Immunologic Response to SARS-CoV-2 Vaccination in Pediatric Kidney Transplant Recipients: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Data Extraction
2.3. Statistical Analysis
3. Results
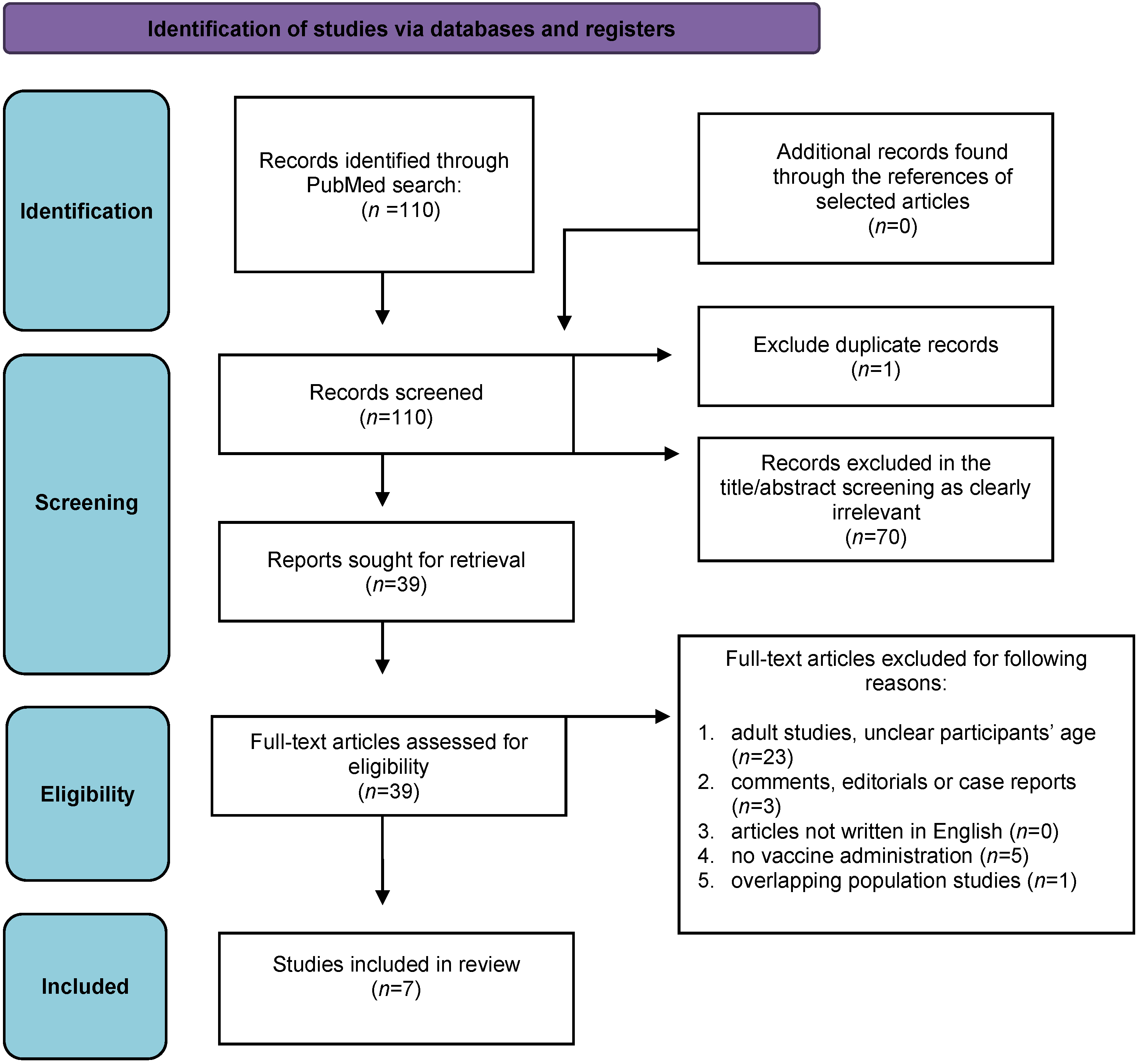
3.1. Study Selection
3.2. Characteristics of the Included Studies
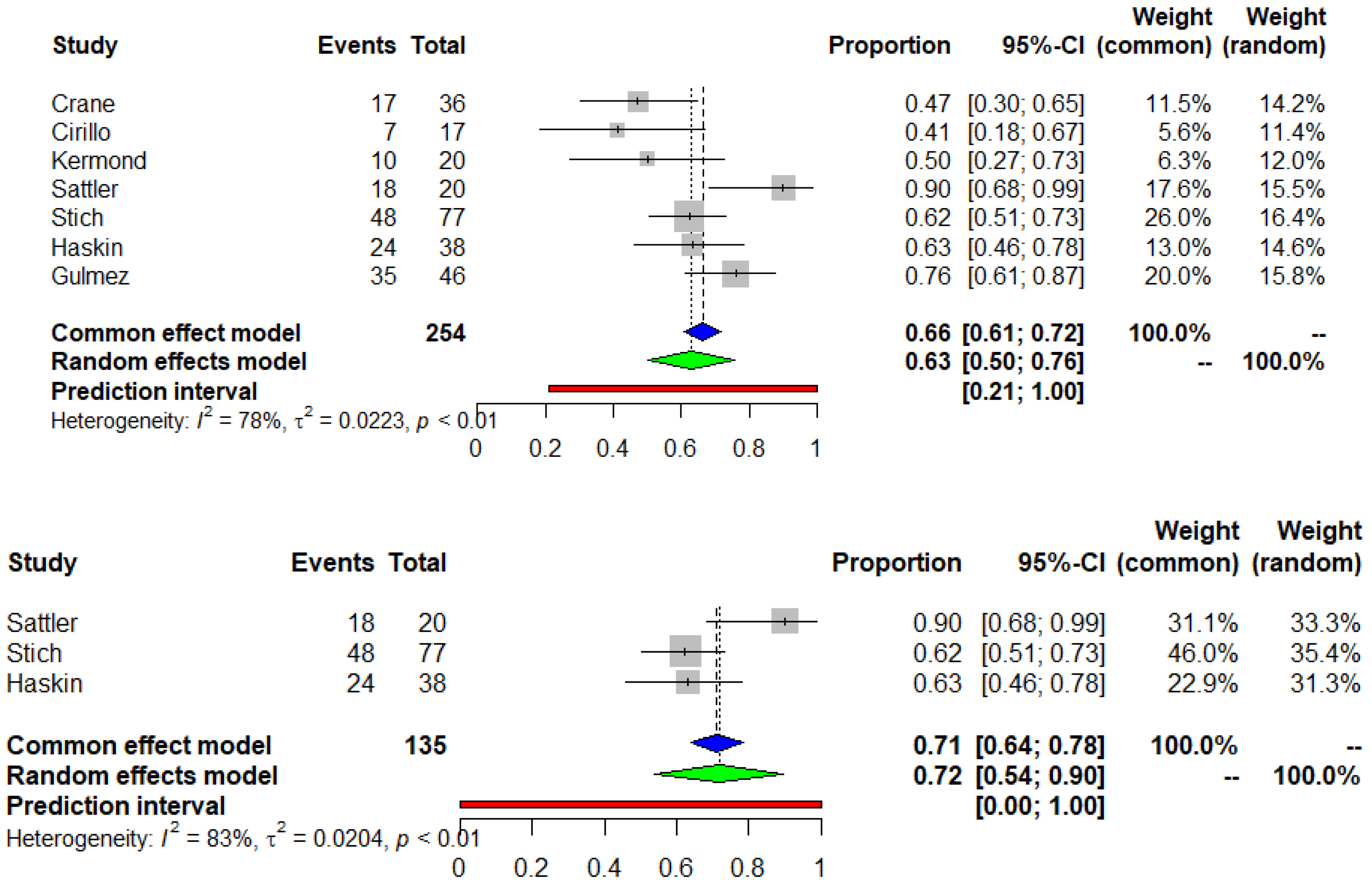
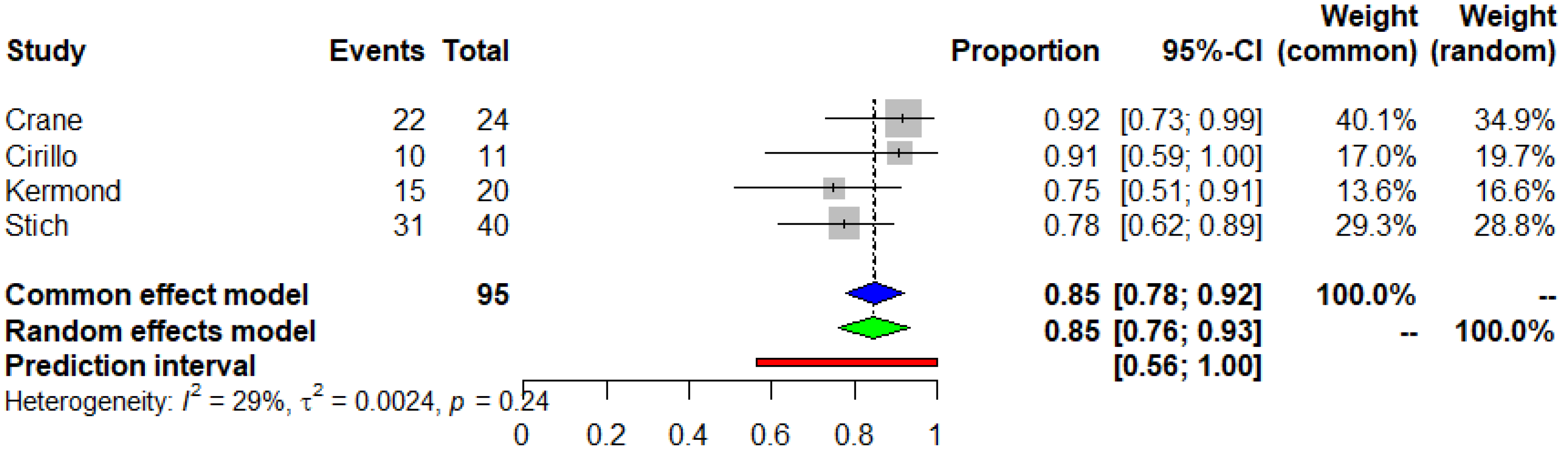
3.3. Seroconversion after the Second and the Third Dose of COVID-19 mRNA Vaccine
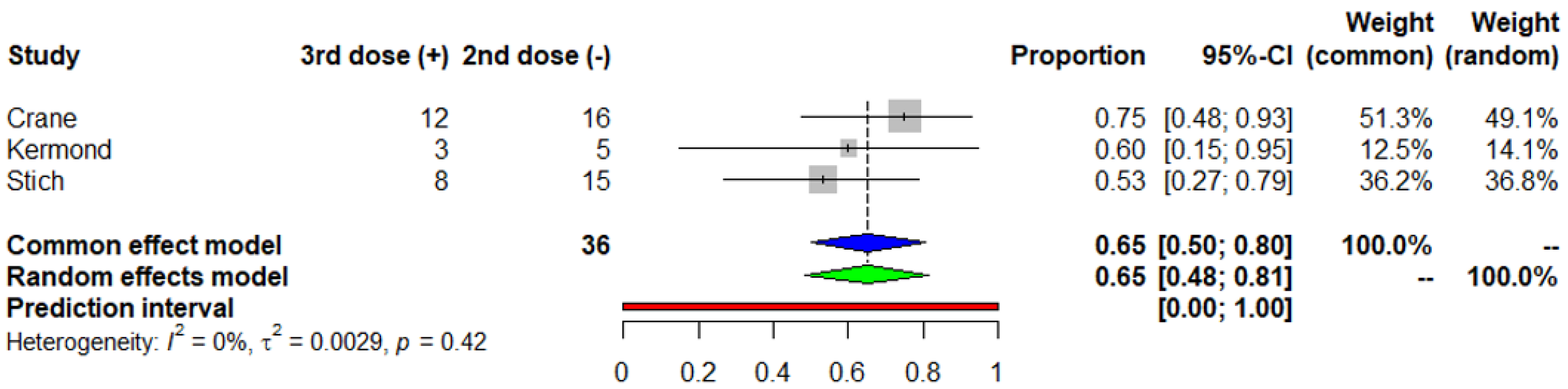
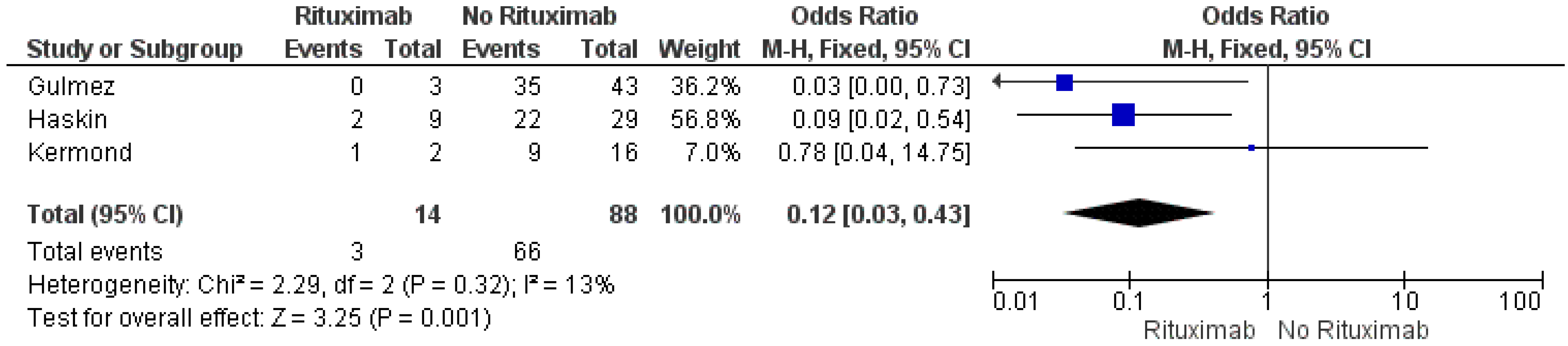
3.4. Risk Factors of no Seroconversion after the Second Dose of the COVID-19 mRNA Vaccine
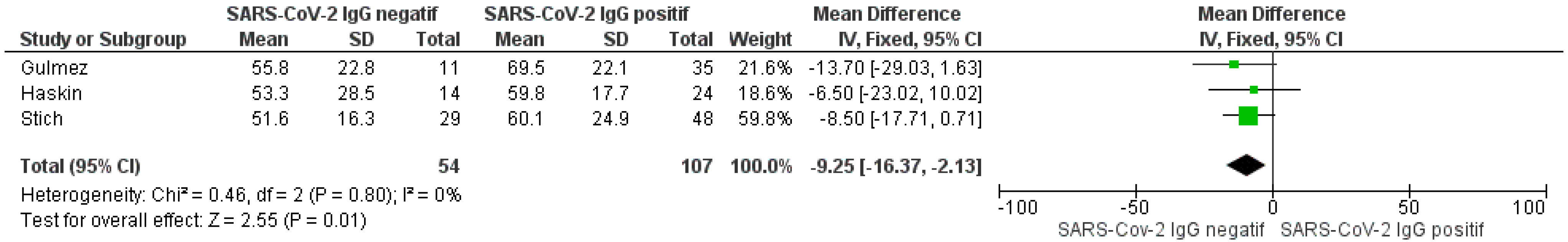
3.5. Neutralization Activity
3.6. Adverse Events and Renal Outcomes
3.7. Comparison of Immune Response in KTRs and Controls
3.8. Comparison of Serologic Response between Vaccinated and Naturally Infected Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oomen, L.; Bootsma-Robroeks, C.; Cornelissen, E.; de Wall, L.; Feitz, W. Pearls and Pitfalls in Pediatric Kidney Transplantation After 5 Decades. Front Pediatr. 2022, 10, 856630. [Google Scholar] [CrossRef] [PubMed]
- Verghese, P. Pediatric kidney transplantation: A historical review. Pediatr. Res. 2017, 81, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Bonthuis, M.; Vidal, E.; Bjerre, A.; Aydoğ, Ö.; Baiko, S.; Garneata, L.; Guzzo, I.; Heaf, J.G.; Jahnukainen, T.; Lilien, M.; et al. Ten-year trends in epidemiology and outcomes of pediatric kidney replace-ment therapy in Europe: Data from the ESPN/ERA-EDTA Registry. Pediatr. Nephrol. 2021, 36, 2337–2348. [Google Scholar] [CrossRef] [PubMed]
- Lentine, K.L.; Smith, J.M.; Miller, J.M.; Bradbrook, K.; Larkin, L.; Weiss, S.; Handarova, D.K.; Temple, K.; Israni, A.K.; Snyder, J.J. OPTN/SRTR 2021 Annual Data Report: Kidney. Am. J. Transplant. 2023, 23 (Suppl. 1), S21–S120. [Google Scholar] [CrossRef]
- Craig-Schapiro, R.; Salinas, T.; Lubetzky, M.; Abel, B.T.; Sultan, S.; Lee, J.R.; Kapur, S.; Aull, M.J.; Dadhania, D.M. COVID-19 outcomes in patients waitlisted for kidney transplantation and kidney transplant recipients. Am. J. Transplant. 2021, 21, 1576–1585. [Google Scholar] [CrossRef]
