MicroRNA-27b-3p Targets the Myostatin Gene to Regulate Myoblast Proliferation and Is Involved in Myoblast Differentiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Experimental Animals and Tissues
2.3. Cell Culture
2.4. Total RNA Extraction and Quality Detection, Complementary DNA (cDNA) Synthesis, and Quantitative Real-Time PCR (qRT-PCR)
2.5. Primers for Quantitative Real-Time PCR (qRT-PCR)
2.6. RNA Oligonucleotides and Plasmids Construction
2.7. Cell Transfection
2.8. Dual-Luciferase Reporter Assay
2.9. CCK-8 Assay
2.10. EdU Assay
2.11. Cell Cycle was Detected by Flow Cytometry
2.12. Immunofluorescence
2.13. Western Blot Assay
2.14. Statistical Analysis
3. Results
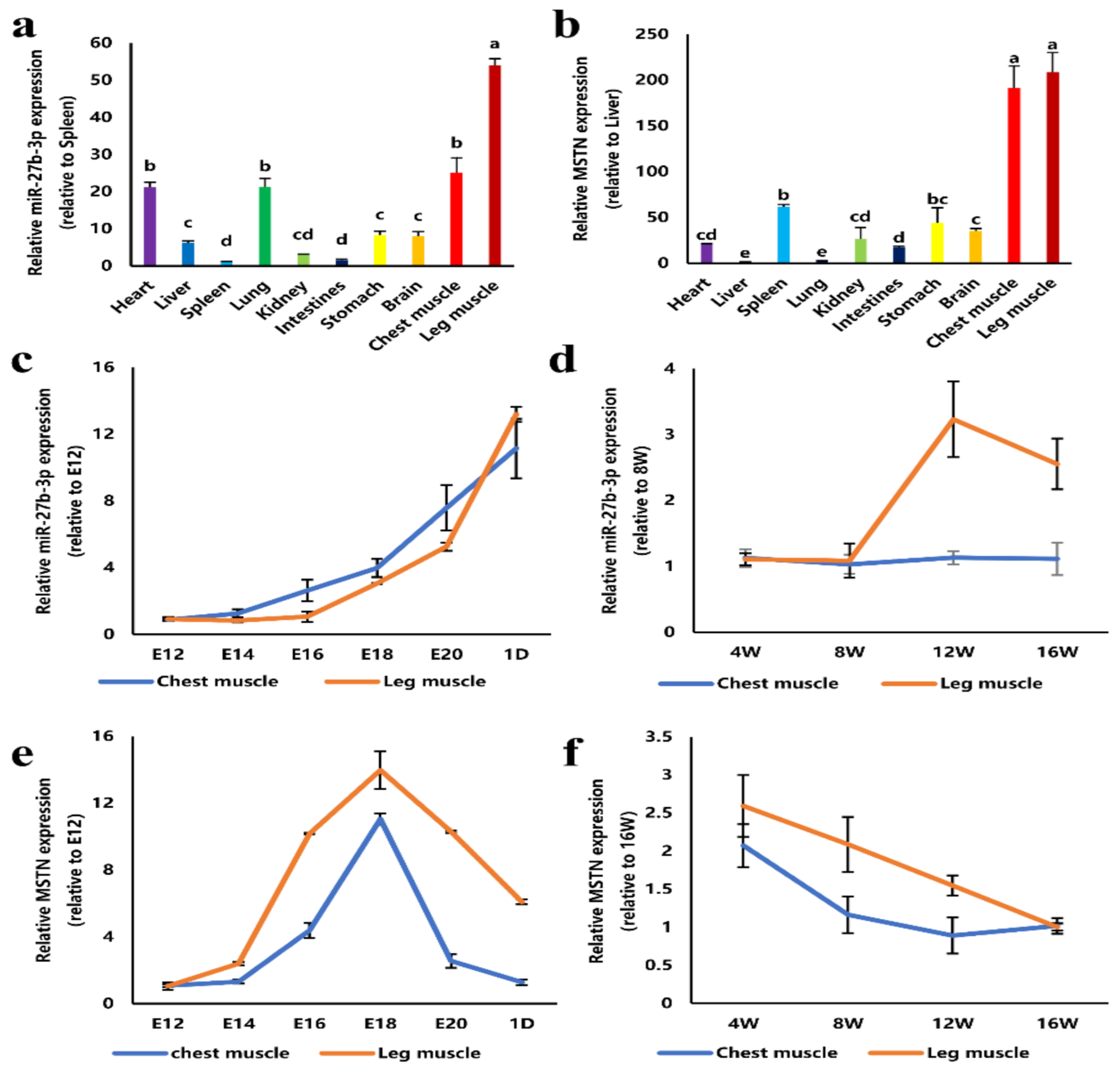
3.1. Expression of miR-27b-3p and MSTN in Tissues of Chicken
3.2. MiR-27b-3p Promotes CPMs Proliferation
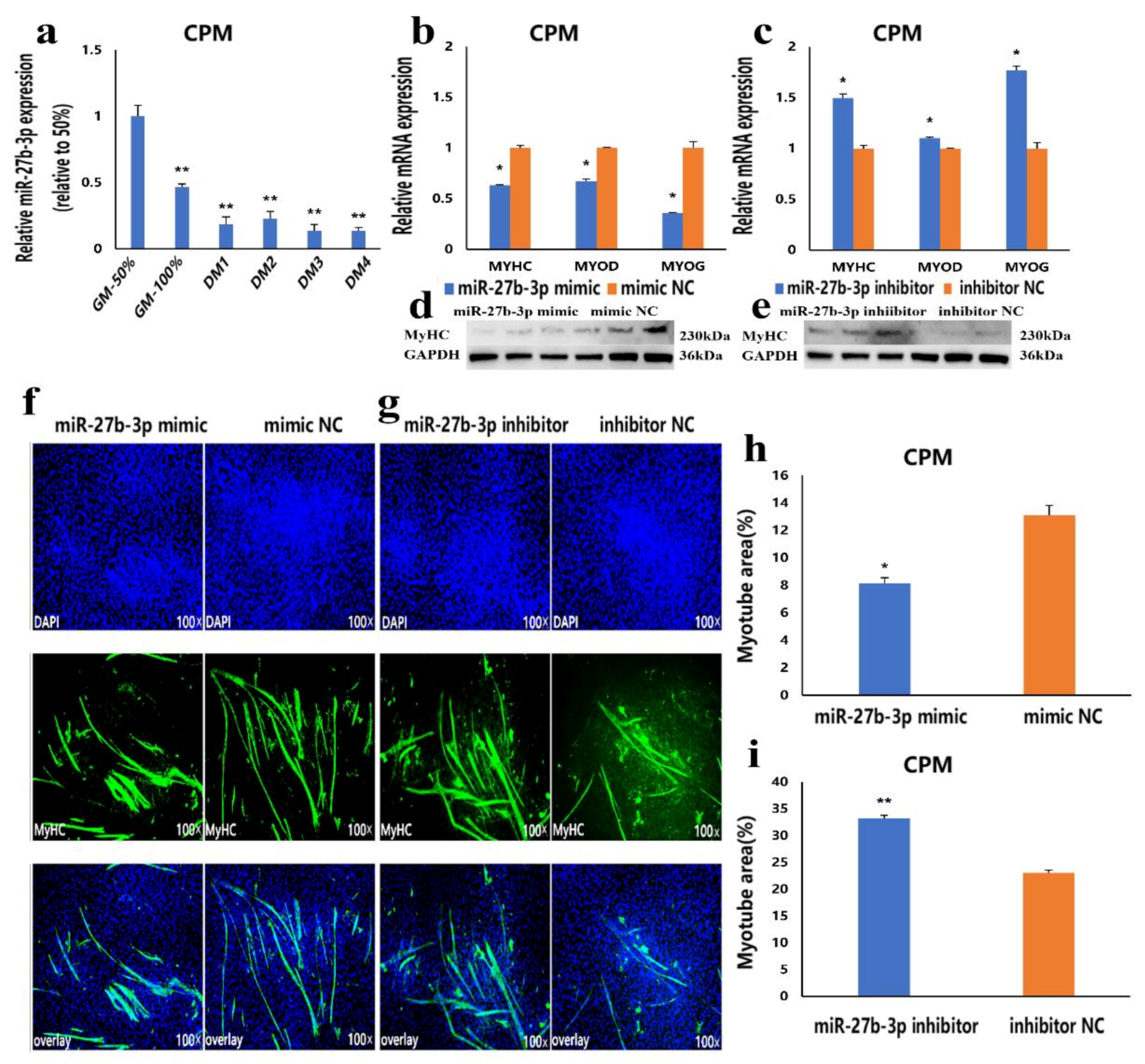
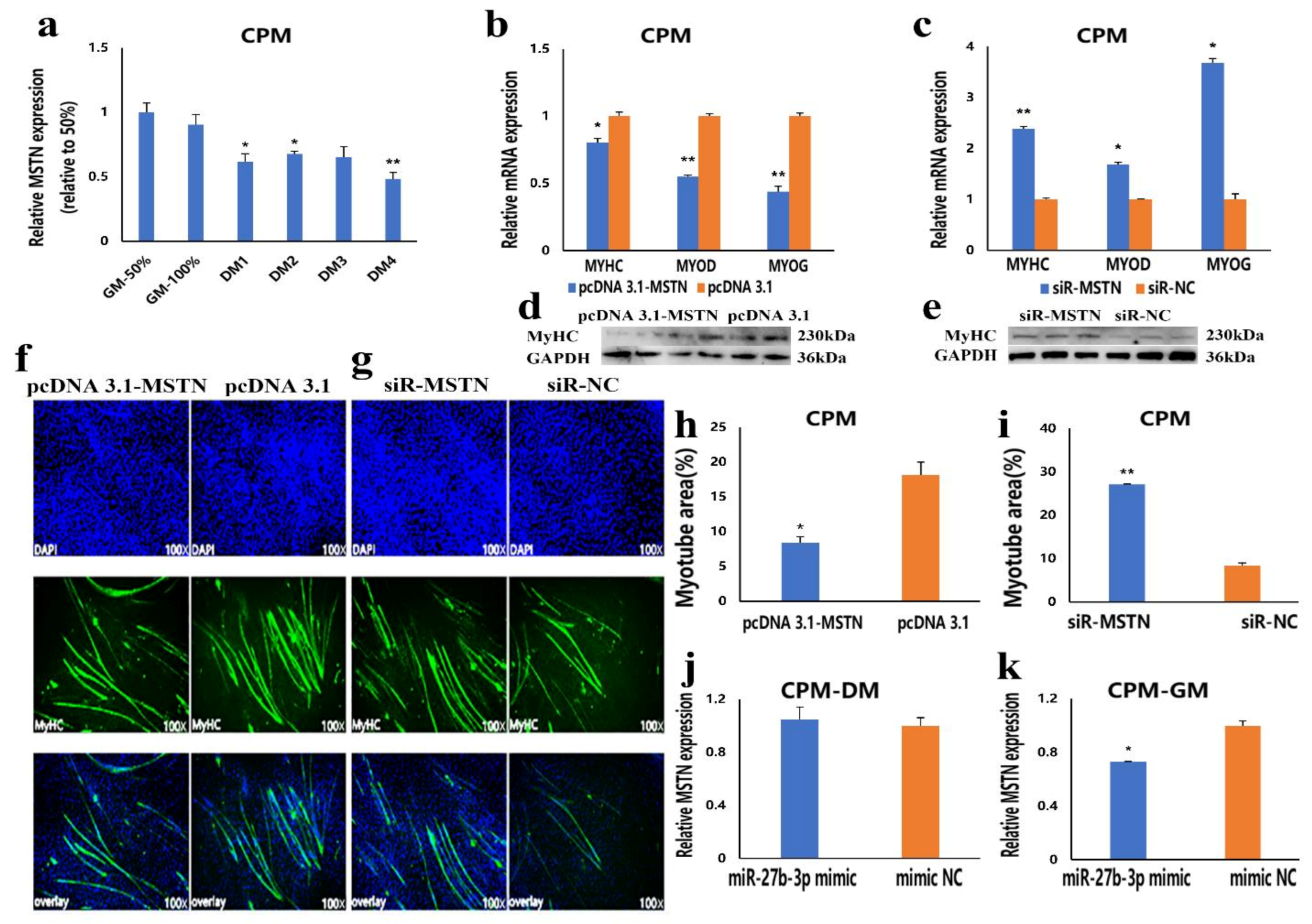
3.3. MiR-27b-3p Inhibits CPMs Differentiation
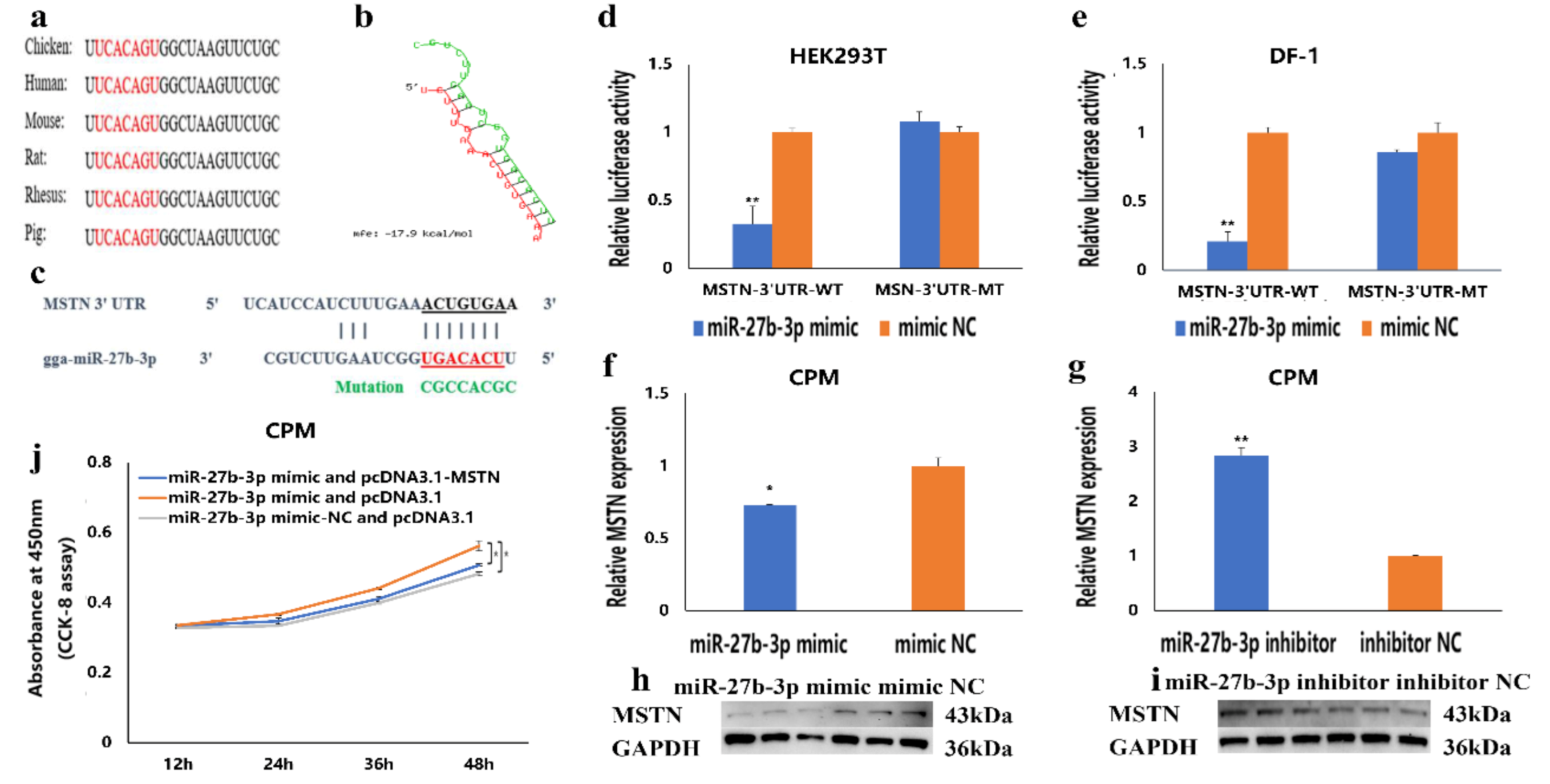
3.4. MSTN Is a Target Gene of miR-27b-3p
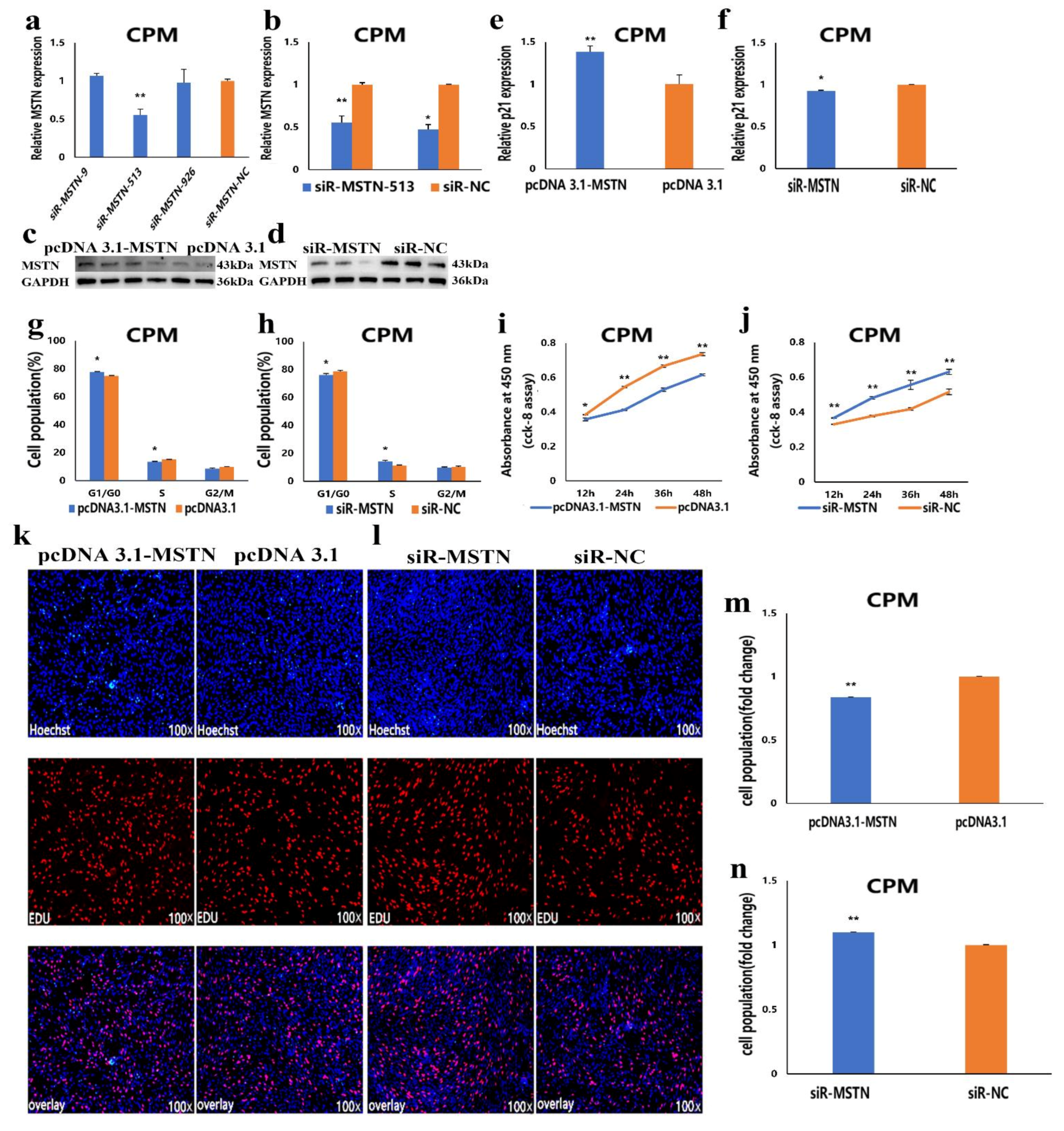
3.5. The Facilitation of miR-27b-3p on CPMs Proliferation was Achieved by Its Target Gene MSTN
3.6. The Inhibition of miR-27b-3p on MSTN Is Different between CPMs Proliferation and Differentiation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lau, N.C.; Lim, L.P.; Weinstein, E.G.; Bartel, D.P. An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science 2001, 294, 858–862. [Google Scholar] [CrossRef]
- Li, X.; Lian, L.; Zhang, D.; Qu, L.; Yang, N. gga-miR-26a targets NEK6 and suppresses Marek’s disease lymphoma cell prolif-eration. Poult. Sci. 2014, 93, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Wahid, F.; Shehzad, A.; Khan, T.; Kim, Y.Y. MicroRNAs: Synthesis, mechanism, function, and recent clinical trials. Biochim. Biophys. Acta BBA Bioenerg. 2010, 1803, 1231–1243. [Google Scholar] [CrossRef]
