Time-Dependent Changes in Performance, Biochemistry, and Histology in Dairy Calves with Acute Aflatoxicosis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Herd Management
2.2. Aflatoxins Production and Quantification
2.3. Biochemical and Pathological Analyses
2.4. Statistical Analysis
3. Results
3.1. Animal Performance
3.2. Biochemistry
3.3. Tissue Biochemistry
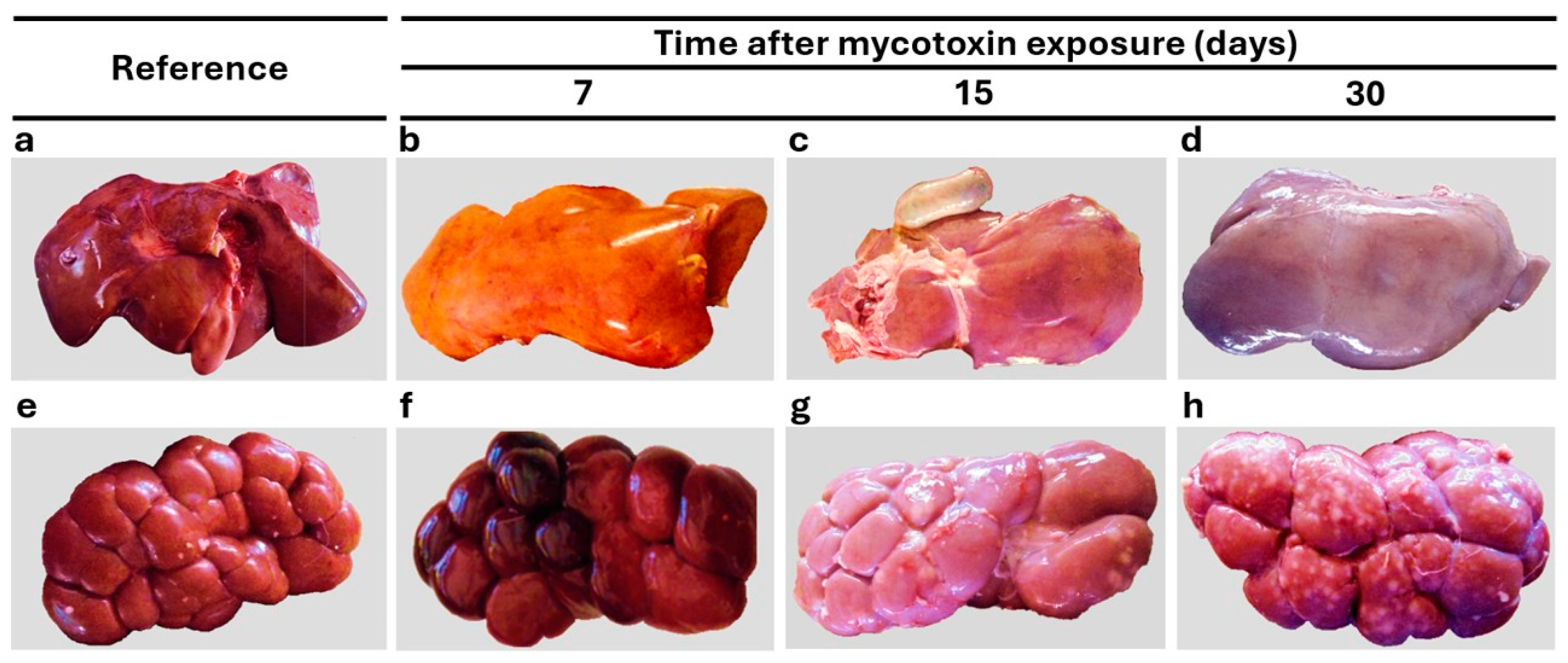
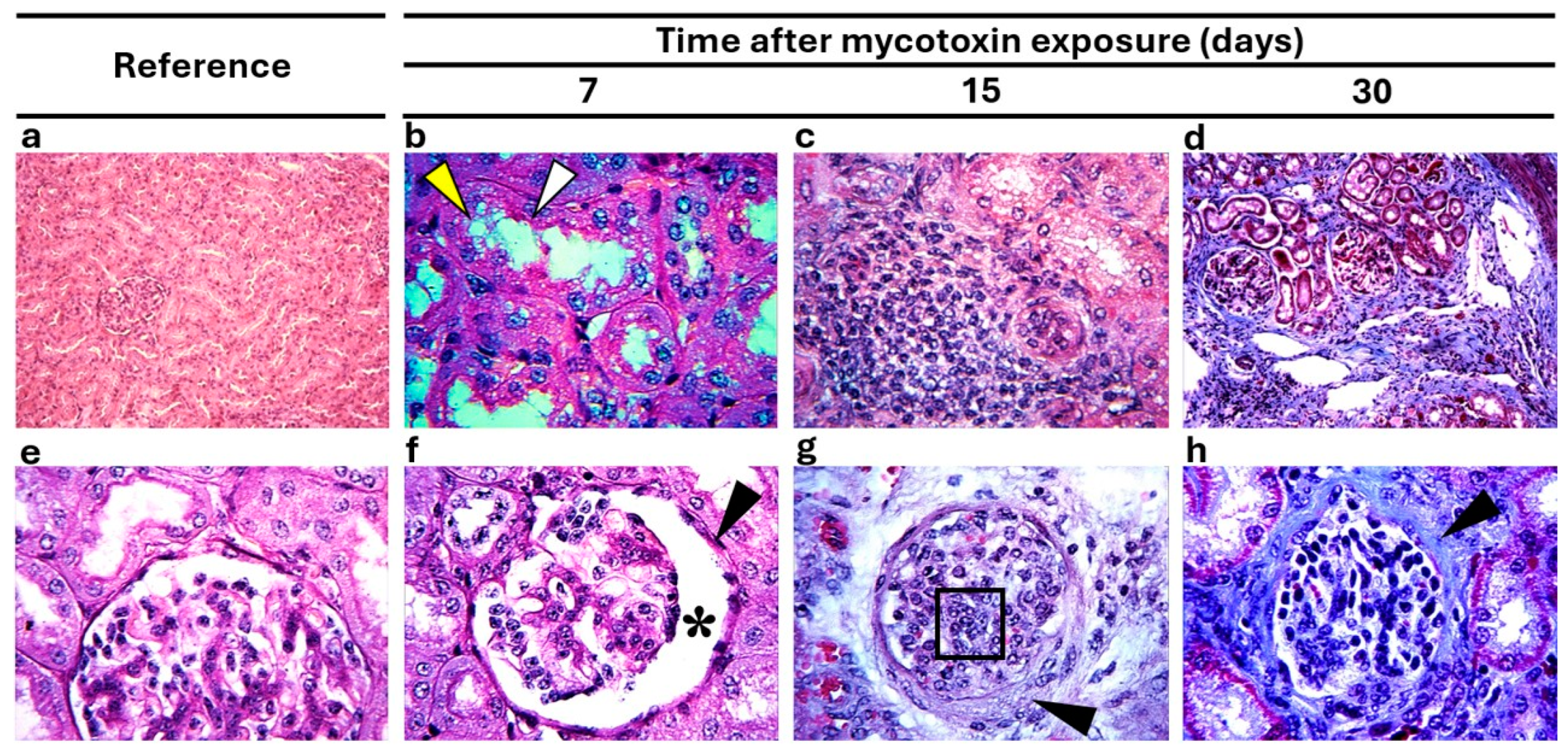
3.4. Morphological Findings
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Luna-López, M.C.; Valdivia-Flores, A.G.; Jaramillo-Juárez, F.; Reyes, J.L.; Ortiz-Martínez, R.; Quezada-Tristán, T. Association between Aspergillus flavus colonization and aflatoxins production in immature grains of maize genotypes. J. Food Sci. Eng. 2013, 12, 688–698. [Google Scholar] [CrossRef]
