Microorganisms: A Potential Source of Bioactive Molecules for Antioxidant Applications
Abstract
:1. Introduction
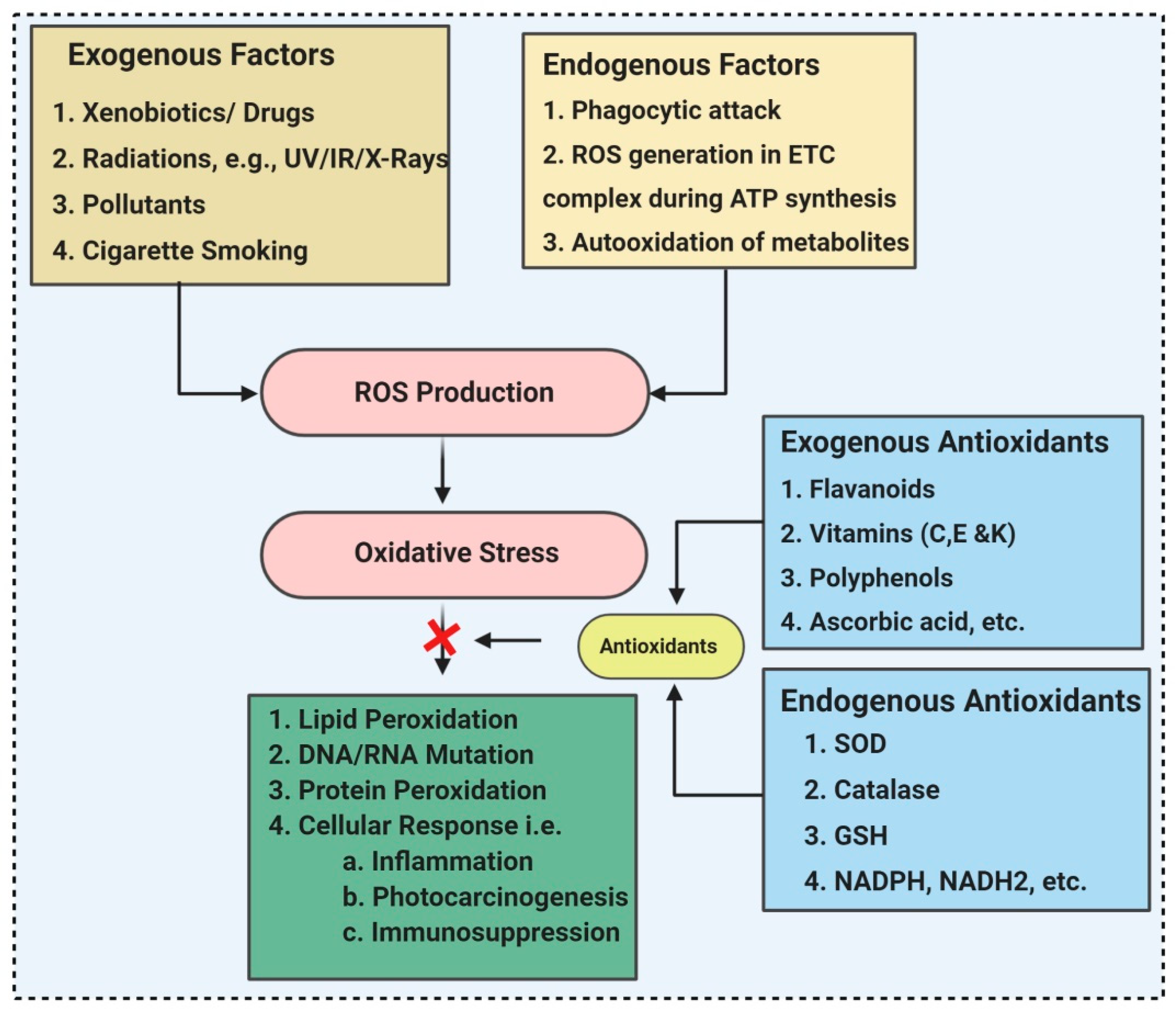
2. ROS (Reactive Oxygen Species) and Antioxidants
3. Microorganisms as a Source of Antioxidant Compounds
3.1. Actinomycetes
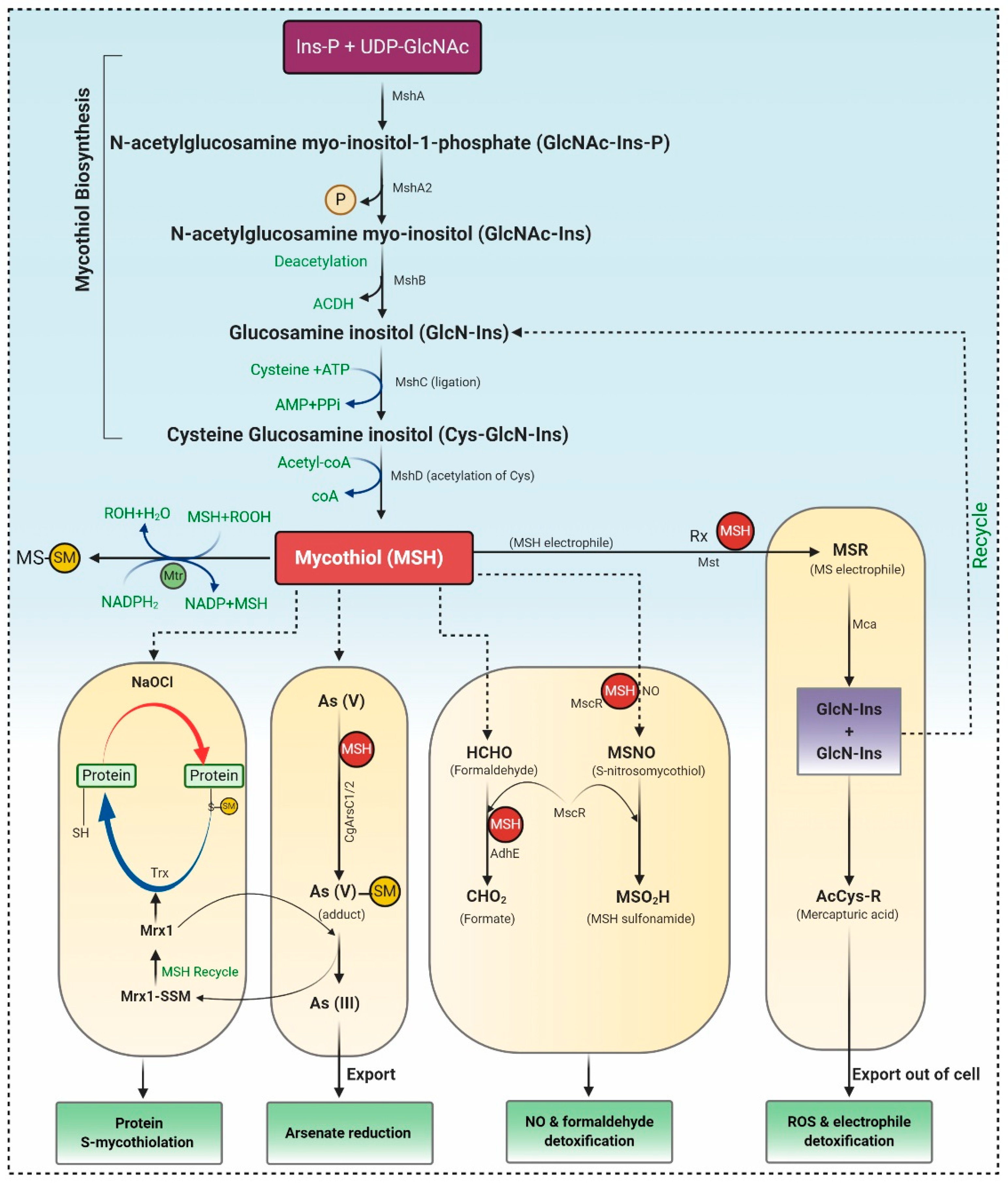
3.1.1. Biosynthesis of MSH
3.1.2. The Catalytic Action of the Mycothiol Disulfide Reductase (Mtr)
3.1.3. Mycothiol-Dependent Detoxification of Electrophiles
3.1.4. Detoxification of Nitric Oxide (NO)
3.1.5. Detoxification of Arsenate
3.1.6. Protein S-Mycothiolation under NaClO and H2O2 Stress
3.2. Archaea
3.3. Bacteria
3.4. Fungi
3.4.1. Mushrooms
3.4.2. Yeast
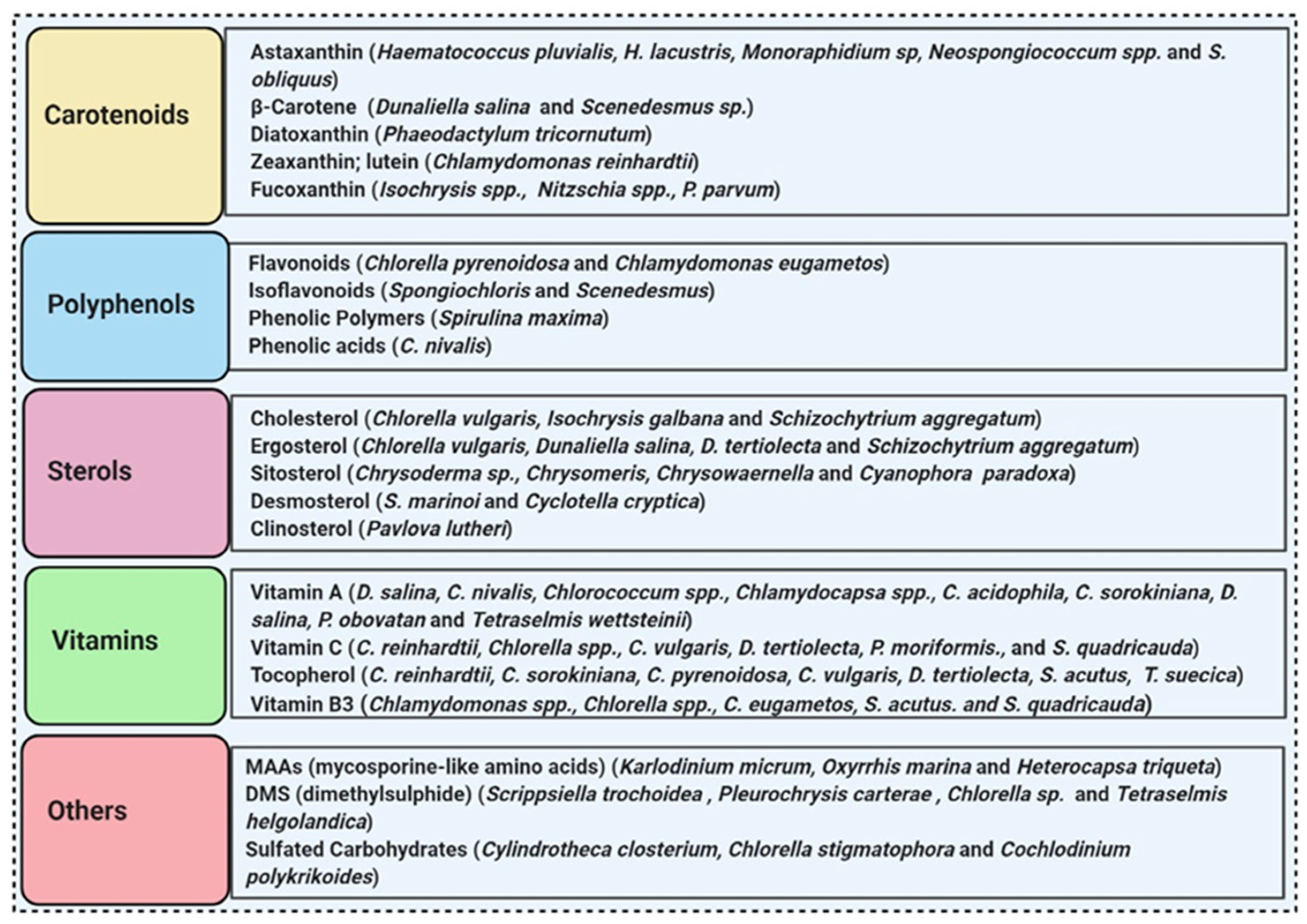
3.5. Microalgae
3.6. Protozoa
4. Future Prospectus
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Castro, I.; Mendo, S.; Caetano, T. Antibiotics from Haloarchaea: What Can We Learn from Comparative Genomics? Mar. Biotechnol. 2020, 22, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Demain, A.L. Importance of microbial natural products and the need to revitalize their discovery. J. Ind. Microbiol. Biotechnol. 2014, 41, 185–201. [Google Scholar] [CrossRef] [PubMed]
- Antibiotics. Antibiotics: Technologies and Global Markets (PHM025D). 2016. Available online: https://www.bccresearch.com/ (accessed on 22 September 2020).
