Adverse Events Following Immunization Associated with the First and Second Doses of the ChAdOx1 nCoV-19 Vaccine among Healthcare Workers in Korea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Adverse Events Reporting System
2.3. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 27 June 2021).
- Korea Centers for Disease Control and Prevention. COVID-19 Domestic Occurrence and Vaccination Status. Available online: https://ncv.kdca.go.kr/ (accessed on 27 June 2021).
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Ramasamy, M.N.; Minassian, A.M.; Ewer, K.J.; Flaxman, A.L.; Folegatti, P.M.; Owens, D.R.; Voysey, M.; Aley, P.K.; Angus, B.; Babbage, G.; et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet 2020, 396, 1979–1993. [Google Scholar] [CrossRef]
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N. Engl. J Med. 2021, 384, 2092–2101. [Google Scholar] [CrossRef] [PubMed]
- AstraZeneca’s COVID-19 Vaccine: EMA Finds Possible Link to Very Rare Cases of Unusual Blood Clots with Low Blood Platelets. Available online: https://www.ema.europa.eu/en/news/astrazenecas-covid-19-vaccine-ema-finds-possible-link-very-rare-cases-unusual-blood-clots-low-blood (accessed on 20 June 2021).
- Aw, J.; Seng, J.J.B.; Seah, S.S.Y.; Low, L.L. COVID-19 Vaccine Hesitancy—A Scoping Review of Literature in High-Income Countries. Vaccines 2021, 9, 900. [Google Scholar] [CrossRef] [PubMed]
- Oldenburg, J.; Klamroth, R.; Langer, F.; Albisetti, M.; von Auer, C.; Ay, C.; Korte, W.; Scharf, R.E.; Pötzsch, B.; Greinacher, A. Diagnosis and Management of Vaccine-Related Thrombosis following AstraZeneca COVID-19 Vaccination: Guidance Statement from the GTH. Hamostaseologie 2021, 41, 184–189. [Google Scholar] [PubMed]
- Korea Centers for Disease Control and Prevention. Press Releases of COVID-19 Vaccination. Available online: http://ncov.mohw.go.kr/tcmBoardView.do?brdId=3&brdGubun=31&dataGubun=&ncvContSeq=5156&contSeq=5156&board_id=312&gubun=BDJ# (accessed on 22 June 2021).
- U.S. Food & Drug Administration. Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/toxicity-grading-scale-healthy-adult-and-adolescent-volunteers-enrolled-preventive-vaccine-clinical (accessed on 26 March 2021).
