Discovery of Antivirals Using Phage Display
Abstract
1. Introduction
2. Phage Display Libraries Used for Antiviral Discovery
2.1. Peptide Libraries
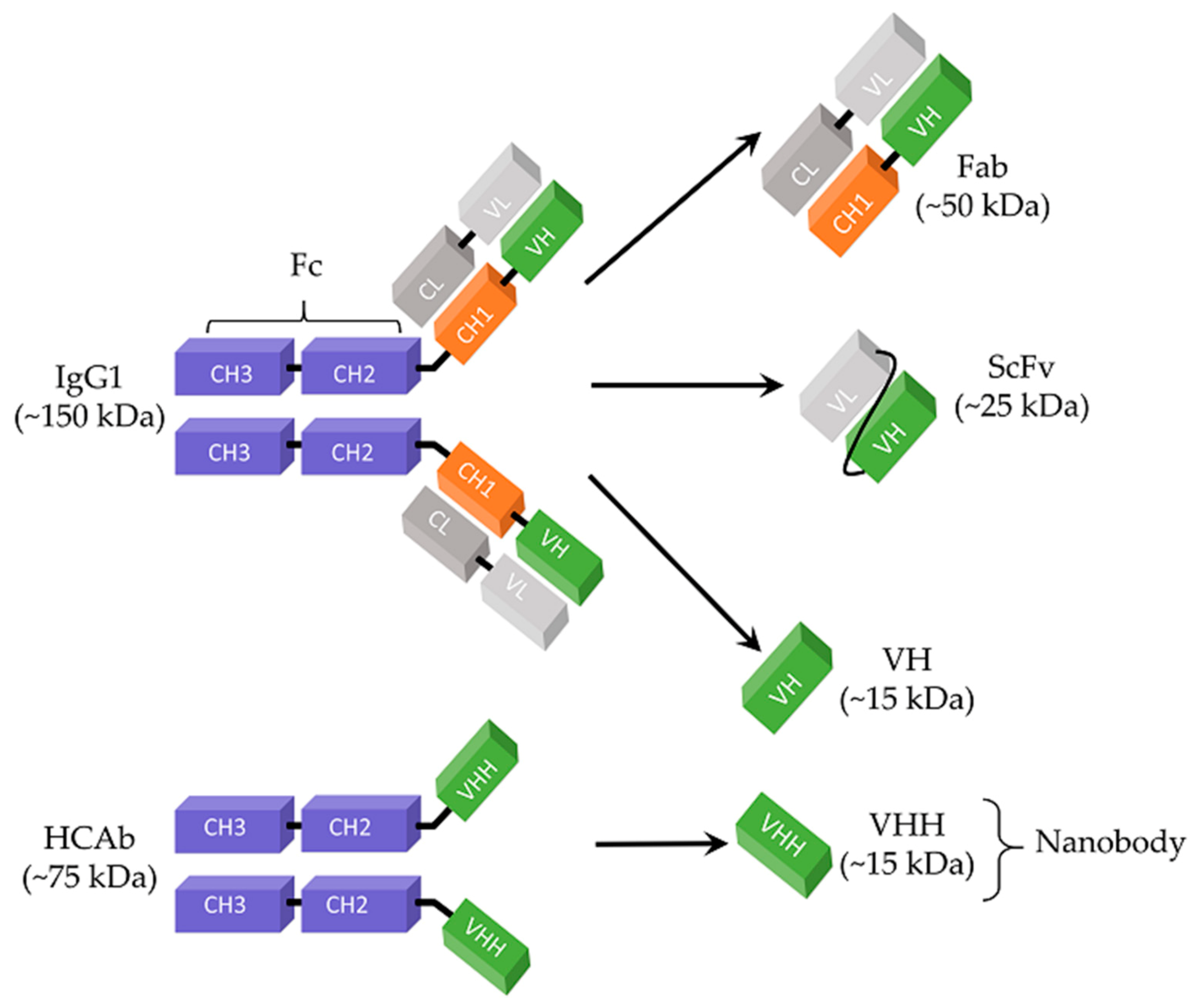
2.2. Antigen-Binding Fragment (Fab) Libraries
2.3. Single-Chain Variable Fragments (ScFv)
2.4. Nanobodies
2.5. Other Protein Scaffolds
3. Strategies to Increase the Potency of Phage Display-Selected Antivirals
3.1. Polyvalent Presentation of Antiviral Peptides
3.2. Modification of the Peptide Backbone to Enhance Stability
3.3. Conversion of Antibody Fragments into Whole IgG Antibodies
3.4. Modification of Antiviral Peptides/Antibodies for Intracellular Delivery
4. Perspective
Funding
Conflicts of Interest
References
- Morens, D.M.; Folkers, G.K.; Fauci, A.S. The challenge of emerging and re-emerging infectious diseases. Nature 2004, 430, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Fauci, A.S. Infectious diseases: Considerations for the 21st century. Clin. Infect. Dis. 2001, 32, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.X.; Bishop-Hurley, S.L.; Cooper, M.A. Development of Anti-Infectives Using Phage Display: Biological Agents against Bacteria, Viruses, and Parasites. Antimicrob. Agents Chemother. 2012, 56, 4569. [Google Scholar] [CrossRef] [PubMed]
- Kilbourne, E.D. Influenza pandemics of the 20th century. Emerg. Infect. Dis. 2006, 12, 9–14. [Google Scholar] [CrossRef]
- Afrough, B.; Dowall, S.; Hewson, R. Emerging viruses and current strategies for vaccine intervention. Clin. Exp. Immunol. 2019, 196, 157–166. [Google Scholar] [CrossRef]
- Ryu, W.-S. New Emerging Viruses. Mol. Virol. Hum. Pathog. Viruses 2017, 289–302. [Google Scholar] [CrossRef]
- Jin, Y.; Lei, C.; Hu, D.; Dimitrov, D.S.; Ying, T. Human monoclonal antibodies as candidate therapeutics against emerging viruses. Front. Med. 2017, 11, 462–470. [Google Scholar] [CrossRef]
- Rohan, H.; McKay, G. The Ebola outbreak in the Democratic Republic of the Congo: Why there is no ‘silver bullet’. Nat. Immunol. 2020, 21, 591–594. [Google Scholar] [CrossRef]
- Delhalle, S.; Schmit, J.C.; Chevigné, A. Phages and HIV-1: From display to interplay. Int. J. Mol. Sci. 2012, 13, 4727–4794. [Google Scholar] [CrossRef]
- Reperant, L.A.; Osterhaus, A. AIDS, Avian flu, SARS, MERS, Ebola, Zika… what next? Vaccine 2017, 35 Pt A, 4470–4474. [Google Scholar] [CrossRef]
- Fauci, A.S. HIV and AIDS: 20 years of science. Nat. Med. 2003, 9, 839–843. [Google Scholar] [CrossRef]
- World Health Organization. Estimated number of people (all ages) living with HIV. In Data on the Size of the HIV/AIDS Epidemic; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Sørensen, M.D.; Sørensen, B.; Gonzalez-Dosal, R.; Melchjorsen, C.J.; Weibel, J.; Wang, J.; Jun, C.W.; Huanming, Y.; Kristensen, P. Severe acute respiratory syndrome (SARS): Development of diagnostics and antivirals. Ann. N. Y. Acad. Sci. 2006, 1067, 500–505. [Google Scholar] [CrossRef]
- Graham, R.L.; Donaldson, E.F.; Baric, R.S. A decade after SARS: Strategies for controlling emerging coronaviruses. Nat. Rev. Microbiol. 2013, 11, 836–848. [Google Scholar] [CrossRef]
- Ying, T.; Li, H.; Lu, L.; Dimitrov, D.S.; Jiang, S. Development of human neutralizing monoclonal antibodies for prevention and therapy of MERS-CoV infections. Microbes Infect. 2015, 17, 142–148. [Google Scholar] [CrossRef]
- Peeri, N.C.; Shrestha, N.; Rahman, M.S.; Zaki, R.; Tan, Z.; Bibi, S.; Baghbanzadeh, M.; Aghamohammadi, N.; Zhang, W.; Haque, U. The SARS, MERS and novel coronavirus (COVID-19) epidemics, the newest and biggest global health threats: What lessons have we learned? Int. J. Epidemiol. 2020, 49, 717–726. [Google Scholar] [CrossRef]
- Fernandez-Garcia, L.; Pacios, O.; González-Bardanca, M.; Blasco, L.; Bleriot, I.; Ambroa, A.; López, M.; Bou, G.; Tomás, M. Viral Related Tools against SARS-CoV-2. Viruses 2020, 12, 1172. [Google Scholar] [CrossRef]
- Datta, P.K.; Liu, F.; Fischer, T.; Rappaport, J.; Qin, X. SARS-CoV-2 pandemic and research gaps: Understanding SARS-CoV-2 interaction with the ACE2 receptor and implications for therapy. Theranostics 2020, 10, 7448–7464. [Google Scholar] [CrossRef]
- Serrano-Aroca, Á.; Takayama, K.; Tuñón-Molina, A.; Seyran, M.; Hassan, S.S.; Pal Choudhury, P.; Uversky, V.N.; Lundstrom, K.; Adadi, P.; Palù, G.; et al. Carbon-Based Nanomaterials: Promising Antiviral Agents to Combat COVID-19 in the Microbial-Resistant Era. ACS Nano 2021, 15, 8069–8086. [Google Scholar] [CrossRef]
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Tulsiani, S.M.; Graham, G.C.; Moore, P.R.; Jansen, C.C.; Van Den Hurk, A.F.; Moore, F.A.; Simmons, R.J.; Craig, S.B. Emerging tropical diseases in Australia: Part 5. Hendra virus. Ann. Trop. Med. Parasitol. 2011, 105, 1–11. [Google Scholar] [CrossRef]
- Sharma, V.; Kaushik, S.; Kumar, R.; Yadav, J.P.; Kaushik, S. Emerging trends of Nipah virus: A review. Rev. Med. Virol. 2019, 29, e2010. [Google Scholar] [CrossRef]
- Letko, M.; Seifert, S.N.; Olival, K.J.; Plowright, R.K.; Munster, V.J. Bat-borne virus diversity, spillover and emergence. Nat. Rev. Microbiol. 2020, 18, 461–471. [Google Scholar] [CrossRef]
- Graham, B.S. Advances in antiviral vaccine development. Immunol. Rev. 2013, 255, 230–242. [Google Scholar] [CrossRef]
- Evans, M.R.; Watson, P.A. Why do older people not get immunised against influenza? A community survey. Vaccine 2003, 21, 2421–2427. [Google Scholar] [CrossRef]
- Schmidt, A.C. Antiviral therapy for influenza: A clinical and economic comparative review. Drugs 2004, 64, 2031–2046. [Google Scholar] [CrossRef]
- De Clercq, E.; Herdewijn, P. Strategies in the design of antiviral drugs. In Pharmaceutical Sciences Encyclopedia; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 1–56. [Google Scholar]
- Pardi, N.; Weissman, D. Development of vaccines and antivirals for combating viral pandemics. Nat. Biomed. Eng. 2020, 4, 1128–1133. [Google Scholar] [CrossRef]
- De Clercq, E.; Li, G. Approved Antiviral Drugs over the Past 50 Years. Clin. Microbiol. Rev. 2016, 29, 695–747. [Google Scholar] [CrossRef]
- Espiritu, M.J.; Collier, A.C.; Bingham, J.-P. A 21st-century approach to age-old problems: The ascension of biologics in clinical therapeutics. Drug Discov. Today 2014, 19, 1109–1113. [Google Scholar] [CrossRef]
- Chaudhuri, S.; Symons, J.A.; Deval, J. Innovation and trends in the development and approval of antiviral medicines: 1987–2017 and beyond. Antivir. Res. 2018, 155, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Oldham, R.K.; Dillman, R.O. Monoclonal antibodies in cancer therapy: 25 years of progress. J. Clin. Oncol. 2008, 26, 1774–1777. [Google Scholar] [CrossRef]
