Efficacy of High-Dose Polyclonal Intravenous Immunoglobulin in COVID-19: A Systematic Review
Abstract
:1. Introduction
2. Material and Methods
2.1. Review Question/Objective
2.2. Inclusion and Exclusion Criteria
2.3. Clinical Setting and Participants
2.4. Intervention and Outcomes
2.5. Search Methods
2.6. Study Selection and Data Extraction
2.7. Assessment of the Methodological Quality of Published Clinical Studies
2.8. Effect of Intervention
2.9. Subgroup Analyses
2.10. ‘Summary of Findings’ Tables
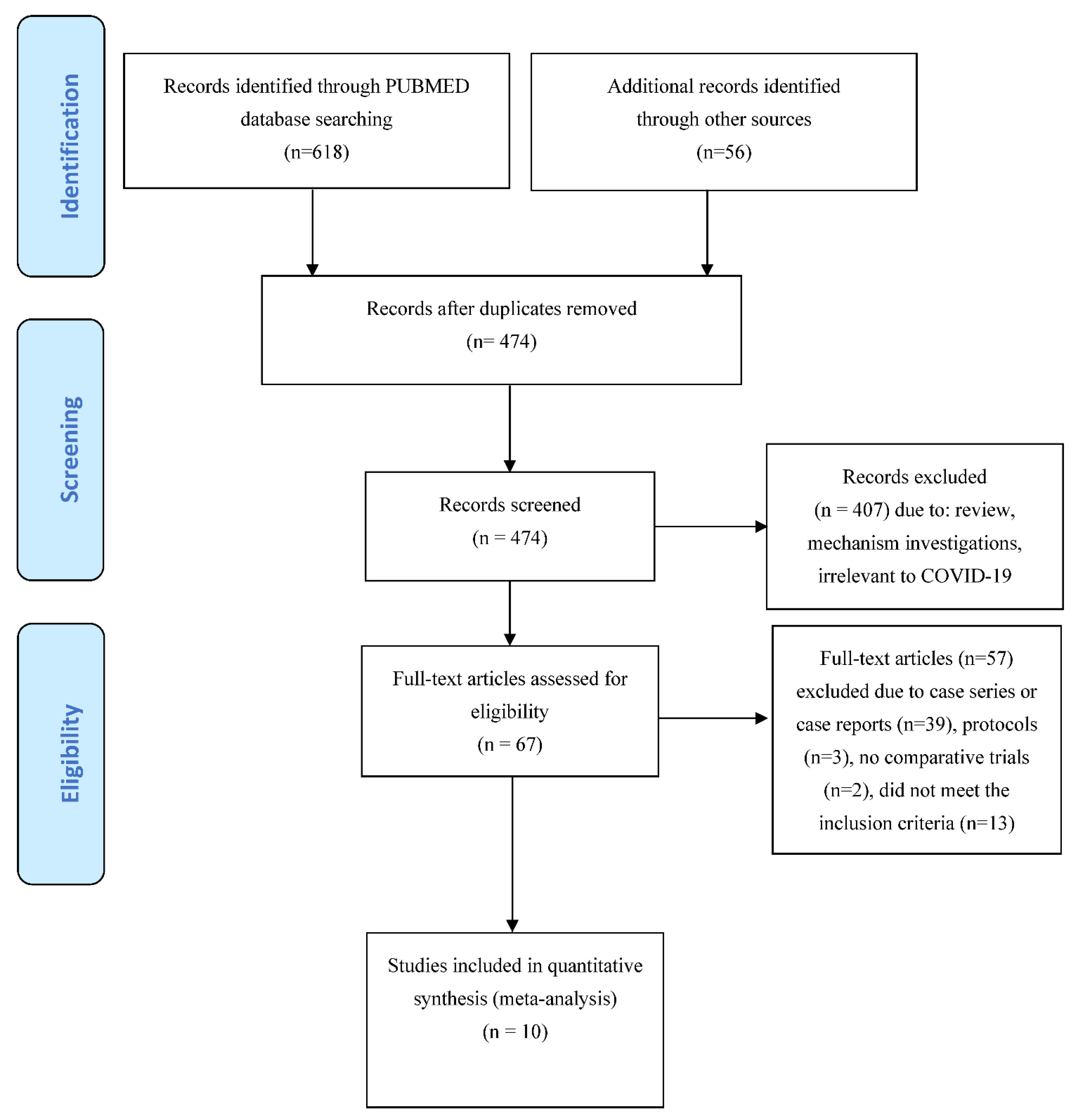
3. Results
| First Author, Year [Ref.] | Type of Study | Disease Severity | Population Size (Intervention/Control) | Single IVIG Dose per Day (Intervention/Control) | Duration (Days) | Cumulative Dose | Control | Safety | Main Results |
|---|---|---|---|---|---|---|---|---|---|
| Cao, 2021 [23] | RCS | Severe COVID-19 | 26/89 | 0.4–1 g/kg | 2–5 days | 2 g/kg | ST | No AEs | High-dose IVIG reduced 28-day mortality (HR 0.24, 95% CI 0.06–0.99; p < 0.001). Early treatment (within 7 days of onset) was associated with greater benefit |
| Esen, 2021 [24] | RCS | Severe COVID-19 | 51/42 | 0.4 g/kg * | 5 days | 2 g/kg | ST | NR | IVIG significantly prolonged median survival time (68 versus 18 days, p = 0.014) |