- Jeon, M.; Kim, J.; Oh, C.E.; Lee, J.Y. Adverse Events Following Immunization Associated with Coronavirus Disease 2019 Vaccination Reported in the Mobile Vaccine Adverse Events Reporting System. J. Korean Med. Sci. 2021, 36, e114. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Park, S.Y.; Yu, S.; Park, J.W.; Lee, E.; Jeon, M.H.; Kim, T.H.; Choo, E.J. Impacts of Side Effects to BNT162b2 and the First Dose of ChAdOx1 Anti-SARS-CoV-2 Vaccination on Work Productivity, the Need for Medical Attention, and Vaccine Acceptance: A Multicenter Survey on Healthcare Workers in Referral Teaching Hospitals in the Republic of Korea. Vaccines 2021, 9, 648. [Google Scholar] [PubMed]
- Riad, A.; Pokorná, A.; Mekhemar, M.; Conrad, J.; Klugarová, J.; Koščík, M.; Klugar, M.; Attia, S. Safety of ChAdOx1 nCoV-19 Vaccine: Independent Evidence from Two EU States. Vaccines 2021, 9, 673. [Google Scholar] [CrossRef] [PubMed]
- Hatmal, M.M.; Al-Hatamleh, M.A.I.; Olaimat, A.N.; Hatmal, M.; Alhaj-Qasem, D.M.; Olaimat, T.M.; Mohamud, R. Side Effects and Perceptions Following COVID-19 Vaccination in Jordan: A Randomized, Cross-Sectional Study Implementing Machine Learning for Predicting Severity of Side Effects. Vaccines 2021, 9, 556. [Google Scholar] [CrossRef] [PubMed]
- Alhazmi, A.; Alamer, E.; Daws, D.; Hakami, M.; Darraj, M.; Abdelwahab, S.; Maghfuri, A.; Algaissi, A. Evaluation of Side Effects Associated with COVID-19 Vaccines in Saudi Arabia. Vaccines 2021, 9, 674. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Wi, Y.M.; Yun, S.Y.; Ryu, J.S.; Shin, J.M.; Lee, E.H.; Seo, K.H.; Lee, S.H.; Peck, K.R. Adverse Events in Healthcare Workers after the First Dose of ChAdOx1 nCoV-19 or BNT162b2 mRNA COVID-19 Vaccination: A Single Center Experience. J. Korean Med. Sci. 2021, 36, e107. [Google Scholar] [CrossRef] [PubMed]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- Korea Centers for Disease Control and Prevention. Weekly Report of Adverse Reaction after COVID-19 Vaccination (23th Week). Available online: https://ncv.kdca.go.kr/board.es?mid=a11707010000&bid=0032#content (accessed on 17 August 2021).
- Korea Centers for Disease Control and Prevention. COVID-19 Vaccination Strategy and Implementation Plan of July. Available online: http://ncov.mohw.go.kr/tcmBoardView.do?brdId=3&brdGubun=31&dataGubun=&ncvContSeq=5626&contSeq=5626&board_id=312&gubun=ALL (accessed on 1 July 2021).