- Ajmal, M.; Bedale, W.; Akram, A.; Yu, J.-H. Comprehensive review of aflatoxin contamination, impact on health and food security, and management strategies in Pakistan. Toxins 2022, 14, 845. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Cao, S.; Wan, S.F.; Chen, Z.; Saleemi, M.K.; Wang, N.; Naseem, M.N.; Munawar, J. Mycotoxins—A global one health concern: A review. Agrobiol. Rec. 2020, 2, 1–16. [Google Scholar] [CrossRef]
- Guo, W.; Fan, Z.; Fan, K.; Meng, J.; Nie, D.; Tangni, E.K.; Li, Z.; Zhao, Z.; Han, Z. In vivo kinetics and biotransformation of aflatoxin B1 in dairy cows based on the establishment of a reliable UHPLCMS/MS method. Front. Chem. 2021, 9, 809480. [Google Scholar] [CrossRef]
- Board, P.G.; Menon, D. Glutathione transferases, regulators of cellular metabolism and physiology. BBA Gen. Subj. 2013, 1830, 3267–3288. [Google Scholar] [CrossRef] [PubMed]
- Diaz, D.E.; Hagler, W.M., Jr.; Blackwelder, J.T.; Eve, J.A.; Hopkins, B.A.; Anderson, K.L.; Jones, F.T.; Whitlow, L.W. Aflatoxin binders II: Reduction of aflatoxin M1 in milk by sequestering agents of cows consuming aflatoxin in feed. Mycopathologia 2004, 157, 233–241. [Google Scholar] [CrossRef]
- Rangel-Muñoz, E.J.; Valdivia-Flores, A.G.; Moreno-Rico, O.; Hernández-Delgado, S.; Cruz-Vázquez, C.; de-Luna-López, M.C.; Quezada-Tristán, T.; Ortiz-Martínez, R.; Máyek-Pérez, N. Characterization of Aspergillus flavus and quantification of aflatoxins in feed and raw milk of cows in Aguascalientes, Mexico. Rev. Mex. Cienc. Pecu. 2020, 11, 435–454. [Google Scholar] [CrossRef]
- Zentai, A.; Jóźwiak, Á.; Süth, M.; Farkas, Z. Carry-over of aflatoxin B1 from feed to cow milk-a review. Toxins 2023, 15, 195. [Google Scholar] [CrossRef] [PubMed]
- Obremski, K.; Zielonka, Ł.; Gajęcka, M.; Jakimiuk, E.; Gajęcki, M. Mycotoxins-dairy cattle breeding problem. A case report. Bull. Vet. Inst. Pulawy. 2009, 53, 221–224. Available online: https://api.semanticscholar.org/CorpusID:56349162 (accessed on 29 January 2025).