- Cui, M.; Yao, X.; Lin, Y.; Zhang, D.; Cui, R.; Zhang, X. Interactive functions of microRNAs in the miR-23a-27a-24-2 cluster and the potential for targeted therapy in cancer. J. Cell Physiol. 2020, 235, 6–16. [Google Scholar] [CrossRef]
- Guo, H.; Ingolia, N.T.; Weissman, J.S.; Bartel, D.P. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nat. Cell Biol. 2010, 466, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Wang, Y.; Li, Y.; Cui, L.; Zhao, Y.; Zhao, B.; Li, K. MiR-206, a Key Modulator of Skeletal Muscle Development and Disease. Int. J. Biol. Sci. 2015, 11, 345–352. [Google Scholar] [CrossRef]
- Sempere, L.F.; Freemantle, S.; Pitha-Rowe, I.; Moss, E.; Dmitrovsky, E.; Ambros, V. Expression profiling of mammalian mi-croRNAs uncovers a subset of brain-expressed microRNAs with possible roles in murine and human neuronal differentiation. Genome Biol. 2004, 5, R13. [Google Scholar] [CrossRef]
- Chen, J.F.; Mandel, E.M.; Thomson, J.M.; Wu, Q.; Callis, T.E.; Hammond, S.M.; Conlon, F.L.; Wang, D.Z. The role of mi-croRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat. Genet. 2006, 38, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Wu, H.; Ye, Y.; Li, Z.; Hao, S.; Kong, L.; Zheng, X.; Lin, S.; Nie, Q.; Zhang, X. The transient expression of miR-203 and its inhibiting effects on skeletal muscle cell proliferation and differentiation. Cell Death Dis. 2014, 5, e1347. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Nie, Q.; Zhang, X. MicroRNAs Involved in Skeletal Muscle Differentiation. J. Genet. Genom. 