- Mimmi, S.; Maisano, D.; Quinto, I.; Iaccino, E. Phage Display: An Overview in Context to Drug Discovery. Trends Pharmacol. Sci. 2019, 40, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Hamzeh-Mivehroud, M.; Alizadeh, A.A.; Morris, M.B.; Bret Church, W.; Dastmalchi, S. Phage display as a technology delivering on the promise of peptide drug discovery. Drug Discov. Today 2013, 18, 1144–1157. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, G.; Gabrani, R. Antiviral Peptides: Identification and Validation. Int. J. Pept. Res. Ther. 2021, 27, 149–168. [Google Scholar] [CrossRef] [PubMed]
- Casey, J.P.; Barbero, R.J.; Heldman, N.; Belcher, A.M. Versatile de Novo Enzyme Activity in Capsid Proteins from an Engineered M13 Bacteriophage Library. J. Am. Chem. Soc. 2014, 136, 16508–16514. [Google Scholar] [CrossRef] [PubMed]
- Sioud, M. Phage Display Libraries: From Binders to Targeted Drug Delivery and Human Therapeutics. Mol. Biotechnol. 2019, 61, 286–303. [Google Scholar] [CrossRef]
- Rothe, A.; Hosse, R.J.; Power, B.E. In vitro display technologies reveal novel biopharmaceutics. FASEB J. 2006, 20, 1599–1610. [Google Scholar] [CrossRef]
- Huang, Y.; Chiang, C.Y.; Lee, S.K.; Gao, Y.; Hu, E.L.; De Yoreo, J.; Belcher, A.M. Programmable assembly of nanoarchitectures using genetically engineered viruses. Nano Lett. 2005, 5, 1429–1434. [Google Scholar] [CrossRef]
- Smith, G.P. Filamentous fusion phage: Novel expression vectors that display cloned antigens on the virion surface. Science 1985, 228, 1315–1317. [Google Scholar] [CrossRef]
- Castel, G.; Chtéoui, M.; Heyd, B.; Tordo, N. Phage display of combinatorial peptide libraries: Application to antiviral research. Molecules 2011, 16, 3499–3518. [Google Scholar] [CrossRef]
- Saw, P.E.; Song, E.W. Phage display screening of therapeutic peptide for cancer targeting and therapy. Protein Cell 2019, 10, 787–807. [Google Scholar] [CrossRef]
- Nagano, K.; Tsutsumi, Y. Phage Display Technology as a Powerful Platform for Antibody Drug Discovery. Viruses 2021, 13, 178. [Google Scholar] [CrossRef]
- Sokullu, E.; Soleymani Abyaneh, H.; Gauthier, M.A. Plant/Bacterial Virus-Based Drug Discovery, Drug Delivery, and Therapeutics. Pharmaceutics 2019, 11, 211. [Google Scholar] [CrossRef]
- Nixon, A.E.; Sexton, D.J.; Ladner, R.C. Drugs derived from phage display: From candidate identification to clinical practice. MAbs 2014, 6, 73–85. [Google Scholar] [CrossRef]
- Lee, A.C.; Harris, J.L.; Khanna, K.K.; Hong, J.H. A Comprehensive Review on Current Advances in Peptide Drug Development and Design. Int. J. Mol. Sci. 2019, 20, 2383. [Google Scholar] [CrossRef]
- Ladner, R.C.; Sato, A.K.; Gorzelany, J.; de Souza, M. Phage display-derived peptides as therapeutic alternatives to antibodies. Drug Discov. Today 2004, 9, 525–529. [Google Scholar] [CrossRef]
- Molek, P.; Strukelj, B.; Bratkovic, T. Peptide phage display as a tool for drug discovery: Targeting membrane receptors. Molecules 2011, 16, 857–887. [Google Scholar] [CrossRef]
- Omidfar, K.; Daneshpour, M. Advances in phage display technology for drug discovery. Expert Opin. Drug Discov. 2015, 10, 651–669. [Google Scholar] [CrossRef]
- Ozawa, M.; Ohashi, K.; Onuma, M. Identification and characterization of peptides binding to newcastle disease virus by phage display. J. Vet. Med. Sci. 2005, 67, 1237–1241. [Google Scholar] [CrossRef]
- Yin, L.; Luo, Y.; Liang, B.; Wang, F.; Du, M.; Petrenko, V.A.; Qiu, H.J.; Liu, A. Specific ligands for classical swine fever virus screened from landscape phage display library. Antivir. Res. 2014, 109, 68–71. [Google Scholar] [CrossRef]
- Yi, G.; Qian, J.; Wang, Z.; Qi, Y. A phage-displayed peptide can inhibit infection by white spot syndrome virus of shrimp. J. Gen. Virol. 2003, 84 Pt 9, 2545–2553. [Google Scholar] [CrossRef]
- Peng, B.; Chen, H.; Tan, Y.; Jin, M.; Chen, H.; Guo, A. Identification of one peptide which inhibited infectivity of avian infectious bronchitis virus in vitro. Sci. China C Life Sci. 2006, 49, 158–163. [Google Scholar] [CrossRef]
- Thong, Q.X.; Wong, C.L.; Ooi, M.K.; Kueh, C.L.; Ho, K.L.; Alitheen, N.B.; Tan, W.S. Peptide inhibitors of Macrobrachium rosenbergii nodavirus. J. Gen. Virol. 2018, 99, 1227–1238. [Google Scholar] [CrossRef]
- Zu, X.; Liu, Y.; Wang, S.; Jin, R.; Zhou, Z.; Liu, H.; Gong, R.; Xiao, G.; Wang, W. Peptide inhibitor of Japanese encephalitis virus infection targeting envelope protein domain III. Antivir. Res. 2014, 104, 7–14. [Google Scholar] [CrossRef]
- Wei, J.; Hameed, M.; Wang, X.; Zhang, J.; Guo, S.; Anwar, M.N.; Pang, L.; Liu, K.; Li, B.; Shao, D.; et al. Antiviral activity of phage display-selected peptides against Japanese encephalitis virus infection in vitro and in vivo. Antivir. Res. 2020, 174, 104673. [Google Scholar] [CrossRef]
- Wang, H.; Liu, R.; Cui, J.; Deng, S.; Xie, J.; Nin, Z.; Zhang, G. Characterization and utility of phages bearing peptides with affinity to porcine reproductive and respiratory syndrome virus nsp7 protein. J. Virol. Methods 2015, 222, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Zarlenga, D.S.; Sestak, K.; Suo, S.; Ren, X. Transmissible gastroenteritis virus: Identification of M protein-binding peptide ligands with antiviral and diagnostic potential. Antivir. Res. 2013, 99, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, Y.; Ji, Q.; Gu, J.; Liu, S.; Feng, X.; Sun, C.; Li, Y.; Lei, L. Selection of antiviral peptides against mink enteritis virus using a phage display Peptide library. Curr. Microbiol. 2013, 66, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Chew, M.F.; Tham, H.W.; Rajik, M.; Sharifah, S.H. Anti-dengue virus serotype 2 activity and mode of action of a novel peptide. J. Appl. Microbiol. 2015, 119, 1170–1180. [Google Scholar] [CrossRef]
- de la Guardia, C.; Quijada, M.; Lleonart, R. Phage-Displayed Peptides Selected to Bind Envelope Glycoprotein Show Antiviral Activity against Dengue Virus Serotype 2. Adv. Virol. 2017, 2017, 1827341. [Google Scholar] [CrossRef]
- Ojeda, N.; Cárdenas, C.; Guzmán, F.; Marshall, S.H. Chemical Synthesis and In Vitro Evaluation of a Phage Display-Derived Peptide Active against Infectious Salmon Anemia Virus. Appl. Environ. Microbiol. 2016, 82, 2563–2571. [Google Scholar] [CrossRef]
- Hou, P.; Zhao, G.; He, C.; Wang, H.; He, H. Biopanning of polypeptides binding to bovine ephemeral fever virus G(1) protein from phage display peptide library. BMC Vet. Res. 2018, 14, 3. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, H.; Wu, N.; Shi, H.; Wang, X.; Wang, T. Monoclonal antibody, but not synthetic peptide, targeting the ectodomain of influenza B virus M2 proton channel has antiviral activity. New Microbiol. 2010, 33, 311–317. [Google Scholar]