- Tangni, E.K.; Pussemier, L.; Van Hove, F. Mycotoxin contaminating maize and grass silages for dairy cattle feeding: Current state and challenges. J. Anim. Sci. Adv. 2013, 3, 492–511. [Google Scholar]
- Fink-Gremmels, J. The role of mycotoxins in the health and performance of dairy cows. Vet. J. 2008, 176, 84–92. [Google Scholar] [CrossRef]
- Kaleibar, M.T.; Helan, J. A field outbreak of aflatoxicosis with high fatality rate in feedlot calves in Iran. Comp. Clin. Pathol. 2013, 22, 1155–1163. [Google Scholar] [CrossRef]
- Pierezan, F.; Oliveira-Filho, J.C.; Carmo, P.M.; Aires, A.R.; Leal, M.L.R.; Souza, T.M.; Mallmann, C.A.; Barros, C.S.L. Intoxicação experimental por aflatoxina em bezerros. Pesqui. Veterinária Bras. 2012, 32, 607–618. [Google Scholar] [CrossRef]
- Hernandez-Valdivia, E.; Valdivia-Flores, A.G.; Cruz-Vazquez, C.; Martinez-Saldaña, M.C.; Quezada-Tristan, T.; Rangel-Muñoz, E.J.; Ortiz-Martinez, R.; Medina-Esparza, L.E.; Jaramillo-Juarez, F. Diagnosis of subclinical aflatoxicosis by biochemical changes in dairy cows under field conditions. Pak. Vet. J. 2021, 41, 33–38. [Google Scholar] [CrossRef]
- Álvarez-Días, F.; Torres-Parga, B.; Valdivia-Flores, A.G.; Quezada-Tristán, T.; Alejos-De La Fuente, J.I.; Sosa-Ramírez, J.; Rangel-Muñoz, E.J. Aspergillus flavus and total aflatoxins occurrence in dairy feed and aflatoxin M1 in bovine milk in Aguascalientes, Mexico. Toxins 2022, 14, 292. [Google Scholar] [CrossRef]
- González-Padilla, E.; Lassala, A.; Pedernera, M.; Gutiérrez, C.G. Cow-calf management practices in Mexico: Farm organization and infrastructure. Vet. Méx. 2019, 6, 1–17. [Google Scholar] [CrossRef]
- Trucksess, M.W. Natural toxins. In Official Methods of Analysis of AOAC International, 17th ed.; Horwitz, W., Ed.; AOAC International: Gaithersburg, MD, USA, 2000; Volume 2, pp. 1–64. Available online: https://scholar.google.com/scholar_lookup?title=Official%20Methods%20of%20Analysis&author=M.W.%20Trucksess&publication_year=2000& (accessed on 2 January 2025).
- Hissin, P.J.; Hilf, R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal. Biochem. 1976, 74, 214–226. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Diario Oficial de la Federación. NOM-033-SAG/ZOO-2014 Mexican Official Standard, Methods of Slaughtering Domestic and Wild Animals. 26 August 2015. Available online: https://dof.gob.mx/nota_detalle.php?codigo=5405210&fecha=26/08/2015#gsc.tab=0 (accessed on 2 January 2025).
- Umar, S.; Munir, M.T.; Shah, M.A.; Shahzad, M.; Sohoo, M.-U.-R.; Khan, R.A.; Khan, A.U.; Ameen, K.; Rafia-Munir, A.; Saleem, F. Outbreak of aflatoxicosis on a local cattle farm in Pakistan. Veterinaria 2015, 3, 13–17. Available online: https://hal.science/hal-03160934v1 (accessed on 15 January 2025).
- Elgioushy, M.M.; Elgaml, S.A.; El-Adl, M.M.; Hegazy, A.M.; Hashish, E.A. Aflatoxicosis in cattle: Clinical findings and biochemical alterations. Environ. Sci. Pollut. Res. 2020, 27, 35526–35534. [Google Scholar] [CrossRef]
- Hamali, H.; Ashrafi-Helan, J.; Khordadmehr, M. Natural aflatoxicosis in neonatal calves in a dairy herd–pathological diagnosis. Bulg. J. Vet. Med. 2021, 24, 291–296. [Google Scholar] [CrossRef]
- Allameh, A.; Safamehr, A.; Mirhadi, S.A.; Shivazad, M.; Razzaghi-Abyaneh, M.; Afshar-Naderi, A. Evaluation of biochemical and production parameters of broiler chicks fed ammonia treated aflatoxin contaminated maize grains. Anim. Feed. Sci. Technol. 2005, 122, 289–301. [Google Scholar] [CrossRef]
- Verma, J.; Johri, T.S.; Swain, B.K. Effect of aflatoxin, ochratoxin and their combination on protein and energy utilization in white leghorn laying hens. J. Sci. Food Agric. 2007, 87, 760–764. [Google Scholar] [CrossRef]
