Assessment of Conventional Solvent Extraction vs. Supercritical Fluid Extraction of Khella (Ammi visnaga L.) Furanochromones and Their Cytotoxicity
Abstract
:1. Introduction
2. Results
2.1. Conventional Solvent Extraction vs. Supercritical Fluid Extraction of Furanochromones
2.2. HPLC Analysis of Conventional Solvents and SCF Extracts
2.3. In-Vitro Cytotoxic Activity of A. visnaga Extracts, Standard Khellin and Visnagin
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Preparation of the Plant Extracts
4.2.1. Conventional Solvent Extraction
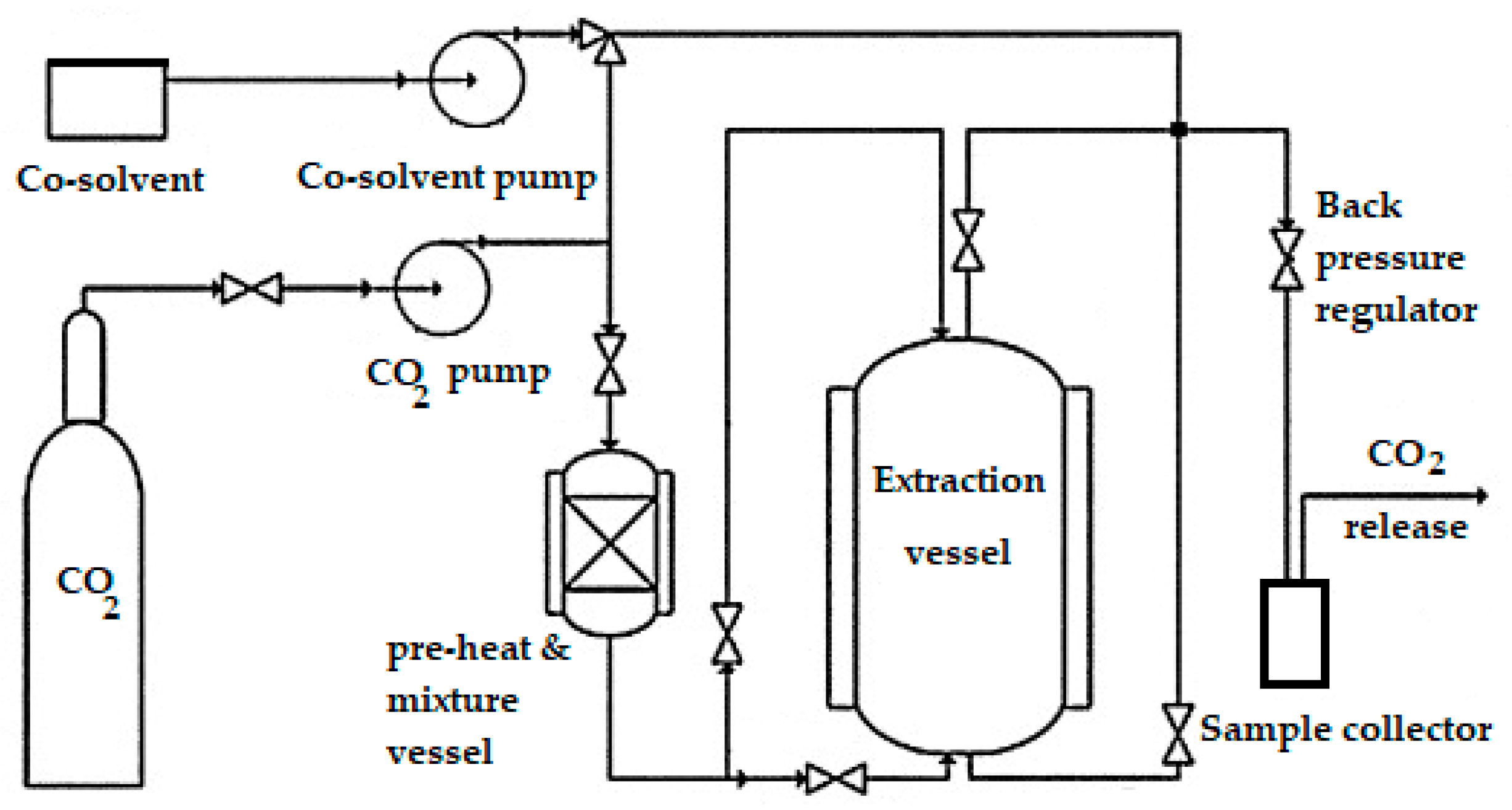
4.2.2. Pilot Supercritical Fluid Carbon Dioxide Extraction Method
4.3. HPLC Analysis
4.4. Cytotoxic Activity of A. visnaga Extracts, Standard Khellin, Visnagin and Doxorubocin
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Sample Availability
References
- Batanouny, K. Wild Medicinal Plants in Egypt: An Inventory to Support Conservation and Sustainable Use; Palm Press: Cairo, Egypt, 1999. [Google Scholar]
- Khalil, N.; Bishr, M.; Desouky, S.; Salama, O. Ammi Visnaga L., a Potential Medicinal Plant: A Review. Molecules 2020, 25, 301. [Google Scholar] [CrossRef] [Green Version]
- Mucklow, J.C. Martindale: The complete drug reference. Br. J. Clin. Pharmacol. 2000, 49, 613. [Google Scholar] [CrossRef]
- Vanachayangkul, P.; Chow, N.; Khan, S.R.; Butterweck, V. Prevention of renal crystal deposition by an extract of Ammi visnaga L. and its constituents khellin and visnagin in hyperoxaluric rats. Urol. Res. 2011, 39, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Vanachayangkul, P.; Byer, K.; Khan, S.; Butterweck, V. An aqueous extract of Ammi visnaga fruits and its constituents khellin and visnagin prevent cell damage caused by oxalate in renal epithelial cells. Phytomed. Int. J. Phytother. Phytopharm. 2010, 17, 638–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, Z.A.; Assiri, A.M.; Al-Afghani, H.M.; Maghrabi, T.M. Inhibition of oxalate nephrolithiasis with Ammi visnaga (AI-Khillah). Int. Urol. Nephrol. 2001, 33, 605–608. [Google Scholar] [CrossRef]
- Gunaydin, K.; Beyazit, N. The chemical investigations on the ripe fruits of Ammi visnaga (Lam.) Lamarck growing in Turkey. Nat. Prod. Res. 2004, 18, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Duarte, J.; Vallejo, I.; Perez-Vizcaino, F.; Jimenez, R.; Zarzuelo, A.; Tamargo, J. Effects of visnadine on rat isolated vascular smooth muscles. Planta Med. 1997, 63, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Bhagavathula, A.; Mahmoud Al-Khatib, A.; Elnour, A.; Al Kalbani, N.; Shehab, A. Ammi Visnaga in treatment of urolithiasis and hypertriglyceridemia. Pharmacogn. Res. 2015, 7, 397–400. [Google Scholar]