- Cao, L.; Ge, X.; Gao, Y.; Zarlenga, D.S.; Wang, K.; Li, X.; Qin, Z.; Yin, X.; Liu, J.; Ren, X.; et al. Putative phage-display epitopes of the porcine epidemic diarrhea virus S1 protein and their anti-viral activity. Virus Genes 2015, 51, 217–224. [Google Scholar] [CrossRef]
- Lü, X.; Yao, M.; Zhang, J.M.; Yang, J.; Lei, Y.F.; Huang, X.J.; Jia, Z.S.; Ma, L.; Lan, H.Y.; Xu, Z.K.; et al. Identification of peptides that bind hepatitis C virus envelope protein E2 and inhibit viral cellular entry from a phage-display peptide library. Int. J. Mol. Med. 2014, 33, 1312–1318. [Google Scholar] [CrossRef]
- Zhang, Q.; Bai, G.; Chen, J.-Q.; Tian, W.; Cao, Y.; Pan, P.-W.; Wang, C. Identification of antiviral mimetic peptides with interferon α-2b-like activity from a random peptide library using a novel functional biopanning method. Acta Pharmacol. Sin. 2008, 29, 634–640. [Google Scholar] [CrossRef]
- Ramanujam, P.; Tan, W.S.; Nathan, S.; Yusoff, K. Novel peptides that inhibit the propagation of Newcastle disease virus. Arch. Virol. 2002, 147, 981–993. [Google Scholar] [CrossRef]
- Heiskanen, T.; Lundkvist, A.; Vaheri, A.; Lankinen, H. Phage-displayed peptide targeting on the Puumala hantavirus neutralization site. J. Virol. 1997, 71, 3879–3885. [Google Scholar] [CrossRef]
- Rajik, M.; Jahanshiri, F.; Omar, A.R.; Ideris, A.; Hassan, S.S.; Yusoff, K. Identification and characterisation of a novel anti-viral peptide against avian influenza virus H9N2. Virol. J. 2009, 6, 74. [Google Scholar] [CrossRef]
- Rajik, M.; Omar, A.R.; Ideris, A.; Hassan, S.S.; Yusoff, K. A novel peptide inhibits the influenza virus replication by preventing the viral attachment to the host cells. Int. J. Biol. Sci. 2009, 5, 543–548. [Google Scholar] [CrossRef]
- Simonetti, L.; Ivarsson, Y. Genetically Encoded Cyclic Peptide Phage Display Libraries. ACS Cent. Sci. 2020, 6, 336–338. [Google Scholar] [CrossRef]
- Roxin, Á.; Zheng, G. Flexible or fixed: A comparative review of linear and cyclic cancer-targeting peptides. Futur. Med. Chem. 2012, 4, 1601–1618. [Google Scholar] [CrossRef]
- Desimmie, B.A.; Humbert, M.; Lescrinier, E.; Hendrix, J.; Vets, S.; Gijsbers, R.; Ruprecht, R.M.; Dietrich, U.; Debyser, Z.; Christ, F. Phage Display-Directed Discovery of LEDGF/p75 Binding Cyclic Peptide Inhibitors of HIV Replication. Mol. Ther. 2021, 29, 887. [Google Scholar] [CrossRef] [PubMed]
- Hall, P.R.; Hjelle, B.; Njus, H.; Ye, C.; Bondu-Hawkins, V.; Brown, D.C.; Kilpatrick, K.A.; Larson, R.S. Phage display selection of cyclic peptides that inhibit Andes virus infection. J. Virol. 2009, 83, 8965–8969. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.; Zaccardi, J.; Mullen, S.; Olland, S.; Orlowski, M.; Feld, B.; Labonte, P.; Mak, P. Identification of constrained peptides that bind to and preferentially inhibit the activity of the hepatitis C viral RNA-dependent RNA polymerase. Virology 2003, 313, 158–169. [Google Scholar] [CrossRef]
- Bai, F.; Town, T.; Pradhan, D.; Cox, J.; Ashish; Ledizet, M.; Anderson, J.F.; Flavell, R.A.; Krueger, J.K.; Koski, R.A.; et al. Antiviral Peptides Targeting the West Nile Virus Envelope Protein. J. Virol. 2007, 81, 2047. [Google Scholar] [CrossRef] [PubMed]
- Schulze, A.; Gripon, P.; Urban, S. Hepatitis B virus infection initiates with a large surface protein–dependent binding to heparan sulfate proteoglycans. Hepatology 2007, 46, 1759–1768. [Google Scholar] [CrossRef]
- Blanchet, M.; Sureau, C. Infectivity Determinants of the Hepatitis B Virus Pre-S Domain Are Confined to the N-Terminal 75 Amino Acid Residues. J. Virol. 2007, 81, 5841. [Google Scholar] [CrossRef]
- Deng, Q.; Zhai, J.W.; Michel, M.L.; Zhang, J.; Qin, J.; Kong, Y.Y.; Zhang, X.X.; Budkowska, A.; Tiollais, P.; Wang, Y.; et al. Identification and characterization of peptides that interact with hepatitis B virus via the putative receptor binding site. J. Virol. 2007, 81, 4244–4254. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; Zu, X.; Jin, R.; Xiao, G. Blocking peptides against HBV: preS1 protein selected from a phage display library. Biochem. Biophys. Res. Commun. 2011, 412, 633–637. [Google Scholar] [CrossRef]
- He, Y.; Ye, X.; Tiollais, P.; Zhang, J.; Zhang, J.; Liu, J.; Xie, Y. Selection of HBV preS1-binding penta-peptides by phage display. Acta Biochim. Biophys. Sin. 2014, 46, 691–698. [Google Scholar] [CrossRef]
- Ho, K.L.; Yusoff, K.; Seow, H.F.; Tan, W.S. Selection of high affinity ligands to hepatitis B core antigen from a phage-displayed cyclic peptide library. J. Med. Virol. 2003, 69, 27–32. [Google Scholar] [CrossRef]
- Dyson, M.R.; Murray, K. Selection of peptide inhibitors of interactions involved in complex protein assemblies: Association of the core and surface antigens of hepatitis B virus. Proc. Natl. Acad. Sci. USA 1995, 92, 2194–2198. [Google Scholar] [CrossRef]
- Lopes, R.S.; Queiroz, M.A.F.; Gomes, S.T.M.; Vallinoto, A.C.R.; Goulart, L.R.; Ishak, R. Phage display: An important tool in the discovery of peptides with anti-HIV activity. Biotechnol. Adv. 2018, 36, 1847–1854. [Google Scholar] [CrossRef]
- Sticht, J.; Humbert, M.; Findlow, S.; Bodem, J.; Müller, B.; Dietrich, U.; Werner, J.; Kräusslich, H.G. A peptide inhibitor of HIV-1 assembly in vitro. Nat. Struct. Mol. Biol. 2005, 12, 671–677. [Google Scholar] [CrossRef]
- Desjobert, C.; de Soultrait, V.R.; Faure, A.; Parissi, V.; Litvak, S.; Tarrago-Litvak, L.; Fournier, M. Identification by phage display selection of a short peptide able to inhibit only the strand transfer reaction catalyzed by human immunodeficiency virus type 1 integrase. Biochemistry 2004, 43, 13097–13105. [Google Scholar] [CrossRef]
- Yoon, V.; Fridkis-Hareli, M.; Munisamy, S.; Lee, J.; Anastasiades, D.; Stevceva, L. The GP120 molecule of HIV-1 and its interaction with T cells. Curr. Med. Chem. 2010, 17, 741–749. [Google Scholar] [CrossRef]
- Davenport, M.P.; Zaunders, J.J.; Hazenberg, M.D.; Schuitemaker, H.; van Rij, R.P. Cell turnover and cell tropism in HIV-1 infection. Trends Microbiol. 2002, 10, 275–278. [Google Scholar] [CrossRef]
- Chevigne, A.; Delhalle, S.; Counson, M.; Beaupain, N.; Rybicki, A.; Verschueren, C.; Staub, T.; Schmit, J.C.; Seguin-Devaux, C.; Deroo, S. Isolation of an HIV-1 neutralizing peptide mimicking the CXCR4 and CCR5 surface from the heavy-chain complementary determining region 3 repertoire of a viremic controller. AIDS 2016, 30, 377–382. [Google Scholar] [CrossRef]
- Berger, E.A.; Murphy, P.M.; Farber, J.M. Chemokine receptors as HIV-1 coreceptors: Roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 1999, 17, 657–700. [Google Scholar] [CrossRef]
- Veljkovic, V.; Veljkovic, N.; Esté, J.A.; Hüther, A.; Dietrich, U. Application of the EIIP/ISM bioinformatics concept in development of new drugs. Curr. Med. Chem. 2007, 14, 441–453. [Google Scholar] [CrossRef]