- Medicines & Healthcare Products Regulatory Agency. Coronavirus Vaccine—Weekly Summary of Yellow Card Reporting. Available online: https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting (accessed on 14 August 2021).
- Paul-Ehrlich-Institut. Reports on Suspected Cases of Adverse Effects and Vaccination Complications Following a Vaccination for the Protection against COVID-19 (Reporting Period 27 December 2020 to 30 June 2021). Available online: https://www.pei.de/SharedDocs/Downloads/EN/newsroom-en/dossiers/safety-reports/safety-report-27-december-30-june-2021.pdf?__blob=publicationFile&v=6 (accessed on 14 August 2021).
- Bunn, C. ‘Getting a Clearer Picture’: Black Americans on the Factors That Overcame Their Vaccine Hesitancy. NBC News, 13 April 2021. [Google Scholar]
- Enwezor, C.H.; Peacock, J.E.; Seals, A.L.; Edelstein, S.L.; Hinkelman, A.N.; Wierzba, T.F.; Munawar, I.; Maguire, P.D.; Lagarde, W.H.; Runyon, M.S.; et al. Changing Attitudes toward the COVID-19 Vaccine among North Carolina Participants in the COVID-19 Community Research Partnership. Vaccines 2021, 9, 916. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Peng, Z.; Luo, W.; Si, S.; Mo, M.; Zhou, H.; Xin, X.; Liu, H.; Yu, Y. Efficacy and Safety of COVID-19 Vaccines in Phase III Trials: A Meta-Analysis. Vaccines 2021, 9, 582. [Google Scholar] [CrossRef] [PubMed]
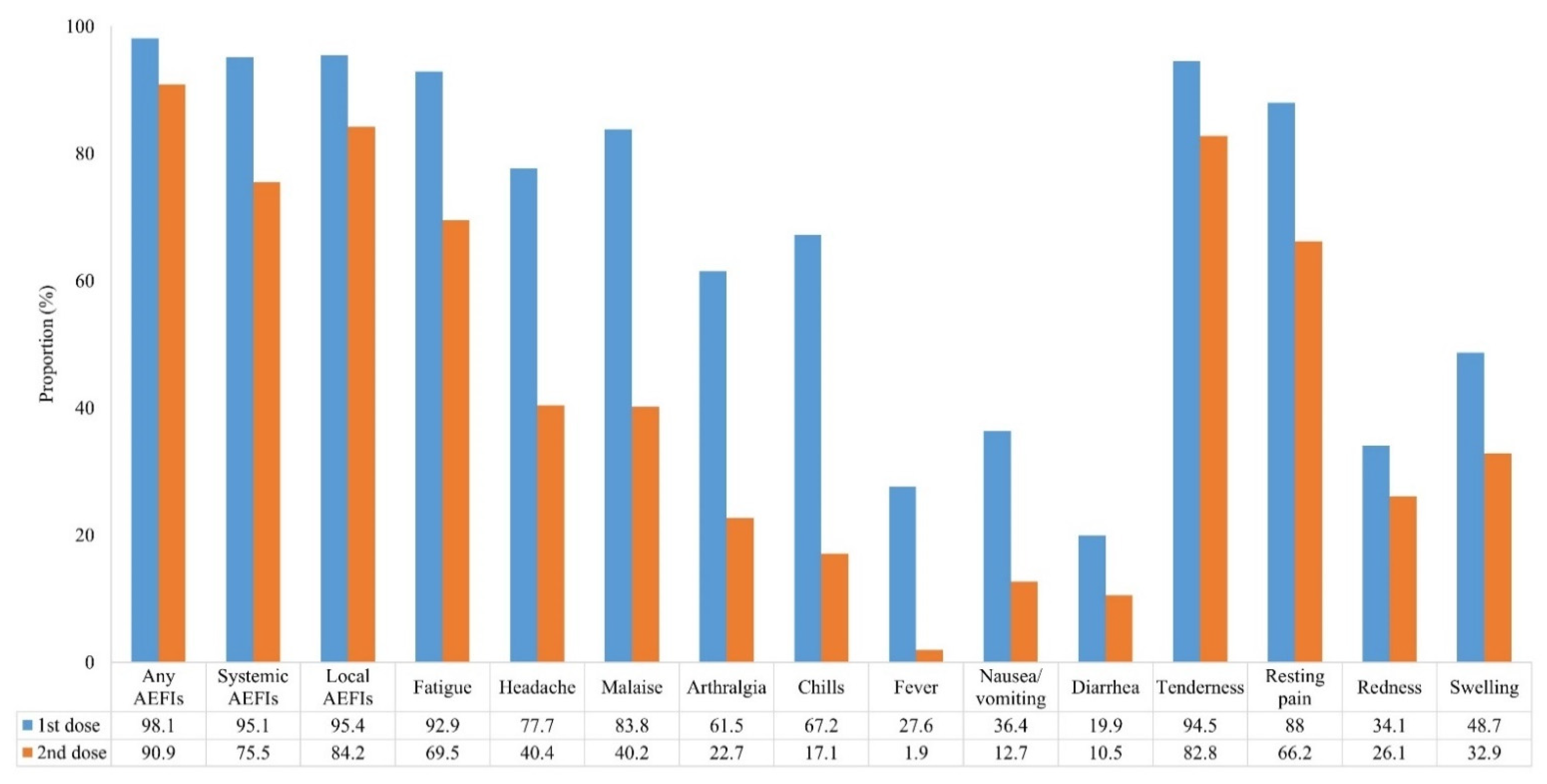


| Adverse Events | 1st Dose (n = 994) | 2nd Dose (n = 727) | p Value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any | None | G1 | G2 | G3 | G4 a | Any | None | G1 | G2 | G3 | G4 a | ||
| Age, mean ± SD | 35.66 ± 10.46 (range, 19–63) | 36.75 ± 10.45 (range, 20–63) | 0.034 | ||||||||||
| Female sex | 762 (76.7) | 559 (76.9%) | 0.911 | ||||||||||
| Any AEFIs | 975 (98.1) | 19 (1.9) | 108 (10.9) | 422 (42.5) | 428 (43.1) | 17 (1.7) | 661 (90.9) | 66 (9.1) | 221 (30.4) | 303 (41.7) | 134 (18.4) | 3 (0.4) | <0.001 |
| Local AEFIs | 948 (95.4) | 46 (4.6) | 189 (19.0) | 547 (55.0) | 207 (20.8) | 5 0.5) | 612 (84.2) | 115 (15.8) | 242 (33.3) | 252 (34.7) | 115 (15.8) | 3 (0.4) | <0.001 |
