Preliminary Evaluation of NT-proBNP and cTnI as Predictors of Procedure Safety in Dogs Undergoing Transcatheter Edge-to-Edge Mitral Valve Repair
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Measurement of the Blood Concentration of NT-proBNP and cTnI
2.3. TEER Procedure
2.4. Postoperative Follow up
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Concurrent Diseases
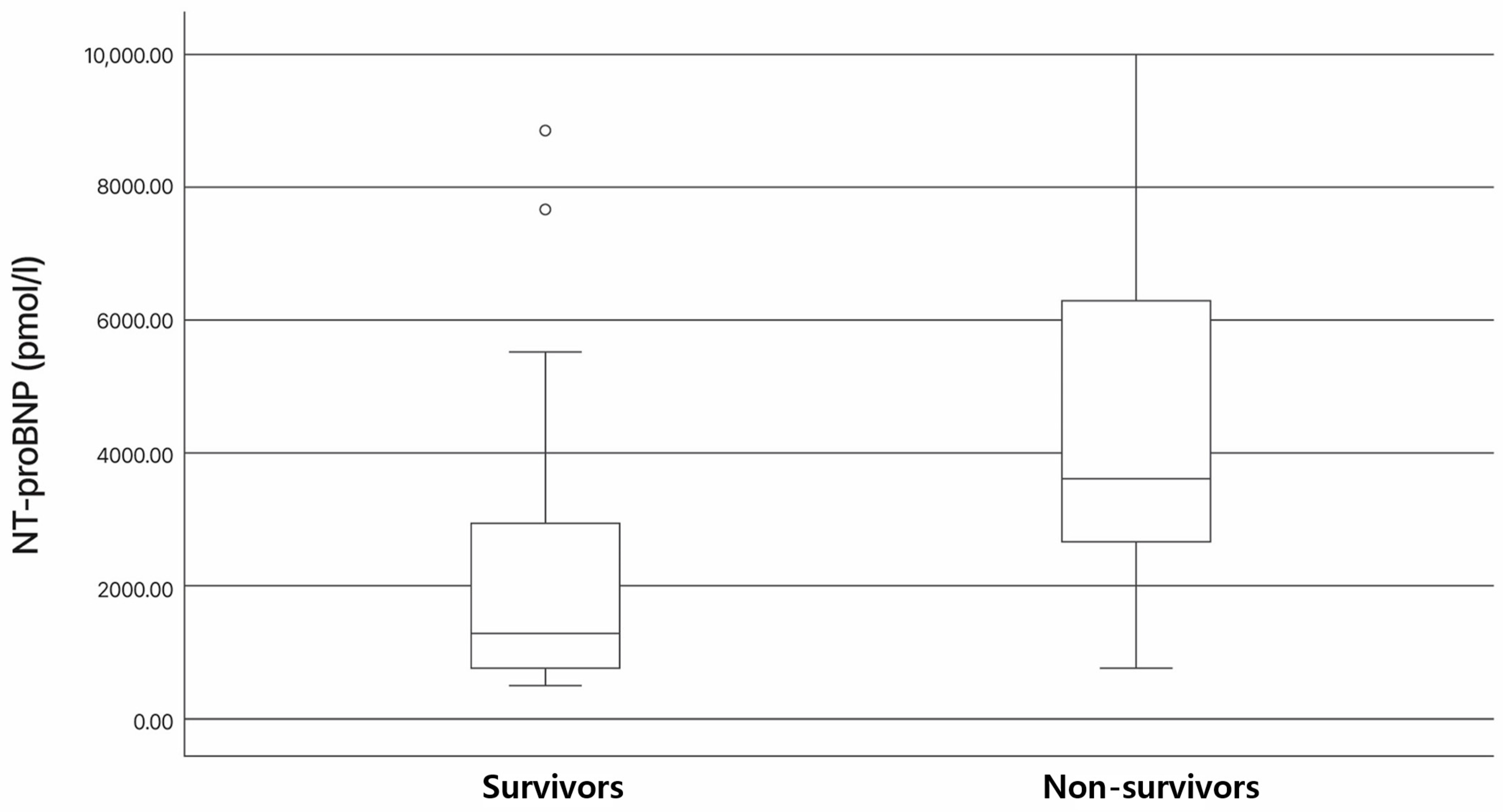
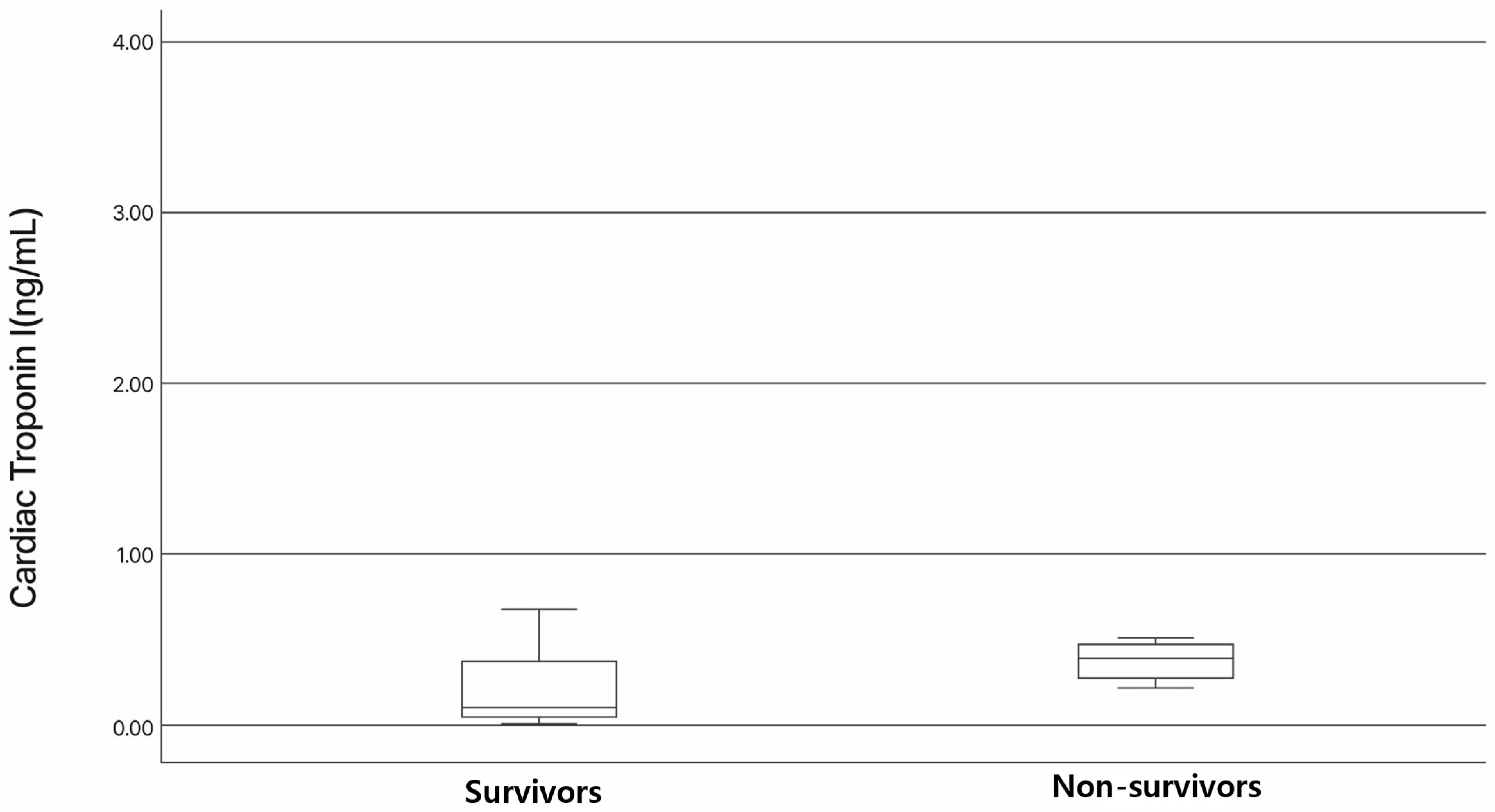
3.3. Association of NT-proBNP and cTnI Levels with Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MMVD | myxomatous mitral valve disease |
| TEER | transcatheter edge-to-edge mitral valve repair |
| NT-proBNP | N-terminal pro-brain natriuretic peptide |
| cTnI | cardiac troponin I |
| CKD | chronic kidney disease |
| TEE | transesophageal echocardiography |
| AKI | acute kidney injury |
References
- Keene, B.W.; Atkins, C.E.; Bonagura, J.D.; Fox, P.R.; Häggström, J.; Fuentes, V.L.; Oyama, M.A.; Rush, J.E.; Stepien, R.; Uechi, M. ACVIM Consensus Guidelines for the Diagnosis and Treatment of Myxomatous Mitral Valve Disease in Dogs. J. Vet. Intern. Med. 2019, 33, 1127–1140. [Google Scholar] [CrossRef]
- Menciotti, G.; Borgarelli, M. Review of Diagnostic and Therapeutic Approach to Canine Myxomatous Mitral Valve Disease. Vet. Sci. 2017, 4, 47. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.J.; Kim, J.H. Prognostic Efficacy of Complete Blood Count Indices for Assessing the Presence and the Progression of Myxomatous Mitral Valve Disease in Dogs. Animals 2023, 13, 2821. [Google Scholar] [CrossRef]