- Hartley, O.; Dorgham, K.; Perez-Bercoff, D.; Cerini, F.; Heimann, A.; Gaertner, H.; Offord, R.E.; Pancino, G.; Debré, P.; Gorochov, G. Human immunodeficiency virus type 1 entry inhibitors selected on living cells from a library of phage chemokines. J. Virol. 2003, 77, 6637–6644. [Google Scholar] [CrossRef]
- Craigie, R. The molecular biology of HIV integrase. Futur. Virol. 2012, 7, 679–686. [Google Scholar] [CrossRef]
- Köhler, G.; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256, 495–497. [Google Scholar] [CrossRef]
- Ahangarzadeh, S.; Payandeh, Z.; Arezumand, R.; Shahzamani, K.; Yarian, F.; Alibakhshi, A. An update on antiviral antibody-based biopharmaceuticals. Int. Immunopharmacol. 2020, 86, 106760. [Google Scholar] [CrossRef]
- Plückthun, A. Alternative scaffolds: Expanding the options of antibodies. In Recombinant Antibodies for Immunotherapy; Little, M., Ed.; Cambridge University Press: Cambridge, MA, USA, 2009; pp. 243–272. [Google Scholar]
- Gray, A.; Bradbury, A.R.M.; Knappik, A.; Plückthun, A.; Borrebaeck, C.A.K.; Dübel, S. Animal-free alternatives and the antibody iceberg. Nat. Biotechnol. 2020, 38, 1234–1239. [Google Scholar] [CrossRef]
- McCafferty, J.; Griffiths, A.D.; Winter, G.; Chiswell, D.J. Phage antibodies: Filamentous phage displaying antibody variable domains. Nature 1990, 348, 552–554. [Google Scholar] [CrossRef]
- Ledsgaard, L.; Kilstrup, M.; Karatt-Vellatt, A.; McCafferty, J.; Laustsen, A.H. Basics of Antibody Phage Display Technology. Toxins 2018, 10, 236. [Google Scholar] [CrossRef]
- Nelson, A.L. Antibody fragments: Hope and hype. MAbs 2010, 2, 77–83. [Google Scholar] [CrossRef]
- Bates, A.; Power, C.A. David vs. Goliath: The Structure, Function, and Clinical Prospects of Antibody Fragments. Antibodies 2019, 8, 28. [Google Scholar] [CrossRef]
- Griffiths, A.D.; Duncan, A.R. Strategies for selection of antibodies by phage display. Curr. Opin. Biotechnol. 1998, 9, 102–108. [Google Scholar] [CrossRef]
- Hoogenboom, H.R.; de Bruïne, A.P.; Hufton, S.E.; Hoet, R.M.; Arends, J.-W.; Roovers, R.C. Antibody phage display technology and its applications. Immunotechnology 1998, 4, 1–20. [Google Scholar] [CrossRef]
- Plaisant, P.; Burioni, R.; Manzin, A.; Solforosi, L.; Candela, M.; Gabrielli, A.; Fadda, G.; Clementi, M. Human monoclonal recombinant Fabs specific for HCV antigens obtained by repertoire cloning in phage display combinatorial vectors. Res. Virol. 1997, 148, 165–169. [Google Scholar] [CrossRef]
- Burioni, R.; Plaisant, P.; Manzin, A.; Rosa, D.; Delli Carri, V.; Bugli, F.; Solforosi, L.; Abrignani, S.; Varaldo, P.E.; Fadda, G.; et al. Dissection of human humoral immune response against hepatitis C virus E2 glycoprotein by repertoire cloning and generation of recombinant Fab fragments. Hepatology 1998, 28, 810–814. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Y.; Shu, Y.; Phogat, S.; Xiao, X.; Cham, F.; Bouma, P.; Choudhary, A.; Feng, Y.R.; Sanz, I.; Rybak, S.; et al. Broadly cross-reactive HIV neutralizing human monoclonal antibody Fab selected by sequential antigen panning of a phage display library. J. Immunol. Methods 2003, 283, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Schofield, D.J.; Glamann, J.; Emerson, S.U.; Purcell, R.H. Identification by phage display and characterization of two neutralizing chimpanzee monoclonal antibodies to the hepatitis E virus capsid protein. J. Virol. 2000, 74, 5548–5555. [Google Scholar] [CrossRef]
- Weisser, N.E.; Hall, J.C. Applications of single-chain variable fragment antibodies in therapeutics and diagnostics. Biotechnol. Adv. 2009, 27, 502–520. [Google Scholar] [CrossRef]
- Wörn, A.; Plückthun, A. Stability engineering of antibody single-chain Fv fragments. J. Mol. Biol. 2001, 305, 989–1010. [Google Scholar] [CrossRef]
- Saerens, D.; Ghassabeh, G.H.; Muyldermans, S. Single-domain antibodies as building blocks for novel therapeutics. Curr. Opin. Pharm. 2008, 8, 600–608. [Google Scholar] [CrossRef]
- Ahamadi-Fesharaki, R.; Fateh, A.; Vaziri, F.; Solgi, G.; Siadat, S.D.; Mahboudi, F.; Rahimi-Jamnani, F. Single-Chain Variable Fragment-Based Bispecific Antibodies: Hitting Two Targets with One Sophisticated Arrow. Mol. Ther. Oncolytics 2019, 14, 38–56. [Google Scholar] [CrossRef]
- Zemel, R.; Berdichevsky, Y.; Bachmatov, L.; Benhar, I.; Tur-Kaspa, R. Inhibition of hepatitis C virus NS3-mediated cell transformation by recombinant intracellular antibodies. J. Hepatol. 2004, 40, 1000–1007. [Google Scholar] [CrossRef]
- Poungpair, O.; Chaicumpa, W.; Kulkeaw, K.; Maneewatch, S.; Thueng-in, K.; Srimanote, P.; Tongtawe, P.; Songserm, T.; Lekcharoensuk, P.; Tapchaisri, P. Human single chain monoclonal antibody that recognizes matrix protein of heterologous influenza A virus subtypes. J. Virol. Methods 2009, 159, 105–111. [Google Scholar] [CrossRef]
- Zhang, X.; Qi, X.; Zhang, Q.; Zeng, X.; Shi, Z.; Jin, Q.; Zhan, F.; Xu, Y.; Liu, Z.; Feng, Z.; et al. Human 4F5 single-chain Fv antibody recognizing a conserved HA1 epitope has broad neutralizing potency against H5N1 influenza A viruses of different clades. Antivir. Res. 2013, 99, 91–99. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, C.; Xiao, X.; Pang, L.; Shen, S.; Zhang, J.; Cen, S.; Yang, B.B.; Huang, Y.; Sheng, W.; et al. Phage Display-Derived Cross-Reactive Neutralizing Antibody against Enterovirus 71 and Coxsackievirus A16. Jpn. J. Infect. Dis. 2016, 69, 66–74. [Google Scholar] [CrossRef]
- Flego, M.; Frau, A.; Accardi, L.; Mallano, A.; Ascione, A.; Gellini, M.; Fanunza, E.; Vella, S.; Di Bonito, P.; Tramontano, E. Intracellular human antibody fragments recognizing the VP35 protein of Zaire Ebola filovirus inhibit the protein activity. BMC Biotechnol. 2019, 19, 64. [Google Scholar] [CrossRef]
- Amatya, P.; Wagner, N.; Chen, G.; Luthra, P.; Shi, L.; Borek, D.; Pavlenco, A.; Rohrs, H.; Basler, C.F.; Sidhu, S.S.; et al. Inhibition of Marburg Virus RNA Synthesis by a Synthetic Anti-VP35 Antibody. ACS Infect. Dis. 2019, 5, 1385–1396. [Google Scholar] [CrossRef]
- Maneewatch, S.; Thanongsaksrikul, J.; Songserm, T.; Thueng-In, K.; Kulkeaw, K.; Thathaisong, U.; Srimanote, P.; Tongtawe, P.; Tapchaisri, P.; Chaicumpa, W. Human single-chain antibodies that neutralize homologous and heterologous strains and clades of influenza A virus subtype H5N1. Antivir. Ther. 2009, 14, 221–230. [Google Scholar]
- Zhao, X.L.; Yin, J.; Chen, W.Q.; Jiang, M.; Yang, G.; Yang, Z.H. Generation and characterization of human monoclonal antibodies to G5, a linear neutralization epitope on glycoprotein of rabies virus, by phage display technology. Microbiol. Immunol. 2008, 52, 89–93. [Google Scholar] [CrossRef]