| Tenderness | 939 (94.5) | 55 (5.5) | 208 (20.9) | 400 (40.2) | 331 (33.3) | 602 (82.8) | 125 (17.2) | 240 (33.0) | 250 (34.4) | 112 (15.4) | <0.001 | ||
| Resting pain | 875 (88.0) | 119 (12.0) | 543 (54.6) | 328 (33.0) | 4 (0.4) | 481 (66.2) | 246 (33.8) | 413 (56.8) | 68 (9.4) | 0 (0.0) | <0.001 | ||
| Redness | 339 (34.1) | 655 (65.9) | 251 (25.3) | 49 (4.9) | 31 (3.1) | 8 (0.8) | 190 (26.1) | 537 (73.9) | 172 (23.7) | 12 (1.7) | 5 (0.7) | 1 (0.1) | <0.001 |
| Swelling | 484 (48.7) | 510 (51.3) | 326 (32.8) | 108 (10.9) | 40 (4.0) | 10 (1.0) | 239 (32.9) | 488 (67.1) | 186 (25.6) | 42 (5.8) | 8 (1.1) | 3 (0.4) | <0.001 |
| Systemic AEFIs | 945 (95.1) | 49 (4.9) | 191 (19.2) | 405 (40.7) | 337 (33.9) | 12 (1.2) | 549 (75.5) | 178 (24.5) | 294 (40.4) | 223 (30.7) | 32 (4.4) | 0 (0.0) | <0.001 |
| Fatigue | 923 (92.9) | 71 (7.1) | 276 (27.8) | 454 (45.7) | 193 (19.4) | 505 (69.5) | 222 (30.5) | 310 (42.6) | 165 (22.7) | 30 (4.1) | <0.001 | ||
| Headache | 772 (77.7) | 222 (22.3) | 262 (26.4) | 502 (50.5) | 8 (0.8) | 294 (40.4) | 433 (59.6) | 175 (24.1) | 118 (16.2) | 1 (0.1) | <0.001 | ||
| Malaise | 833 (83.8) | 161 (16.2) | 236 (23.7) | 580 (58.4) | 17 (1.7) | 292 (40.2) | 435 (59.8) | 196 (27.0) | 94 (12.9) | 2 (0.3) | <0.001 | ||
| Arthralgia | 611 (61.5) | 383 (38.5) | 227 (22.8) | 377 (37.9) | 7 (0.7) | 165 (22.7) | 562 (77.3) | 114 (15.7) | 51 (7.0) | 0 (0.0) | <0.001 | ||
| Chills | 668 (67.2) | 326 (32.8) | 232 (23.3) | 421 (42.4) | 15 (1.5) | 124 (17.1) | 603 (82.9) | 86 (11.8) | 35 (4.8) | 3(0.4) | <0.001 | ||
| Fever | 274 27.6) | 720 (72.4) | 181 (18.2) | 74 (7.4) | 17 (1.7) | 2 (0.2) | 14 (1.9) | 713 (98.1) | 11 (1.5) | 2 (0.3) | 1 (0.1) | 0 (0.0) | <0.001 |
| Nausea/ vomiting | 362 (36.4) | 632 (63.6) | 288 (29.0) | 67 (6.7) | 7 (0.7) | 92 (12.7) | 635 (87.3) | 84 (11.6) | 6 (0.8) | 2 (0.3) | <0.001 | ||
| Diarrhea | 198 19.9) | 796 (80.1) | 119 (12.0) | 66 (6.6) | 10 (1.0) | 3 (0.3) | 76 (10.5) | 651 (89.5) | 43 (5.9) | 29 (4.0) | 4 (0.6) | 0 (0.0) | <0.001 |
| 1st Dose | 2nd Dose | |||||||
|---|---|---|---|---|---|---|---|---|
| Adverse Events | <30 Years (n = 394) | 30–50 Years (n = 471) | >50 Years (n = 129) | p Value for Trend | <30 Years (n = 244) | 30–50 Years (n = 378) | >50 Years (n = 105) | p Value for Trend |