2013, 40, 107–116. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, G.; Zhang, B.; Liu, C.; Yu, Y.; Jin, Y. miR-27b-3p suppresses cell proliferation, migration and invasion by tar-geting LIMK1 in colorectal cancer. Int. J. Clin. Exp. Pathol. 2017, 10, 9251–9261. [Google Scholar]
- Allen, D.L.; Loh, A.S. Posttranscriptional mechanisms involving microRNA-27a and b contribute to fast-specific and gluco-corticoid-mediated myostatin expression in skeletal muscle. Am. J. Physiol. Cell Physiol. 2011, 300, C124–C137. [Google Scholar] [CrossRef] [PubMed]
- Lui, W.-O.; Pourmand, N.; Patterson, B.K.; Fire, A. Patterns of Known and Novel Small RNAs in Human Cervical Cancer. Cancer Res. 2007, 67, 6031–6043. [Google Scholar] [CrossRef]
- Michael, M.Z.; Connor, S.M.O.; Pellekaan, N.G.V.H.; Young, G.P.; James, R.J. Reduced accumulation of specific microRNAs in colorectal neoplasia. Mol. Cancer Res. 2003, 1, 882–891. [Google Scholar] [PubMed]
- Tao, J.; Zhi, X.; Zhang, X.; Fu, M.; Huang, H.; Fan, Y.; Guan, W.; Zou, C. miR-27b-3p suppresses cell proliferation through targeting receptor tyrosine kinase like orphan receptor 1 in gastric cancer. J. Exp. Clin. Cancer Res. 2015, 34, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Tang, Y.; Liu, B.; Cong, W.; Liu, C.; Xiao, J. Retinoid acid-induced microRNA-27b-3p impairs C2C12 myoblast prolifer-ation and differentiation by suppressing alpha-dystrobrevin. Exp. Cell Res. 2017, 350, 301–311. [Google Scholar] [CrossRef]
- Langley, B.; Thomas, M.; Bishop, A.; Sharma, M.; Gilmour, S.; Kambadur, R. Myostatin Inhibits Myoblast Differentiation by Down-regulating MyoD Expression. J. Biol. Chem. 2002, 277, 49831–49840. [Google Scholar] [CrossRef]
