [Google Scholar] [CrossRef]
- Turnau, K.; Mesjasz-Przybylowicz, J. Arbuscular mycorrhiza of Berkheya coddii and other Ni-hyperaccumulating members of Asteraceae from ultramafic soils in South Africa. Mycorrhiza 2003, 13, 185–190. [Google Scholar] [CrossRef]
- Alves, J.C.; De Souza, A.P.; Pôrto, M.L.A.; Fontes, R.L.F.; De Arruda, J.; Marques, L.F. Potential of sunflower, castor bean, common buckwheat and vetiver as lead phytoaccumulators. Rev. Braz. Eng. Agrícola Ambient. 2016, 20, 243–249. [Google Scholar] [CrossRef]
- Warwick, S.I.; Al-Shehbaz, I.A. Brassicaceae: Chromosome number index and database on CD-Rom. Plant Syst. Evol. 2006, 259, 237–248. [Google Scholar] [CrossRef]
- Roy, S.; Mondal, S. Brassicaceae Plants Response and Tolerance to Metal/Metalloid Toxicity. In The Plant Family Brassicaceae; Hasanuzzaman, M., Ed.; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Govaerts, R.; Simpson, D.A.; Bruhl, J.; Egorova, T.; Goetghebeur, P.; Wilson, K. Word checklist of Cyperaceae Sedges. Royal Botanic Gardens, Kew. 2007. Available online: www.kew.org/neotropikey (accessed on 15 October 2019).
- Alves, M.; Araújocambiar, A.C.; Prata, A.P.; Vitta, F.; Hefler, S.; Trevisan, R.; Gil, A.B.; Martins, S.; Thomas, W.W. Di-versidade de Cyperaceae no Brazil. In A Botânica no Brazil: Pesquisa, Ensino e Políticas Públicas Ambientais; Barbosa, L., Santos Junior, N., Eds.; SBB: São Paulo, Brazil, 2007; pp. 286–290. [Google Scholar]
- Kaewtubtim, P. Heavy metal phytoremediation potential of plant species in a mangrove ecosystem in Pattani Bay, Thailand. Appl. Ecol. Environ. Res. 2016, 14, 367–382. [Google Scholar] [CrossRef]
- Garba, S.T.; Gudusu, M.; Inuwa, L.B. Accumulation Ability of the Native Grass Species, Cyperus rotundus for the Heavy Metals; Zinc (Zn), Cadmium (Cd), Nickel (Ni) and Lead (Pb). Int. Res. J. Pure Appl. Chem. 2018, 17, 1–15. [Google Scholar] [CrossRef]
- Webster, G.L. Synopsis of the Genera and Suprageneric Taxa of Euphorbiaceae. Ann. Mo. Bot. Gard. 1994, 81, 33. [Google Scholar] [CrossRef]
- Radcliffe-Smith, A. Genera Euphorbiacearum; Royal Botanic Gardens: Kew, UK, 2001; p. 464.
- Heller, J. Physic Nut, Jatropha curcas L.; Bioversity International: Rome, Italy, 1996; Volume 1. [Google Scholar]
- García-Martín, J.F.; Caro, M.D.C.G.; Barrera, M.D.C.L.; Torres-García, M.; Barbin, D.F.; Álvarez-Mateos, P. Metal Accumulation by Jatropha curcas L. Adult Plants Grown on Heavy Metal-Contaminated Soil. Plants 2020, 9, 418. [Google Scholar] [CrossRef]
- Farruggia, F.T.; Lavin, M.; Wojciechowski, M.F. Phylogenetic Systematics and Biogeography of the Pantropical Genus Sesbania (Leguminosae). Syst. Bot. 2018, 43, 414–429. [Google Scholar] [CrossRef]
- Sahi, S.V.; Bryant, N.L.; Sharma, N.C.; Singh, S.R. Characterization of a Lead Hyperaccumulator Shrub, Sesbania drummondii. Environ. Sci. Technol. 2002, 36, 4676–4680. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Fang, W.; Yang, Z.; Yuan, J. Cadmium and copper uptake and accumulation by Sesbania rostrata seedling, a N-fixing annual plant: Implications for the mechanism of heavy metal tolerance. Front. Biol. China 2009, 4, 200–206. [Google Scholar] [CrossRef]
- Kress, W.J.; Betancu, J.; Echeverry, B. Heliconias, Llamaradas de la Selva Colombiana, 1st ed.; Gufa de Campo; Uribe, C., Ed.; Santafé de Bogotá: Bogota, Colombia, 1999; p. 200. [Google Scholar]
- Jerez, E. El cultivo de las heliconias. Cultiv. Trop. 2007, 28, 29–35. Available online: https://www.redalyc.org/pdf/1932/193215858005.pdf (accessed on 27 August 2019).