| Any AEFIs | 389 (98.7) | 464 (98.5) | 122 (94.6) | 0.017 | 223 (91.4) | 346 (91.5) | 92 (87.6) | 0.384 |
| Local AEFIs | 382 (97.0) | 450 (95.5) | 113 (87.6) | <0.001 | 205 (84.0) | 326 (86.2) | 81 (77.1) | 0.321 |
| Tenderness | 382 (97.0) | 448 (95.1) | 109 (84.5) | <0.001 | 198 (81.1) | 324 (85.7) | 80 (76.2) | 0.712 |
| Resting pain | 355 (90.1) | 424 (90.0) | 96 (74.4) | <0.001 | 157 (64.3) | 259 (68.5) | 65 (61.9) | >0.999 |
| Redness | 145 (36.8) | 159 (33.8) | 35 (27.1) | 0.053 | 51 (20.9) | 110 (29.1) | 29 (27.6) | 0.076 |
| Swelling | 209 (53.0) | 221 (46.9) | 54 (41.9) | 0.016 | 72 (29.5) | 129 (34.1) | 38 (36.2) | 0.173 |
| Systemic AEFIs | 383 (97.2) | 452 (96.0) | 113 (87.6) | <0.001 | 182 (74.6) | 290 (76.7) | 77 (73.3) | >0.999 |
| Fatigue | 374 (94.9) | 440 (93.4) | 109(84.5) | 0.001 | 163 (66.8) | 274 (72.5) | 68 (64.8) | 0.856 |
| Headache | 334 (84.8) | 356 (75.6) | 82 (63.6) | <0.001 | 104 (42.6) | 149 (39.4) | 41 (39.0) | 0.461 |
| Malaise | 343 (87.1) | 397 (84.3) | 93 (72.1) | <0.001 | 88 (36.1) | 160 (42.3) | 44 (41.9) | 0.192 |
| Arthralgia | 240 (60.9) | 302 (64.1) | 69 (53.5) | 0.440 | 47 (19.3) | 85 (22.5) | 33 (31.4) | 0.020 |
| Chills | 277 (70.3) | 322 (68.4) | 69 (53.5) | 0.003 | 38 (15.6) | 70 (18.5) | 16 (15.2) | 0.825 |
| Fever | 130 (33.0) | 134 (28.5) | 10 (7.8) | <0.001 | 1 (0.4) | 11 (2.9) | 2 (1.9) | 0.158 |
| Nausea/vomiting | 177 (44.9) | 160 (34.0) | 25 (19.4) | <0.001 | 36 (14.8) | 45 (11.9) | 11 (10.5) | 0.241 |
| Diarrhea | 92 (23.4) | 85 (18.0) | 21 (16.3) | 0.034 | 25 (10.2) | 45 (11.9) | 6 (5.7) | 0.467 |
| Adverse Events | 1st Dose (n = 652) | 2nd Dose (n = 652) | p Value b | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any | None | G1 | G2 | G3 | G4 a | Any | None | G1 | G2 | G3 | G4 a | ||
| Age, mean ± SD | 36.75 ± 10.41 (range, 20–63) | ||||||||||||
| Female sex | 513 (78.7) | ||||||||||||
| Any AEFIs | 642 (98.5) | 10 (1.5) | 82 (12.6) | 256 (39.3) | 293 (44.9) | 11 (1.7) | 598 (91.7) | 54 (8.3) | 194 (29.8) | 275 (42.2) | 127 (19.5) | 2 (0.3) | <0.001 |
| Local AEFIs | 626 (96.0) | 26 (4.0) | 132 (20.2) | 252 (38.7) | 234 (35.9) | 8 (1.2) | 557 (85.4) | 95 (14.6) | 213 (32.7) | 232 (35.6) | 110 (16.9) | 2 (0.3) | <0.001 |
