Transformation of Biowaste for Medical Applications: Incorporation of Biologically Derived Silver Nanoparticles as Antimicrobial Coating
Abstract
1. Introduction
2. Silver Nanoparticles (AgNPs)
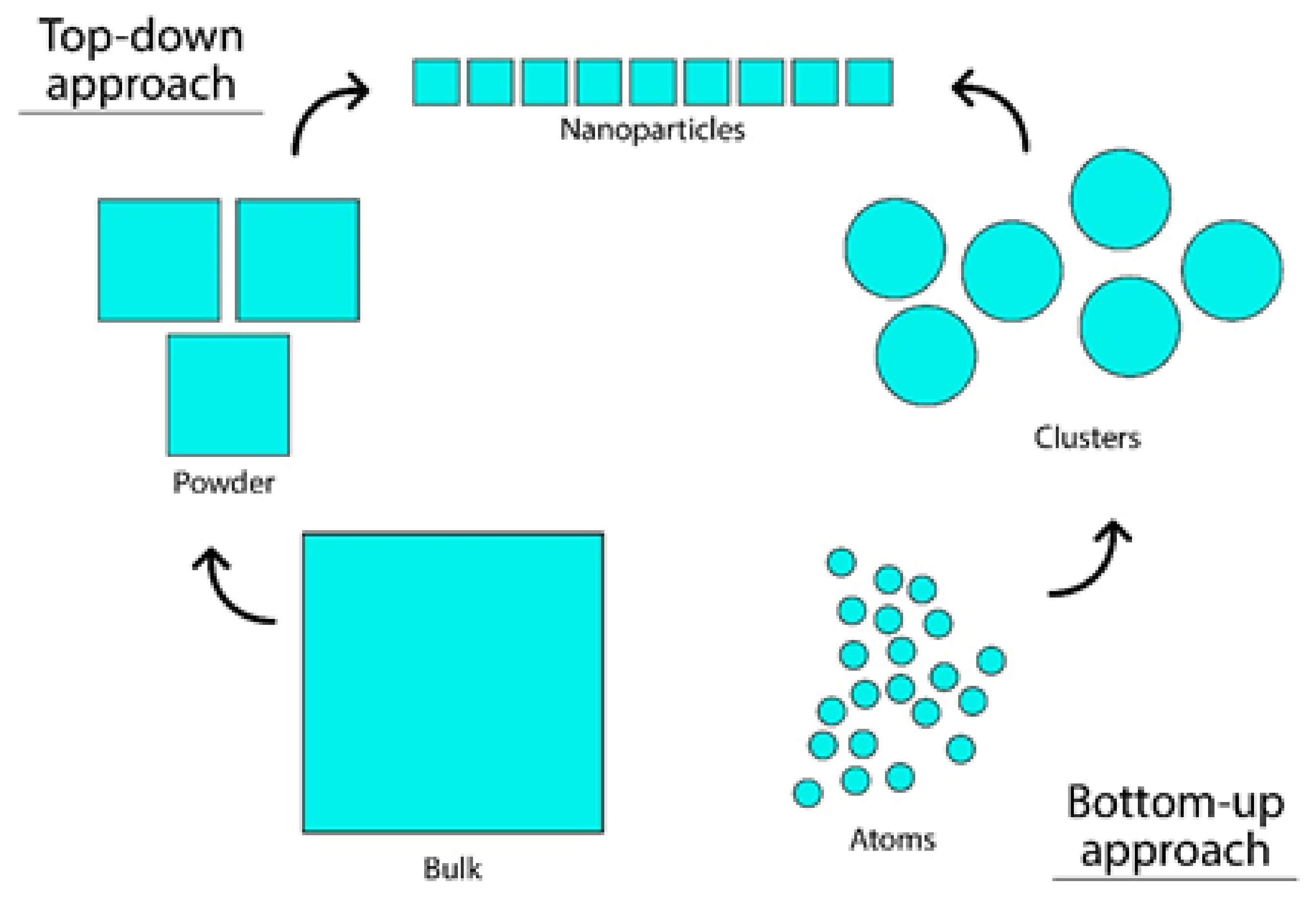
3. Production of AgNPs
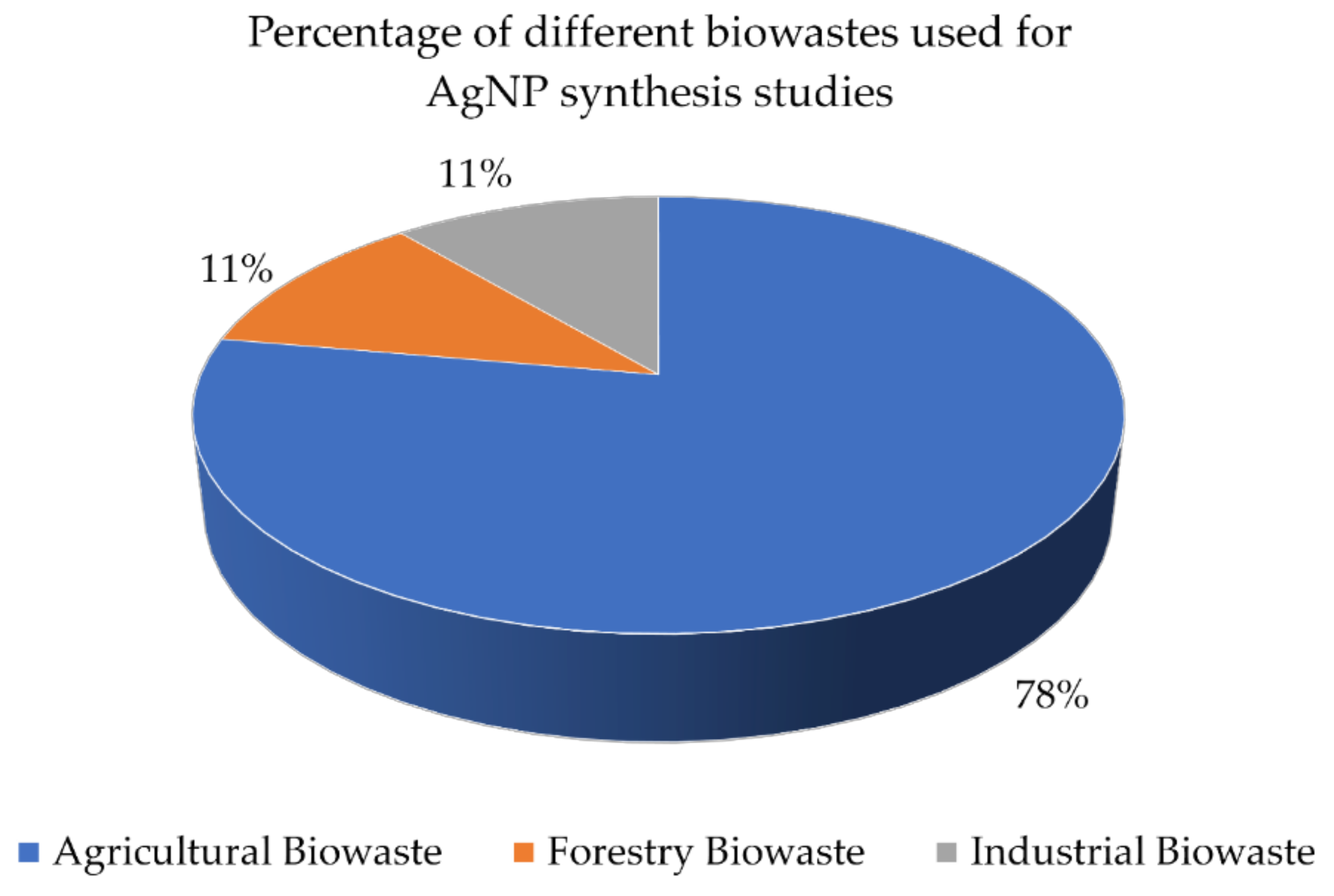
Biological Approach of Nanoparticle Synthesis
4. Antimicrobial Properties of AgNPs
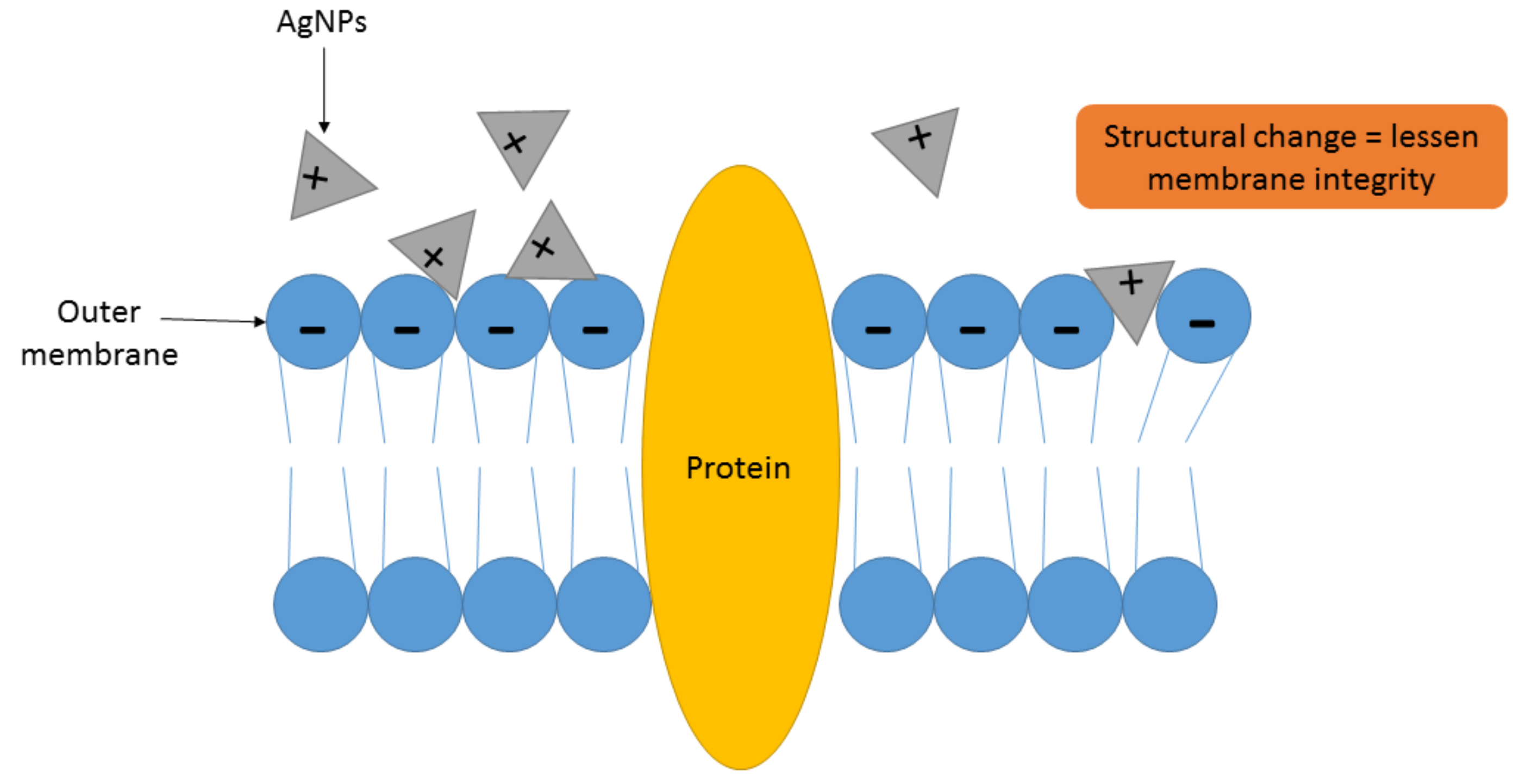
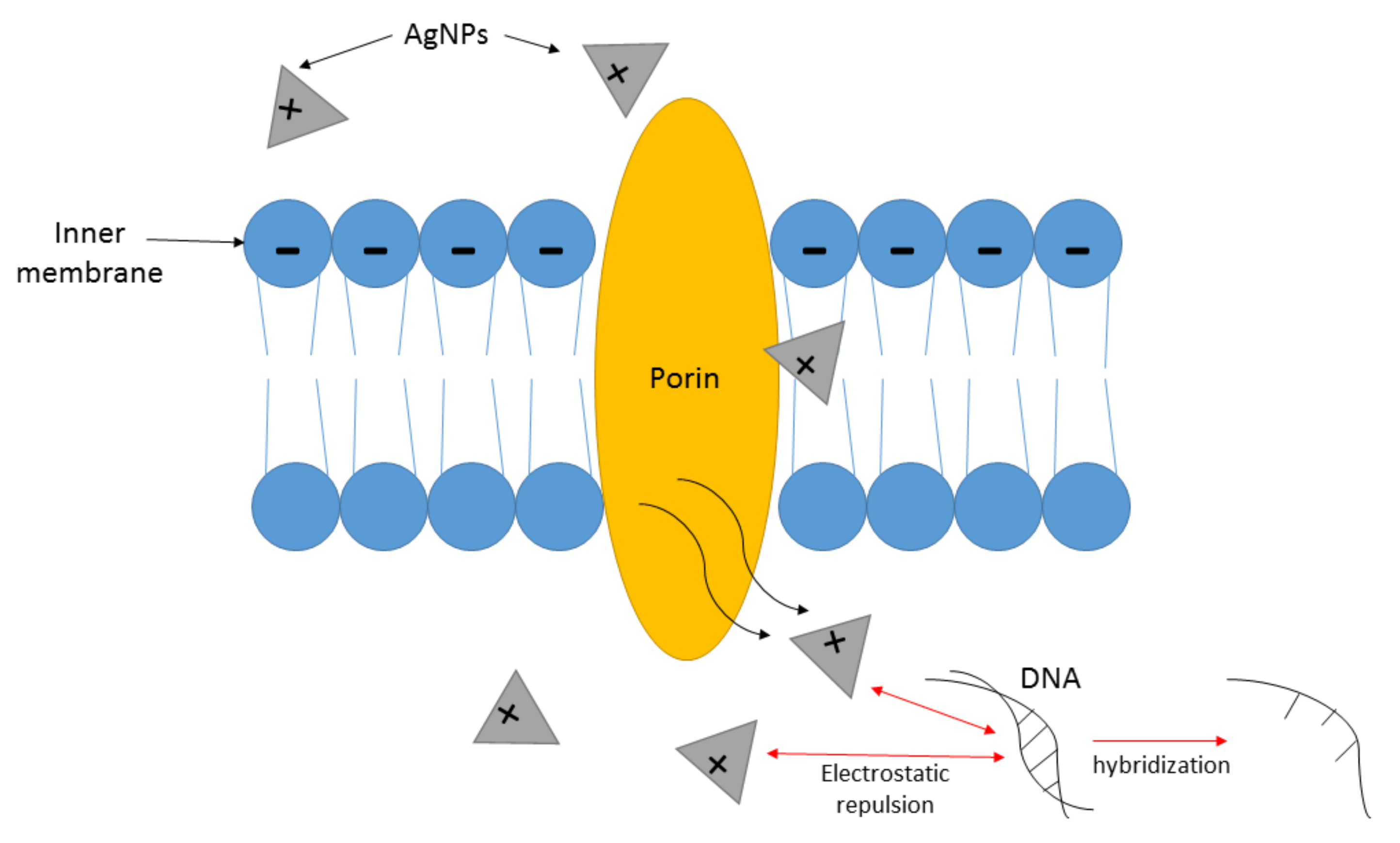