- Polizopoulou, Z.S.; Koutinas, C.K.; Dasopoulou, A.; Patsikas, M.; York, M.; Roman, I.; Gandhi, M.; Patel, S.; Koutinas, A.F.; O’Brien, P.J. Serial Analysis of Serum Cardiac Troponin I Changes and Correlation with Clinical Findings in 46 Dogs with Mitral Valve Disease. Vet. Clin. Pathol. 2014, 43, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Takemura, N.; Toda, N.; Miyagawa, Y.; Asano, K.; Tejima, K.; Kanno, N.; Arisawa, K.; Kurita, T.; Nunokawa, K.; Hirakawa, A.; et al. Evaluation of Plasma N-Terminal Pro-Brain Natriuretic Peptide (NT-ProBNP) Concentrations in Dogs with Mitral Valve Insufficiency. J. Vet. Med. Sci. 2009, 71, 925–929. [Google Scholar] [CrossRef] [PubMed]
- Langhorn, R.; Willesen, J.L. Cardiac Troponins in Dogs and Cats. J. Vet. Intern. Med. 2016, 30, 36–50. [Google Scholar] [CrossRef]
- Bristow, P.; Markovic, L.E. Mitral Valve Repair–The Development and Rise of Options in the Veterinary World. Vet. Clin. North Am. Small Anim. Pract. 2023, 53, 1343–1352. [Google Scholar] [CrossRef]
- Liu, B.; Leach, S.B.; Pan, W.; Zheng, F.; Jia, L.; Zhou, X.; Li, J. Preliminary Outcome of a Novel Edge-to-Edge Closure Device to Manage Mitral Regurgitation in Dogs. Front. Vet. Sci. 2020, 7, 597879. [Google Scholar] [CrossRef]
- Potter, B.M.; Orton, E.C.; Scansen, B.A.; Abbott-Johnson, K.M.; Visser, L.C.; Chi, I.-J.B.; Ross, E.S.; Del Nero, B.; Tantisuwat, L.; Krause, E.T.; et al. Clinical Feasibility Study of Transcatheter Edge-to-Edge Mitral Valve Repair in Dogs with the Canine V-Clamp Device. Front. Vet. Sci. 2024, 11, 1448828. [Google Scholar] [CrossRef] [PubMed]
- Orton, C.; Potter, B.; Bussadori, C. Textbook of Cardiovascular Medicine in Dogs and Cats, 1st ed.; Edrapublis: New York, NY, USA, 2023; Volume 1. [Google Scholar]