- Singh, R.; Kumar, M.; Mittal, A.; Mehta, P.K. Microbial metabolites in nutrition, healthcare and agriculture. 3 Biotech 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sen, T.; Barrow, C.J.; Deshmukh, S.K. Microbial Pigments in the Food Industry—Challenges and the Way Forward. Front. Nutr. 2019, 6, 7. [Google Scholar] [CrossRef] [Green Version]
- Chandra, P.; Sharma, R.K.; Arora, D.S. Antioxidant compounds from microbial sources: A review. Food Res. Int. 2020, 129, 108849. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Shah, C.; Mokashe, N.; Chavan, R.; Yadav, H.; Prajapati, J. Probiotics as Potential Antioxidants: A Systematic Review. J. Agric. Food Chem. 2015, 63, 3615–3626. [Google Scholar] [CrossRef]
- Feng, T.; Wang, J. Oxidative stress tolerance and antioxidant capacity of lactic acid bacteria as probiotic: A systematic review. Gut Microbes 2020, 12. [Google Scholar] [CrossRef]
- Young, A.J.; Lowe, G.L. Carotenoids—Antioxidant properties. Antioxidants 2018, 7, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekpe, L.; Inaku, K.; Ekpe, V. Antioxidant effects of astaxanthin in various diseases—A review. J. Mol. Pathophysiol. 2018, 7, 1–6. [Google Scholar] [CrossRef]
- Davinelli, S.; Nielsen, M.E.; Scapagnini, G. Astaxanthin in Skin Health, Repair, and Disease: A Comprehensive Review. Nutrients 2018, 10, 522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khatua, S.; Paul, S.; Acharya, K. Mushroom as the potential source of new generation of antioxidant: A review. Res. J. Pharm. Technol. 2013, 6, 3. [Google Scholar]
- Gupta, S.; Chaturvedi, P.; Kulkarni, M.G.; Van Staden, J. A critical review on exploiting the pharmaceutical potential of plant endophytic fungi. Biotechnol. Adv. 2020, 39, 107462. [Google Scholar] [CrossRef]
- Vitale, G.A.; Coppola, D.; Esposito, F.P.; Buonocore, C.; Ausuri, J.; Tortorella, E.; De Pascale, D. Antioxidant Molecules from Marine Fungi: Methodologies and Perspectives. Antioxidants 2020, 9, 1183. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Wang, Y.; Li, W. Antioxidant Properties of Probiotic Bacteria. Nutrients 2017, 9, 521. [Google Scholar] [CrossRef]
- Shah, M.R.; Liang, Y.; Cheng, J.J.; Daroch, M. Astaxanthin-Producing Green Microalga Haematococcus pluvialis: From Single Cell to High Value Commercial Products. Front. Plant Sci. 2016, 7, 531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, H.; Yılmaz, H.; Gülçin, I. Antioxidant Activity of Flaxseed (Linum usitatissimum L.) shell and Analysis of Its Polyphenol Contents by LC-MS/MS. Rec. Nat. Prod. 2018, 12, 397–402. [Google Scholar] [CrossRef]
- Bulut, N.; Kocyigit, U.M.; Gecibesler, I.H.; Dastan, T.; Karci, H.; Taslimi, P.; Dastan, S.D.; Gulcin, I.; Cetin, A. Synthesis of some novel pyridine compounds containing bis-1,2,4-triazole/thiosemicarbazide moiety and investigation of their antioxidant properties, carbonic anhydrase, and acetylcholinesterase enzymes inhibition profiles. J. Biochem. Mol. Toxicol. 2018, 32, e22006. [Google Scholar] [CrossRef]
- Krawczyk, H. The stilbene derivatives, nucleosides, and nucleosides modified by stilbene derivatives. Bioorganic Chem. 2019, 90, 103073. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A. Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem. 2020, 44. [Google Scholar] [CrossRef] [PubMed]
- Neha, K.; Haider, R.; Pathak, A.; Yar, M.S. Medicinal prospects of antioxidants: A review. Eur. J. Med. Chem. 2019, 178, 687–704. [Google Scholar] [CrossRef]
- Mitra, A.K. Antioxidants: A Masterpiece of Mother Nature to Prevent Illness. J. Chem. Rev. 2020, 2, 243–256. [Google Scholar]
- Yashin, A.; Yashin, Y.; Xia, X.; Nemzer, B. Antioxidant Activity of Spices and Their Impact on Human Health: A Review. Antioxidants 2017, 6, 70. [Google Scholar] [CrossRef] [Green Version]
- Amany, M.; Abdel-Raheam, H.E. Red and yellow monascus pigments as potential natural antioxidants for fatty foods. Plant Arch. 2020, 20, 444–449. [Google Scholar]
- Tripathi, V.C.; Horam, S.; Singh, A.; Lata, M.; Reddy, T.J.; Arockiaraj, J.; Pasupuleti, M. The discovery of antioxidants in marine microorganisms and their protective effects on the hepatic cells from chemical-induced oxidative stress. Free. Radic. Res. 2020, 54, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Widowati, I.; Zainuri, M.; Kusumaningrum, H.P.; Susilowati, R.; Hardivillier, Y.; Leignel, V.; Bourgougnon, N.; Mouget, J.-L. (Eds.) Antioxidant Activity of Three Microalgae Dunaliella Salina, Tetraselmis Chuii and Isochrysis Galbana Clone Tahiti; IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2017. [Google Scholar]
- Wali, A.F.; Al Dhaheri, Y.; Pillai, J.R.; Mushtaq, A.; Rao, P.G.M.; Rabbani, S.A.; Firdous, A.; Elshikh, M.S.; Al Farraj, D.A. LC-MS Phytochemical Screening, In Vitro Antioxidant, Antimicrobial and Anticancer Activity of Microalgae Nannochloropsis oculata Extract. Separations 2020, 7, 54. [Google Scholar] [CrossRef]
- NCBI. (National Center for Biotechnology Information) PubChem Compound 2021. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Astaxanthin (accessed on 31 December 2020).