4.1. Mechanism of Action of AgNPs as Antimicrobial Agent
4.2. Antibacterial Effects of AgNPs
- Adherence onto the cell membrane of microorganisms.
- Perforation of cells by AgNPs, interrupting cell molecules and causing intracellular destruction.
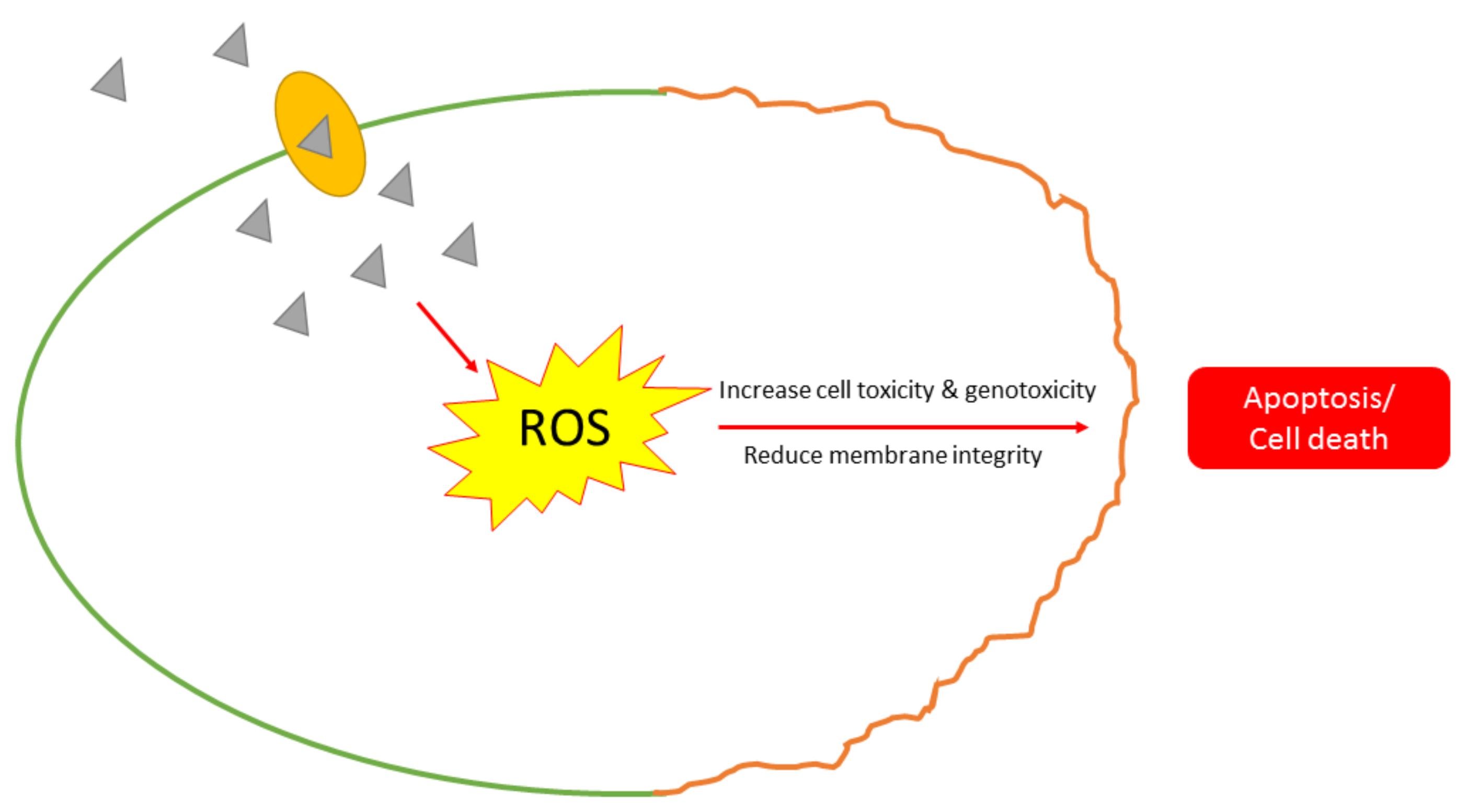
- Effecting toxicity of microbial cells through the synthesis of ROS that stimulates cell oxidative stress.
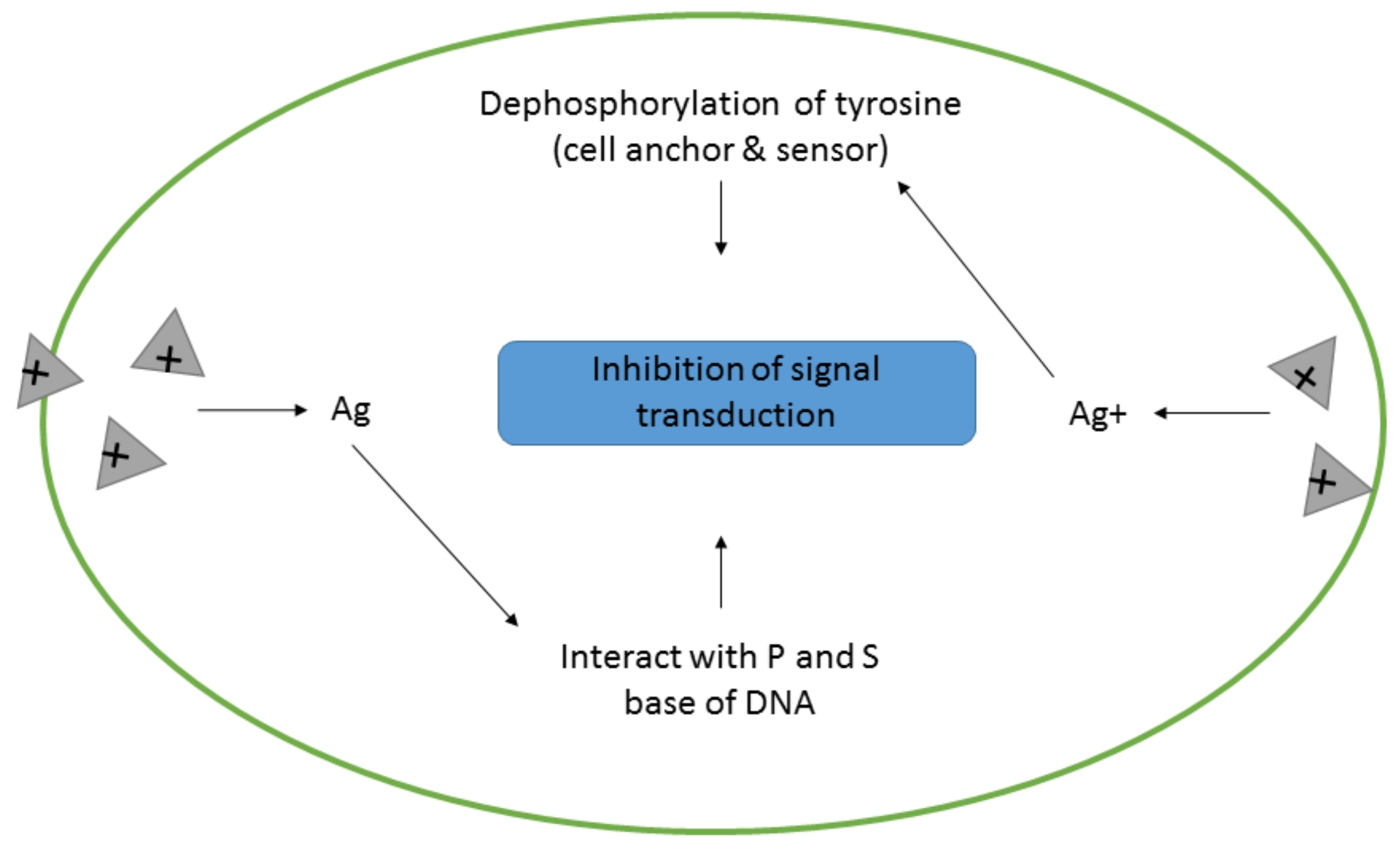
- Obstruction of cell signal transduction pathways.