- Clarke, C.; Lucisano, G.; Prendecki, M.; Gleeson, S.; Martin, P.; Ali, M.; McAdoo, S.P.; Lightstone, L.; Ashby, D.; Charif, R.; et al. Informing the Risk of Kidney Transplantation Versus Remaining on the Waitlist in the Coronavirus Disease 2019 Era. Kidney Int. Rep. 2021, 6, 46–55. [Google Scholar] [CrossRef]
- Hilbrands, L.B.; Duivenvoorden, R.; Vart, P.; Franssen, C.F.M.; Hemmelder, M.H.; Jager, K.J.; Kieneker, L.M.; Noordzij, M.; Pena, M.J.; Vries, H.; et al. COVID-19-related mortality in kidney transplant and dialysis patients: Results of the ERACODA collaboration. Nephrol. Dial. Transplant. 2020, 35, 1973–1983. [Google Scholar] [CrossRef]
- Gagliardi, I.; Patella, G.; Michael, A.; Serra, R.; Provenzano, M.; Andreucci, M. COVID-19 and the Kidney: From Epidemiology to Clinical Practice. J. Clin. Med. 2020, 9, 2506. [Google Scholar] [CrossRef]
- Brodin, P. SARS-CoV-2 infections in children: Understanding diverse outcomes. Immunity 2022, 55, 201–209. [Google Scholar] [CrossRef]
- Hogan, J.; Kwon, T.; Paye-Jaouen, A.; Fait, C.; Cointe, A.; Baudouin, V. Kidney Transplantation in a COVID-19-positive Pediatric Recipient. Transplantation 2021, 105, e74–e75. [Google Scholar] [CrossRef]
- Teoh, C.W.; Gaudreault-Tremblay, M.M.; Blydt-Hansen, T.D.; Goldberg, A.; Arora, S.; Feber, J.; Langlois, V.; Ruhl, M.; Phan, V.; Morgan, C.; et al. Management of Pediatric Kidney Transplant Patients During the COVID-19 Pandemic: Guidance From the Canadian Society of Transplantation Pediatric Group. Can. J. Kidney Health Dis. 2020, 7, 2054358120967845. [Google Scholar] [CrossRef] [PubMed]
- Marlais, M.; Wlodkowski, T.; Vivarelli, M.; Pape, L.; Tönshoff, B.; Schaefer, F.; Tullus, K. The severity of COVID-19 in children on immunosuppressive medication. Lancet Child. Adolesc. Health. 2020, 4, e17–e18. [Google Scholar] [CrossRef]