- McPherron, A.C.; Lee, S.-J. Double muscling in cattle due to mutations in the myostatin gene. Proc. Natl. Acad. Sci. USA 1997, 94, 12457–12461. [Google Scholar] [CrossRef]
- Mott, I.; Ivarie, R. Expression of myostatin is not altered in lines of poultry exhibiting myofiber hyper- and hypoplasia. Poult. Sci. 2002, 81, 799–804. [Google Scholar] [CrossRef]
- Ji, S.; Losinski, R.L.; Cornelius, S.G.; Frank, G.R.; Willis, G.M.; Gerrard, D.E.; Depreux, F.F.S.; Spurlock, M.E. Myostatin expression in porcine tissues: Tissue specificity and developmental and postnatal regulation. Am. J. Physiol. Content 1998, 275, R1265–R1273. [Google Scholar] [CrossRef] [PubMed]
- McFarland, D.C.; Velleman, S.G.; Pesall, J.E.; Liu, C. The role of myostatin in chicken (Gallus domesticus) myogenic satellite cell proliferation and differentiation. Gen. Comp. Endocrinol. 2007, 151, 351–357. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.; Ma, M.; Chen, B.; Li, Z.; Abdalla, B.A.; Nie, Q.; Zhang, X. MiR-16-5p targets SESN1 to regulate the p53 signaling pathway, affecting myoblast proliferation and apoptosis, and is involved in myoblast differentiation. Cell Death Dis. 2018, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Darnell, D.K.; Kaur, S.; Stanislaw, S.; Konieczka, J.K.; Yatskievych, T.A.; Antin, P.B. MicroRNA expression during chick embryo development. Dev. Dyn. 2006, 235, 3156–3165. [Google Scholar] [CrossRef]
- Lie, S.; Morrison, J.L.; Williams-Wyss, O.; Suter, C.M.; Humphreys, D.T.; Ozanne, S.E.; Zhang, S.; MacLaughlin, S.M.; Kleemann, D.O.; Walker, S.K. Impact of periconceptional and preimplantation undernutrition on factors regulating myo-genesis and protein synthesis in muscle of singleton and twin fetal sheep. Physiol. Rep. 2015, 3, e12495. [Google Scholar] [CrossRef]