5. Applications of AgNPs in Antimicrobial Coatings
6. Future Outlook and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eskin, N.A.M.; Robinson, D.S. Food Shelf Life Stability; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Keat, C.L.; Aziz, A.; Eid, A.M.; Elmarzugi, N.A. Biosynthesis of nanoparticles and silver nanoparticles. Bioresour. Bioprocess. 2015, 2, 47. [Google Scholar] [CrossRef]
- Vega-Baudrit, J.; Gamboa, S.M.; Rojas, E.R.; Martinez, V.V. Synthesis and characterization of silver nanoparticles and their application as an antibacterial agent. Int. J. Biosens. Bioelectron. 2019, 5, 166–173. [Google Scholar] [CrossRef]
- Firdhouse, M.J.; Lalitha, P. Biosynthesis of silver nanoparticles and its applications. J. Nanotechnol. 2015, 2015, 47. [Google Scholar] [CrossRef]
- Castillo-Henríquez, L.; Alfaro-Aguilar, K.; Ugalde-Álvarez, J.; Vega-Fernández, L.; Montes de Oca-Vásquez, G.; Vega-Baudrit, J.R. Green synthesis of gold and silver nanoparticles from plant extracts and their possible applications as antimicrobial agents in the agricultural area. Nanomaterials 2020, 10, 1763. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Liu, Z.G.; Shen, W.; Gurunathan, S. Silver nanoparticles: Synthesis, characterization, properties, applications, and therapeutic approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Nasiriboroumand, M.; Montazer, M.; Barani, H. Preparation and characterization of biocompatible silver nanoparticles using pomegranate peel extract. J. Photochem. Photobiol. B Biol. 2018, 179, 98–104. [Google Scholar] [CrossRef]
- Barros, C.H.N.; Cruz, G.C.F.; Mayrink, W.; Tasic, L. Bio-based synthesis of silver nanoparticles from orange waste: Effects of distinct biomolecule coatings on size, morphology, and antimicrobial activity. Nanotechnol. Sci. Appl. 2018, 11, 1–14. [Google Scholar] [CrossRef]
- Kee, S.H.; Chiongson, J.B.V.; Saludes, J.P.; Vigneswari, S.; Ramakrishna, S.; Bhubalan, K. Bioconversion of agro-industry sourced biowaste into biomaterials via microbial factories—A viable domain of circular economy. Environ. Pollut. 2021, 271, 116311. [Google Scholar] [CrossRef]
- Jeremiah, S.S.; Miyakawa, K.; Morita, T.; Yamaoka, Y.; Ryo, A. Potent antiviral effect of silver nanoparticles on SARS-CoV-2. Biochem. Biophys. Res. Commun. 2020, 533, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Volkova, N.A.; Yukhta, M.S.; Pavlovich, E.V.; Goltsev, A.N. Change in functional state of bone marrow-derived mesenchymal stem cells after incubation with silver nanoparticles. In Nanophotonics, Nanooptics, Nanobiotechnology, and Their Applications; Fesenko, O., Yatsenko, L., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 273–282. [Google Scholar]
- Nadtoka, O.; Kutsevol, N.; Linnik, O.; Nikiforov, M. Nanocomposite hydrogels containing silver nanoparticles as materials for wound dressings. In Nanophotonics, Nanooptics, Nanobiotechnology, and Their Applications; Fesenko, O., Yatsenko, L., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 375–387. [Google Scholar]
- Pylypchuk, I.V.; Mukha, I.P.; Vityuk, N.V.; Szczepanowicz, K.; Storozhuk, L.P.; Eremenko, A.M.; Warszyński, P.; Gorbyk, P.P. Tryptophan-stabilized plasmonic Fe3O4/Ag nanoparticles. In Nanophotonics, Nanooptics, Nanobiotechnology, and Their Applications; Fesenko, O., Yatsenko, L., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 417–430. [Google Scholar]
- Sundar, A.; Arunachalam, S.; Jayavel, S.; Muthulakshmi, L. Encapsulation of amphotericin B into quercetin based silver nanoparticles: Preparation, characterization and preliminary investigation of antiparasitic activity. In Proceedings of the International Conference on Nanomedicine (ICON–2019); Rajan, M., Anand, K., Chuturgoon, A., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 63–71. [Google Scholar]
- Ganesan, S.; Mehalingam, P.; Selvam, G.S. Green synthesis of silver nanoparticles from de-oiled rhizomes of Curcuma longa L. and its biomedical potential. In Proceedings of the International Conference on Nanomedicine (ICON–2019); Rajan, M., Anand, K., Chuturgoon, A., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 94–106. [Google Scholar]
- Soneya, S.; Reddy, N.V.; Saritha, K.V.; Kotakadi, V.S.; Vijaya, T. Phytosynthesis of silver nanoparticles using Rhynchosia heynei wight & arn leaf extract: Characterization and in vitro assessment of antimicrobial, antioxidant and anticancer activities. In Proceedings of the International Conference on Nanomedicine (ICON–2019); Rajan, M., Anand, K., Chuturgoon, A., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 120–140. [Google Scholar]
- Aswini, R.; Meimozhi, S.; Tamilmozhi, R.; Kowsalya, M.; Murugesan, S. Green synthesis of silver nanoparticles using Ledebouria revoluta bulb extract and its biological activity. In Proceedings of the International Conference on Nanomedicine (ICON–2019); Rajan, M., Anand, K., Chuturgoon, A., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 1–10. [Google Scholar]
- Banik, M.; Patra, M.; Dutta, D.; Mukherjee, R.; Basu, T. A simple robust method of synthesis of copper-silver core shell nano-particle: Evaluation of its structural and chemical properties with anticancer potency. Nanotechnology 2018, 29, 325102. [Google Scholar] [CrossRef] [PubMed]
- Edis, Z.; Bloukh, S.H.; Ashames, A.; Ibrahim, M. Copper-based nanoparticles, their chemistry and antibacterial properties: A review. In Chemistry for a Clean and Healthy Planet; Ramasami, P., Bhowon, M.G., Laulloo, S.J., Wah, H.L.K., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 401–428. [Google Scholar]
- Khare, P.; Sharma, A.; Verma, N. Synthesis of phenolic precursor-based porous carbon beads in situ dispersed with copper-silver bimetal nanoparticles for antibacterial applications. J. Colloid Interface Sci. 2014, 418, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Takamiya, A.S.; Monteiro, D.R.; Gorup, L.F.; Silva, E.A.; Camargo, E.R.; Gomes-Filho, J.E.; Oliveira, S.H.P.; Barbosa, D.B. Biocompatible silver nanoparticles incorporated in acrylic resin for dental application inhibit Candida albicans biofilm. Mater. Sci. Eng. C 2021, 118, 111341. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Luo, L.-J.; Lai, J.-Y. Toward understanding the purely geometric effects of silver nanoparticles on potential application as ocular therapeutics via treatment of bacterial keratitis. Mater. Sci. Eng. C 2021, 119, 111497. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.; Dipallini, S.; Lata, S.; Mohanty, S.; Pradhan, P.K.; Patel, P.; Makkar, H.; Verma, S.K. Oxidative stress induced antimicrobial efficacy of chitosan and silver nanoparticles coated Gutta-percha for endodontic applications. Mater. Today Chem. 2020, 17, 100299. [Google Scholar] [CrossRef]
- Sofi, H.S.; Akram, T.; Shabir, N.; Vasita, R.; Jadhav, A.H.; Sheikh, F.A. Regenerated cellulose nanofibers from cellulose acetate: Incorporating hydroxyapatite (HAp) and silver (Ag) nanoparticles (NPs), as a scaffold for tissue engineering applications. Mater. Sci. Eng. C 2021, 118, 111547. [Google Scholar] [CrossRef]
- Barot, T.; Rawtani, D.; Kulkarni, P. Physicochemical and biological assessment of silver nanoparticles immobilized Halloysite nanotubes-based resin composite for dental applications. Heliyon 2020, 6, e03601. [Google Scholar] [CrossRef]
- Rodrigues, M.C.; Rolim, W.R.; Viana, M.M.; Rodrigues Souza, T.; Gonçalves, F.; Tanaka, C.J.; Bueno-Silva, B.; Seabra, A.B. Biogenic synthesis and antimicrobial activity of silica-coated silver nanoparticles for esthetic dental applications. J. Dent. 2020, 96, 103327. [Google Scholar] [CrossRef]
- Hosseini, H.; Zirakjou, A.; Goodarzi, V.; Mousavi, S.M.; Khonakdar, H.A.; Zamanlui, S. Lightweight aerogels based on bacterial cellulose/silver nanoparticles/polyaniline with tuning morphology of polyaniline and application in soft tissue engineering. Int. J. Biol. Macromol. 2020, 152, 57–67. [Google Scholar] [CrossRef]
- LewisOscar, F.; Nithya, C.; Vismaya, S.; Arunkumar, M.; Pugazhendhi, A.; Nguyen-Tri, P.; Alharbi, S.A.; Alharbi, N.S.; Thajuddin, N. In vitro analysis of green fabricated silver nanoparticles (AgNPs) against Pseudomonas aeruginosa PA14 biofilm formation, their application on urinary catheter. Prog. Org. Coat 2021, 151, 106058. [Google Scholar] [CrossRef]
- Maghimaa, M.; Alharbi, S.A. Green synthesis of silver nanoparticles from Curcuma longa L. and coating on the cotton fabrics for antimicrobial applications and wound healing activity. J. Photochem. Photobiol. B 2020, 204, 111806. [Google Scholar] [CrossRef]
- Salve, M.; Mandal, A.; Amreen, K.; Pattnaik, P.K.; Goel, S. Greenly synthesized silver nanoparticles for supercapacitor and electrochemical sensing applications in a 3D printed microfluidic platform. Microchem. J. 2020, 157, 104973. [Google Scholar] [CrossRef]