- Zhang, C.; Chen, X.; Too, H.-P. Microbial astaxanthin biosynthesis: Recent achievements, challenges, and commercialization outlook. Appl. Microbiol. Biotechnol. 2020, 104, 5725–5737. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-R.; Ng, I.-S. Development of CRISPR/Cas9 system in Chlorella vulgaris FSP-E to enhance lipid accumulation. Enzym. Microb. Technol. 2020, 133, 109458. [Google Scholar] [CrossRef]
- Rani, D.J.; Mala, R.; Mohan, P.; Keerthana, R.; Prasath, N.H.; Celsia, A.S.R. Chitosan nanoparticle-mediated delivery of curcumin and phycocyanin for photodynamic therapy against biofilm forming bacteria. Mater. Express 2020, 10, 1854–1870. [Google Scholar] [CrossRef]
- Nawaz, A.; Chaudhary, R.; Shah, Z.; Dufossé, L.; Fouillaud, M.; Mukhtar, H.; Haq, I.U. An Overview on Industrial and Medical Applications of Bio-Pigments Synthesized by Marine Bacteria. Microorganisms 2020, 9, 11. [Google Scholar] [CrossRef]
- Cauz, A.C.G.; Carretero, G.P.B.; Saraiva, G.K.V.; Park, P.; Mortara, L.; Cuccovia, I.M.; Brocchi, M.; Gueiros-Filho, F.J. Violacein Targets the Cytoplasmic Membrane of Bacteria. Acs Infect. Dis. 2019, 5, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Kupriyanova, E.V.; Sinetova, M.A.; Bedbenov, V.S.; Pronina, N.A.; Los, D.A. Putative extracellular α-class carbonic anhydrase, EcaA, of Synechococcus elongatus PCC 7942 is an active enzyme: A sequel to an old story. Microbiologyresearch 2018, 164, 576–586. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhao, J.; Wang, Y.; Qin, S.; Lu, Y. Characterization and engineering of a dual-function diacylglycerol acyltransferase in the oleaginous marine diatom Phaeodactylum tricornutum. Biotechnol. Biofuels 2018, 11, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Sun, Z.; Gerken, H.; Huang, J.; Jiang, Y.; Chen, F. Genetic engineering of the green alga Chlorella zofingiensis: A modified norflurazon-resistant phytoene desaturase gene as a dominant selectable marker. Appl. Microbiol. Biotechnol. 2014, 98, 5069–5079. [Google Scholar] [CrossRef]
- Haslam, R.P.; Hamilton, M.L.; Economou, C.K.; Smith, R.; Hassall, K.L.; Napier, J.A.; Sayanova, O. Overexpression of an endogenous type 2 diacylglycerol acyltransferase in the marine diatom Phaeodactylum tricornutum enhances lipid production and omega-3 long-chain polyunsaturated fatty acid content. Biotechnol. Biofuels 2020, 13, 1–17. [Google Scholar] [CrossRef]
- Hameed, A.; Hussain, S.A.; Yang, J.; Ijaz, M.U.; Liu, Q.; Suleria, H.A.R.; Song, Y. Antioxidants Potential of the Filamentous Fungi (Mucor circinelloides). Nutrients 2017, 9, 1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozarski, M.; Klaus, A.; Jakovljevic, D.; Todorovic, N.; Vunduk, J.; Petrović, P.; Niksic, M.; Vrvic, M.M.; van Griensven, L. Antioxidants of Edible Mushrooms. Molecules 2015, 20, 19489–19525. [Google Scholar] [CrossRef] [Green Version]
- Kalaras, M.D.; Richie, J.P.; Calcagnotto, A.; Beelman, R.B. Mushrooms: A rich source of the antioxidants ergothioneine and glutathione. Food Chem. 2017, 233, 429–433. [Google Scholar] [CrossRef]
- Yang, X.; Yang, Y.; Peng, T.; Yang, F.; Zhou, H.; Zhao, L.; Xu, L.; Ding, Z. A New Cyclopeptide from Endophytic Streptomyces sp. YIM 64018. Nat. Prod. Commun. 2013, 8, 1753–1754. [Google Scholar] [CrossRef] [Green Version]
- Álvarez-Pérez, J.M.; González-García, S.; Cobos, R.; Olego, M.Á.; Ibañez, A.; Díez-Galán, A.; Garzón-Jimeno, E.; Coque, J.J.R. Use of Endophytic and Rhizosphere Actinobacteria from Grapevine Plants To Reduce Nursery Fungal Graft Infections That Lead to Young Grapevine Decline. Appl. Environ. Microbiol. 2017, 83. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Fan, C.; Chen, Y.; Wang, J.; Yin, W.; Wang, R.R.C.; Hu, Z. Identification and characterization of an efficient acyl-CoA: Diacylglycerol acyltransferase 1 (DGAT1) gene from the microalga Chlorella ellipsoidea. BMC Plant Biol. 2017, 17, 48. [Google Scholar] [CrossRef] [Green Version]
- Manimaran, M.; Gopal, J.V.; Kannabiran, K. Antibacterial Activity of Streptomyces sp. VITMK1 Isolated from Mangrove Soil of Pichavaram, Tamil Nadu, India. Proc. Natl. Acad. Sci. India Sect. B Boil. Sci. 2015, 87, 499–506. [Google Scholar] [CrossRef]
- Wang, P.; Xi, L.; Liu, P.; Wang, Y.; Wang, W.; Huang, Y.; Zhu, W. Diketopiperazine Derivatives from the Marine-Derived Actinomycete Streptomyces sp. FXJ7.328. Mar. Drugs 2013, 11, 1035–1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grasso, L.L.; Martino, D.C.; Alduina, R. Production of antibacterial compounds from Actinomycetes. In Actinobacteria-Basics and Biotechnological Applications; InTechOpen: London, UK, 2016; pp. 177–198. [Google Scholar]
- Malve, H. Exploring the ocean for new drug developments: Marine pharmacology. J. Pharm. Bioallied Sci. 2016, 8, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Daniel, T. Avicennia (Acanthaceae: Avicennioideae) in North America and Mesoamerica. Proc. Calif. Acad. Sci. 2016, 63, 163–189. [Google Scholar]
- Noor-Syaheera, M.; Noraini, T.; Radhiah, A.; Che-Nurul-Aini, C. Leaf anatomical characteristics of Avicennia L. and some selected taxa in Acanthaceae. Malay. Nat. J. 2015, 67, 81–94. [Google Scholar]
- Li, X.; Wang, X.; Duan, C.; Yi, S.; Gao, Z.; Xiao, C.; Agathos, S.N.; Wang, G.; Li, J. Biotechnological production of astaxanthin from the microalga Haematococcus pluvialis. Biotechnol. Adv. 2020, 43, 107602. [Google Scholar] [CrossRef] [PubMed]
- Harvey, P.J.; Ben-Amotz, A. Towards a sustainable Dunaliella salina microalgal biorefinery for 9-cis β-carotene production. Algal Res. 2020, 50, 102002. [Google Scholar] [CrossRef]
- Ardiles, P.; Cerezal-Mezquita, P.; Salinas-Fuentes, F.; Órdenes, D.; Renato, G.; Ruiz-Domínguez, M.C. Biochemical Composition and Phycoerythrin Extraction from Red Microalgae: A Comparative Study Using Green Extraction Technologies. Processes 2020, 8, 1628. [Google Scholar] [CrossRef]
- Spinola, M.V.; Díaz-Santos, E. Microalgae Nutraceuticals: The Role of Lutein in Human Health; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 243–263. [Google Scholar]
- García-López, D.; Olguín, E.; González-Portela, R.; Sánchez-Galván, G.; De Philippis, R.; Lovitt, R.; Llewellyn, C.; Fuentes-Grünewald, C.; Saldívar, R.P. A novel two-phase bioprocess for the production of Arthrospira (Spirulina) maxima LJGR1 at pilot plant scale during different seasons and for phycocyanin induction under controlled conditions. Bioresour. Technol. 2020, 298, 122548. [Google Scholar] [CrossRef]
- Quach, N.T. Functional Analyses of Thiol Switches and Their Impact on the Mycothiol Redox Potential in Actinomycetes. Ph.D. Thesis, Freie University of Berlin, Berlin, Germany, 2020. [Google Scholar]