- Hashim, S.; Jan, A.; Marwat, K.; Khan, M. Phytochemistry and medicinal properties of Ammi visnaga (Apiacae). Pak. J. Bot. 2014, 46, 861–867. [Google Scholar]
- Abu-Hashem, A.; El-Shazly, M. Synthesis, reactions and biological activities of furochromones: A review. Eur. J. Med. Chem 2015, 90, 633–665. [Google Scholar] [CrossRef]
- Winderl, B.; Schwaiger, S.; Ganzera, M. Fast and improved separation of major coumarins in Ammi visnaga (L.) Lam. by supercritical fluid chromatography. J. Sep. Sci. 2016, 39, 4042–4048. [Google Scholar] [CrossRef] [PubMed]
- Zgorka, G.; Dragan, T.; Glowniak, K.; Basiura, E. Determination of furanochromones and pyranocoumarins in drugs and Ammi visnaga fruits by combined solid-phase extraction-high-performance liquid chromatography and thin-layer chromatography-high-performance liquid chromatography. J. Chromatogr. A 1998, 797, 305–309. [Google Scholar] [CrossRef]
- Abu-Hashem, A.A.; Youssef, M.M. Synthesis of new visnagen and khellin furochromone pyrimidine derivatives and their anti-inflammatory and analgesic activity. Molecules 2011, 16, 1956–1972. [Google Scholar] [CrossRef] [Green Version]
- Ragab, F.A.; El-Sayed, N.A.; Eissa, A.A.; El Kerdawy, A.M. Synthesis and anticonvulsant activity of certain substituted furochromone, benzofuran and flavone derivatives. Chem. Pharm. Bull. 2010, 58, 1148–1156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sellami, H.K.; Napolitano, A.; Masullo, M.; Smiti, S.; Piacente, S.; Pizza, C. Influence of growing conditions on metabolite profile of Ammi visnaga umbels with special reference to bioactive furanochromones and pyranocoumarins. Phytochemistry 2013, 95, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Harvengt, C.; Desager, J. HDL-cholesterol increase in normolipaemic subjects on khellin: A pilot study. Int. J. Clin. Pharmacol. Res. 1983, 3, 363–366. [Google Scholar] [PubMed]
- Duarte, J.; Torres, A.I.; Zarzuelo, A. Cardiovascular effects of visnagin on rats. Planta Med. 2000, 66. [Google Scholar] [CrossRef]
- Voros, V.; Drioli, E.; Fonte, C.; Szekely, G. Process Intensification via Continuous and Simultaneous Isolation of Antioxidants: An Upcycling Approach for Olive Leaf Waste. ACS Sustain. Chem. Eng. 2019, 7, 18444–18452. [Google Scholar] [CrossRef]