- Makhuvele, R.; Naidu, K.; Gbashi, S.; Thipe, V.C.; Adebo, O.A.; Njobeh, P.B. The use of plant extracts and their phytochemicals for control of toxigenic fungi and mycotoxins. Heliyon 2020, 6, e05291. [Google Scholar] [CrossRef]
- Yunus, A.W.; Razzazi-Fazeli, E.; Bohm, J. Aflatoxin B1 in affecting broiler’s performance, immunity, and gastrointestinal tract: A review of history and contemporary issues. Toxins 2011, 3, 566–590. [Google Scholar] [CrossRef]
- Fatemi, F.; Allameh, A.; Dadkhah, A.; Forouzandeh, M.; Kazemnejad, S.; Sharifi, R. Changes in hepatic cytosolic glutathione S-transferase activity and expression of its class-P during prenatal and postnatal period in rats treated with aflatoxin B1. Arch. Toxicol. 2006, 80, 572–579. [Google Scholar] [CrossRef]
- Yarru, L.P.; Settivari, R.S.; Antoniou, E.; Ledoux, D.R.; Rottinghaus, G.E. Toxicological and gene expression analysis of the impact of aflatoxin B1 on hepatic function of male broiler chicks. Poult. Sci. 2009, 88, 163–171. [Google Scholar] [CrossRef]
- Włodek, P.; Sokołowska, M.; Smoleński, O.; Włodek, L. The γ-glutamyltransferase activity and non-protein sulfhydryl compounds levels in rat kidney of different age groups. Acta Biochim. Pol. 2002, 49, 501–507. Available online: https://www.frontierspartnerships.org/articles/10.18388/abp.2002_3809/pdf (accessed on 2 January 2025). [CrossRef]
- Maxie, M.G. Jubb, Kennedy & Palmer’s Pathology of Domestic Animals, 5th ed.; Elsevier Saunders: Edinburgh, UK, 2007; Volume 2. [Google Scholar] [CrossRef]
- Rauber, R.H.; Dilkin, P.; Giacomini, L.Z.; Araújo de Almeida, C.A.; Mallmann, C.A. Performance of turkey poults fed different doses of aflatoxins in the diet. Poult. Sci. 2007, 86, 1620–1624. [Google Scholar] [CrossRef]
- Prabu, P.C.; Dwivedi, P.; Sharma, A.K. Toxicopathological studies on the effects of aflatoxin B1, ochratoxin A and their interaction in New Zealand White rabbits. Exp. Toxicol. Pathol. 2013, 65, 277–286. [Google Scholar] [CrossRef]
- Meissonnier, G.M.; Laffitte, J.; Loiseau, N.; Benoit, E.; Raymond, I.; Pinton, P.; Cossalter, A.-M.; Bertin, G.; Oswald, I.P.; Galtier, P. Selective impairment of drug-metabolizing enzymes in pig liver during subchronic dietary exposure to aflatoxin B1. Food Chem. Toxicol. 2007, 45, 2145–2154. [Google Scholar] [CrossRef]
- Ertor, B.; Topaloglu, S.; Calik, A.; Cobanoglu, U.; Ahmetoglu, A.; Ak, H.; Karabulut, E.; Arslan, M.K. The effects of bile duct obstruction on liver volume: An experimental study. Int. Sch. Res. Not. 2013, 2013, 156347. [Google Scholar] [CrossRef]
- Martínez-de-Anda, A.; Valdivia, A.G.; Jaramillo-Juárez, F.; Reyes, J.L.; Ortiz, R.; Quezada, T.; de Luna, M.C.; Rodríguez, M.L. Effects of aflatoxin chronic intoxication in renal function of laying hens. Poult. Sci. 2010, 89, 1622–1628. [Google Scholar] [CrossRef]
- Jaćević, V.; Dumanović, J.; Alomar, S.Y.; Resanović, R.; Milovanović, Z.; Nepovimova, E.; Wu, Q.; Franca, T.C.C.; Wu, W.; Kuča, K. Research update on aflatoxins toxicity, metabolism, distribution, and detection: A concise overview. Toxicology 2023, 492, 153549. [Google Scholar] [CrossRef]
- Al-Habib, M.F.M.; Jaffar, A.A.; Abdul-Ameer, H.H. Aflatoxin B1-induced kidney damage in rats. J. Fac. Med. Baghdad 2007, 49, 147–150. [Google Scholar] [CrossRef]
- Kumar, R.; Balachandran, C. Histopathological changes in broiler chickens fed aflatoxin and cyclopiazonic acid. Vet. Arh. 2009, 79, 31–40. Available online: https://hrcak.srce.hr/32935 (accessed on 21 January 2025).