- Canpolat, N.; Yıldırım, Z.Y.; Yıldız, N.; Taşdemir, M.; Göknar, N.; Evrengül, H.; Gülmez, R.; Aksu, B.; Dursun, H.; Özçelik, G.; et al. COVID-19 in pediatric patients undergoing chronic dialysis and kidney transplantation. Eur. J. Pediatr. 2022, 181, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Rana, R.; Rana, D.S.; Gupta, A.; Sachdeva, M.P. SARS-CoV-2 in Kidney Transplant Recipients: A Systematic Review. Transplantology 2022, 3, 33–48. [Google Scholar] [CrossRef]
- Kotton, C.N. Immunization after kidney transplantation—What is necessary and what is safe? Nat. Rev. Nephrol. 2014, 10, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Grupper, A.; Rabinowich, L.; Schwartz, D.; Schwartz, I.F.; Ben-Yehoyada, M.; Shashar, M.; Katchman, E.; Halperin, T.; Turner, D.; Goykhman, Y.; et al. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am. J. Transplant. 2021, 21, 2719–2726. [Google Scholar] [CrossRef]
- Banerjee, S.; Dissanayake, P.V.; Abeyagunawardena, A.S. Vaccinations in children on immunosuppressive medications for renal disease. Pediatr. Nephrol. 2016, 31, 1437–1448. [Google Scholar] [CrossRef]
- Crane, C.; Phebus, E.; Ingulli, E. Antibody response to 2- and 3-dose SARS-CoV-2 mRNA vaccination in pediatric and adolescent kidney transplant recipients. Pediatr. Nephrol. 2023, 38, 611–614. [Google Scholar] [CrossRef]
- Cirillo, L.; Citera, F.; Mazzierli, T.; Becherucci, F.; Terlizzi, V.; Lodi, L.; Buti, E.; Romagnani, P. Response to Third Dose of Vaccine Against SARS-CoV-2 in Adolescent and Young Adult Kidney Transplant Recipients. Transplantation 2022, 106, E386–E387. [Google Scholar] [CrossRef]
- Kermond, R.F.; Ozimek-Kulik, J.E.; Kim, S.; Alexander, S.I.; Hahn, D.; Kesson, A.; Wood, N.; McCarthy, H.J.; Durkan, A.M. Immunologic response to SARS-CoV-2 mRNA vaccination in pediatric kidney transplant recipients. Pediatr. Nephrol. 2023, 38, 859–866. [Google Scholar] [CrossRef]
- Sattler, A.; Thumfart, J.; Tóth, L.; Schrezenmeier, E.; Proß, V.; Stahl, C.; Siegle, J.; He, A.; Thole, L.M.L.; Ludwig, C.; et al. SARS-CoV2 mRNA Vaccine-Specific B-, T- and Humoral Responses in Adolescents After Kidney Transplantation. Transpl. Int. 2022, 35, 10677. [Google Scholar] [CrossRef] [PubMed]