- Qayyum, H.; Ahmed, W.; Hussain, S.; Khan, G.A.; Rehman, Z.U.; Ullah, S.; Rahman, T.U.; Dogar, A.H. Laser synthesis of surfactant-free silver nanoparticles for toxic dyes degradation and SERS applications. Opt. Laser Technol. 2020, 129, 106313. [Google Scholar] [CrossRef]
- Rajesh, K.; Sakthivel, P.; Santhanam, A.; Venugobal, J. Incorporation of silver ion on structural and optical characteristics of CeO2 nanoparticles: White LED applications. Optik 2020, 216, 164800. [Google Scholar] [CrossRef]
- Munir, T.; Mahmood, A.; Imran, M.; Sohail, A.; Fakhar-e-Alam, M.; Sharif, M.; Masood, T.; Bajwa, S.Z.; Shafiq, F.; Latif, S. Quantitative analysis of glucose by using (PVP and MA) capped silver nanoparticles for biosensing applications. Physica B Condens. Matter 2021, 602, 412564. [Google Scholar] [CrossRef]
- He, Y.; Li, H.; Fei, X.; Peng, L. Carboxymethyl cellulose/cellulose nanocrystals immobilized silver nanoparticles as an effective coating to improve barrier and antibacterial properties of paper for food packaging applications. Carbohydr. Polym. 2021, 252, 117156. [Google Scholar] [CrossRef] [PubMed]
- Affes, S.; Maalej, H.; Aranaz, I.; Kchaou, H.; Acosta, N.; Heras, A.; Nasri, M. Controlled size green synthesis of bioactive silver nanoparticles assisted by chitosan and its derivatives and their application in biofilm preparation. Carbohydr. Polym. 2020, 236, 116063. [Google Scholar] [CrossRef] [PubMed]
- Gol, F.; Aygun, A.; Seyrankaya, A.; Gur, T.; Yenikaya, C.; Sen, F. Green synthesis and characterization of Camellia sinensis mediated silver nanoparticles for antibacterial ceramic applications. Mater. Chem. Phys. 2020, 250, 123037. [Google Scholar] [CrossRef]
- Rajkumar, R.; Ezhumalai, G.; Gnanadesigan, M. A green approach for the synthesis of silver nanoparticles by Chlorella vulgaris and its application in photocatalytic dye degradation activity. Environ. Technol. Innov. 2021, 21, 101282. [Google Scholar] [CrossRef]
- Tawil, N.; Katsarava, R.; Tugushi, D.; Beridze, V. Composition Comprising Amino acid Polymers and a Bioactive Agent and Method of Preparing Thereof. U.S. Patent No. US20200368176A1, 26 November 2020. [Google Scholar]
- Jongpaiboonkit, L.; William, L.; Murphy, S.V.S. Coating Scaffolds. Australian Patent No. AU2020250274A1, 11 May 2020. [Google Scholar]
- Jiang, G.; Zou, J.; Qin, P. Microcellular foamed HIPS Electromagnetic Shielding Material and Preparation Method and Application thereof. Chinese Patent No. CN11196105A, 20 November 2020. [Google Scholar]
- Li, Y.; Sun, M.; Zhong, Y.; Huang, Y.; Zhang, H.; Tang, H. Preparation Method of Nano-Silver Slow-Release Antibacterial Agent. Chinese Patent No. CN111973794A, 24 November 2020. [Google Scholar]
- Munch, E. Mobile Device for Cleaning and Disinfecting Room Air That Can Be Operated Using a Temperature Difference. German Patent No. DE202020105700U1, 16 October 2020. [Google Scholar]
- Sedic, F. Skin Cleanser (09-24-2020). Australian Patent No. AU2020227091A1, 24 September 2020. [Google Scholar]
- Song, C. Synthetic Fiber with Semi-Permanent Antibacterial and Anti-Fungal Properties and Uses Thereof. Korean Patent No. KR102163245B1, 8 October 2020. [Google Scholar]
- Base, A. A System that Provides Local Cooling to the Brain and Spinal Cord. Japanese Patent No. JP2020171791A, 22 October 2020. [Google Scholar]
- Kamal, T.; Abdullah, M.; Asiri, S.B.K. Method of Reducing an Organic Pollutant in Contaminated Water. U.S. Patent No. US10793684B1, 6 October 2020. [Google Scholar]
- Wang, Z. Medicine for Treating ant Bite and Its Medicine Applying Plaster. Chinese Patent No. CN111671844A, 18 September 2020. [Google Scholar]
- Zhang, S.; Duan, X. Trypsin Detection film, Preparation Method and Application Thereof and Trypsin Detection Kit. Chinese Patent No. CN111,808,916A, 23 October 2020. [Google Scholar]
- Nikolopoulou, S.G.; Boukos, N.; Sakellis, E.; Efthimiadou, E.K. Synthesis of biocompatible silver nanoparticles by a modified polyol method for theranostic applications: Studies on red blood cells, internalization ability and antibacterial activity. J. Inorg. Biochem. 2020, 211, 111177. [Google Scholar] [CrossRef]
- Sadasivuni, K.K.; Rattan, S.; Waseem, S.; Bramhe, S.K.; Kondawar, S.B.; Ghosh, S.; Das, A.P.; Chakraborty, P.K.; Adhikari, J.; Saha, P.; et al. Silver nanoparticles and its polymer nanocomposites—Synthesis, optimization, biomedical usage, and its various applications. In Polymer Nanocomposites in Biomedical Engineering; Sadasivuni, K.K., Ponnamma, D., Rajan, M., Ahmed, B., Al-Maadeed, M.A.S.A., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 331–373. [Google Scholar]
- Mishra, V.K.; Husen, A.; Rahman, Q.I.; Iqbal, M.; Sohrab, S.S.; Yassin, M.O. Plant-based fabrication of silver nanoparticles and their application. In Nanomaterials and Plant Potential; Husen, A., Iqbal, M., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 135–175. [Google Scholar]
- Adekoya, J.A.; Ogunniran, K.O.; Siyanbola, T.O.; Dare, E.O.; Revaprasadu, N. Band structure, morphology, functionality, and size-dependent properties of metal nanoparticles. In Noble and Precious Metals-Properties, Nanoscale Effects and Applications; IntechOpen: London, UK, 2018; pp. 15–42. [Google Scholar]
- Govindarajan, M.; Rajeswary, M.; Veerakumar, K.; Muthukumaran, U.; Hoti, S.L.; Benelli, G. Green synthesis and characterization of silver nanoparticles fabricated using Anisomeles indica: Mosquitocidal potential against malaria, dengue and Japanese encephalitis vectors. Exp. Parasitol. 2016, 161, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Manukyan, A.; Gyulasaryan, H.; Ginoyan, A.; Kaniukov, E.; Petrov, A.; Yakimchuk, D.; Shashov, S.; Nurijanyan, M.; Mirzakhanyan, A. Structural, morphological and magnetic properties of nickel-carbon nanocomposites prepared by solid-phase pyrolysis of Ni phthalocyanine. Fundam. Appl. Nano Electromagn. 2016, 1, 273–290. [Google Scholar]
- Yuan, Z.; Zhao, Y.; Yang, W.; Hu, Y.; Cai, K.; Liu, P.; Ding, H. Fabrication of antibacterial surface via UV-inducing dopamine polymerization combined with co-deposition Ag nanoparticles. Mater. Lett. 2016, 183, 85–89. [Google Scholar] [CrossRef]
- You, C.; Han, C.; Wang, X.; Zheng, Y.; Li, Q.; Hu, X.; Sun, H. The progress of silver nanoparticles in the antibacterial mechanism, clinical application and cytotoxicity. Mol. Biol. Rep. 2012, 39, 9193–9201. [Google Scholar] [CrossRef]
- Relinque, J.J.; de León, A.S.; Hernández-Saz, J.; García-Romero, M.G.; Navas-Martos, F.J.; Morales-Cid, G.; Molina, S.I. Development of surface-coated polylactic acid/polyhydroxyalkanoate (PLA/PHA) nanocomposites. Polymers 2019, 11, 400. [Google Scholar] [CrossRef]
- Mukheem, A.; Muthoosamy, K.; Manickam, S.; Sudesh, K.; Shahabuddin, S.; Saidur, S.; Akbar, N.; Sridewi, N. Fabrication and characterization of an electrospun PHA/graphene silver nanocomposite scaffold for antibacterial applications. Materials 2018, 11, 1673. [Google Scholar] [CrossRef]
- Temgire, M.K.; Joshi, S.S. Optical and structural studies of silver nanoparticles. Radiat. Phys. Chem. 2003, 71, 1039–1044. [Google Scholar] [CrossRef]
- Castro-Mayorga, J.L.; Martínez-Abad, A.; Fabra, M.F.; Lagarón, J.M.; Ocio, M.J.; Sánchez, G. Silver-based antibacterial and virucide biopolymers: Usage and potential in antimicrobial packaging. In Antimicrobial Food Packaging; Elsevier: Amsterdam, The Netherlands, 2016; pp. 407–416. [Google Scholar]
- Li, Y.; Fu, Z.-Y.; Su, B.-L. Hierarchically structured porous materials for energy conversion and storage. Adv. Funct. Mater. 2012, 22, 4634–4667. [Google Scholar] [CrossRef]
- Qiu, R.; Cha, H.G.; Noh, H.B.; Shim, Y.B.; Zhang, X.L.; Qiao, R.; Zhang, D.; Kim, Y.I.; Pal, U.; Kang, Y.S. Preparation of dendritic copper nanostructures and their characterization for electroreduction. J. Phys. Chem. C 2009, 113, 15891–15896. [Google Scholar] [CrossRef]
- Jain, P.; Pradeep, T. Potential of silver nanoparticle-coated polyurethane foam as an antibacterial water filter. Biotechnol. Bioeng. 2005, 90, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Farkhari, N.; Abbasian, S.; Moshaii, A.; Nikkhah, M. Mechanism of adsorption of single and double stranded DNA on gold and silver nanoparticles: Investigating some important parameters in bio-sensing applications. Colloids Surf. B Biointerfaces 2016, 148, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, D.; Zhang, Q.-Y.; Tang, X. Surface enhanced Raman scattering substrate with high-density hotspots fabricated by depositing Ag film on TiO2-catalyzed Ag nanoparticles. J. Alloys Compd. 2016, 689, 439–445. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385. [Google Scholar]