- Li, W.; Chang, N.; Tian, L.; Yang, J.; Ji, X.; Xie, J.; Yang, L.; Li, L. miR-27b-3p, miR-181a-1-3p, and miR-326-5p are involved in the inhibition of macrophage activation in chronic liver injury. J. Mol. Med. 2017, 95, 1091–1105. [Google Scholar] [CrossRef]
- Cheng, X.; Kan, P.; Ma, Z.; Wang, Y.; Song, W.; Huang, C.; Zhang, B. Exploring the potential value of miR-148b-3p, miR-151b and miR-27b-3p as biomarkers in acute ischemic stroke. Biosci. Rep. 2018, 38, 38. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhang, G.; Wu, P.; Duan, L.; Li, G.; Liu, Q.; Wang, J. Dissection of Myogenic Differentiation Signatures in Chickens by RNA-Seq Analysis. Genes 2018, 9, 34. [Google Scholar] [CrossRef]
- Gu, S.; Jin, L.; Zhang, F.; Sarnow, P.; Kay, M.A. Biological basis for restriction of microRNA targets to the 3′ untranslated region in mammalian mRNAs. Nat. Struct. Mol. Biol. 2009, 16, 144–150. [Google Scholar] [CrossRef]
- Yin, H.; He, H.; Shen, X.; Zhao, J.; Cao, X.; Han, S.; Cui, C.; Chen, Y.; Wei, Y.; Xia, L.; et al. miR-9-5p Inhibits Skeletal Muscle Satellite Cell Proliferation and Differentiation by Targeting IGF2BP3 through the IGF2-PI3K/Akt Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 1655. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, T.; Cao, Y.-W.; Ding, X.-Q. Antitumor effect of miR-27b-3p on lung cancer cells via targeting Fzd7. Eur. Rev. Med Pharmacol. Sci. 2017, 21, 4113–4123. [Google Scholar] [PubMed]