- Haskin, O.; Ashkenazi-Hoffnung, L.; Ziv, N.; Borovitz, Y.; Dagan, A.; Levi, S.; Koren, G.; Hamdani, G.; Levi-Erez, D.; Landau, D.; et al. Serological Response to the BNT162b2 COVID-19 mRNA Vaccine in Adolescent and Young Adult Kidney Transplant Recipients. Transplantation 2021, 105, E226–E233. [Google Scholar] [CrossRef] [PubMed]
- Stich, M.; Di Cristanziano, V.; Tönshoff, B.; Weber, L.T.; Dötsch, J.; Rammer, M.T.; Rieger, S.; Heger, E.; Garbade, S.F.; Burgmaier, K.; et al. Humoral immune response and live-virus neutralization of the SARS-CoV-2 omicron (BA.1) variant after COVID-19 mRNA vaccination in children and young adults with chronic kidney disease. Pediatr Nephrol. 2023, 38, 1935–1948. [Google Scholar] [CrossRef] [PubMed]
- Gulmez, R.; Ozbey, D.; Agbas, A.; Aksu, B.; Yildiz, N.; Uckardes, D.; Saygili, S.; Yilmaz, E.K.; Yildirim, Z.Y.; Tasdemir, M.; et al. Humoral and cellular immune response to SARS-CoV-2 mRNA BNT162b2 vaccine in pediatric kidney transplant recipients compared with dialysis patients and healthy children. Pediatr Nephrol. 2023, 38, 2199–2208. [Google Scholar] [CrossRef] [PubMed]
- Benning, L.; Morath, C.; Bartenschlager, M.; Nusshag, C.; Kälble, F.; Buylaert, M.; Schaier, M.; Beimler, J.; Klein, K.; Grenz, J.; et al. Neutralization of SARS-CoV-2 Variants of Concern in Kidney Transplant Recipients after Standard COVID-19 Vaccination. Clin. J. Am. Soc. Nephrol. 2022, 17, 98–106. [Google Scholar] [CrossRef]
- Rozen-Zvi, B.; Yahav, D.; Agur, T.; Zingerman, B.; Ben-Zvi, H.; Atamna, A.; Tau, N.; Mashraki, T.; Nesher, E.; Rahamimov, R. Antibody response to SARS-CoV-2 mRNA vaccine among kidney transplant recipients: A prospective cohort study. Clin. Microbiol. Infect. 2021, 27, 1173.e1–1173.e4. [Google Scholar] [CrossRef]
- Boyarsky, B.J.; Werbel, W.A.; Avery, R.K.; Tobian, A.A.R.; Massie, A.B.; Segev, D.L.; Garonzik-Wang, J.M. Antibody Response to 2-Dose SARS-CoV-2 mRNA Vaccine Series in Solid Organ Transplant Recipients. JAMA 2021, 325, 2204–2206. [Google Scholar] [CrossRef]
- Ślizień, Z.; Muchlado, M.; Kubanek, A.; Biedunkiewicz, B.; Renke, M.; Komorowska, K.; Dębska-Ślizień, A.; Tylicki, L. Safety and Tolerability of mRNA COVID-19 Vaccines in Kidney Transplant Recipients. Transplant. Proc. 2022, 54, 878–883. [Google Scholar] [CrossRef]