- Taramasso, M.; Maisano, F.; Latib, A.; Denti, P.; Buzzatti, N.; Cioni, M.; La Canna, G.; Colombo, A.; Alfieri, O. Clinical Outcomes of MitraClip for the Treatment of Functional Mitral Regurgitation. EuroIntervention 2014, 10, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Tabata, N.; Weber, M.; Sugiura, A.; Ishii, M.; Öztürk, C.; Tsujita, K.; Sinning, J.M.; Werner, N.; Nickenig, G. Impact of Combined Baseline and Postprocedural Troponin Values on Clinical Outcome Following the MitraClip Procedure. Catheter. Cardiovasc. Interv. 2020, 96, E735–E743. [Google Scholar] [CrossRef] [PubMed]
- International Renal Interest Society (IRIS) Ltd. IRIS. Staging of CKD (Modified in 2023). Available online: https://iris-kidney.com/guidelines/grading.html (accessed on 25 January 2025).
- Peacock, W.F.; De Marco, T.; Fonarow, G.C.; Diercks, D.; Wynne, J.; Apple, F.S.; Wu, A.H.B. Cardiac Troponin and Outcome in Acute Heart Failure. N. Engl. J. Med. 2008, 358, 2117–2126. [Google Scholar] [CrossRef] [PubMed]
- Arenja, N.; Reichlin, T.; Drexler, B.; Oshima, S.; Denhaerynck, K.; Haaf, P.; Potocki, M.; Breidthardt, T.; Noveanu, M.; Stelzig, C.; et al. Sensitive Cardiac Troponin in the Diagnosis and Risk Stratification of Acute Heart Failure. J. Intern. Med. 2012, 271, 598–607. [Google Scholar] [CrossRef]
- Schillinger, W.; Athanasiou, T.; Weicken, N.; Berg, L.; Tichelbäcker, T.; Puls, M.; Hünlich, M.; Wachter, R.; Helms, H.; Seipelt, R.; et al. Impact of the Learning Curve on Outcomes after Percutaneous Mitral Valve Repair with MitraClip® and Lessons Learned after the First 75 Consecutive Patients. Eur. J. Heart Fail. 2011, 13, 1331–1339. [Google Scholar] [CrossRef] [PubMed]
- Eggers, K.M.; Jernberg, T.; Lindahl, B. Cardiac Troponin Elevation in Patients Without a Specific Diagnosis. J. Am. Coll. Cardiol. 2019, 73, 1–9. [Google Scholar] [CrossRef]