- El-Nahla, S.M.; Imam, H.M.; Moussa, E.A.; Ibrahim, A.M.; Ghanam, A.R. Teratogenic effects of aflatoxin in Rabbits (Oryctolagus cuniculus). J. Vet. Anat. 2013, 6, 67–85. [Google Scholar] [CrossRef]
- Kemboi, D.C.; Antonissen, G.; Ochieng, P.E.; Croubels, S.; Okoth, S.; Kangethe, E.K.; Faas, J.; Lindahl, J.F.; Gathumbi, J.K. A Review of the impact of mycotoxins on dairy cattle health: Challenges for food safety and dairy production in Sub-Saharan Africa. Toxins 2020, 12, 222. [Google Scholar] [CrossRef]

| Performance a | Reference b | Time After Mycotoxin Exposure (Days) | |||
|---|---|---|---|---|---|
| 1 | 7 | 15 | 30 | ||
| DMI (kg/d) | 7.0–7.6 | 4.9 ± 0.42 ** | 5.71 ± 1.14 * | 5.3 ± 1.17 * | 4.54 ± 0.08 ** |
| Milk (kg/d) | 6.0–6.1 | 2.9 ± 0.1 4 ** | 5.8 ± 0.3 | 4.6 ± 0.6 ** | 4.2 ± 0.3 ** |
| BW (kg) | 59.5–62.3 | 50 ± 2.17 ** | 58.3 ± 7.8 | 59.4 ± 11.6 | 48.2 ± 4.8 ** |
| CI (DMI/BW) | 0.91–1.37 | 0.73 ± 0.23 * | 1.36 ± 1.14 | 4.82 ± 1.17 ** | −4.54 ± 0.08 ** |
| Biochemistry a | Reference b | Time After Mycotoxin Exposure (Days) | |||
|---|---|---|---|---|---|
| 1 | 7 | 15 | 30 | ||
| Plasma | |||||
| ALB (g/dL) | 2.7–2.7 | 2.0 ± 0.28 * | 1.34 ± 0.3 * | 1.6 ± 0.2 * | 1.53 ± 0.04 * |
| TP (g/dL) | 15.6–16.7 | 7.3 ± 0.49 ** | 6.1 ± 0.1 ** | 6.9 ± 0.8 ** | 11.5 ± 1.20 ** |
| DB (mg/dL) | 0.31–0.37 | 1.30 ± 0.12 * | 5.3 ± 0.0 ** | 6.4 ± 0.5 ** | 9.4 ± 0.79 ** |
| TB (mg/dL) | 1.75–1.82 | 2.9 ± 0.14 * | 35.1 ± 0.3 ** | 36.6 ± 0.3 ** | 44.2 ± 1.65 ** |
| PT (s) | 19.0–19.3 | 20.9 ± 0.73 | 19.7 ± 0.8 | 20.6 ± 1.0 | 22.5 ± 1.00 |
| ALT (EA, U/L) | 15.0–23.8 | 49.7 ± 6.2 * | 58.0 ± 12.4 * | 41.6 ± 9.4 * | 41.9 ± 11.9 * |
| AST (EA, U/L) | 56.5–64.5 | 49.7 ± 21.2 | 286 ± 61.0 ** | 228 ± 34.3 ** | 218 ± 0.00 ** |
| ALP (EA, U/L) | 71.9–80.0 | 116 ± 13.5 * | 186 ± 14.4 ** | 144 ± 21.2 ** | 104 ± 4.2 * |
| GGT (EA, U/L) | 118–142 | 157 ± 22.7 * | 304 ± 58.1 ** | 185 ± 31.4 * | 150 ± 6.0 * |
| Liver | |||||
| GSH (μmol/g) | 0.66 ± 0.11 | 0.87 ± 0.20 | 0.98 ± 0.41 * | 1.26 ± 0.15 ** | 1.26 ± 0.15 ** |
| GST (μmol CDNB/min) | 45.6 ± 0.11 | 43.0 ± 0.15 | 42.8 ± 2.68 | 45.5 ± 2.3 | 36.7 ± 0.15 * |