- Muyldermans, S. Nanobodies: Natural single-domain antibodies. Annu. Rev. Biochem. 2013, 82, 775–797. [Google Scholar] [CrossRef]
- Vincke, C.; Muyldermans, S. Introduction to heavy chain antibodies and derived Nanobodies. Methods Mol. Biol. 2012, 911, 15–26. [Google Scholar]
- Bannas, P.; Koch-Nolte, F. Perspectives for the Development of CD38-Specific Heavy Chain Antibodies as Therapeutics for Multiple Myeloma. Front. Immunol. 2018, 9, 2559. [Google Scholar] [CrossRef]
- Yang, E.Y.; Shah, K. Nanobodies: Next Generation of Cancer Diagnostics and Therapeutics. Front. Oncol. 2020, 10, 1182. [Google Scholar] [CrossRef]
- Bannas, P.; Hambach, J.; Koch-Nolte, F. Nanobodies and Nanobody-Based Human Heavy Chain Antibodies As Antitumor Therapeutics. Front. Immunol. 2017, 8, 1603. [Google Scholar] [CrossRef]
- Hassanzadeh-Ghassabeh, G.; Devoogdt, N.; De Pauw, P.; Vincke, C.; Muyldermans, S. Nanobodies and their potential applications. Nanomedicine 2013, 8, 1013–1026. [Google Scholar] [CrossRef]
- Van Audenhove, I.; Gettemans, J. Nanobodies as Versatile Tools to Understand, Diagnose, Visualize and Treat Cancer. EBioMedicine 2016, 8, 40–48. [Google Scholar] [CrossRef]
- Morrison, C. Nanobody approval gives domain antibodies a boost. Nat. Rev. Drug Discov. 2019, 18, 485–487. [Google Scholar] [CrossRef]
- Doshi, R.; Chen, B.R.; Vibat, C.R.T.; Huang, N.; Lee, C.-W.; Chang, G. In vitro nanobody discovery for integral membrane protein targets. Sci. Rep. 2014, 4, 6760. [Google Scholar] [CrossRef]
- Zhu, S.; Huang, A.-G.; Luo, F.; Li, J.; Li, J.; Zhu, L.; Zhao, L.; Zhu, B.; Ling, F.; Wang, G.-X. Application of Virus Targeting Nanocarrier Drug Delivery System in Virus-Induced Central Nervous System Disease Treatment. ACS Appl. Mater. Interfaces 2019, 11, 19006–19016. [Google Scholar] [CrossRef]
- Li, T.; Huang, M.; Xiao, H.; Zhang, G.; Ding, J.; Wu, P.; Zhang, H.; Sheng, J.; Chen, C. Selection and characterization of specific nanobody against bovine virus diarrhea virus (BVDV) E2 protein. PLoS ONE 2017, 12, e0178469. [Google Scholar] [CrossRef]
- Thys, B.; Schotte, L.; Muyldermans, S.; Wernery, U.; Hassanzadeh-Ghassabeh, G.; Rombaut, B. In vitro antiviral activity of single domain antibody fragments against poliovirus. Antivir. Res. 2010, 87, 257–264. [Google Scholar] [CrossRef]
- Tarr, A.W.; Lafaye, P.; Meredith, L.; Damier-Piolle, L.; Urbanowicz, R.A.; Meola, A.; Jestin, J.L.; Brown, R.J.; McKeating, J.A.; Rey, F.A.; et al. An alpaca nanobody inhibits hepatitis C virus entry and cell-to-cell transmission. Hepatology 2013, 58, 932–939. [Google Scholar] [CrossRef]
- Lo, A.S.; Zhu, Q.; Marasco, W.A. Intracellular antibodies (intrabodies) and their therapeutic potential. Handb. Exp. Pharm. 2008, 181, 343–373. [Google Scholar]
- Liu, H.; Wang, Y.; Duan, H.; Zhang, A.; Liang, C.; Gao, J.; Zhang, C.; Huang, B.; Li, Q.; Li, N.; et al. An intracellularly expressed Nsp9-specific nanobody in MARC-145 cells inhibits porcine reproductive and respiratory syndrome virus replication. Vet. Microbiol. 2015, 181, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liang, C.; Duan, H.; Zhang, X.; Wang, X.; Xiao, S.; Zhou, E.M. Intracellularly expressed nanobodies against non-structural protein 4 of porcine reproductive and respiratory syndrome virus inhibit virus replication. Biotechnol. Lett. 2016, 38, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Ma, Z.; Xu, L.; Zhang, A.; Li, Z.; Xiao, S. A novel intracellularly expressed NS5B-specific nanobody suppresses bovine viral diarrhea virus replication. Vet. Microbiol. 2020, 240, 108449. [Google Scholar] [CrossRef] [PubMed]
- Riechmann, L.; Muyldermans, S. Single domain antibodies: Comparison of camel VH and camelised human VH domains. J. Immunol. Methods 1999, 231, 25–38. [Google Scholar] [CrossRef]
- Davies, J.; Riechmann, L. Antibody VH domains as small recognition units. Biotechnology 1995, 13, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Rouet, R.; Dudgeon, K.; Christie, M.; Langley, D.; Christ, D. Fully Human VH Single Domains That Rival the Stability and Cleft Recognition of Camelid Antibodies. J. Biol. Chem. 2015, 290, 11905–11917. [Google Scholar] [CrossRef]
- Chen, W.; Zhu, Z.; Xiao, X.; Dimitrov, D.S. Construction of a human antibody domain (VH) library. Methods Mol. Biol. 2009, 525, 81–99. [Google Scholar]
- Davies, J.; Riechmann, L. Single antibody domains as small recognition units: Design and in vitro antigen selection of camelized, human VH domains with improved protein stability. Protein Eng. Des. Sel. 1996, 9, 531–537. [Google Scholar] [CrossRef]
- Wu, Y.; Li, C.; Xia, S.; Tian, X.; Kong, Y.; Wang, Z.; Gu, C.; Zhang, R.; Tu, C.; Xie, Y.; et al. Identification of Human Single-Domain Antibodies against SARS-CoV-2. Cell Host Microbe 2020, 27, 891–898.e5. [Google Scholar] [CrossRef]
- Martin, F.; Toniatti, C.; Salvati, A.L.; Venturini, S.; Ciliberto, G.; Cortese, R.; Sollazzo, M. The affinity-selection of a minibody polypeptide inhibitor of human interleukin-6. EMBO J. 1994, 13, 5303–5309. [Google Scholar] [CrossRef]
- Tramontano, A.; Bianchi, E.; Venturini, S.; Martin, F.; Pessi, A.; Sollazzo, M. The making of the minibody: An engineered beta-protein for the display of conformationally constrained peptides. J. Mol. Recognit. 1994, 7, 9–24. [Google Scholar] [CrossRef]
- Martin, F.; Toniatti, C.; Salvati, A.L.; Ciliberto, G.; Cortese, R.; Sollazzo, M. Coupling protein design and in vitro selection strategies: Improving specificity and affinity of a designed beta-protein IL-6 antagonist. J. Mol. Biol. 1996, 255, 86–97. [Google Scholar] [CrossRef]
- Pessi, A.; Bianchi, E.; Crameri, A.; Venturini, S.; Tramontano, A.; Sollazzo, M. A designed metal-binding protein with a novel fold. Nature 1993, 362, 367–369. [Google Scholar]
- Röttgen, P.; Collins, J. A human pancreatic secretory trypsin inhibitor presenting a hypervariable highly constrained epitope via monovalent phagemid display. Gene 1995, 164, 243–250. [Google Scholar] [CrossRef]
- Szardenings, M.; Vasel, B.; Hecht, H.J.; Collins, J.; Schomburg, D. Highly effective protease inhibitors from variants of human pancreatic secretory trypsin inhibitor (hPSTI): An assessment of 3-D structure-based protein design. Protein Eng. 1995, 8, 45–52. [Google Scholar]
- Dimasi, N.; Martin, F.; Volpari, C.; Brunetti, M.; Biasiol, G.; Altamura, S.; Cortese, R.; De Francesco, R.; Steinkühler, C.; Sollazzo, M. Characterization of engineered hepatitis C virus NS3 protease inhibitors affinity selected from human pancreatic secretory trypsin inhibitor and minibody repertoires. J. Virol. 1997, 71, 7461. [Google Scholar] [CrossRef]