- Didaskalou, C.; Buyuktiryaki, S.; Kecili, R.; Fonte, C.P.; Szekely, G. Valorisation of agricultural waste with an adsorption/nanofiltration hybrid process: From materials to sustainable process design. Green Chem. 2017, 19, 3116–3125. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Jing, W.; Tian, H.; Liu, M.; Yan, H.; Bi, W.; Chen, D.D.Y. Investigation of Deep Eutectic Solvent-Based Microwave-Assisted Extraction and Efficient Recovery of Natural Products. ACS Sustain. Chem. Eng. 2020, 8, 12080–12088. [Google Scholar] [CrossRef]
- Cao, D.; Liu, Q.; Jing, W.; Tian, H.; Yan, H.; Bi, W.; Jiang, Y.; Chen, D.D.Y. Insight into the Deep Eutectic Solvent Extraction Mechanism of Flavonoids from Natural Plant. ACS Sustain. Chem. Eng. 2020, 8, 19169–19177. [Google Scholar] [CrossRef]
- Nahar, L.; Sarker, S.D. Supercritical fluid extraction in natural products analyses. Methods Mol. Biol. 2012, 864, 43–74. [Google Scholar] [PubMed]
- Ashraf-Khorassani, M.; Combs, M.; Taylor, L. Supercritical fluid extraction of metal ions and metal chelates from different environments. J. Chromatogr. A 1997, 774, 37–49. [Google Scholar] [CrossRef]
- Zougagh, M.; Valcárcel, M.; Ríos, A. Supercritical fluid extraction: A critical review of its analytical usefulness. Trend. Anal. Chem. 2004, 23, 399–405. [Google Scholar] [CrossRef]
- Pourmortazavi, S.; Hajimirsadeghi, S. Supercritical fluid extraction in plant essential and volatile oil analysis. J. Chromatogr. A 2007, 1163, 2–24. [Google Scholar] [CrossRef]
- Careri, M.; Furlattini, L.; Mangia, A.; Musc, M.; Anklam, E.; Theobald, A.; von Holst, C. Supercritical fluid extraction for liquid chromatographic determination of carotenoids in Spirulina pacifica algae: A chemometric approach. J. Chromatogr. A 2001, 912, 61–71. [Google Scholar] [CrossRef]
- Brachet, A.; Mateus, L.; Cherkaoui, S.; Christen, P.; Gauvrit, J.-Y.; Lantéri, P.; Veuthey, J.-L. Application of central composite designs in the supercritical fluid extraction of tropane alkaloids in plant extracts. Analusis 1999, 27, 772–778. [Google Scholar] [CrossRef] [Green Version]
- Egydio, J.; Moraes, Â.; Rosa, P. Supercritical fluid extraction of lycopene from tomato juice and characterization of its antioxidation activity. J Supercrit. Fluids 2010, 54, 159–164. [Google Scholar] [CrossRef]
- Ambrosino, P.; Fresa, R.; Fogliano, V.; Monti, S.M.; Ritieni, A. Extraction of azadirachtin A from neem seed kernels by supercritical fluid and its evaluation by HPLC and LC/MS. J. Agric. Food Chem. 1999, 47, 5252–5256. [Google Scholar] [CrossRef]