| GGT (AE, U/g) | 49.2 ± 1.25 | 35.0 ± 2.0 * | 50.1 ± 10.4 | 363 ± 178 ** | 230 ± 23.5 ** |
| Kidney | |||||
| GSH (μmol/g) | 0.76 ± 0.01 | 0.78 ± 0.03 | 1.23 ± 0.37 * | 1.26 ± 0.05 ** | 0.76 ± 0.01 |
| GST (μmol CDNB/min) | 44.4 ± 0.53 | 45.0 ± 0.90 | 44.8 ± 2.2 | 38.2 ± 3.5 | 29.0 ± 0.75 * |
| GGT (AE, U/g) | 95.8 ± 1.50 | 58.0 ± 5.3 * | 250 ± 104 ** | 597 ± 124 ** | 776 ± 73.5 ** |
| Morphological Findings | Nonexposed Group | Exposed Group | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Days Postexposure | (No.) | (%) | Days Postexposure | (No.) | (%) | |||||
| 7 | 15 | 30 | 7 | 15 | 30 | |||||
| Liver | ||||||||||
| Pallor | 0 | 0 | 0 | 0/15 | 0.0 | 5 | 5 | 5 | 15/15 | 100 |
| Hepatomegaly | 1 | 0 | 0 | 1/15 | 6.7 | 0 | 5 | 2 | 7/15 | 46.7 |
| Hemorrhages | 1 | 0 | 0 | 1/15 | 6.7 | 5 | 5 | 5 | 15/15 | 100 |
| Friable consistency | 0 | 0 | 0 | 0/15 | 0.0 | 5 | 5 | 5 | 15/15 | 100 |
| Kidney | ||||||||||
| Pallor | 0 | 0 | 0 | 0/15 | 0.0 | 0 | 1 | 3 | 4/15 | 26.7 |
| Greyish-white foci | 0 | 0 | 1 | 1/15 | 6.7 | 5 | 4 | 0 | 9/15 | 60.0 |
| Hemorrhages | 1 | 0 | 0 | 1/15 | 6.7 | 5 | 4 | 0 | 9/15 | 60.0 |
| Friable consistency | 0 | 0 | 0 | 0/15 | 0.0 | 5 | 5 | 0 | 10/15 | 66.7 |
| Morphological Findings | Nonexposed Group a | Exposed Group a | χ2 | p- Value | OR | ||
|---|---|---|---|---|---|---|---|
| (No.) | (%) | (No.) | (%) | ||||
| Liver | |||||||
| Bile duct obstruction | 1 | 2.2 | 43 | 95.6 | 78.4 | <0.01 | 946 |
| Cell degeneration | 3 | 6.7 | 43 | 95.6 | 71.1 | <0.01 | 301 |
| Cholestasis | 0 | 0 | 30 | 66.7 | 45.0 | <0.01 | * |
| Eosinophilic material | 1 | 2.2 | 23 | 85.2 | 27.5 | <0.01 | 46.0 |
| Fatty degeneration | 0 | 0 | 30 | 66.7 | 45.0 | <0.01 | * |
| Fibrosis | 0 | 0 | 39 | 86.7 | 68.8 | <0.01 | * |
| Lymphocytic infiltration | 1 | 2.2 | 24 | 88.9 | 29.3 | <0.01 | 50.3 |
| Kidney | |||||||
| Brush border edge loss | 0 | 0 | 45 | 100 | 90.0 | <0.01 | * |
| Cell degeneration | 1 | 2.2 | 40 | 88.9 | 68.1 | <0.01 | 352 |
| Eosinophilic material | 3 | 6.7 | 42 | 93.3 | 67.6 | <0.01 | 196 |