- Chen, D.; Si, W.; Shen, J.; Du, C.; Lou, W.; Bao, C.; Zheng, H.; Pan, J.; Zhong, G.; Xu, L.; et al. miR-27b-3p inhibits proliferation and potentially reverses multi-chemoresistance by targeting CBLB/GRB2 in breast cancer cells. Cell Death Dis. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Zhang, C.; Zou, Y.; Dai, D.-Q. Downregulation of microRNA-27b-3p via aberrant DNA methylation contributes to malignant behavior of gastric cancer cells by targeting GSPT1. Biomed. Pharmacother. 2019, 119, 109417. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Chen, F.; Wu, P.; Li, T.; He, M.; Yin, X.; Shi, H.; Duan, Y.; Zhang, T.; Wang, J.; et al. MicroRNA-7 Targets the KLF4 Gene to Regulate the Proliferation and Differentiation of Chicken Primary Myoblasts. Front. Genet. 2020, 11, 842. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Yulin, B.; Tang, B.; Wang, M.; Zhang, C.; Zhang, W.; Jin, J.; Li, T.; Zhao, R.; et al. CRISPR/Cas9-mediated sheep MSTN gene knockout and promote sSMSCs differentiation. J. Cell. Biochem. 2019, 120, 1794–1806. [Google Scholar] [CrossRef]
- Taylor, W.E.; Bhasin, S.; Artaza, J.; Byhower, F.; Azam, M.; Willard, D.H., Jr.; Kull, F.C., Jr.; Gonzalez-Cadavid, N. Myostatin inhibits cell proliferation and protein synthesis in C2C12 muscle cells. Am. J. Physiol. Metab. 2001, 280, E221–E228. [Google Scholar] [CrossRef]
- Ríos, R.; Carneiro, I.; Arce, V.M.; Devesa, J.; Rios, R. Myostatin is an inhibitor of myogenic differentiation. Am. J. Physiol. Physiol. 2002, 282, C993–C999. [Google Scholar] [CrossRef]
- Berkes, C.A.; Tapscott, S.J. MyoD and the transcriptional control of myogenesis. Semin. Cell Dev. Biol. 2005, 16, 585–595. [Google Scholar] [CrossRef]
- Edmondson, D.G.; Olson, E.N. A gene with homology to the myc similarity region of MyoD1 is expressed during myogenesis and is sufficient to activate the muscle differentiation program. Genes Dev. 1990, 4, 1450. [Google Scholar] [CrossRef]