- Campos, L.; Michielin, E.; Danielski, L.; Ferreira, S. Experimental data and modeling the supercritical fluid extraction of marigold (Calendula officinalis) oleoresin. J. Supercrit. Fluids 2005, 34, 163–170. [Google Scholar] [CrossRef]
- Han, Z.; Wang, X.; Xu, M.; Wang, Y.; Yang, L.; Han, M. Optimization of Supercritical Fluid Extraction and Rapid Resolution LC-MS/ESI Identification of Chromones from Saposhnikoviae radix through Orthogonal Array Design. Chin. Herb. Med. 2016, 8, 314–322. [Google Scholar] [CrossRef]
- Khaw, K.Y.; Parat, M.O.; Shaw, P.N.; Falconer, J.R. Solvent supercritical fluid technologies to extract bioactive compounds from natural sources: A Review. Molecules 2017, 22, 1186. [Google Scholar] [CrossRef]
- Herrero, M.; Cifuentes, A.; Ibañez, E. Sub- and supercritical fluid extraction of functional ingredients from different natural sources: Plants, food-by-products, algae and microalgae: A review. Food Chem. 2006, 98, 136–148. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, R.P.F.F.; Rocha-Santos, T.A.P.; Duarte, A.C. Supercritical fluid extraction of bioactive compounds. TrAC Trends Anal. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef] [Green Version]
- Vlietinck, A.J. Screening Methods for Detection and Evaluation of Biological Activities of Plant Preparations. In Bioassay Methods in Natural Product Research and Drug Development; Bohlin, L., Bruhn, J.G., Eds.; Springer: Dordrecht, The Netherlands, 1999; pp. 37–52. [Google Scholar]
- Cordero, C.P.; Gómez-González, S.; León-Acosta, C.J.; Morantes-Medina, S.J.; Aristizabal, F.A. Cytotoxic activity of five compounds isolated from Colombian plants. Fitoterapia 2004, 75, 225–227. [Google Scholar] [CrossRef]
- Ashour, A.; El Sharkawy, S.; Amer, M.; Abdelbar, F.; Kondo, R. Melanin Biosynthesis Inhibitory Activity of Compounds Isolated from Unused Parts of Ammi visinaga. J. Cosmet. Dermatol. Sci. Appl. 2013, 3, 40–43. [Google Scholar]
- Beltagy, A.M.; Beltagy, D.M. Chemical composition of Ammi visnaga L. and new cytotoxic activity of its constituents Khellin and Visnagin. J. Pharm. Sci. Res. 2015, 7, 285–291. [Google Scholar]
- Bishr, M.; El-Degwy, M.; Abdel Hady, M.; Amin, M.; Salama, O. Supercritical fluid extraction of γ-Pyrones from Ammi visnaga L. fruits. Future J. Pharm. Sci. 2018, 4, 57–62. [Google Scholar] [CrossRef]
- Bishr, M.; El Degwy, M.; AbdelHady, M.; Amin, M.; Salama, O. HPLC simultaneous determination of khellin and visnagin in Ammi visnaga L. fruits. IOSR J. Pharm. Biol. Sci. 2016, 11, 110–115. [Google Scholar]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]