| Gharebaghi, 2020 [25] | RCT | Severe COVID-19 | 30/29 | 0.3 g/kg * | 3 days | 0.9 g/kg | placebo | NR | IVIG significantly reduced mortality rate (aOR 0.003, 95% CI 0.001–0.815; p = 0.042) |
| Hou, 2021 [26] | RCS | Severe COVID-19 | 47/66 | 0.5 g/kg | NR | NR | ST | NR | IVIG did not improve in-hospital mortality rates or the need for mechanical ventilation |
| Huang, 2021 [27] | RCS | Non-severe COVID-19 | 45/594 | 0.13 g/kg (8 patients) * | 3 days | 0.5 g/kg | ST | NR | No benefit was observed with IVIG in terms of mortality rate, progression to severe disease or length of hospital stay |
| 0.13 g/kg (13 patients) * | 5 days | 0.7 g/kg | |||||||
| 0.26 g/kg (16 patients) * | 3 days | 0.8 g/kg | |||||||
| 0.26 g/kg (8 patients) * | 5 days | 1.3 g/kg | |||||||
| Liu, 2021 [28] | RCS | Severe COVID-19 | 421/429 | 0.13 g/day | 9.5 days | 1.3 g/kg | ST | NR | IVIG was not associated with significant changes in 28-day mortality in severe COVID-19 patients |
| Raman, 2021 [29] | RCT | Non-severe COVID-19 | 50/50 | 0.4 g/kg | 5 days | 2 g/kg | ST | 17 (34%) mild to moderate | Duration of hospital stay was significantly lower in IVIG group (7.7 vs. 17.5 days, p = 0.0001) |