- Toniolo, L.; Maccatrozzo, L.; Patruno, M.; Pavan, E.; Caliaro, F.; Rossi, R.; Rinaldi, C.; Canepari, M.; Reggiani, C.; Mascarello, F. Fiber types in canine muscles: Myosin isoform expression and functional characterization. Am. J. Physiol. Physiol. 2007, 292, C1915–C1926. [Google Scholar] [CrossRef] [PubMed]
- Brennecke, J.; Stark, A.; Russell, R.B.; Cohen, S.M. Principles of microRNA-target recognition. PLoS Biol. 2005, 3, e85. [Google Scholar] [CrossRef] [PubMed]

| Gene | Primer Sequence (5′-3′) | Annealing Temperature (°C) |
|---|---|---|
| Gga-miR-27b-3p | F:GCGCGTTCACAGTGGCTAAG | 60 |
| R:AGTGCAGGGTCCGAGGTATT | ||
| Stem-loop primer | GTCGTATCCAGTGCAGGGTCCGAGG | |
| TATTCGCACTGGATACGACGCAGAA | ||
| U6 | F:GTCACTTCTGGTGGCGGTAA | 60 |
| R:GTTCAGTAGAGGGTCAAA |
| Gene | Primer Sequence (5′-3′) | Product Size (bp) | Annealing Temperature (°C) | Accession Number |
|---|---|---|---|---|
| MSTN | F:GCTTTTACCCAAAGCTCCTCCAC | 179 | 60 | NM_001001461.1 |
| R:AGCAACATTTTGGTTTTCCCTCC | ||||
| P21 | F:GAGATGCTGAAGGAGATCAATGAG | 102 | 60 | NM_204396.1 |
| R:GTGGTCAGTCCGAGCCTTTT | ||||
| MYOD1 | F:GCTACTACACGGAATCACCAAAT | 200 | 60 | NM_204214.2 |
| R:CTGGGCTCCACTGTCACTCA | ||||
| MYHC | F:CTCCTCACGCTTTGGTAA | 213 | 60 | NM_001319304.1 |
| MYOG | R:TGATAGTCGTATGGGTTGGT | 320 | 60 | NM_204184.1 |
| F:CGGAGGCTGAAGAAGGTGAA | ||||
| R:CGGTCCTCTGCCTGGTCAT | ||||
| β-actin | F:CAGCCATCTTTCTTGGGTAT | 169 | 60 | NM_205518.1 |
| R:CTGTGATCTCCTTCTGCATCC |
| Fragment Name | Sequence (5′-3′) |
|---|---|
| miR-27b-3p mimic | UUCACAGUGGCUAAGUUCUGC |
| AGAACUUAGCCACUGUGAAUU | |
| mimic-NC | UUCUCCGAACGUGUCACGUTT |
| ACGUGACACGUUCGGAGAATT | |
| miR-27b-3p inhibitor | GCAGAACUUAGCCACUGUGAA |