- Zhang, Y.; Huang, R.; Zhu, X.; Wang, L.; Wu, C. Synthesis, properties, and optical applications of noble metal nanoparticle-biomolecule conjugates. Chin. Sci. Bull. 2012, 57, 238–246. [Google Scholar] [CrossRef]
- Lee, K.S.; El-Sayed, M.A. Gold and silver nanoparticles in sensing and imaging: Sensitivity of plasmon response to size, shape, and metal composition. J. Phys. Chem. B 2006, 110, 19220–19225. [Google Scholar] [CrossRef]
- Penn, S.G.; He, L.; Natan, M.J. Nanoparticles for bioanalysis. Curr. Opin. Chem. Biol. 2003, 7, 609–615. [Google Scholar] [CrossRef]
- Plowman, B.J.; Jones, L.A.; Bhargava, S.K. Building with bubbles: The formation of high surface area honeycomb-like films via hydrogen bubble templated electrodeposition. Chem. Commun. 2015, 51, 4331–4346. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Kaempgen, M.; Nopphawan, P.; Wee, G.; Mhaisalkar, S.; Srinivasan, M. Silver nanoparticle-decorated carbon nanotubes as bifunctional gas-diffusion electrodes for zinc-air batteries. J. Power Sourc. 2010, 195, 4350–4355. [Google Scholar] [CrossRef]
- Edison, T.N.J.I.; Atchudan, R.; Kamal, C.; Lee, Y.R. Caulerpa racemosa: A marine green alga for eco-friendly synthesis of silver nanoparticles and its catalytic degradation of methylene blue. Bioprocess Biosyst. Eng. 2016, 39, 1401–1408. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Smita, K.; Cumbal, L.; Debut, A. Ficus carica (fig) fruit mediated green synthesis of silver nanoparticles and its antioxidant activity: A comparison of thermal and ultrasonication approach. BioNanoScience 2016, 6, 15–21. [Google Scholar] [CrossRef]
- Vinković, T.; Novák, O.; Strnad, M.; Goessler, W.; Jurašin, D.D.; Parađiković, N.; Vrček, I.V. Cytokinin response in pepper plants (Capsicum annuum L.) exposed to silver nanoparticles. Environ. Res. 2017, 156, 10–18. [Google Scholar]
- Siddiqi, K.S.; Husen, A. Plant response to engineered metal oxide nanoparticles. Nano Res. Lett 2017, 12, 92. [Google Scholar] [CrossRef] [PubMed]
- Cvjetko, P.; Milošić, A.; Domijan, A.M.; Vrček, I.V.; Tolić, S.; Štefanić, P.P.; Letofsky-Papst, I.; Tkalec, M.; Balen, B. Toxicity of silver ions and differently coated silver nanoparticles in Allium cepa roots. Ecotoxicol. Environ. Saf. 2017, 137, 18–28. [Google Scholar] [CrossRef]
- Rui, M.; Ma, C.; Tang, X.; Yang, J.; Jiang, F.; Pan, Y.; Xiang, Z.; Hao, Y.; Rui, Y.; Cao, W.; et al. Phytotoxicity of silver nanoparticles to peanut (Arachis hypogaea L.): Physiological responses and food safety. ACS Sustain. Chem. Eng. 2017, 5, 6557–6567. [Google Scholar] [CrossRef]
- Huang, T.; Sui, M.; Yan, X.; Zhang, X.; Yuan, Z. Anti-algae efficacy of silver nanoparticles to Microcystis aeruginosa: Influence of NOM, divalent cations, and pH. Colloids Surf. A Physicochem. Eng. Asp. 2016, 509, 492–503. [Google Scholar] [CrossRef]
- Qian, H.; Zhu, K.; Lu, H.; Lavoie, M.; Chen, S.; Zhou, Z.; Deng, Z.; Chen, J.; Fu, Z. Contrasting silver nanoparticle toxicity and detoxification strategies in Microcystis aeruginosa and Chlorella vulgaris: New insights from proteomic and physiological analyses. Sci. Total Environ. 2016, 572, 1213–1221. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Ahmed, S. Recent advances in edible polymer based hydrogels as a sustainable alternative to conventional polymers. J. Agric. Food Chem. 2018, 66, 6940–6967. [Google Scholar] [CrossRef] [PubMed]
- Amini, M.; Yousefi-Massumabad, H.; Younesi, H.; Abyar, H.; Bahramifar, N. Production of the polyhydroxyalkanoate biopolymer by Cupriavidus necator using beer brewery wastewater containing maltose as a primary carbon source. J. Environ. Chem. Eng. 2020, 8, 103588. [Google Scholar] [CrossRef]
- Annu, A.S.; Kaur, G.; Sharma, P.; Singh, S.; Ikram, S. Fruit waste (peel) as bio-reductant to synthesize silver nanoparticles with antimicrobial, antioxidant and cytotoxic activities. J. Appl. Biomed. 2018, 16, 221–231. [Google Scholar] [CrossRef]
- Ahvenainen, R. New approaches in improving the shelf life of minimally processed fruit and vegetables. Trends Food Sci. Technol. 1996, 7, 179–187. [Google Scholar] [CrossRef]
- Alhendi, A.S.; Choudhary, R. Current practices in bread packaging and possibility of improving bread shelf life by nanotechnology. Mater. Sci. 2013, 3, 55–60. [Google Scholar]
- Alsalhi, M.S.; Devanesan, S.; Alfuraydi, A.A.; Vishnubalaji, R.; Munusamy, M.A.; Murugan, K.; Nicoletti, M.; Benelli, G. Green synthesis of silver nanoparticles using Pimpinella anisum seeds: Antimicrobial activity and cytotoxicity on human neonatal skin stromal cells and colon cancer cells. Int. J. Nanomed. 2016, 11, 4439–4449. [Google Scholar] [CrossRef]
- Amado, D.A.V.; Helmann, G.A.B.; Detoni, A.M.; de Carvalho, S.L.C.; de Aguiar, C.M.; Martin, C.A.; Tiuman, T.S.; Cottica, S.M. Antioxidant and antibacterial activity and preliminary toxicity analysis of four varieties of avocado (Persea americana Mill.). Braz. J. Food Technol. 2019, 22. [Google Scholar] [CrossRef]
- Devanesan, S.; AlSalhi, M.S.; Balaji, R.V.; Ranjitsingh, A.J.A.; Ahamed, A.; Alfuraydi, A.A.; AlQahtani, F.Y.; Aleanizy, F.S.; Othman, A.H. Antimicrobial and cytotoxicity effects of synthesized silver nanoparticles from Punica granatum peel extract. Nanoscale Res. Lett. 2018, 13, 315. [Google Scholar] [CrossRef]
- Vu, B.; Chen, M.; Crawford, R.J.; Ivanova, E.P. Bacterial extracellular polysaccharides involved in biofilm formation. Molecules 2009, 14, 2535–2554. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Yong, K.T.; Roy, I.; Dinh, X.Q.; Yu, X.; Luan, F. A review on functionalized gold nanoparticles for biosensing applications. Plasmonics 2011, 6, 491–506. [Google Scholar] [CrossRef]
- Behzad, F.; Naghib, S.M.; Kouhbanani, M.A.J.; Tabatabaei, S.N.; Zare, Y.; Rhee, K.Y. An overview of the plant-mediated green synthesis of noble metal nanoparticles for antibacterial applications. J. Ind. Eng. Chem. 2021, 94, 92–104. [Google Scholar] [CrossRef]
- Garg, D.; Sarkar, A.; Chand, P.; Bansal, P.; Gola, D.; Sharma, S.; Khantwal, S.; Surabhi; Mehrotra, R.; Chauhan, N.; et al. Synthesis of silver nanoparticles utilizing various biological systems: Mechanisms and applications—A review. Prog. Biomater. 2020, 9, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Hamouda, R.A.; Hussein, M.H.; Abo-Elmagd, R.A.; Bawazir, S.S. Synthesis and biological characterization of silver nanoparticles derived from the cyanobacterium Oscillatoria limnetica. Sci. Rep. 2019, 9, 13071. [Google Scholar] [CrossRef]
- Mangindaan, D.; Lin, G.Y.; Kuo, C.J.; Chien, H.W. Biosynthesis of silver nanoparticles as catalyst by spent coffee ground/recycled poly(ethylene terephthalate) composites. Food Bioprod. Process. 2020, 121, 193–201. [Google Scholar] [CrossRef]
- Shivakumar, M.; Nagashree, K.L.; Yallappa, S.; Manjappa, S.; Manjunath, K.S.; Dharmaprakash, M.S. Biosynthesis of silver nanoparticles using pre-hydrolysis liquor of Eucalyptus wood and its effective antimicrobial activity. Enzyme Microb. Technol. 2017, 97, 55–62. [Google Scholar] [CrossRef]
- Aguilar, N.M.; Arteaga-Cardona, F.; Estévez, J.O.; Silva-González, N.R.; Benítez-Serrano, J.C.; Salazar-Kuri, U. Controlled biosynthesis of silver nanoparticles using sugar industry waste, and its antimicrobial activity. J. Environ. Chem. Eng. 2018, 6, 6275–6281. [Google Scholar] [CrossRef]
- Nesrin, K.; Yusuf, C.; Ahmet, K.; Ali, S.B.; Muhammad, N.A.; Suna, S.; Fatih, Ş. Biogenic silver nanoparticles synthesized from Rhododendron ponticum and their antibacterial, antibiofilm and cytotoxic activities. J. Pharm. Biomed. Anal. 2020, 179. [Google Scholar] [CrossRef] [PubMed]
- Gul, A.R.; Shaheen, F.; Rafique, R.; Bal, J.; Waseem, S.; Park, T.J. Grass-mediated biogenic synthesis of silver nanoparticles and their drug delivery evaluation: A biocompatible anti-cancer therapy. Chem. Eng. J. 2021, 407, 127202. [Google Scholar] [CrossRef]
- He, Y.; Du, Z.; Ma, S.; Cheng, S.; Jiang, S.; Liu, Y.; Li, D.; Huang, H.; Zhang, K.; Zheng, X. Biosynthesis, antibacterial activity and anticancer effects against prostate cancer (PC-3) cells of silver nanoparticles using dimocarpus longan lour. peel extract. Nanoscale Res. Lett. 2016, 11, 300. [Google Scholar] [CrossRef] [PubMed]
- Jalani, N.S.; Michell, W.; Lin, W.E.; Hanani, S.Z.; Hashim, U.; Abdullah, R. Biosynthesis of silver nanoparticles using Citrus grandis peel extract. Malays. J. Anal. Sci. 2018, 22, 676–683. [Google Scholar]
- Kowsalya, E.; MosaChristas, K.; Balashanmugam, P.; Rani, J.C. Biocompatible silver nanoparticles/poly (vinyl alcohol) electrospun nanofibers for potential antimicrobial food packaging applications. Food Packag. Shelf Life 2019, 21, 100379. [Google Scholar]