- Sedgwick, S.G.; Smerdon, S.J. The ankyrin repeat: A diversity of interactions on a common structural framework. Trends Biochem. Sci. 1999, 24, 311–316. [Google Scholar]
- Plückthun, A. Designed ankyrin repeat proteins (DARPins): Binding proteins for research, diagnostics, and therapy. Annu. Rev. Pharm. Toxicol. 2015, 55, 489–511. [Google Scholar] [CrossRef]
- Nangola, S.; Urvoas, A.; Valerio-Lepiniec, M.; Khamaikawin, W.; Sakkhachornphop, S.; Hong, S.-S.; Boulanger, P.; Minard, P.; Tayapiwatana, C. Antiviral activity of recombinant ankyrin targeted to the capsid domain of HIV-1 Gag polyprotein. Retrovirology 2012, 9, 17. [Google Scholar] [CrossRef]
- Mammen, M.; Choi, S.K.; Whitesides, G.M. Polyvalent Interactions in Biological Systems: Implications for Design and Use of Multivalent Ligands and Inhibitors. Angew Chem. Int. Ed. 1998, 37, 2754–2794. [Google Scholar] [CrossRef]
- Mammen, M.; Dahmann, G.; Whitesides, G.M. Effective Inhibitors of Hemagglutination by Influenza Virus Synthesized from Polymers Having Active Ester Groups. Insight into Mechanism of Inhibition. J. Med. Chem. 1995, 38, 4179–4190. [Google Scholar] [CrossRef]
- Hall, P.R.; Hjelle, B.; Brown, D.C.; Ye, C.; Bondu-Hawkins, V.; Kilpatrick, K.A.; Larson, R.S. Multivalent presentation of antihantavirus peptides on nanoparticles enhances infection blockade. Antimicrob. Agents Chemother. 2008, 52, 2079–2088. [Google Scholar] [CrossRef]
- Matsubara, T.; Onishi, A.; Saito, T.; Shimada, A.; Inoue, H.; Taki, T.; Nagata, K.; Okahata, Y.; Sato, T. Sialic acid-mimic peptides as hemagglutinin inhibitors for anti-influenza therapy. J. Med. Chem. 2010, 53, 4441–4449. [Google Scholar] [CrossRef]
- Matsubara, T.; Sumi, M.; Kubota, H.; Taki, T.; Okahata, Y.; Sato, T. Inhibition of influenza virus infections by sialylgalactose-binding peptides selected from a phage library. J. Med. Chem. 2009, 52, 4247–4256. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, J.; Huang, R.; Huang, S.; Wu, Y.; Huang, L.; He, J.; Xie, J. An affinity peptide exerts antiviral activity by strongly binding nervous necrosis virus to block viral entry. Fish Shellfish Immunol. 2019, 86, 465–473. [Google Scholar] [CrossRef]
- Evans, B.J.; King, A.T.; Katsifis, A.; Matesic, L.; Jamie, J.F. Methods to Enhance the Metabolic Stability of Peptide-Based PET Radiopharmaceuticals. Molecules 2020, 25, 2314. [Google Scholar] [CrossRef] [PubMed]
- Werner, H.M.; Cabalteja, C.C.; Horne, W.S. Peptide Backbone Composition and Protease Susceptibility: Impact of Modification Type, Position, and Tandem Substitution. ChemBioChem 2016, 17, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Lu, W. Mirror image proteins. Curr. Opin. Chem. Biol. 2014, 22, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Garton, M.; Nim, S.; Stone, T.A.; Wang, K.E.; Deber, C.M.; Kim, P.M. Method to generate highly stable D-amino acid analogs of bioactive helical peptides using a mirror image of the entire PDB. Proc. Natl. Acad. Sci. USA 2018, 115, 1505. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Feng, X.; Ma, Z.; Luo, C.; Zhou, B.; Cao, R.; Huang, L.; Miao, D.; Pang, R.; He, D.; et al. Antiviral activity of phage display selected peptides against Porcine reproductive and respiratory syndrome virus in vitro. Virology 2012, 432, 73–80. [Google Scholar] [CrossRef][Green Version]
- Funke, S.A.; Willbold, D. Mirror image phage display—A method to generate D-peptide ligands for use in diagnostic or therapeutical applications. Mol. Biosyst. 2009, 5, 783–786. [Google Scholar] [CrossRef]
- Eckert, D.M.; Malashkevich, V.N.; Hong, L.H.; Carr, P.A.; Kim, P.S. Inhibiting HIV-1 entry: Discovery of D-peptide inhibitors that target the gp41 coiled-coil pocket. Cell 1999, 99, 103–115. [Google Scholar] [CrossRef]
- Schumacher, T.N.; Mayr, L.M.; Minor, D.L., Jr.; Milhollen, M.A.; Burgess, M.W.; Kim, P.S. Identification of D-peptide ligands through mirror-image phage display. Science 1996, 271, 1854–1857. [Google Scholar] [CrossRef]
- Welch, B.D.; VanDemark, A.P.; Heroux, A.; Hill, C.P.; Kay, M.S. Potent D-peptide inhibitors of HIV-1 entry. Proc. Natl. Acad. Sci. USA 2007, 104, 16828–16833. [Google Scholar] [CrossRef]
- Welch, B.D.; Francis, J.N.; Redman, J.S.; Paul, S.; Weinstock, M.T.; Reeves, J.D.; Lie, Y.S.; Whitby, F.G.; Eckert, D.M.; Hill, C.P.; et al. Design of a potent D-peptide HIV-1 entry inhibitor with a strong barrier to resistance. J. Virol. 2010, 84, 11235–11244. [Google Scholar] [CrossRef]
- Fievez, V.; Szpakowska, M.; Mosbah, A.; Arumugam, K.; Mathu, J.; Counson, M.; Beaupain, N.; Seguin-Devaux, C.; Deroo, S.; Baudy-Floc’h, M.; et al. Development of Mimokines, chemokine N terminus-based CXCR4 inhibitors optimized by phage display and rational design. J. Leukoc. Biol. 2018, 104, 343–357. [Google Scholar] [CrossRef]
- Laurencin, M.; Amor, M.; Fleury, Y.; Baudy-Floc’h, M. De Novo Cyclic Pseudopeptides Containing Aza-β3-amino Acids Exhibiting Antimicrobial Activities. J. Med. Chem. 2012, 55, 10885–10895. [Google Scholar] [CrossRef]
- Kisseljova, K.; Kuznetsov, A.; Baudy-Floc’h, M.; Järv, J. Aza-beta(3)-amino acid containing peptidomimetics as cAMP-dependent protein kinase substrates. Bioorg. Chem. 2010, 38, 229–233. [Google Scholar]
- Koellhoffer, J.F.; Chen, G.; Sandesara, R.G.; Bale, S.; Saphire, E.O.; Chandran, K.; Sidhu, S.S.; Lai, J.R. Two synthetic antibodies that recognize and neutralize distinct proteolytic forms of the ebola virus envelope glycoprotein. ChemBioChem 2012, 13, 2549–2557. [Google Scholar] [CrossRef]
- Li, W.; Chen, C.; Drelich, A.; Martinez, D.R.; Gralinski, L.E.; Sun, Z.; Schäfer, A.; Kulkarni, S.S.; Liu, X.; Leist, S.R.; et al. Rapid identification of a human antibody with high prophylactic and therapeutic efficacy in three animal models of SARS-CoV-2 infection. Proc. Natl. Acad. Sci. USA 2020, 117, 29832–29838. [Google Scholar]
- Bugli, F.; Graffeo, R.; Pescatori, M.; Paroni Sterbini, F.; Torelli, R.; Masucci, L.; Manzara, S.; Fadda, G. Human antibodies from phage display libraries: Expression of recombinant full length immunoglobulin G specific to the hepatitis C virus E2 glycoprotein. New Microbiol. 2009, 32, 341–349. [Google Scholar]
- Ohta, A.; Fujita, A.; Murayama, T.; Iba, Y.; Kurosawa, Y.; Yoshikawa, T.; Asano, Y. Recombinant human monoclonal antibodies to human cytomegalovirus glycoprotein B neutralize virus in a complement-dependent manner. Microbes Infect. 2009, 11, 1029–1036. [Google Scholar] [CrossRef]
- Zhu, Z.; Dimitrov, A.S.; Bossart, K.N.; Crameri, G.; Bishop, K.A.; Choudhry, V.; Mungall, B.A.; Feng, Y.R.; Choudhary, A.; Zhang, M.Y.; et al. Potent neutralization of Hendra and Nipah viruses by human monoclonal antibodies. J. Virol. 2006, 80, 891–899. [Google Scholar] [CrossRef]