| Sakoulas, 2020 [30] | RCT | Severe COVID-19 | 16/17 | 0.5 g/kg | 3 days | 1.5 g/kg | ST | No AEs | IVIG improved hypoxia and reduced hospital length of stay and progression to mechanical ventilation |
| Shao, 2020 [31] | RCS | Severe or critical COVID-19 | 174/151 | 0.1 g/kg (100 patients) 0.5 g/kg (74 patients) | 5–15 days (not specified according to daily dose) | 0.5–5 g/kg (not specified according to daily dose) | ST | NR | Early administration (≤7 days after hospital admission) with high dose (>15 g/day) of IVIG significantly reduced 60-day mortality |
| Tabarsi, 2021 [32] | RCT | Severe COVID-19 | 52/32 | 0.4 g/kg | 3 days | 1.2 g/kg | ST | NR | No benefit was observed with IVIG in terms of mortality rate and need for mechanical ventilation |
3.1. Risk of Bias in Included Studies
3.2. Effects of Interventions
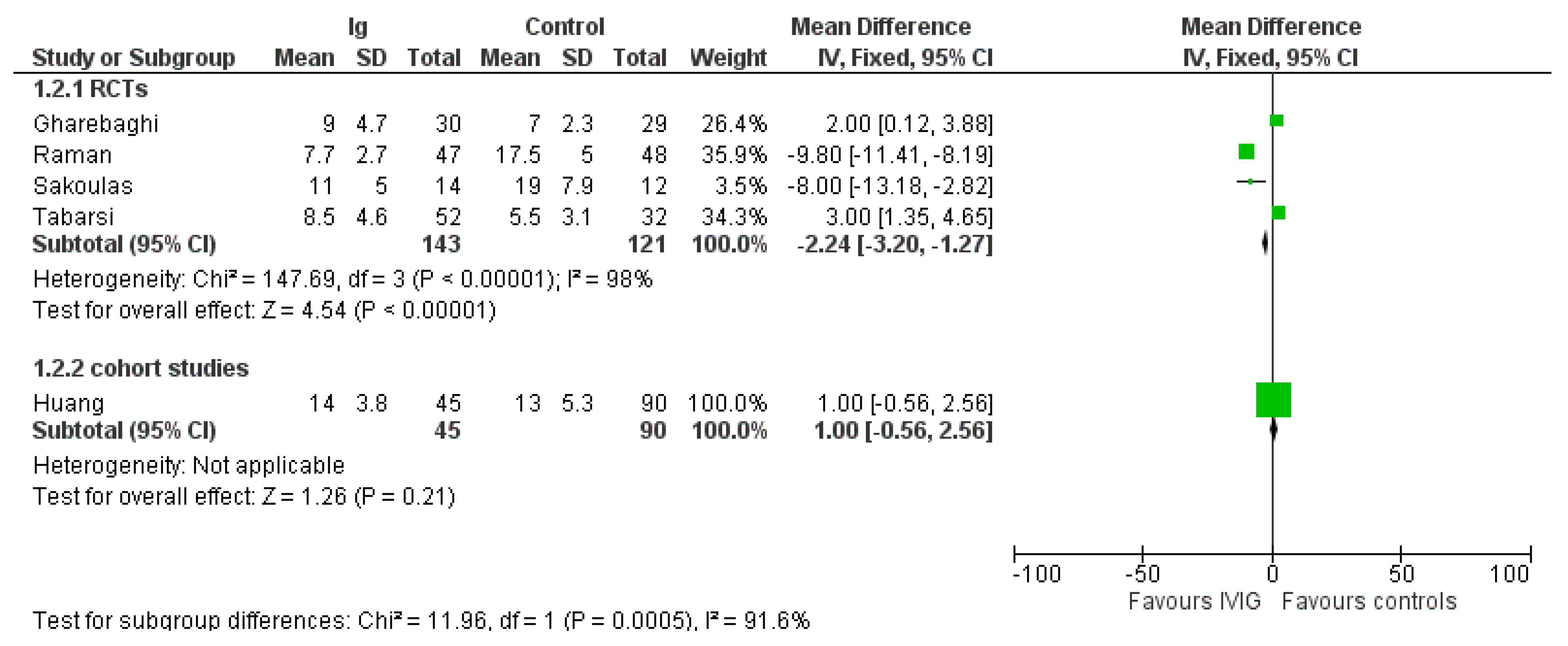
3.3. Mortality
3.4. Length of Hospital Stay
3.5. Adverse Events
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
References
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- WHO. Coronavirus Disease (COVID-19). Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 31 August 2021).