| Solvent Used | Solvent Temperature (°C) | Physical Properties Of The Obtained Extract | Total Solid Extract Yield (G) | Total Solid Extract Yield (%) | HPLC Analysis | |||
|---|---|---|---|---|---|---|---|---|
| Kh. (%) | Vis. (%) | Kh. + Vis. (g) | Kh. + Vis.% | |||||
| Boiling water | 100 | Soft brown sticky | 1380.7 ± 30.22 | 13.807 ± 0.32 | 5.03 ± 0.06 | 0.92 ± 0.08 | 82.11 ± 2.33 | 5.95 ± 0.14 |
| 95% Ethanol | 45–50 | Soft light brown | 1350.6 ± 25.67 | 13.506 ± 0.25 | 6.03 ± 0.07 | 2.20 ± 0.02 | 111.1 ± 3.58 | 8.23 ± 0.09 |
| 30% Ethanol | 45–50 | Soft dark brown | 1544.1 ± 51.25 | 15.441 ± 0.51 | 5.03 ± 0.03 | 1.56 ± 0.02 | 101.74 ± 2.01 | 6.59 ± 0.05 |
| Acetone | 45–50 | Soft fatty greenish | 507.5 ± 11.69 | 5.075 ± 0.11 | 13.71 ± 0.12 | 4.78 ± 0.09 | 93.74 ± 3.45 | 18.49 ± 0.21 |
| SCFE | 45 | Yellowish white, slightly fatty, strong characteristic odor | 450.8 ± 8.58 | 4.508 ± 0.81 | 28.8 ± 1.22 | 2.01 ± 0.04 | 135 ± 3.22 | 30.1 ± 1.56 |
| Test | Hep G2 | SI | MCF-7 | SI |
|---|---|---|---|---|
| IC50 (μg/mL) | IC50 (μg/mL) | |||
| Boiling water extract | 112.58 ± 5.69 aB | 2.11 | 123.87 ± 6.22 aB | 2.69 |
| 30% Ethanol extract | 51.22 ± 3.12 aB | 2.89 | 33.96 ± 2.45 aB | 3.11 |
| 95% Ethanol extract | 45.28 ± 2.33 aB | 2.14 | 59.15 ± 4.25 aB | 2.55 |
| Acetone extract | 89.12 ± 4.23 aB | 1.23 | 88.14 ± 3.45 aB | 1.55 |
| SCFE | 17.53 ± 1.03 bB | 3.15 | 15.39 ± 0.58 bB | 4.57 |
| Khellin | 13.86 ± 0.88 bB | 3.96 | 12.54 ± 0.57 bB | 3.24 |
| Visnagin | 15.98 ± 0.66 bB | 3.78 | 13.79 ± 0.43 bB | 2.78 |
| Doxorubicin | 4.93 ± 0.26 A | 3.58 ± 0.31 A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalil, N.; Bishr, M.; El-Degwy, M.; Abdelhady, M.; Amin, M.; Salama, O. Assessment of Conventional Solvent Extraction vs. Supercritical Fluid Extraction of Khella (Ammi visnaga L.) Furanochromones and Their Cytotoxicity. Molecules 2021, 26, 1290. https://doi.org/10.3390/molecules26051290
Khalil N, Bishr M, El-Degwy M, Abdelhady M, Amin M, Salama O. Assessment of Conventional Solvent Extraction vs. Supercritical Fluid Extraction of Khella (Ammi visnaga L.) Furanochromones and Their Cytotoxicity. Molecules. 2021; 26(5):1290. https://doi.org/10.3390/molecules26051290
Chicago/Turabian StyleKhalil, Noha, Mokhtar Bishr, Mohamed El-Degwy, Mohamed Abdelhady, Mohamed Amin, and Osama Salama. 2021. "Assessment of Conventional Solvent Extraction vs. Supercritical Fluid Extraction of Khella (Ammi visnaga L.) Furanochromones and Their Cytotoxicity" Molecules 26, no. 5: 1290. https://doi.org/10.3390/molecules26051290