- Dorr, L.J.; Berry, F.; Kress, W.J. Heliconia An Identification Guide. Brittonia 1991, 43, 170. [Google Scholar] [CrossRef][Green Version]
- Gómez-Merino, F.C.; Trejo-Tellez, L.I.; García-Albarado, J.C.; Pérez-Sato, J.A. Diversidad, distribución y reproduc-ción de heliconias. Agro Product. 2018, 11, 33–40. [Google Scholar] [CrossRef]
- Guerra, B.E.; Aguillón, A.D.; García, S.; Muñoz, G.J. Crecimiento de Heliconia pssitacorum (Heliconiaceae) en suelos contaminados por cadmio, asociados a simbiosis micorrizica y biomasas de Aspergillus niger. Simposio -Hongos en Biorremediación: SIMP 21.2. IX Congreso Latinoamericano de Micología, Libro de Resumenes, Lima, Perú, 22–25 August 2017; Available online: https://drive.google.com/file/d/1CqYnnLwmNE4FPtvc-MESd6L1_aSQ1KNz/view (accessed on 15 July 2019).
- Lu, F.; Rodriguez-Garcia, J.; Van Damme, I.; Lebl, T.; Shaw, L.; Robinson, J.S.; Warren, G.; Chatzifragkou, A.; Mason, S.M.; Gomez, L.; et al. Valorisation strategies for cocoa pod husk and its fractions. Curr. Opin. Green Sustain. Chem. 2018, 14, 80–88. [Google Scholar] [CrossRef]
- Meter, A.; Atkinson, R.J.; Laliberte, B. Cadmium in Cacao from Latin America and The Caribbean. A Review of Re-search and Potential Mitigation Solutions; CAF: Caracas, Venezuela, 2019. [Google Scholar]
- Merkl, N.; Schultze-Kraft, R.; Infante, C. Phytoremediation in the tropics—Influence of heavy crude oil on root morphological characteristics of graminoids. Environ. Pollut. 2005, 138, 86–91. [Google Scholar] [CrossRef]
- Ortega-Ortega, R.E.; Beltrá, H.; Javier, D.; Marrugo, N.; José, L. Acumulación de mercurio (Hg) por caña flecha (Gynerium sagittatum) (Aubl) Beauv. in vitro. Rev. Colomb. Biotechnol. 2011, 13, 33–41. Available online: http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0123-34752011000100005&lng=en&tlng=es (accessed on 22 May 2020).