- Fan, F.; Vetting, M.W.; Frantom, P.A.; Blanchard, J.S. Structures and mechanisms of the mycothiol biosynthetic enzymes. Curr. Opin. Chem. Biol. 2009, 13, 451–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loi, V.V.; Rossius, M.; Antelmann, H. Redox regulation by reversible protein S-thiolation in bacteria. Front. Microbiol. 2015, 6, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imber, M.; Pietrzyk-Brzezinska, A.J.; Antelmann, H. Redox regulation by reversible protein S-thiolation in Gram-positive bacteria. Redox Biol. 2019, 20, 130–145. [Google Scholar] [CrossRef]
- Venkataraman, K.; Raghunathan, C. Coastal and Marine Biodiversity of India. Mar. Faunal Divers. India 2015, 303–348. [Google Scholar] [CrossRef]
- Kumar, V.; Tiwari, S.K. Halocin HA1: An archaeocin produced by the haloarchaeon Haloferax larsenii HA1. Process. Biochem. 2017, 61, 202–208. [Google Scholar] [CrossRef]
- Tossounian, M.-A.; Pedre, B.; Wahni, K.; Erdogan, H.; Vertommen, D.; Van Molle, I.; Messens, J. Corynebacterium diphtheriae Methionine Sulfoxide Reductase A Exploits a Unique Mycothiol Redox Relay Mechanism. J. Biol. Chem. 2015, 290, 11365–11375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Yang, Y.; Peng, T.; Li, W.; Zhao, L.; Xu, L.; Ding, Z. Metabolites of Streptomyces sp., an endophytic actinomycete from Alpinia oxyphylla. Nat. Prod. Res. 2014, 28, 265–267. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Shindo, K.; Yamagishi, Y.; Matsuoka, M.; Kawai, H.; Mochizuki, J. Phenazoviridin, a novel free radical scavenger from Streptomyces sp. Taxonomy, fermentation, isolation, structure elucidation and biological properties. J. Antibiot. 1993, 46, 1485–1493. [Google Scholar] [CrossRef]
- Guimarães, T.C.; Gomes, T.S.; Fernandes, C.D.; Barros, F.D.; Oliveira, K.V.; Bilal, M.; Bharagava, R.N.; Ferreira, L.F.R.; Hollanda, L.M. Antitumor Microbial Products by Actinomycetes Isolated from Different Environments. Role Microb. Communities Sustain. 2020, 113–160. [Google Scholar] [CrossRef]
- Shindo, K.; Misawa, N. New and Rare Carotenoids Isolated from Marine Bacteria and Their Antioxidant Activities. Mar. Drugs 2014, 12, 1690–1698. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, M.; Kuzuyama, T. Recent Advances in the Biosynthesis of Carbazoles Produced by Actinomycetes. Biomolecules 2020, 10, 1147. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.T.-H.; Chan, K.-G.; Khan, T.M.; Bukhari, S.I.; Saokaew, S.; Duangjai, A.; Pusparajah, P.; Lee, L.-H.; Goh, B.-H. Streptomyces sp. MUM212 as a Source of Antioxidants with Radical Scavenging and Metal Chelating Properties. Front. Pharm. 2017, 8, 276. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.T.-H.; Chan, K.-G.; Pusparajah, P.; Yin, W.-F.; Khan, T.M.; Lee, L.-H.; Goh, B.-H. Mangrove derived Streptomyces sp. MUM265 as a potential source of antioxidant and anticolon-cancer agents. BMC Microbiol. 2019, 19, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Praptiwi; Fathoni, A.; Putri, A.L.; Wulansari, D.; Agusta, A. (Eds.) Assessment of actinomycetes isolated from soils on Simeuleu Island as antibacterial and antioxidant. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2019. [Google Scholar]
- Giani, M.; Garbayo, I.; Vílchez, C.; Martínez-Espinosa, R.M. Haloarchaeal Carotenoids: Healthy Novel Compounds from Extreme Environments. Mar. Drugs 2019, 17, 524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigo-Baños, M.; Garbayo, I.; Vílchez, C.; Bonete, M.-J.; Martínez-Espinosa, R.M. Carotenoids from Haloarchaea and Their Potential in Biotechnology. Mar. Drugs 2015, 13, 5508–5532. [Google Scholar] [CrossRef] [Green Version]
- Madern, D.; Zaccai, G.; Camacho, M.; Rodríguez-Arnedo, A.; Bonete, M.-J. Salt-dependent studies of NADP-dependent isocitrate dehydrogenase from the halophilic archaeon Haloferax volcanii. Extremophiles 2004, 8, 377–384. [Google Scholar] [CrossRef]
- Yatsunami, R.; Ando, A.; Yang, Y.; Takaichi, S.; Kohno, M.; Matsumura, Y.; Ikeda, H.; Fukui, T.; Nakasone, K.; Fujita, N.; et al. Identification of carotenoids from the extremely halophilic archaeon Haloarcula japonica. Front. Microbiol. 2014, 5, 100. [Google Scholar] [CrossRef]
- Sahli, K.; Gomri, M.A.; Esclapez, J.; Gómez-Villegas, P.; Ghennai, O.; Bonete, M.-J.; León, R.; Kharroub, K. Bioprospecting and characterization of pigmented halophilic archaeal strains from Algerian hypersaline environments with analysis of carotenoids produced by Halorubrum sp. BS2. J. Basic Microbiol. 2020, 60, 624–638. [Google Scholar] [CrossRef]
- Newton, G.L.; Javor, B. gamma-Glutamylcysteine and thiosulfate are the major low-molecular-weight thiols in halobacteria. J. Bacteriol. 1985, 161, 438–441. [Google Scholar] [CrossRef] [Green Version]
- Rawat, M.; Maupin-Furlow, J.A. Redox and Thiols in Archaea. Antioxidants 2020, 9, 381. [Google Scholar] [CrossRef]
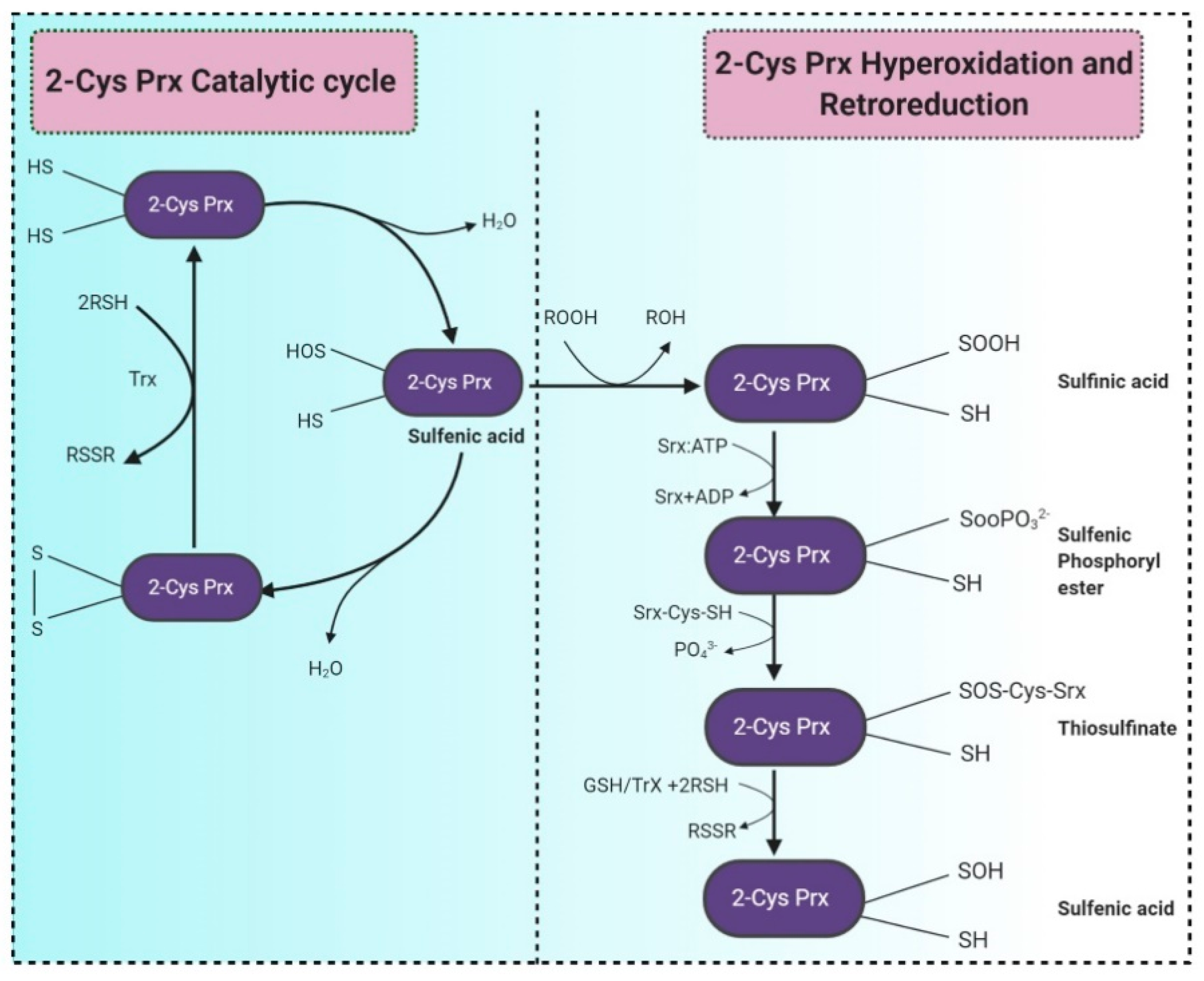
- Pedone, E.; Fiorentino, G.; Bartolucci, S.; Limauro, D. Enzymatic Antioxidant Signatures in Hyperthermophilic Archaea. Antioxidants 2020, 9, 703. [Google Scholar] [CrossRef]
- Sarcinelli, C.; Fiorentino, G.; Pizzo, E.; Bartolucci, S.; Limauro, D. Discovering Antioxidant Molecules in the Archaea Domain: Peroxiredoxin Bcp1 from Sulfolobus solfataricus Protects H9c2 Cardiomyoblasts from Oxidative Stress. Archaea 2016, 2016, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiedenheft, B.; Mosolf, J.; Willits, D.; Yeager, M.; Dryden, K.A.; Young, M.; Douglas, T. From The Cover: An archaeal antioxidant: Characterization of a Dps-like protein from Sulfolobus solfataricus. Proc. Natl. Acad. Sci. USA 2005, 102, 10551–10556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subramanian, D.; Kim, M.-S.; Kim, D.-H.; Heo, M.-S. Isolation, Characterization, Antioxidant, Antimicrobial and Cytotoxic Effect of Marine Actinomycete, Streptomyces Carpaticus MK-01, against Fish Pathogens. Braz. Arch. Biol. Technol. 2017, 60. [Google Scholar] [CrossRef]
- Correa-Llantén, D.N.; Amenábar, M.J.; Blamey, J.M. Antioxidant capacity of novel pigments from an Antarctic bacterium. J. Microbiol. 2012, 50, 374–379. [Google Scholar] [CrossRef]
- Yokoyama, A.; Adachi, K.; Shizuri, Y. New Carotenoid Glucosides, Astaxanthin Glucoside and Adonixanthin Glucoside, Isolated from the Astaxanthin-Producing Marine Bacterium, Agrobacterium aurantiacum. J. Nat. Prod. 1995, 58, 1929–1933. [Google Scholar] [CrossRef]
- Yokoyama, A.; Miki, W.; Izumida, H.; Shizuri, Y. New Trihydroxy-keto-carotenoids Isolated from an Astaxanthin-producing Marine Bacterium. Biosci. Biotechnol. Biochem. 1996, 60, 200–203. [Google Scholar] [CrossRef] [Green Version]
- Yokoyama, A.; Izumida, H.; Shizuri, Y. New Carotenoid Sulfates Isolated from a Marine Bacterium. Biosci. Biotechnol. Biochem. 1996, 60, 1877–1878. [Google Scholar] [CrossRef]
- Mishra, S.; Reddy, D.S.K.; Jamwal, V.S.; Bansal, D.D.; Patel, D.D.; Malhotra, P.; Gupta, A.K.; Singh, P.K.; Jawed, S.; Kumar, R. Semiquinone derivative isolated from Bacillus sp. INM-1 protects cellular antioxidant enzymes from γ-radiation-induced renal toxicity. Mol. Cell. Biochem. 2013, 379, 19–27. [Google Scholar] [CrossRef]
- Sy, C.; Dangles, O.; Borel, P.; Caris-Veyrat, C. Interactions between Carotenoids from Marine Bacteria and Other Micronutrients: Impact on Stability and Antioxidant Activity. Mar. Drugs 2015, 13, 7020–7039. [Google Scholar] [CrossRef]