- Kokila, T.; Ramesh, P.S.; Geetha, D. Biosynthesis of silver nanoparticles from Cavendish banana peel extract and its antibacterial and free radical scavenging assay: A novel biological approach. Appl. Nanosci. 2015, 5, 911–920. [Google Scholar] [CrossRef]
- Ahmad, N.; Sharma, S.; Rai, R. Rapid green synthesis of silver and gold nanoparticles using peels of Punica granatum. Adv. Mater. Lett. 2012, 3, 376–380. [Google Scholar] [CrossRef]
- Das, G.; Patra, J.K.; Debnath, T.; Ansari, A.; Shin, H.S. Investigation of antioxidant, antibacterial, antidiabetic, and cytotoxicity potential of silver nanoparticles synthesized using the outer peel extract of Ananas comosus (L.). PLoS ONE 2019, 14. [Google Scholar] [CrossRef] [PubMed]
- Sarvamangala, D.; Kondala, K.; Murthy, U.S.N.; Rao, B.N.; Sharma, G.V.R.; Satyanarayana, R. Biogenic synthesis of AGNP’s using Pomelo fruit—characterization and antimicrobial activity against gram +Ve and gram −Ve bacteria. Int. J. Pharm. Sci. Rev. Res. 2013, 19, 30–35. [Google Scholar]
- Vinay, C.H.; Goudanavar, P.; Acharya, A. Development and characterization of pomegranate and orange fruit peel extract based silver nanoparticles. J. Manmohan Meml. Inst. Heal. Sci. 2018, 4, 72–85. [Google Scholar] [CrossRef]
- Soto, K.M.; Quezada-Cervantes, C.T.; Hernández-Iturriaga, M.; Luna-Bárcenas, G.; Vazquez-Duhalt, R.; Mendoza, S. Fruit peels waste for the green synthesis of silver nanoparticles with antimicrobial activity against foodborne pathogens. LWT 2019, 103, 293–300. [Google Scholar] [CrossRef]
- Kahrilas, G.A.; Wally, L.M.; Fredrick, S.J.; Hiskey, M.; Prieto, A.L.; Owens, J.E. Microwave-assisted green synthesis of silver nanoparticles using orange peel extract. ACS Sustain. Chem. Eng. 2014, 2, 367–376. [Google Scholar] [CrossRef]
- Radwan, R.A.; El-Sherif, Y.A.; Salama, M.M. A novel biochemical study of anti-ageing potential of Eucalyptus camaldulensis bark waste standardized extract and silver nanoparticles. Colloids Surf. B Biointerfaces 2020, 191, 111004. [Google Scholar] [CrossRef]
- Abdullah, H.S.T.S.H.; Asseri, S.N.A.R.M.; Mohamad, W.N.K.W.; Kan, S.Y.; Azmi, A.A.; Julius, F.S.Y.; Chia, P.W. Green synthesis, characterization and applications of silver nanoparticle mediated by the aqueous extract of red onion peel. Environ. Pollut. 2021, 271. [Google Scholar] [CrossRef]
- Doan, V.D.; Phung, M.T.; Nguyen, T.L.H.; Mai, T.C.; Nguyen, T.D. Noble metallic nanoparticles from waste Nypa fruticans fruit husk: Biosynthesis, characterization, antibacterial activity and recyclable catalysis. Arab. J. Chem. 2020, 13, 7490–7503. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Kumar, A.; Ansari, A.Z.; Kim, H.; Shin, H.S. Photo-mediated biosynthesis of silver nanoparticles using the non-edible accrescent fruiting calyx of Physalis peruviana L. Fruits and investigation of its radical scavenging potential and cytotoxicity activities. J. Photochem. Photobiol. B Biol. 2018, 188, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Shin, H.S.; Kumar, A.; Vishnuprasad, C.N.; Patra, J.K. Photo-mediated optimized synthesis of silver nanoparticles using the extracts of outer shell fibre of Cocos nucifera L. fruit and detection of its antioxidant, cytotoxicity and antibacterial potential. Saudi J. Biol. Sci. 2020, 28, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Baek, K.H. Phyto-mediated biosynthesis of silver nanoparticles using the rind extract of watermelon (Citrullus lanatus) under photo-catalyzed condition and investigation of its antibacterial, anticandidal and antioxidant efficacy. J. Photochem. Photobiol. B Biol. 2016, 161, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Pirathiba, S.; Dayananda, B.S. Potato peel waste as reductant for the biogenesis of gold and silver ultrafine particles. Mater. Today Proc. 2021. [Google Scholar] [CrossRef]
- Liu, Y.S.; Chang, Y.C.; Chen, H.H. Silver nanoparticle biosynthesis by using phenolic acids in rice husk extract as reducing agents and dispersants. J. Food Drug Anal. 2018, 26, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 32. [Google Scholar] [CrossRef]
- Salleh, A.; Naomi, R.; Utami, N.D.; Mohammad, A.W.; Mahmoudi, E.; Mustafa, N.; Fauzi, M.B. The potential of silver nanoparticles for antiviral and antibacterial applications: A mechanism of action. Nanomaterials 2020, 10, 1566. [Google Scholar] [CrossRef]
- Marambio-Jones, C.; Hoek, E.M. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanoparticle Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Barkat, M.A.; Beg, S.; Naim, M.; Pottoo, F.H.; Singh, S.P.; Ahmad, F.J. Current progress in synthesis, characterization and applications of silver nanoparticles: Precepts and prospects. Recent Pat. Anti Infect. Drug Disc. 2018, 13, 53–69. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, S.E.; Kim, J.E.; Lee, J.C.; Yoon, J.Y. The biocidal activity of nano-sized silver particles comparing with silver ion. J. Korean Soc. Environ. Eng. 2005, 27, 771–776. [Google Scholar]
- Chen, X.; Schluesener, H.J. Nanosilver: A nanoproduct in medical application. Toxicol. Lett. 2008, 176, 1–12. [Google Scholar] [CrossRef]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic basis of antimicrobial actions of silver nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef]
- Mokhena, T.C.; Luyt, A.S. Electrospun alginate nanofibres impregnated with silver nanoparticles: Preparation, morphology and antibacterial properties. Carbohydr. Polym. 2017, 165, 304–312. [Google Scholar] [CrossRef]
- Gudikandula, K.; Vadapally, P.; Singara Charya, M.A. Biogenic synthesis of silver nanoparticles from white rot fungi: Their characterization and antibacterial studies. OpenNano 2017, 2, 64–78. [Google Scholar] [CrossRef]
- Jena, P.; Bhattacharya, M.; Bhattacharjee, G.; Satpati, B.; Mukerjee, P.; Senapati, D.; Srinivasan, R. Bimetallic gold-silver nanoparticles mediate bacterial killing by disrupting the actin cytoskeleton MreB. Nanoscale 2020, 12, 3731–3749. [Google Scholar] [CrossRef]
- Veeraputhiran, V. Bio-catalytic synthesis of silver nanoparticles. Int. J. Chem. Tech. Res. 2013, 5, 255–2562. [Google Scholar]
- Guan, Q.; Xia, C.; Li, W. Bio-friendly controllable synthesis of silver nanoparticles and their enhanced antibacterial property. Catal. Today 2019, 327, 196–202. [Google Scholar] [CrossRef]
- Prasher, P.; Singh, M.; Mudila, H. Oligodynamic effect of silver nanoparticles: A review. Bionanoscience 2018, 8, 951–962. [Google Scholar] [CrossRef]
- Lee, J.H.; Lim, J.M.; Velmurugan, P.; Park, Y.J.; Park, Y.J.; Bang, K.S.; Oh, B.T. Photobiologic-mediated fabrication of silver nanoparticles with antibacterial activity. J. Photochem. Photobiol. B Biol. 2016, 162, 93–99. [Google Scholar] [CrossRef]
- Ghiuță, I.; Cristea, D.; Croitoru, C.; Kost, J.; Wenkert, R.; Vyrides, I.; Anayiotos, A.; Munteanu, D. Characterization and antimicrobial activity of silver nanoparticles, biosynthesized using Bacillus species. Appl. Surf. Sci. 2018, 438, 66–73. [Google Scholar] [CrossRef]
- Amooaghaie, R.; Saeri, M.R.; Azizi, M. Synthesis, characterization and biocompatibility of silver nanoparticles synthesized from Nigella sativa leaf extract in comparison with chemical silver nanoparticles. Ecotoxicol. Environ. Saf. 2015, 120, 400–408. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, K.; Guo, Z.; Fang, K.; Wang, X.; Yang, F.; Gu, N. PLLA microcapsules combined with silver nanoparticles and chlorhexidine acetate showing improved antibacterial effect. Mater. Sci. Eng. C 2017, 78, 349–353. [Google Scholar] [CrossRef]
- Faria, A.F.; Martinez, D.S.T.; Meira, S.M.M.; de Moraes, A.C.M.; Brandelli, A.; Filho, A.G.S.; Alves, O.L. Anti-adhesion and antibacterial activity of silver nanoparticles supported on graphene oxide sheets. Colloids Surf. B Biointerfaces 2014, 113, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Setyawati, M.I.; Leong, D.T.; Xie, J. Antimicrobial silver nanomaterials. Coord. Chem. Rev. 2018, 357, 1–17. [Google Scholar] [CrossRef]
- Koduru, J.R.; Kailasa, S.K.; Bhamore, J.R.; Kim, K.H.; Dutta, T.; Vellingiri, K. Phytochemical-assisted synthetic approaches for silver nanoparticles antimicrobial applications: A review. Adv. Colloid Interface Sci. 2018, 256, 326–339. [Google Scholar] [CrossRef]
- Choi, O.; Yu, C.P.; Esteban Fernández, G.; Hu, Z. Interactions of nanosilver with Escherichia coli cells in planktonic and biofilm cultures. Water Res. 2010, 44, 6095–6103. [Google Scholar] [CrossRef]
- Abdalla, S.S.I.; Katas, H.; Chan, J.Y.; Ganasan, P.; Azmi, F.; Busra, M.F.M. Antimicrobial activity of multifaceted lactoferrin or graphene oxide functionalized silver nanocomposites biosynthesized using mushroom waste and chitosan. RSC Adv. 2020, 10, 4969–4983. [Google Scholar] [CrossRef]