- Tobaiqy, M.; Qashqary, M.; Al-Dahery, S.; Mujallad, A.; Hershan, A.; Kamal, M.; Helmi, N. Therapeutic management of patients with COVID-19: A systematic review. Infect. Prev. Pract. 2020, 2, 100061. [Google Scholar] [CrossRef]
- Heustess, A.M.; Allard, M.A.; Thompson, D.K.; Fasinu, P.S. Clinical Management of COVID-19: A Review of pharmacological treatment options. Pharmaceuticals 2021, 14, 520. [Google Scholar] [CrossRef] [PubMed]
- Franchini, M.; Liumbruno, G.M.; Piacentini, G.; Glingani, C.; Zaffanello, M. The three pillars of COVID-19 convalescent plasma therapy. Life 2021, 11, 354. [Google Scholar] [CrossRef]
- Focosi, D.; Franchini, M. COVID-19 convalescent plasma therapy: Hit fast, hit hard! Vox Sang. 2021, 116, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-T.; Sheng, W.-H.; Fang, C.-T.; Chen, Y.-C.; Wang, J.-L.; Yu, C.-J.; Chang, S.-C.; Yang, P.-C. Clinical manifestations, laboratory findings, and treatment outcomes of SARS patients. Emerg. Infect. Dis. 2004, 10, 818–824. [Google Scholar] [CrossRef]
- Liu, X.; Cao, W.; Li, T. High-Dose Intravenous Immunoglobulins in the Treatment of Severe Acute Viral Pneumonia: The Known Mechanisms and Clinical Effects. Front. Immunol. 2020, 11, 1660. [Google Scholar] [CrossRef]
- Soy, M.; Keser, G.; Atagündüz, P.; Tabak, F.; Atagündüz, I.; Kayhan, S. Cytokine storm in COVID-19: Pathogenesis and overview of anti-inflammatory agents used in treatment. Clin. Rheumatol. 2020, 39, 2085–2094. [Google Scholar] [CrossRef] [PubMed]
- Yaqinuddin, A.; Ambia, A.R.; Elgazzar, T.A.; AlSaud, M.B.M.; Kashir, J. Application of intravenous immunoglobulin (IVIG) to modulate inflammation in critical COVID-19-A theoretical perspective. Med. Hypotheses 2021, 151, 110592. [Google Scholar] [CrossRef]
- Xiang, H.R.; Cheng, X.; Li, Y.; Luo, W.W.; Zhang, Q.Z.; Peng, W.X. Efficacy of IVIG (intravenous immunoglobulin) for corona virus disease 2019 (COVID-19): A meta-analysis. Int. Immunopharmacol. 2021, 96, 107732. [Google Scholar] [CrossRef]
- Moradimajd, P.; Samaee, H.; Sedigh-Maroufi, S.; Kourosh-Aami, M.; Mohsenzadagan, M. Administration of intravenous immunoglobulin in the treatment of COVID-19: A review of available evidence. J. Med. Virol. 2021, 93, 2675–2682. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions–Version 5.1.0 [Updated March 2011]. The Cochrane Collaboration: London, UK, 2011; Available online: www.handbook.cochrane.org (accessed on 1 December 2021).
- Reeves, B.C.; Deeks, J.J.; Higgins, J.P.T.; Wells, G.A. Chapter 13: Including non-randomized studies. In Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (Updated March 2011); Higgins, J.P.T., Green, S., Eds.; The Cochrane Collaboration: London, UK, 2011; Available online: www.handbook.cochrane.org (accessed on 1 December 2021).
- Sterne, J.A.C.; Hernán, M.A.; McAleenan, A.; Reeves, B.C.; Higgins, J.P.T. Chapter 25: Assessing risk of bias in a non-randomized study. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (Updated July 2019); Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: London, UK, 2019; Available online: www.training.cochrane.org/handbook (accessed on 1 December 2021).
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, 4–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.; Thompson, S.; Deeks JAltman, D.T.I. Measuring inconsistency in meta-analyses. BMJ 2003, 32, 557–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schünemann, H.J.; Oxman, A.D.; Higgins, J.P.; Vist, G.E.; Glasziou, P.; Guyatt, G.H. Chapter 11: Presenting results and ’Summary of findings’ tables. In Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (Updated March 2011); Higgins, J.P.T., Green, S., Eds.; The Cochrane Collaboration: London, UK, 2011; Available online: www.handbook.cochrane.org (accessed on 1 December 2021).