| Fibrosis | 0 | 0 | 35 | 77.8 | 57.3 | <0.01 | * |
| Foci of bleeding | 2 | 4.4 | 40 | 88.9 | 64.5 | <0.01 | 172 |
| Glomerular atrophy | 0 | 0 | 25 | 55.6 | 34.6 | <0.01 | * |
| Glomerulonephritis | 0 | 0 | 23 | 51.1 | 30.9 | <0.01 | * |
| Lymphocytic infiltration | 1 | 3.7 | 27 | 60 | 35.0 | <0.01 | 66.0 |
| Mesangial proliferation | 0 | 0 | 43 | 95.6 | 82.3 | <0.01 | * |
| Pigmentation | 0 | 0 | 39 | 86.7 | 68.8 | <0.01 | * |
| Thickening of blood vessels | 1 | 2.2 | 42 | 93.3 | 74.9 | <0.01 | 616 |
| Thickening of Bowman’s capsules | 0 | 0 | 43 | 95.6 | 82.3 | <0.01 | * |
| Vacuolization | 0 | 0 | 20 | 44.4 | 25.7 | <0.01 | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Luna-López, M.C.; Valdivia-Flores, A.G.; Rangel-Muñoz, E.J.; Hernández-Valdivia, E.; Quezada-Tristán, T.; Jaramillo-Juárez, F.; Ortiz-Martínez, R. Time-Dependent Changes in Performance, Biochemistry, and Histology in Dairy Calves with Acute Aflatoxicosis. Vet. Sci. 2025, 12, 273. https://doi.org/10.3390/vetsci12030273
de Luna-López MC, Valdivia-Flores AG, Rangel-Muñoz EJ, Hernández-Valdivia E, Quezada-Tristán T, Jaramillo-Juárez F, Ortiz-Martínez R. Time-Dependent Changes in Performance, Biochemistry, and Histology in Dairy Calves with Acute Aflatoxicosis. Veterinary Sciences. 2025; 12(3):273. https://doi.org/10.3390/vetsci12030273
Chicago/Turabian Stylede Luna-López, María Carolina, Arturo G. Valdivia-Flores, Erika Janet Rangel-Muñoz, Emmanuel Hernández-Valdivia, Teódulo Quezada-Tristán, Fernando Jaramillo-Juárez, and Raúl Ortiz-Martínez. 2025. "Time-Dependent Changes in Performance, Biochemistry, and Histology in Dairy Calves with Acute Aflatoxicosis" Veterinary Sciences 12, no. 3: 273. https://doi.org/10.3390/vetsci12030273
APA Stylede Luna-López, M. C., Valdivia-Flores, A. G., Rangel-Muñoz, E. J., Hernández-Valdivia, E., Quezada-Tristán, T., Jaramillo-Juárez, F., & Ortiz-Martínez, R. (2025). Time-Dependent Changes in Performance, Biochemistry, and Histology in Dairy Calves with Acute Aflatoxicosis. Veterinary Sciences, 12(3), 273. https://doi.org/10.3390/vetsci12030273