- Danh, L.T.; Truong, P.; Mammucari, R.; Tran, T.; Foster, N. Vetiver grass, vetiveria zizanioides: A choice plant for phytoremediation of heavy metals and organic wastes. Int. J. Phytoremediat. 2009, 11, 664–691. [Google Scholar] [CrossRef]
- Das, A.; Belgaonkar, P.; Raman, A.S.; Banu, S.; Osborne, W.J. Bioremoval of lead using Pennisetum purpureum augmented with Enterobacter cloacae-VITPASJ1: A pot culture approach. Environ. Sci. Pollut. Res. 2017, 24, 15444–15453. [Google Scholar] [CrossRef]
- Vidal Durango, J.V.; Marrugo Negrete, J.L.; Jaramillo Colorado, B.; Pérez Castro, L.M. Remediación de suelos con-taminados con mercurio utilizando guarumo (Cecropia peltata). Ing. Desarro. 2010, 27, 113–129. [Google Scholar]
- Simpson, M.G. 2010. Diversity and Classification of Flowering Plants: Eudicots. Plant Systematics, 2nd ed.; The Plant List, Version 1; Academic Press: San Diego, CA, USA, 2013; pp. 275–448. Available online: http://www.theplantlist.org/ (accessed on 27 February 2020).
- Van Oosten, M.J.; Maggio, A. Functional biology of halophytes in the phytoremediation of heavy metal contaminated soils. Environ. Exp. Bot. 2015, 111, 135–146. [Google Scholar] [CrossRef]
- Dos Santos, M.N.; Utmazian, M.N.; Wenzel, W.W. Phytoextraction of Metal Polluted Soils in Latin America; Department of Forest and Soil Sciences, University of Natural Resources and Applied Life Science: Vienna, Austria, 2006. [Google Scholar]
- Chen, F.; Yang, Y.; Mi, J.; Liu, R.; Hou, H.; Zhang, S. Effects of Vegetation Pattern and Spontaneous Succession on Remediation of Potential Toxic Metal-Polluted Soil in Mine Dumps. Sustainability 2019, 11, 397. [Google Scholar] [CrossRef]
- Chen, J.; Huang, J. Role of pH in Phytoremediation of Contaminated Soils. In Handbook of Soil Acidity; CRC Press: Boca Raton, FL, USA, 2003; pp. 449–472. [Google Scholar]
- Hauptvogl, M.; Kotrla, M.; Prčík, M.; Pauková, Ž.; Kováčik, M.; Lošák, T. Phytoremediation Potential of Fast-Growing Energy Plants: Challenges and Perspectives—A Review. Pol. J. Environ. Stud. 2019, 29, 505–516. [Google Scholar] [CrossRef]
- Nouri, J.; Khorasani, N.; Lorestani, B.; Karami, M.; Hassani, A.H.; Yousefi, N. Accumulation of heavy metals in soil and uptake by plant species with phytoremediation potential. Environ. Earth Sci. 2009, 59, 315–323. [Google Scholar] [CrossRef]
- Becerril, J.; Barrutia, O.; Garcia, J.P.; Hernandez, A.; Olano, J.; Garbisu, C. Especies nativas de suelos contaminados por metales: Aspectos ecofisiologicos y su uso en fitorremediacion. Ecosistemas 2007, 16, 50–55. [Google Scholar]
- Leštan, D.; Luo, C.-L.; Li, X.-D. The use of chelating agents in the remediation of metal-contaminated soils: A review. Environ. Pollut. 2008, 153, 3–13. [Google Scholar] [CrossRef]
- Ojuederie, O.B.; Babalola, O.O. Microbial and Plant-Assisted Bioremediation of Heavy Metal Polluted Environments: A Review. Int. J. Environ. Res. Public Health 2017, 14, 1504. [Google Scholar] [CrossRef] [PubMed]
| Classification | Description |
|---|---|
| Oxisols | Highly degraded tropical soils. These are located in hot and humid areas, have undergone major weathering processes, and accumulate kaolinite and sesquioxides necessary for the formation of the characteristic oxic horizon of this order. All Oxisols have relatively low cation exchange values and some have a net positive charge in the subsoil. Natural limitations due to acidity and low nutrient content have been overcome economically with lime and fertilizer applications. |