| Tenderness | 622 (95.4) | 30 (4.6) | 144 (22.1) | 251 (38.5) | 227 (34.8) | 548 (84.0) | 104 (16.0) | 212 (32.5) | 229 (35.1) | 107 (16.4) | <0.001 | ||
| Resting pain | 583 (89.4) | 69 (10.6) | 355 (54.4) | 226 (34.7) | 2 (0.3) | 445 (68.3) | 207 (31.7) | 380 (58.3) | 65 (10.0) | 0 (0.0) | <0.001 | ||
| Redness | 222 (34.0) | 430 (66.0) | 159 (24.4) | 35 (5.4) | 23 (3.5) | 5 (0.8) | 177 (27.1) | 475 (72.9) | 161 (24.7) | 12 (1.8) | 4 (0.6) | 0 (0.0) | <0.001 |
| Swelling | 327 (50.2) | 325 (49.8) | 216 (33.1) | 73 (11.2) | 31 (4.8) | 7 (1.1) | 223 (34.2) | 429 (65.8) | 173 (26.5) | 42 (6.4) | 6 (0.9) | 2 (0.3) | <0.001 |
| Systemic AEFIs | 621 (95.2) | 31 (4.8) | 130 (19.9) | 354 (54.3) | 134 (20.6) | 3 (0.5) | 497 (76.2) | 155 (23.8) | 265 (40.6) | 202 (31.0) | 30 (4.6) | 0 (0.0) | <0.001 |
| Fatigue | 604 (92.6) | 48 (7.4) | 178 (27.3) | 301 (46.2) | 125 (19.2) | 458 (70.2) | 194 (29.8) | 282 (43.3) | 148 (22.7) | 28 (4.3) | <0.001 | ||
| Headache | 504 (77.3) | 148 (22.7) | 175 (26.8) | 323 (49.5) | 6 (0.9) | 271 (41.6) | 381 (58.4) | 161 (24.7) | 109 (16.7) | 1 (0.2) | <0.001 | ||
| Malaise | 542 (83.1) | 110 (16.9) | 156 (23.9) | 373 (57.2) | 13 (2.0) | 262 (40.2) | 390 (59.8) | 177 (27.1) | 83 (12.7) | 2 (0.3) | <0.001 | ||
| Arthralgia | 395 (60.6) | 257 (39.4) | 151 (23.2) | 240 (36.8) | 4 (0.6) | 150 (23.0) | 502 (77.0) | 103 (15.8) | 47 (7.2) | 0 (0.0) | <0.001 | ||
| Chills | 418 (64.1) | 243 (35.9) | 138 (21.2) | 271 (41.6) | 9 (1.4) | 112 (17.2) | 540 (82.8) | 75 (11.5) | 34 (5.2) | 3 (0.5) | <0.001 | ||
| Fever | 171 (26.2) | 481 (73.8) | 116 (17.8) | 41 (6.3) | 12 (1.8) | 2 (0.3) | 12 (1.8) | 640 (98.2) | 10 (1.5) | 1 (0.2) | 1 (0.2) | 0 (0.0) | <0.001 |
| Nausea/vomiting | 226 (34.7) | 426 (65.3) | 185 (28.4) | 36 (5.5) | 5 (0.8) | 86 (13.2) | 566 (86.8) | 79 (12.1) | 5 (0.8) | 2 (0.3) | <0.001 | ||
| Diarrhea | 127 (19.5) | 525 (80.5) | 82 (12.6) | 39 (6.0) | 5 (0.8) | 1 (0.2) | 70 (10.7) | 582 (89.3) | 41 (6.3) | 27 (4.1) | 2 (0.3) | 0 (0.0) | <0.001 |
| Adverse Events | 1st Dose (n = 217) | 2nd Dose (n = 217) | p Value a | ||
|---|---|---|---|---|---|
| Age, Mean ± SD | 26.1 ± 2.1 (Range, 20–29) | ||||
| Female sex | 192 (88.5) | ||||