- Grupper, A.; Katchman, E.; Ben-Yehoyada, M.; Rabinowich, L.; Schwartz, D.; Schwartz, I.F.; Shashar, M.; Halperin, T.; Turner, D.; Goykhman, Y.; et al. Kidney transplant recipients vaccinated before transplantation maintain superior humoral response to SARS-CoV-2 vaccine. Clin. Transplant. 2021, 35, e14478. [Google Scholar] [CrossRef]
- Hookham, L.; Lee, H.C.; Patel, D.A.; Coelho, M.; Giglio, N.; Le Doare, K.; Pannaraj, P.S. Vaccinating Children against SARS-CoV-2: A Literature Review and Survey of International Experts to Assess Safety, Efficacy and Perceptions of Vaccine Use in Children. Vaccines 2022, 11, 78. [Google Scholar] [CrossRef]
- Mehrabi Nejad, M.M.; Shobeiri, P.; Dehghanbanadaki, H.; Tabary, M.; Aryannejad, A.; Haji Ghadery, A.; Shabani, M.; Moosaie, F.; SeyedAlinaghi, S.; Rezaei, N. Seroconversion following the first, second, and third dose of SARS-CoV-2 vaccines in immunocompromised population: A systematic review and meta-analysis. Virol. J. 2022, 19, 132. [Google Scholar] [CrossRef] [PubMed]
- Kreuzberger, N.; Hirsch, C.; Andreas, M.; Böhm, L.; Bröckelmann, P.J.; Di Cristanziano, V.; Golinski, M.; Hausinger, R.I.; Mellinghoff, S.; Lange, B.; et al. Immunity after COVID-19 vaccination in people with higher risk of compromised immune status: A scoping review. Cochrane Database Syst. Rev. 2022, 8, CD015021. [Google Scholar] [PubMed]
- Van Assen, S.; Holvast, A.; Benne, C.A.; Posthumus, M.D.; Van Leeuwen, M.A.; Voskuyl, A.E.; Blom, M.; Risselada, A.P.; de Haan, A.; Westra, J.; et al. Humoral responses after influenza vaccination are severely reduced in patients with rheumatoid arthritis treated with rituximab. Arthritis Rheum. 2010, 62, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Arad, U.; Tzadok, S.; Amir, S.; Mandelboim, M.; Mendelson, E.; Wigler, I.; Sarbagil-Maman, H.; Paran, D.; Caspi, D.; Elkayam, O. The cellular immune response to influenza vaccination is preserved in rheumatoid arthritis patients treated with rituximab. Vaccine 2011, 29, 1643–1648. [Google Scholar] [CrossRef]