- Photolo, M.M.; Mavumengwana, V.; Sitole, L.; Tlou, M.G. Antimicrobial and Antioxidant Properties of a Bacterial Endophyte, Methylobacterium radiotolerans MAMP 4754, Isolated from Combretum erythrophyllum Seeds. Int. J. Microbiol. 2020, 2020, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurjogi, M.; Sanakal, R.; Kaliwal, B. Antibiotic susceptibility and antioxidant activity of Staphylococcus aureus pigment staphyloxanthin on carbon tetrachloride (ccl4) induced stress in swiss albino mice. Int. J. Biotechnol. Appl. 2010, 2, 33–40. [Google Scholar]
- Wolter, A.; Hager, A.-S.; Zannini, E.; Galle, S.; Gänzle, M.; Waters, D.; Arendt, E. Evaluation of exopolysaccharide producing Weissella cibaria MG1 strain for the production of sourdough from various flours. Food Microbiol. 2014, 37, 44–50. [Google Scholar] [CrossRef]
- Yu, Y.-J.; Chen, Z.; Chen, P.T.; Ng, I.-S. Production, characterization and antibacterial activity of exopolysaccharide from a newly isolated Weissella cibaria under sucrose effect. J. Biosci. Bioeng. 2018, 126, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Jung, J.-H.; Blanchard, L.; de Groot, A. Conservation and diversity of radiation and oxidative stress resistance mechanisms in Deinococcus species. FEMS Microbiol. Rev. 2019, 43, 19–52. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.M.; Baek, C.Y.; Jung, J.-H.; Kim, W.S.; Song, H.-Y.; Lee, J.H.; Ji, H.J.; Zhi, Y.; Kang, B.S.; Bahn, Y.-S.; et al. Antioxidant Activities of an Exopolysaccharide (DeinoPol) Produced by the Extreme Radiation-Resistant Bacterium Deinococcus radiodurans. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Hu, S.; Nie, S.; Yu, Q.; Xie, M. Reviews on Mechanisms ofIn VitroAntioxidant Activity of Polysaccharides. Oxidative Med. Cell. Longev. 2015, 2016, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aarti, C.; Khusro, A.; Varghese, R.; Arasu, M.V.; Agastian, P.; Al-Dhabi, N.A.; Ilavenil, S.; Choi, K.C. In vitro studies on probiotic and antioxidant properties of Lactobacillus brevis strain LAP2 isolated from Hentak, a fermented fish product of North-East India. LWT 2017, 86, 438–446. [Google Scholar] [CrossRef]
- Son, S.-H.; Jeon, H.-L.; Jeon, E.B.; Lee, N.-K.; Park, Y.-S.; Kang, D.-K.; Paik, H.-D. Potential probiotic Lactobacillus plantarum Ln4 from kimchi: Evaluation of β-galactosidase and antioxidant activities. LWT 2017, 85, 181–186. [Google Scholar] [CrossRef]
- Son, S.-H.; Yang, S.-J.; Jeon, H.-L.; Yu, H.-S.; Lee, N.-K.; Park, Y.-S.; Paik, H.-D. Antioxidant and immunostimulatory effect of potential probiotic Lactobacillus paraplantarum SC61 isolated from Korean traditional fermented food, jangajji. Microb. Pathog. 2018, 125, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Ayyanna, R.; Ankaiah, D.; Arul, V. Anti-inflammatory and Antioxidant Properties of Probiotic Bacterium Lactobacillus mucosae AN1 and Lactobacillus fermentum SNR1 in Wistar Albino Rats. Front. Microbiol. 2018, 9, 3063. [Google Scholar] [CrossRef] [PubMed]
- Bonfili, L.; Cecarini, V.; Cuccioloni, M.; Angeletti, M.; Berardi, S.; Scarpona, S.; Rossi, G.; Eleuteri, A.M. SLAB51 Probiotic Formulation Activates SIRT1 Pathway Promoting Antioxidant and Neuroprotective Effects in an AD Mouse Model. Mol. Neurobiol. 2018, 55, 7987–8000. [Google Scholar] [CrossRef] [Green Version]
- Li, J.-Y.; Jin, M.-M.; Meng, J.; Gao, S.-M.; Lu, R.-R. Exopolysaccharide from Lactobacillus planterum LP6: Antioxidation and the effect on oxidative stress. Carbohydr. Polym. 2013, 98, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, C.; Li, D.; Zhao, Y.; Zhang, X.; Zeng, X.; Yang, Z.; Li, S. Antioxidant activity of an exopolysaccharide isolated from Lactobacillus plantarum C88. Int. J. Biol. Macromol. 2013, 54, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Seth, A.; Yan, F.; Polk, D.B.; Rao, R.K. Probiotics ameliorate the hydrogen peroxide-induced epithelial barrier disruption by a PKC- and MAP kinase-dependent mechanism. Am. J. Physiol. Liver Physiol. 2008, 294, G1060–G1069. [Google Scholar] [CrossRef] [Green Version]
- Segawa, S.; Fujiya, M.; Konishi, H.; Ueno, N.; Kobayashi, N.; Shigyo, T.; Kohgo, Y. Probiotic-Derived Polyphosphate Enhances the Epithelial Barrier Function and Maintains Intestinal Homeostasis through Integrin–p38 MAPK Pathway. PLoS ONE 2011, 6, e23278. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.H.; Jang, S.; Jung, B.J.; Jang, K.-S.; Kim, B.-G.; Chung, D.K.; Kim, H. Differential immune-stimulatory effects of LTAs from different lactic acid bacteria via MAPK signaling pathway in RAW 264.7 cells. Immunobiology 2015, 220, 460–466. [Google Scholar] [CrossRef]
- Fujiya, M.; Musch, M.W.; Nakagawa, Y.; Hu, S.; Alverdy, J.; Kohgo, Y.; Schneewind, O.; Jabri, B.; Chang, E.B. The Bacillus subtilis Quorum-Sensing Molecule CSF Contributes to Intestinal Homeostasis via OCTN2, a Host Cell Membrane Transporter. Cell Host Microbe 2007, 1, 299–308. [Google Scholar] [CrossRef] [Green Version]
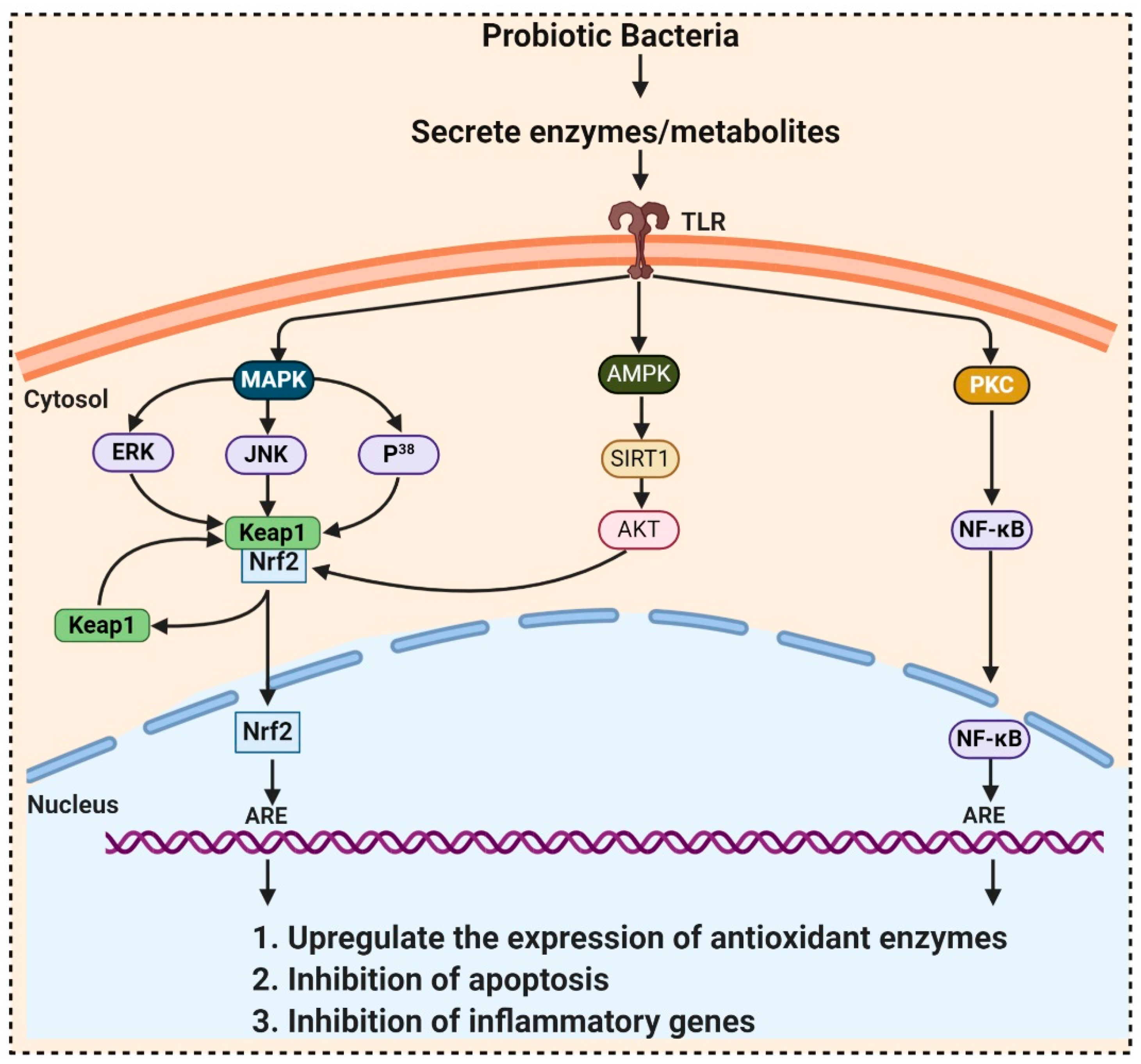
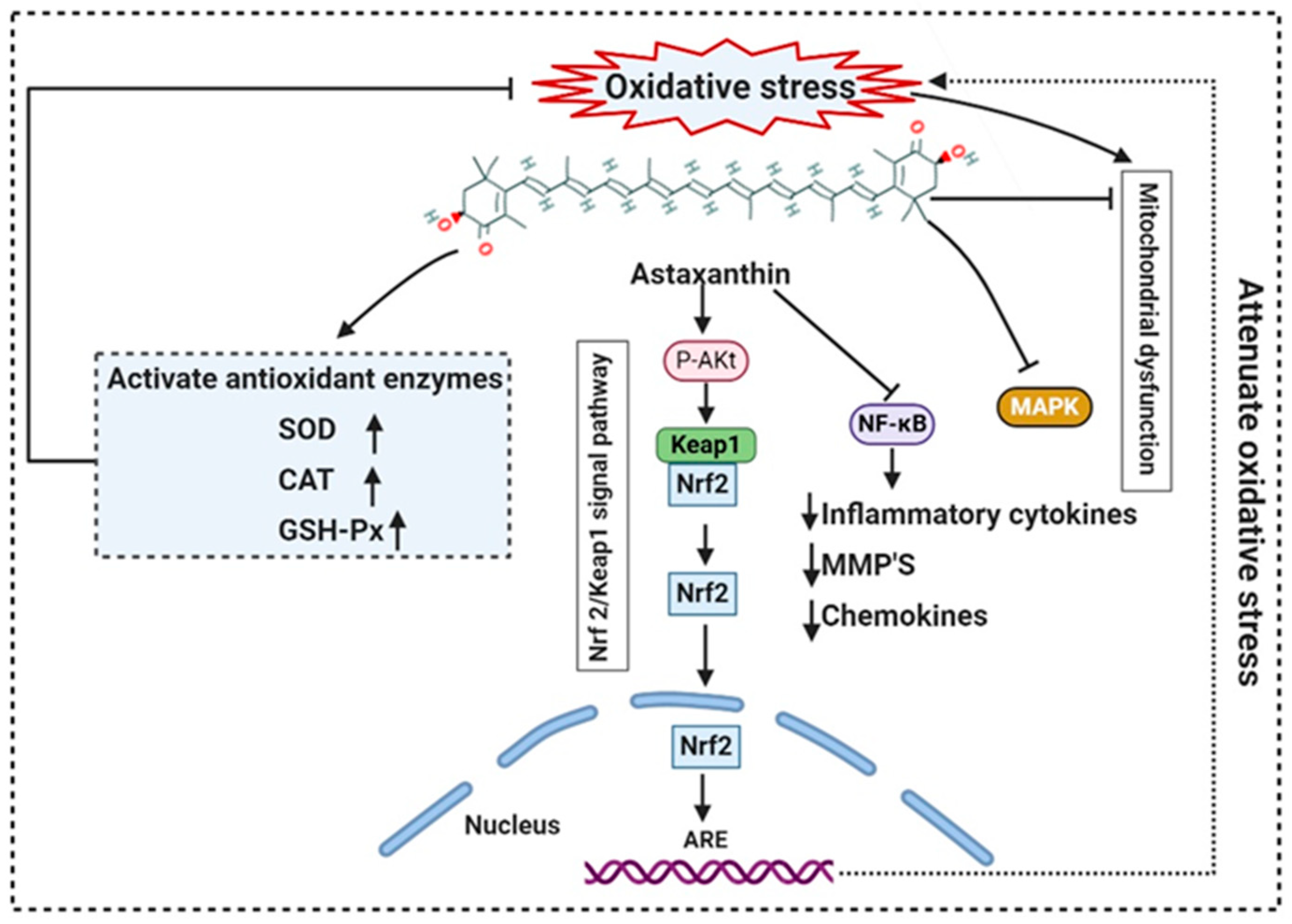
- Wang, Y.; Wu, Y.; Wang, Y.; Fu, A.; Gong, L.; Li, W.; Li, Y. Bacillus amyloliquefaciens SC06 alleviates the oxidative stress of IPEC-1 via modulating Nrf2/Keap1 signaling pathway and decreasing ROS production. Appl. Microbiol. Biotechnol. 2017, 101, 3015–3026. [Google Scholar] [CrossRef] [PubMed]
- Endo, H.; Niioka, M.; Kobayashi, N.; Tanaka, M.; Watanabe, T. Butyrate-Producing Probiotics Reduce Nonalcoholic Fatty Liver Disease Progression in Rats: New Insight into the Probiotics for the Gut-Liver Axis. PLoS ONE 2013, 8, e63388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, D.; Gao, Z.; Zhu, G. Antioxidant effects of Lactobacillus plantarum via activation of transcription factor Nrf2. Food Funct. 2013, 4, 982–989. [Google Scholar] [CrossRef]
- Kim, Y.-G.; Ohta, T.; Takahashi, T.; Kushiro, A.; Nomoto, K.; Yokokura, T.; Okada, N.; Danbara, H. Probiotic Lactobacillus casei activates innate immunity via NF-κB and p38 MAP kinase signaling pathways. Microbes Infect. 2006, 8, 994–1005. [Google Scholar] [CrossRef] [PubMed]
- Diao, Y.; Xin, Y.; Zhou, Y.; Li, N.; Pan, X.; Qi, S.; Qi, Z.; Xu, Y.; Luo, L.; Wan, H. Extracellular polysaccharide from Bacillus sp. strain LBP32 prevents LPS-induced inflammation in RAW 264.7 macrophages by inhibiting NF-κB and MAPKs activation and ROS production. Int. Immunopharmacol. 2014, 18, 12–19. [Google Scholar] [CrossRef]
- Jang, S.-E.; Han, M.J.; Kim, S.-Y.; Kim, D.-H. Lactobacillus plantarum CLP-0611 ameliorates colitis in mice by polarizing M1 to M2-like macrophages. Int. Immunopharmacol. 2014, 21, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Kullisaar, T.; Zilmer, M.; Mikelsaar, M.; Vihalemm, T.; Annuk, H.; Kairane, C.; Kilk, A. Two antioxidative lactobacilli strains as promising probiotics. Int. J. Food Microbiol. 2002, 72, 215–224. [Google Scholar] [CrossRef]