- Wakshlak, R.B.K.; Pedahzur, R.; Avnir, D. Antibacterial activity of silver-killed bacteria: The “zombies” effect. Sci. Rep. 2015, 5, 1–5. [Google Scholar] [CrossRef]
- Vazquez-Muñoz, R.; Meza-Villezcas, A.; Fournier, P.G.J.; Soria-Castro, E.; Juarez-Moreno, K.; Gallego-Hernández, A.L.; Bogdanchikova, N.; Vazquez-Duhalt, R.; Huerta-Saquero, A. Enhancement of antibiotics antimicrobial activity due to the silver nanoparticles impact on the cell membrane. PLoS ONE 2019, 14, 1–18. [Google Scholar] [CrossRef]
- Li, W.R.; Xie, X.B.; Shi, Q.S.; Zeng, H.Y.; Ou-Yang, Y.S.; Chen, Y.-B. Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl. Microbiol. Biotechnol. 2010, 85, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Katas, H.; Raja, M.A.G.; Lam, K.L. Development of chitosan nanoparticles as a stable drug delivery system for protein/siRNA. Int. J. Biomater. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Yi, T.; Huang, Z.; Liu, B.; Wang, J.; Yi, X.; Liu, J. Etching silver nanoparticles using DNA. Mater. Horizons 2019, 6, 155–159. [Google Scholar] [CrossRef]
- Sadoon, A.A.; Khadka, P.; Freeland, J.; Gundampati, R.K.; Manso, R.H.; Ruiz, M.; Krishnamurthi, V.R.; Thallapuranam, S.K.; Chen, J.; Wang, Y. Silver ions caused faster diffusive dynamics of histone-like nucleoid-structuring proteins in live bacteria. Appl. Environ. Microbiol. 2020, 86. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef]
- Su, H.L.; Chou, C.C.; Hung, D.J.; Lin, S.H.; Pao, I.C.; Lin, J.H.; Huang, F.L.; Dong, R.X.; Lin, J.J. The disruption of bacterial membrane integrity through ROS generation induced by nanohybrids of silver and clay. Biomaterials 2009, 30, 5979–5987. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, L.; Mi, Y.; Si, Y. Silver nanoparticles induced cell apoptosis, membrane damage of Azotobacter vinelandii and Nitrosomonas europaea via generation of reactive oxygen species. Bull. Environ. Contam. Toxicol. 2019, 103, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, S.; Chatterjee, S.; Saha, A.; Devi, P.S.; Suresh Kumar, G. Unraveling the interaction of silver nanoparticles with mammalian and bacterial DNA. J. Phys. Chem. B 2016, 120, 5313–5324. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Dash, S.K.; Mandal, D.; Ghosh, T.; Chattopadhyay, S.; Tripathy, S.; Das, S.; Dey, S.K.; Das, D.; Roy, S. Green synthesized silver nanoparticles destroy multidrug resistant bacteria via reactive oxygen species mediated membrane damage. Arab. J. Chem. 2017, 10, 862–876. [Google Scholar] [CrossRef]
- Ji, H.; Zhou, S.; Fu, Y.; Wang, Y.; Mi, J.; Lu, T.; Wang, X.; Lü, C. Size-controllable preparation and antibacterial mechanism of thermo-responsive copolymer-stabilized silver nanoparticles with high antimicrobial activity. Mater. Sci. Eng. C 2020, 110, 110735. [Google Scholar] [CrossRef]
- Adeyemi, O.S.; Shittu, E.O.; Akpor, O.B.; Rotimi, D.; Batiha, G.E.S. Silver nanoparticles restrict microbial growth by promoting oxidative stress and DNA damage. EXCLI J. 2020, 19, 492–500. [Google Scholar] [PubMed]
- Belluco, S.; Losasso, C.; Patuzzi, I.; Rigo, L.; Conficoni, D.; Gallocchio, F.; Cibin, V.; Catellani, P.; Segato, S.; Ricci, A. Silver as antibacterial toward Listeria monocytogenes. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef]
- Nalwade, A.R.; Jadhav, A.A. Biosynthesis of silver nanoparticles using leaf extract of Datura alba Nees and evaluation of their antibacterial activity. Arch. Appl. Sci. Res. 2013, 5, 45–49. [Google Scholar]
- Ouda Sahar, M. Some nanoparticles effects on Proteus sp. and Klebsiella sp. Isolated from water. Am. J. Infect. Dis. Microbiol. 2014, 2, 4–10. [Google Scholar]
- Radzig, M.A.; Nadtochenko, V.A.; Koksharova, O.A.; Kiwi, J.; Lipasova, V.A.; Khmel, I.A. Antibacterial effects of silver nanoparticles on gram-negative bacteria: Influence on the growth and biofilms formation, mechanisms of action. Colloids Surf. B Biointerfaces 2013, 102, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Kora, A.J.; Arunachalam, J. Assessment of antibacterial activity of silver nanoparticles on Pseudomonas aeruginosa and its mechanism of action. World J. Microbiol. Biotechnol. 2011, 27, 1209–1216. [Google Scholar] [CrossRef]
- Salem, W.; Leitner, D.R.; Zingl, F.G.; Schratter, G.; Prassl, R.; Goessler, W.; Reidl, J.; Schild, S. Antibacterial activity of silver and zinc nanoparticles against Vibrio cholerae and enterotoxic Escherichia coli. Int. J. Med. Microbiol. 2015, 305, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Rajawat, S.; Qureshi, M.S. Comparative study on bactericidal effect of silver nanoparticles, synthesized using green technology, in combination with antibiotics on Salmonella typhi. J. Biomater. Nanobiotechnol. 2012, 3, 480–485. [Google Scholar] [CrossRef]
- Faunce, T.; Watal, A. Nanosilver and global public health: International regulatory issues. Nanomedicine 2010, 5, 617–632. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; He, J.; Zhang, Z. Selective growth of metallic nanostructures on microstructured copper substrate in solution. CrystEngComm 2015, 17, 7262–7269. [Google Scholar] [CrossRef]
- Ghodake, G.; Kim, M.; Sung, J.S.; Shinde, S.; Yang, J.; Hwang, K.; Kim, D.Y. Extracellular synthesis and characterization of silver nanoparticles—Antibacterial activity against multidrug-resistant bacterial strains. Nanomaterials 2020, 10, 360. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.P.; Bandyopadhyay, A.; Harsha, S.N.; Policegoudra, R.S.; Bhattacharya, S.; Karak, N.; Chattopadhyay, A. Mentha arvensis (Linn.)-mediated green silver nanoparticles trigger caspase 9-dependent cell death in MCF7 and MDA-MB-231 cells. Breast Cancer Targets Ther. 2017, 9, 265–278. [Google Scholar] [CrossRef]
- Gaikwad, S.; Ingle, A.; Gade, A.; Rai, M.; Falanga, A.; Incoronato, N.; Russo, L.; Galdiero, S.; Galdiero, M. Antiviral activity of mycosynthesized silver nanoparticles against herpes simplex virus and human parainfluenza virus type 3. Int. J. Nanomed. 2013, 8, 4303–4314. [Google Scholar]
- Xiang, D.X.; Chen, Q.; Pang, L.; Zheng, C.L. Inhibitory effects of silver nanoparticles on H1N1 influenza A virus in vitro. J. Virol. Methods 2011, 1780, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Jong, W.H.; Van Der Ven, L.T.M.; Sleijffers, A.; Park, M.V.D.Z.; Jansen, E.H.J.M.; Van Loveren, H.; Vandebriel, R.J. Systemic and immunotoxicity of silver nanoparticles in an intravenous 28 days repeated dose toxicity study in rats. Biomaterials 2013, 34, 8333–8343. [Google Scholar] [CrossRef]
- Maretti, L.; Billone, P.S.; Liu, Y.; Scaiano, J.C. Facile photochemical synthesis and characterization of highly fluorescent silver nanoparticles. J. Am. Chem. Soc. 2009, 131, 13972–13980. [Google Scholar] [CrossRef] [PubMed]
- Navaladian, S.; Viswanathan, B.; Viswanath, R.P.; Varadarajan, T.K. Thermal decomposition as route for silver nanoparticles. Nanoscale Res. Lett. 2007, 2, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Song, L.; Liu, Y.; Fang, Y.-E. Synthesis of silver nanoparticles by γ-ray irradiation in acetic water solution containing chitosan. Radiat. Phys. Chem. 2007, 76, 1165–1168. [Google Scholar] [CrossRef]
- Jing, S.; Xing, S.; Yu, L.; Wu, Y.; Zhao, C. Synthesis and characterization of Ag/polyaniline core-shell nanocomposites based on silver nanoparticles colloid. Mater. Lett. 2007, 61, 2794–2797. [Google Scholar] [CrossRef]
- Krystek, P.; Cirtiu, C.M.; Braakhuis, H.; Park, M.; Jong, W.H. Inductively coupled plasma-mass spectrometry in biodistribution studies of (engineered) nanoparticles. In Encyclopedia of Analytical Chemistry; Wiley: Hoboken, NJ, USA, 2019; pp. 1–23. [Google Scholar]
- Wijnhoven, S.W.P.; Peijnenburg, W.J.G.M.; Herberts, C.A.; Hagens, W.I.; Oomen, A.G.; Heugens, E.H.W.; Roszek, B.; Bisschops, J.; Gosens, I.; Van De Meent, D.; et al. Nano-silver—A review of available data and knowledge gaps in human and environmental risk assessment. Nanotoxicology 2009, 3, 109–138. [Google Scholar] [CrossRef]
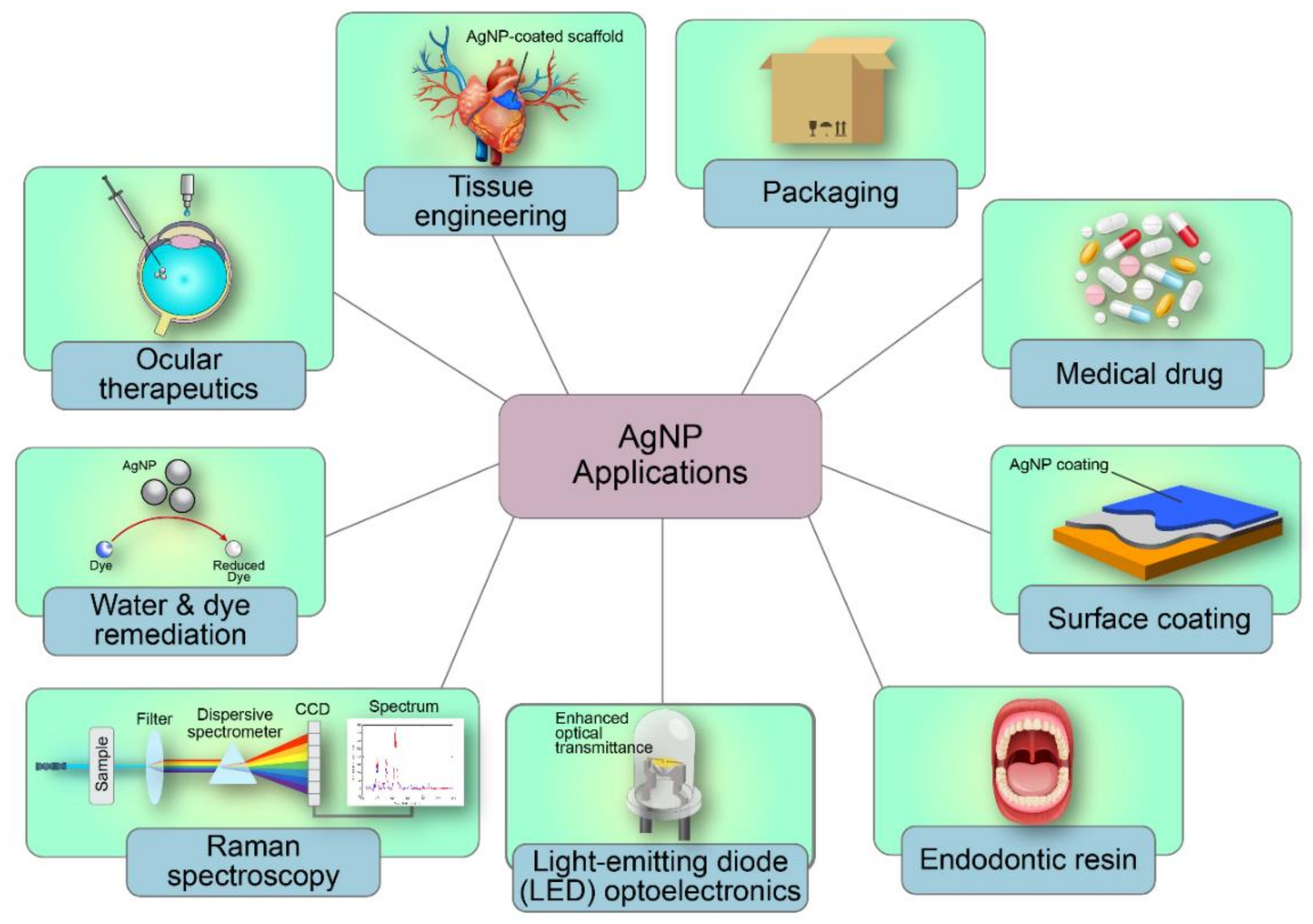
| Industry | Application | Properties | References |
|---|---|---|---|
| Medical | Scaffold | Cyto-compatible and antibacterial scaffold | [21] |
| Aerogel | Antibacterial aerogel | [22] | |
| Endodontics | Antibacterial Gutta-percha | [23] | |
| Dental acrylic resin | Antimicrobial and biocompatible resin | [24,25,26] | |
| Ocular therapeutics | Antiangiogenic and antibacterial therapeutic nanoparticle | [27] | |
| Urinary catheters | Antibiofilm urinary catheter | [28] | |
| Medical dressing | Wound-healing cotton fabric | [29] | |
| Electronics | Supercapacitor and electrochemical sensing | High electrochemical capacity in 3D-printed microfluidic device | [30] |
| Optoelectronic applications | Enhanced optical transmittance | [31] | |