- Guyatt, G.H.; Oxman, A.D.; Kunz, R.; Vist, G.E.; Falck-Ytter, Y.; Schünemann, H.J. What is ’quality of evidence’ and why is it important to clinicians? BMJ 2008, 336, 995–998. [Google Scholar] [CrossRef] [Green Version]
- Omma, A.; Erden, A.; Armağan, B.; Güven, S.C.; Karakaş, Ö.; Şahiner, E.S.; Erdem, D.; Izdeş, S.; Ateş, I.; Küçükşahin, O. A single center experience of intravenous immunoglobulin treatment in Covid-19. Int. Immunopharmacol. 2021, 98, 107891. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Cao, S.; Dong, H.; Li, Q.; Chen, E.; Zhang, W.; Yang, L.; Fu, S.; Wang, R. Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID-19. J. Infect. 2020, 81, 318–356. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Liu, X.; Bai, T.; Fan, H.; Hong, K.; Song, H.; Han, Y.; Lin, L.; Ruan, L.; Li, T. High-Dose Intravenous Immunoglobulin as a therapeutic option for deteriorating patients with Coronavirus Disease 2019. Open Forum Infect. Dis. 2020, 7, ofaa102. [Google Scholar] [CrossRef] [Green Version]
- Esen, F.; Özcan, P.E.; Orhun, G.; Polat, Ö.; Anaklı, İ.; Alay, G.; Tuna, V.; Çeliksoy, E.; Kılıç, M.; Mercan, M.; et al. Effects of adjunct treatment with intravenous immunoglobulins on the course of severe COVID-19: Results from a retrospective cohort study. Curr. Med. Res. Opin. 2021, 37, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Gharebaghi, N.; Nejadrahim, R.; Mousavi, S.J.; Sadat-Ebrahimi, S.R.; Hajizadeh, R. The use of intravenous immunoglobulin gamma for the treatment of severe coronavirus disease 2019: A randomized placebo-controlled double-blind clinical trial. BMC Infect. Dis. 2020, 20, 786. [Google Scholar] [CrossRef]
- Hou, X.; Tian, L.; Zhou, L.; Jia, X.; Kong, L.; Xue, Y.; Hao, H.; Meng, X.; Zhang, F.; Dong, X. Intravenous immunoglobulin-based adjuvant therapy for severe COVID-19: A single-center retrospective cohort study. Virol. J. 2021, 18, 101. [Google Scholar] [CrossRef]
- Huang, C.; Fei, L.; Li, W.; Xu, W.; Xie, X.; Li, Q.; Chen, L. Efficacy evaluation of intravenous immunoglobulin in non-severe patients with COVID-19: A retrospective cohort study based on propensity score matching. Int. J. Infect. Dis. 2021, 105, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, Y.; Li, R.; Wu, Z.; Xu, Q.; Li, Z.; Annane, D.; Feng, H.; Huang, S.; Guo, J.; et al. Intravenous immunoglobulin treatment for patients with severe COVID-19: A retrospective multicentre study. Clin. Microbiol. Infect. 2021, 27, 1488–1493. [Google Scholar] [CrossRef]
- Raman, R.S.; Barge, V.B.; Kumar, D.A.; Dandu, H.; Kartha, R.R.; Bafna, V.; Aravinda, V.T.; Raghuram, T.C. A Phase II Safety and Efficacy Study on Prognosis of Moderate Pneumonia in Coronavirus Disease 2019 Patients With Regular Intravenous Immunoglobulin Therapy. J. Infect. Dis. 2021, 223, 1538–1543. [Google Scholar] [CrossRef] [PubMed]
- Sakoulas, G.; Geriak, M.; Kullar, R.; Greenwood, K.L.; Habib, M.; Vyas, A.; Ghafourian, M.; Dintyala, V.N.K.; Haddad, F. Intravenous Immunoglobulin plus methylprednisolone mitigate respiratory morbidity in Coronavirus Disease 2019. Crit. Care Explor. 2020, 2, e0280. [Google Scholar] [CrossRef]