- Leblanc, J.G.; Del Carmen, S.; Miyoshi, A.; Azevedo, V.; Sesma, F.; Langella, P.; Bermúdez-Humarán, L.G.; Watterlot, L.; Perdigon, G.; Leblanc, A.D.M.D. Use of superoxide dismutase and catalase producing lactic acid bacteria in TNBS induced Crohn’s disease in mice. J. Biotechnol. 2011, 151, 287–293. [Google Scholar] [CrossRef]
- Leblanc, A.D.M.D.; Leblanc, J.G.; Perdigón, G.; Miyoshi, A.; Langella, P.; Azevedo, V.; Sesma, F. Oral administration of a catalase-producing Lactococcus lactis can prevent a chemically induced colon cancer in mice. J. Med. Microbiol. 2008, 57, 100–105. [Google Scholar] [CrossRef]
- Tan, R.X.; Zou, W.X. Endophytes: A rich source of functional metabolites (1987 to 2000). Nat. Prod. Rep. 2001, 18, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Jalgaonwala, R.E.; Mohite, B.V.; Mahajan, R.T. A review: Natural products from plant associated endophytic fungi. J. Microbiol. Biotechnol. Res. 2011, 1, 21–32. [Google Scholar]
- Zeng, P.; Wu, J.; Liao, L.; Chen, T.-Q.; Wu, J.; Wong, K.H. In vitro antioxidant activities of endophytic fungi isolated from the liverwort Scapania verrucosa. Genet. Mol. Res. 2011, 10, 3169–3179. [Google Scholar] [CrossRef] [PubMed]
- Prihantini, A.I.; Tachibana, S. Antioxidant compounds produced by Pseudocercospora sp. ESL 02, an endophytic fungus isolated from Elaeocarpus sylvestris. Asian Pac. J. Trop. Biomed. 2017, 7, 110–115. [Google Scholar] [CrossRef]
- Nuraini, F.R.; Setyaningsih, R.; Susilowati, A. Antioxidant activity of bioactive compound produced by endophytic fungi isolated from endemic plant of South Kalimantan Mangifera casturi Kosterm. In Proceedings of the International Conference on Biology and Applied Science (ICOBAS), Malang, Indonesia, 13–14 March 2019; AIP Publishing: Melville, NY, USA, 2019; Volume 2120, p. 080013. [Google Scholar]
- Caicedo, N.H.; Davalos, A.F.; Puente, P.A.; Rodríguez, A.Y.; Caicedo, P.A. Antioxidant activity of exo-metabolites produced by Fusarium oxysporum: An endophytic fungus isolated from leaves of Otoba gracilipes. MicrobiologyOpen 2019, 8, e903. [Google Scholar] [CrossRef] [Green Version]
- Smith, H.; Doyle, S.; Murphy, R. Filamentous fungi as a source of natural antioxidants. Food Chem. 2015, 185, 389–397. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Cai, Y.-Z.; Hyde, K.D.; Corke, H.; Sun, M. Endophytic fungi from Nerium oleander L (Apocynaceae): Main constituents and antioxidant activity. World J. Microbiol. Biotechnol. 2007, 23, 1253–1263. [Google Scholar] [CrossRef] [Green Version]
- Fakruddin; Hossain, N.; Ahmed, M.M. Antimicrobial and antioxidant activities of Saccharomyces cerevisiae IFST062013, a potential probiotic. BMC Complement. Altern. Med. 2017, 17, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.-Y.; Ho, K.-J.; Hsieh, Y.-J.; Wang, L.-T.; Mau, J.-L. Contents of lovastatin, γ-aminobutyric acid and ergothioneine in mushroom fruiting bodies and mycelia. LWT 2012, 47, 274–278. [Google Scholar] [CrossRef]
- Kozarski, M.; Klaus, A.; Vunduk, J.; Zizak, Z.; Niksic, M.; Jakovljevic, D.; Vrvic, M.M.; Van Griensven, L.J.L.D. Nutraceutical properties of the methanolic extract of edible mushroom Cantharellus cibarius (Fries): Primary mechanisms. Food Funct. 2015, 6, 1875–1886. [Google Scholar] [CrossRef] [Green Version]
- Batbayar, S.; Lee, D.-H.; Kim, H.-W. Immunomodulation of Fungal β-Glucan in Host Defense Signaling by Dectin-1. Biomol. Ther. 2012, 20, 433–445. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.K.; Seok, S.J.; Kim, W.K.; Yun, B.S. Hispidin derivatives from the mushroom Inonotus xeranticus and their antioxidant activity. J. Nat. Prod. 2006, 69, 299–301. [Google Scholar] [CrossRef]
- Zan, L.-F.; Qin, J.; Zhang, Y.; Yao, Y.-H.; Bao, H.-Y.; Li, X. Antioxidant hispidin derivatives from medicinal mushroom Inonotus hispidus. Chem. Pharm. Bull. 2011, 59, 770–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, N.; Zhao, L.; Ng, T.B.; Wong, J.H.; Yan, Y.; Shi, Z.; Liu, F. Separation and purification of the antioxidant compound hispidin from mushrooms by molecularly imprinted polymer. Appl. Microbiol. Biotechnol. 2015, 99, 7569–7577. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Diao, Q.-P.; Zhang, B.; Feng, T. Two new illudane sesquiterpenoids and one new menthane monoterpene from cultures of Craterellus cornucopioides. J. Asian Nat. Prod. Res. 2017, 21, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-W.; Wang, L.-M.; Gong, L.-L.; Lu, Y.-M.; Pan, W.-J.; Wang, Y.; Zhang, W.-N.; Chen, Y. Structural characterization and antioxidant activities of a novel polysaccharide fraction from the fruiting bodies of Craterellus cornucopioides. Int. J. Biol. Macromol. 2018, 117, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Ao, T.; Deb, C.R. Nutritional and antioxidant potential of some wild edible mushrooms of Nagaland, India. J. Food Sci. Technol. 2019, 56, 1084–1089. [Google Scholar] [CrossRef]
- Bach, F.; Zielinski, A.A.F.; Helm, C.V.; Maciel, G.M.; Pedro, A.C.; Stafussa, A.P.; Ávila, S.; Haminiuk, C.W.I. Bio compounds of edible mushrooms: In vitro antioxidant and antimicrobial activities. LWT 2019, 107, 214–220. [Google Scholar] [CrossRef]
- Krüzselyi, D.; Móricz, Á.M.; Vetter, J. Comparison of different morphological mushroom parts based on the antioxidant activity. LWT 2020, 127, 109436. [Google Scholar] [CrossRef]
- Angelini, P.; Venanzoni, R.; Flores, G.A.; Tirillini, B.; Orlando, G.; Recinella, L.; Chiavaroli, A.; Brunetti, L.; Leone, S.; Di Simone, S.C.; et al. Evaluation of Antioxidant, Antimicrobial and Tyrosinase Inhibitory Activities of Extracts from Tricholosporum goniospermum, an Edible Wild Mushroom. Antibiotics 2020, 9, 513. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on the safety of ‘yeast beta-glucans’ as a Novel Food ingredient. EFSA J. 2011, 9, 2137. [Google Scholar] [CrossRef]
- Pedrajas, J.R.; Miranda-Vizuete, A.; Javanmardy, N.; Gustafsson, J.-Å.; Spyrou, G. Mitochondria of Saccharomyces cerevisiae Contain One-conserved Cysteine Type Peroxiredoxin with Thioredoxin Peroxidase Activity. J. Biol. Chem. 2000, 275, 16296–16301. [Google Scholar] [CrossRef] [Green Version]
- Garrigós, V.; Picazo, C.; Matallana, E.; Aranda, A. Wine yeast peroxiredoxin TSA1 plays a role in growth, stress response and trehalose metabolism in biomass propagation. Microorganisms 2020, 8, 1537. [Google Scholar] [CrossRef]
- Weids, A.J.; Grant, C.M. The yeast peroxiredoxin Tsa1 protects against protein-aggregate-induced oxidative stress. J. Cell Sci. 2014, 127, 1327–1335. [Google Scholar] [CrossRef] [Green Version]
- Espinosa-Diez, C.; Miguel, V.; Mennerich, D.; Kietzmann, T.; Sánchez-Pérez, P.; Cadenas, S.; Lamas, S. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015, 6, 183–197. [Google Scholar] [CrossRef] [Green Version]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2015, 32, 116–211. [Google Scholar] [CrossRef] [Green Version]