- Zhu, Z.; Bossart, K.N.; Bishop, K.A.; Crameri, G.; Dimitrov, A.S.; McEachern, J.A.; Feng, Y.; Middleton, D.; Wang, L.F.; Broder, C.C.; et al. Exceptionally potent cross-reactive neutralization of Nipah and Hendra viruses by a human monoclonal antibody. J. Infect. Dis. 2008, 197, 846–853. [Google Scholar] [CrossRef]
- Bossart, K.N.; Zhu, Z.; Middleton, D.; Klippel, J.; Crameri, G.; Bingham, J.; McEachern, J.A.; Green, D.; Hancock, T.J.; Chan, Y.P.; et al. A neutralizing human monoclonal antibody protects against lethal disease in a new ferret model of acute nipah virus infection. PLoS Pathog. 2009, 5, e1000642. [Google Scholar] [CrossRef]
- Bossart, K.N.; Geisbert, T.W.; Feldmann, H.; Zhu, Z.; Feldmann, F.; Geisbert, J.B.; Yan, L.; Feng, Y.R.; Brining, D.; Scott, D.; et al. A neutralizing human monoclonal antibody protects african green monkeys from hendra virus challenge. Sci. Transl. Med. 2011, 3, 105ra103. [Google Scholar] [CrossRef]
- O’Brien, L.M.; Underwood-Fowler, C.D.; Goodchild, S.A.; Phelps, A.L.; Phillpotts, R.J. Development of a novel monoclonal antibody with reactivity to a wide range of Venezuelan equine encephalitis virus strains. Virol. J. 2009, 6, 206. [Google Scholar] [CrossRef][Green Version]
- Tian, P.; Wang, Y.; Liu, H.; Yang, Y.; Wu, X.; Wei, H.; Chen, T. Preparation and Evaluation of the Fully Humanized Monoclonal Antibody GD-mAb Against Respiratory Syncytial Virus. Front. Cell. Infect. Microbiol. 2019, 9, 275. [Google Scholar] [CrossRef]
- Lim, A.P.; Chan, C.E.; Wong, S.K.; Chan, A.H.; Ooi, E.E.; Hanson, B.J. Neutralizing human monoclonal antibody against H5N1 influenza HA selected from a Fab-phage display library. Virol. J. 2008, 5, 130. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Xiao, X.; Sidorov, I.A.; Choudhry, V.; Cham, F.; Zhang, P.F.; Bouma, P.; Zwick, M.; Choudhary, A.; Montefiori, D.C.; et al. Identification and characterization of a new cross-reactive human immunodeficiency virus type 1-neutralizing human monoclonal antibody. J. Virol. 2004, 78, 9233–9242. [Google Scholar] [CrossRef]
- Prabakaran, P.; Gan, J.; Feng, Y.; Zhu, Z.; Choudhry, V.; Xiao, X.; Ji, X.; Dimitrov, D.S. Structure of severe acute respiratory syndrome coronavirus receptor-binding domain complexed with neutralizing antibody. J. Biol. Chem. 2006, 281, 15829–15836. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Chakraborti, S.; He, Y.; Roberts, A.; Sheahan, T.; Xiao, X.; Hensley, L.E.; Prabakaran, P.; Rockx, B.; Sidorov, I.A.; et al. Potent cross-reactive neutralization of SARS coronavirus isolates by human monoclonal antibodies. Proc. Natl. Acad. Sci. USA 2007, 104, 12123. [Google Scholar] [CrossRef] [PubMed]
- Ying, T.; Du, L.; Ju, T.W.; Prabakaran, P.; Lau, C.C.; Lu, L.; Liu, Q.; Wang, L.; Feng, Y.; Wang, Y.; et al. Exceptionally potent neutralization of Middle East respiratory syndrome coronavirus by human monoclonal antibodies. J. Virol. 2014, 88, 7796–7805. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.S.; Ying, T.; Tao, X.; Garron, T.; Algaissi, A.; Wang, Y.; Wang, L.; Peng, B.H.; Jiang, S.; Dimitrov, D.S.; et al. Passive Transfer of A Germline-like Neutralizing Human Monoclonal Antibody Protects Transgenic Mice Against Lethal Middle East Respiratory Syndrome Coronavirus Infection. Sci. Rep. 2016, 6, 31629. [Google Scholar] [CrossRef]
- Noy-Porat, T.; Makdasi, E.; Alcalay, R.; Mechaly, A.; Levy, Y.; Bercovich-Kinori, A.; Zauberman, A.; Tamir, H.; Yahalom-Ronen, Y.; Israeli, M.; et al. A panel of human neutralizing mAbs targeting SARS-CoV-2 spike at multiple epitopes. Nat. Commun. 2020, 11, 4303. [Google Scholar] [CrossRef]
- Li, W.; Schäfer, A.; Kulkarni, S.S.; Liu, X.; Martinez, D.R.; Chen, C.; Sun, Z.; Leist, S.R.; Drelich, A.; Zhang, L.; et al. High Potency of a Bivalent Human V(H) Domain in SARS-CoV-2 Animal Models. Cell 2020, 183, 429–441.e16. [Google Scholar] [CrossRef]
- Temsamani, J.; Vidal, P. The use of cell-penetrating peptides for drug delivery. Drug Discov. Today 2004, 9, 1012–1019. [Google Scholar] [CrossRef]
- Marschall, A.L.; Frenzel, A.; Schirrmann, T.; Schüngel, M.; Dübel, S. Targeting antibodies to the cytoplasm. MAbs 2011, 3, 3–16. [Google Scholar] [CrossRef]
- Xie, J.; Bi, Y.; Zhang, H.; Dong, S.; Teng, L.; Lee, R.J.; Yang, Z. Cell-Penetrating Peptides in Diagnosis and Treatment of Human Diseases: From Preclinical Research to Clinical Application. Front. Pharmacol. 2020, 11, 697. [Google Scholar] [CrossRef]
- Balayssac, S.; Burlina, F.; Convert, O.; Bolbach, G.; Chassaing, G.; Lequin, O. Comparison of Penetratin and Other Homeodomain-Derived Cell-Penetrating Peptides: Interaction in a Membrane-Mimicking Environment and Cellular Uptake Efficiency. Biochemistry 2006, 45, 1408–1420. [Google Scholar] [CrossRef]
- Yang, B.; Gao, L.; Li, L.; Lu, Z.; Fan, X.; Patel, C.A.; Pomerantz, R.J.; DuBois, G.C.; Zhang, H. Potent suppression of viral infectivity by the peptides that inhibit multimerization of human immunodeficiency virus type 1 (HIV-1) Vif proteins. J. Biol. Chem. 2003, 278, 6596–6602. [Google Scholar] [CrossRef]
- Glab-Ampai, K.; Malik, A.A.; Chulanetra, M.; Thanongsaksrikul, J.; Thueng-In, K.; Srimanote, P.; Tongtawe, P.; Chaicumpa, W. Inhibition of HCV replication by humanized-single domain transbodies to NS4B. Biochem. Biophys. Res. Commun. 2016, 476, 654–664. [Google Scholar] [CrossRef]
- Rizzuti, M.; Nizzardo, M.; Zanetta, C.; Ramirez, A.; Corti, S. Therapeutic applications of the cell-penetrating HIV-1 Tat peptide. Drug Discov. Today 2015, 20, 76–85. [Google Scholar] [CrossRef]
- Zou, L.; Peng, Q.; Wang, P.; Zhou, B. Progress in Research and Application of HIV-1 TAT-Derived Cell-Penetrating Peptide. J. Membr. Biol. 2017, 250, 115–122. [Google Scholar] [CrossRef]
- Zhuang, X.; Stahl, S.J.; Watts, N.R.; DiMattia, M.A.; Steven, A.C.; Wingfield, P.T. A cell-penetrating antibody fragment against HIV-1 Rev has high antiviral activity: Characterization of the paratope. J. Biol. Chem. 2014, 289, 20222–20233. [Google Scholar] [CrossRef]
- Songprakhon, P.; Thaingtamtanha, T.; Limjindaporn, T.; Puttikhunt, C.; Srisawat, C.; Luangaram, P.; Dechtawewat, T.; Uthaipibull, C.; Thongsima, S.; Yenchitsomanus, P.-T.; et al. Peptides targeting dengue viral nonstructural protein 1 inhibit dengue virus production. Sci. Rep. 2020, 10, 12933. [Google Scholar] [CrossRef]
- Glab-ampai, K.; Chulanetra, M.; Malik, A.A.; Juntadech, T.; Thanongsaksrikul, J.; Srimanote, P.; Thueng-in, K.; Sookrung, N.; Tongtawe, P.; Chaicumpa, W. Human single chain-transbodies that bound to domain-I of non-structural protein 5A (NS5A) of hepatitis C virus. Sci. Rep. 2017, 7, 15042. [Google Scholar] [CrossRef]