| inhibitor-NC | CAGUACUUUUGUGUAGUACAA |
| siR-MSTN | GCAGAUCCUGAGACUCAUUTT |
| AAUGAGUCUCAGGAUCUGCTT | |
| siR-NC | UUCUCCGAACGUGUCACGUTT |
| ACGUGACACGUUCGGAGAATT |
| Primer Name | Primer Sequence (5′-3′) | Product Size (bp) | Annealing Temperature (°C) |
|---|---|---|---|
| MSTN-3′ UTR-WT | F:AAAGATCCTTTATTAAGCTTCTGTCGTGAGATCCACCATT | 298 | 62 |
| R:ATAGGCCGGCATAGACGCGTCCCATTTGTTAAATCCGGTG | |||
| MSTN-3′ UTR-MT | F:TTGAACGCCACGCATTACGTACGCTAGGCATTGCC | 6742 | 68 |
| R: GTAATGCGTGGCGTTCAAAGATGGATGAGGGGATATAG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; He, M.; Wu, P.; Zhang, X.; Zhou, K.; Li, T.; Zhang, T.; Xie, K.; Dai, G.; Wang, J. MicroRNA-27b-3p Targets the Myostatin Gene to Regulate Myoblast Proliferation and Is Involved in Myoblast Differentiation. Cells 2021, 10, 423. https://doi.org/10.3390/cells10020423
Zhang G, He M, Wu P, Zhang X, Zhou K, Li T, Zhang T, Xie K, Dai G, Wang J. MicroRNA-27b-3p Targets the Myostatin Gene to Regulate Myoblast Proliferation and Is Involved in Myoblast Differentiation. Cells. 2021; 10(2):423. https://doi.org/10.3390/cells10020423
Chicago/Turabian StyleZhang, Genxi, Mingliang He, Pengfei Wu, Xinchao Zhang, Kaizhi Zhou, Tingting Li, Tao Zhang, Kaizhou Xie, Guojun Dai, and Jinyu Wang. 2021. "MicroRNA-27b-3p Targets the Myostatin Gene to Regulate Myoblast Proliferation and Is Involved in Myoblast Differentiation" Cells 10, no. 2: 423. https://doi.org/10.3390/cells10020423
APA StyleZhang, G., He, M., Wu, P., Zhang, X., Zhou, K., Li, T., Zhang, T., Xie, K., Dai, G., & Wang, J. (2021). MicroRNA-27b-3p Targets the Myostatin Gene to Regulate Myoblast Proliferation and Is Involved in Myoblast Differentiation. Cells, 10(2), 423. https://doi.org/10.3390/cells10020423