- Park, S.Y.; Binkley, R.M.; Kim, W.J.; Lee, M.H.; Lee, S.Y. Metabolic engineering of Escherichia coli for high-level astaxanthin production with high productivity. Metab. Eng. 2018, 49, 105–115. [Google Scholar] [CrossRef]
- Besse, A.; Peduzzi, J.; Rebuffat, S.; Carré-Mlouka, A. Antimicrobial peptides and proteins in the face of extremes: Lessons from archaeocins. Biochimie 2015, 118, 344–355. [Google Scholar] [CrossRef]
- Levasseur, W.; Perré, P.; Pozzobon, V. A review of high value-added molecules production by microalgae in light of the classification. Biotechnol. Adv. 2020, 41, 107545. [Google Scholar] [CrossRef]
- Sztretye, M.; Dienes, B.; Gönczi, M.; Czirják, T.; Csernoch, L.; Dux, L.; Szentesi, P.; Keller-Pintér, A. Astaxanthin: A Potential Mitochondrial-Targeted Antioxidant Treatment in Diseases and with Aging. Oxidative Med. Cell. Longev. 2019, 2019, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Koller, M.; Muhr, A.; Braunegg, G. Microalgae as versatile cellular factories for valued products. Algal Res. 2014, 6, 52–63. [Google Scholar] [CrossRef]
- Pereira, C.P.M.; Souza, A.C.R.; Vasconcelos, A.R.; Prado, P.S.; Name, J.J. Antioxidant and anti-inflammatory mechanisms of action of astaxanthin in cardiovascular diseases (Review). Int. J. Mol. Med. 2020, 47, 37–48. [Google Scholar] [CrossRef]
- Kindlund, P.J. Astaxanthin. Nutrafoods 2011, 10, 27–31. [Google Scholar] [CrossRef]
- Chen, Z.; Xiao, J.; Liu, H.; Yao, K.; Hou, X.; Cao, Y.; Liu, X. Astaxanthin attenuates oxidative stress and immune impairment in d-galactose-induced aging in rats by activating the Nrf2/Keap1 pathway and suppressing the NF-κB pathway. Food Funct. 2020, 11, 8099–8111. [Google Scholar] [CrossRef]
- Pongkan, W.; Takatori, O.; Ni, Y.; Xu, L.; Nagata, N.; Chattipakorn, S.C.; Usui, S.; Kaneko, S.; Takamura, M.; Sugiura, M.; et al. β-Cryptoxanthin exerts greater cardioprotective effects on cardiac ischemia-reperfusion injury than astaxanthin by attenuating mitochondrial dysfunction in mice. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef]
- Dose, J.; Matsugo, S.; Yokokawa, H.; Koshida, Y.; Okazaki, S.; Seidel, U.; Eggersdorfer, M.; Rimbach, G.; Esatbeyoglu, T. Free Radical Scavenging and Cellular Antioxidant Properties of Astaxanthin. Int. J. Mol. Sci. 2016, 17, 103. [Google Scholar] [CrossRef] [PubMed]
- Mohammadpourfard, I.; Khanjari, A.; Basti, A.A.; Herrero-Latorre, C.; Shariatifar, N.; Hosseini, H. Evaluation of microbiological, chemical, and sensory properties of cooked probiotic sausages containing different concentrations of astaxanthin, thymol, and nitrite. Food Sci. Nutr. 2021, 9, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Spiller, G.A.; Dewell, A. Safety of an Astaxanthin-RichHaematococcus pluvialisAlgal Extract: A Randomized Clinical Trial. J. Med. Food 2003, 6, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Liebler, D.C. Antioxidant Reactions of Carotenoids. Ann. N. Y. Acad. Sci. 1993, 691, 20–31. [Google Scholar] [CrossRef]
- Gastineau, R.; Turcotte, F.; Pouvreau, J.-B.; Morançais, M.; Fleurence, J.; Windarto, E.; Prasetiya, F.S.; Arsad, S.; Jaouen, P.; Babin, M.; et al. Marennine, Promising Blue Pigments from a Widespread Haslea Diatom Species Complex. Mar. Drugs 2014, 12, 3161–3189. [Google Scholar] [CrossRef] [Green Version]
- Lauritano, C.; Andersen, J.H.; Hansen, E.; Albrigtsen, M.; Escalera, L.; Esposito, F.; Helland, K.; Hanssen, K.Ø.; Romano, G.; Ianora, A. Bioactivity Screening of Microalgae for Antioxidant, Anti-Inflammatory, Anticancer, Anti-Diabetes, and Antibacterial Activities. Front. Mar. Sci. 2016, 3. [Google Scholar] [CrossRef] [Green Version]
- Miranda, M.; Cintra, R.; Barros, S.; Mancini-Filho, J. Antioxidant activity of the microalga Spirulina maxima. Braz. J. Med. Biol. Res. 1998, 31, 1075–1079. [Google Scholar] [CrossRef] [PubMed]
- Elshobary, M.E.; El-Shenody, R.A.; Ashour, M.; Zabed, H.M.; Qi, X. Antimicrobial and antioxidant characterization of bioactive components from Chlorococcum minutum. Food Biosci. 2020, 35, 100567. [Google Scholar] [CrossRef]
- M, P.; Y, S.; B, M.V. Response of Anaerobic Protozoa to Oxygen Tension in Anaerobic System. Int. Microbiol. 2019, 22, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Mastronicola, D.; Falabella, M.; Forte, E.; Testa, F.; Sarti, P.; Giuffrè, A. Antioxidant defence systems in the protozoan pathogen Giardia intestinalis. Mol. Biochem. Parasitol. 2016, 206, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Joardar, N.; Babu, S.P.S. A review on the druggability of a thiol-based enzymatic antioxidant thioredoxin reductase for treating filariasis and other parasitic infections. Int. J. Biol. Macromol. 2020, 142, 125–141. [Google Scholar] [CrossRef] [PubMed]
- Jeelani, G.; Nozaki, T. Oxidative Stress and Antioxidant Defense Mechanism in the Human Enteric Protozoan Parasite Entamoeba histolytica. In Oxidative Stress in Microbial Diseases; Springer International Publishing: Singapore, 2019; pp. 209–227. [Google Scholar]
| Biomolecule | Microorganism | Structure | Chemical Nature | Molecular Weight | Chemical Formulae | Bioactivity | References |
|---|---|---|---|---|---|---|---|
| Bacteria | |||||||
| Astaxanthin | Agrobacterium aurantiacum, Paracoccus carotinifaciens | 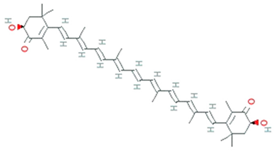 | carotenoid | 596.8 g/mol | C40H52O4 | ROS/RNS, single- and 2-electron oxidants quencher, scavenger of free radicals | [30] |
| Canthaxanthin | Bradyrhizobium sp. Lactobacillus pluvalis |  | terpenoids | 564.8 g/mol | C40H52O2 | increased resistance to lipid peroxidation | [31] |
| Phycocyanin | Pseudomonas sp. | 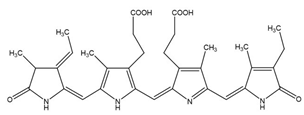 | phycobiliprotein | 30,000 Da | C165H185N20O30 | Cytotoxicity, Neutrophil apoptosis | [32] |
| Zeaxanthin | Staphylococcus aureus, Flavobacterium sp., Paracoccus zeaxanthinifaciens, Sphingobacterium multivorum |  | dihydroxy-carotenoid | 568.88 Da | C40H56O2 | protect cell membranes against oxidative damage | [33] |
| Violacein | Janthinobacterium lividum, Pseudoalteromonas tunicate, Pseudoalteromonas sp., Chromobacterium violaceum | 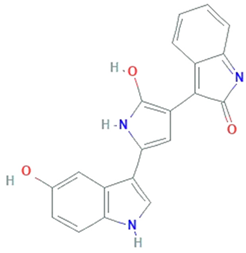 | alkaloid | 343.3 g/mol | C20H13N3O3 | Protect against lipid peroxidation | [34] |
| Granadaene | Streptococcus agalactiae | 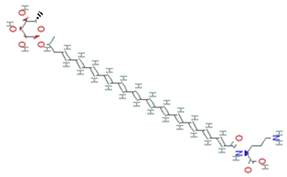 | isoprenoid polyene | 676.8g/mol | C39H52N2O8 | ROS detoxification | [35] |
| Staphyloxanthin | Staphylococcus aureus | 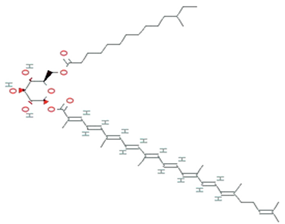 | carotenoid | 819.2 g/mol | C51H78O8 | ROS detoxification | [36] |
| Cyanobacteria | |||||||
| Phycocyanin | Geitlerinema sp. TRV57, spirulina platensis | - | phycobiliprotein | 30,000 Da | C165H185N20O30 | H2O2 scavenging activity | [37,38] |
| Fungi | |||||||
| Canthaxanthin | Monascus sp. | 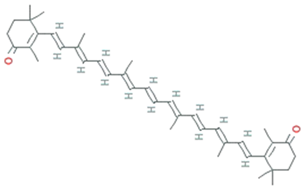 | carotenoid | 564.86 g/mol | C40H52O2 | induction of catalase and superoxide dismutase | [31] |
| β-carotene | Blakeslea trispora, Fusarium sporotrichioides, Mucor, circinelloides, Neurospora crassa, Phycomyces, Blakesleeanus | 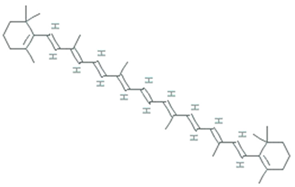 | carotenoid | 536.888 g/mol | C40H56 | Antioxidant | [39] |