| Sensing and biosensing | Stable and rapid photo-optical sensor | [32,33] | |
| Packaging | Paper coating | Extends food shelf-life | [34] |
| Film | Antioxidant and antibacterial film | [35] | |
| Surface coating | Ceramic glaze | Antibacterial ceramic surface | [36] |
| Remediation | Dye degradation | Catalytic ability on dye | [31,37] |
| Application | Patent Title | Patent ID | References |
|---|---|---|---|
| Antimicrobial agent for wound healing | Composition comprising amino acid polymers and a bioactive agent and method of preparing thereof | US20200368176A1 | [38] |
| Scaffold | Coating scaffolds | AU2020250274A1 | [39] |
| Electromagnetic shielding agent | Microcellular foamed HIPS electromagnetic shielding material and preparation method and application thereof | CN111961305A | [40] |
| Slow-release antibacterial agent | Preparation method of nano-silver slow-release antibacterial agent | CN111973794A | [41] |
| Biocidal agent | Mobile device for cleaning and disinfecting room air that can be operated using a temperature difference | DE202020105700U1 | [42] |
| Antioxidative agent | Skin cleanser | AU2020227091A1 | [43] |
| Antimicrobial agent | Synthetic fiber with semi-permanent antibacterial and anti-fungal properties and uses thereof | KR102163245B1 | [44] |
| Antimicrobial coating | A system that provides local cooling to the brain and spinal cord | JP2020171791A | [45] |
| Reducing agent in biopolymer microgel | Method of reducing an organic pollutant in contaminated water | US10793684B1 | [46] |
| Slow-release bactericidal agent | Medicine for treating ant bite and its medicine applying plaster | CN111671844A | [47] |
| Colorimetric sensing of trypsin | Trypsin detection film, preparation method and application thereof and trypsin detection kit | CN111808916A | [48] |
| Source | Size of the Nanoparticle | Inhibited Microbes | Application | References |
|---|---|---|---|---|
| Musa (banana peels) | 50 nm | S. aureus B. subtilis P. aeruginosa E. coli C. albicans | Possess good antimicrobial activity against foodborne microorganisms | [99] |
| Punica granatum (pomegranate peels) | 10–30 nm | Potential application in the biomedical field | [7] | |
| Citrus x sinensis (orange peels) | 10 nm | - | Prospective method of citrus canker control using orange waste | [8] |
| Citrus grandis (pomelo) | 20–30 nm | - | These nanoparticles can be used as a reducing agent | [100] |
| Citrus x limon (lemon) Citrus x sinensis (orange) Citrus limetta (Mosambi peels) | 9–46 nm | E. coli S. aureus | Viable resource for antioxidant extraction; anticancer properties | [80] |
| Vitis (grapes), Carica papaya (papaya) Citrullus lanatus (watermelon) | 50 nm | B. subtilis S. aureus E. coli P. aeruginosa | Active food packaging | [101] |
| Musa (banana peels) | Reducing and stabilizing agent | [102] | ||
| Punica granatum (pomegranate) | 5–10 nm | - | Offers a valuable contribution to green synthesis without adding different physical and chemical steps | [103] |
| Ananas comusus (pineapple) | 9 nm | E. faecium L. monocytogens B. cereus S. aureus | Various biomedical applications, such as management of serious diseases such as diabetes and cancer | [104] |
| Citrus maxima (pomelo) | 2.5–5.7 nm | Useful in extracellular synthesis of silver nanoparticles | [105] | |
| Punica granatum (pomegranate), Citrus x sinensis (orange peels) | 94.5 nm (orange) 74.9 nm (pomegranate) | - | Antimicrobial and wound healing | [106] |
| Vitis vinifera L. (grape pomace), Citrus x sinensis (orange residues) | 90 nm (grape pomace) 96 nm (orange) | E. coli S. aureus P. arginosa | Reduced silver ions acting as capping agents, and also shows highest antimicrobial activity | [107] |
| Citrus x sinensis (orange peels) | 56.1 nm | - | Paves the way for future studies on AgNP toxicity | [108] |
| Punica granatum (Saudi pomegranate fruit) | 34–50 nm | S. aureus S. typhi P. aeruginosa E. coli S. epidermidis K. pneumoniae | An ideal prerequisite for efficient drug delivery | [88] |
| Eucalyptus camaldulensis (river red gum bark) | 468.7 nm | - | Commercial skincare formulations | [109] |
| Rhododendron ponticumu (common rhododendron leaf waste) | 10–21 nm | L. innocua B. subtilis E. aerogenes E. coli | Antibacterial ointment; textile or fabric | [94] |
| Spent coffee grounds | 34.6–54.2 nm | - | Water treatment applications | [95] |
| Eucalyptus sp. (prehydrolysis waste liquor of wood) | 20 nm | P. aeruginosa S. aureus E. coli C. oxysporum P. chrysogenum C. albicans A. niger | Biomedical applications | [96] |
| Poa annua (annual meadow grass leaf) | 36.66 nm | - | Potential drug carrier and therapeutic | [97] |
| Saccharum sp. (sugar cane bagasse) | 6–36 nm | E. coli P. aeruginosa S. aureus | Bactericidal applications without AgCl formation | [98] |
| Allium cepa L. (red onion peels) | 14 nm | - | Medical and agricultural applications | [110] |
| Nypa fruticans (waste husks of Nipa palm) | 10–15 nm | B. cereus | General range of AgNP-related applications | [111] |
| Physalis peruviana L. (outer accrescent fruiting calyx of Cape gooseberry) | 25–55 nm | E. coli S. typhimurium | Antibacterial material, coating, cosmeceutical, and biomedical applications | [112] |
| Cocos nucifera L. (outer shell fibre of coconut) | - | E. coli E. feacium P. acnes L. monocytogenes C. albicans | Biomedical, food, and pharmaceutical applications | [113] |
| Citrullus lanatus (rinds of watermelon) | 20–260 nm | S. aureus E. coli B. cereus L. monocytogenes S. typhimurium | Agricultural, biomedical, cosmeceutical, and pharmaceutical applications | [114] |
| Solanum tuberosum (potato peels) | 20–40 nm | - | General range of AgNP-related applications | [115] |
| Oryza sativa japonica (rice husks) | <47.90 nm | - | General range of AgNP-related applications | [116] |
| Bacterial Strains | Size of AgNPs | Mechanism of Antimicrobial Activity | Reference |
|---|---|---|---|
| Gram-positive | |||
| Multidrug-resistant Staphylococcus aureus (MMC-20) | 18 ± 3 nm | Obstruction of membrane due to ROS formation | [150] |
| Staphylococcus aureus ATCC25923 | 3.91 nm/2.29 nm/1.59 nm | Obstruction of membrane due to ROS formation | [151] |
| Staphylococcus aureus | <100 nm | Oxidative stress caused by modification of kynurenine protein | [152] |
| Bacillus subtilis | <100 nm | Modification of kynurenine protein-mediated kynurenine pathways that inhibited growth | [152] |
| Listeria monocytogenes | 23 ± 2 nm | Increase in ROS levels | [153] |
| Clostridium diphteria | 28.42 nm | Cell wall hostility, denaturation of proteins | [154] |
| Gram-negative | |||
| Escherichia coli | <100 nm | Oxidative stress caused by modification of kynurenine protein | [155] |
| Escherichia coli AB1157 | 8.3 ± 1.9 nm | Destruction of DNA | [156] |
| Escherichia coli ATCC25922 | 3.91 nm/2.29 nm/1.59 nm | Obstruction of membrane due to increased ROS formation | [151] |
| Pseudomonas aeruginosa | 45 nm | Binding of AgNPs to cell wall and synthesis of ROS | [157] |
| Klebsiella pneumoniae | <100 nm | Modification of kynurenine protein-mediated kynurenine pathways that inhibited growth | [152] |
| Proteus sp. | 38 nm | Estrangement of cell membrane and hindered DNA replication | [155] |
| Vibrio cholera | <50 nm | Impeded metabolic pathways | [158] |
| Salmonella thyphii | 2–23 nm | Cell wall rupture | [159] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vigneswari, S.; Amelia, T.S.M.; Hazwan, M.H.; Mouriya, G.K.; Bhubalan, K.; Amirul, A.-A.A.; Ramakrishna, S. Transformation of Biowaste for Medical Applications: Incorporation of Biologically Derived Silver Nanoparticles as Antimicrobial Coating. Antibiotics 2021, 10, 229. https://doi.org/10.3390/antibiotics10030229
Vigneswari S, Amelia TSM, Hazwan MH, Mouriya GK, Bhubalan K, Amirul A-AA, Ramakrishna S. Transformation of Biowaste for Medical Applications: Incorporation of Biologically Derived Silver Nanoparticles as Antimicrobial Coating. Antibiotics. 2021; 10(3):229. https://doi.org/10.3390/antibiotics10030229
Chicago/Turabian StyleVigneswari, Sevakumaran, Tan Suet May Amelia, Mohamad Hazari Hazwan, Govindan Kothandaraman Mouriya, Kesaven Bhubalan, Al-Ashraf Abdullah Amirul, and Seeram Ramakrishna. 2021. "Transformation of Biowaste for Medical Applications: Incorporation of Biologically Derived Silver Nanoparticles as Antimicrobial Coating" Antibiotics 10, no. 3: 229. https://doi.org/10.3390/antibiotics10030229
APA StyleVigneswari, S., Amelia, T. S. M., Hazwan, M. H., Mouriya, G. K., Bhubalan, K., Amirul, A.-A. A., & Ramakrishna, S. (2021). Transformation of Biowaste for Medical Applications: Incorporation of Biologically Derived Silver Nanoparticles as Antimicrobial Coating. Antibiotics, 10(3), 229. https://doi.org/10.3390/antibiotics10030229