- Shao, Z.; Feng, Y.; Zhong, L.; Xie, Q.; Lei, M.; Liu, Z.; Wang, C.; Ji, J.; Liu, H.; Gu, Z.; et al. Clinical efficacy of intravenous immunoglobulin therapy in critical ill patients with COVID-19: A multicenter retrospective cohort study. Clin. Transl. Immunol. 2020, 9, e1192. [Google Scholar] [CrossRef]
- Tabarsi, P.; Barati, S.; Jamaati, H.; Haseli, S.; Marjani, M.; Moniri, A.; Abtahian, Z.; Dastan, A.; Yousefian, S.; Eskandari, R.; et al. Evaluating the effects of Intravenous Immunoglobulin (IVIg) on the management of severe COVID-19 cases: A randomized controlled trial. Int. Immunopharmacol. 2021, 90, 107205. [Google Scholar] [CrossRef] [PubMed]
- Morgenlander, W.R.; Henson, S.N.; Monaco, D.R.; Chen, A.; Littlefield, K.; Bloch, E.M.; Fujimura, E.; Ruczinski, I.; Crowley, A.R.; Natarajan, H.; et al. Antibody responses to endemic coronaviruses modulate COVID-19 convalescent plasma functionality. J. Clin. Investig. 2021, 131, 146927. [Google Scholar] [CrossRef] [PubMed]
- Greenbaum, U.; Klein, K.; Martinez, F.; Song, J.; Thall, P.F.; Ramdial, J.L.; Knape, C.; Aung, F.M.; Scroggins, J.; Knopfelmacher, A.; et al. High levels of common cold coronavirus antibodies in convalescent plasma are associated with improved survival in COVID-19 patients. Front. Immunol. 2021, 12, 675679. [Google Scholar] [CrossRef]
- Siemieniuk, R.A.; Bartoszko, J.J.; Martinez, J.P.D.; Kum, E.; Qasim, A.; Zeraatkar, D.; Izcovich, A.; Mangala, S.; Ge, L.; Han, M.A.; et al. Antibody and cellular therapies for treatment of covid-19: A living systematic review and network meta-analysis. BMJ 2021, 374, n2231. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.W.; Faulkner, N.; Cornish, G.H.; Rosa, A.; Harvey, R.; Hussain, S.; Earl, C.; Wrobel, A.G.; Benton, D.J.; Roustan, C.; et al. Preexisting and de novo humoral immunity to SARS-CoV-2 in humans. Science 2020, 370, 1339–1343. [Google Scholar] [CrossRef] [PubMed]
- Díez, J.-M.; Romero, C.; Gajardo, R. Currently available intravenous immunoglobulin contains antibodies reacting against severe acute respiratory syndrome coronavirus 2 antigens. Immunotherapy 2020, 12, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Focosi, D.; Rosellini, A.; Spezia, P.G.; Macera, L.; Lanza, M.; Paolicchi, A.; Biagini, D.; Baj, A.; Pistello, M.; Maggi, F. Lack of neutralizing activity in nonconvalescent sera, regardless of ABO blood group anti-A isoagglutin titer. JCV Plus 2021, 1, 100035. [Google Scholar] [CrossRef]


| Immunoglobulin Compared with Standard Treatment for COVID-19 | ||||||
|---|---|---|---|---|---|---|
| Patient or Population: Adults with COVID-19 Settings: Both Outpatients and Hospitalized pts Intervention: IVIG Comparison: Standard Treatment | ||||||
| Outcomes | Illustrative Comparative Risks * (95% CI) | Relative Effect (95% CI) | No. of Participants (Studies) | Quality of the Evidence (GRADE) | Comments | |
| Assumed Risk | Corresponding Risk | |||||