- Prendecki, M.; Willicombe, M.; McAdoo, S.P. COVID-19 vaccination in patients with immunity-mediated kidney disease. Nat. Rev. Nephrol. 2021, 17, 790–791. [Google Scholar] [CrossRef]
- Connolly, C.M.; Chiang, T.P.; Boyarsky, B.J.; Ruddy, J.A.; Teles, M.; Alejo, J.L.; Massie, A.; Werbel, W.A.; Shah, A.A.; Christopher-Stine, L.; et al. Temporary hold of mycophenolate augments humoral response to SARS-CoV-2 vaccination in patients with rheumatic and musculoskeletal diseases: A case series. Ann. Rheum Dis. 2022, 81, 293–295. [Google Scholar] [CrossRef]
- Schrezenmeier, E.; Rincon-Arevalo, H.; Jens, A.; Stefanski, A.L.; Hammett, C.; Osmanodja, B.; Koch, N.; Zukunft, B.; Beck, J.; Oellerich, M.; et al. Temporary antimetabolite treatment hold boosts SARS-CoV-2 vaccination-specific humoral and cellular immunity in kidney transplant recipients. JCI Insight 2022, 7, e157836. [Google Scholar] [CrossRef]
- Osmanodja, B.; Ronicke, S.; Budde, K.; Jens, A.; Hammett, C.; Koch, N.; Seelow, E.; Waiser, J.; Zukunft, B.; Bachmann, F.; et al. Serological Response to Three, Four and Five Doses of SARS-CoV-2 Vaccine in Kidney Transplant Recipients. J. Clin. Med. 2022, 11, 2565. [Google Scholar] [CrossRef]
- Schröder, D.; Heinemann, S.; Heesen, G.; Klawonn, F.; Mikuteit, M.; Niewolik, J.; Steffens, S.; Behrens, G.; Jablonka, A.; Müller, F. Who is pausing immunosuppressive medication for COVID-19 vaccination? Results of an exploratory observational trial. Eur. J. Med. Res. 2022, 27, 97. [Google Scholar] [CrossRef]
- Soresina, A.; Moratto, D.; Chiarini, M.; Paolillo, C.; Baresi, G.; Focà, E.; Bezzi, M.; Baronio, B.; Giacomelli, M.; Badolato, R. Two X-linked agammaglobulinemia patients develop pneumonia as COVID-19 manifestation but recover. Pediatr. Allergy Immunol. 2020, 31, 565–569. [Google Scholar] [CrossRef]
- Quinti, I.; Lougaris, V.; Milito, C.; Cinetto, F.; Pecoraro, A.; Mezzaroma, I.; Mastroianni, C.M.; Turriziani, O.; Bondioni, M.P.; Filippini, M.; et al. A possible role for B cells in COVID-19? Lesson from patients with agammaglobulinemia. J. Allergy Clin. Immunol. 2020, 146, 211–213.e4. [Google Scholar] [CrossRef] [PubMed]