| Incidence | Peak grade | Incidence | Peak grade | ||
| Any AEFIs | 215 (99.1) | 3.5 ± 0.7 | 202 (93.1) | 2.8 ± 0.8 | <0.001 |
| Local AEFIs | 212 (97.7) | 3.3 ± 0.8 | 185 (85.3) | 2.5 ± 0.9 | <0.001 |
| Tenderness | 212 (97.7) | 3.2 ± 0.8 | 179 (82.5) | 2.5 ± 0.9 | <0.001 |
| Resting pain | 196 (90.3) | 2.3 ± 0.6 | 143 (65.9) | 1.7 ± 0.6 | <0.001 |
| Redness | 80 (36.9) | 1.5 ± 0.9 | 48 (22.1) | 1.2 ± 0.5 | <0.001 |
| Swelling | 117 (53.9) | 1.8 ± 1.0 | 69 (31.8) | 1.4 ± 0.7 | <0.001 |
| Systemic AEFIs | 212 (97.7) | 3.1 ± 0.7 | 165 (76.0) | 2.2 ± 0.8 | <0.001 |
| Fatigue | 206 (94.9) | 2.9 ± 0.8 | 148 (68.2) | 2.0 ± 0.8 | <0.001 |
| Headache | 186 (85.7) | 2.5 ± 0.7 | 94 (43.3) | 1.6 ± 0.8 | <0.001 |
| Malaise | 190 (87.6) | 2.5 ± 0.7 | 80 (36.9) | 1.5 ± 0.7 | <0.001 |
| Arthralgia | 126 (58.1) | 2.0 ± 0.9 | 42 (19.4) | 1.3 ± 0.6 | <0.001 |
| Chills | 146 (67.3) | 2.2 ± 0.9 | 34 (15.7) | 1.2 ± 0.6 | <0.001 |
| Fever | 73 (33.6) | 1.5 ± 0.7 | 1 (0.5) | 1.0 ± 0.1 | <0.001 |
| Nausea/vomiting | 95 (43.8) | 1.5 ± 0.7 | 33 (15.2) | 1.2 ± 0.4 | <0.001 |
| Diarrhea | 49 (22.6) | 1.3 ± 0.6 | 24 (11.1) | 1.2 ± 0.6 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, M.; Kim, J.; Oh, C.E.; Lee, J.-Y. Adverse Events Following Immunization Associated with the First and Second Doses of the ChAdOx1 nCoV-19 Vaccine among Healthcare Workers in Korea. Vaccines 2021, 9, 1096. https://doi.org/10.3390/vaccines9101096
Jeon M, Kim J, Oh CE, Lee J-Y. Adverse Events Following Immunization Associated with the First and Second Doses of the ChAdOx1 nCoV-19 Vaccine among Healthcare Workers in Korea. Vaccines. 2021; 9(10):1096. https://doi.org/10.3390/vaccines9101096
Chicago/Turabian StyleJeon, Minji, Jehun Kim, Chi Eun Oh, and Jin-Young Lee. 2021. "Adverse Events Following Immunization Associated with the First and Second Doses of the ChAdOx1 nCoV-19 Vaccine among Healthcare Workers in Korea" Vaccines 9, no. 10: 1096. https://doi.org/10.3390/vaccines9101096
APA StyleJeon, M., Kim, J., Oh, C. E., & Lee, J.-Y. (2021). Adverse Events Following Immunization Associated with the First and Second Doses of the ChAdOx1 nCoV-19 Vaccine among Healthcare Workers in Korea. Vaccines, 9(10), 1096. https://doi.org/10.3390/vaccines9101096