| Control | IVIG | |||||
| Mortality—RCTs (28 days) | Overall population with COVID-19 | RR 0.50 (0.18/1.36) RR 0.35 (0.06/2.10) RR 0.54 (0.14/2.09) | 252 (4) 125 (2) 111 (2) | ⊕⊕⊝⊝ low 1 | It is unclear whether IVIG reduces mortality compared to standard treatment in the overall populations of pts with COVID-19 or in moderate or severe COVID-19 pts | |
| Mean mortality was 28.3% | 14.5% (5.0/38.4%) | |||||
| Low-risk population (pts with moderate disease) | ||||||
| Mean mortality was 6.0% | 2.1% (0.3/12.54%) | |||||
| High-risk population (pts with severe/critical disease) | ||||||
| Mean mortality was 59.5% | 32.1% (8.3/124.3%) | |||||
| Mortality—Cohort studies | Overall population with COVID-19 | RR 0.95 (0.61/1.50) | 6 (1630) | ⊕⊕⊝⊝ low 2 | It is unclear whether IVIG reduces mortality compared to standard treatment in COVID-19. The differences were not significant in subgroup analyses of pts with moderate or severe disease either. | |
| Mean mortality was 26.9% | 25.2% (16.4/40.3%) | |||||
| Length of Hospital stay (days) | The mean hospital stay is 12.25 | 10.1 (9.05/10.98) | RD−2.24 (−3.20/−1.27) | 4 (264) | ⊕⊕⊝⊝ low 2 | IVIG reduces LHS compared to standard treatment. The effect was driven mostly by inclusion of pts with moderate COVID-19 infections. Indeed, in the 2 studies enrolling severe pts (see Supplementary File S2), the difference in LHS favored controls compared to IVIG (RD, 2.57; 95% CIs, 1.33/3.80; p < 0.0001; low quality of evidence), while in studies evaluating moderate pts, the difference favored IVIG compared to controls.(RR, −9.64; 95% Cis, −11.18/−8.1; p < 0.00001; low quality of certainty) |
| Adverse events - Overall AE | The mean occurrence of AE was 12.8% | 12.5% (11.6/13.4%) | RD −0.03 (−0.12/0.06) | 3 (248) | ⊕⊝⊝⊝ very-low 3 | Mean occurrence of AE was similar in IVIG recipients and controls |
| - Serious AE | The mean occurrence of serious AE was 5.9% | 5.9% (5.5/6.3%) | RR 0.00 (−0.04/0.04) | 4 (848) | ⊕⊝⊝⊝ very-low 3 | Mean occurrence of serious AE was similar in IVIG recipients and controls |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Focosi, D.; Franchini, M.; Tuccori, M.; Cruciani, M. Efficacy of High-Dose Polyclonal Intravenous Immunoglobulin in COVID-19: A Systematic Review. Vaccines 2022, 10, 94. https://doi.org/10.3390/vaccines10010094
Focosi D, Franchini M, Tuccori M, Cruciani M. Efficacy of High-Dose Polyclonal Intravenous Immunoglobulin in COVID-19: A Systematic Review. Vaccines. 2022; 10(1):94. https://doi.org/10.3390/vaccines10010094
Chicago/Turabian StyleFocosi, Daniele, Massimo Franchini, Marco Tuccori, and Mario Cruciani. 2022. "Efficacy of High-Dose Polyclonal Intravenous Immunoglobulin in COVID-19: A Systematic Review" Vaccines 10, no. 1: 94. https://doi.org/10.3390/vaccines10010094
APA StyleFocosi, D., Franchini, M., Tuccori, M., & Cruciani, M. (2022). Efficacy of High-Dose Polyclonal Intravenous Immunoglobulin in COVID-19: A Systematic Review. Vaccines, 10(1), 94. https://doi.org/10.3390/vaccines10010094