- Porciello, F.; Rishniw, M.; Herndon, W.; Birettoni, F.; Antognoni, M.; Simpson, K. Cardiac Troponin I Is Elevated in Dogs and Cats with Azotaemia Renal Failure and in Dogs with Non-cardiac Systemic Disease. Aust. Vet. J. 2008, 86, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Pelander, L.; Häggström, J.; Ley, C.J.; Ljungvall, I. Cardiac Troponin I and Amino-Terminal Pro B-Type Natriuretic Peptide in Dogs With Stable Chronic Kidney Disease. J. Vet. Intern. Med. 2017, 31, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Miyagawa, Y.; Tominaga, Y.; Toda, N.; Takemura, N. Relationship between Glomerular Filtration Rate and Plasma N-Terminal pro B-Type Natriuretic Peptide Concentrations in Dogs with Chronic Kidney Disease. Vet. J. 2013, 197, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Green, M.D.; Parker, D.M.; Everett, A.D.; Vricella, L.; Jacobs, M.L.; Jacobs, J.P.; Brown, J.R. Cardiac Biomarkers Associated With Hospital Length of Stay After Pediatric Congenital Heart Surgery. Ann. Thorac. Surg. 2021, 112, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Armijo, G.; Estevez-Loureiro, R.; Carrasco-Chinchilla, F.; Arzamendi, D.; Fernández-Vázquez, F.; Jimenez-Quevedo, P.; Freixa, X.; Pascual, I.; Serrador, A.M.; Mesa, D.; et al. Acute Kidney Injury After Percutaneous Edge-to-Edge Mitral Repair. J. Am. Coll. Cardiol. 2020, 76, 2463–2473. [Google Scholar] [CrossRef]
| Variable | Total | Survivals | Non-Survivals |
|---|---|---|---|
| Gender | |||
| Male | 1 (4%) | 1 (5.2%) | 0 (0%) |
| Neutered male | 12 (48%) | 8 (42.1%) | 4 (66.6%) |
| Female | 0 (0%) | 0 (0%) | 0 (0%) |
| Neutered female | 12 (48%) | 10 (52.6%) | 2 (33.3%) |
| Age (year) | 11 (9–14) | 10 (9–14) | 12 (9–13) |
| Weight (kg) | 4.3 (2.1–7.8) | 4.6 (2.3–7.8) | 2.9 (2.1–6.0) |