- Terada, K.; Hagihara, K.; Oishi, T.; Miyata, I.; Akaike, H.; Ogita, S.; Ohno, N.; Ouchi, K. Cellular and humoral immunity after vaccination or natural mumps infection. Pediatr. Int. 2017, 59, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Honeyman, M.C.; Forrest, J.M.; Dorman, D.C. Cell-mediated immune response following natural rubella and rubella vaccination. Clin. Exp. Immunol. 1974, 17, 665–671. [Google Scholar] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]


| First Author | Study Design | Patient Age (Years) | Time Post KT (Years) | Patients | Characteristics of Controls | Type of Vaccine | |||
|---|---|---|---|---|---|---|---|---|---|
| 2nd Dose | 3rd Dose | ||||||||
| N | Time of IgG Measurement (Days) | N | Time of IgG Measurement (Days) | ||||||
| Crane [18] | Retrospective and prospective cohort | Median (IQR):18 (15–20) | Median (IQR): 5 (2–9) | 43 (7 with prior infection) | Median (IQR):56 (30–85) | 26 (2 with prior infection, 16 not responded to 2nd dose) | Median (IQR):39 (28–59) | - | 40 (93%) patients received BNT162b2, 2 (5%) mRNA1273, and 1 patient a mixed vaccine series |
| Cirillo [19] | Retrospective cohort and case-control | Mean (SD):19(2) | NA | 18 (1 with prior infection) | NA | 12 (1 with prior infection) | NA | Healthy controls | mRNA |
| Kermond [20] | Retrospective cohort | Median (IQR):15 (12–16) | Median (range): 11 (2 months–14 years) | 20 | Median (IQR): 38.5 (32.5–57.5) | 20 (5 not responded to 2nd dose) | Median (IQR): 44 (40–52) | - | BNT162b2 |
| Sattler [21] | Retrospective cohort and case-control | Mean (SD):14.17 (1.31) | Mean (SD): 7 (4.1) | 20 | Mean (SD): 39.30 (11.06) | - | - | 13 healthy controls | BNT162b2 |
| Haskin [22] | Prospective cohort and case-control | Mean (SD):18(3) | Mean (SD): 7.3 (5.6) | 38 | Median (IQR): 37 (20.5–53) | - | - | 14 KTRs with prior COVID-19 infection | BNT162b2 |
| Stich [23] | Retrospective cohort and case-control | Median (range): 14.1 (5–30) | NA | 77 | Median (IQR): 34 (22–63) | 40 (15 not responded to 2nd dose) | NA | 26 CKD patients with IS 20 CKD patients without IS | BNT162b2 |