| Ultisols | These form in humid areas. Commonly known as red clay soils, they are extremely eroded and exhibit low fertility. Kaolin, gibbsite, and aluminum-interlayered clays are common in the clay fraction. These soils can become productive with the addition of fertilizers and lime. |
| Alfisols | Soils located in arid climates. Dry soils have low organic content and are sparsely vegetated by drought- or salt-tolerant plants. They accumulate salt, gypsum, calcium carbonate, and other materials that are easily leached. |
| Inceptisols | These are found in a wide range of environments and include a variety of soils in semi-arid and humid areas. They are considered moderately eroded and their mineralogy generally reflects their relative immaturity. They are present in a wide variety of climates. |
| Entisols | Easily erodible soils lacking developed horizons. They are most extensive in floodplains and valleys or on steep slopes where erosion is rapid. Found in dunes, steep slopes, and flood plains. |
| Category | Metals |
|---|---|
| Metals that are essential micronutrients | Cu, Zn, Mn, Fe |
| Nonessential heavy metals | As, Cd, Cr, Co Pb, Hg, Ni, V |
| Industrial Activity | Metals | Associated Processes and Pollutants |
|---|---|---|
| Agriculture and ranching | As, Cd, Cr, Cu, Mn, Mo, Pb, U, V, Zn | Pollution from runoff leads to contamination of surface and ground water. The industry also produces agricultural chemi-cals, which can bioaccumulate in plants and animals |
| Batteries | Cd, Hg, Ni, Pb, Sb, Zn | Residual battery fluids cause contamination of groundwater and soil. |
| Electronic waste | As, Au, Cd, Cr, Hg, Mn, Ni, Pb, Pt | Aqueous and solid metallic waste from the manufacturing and recycling processes results in air and water pollution |
| Electroplating | Cu, Cr, Ni, Zn | Liquid effluents from coating processes pollute water and soil. |
| Ferrous metal mining | Cd, Co, Cu, Cr, Ni, Zn | Leakage of acids from mining. Generation of tailings and sludge. |
| Hydrocarbons | Ag, As, Cu, Cr, Fe, Hg, Ni, Pb, Mn | Exploration, extraction, and refining processes generate pollution of surface water, groundwater, and soil |
| Metallurgy | Cu, Cr, Mn, Zn, Pb, Sb | Thermal treatment of metals. Atmospheric pollution. |
| Paints and pigments | As, Ba, Cr, Pb, Ti, Zn | Aqueous residues from the manufacture of new paint, and the deterioration of old. |
| Steel and alloys | As, Cd, Cu, Mo, Ni, Pb, Te, U, Zn | Manufacture, disposal, and recycling of metals. Includes tailings and cinders. Contaminates water and soils |
| Smelting | As, Cd, Pb, Ti | Processing of ores to extract metals. Atmospheric pollution and solid waste. |
| Waste management | Cd, Cu, Cr, Hg, Ni, Pb, Mn, Zn | Incineration of waste or leaching from landfills can cause atmospheric pollution, as well as contamination of soils, surface water, and groundwater |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerra Sierra, B.E.; Muñoz Guerrero, J.; Sokolski, S. Phytoremediation of Heavy Metals in Tropical Soils an Overview. Sustainability 2021, 13, 2574. https://doi.org/10.3390/su13052574
Guerra Sierra BE, Muñoz Guerrero J, Sokolski S. Phytoremediation of Heavy Metals in Tropical Soils an Overview. Sustainability. 2021; 13(5):2574. https://doi.org/10.3390/su13052574
Chicago/Turabian StyleGuerra Sierra, Beatriz E., Jaider Muñoz Guerrero, and Serge Sokolski. 2021. "Phytoremediation of Heavy Metals in Tropical Soils an Overview" Sustainability 13, no. 5: 2574. https://doi.org/10.3390/su13052574
APA StyleGuerra Sierra, B. E., Muñoz Guerrero, J., & Sokolski, S. (2021). Phytoremediation of Heavy Metals in Tropical Soils an Overview. Sustainability, 13(5), 2574. https://doi.org/10.3390/su13052574