| Breed | |||
| Maltese | 7 (28%) | 4 (21.1%) | 3 (50%) |
| Mix-breeds | 5 (20%) | 4 (21.1%) | 1 (16.7%) |
| Schanuzer | 3 (12%) | 3 (15.8%) | 0 (0%) |
| Shih Tzu | 3 (12%) | 3 (15.8%) | 0 (0%) |
| Poodle | 3 (12%) | 3 (15.8%) | 0 (0%) |
| Pomeranian | 2 (8%) | 1 (5.3%) | 1 (16.7%) |
| Chihuahua | 1 (4%) | 1 (5.3%) | 0 (0%) |
| Dachshund | 1 (4%) | 0 (0%) | 1 (16.7%) |
| Total | 25 | 19 | 6 |
| N (%), Median (Range) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-M.; Lee, S.-K.; Youp, K.-A.; Lee, A.-R.; Cho, Y.-W.; Jung, Y.-S.; Lee, S.-T. Preliminary Evaluation of NT-proBNP and cTnI as Predictors of Procedure Safety in Dogs Undergoing Transcatheter Edge-to-Edge Mitral Valve Repair. Vet. Sci. 2025, 12, 223. https://doi.org/10.3390/vetsci12030223
Lee J-M, Lee S-K, Youp K-A, Lee A-R, Cho Y-W, Jung Y-S, Lee S-T. Preliminary Evaluation of NT-proBNP and cTnI as Predictors of Procedure Safety in Dogs Undergoing Transcatheter Edge-to-Edge Mitral Valve Repair. Veterinary Sciences. 2025; 12(3):223. https://doi.org/10.3390/vetsci12030223
Chicago/Turabian StyleLee, Jeong-Min, Seung-Keun Lee, Kyoung-A Youp, Ah-Ra Lee, Young-Wook Cho, Youn-Seo Jung, and Sun-Tae Lee. 2025. "Preliminary Evaluation of NT-proBNP and cTnI as Predictors of Procedure Safety in Dogs Undergoing Transcatheter Edge-to-Edge Mitral Valve Repair" Veterinary Sciences 12, no. 3: 223. https://doi.org/10.3390/vetsci12030223
APA StyleLee, J.-M., Lee, S.-K., Youp, K.-A., Lee, A.-R., Cho, Y.-W., Jung, Y.-S., & Lee, S.-T. (2025). Preliminary Evaluation of NT-proBNP and cTnI as Predictors of Procedure Safety in Dogs Undergoing Transcatheter Edge-to-Edge Mitral Valve Repair. Veterinary Sciences, 12(3), 223. https://doi.org/10.3390/vetsci12030223