| Gulmez [24] | Prospective cohort and case control | Mean (SD): 15.9(2.86) | NA | 46 | Median (range): 8 weeks (7–14 weeks) | - | - | 19 KTRs with prior COVID-19 infection 19 patients on dialysis 19 healthy controls | BNT162b2 |
| First Authors | Selection | Compatibility | Outcome | Overall NOS Score | ||||
|---|---|---|---|---|---|---|---|---|
| Representativeness of the Exposed Cohort | Ascertainment of Exposure | Absent Outcome at Study Initiation | Assessment of Outcome | Follow-Up Duration | Adequacy of Follow-Up | |||
| Crane [18] | * | * | ** | * | * | * | 7 | |
| Cirillo [19] | * | * | * | * | * | 5 | ||
| Kermond [20] | * | * | ** | * | * | * | 7 | |
| Sattler [21] | * | * | * | ** | * | * | * | 8 |
| Haskin [22] | * | * | * | ** | * | * | * | 8 |
| Stich [23] | * | * | * | ** | * | * | * | 8 |
| Gulmez [24] | * | * | ** | * | * | * | 7 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emmanouilidou-Fotoulaki, E.; Karava, V.; Dotis, J.; Kondou, A.; Printza, N. Immunologic Response to SARS-CoV-2 Vaccination in Pediatric Kidney Transplant Recipients: A Systematic Review and Meta-Analysis. Vaccines 2023, 11, 1080. https://doi.org/10.3390/vaccines11061080
Emmanouilidou-Fotoulaki E, Karava V, Dotis J, Kondou A, Printza N. Immunologic Response to SARS-CoV-2 Vaccination in Pediatric Kidney Transplant Recipients: A Systematic Review and Meta-Analysis. Vaccines. 2023; 11(6):1080. https://doi.org/10.3390/vaccines11061080
Chicago/Turabian StyleEmmanouilidou-Fotoulaki, Elpida, Vasiliki Karava, John Dotis, Antonia Kondou, and Nikoleta Printza. 2023. "Immunologic Response to SARS-CoV-2 Vaccination in Pediatric Kidney Transplant Recipients: A Systematic Review and Meta-Analysis" Vaccines 11, no. 6: 1080. https://doi.org/10.3390/vaccines11061080
APA StyleEmmanouilidou-Fotoulaki, E., Karava, V., Dotis, J., Kondou, A., & Printza, N. (2023). Immunologic Response to SARS-CoV-2 Vaccination in Pediatric Kidney Transplant Recipients: A Systematic Review and Meta-Analysis. Vaccines, 11(6), 1080. https://doi.org/10.3390/vaccines11061080