| Lycopene | Fusarium Sporotrichioides, Blakeslea trispora | 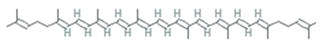 | carotenoid | 536.888 g/mol | C40H56 | singlet oxygen-quencher | [40] |
| Azaphilones | Talaromyces atroroseus, Penicillium purpurogenum | 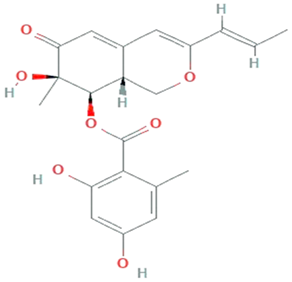 | Polyketides | 386.4 g/mol | C21H22O7 | Activate PKS pathway | [41] |
| Xanthomonadin | Xanthomonas oryzae | 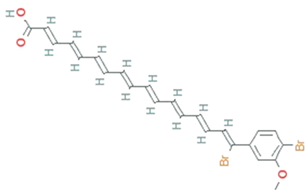 | aryl polyene | 518.2 g/mol | C24H22Br2O3 | photoprotective activity against singlet oxygen analogous | [42] |
| Riboflavin | Ashbya gossypi |  | 7,8-dimethyl-10-(1Υ-D-ribityl) isoalloxazine | 382.32 g/mol | C17H20N4O6 | Inhibit lipid peroxidation | [43] |
| Endophytic fungi | |||||||
| Chaetopyranin | chaetomium globosum, Microsporum sp., Epicoccum sp. | 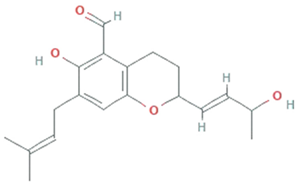 | Benzaldehyde | 316.167459 g/mol | C19H24O4 | ROS scavanger | [44] |
| Flavipin | Chaetomimum sp., Epicoccum nigrum, Aspergillus flavipes and Aspergillus terrus | 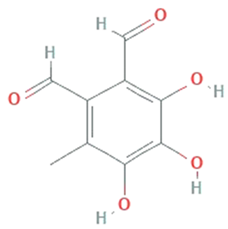 | 3,4,5-Trihydroxy-6-methyl-1,2-benzenedicarbaldehyde;1,2-Benzenedicarboxaldehyde, 3,4,5-trihydroxy-6-methyl | 196.16 g/mol | C9H8O5 | Inhibition of microsomal lipid peroxide | [45] |
| Isopestacin | Pestalotiopsis microspora | 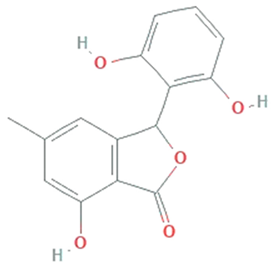 | Isobenzofuranone | 272.25 g/mol | C15H12O5 | scavenge both superoxide and hydroxy free radicals | [46] |
| Pestacin | Pestalotiopsis microspora |  | 1,3-dihydro isobenzofuran | 258.27 g/mol | C15H14O4 | ROS scavenger | [46] |
| Kaempferol | Mucor fragilis, Sinopodophyllum hexandrum | 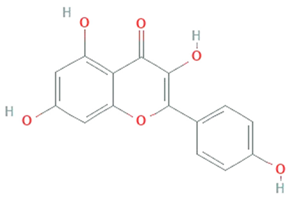 | Tetrahydroxy flavone | 286.24 g/mol | C15H10O6 | ROS scavenger | [47,48] |
| Tyrosol | Diaporthe helianthin, Glomerella cingulata | 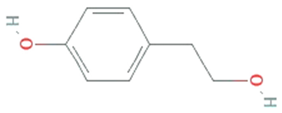 | Phenylated alcohol | 138.164 g/mol | C8H10O2 | scavenging ROS and RNS | [49] |
| Yeast | |||||||
| Melanin | Saccharomyces, Neoformans, Candida albicans, Cryptococcus rajasthanensis | 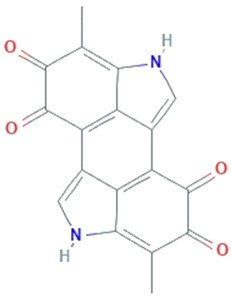 | Natural cutaneous pigment | 318.288 g/mol | C18H10N2O4 | free radical scavenging activity and electron transferring properties | [50] |
| Astaxanthin | Xanthophyllomyces dendrorhous, formerly Phaffia rhodozyma | 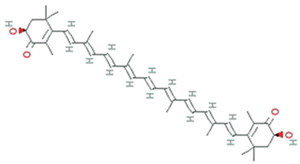 | carotenoid | 596.8 g/mol | C40H52O4 | ROS/RNS, single- and 2-electron oxidants quencher, scavenger of free radicals | |
| Microalgae | |||||||
| Astaxanthin | Haematococcus pluvialis, Acutodesmus obliquus and Coelastrum sp. | 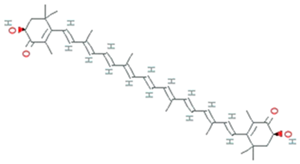 | carotenoid | 596.8 g/mol | C40H52O4 | ROS scavenging and anti-inflammatory properties | [51] |
| β-carotene | Dunaliella Salina, Tetraselmis sp. CTP4, Tetradesmus obliquus | 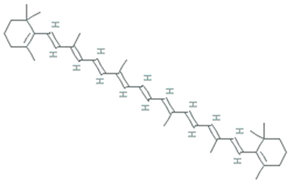 | 536.888 g/mol | C40H56 | Scavenger of lipophilic radicals, inhibits lipid peroxidation | [52] | |
| Phycoerythrin | Porphyridium marinum, P. cruentum and P. purpureum | 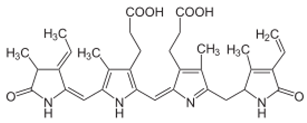 | Natural light-harvesting pigment | ~240,000 Da | - | Activate nuclear factor erythroid 2-related factor 2 (Nrf2)- Superoxide dismutases (SODs) pathways | [53] |
| Lutein | Chlorella sorokiniana, Chlamydomonas sp. | 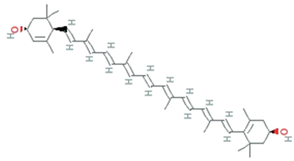 | carotenoid | 568.9g/mol | C40H56O2 | block blue-light damage and quencher oxygen free radicals | [54] |
| Phycocyanin | Spirulina platensis, Arthrospira platensis | 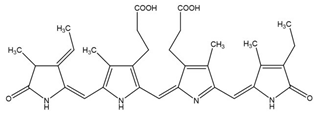 | phycobiliproteins | ~30,000 Da | C165H185N20O30 | Quench ROS | [55] |
| Common Name | Molecular Formulae | Scientific Name | Producers | Mode of Action | References |
|---|---|---|---|---|---|
| Bacterioruberin (BR) | C50H76O4 | 2,2′-bis (3- hydroxy-3-methylbutyl)-3,4,3′,4′-tetradehydro-1,2,1′,2′-tetrahydro-γ,γ-carotene-1,1′-diol | Haloarcula japonica, Halobacterium salinarum, Halorubrum sodomense and Haloarcula vallimortis and Halorubrum sp. TBZ126 | Protection against oxidative stress by arachidonic acid and H2O2 | [71,72] |
| Mono-anhydrobacterioruberin (MABR) | C50H74O3 | 30-(2-hydroxypropan-2-yl)-2,6,10,14,19,23,27,33-octamethyl3-(3-methylbut-2-en-1-yl) tetratriaconta 4,6,8,10,12,14,16,18,20,22,24,26,28-tridecaene-2,33-diol | Haloferax volcanii | ROS scavenging activity | [71,72] |
| Bis-anhydrobacterioruberin (BABR) | C50H72O2 | 2,6,10,14,19,23,27,31-octamethyl3,30-bis (3-methylbut-2- en-1-yl)dotriaconta 4, 6, 8, 10, 12,14,16,18,20,22,24,26,28- tridecaene-2,31-diol | Haloferax volcanii | ROS scavenging activity | [72] |
| Probiotic Bacteria | Pathway | Model Used | Effects | References |
|---|---|---|---|---|
| Probiotic Formulation SLAB51, Bacillus. Longum, Lactobacillus acidophilus | Sirtuin-1 (SIRT1) | Alzheimer’s disease (AD) (3xTg-AD) mice model | Increases antioxidant enzymes activity | [99] |
| L. plantarum LP6 L. plantarum C88 L. rhamnosus GG | PKC (protein kinase C) | Caco-2 cells Caco-2 cell Caco-2 cell | Enhanced activities of SOD and CAT Inhibit malondialdehyde (MDA) formation, raised SOD Ameliorate the oxidative stress-induced disruption of intestinal epithelial tight junction | [100,101] [102] |
| L. brevis SBC8803 | Integrin–p38 MAPK (mitogen activated protein kinase) | Caco2/BBE cells | Induce cytoprotective heat shock proteins (HSP27) maintaining intestinal homeostasis | [103] |
| L. plantarum, L. rhamnosus, Bacillus subtilis JH642 | p38 MAPK | RAW 264.7 cells Caco2bbe cells | Decreases p38, JNK, ERK1/2 phosphorylation Increases p38 phosphorylation | [104] [105] |
| Bacillus amyloliquefaciens SC06 Clostridium butyricum MIYAIRI 588 L. Plantarum FC255 | Nrf2-Keap1-ARE | IPEC-1 cell line rat smice | Increase CAT and glutathione S-transferase (GST) expressions | [106] |
| Activation of ARE-dependent genes i.e., GSTs, and TRX Elevate the activities of SOD and GPX | [107] | |||
| [108] | ||||
| Lactobacillus caseiBacillus LBP32 L. plantarum CLP-0611 | NF-κB | mice RAW 264.7 macrophages mice | Reduce cytokine production Reduce LPS-induced intracellular ROS accumulation Induced expression of M2 macrophage | [109] [110] [111] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rani, A.; Saini, K.C.; Bast, F.; Mehariya, S.; Bhatia, S.K.; Lavecchia, R.; Zuorro, A. Microorganisms: A Potential Source of Bioactive Molecules for Antioxidant Applications. Molecules 2021, 26, 1142. https://doi.org/10.3390/molecules26041142
Rani A, Saini KC, Bast F, Mehariya S, Bhatia SK, Lavecchia R, Zuorro A. Microorganisms: A Potential Source of Bioactive Molecules for Antioxidant Applications. Molecules. 2021; 26(4):1142. https://doi.org/10.3390/molecules26041142
Chicago/Turabian StyleRani, Alka, Khem Chand Saini, Felix Bast, Sanjeet Mehariya, Shashi Kant Bhatia, Roberto Lavecchia, and Antonio Zuorro. 2021. "Microorganisms: A Potential Source of Bioactive Molecules for Antioxidant Applications" Molecules 26, no. 4: 1142. https://doi.org/10.3390/molecules26041142