- Oh, D.; Nasrolahi Shirazi, A.; Northup, K.; Sullivan, B.; Tiwari, R.K.; Bisoffi, M.; Parang, K. Enhanced cellular uptake of short polyarginine peptides through fatty acylation and cyclization. Mol. Pharm. 2014, 11, 2845–2854. [Google Scholar] [CrossRef] [PubMed]
- Koren, E.; Torchilin, V.P. Cell-penetrating peptides: Breaking through to the other side. Trends Mol. Med. 2012, 18, 385–393. [Google Scholar] [CrossRef]
- Phanthong, S.; Densumite, J.; Seesuay, W.; Thanongsaksrikul, J.; Teimoori, S.; Sookrung, N.; Poovorawan, Y.; Onvimala, N.; Guntapong, R.; Pattanapanyasat, K.; et al. Human Antibodies to VP4 Inhibit Replication of Enteroviruses Across Subgenotypes and Serotypes, and Enhance Host Innate Immunity. Front. Microbiol. 2020, 11, 2253. [Google Scholar] [CrossRef]
- Alfaleh, M.A.; Alsaab, H.O.; Mahmoud, A.B.; Alkayyal, A.A.; Jones, M.L.; Mahler, S.M.; Hashem, A.M. Phage Display Derived Monoclonal Antibodies: From Bench to Bedside. Front. Immunol. 2020, 11, 1986. [Google Scholar] [CrossRef]
- Parray, H.A.; Chiranjivi, A.K.; Asthana, S.; Yadav, N.; Shrivastava, T.; Mani, S.; Sharma, C.; Vishwakarma, P.; Das, S.; Pindari, K.; et al. Identification of an anti-SARS-CoV-2 receptor-binding domain-directed human monoclonal antibody from a naïve semisynthetic library. J. Biol. Chem. 2020, 295, 12814–12821. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, S.; Coyle, E.M.; Klenow, L.; Tang, J.; Grubbs, G.; Liu, S.; Wang, T.; Golding, H.; Khurana, S. Antibody signature induced by SARS-CoV-2 spike protein immunogens in rabbits. Sci. Transl. Med. 2020, 12, eabc3539. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Li, L.; Lin, J.; Li, X.; Liu, B.; Kong, Y.; Zeng, S.; Du, J.; Xiao, H.; Zhang, T.; et al. Isolation of a human monoclonal antibody specific for the receptor binding domain of SARS-CoV-2 using a competitive phage biopanning strategy. Antib. Ther. 2020, 3, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Bertoglio, F.; Meier, D.; Langreder, N.; Steinke, S.; Rand, U.; Simonelli, L.; Heine, P.A.; Ballmann, R.; Schneider, K.-T.; Roth, K.D.R.; et al. SARS-CoV-2 neutralizing human recombinant antibodies selected from pre-pandemic healthy donors binding at RBD-ACE2 interface. Nat. Commun. 2021, 12, 1577. [Google Scholar] [CrossRef]
- Gai, J.; Ma, L.; Li, G.; Zhu, M.; Qiao, P.; Li, X.; Zhang, H.; Zhang, Y.; Chen, Y.; Ji, W.; et al. A potent neutralizing nanobody against SARS-CoV-2 with inhaled delivery potential. MedComm 2021, 2, 101–113. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, M.H.; Lee, S.-R.; Chung, H.-Y.; Kim, K.; Lee, T.G.; Kim, D.Y. Neutralizing Human Antibodies against Severe Acute Respiratory Syndrome Coronavirus 2 Isolated from a Human Synthetic Fab Phage Display Library. Int. J. Mol. Sci. 2021, 22, 1913. [Google Scholar] [CrossRef]
- Lim, S.A.; Gramespacher, J.A.; Pance, K.; Rettko, N.J.; Solomon, P.; Jin, J.; Lui, I.; Elledge, S.K.; Liu, J.; Bracken, C.J.; et al. Bispecific VH/Fab antibodies targeting neutralizing and non-neutralizing Spike epitopes demonstrate enhanced potency against SARS-CoV-2. MAbs 2021, 13, 1893426. [Google Scholar] [CrossRef]
- Gupta, V.; Hwang, B.H.; Lee, J.; Anselmo, A.C.; Doshi, N.; Mitragotri, S. Mucoadhesive intestinal devices for oral delivery of salmon calcitonin. J. Control. Release 2013, 172, 753–762. [Google Scholar] [CrossRef]
- Otvos, L., Jr.; Wade, J.D. Current challenges in peptide-based drug discovery. Front. Chem. 2014, 2, 62. [Google Scholar] [CrossRef]
- Maggio, E.T. Intravail™: Highly effective intranasal delivery of peptide and protein drugs. Expert Opin. Drug Deliv. 2006, 3, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.D.; Whitehead, K.A.; Mitragotri, S. Materials for oral delivery of proteins and peptides. Nat. Rev. Mater. 2020, 5, 127–148. [Google Scholar] [CrossRef]
- Lu, R.-M.; Hwang, Y.-C.; Liu, I.J.; Lee, C.-C.; Tsai, H.-Z.; Li, H.-J.; Wu, H.-C. Development of therapeutic antibodies for the treatment of diseases. J. Biomed. Sci. 2020, 27, 1. [Google Scholar] [CrossRef] [PubMed]
- Marasco, W.A.; Sui, J. The growth and potential of human antiviral monoclonal antibody therapeutics. Nat. Biotechnol. 2007, 25, 1421–1434. [Google Scholar] [CrossRef]
- Salazar, G.; Zhang, N.; Fu, T.-M.; An, Z. Antibody therapies for the prevention and treatment of viral infections. NPJ Vaccines 2017, 2, 19. [Google Scholar] [CrossRef]
- Ali, M.G.; Zhang, Z.; Gao, Q.; Pan, M.; Rowan, E.G.; Zhang, J. Recent advances in therapeutic applications of neutralizing antibodies for virus infections: An overview. Immunol. Res. 2020, 68, 325–339. [Google Scholar] [CrossRef]
| Peptides | |||||
| Peptide Sequence | Virus | Targeted Antigen | Binding Affinity | Inhibitory Activity (IC50) | Ref |
| SENRKVPFYSHS | JEV | Domain III of envelope protein | 6.06 × 10−6 M (Kd) | ~1 μM | [55] |
| CNDFRSKTC | H9N2 | Intact virus | NA | 48 μM | [68] |
| ACFPWGNQWCGGK | HCV | RNA-dependent RNA polymerase, NS5B | 34 μM (Kd) | 8.82 μM | [69] |
| CDVIALLACHLNT | WNV | Envelope protein, E | 6 μM (Kd) | 2.60 ± 0.01 μM | [70] |
| KHMHWHPPALNT | HBV | PreS1 protein | 7.21 × 104 ± 4.15 × 104 M−1 (Ka) | NA | [71] |
| ITFEDLLDYYGP | HIV-1 | Capsid domain of Gag polyprotein | 15.0 ± 7.2 μM (Kd) | NA | [72] |
| RAVWRHSVATPSHSV | H1N1 | Neu5Ac | 0.41 μM (Kd) | 6.5 μM | [73] |
| Antibody Fragments | |||||
| Antibody Fragment | Virus | Targeted Antigen | Binding Affinity | Inhibitory Activity (IC50) | Ref |
| Fab | HEV | Putative capsid protein, ORF2 | 1.7 nM (Kd) | NA | [74] |
| Fab | Marburg virus | VP35 protein | 4.9 ± 1 nM (Kd) | NA | [75] |
| Fab | EBOV | Envelope glycoprotein (GP) | NA | 1 μM | [76] |
| Fab | SARS-CoV-2 | RBD | 1.5 nM (Kd) | NA | [77] |
| Fab | HCMV | Glycoprotein B | 9.3 nM (Kd) | NA | [78] |
| Fab | HeV | Envelope glycoprotein, G | 28 nM (Kd) | 4.2 μg/mL | [79] |
| Fab | HIV-1 | Envelope glycoprotein (gp140) | 1.4 nM (Kd) | 8 μg/mL | [80] |
| VH | SARS-CoV-2 | S1 subunit of spike protein | 3.70 ± 0.09 nM (Kd) | 2.6 μg/mL | [81] |
| VH | SARS-CoV-2 | RBD | 19 nM (Kd) | 0.65 μg/mL | [82] |
| VHH | SARS-CoV-2 | RBD | 21.6 nM (Kd) | 0.55 μg/mL | [83] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokullu, E.; Gauthier, M.-S.; Coulombe, B. Discovery of Antivirals Using Phage Display. Viruses 2021, 13, 1120. https://doi.org/10.3390/v13061120
Sokullu E, Gauthier M-S, Coulombe B. Discovery of Antivirals Using Phage Display. Viruses. 2021; 13(6):1120. https://doi.org/10.3390/v13061120
Chicago/Turabian StyleSokullu, Esen, Marie-Soleil Gauthier, and Benoit Coulombe. 2021. "Discovery of Antivirals Using Phage Display" Viruses 13, no. 6: 1120. https://doi.org/10.3390/v13061120
APA StyleSokullu, E., Gauthier, M.-S., & Coulombe, B. (2021). Discovery of Antivirals Using Phage Display. Viruses, 13(6), 1120. https://doi.org/10.3390/v13061120






