Detection and Cellular Tropism of Porcine Astrovirus Type 3 on Breeding Farms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Farm Selection
2.3. Sample Collection
2.4. PoAstV3 RT-qPCR
2.5. Pathology and In Situ Hybridization
2.6. Statistical Analysis
3. Results
3.1. Sow Farm 1(Cross-Sectional Phase-I)
3.2. Sow Farm 1(Cross-Sectional Phase-II)
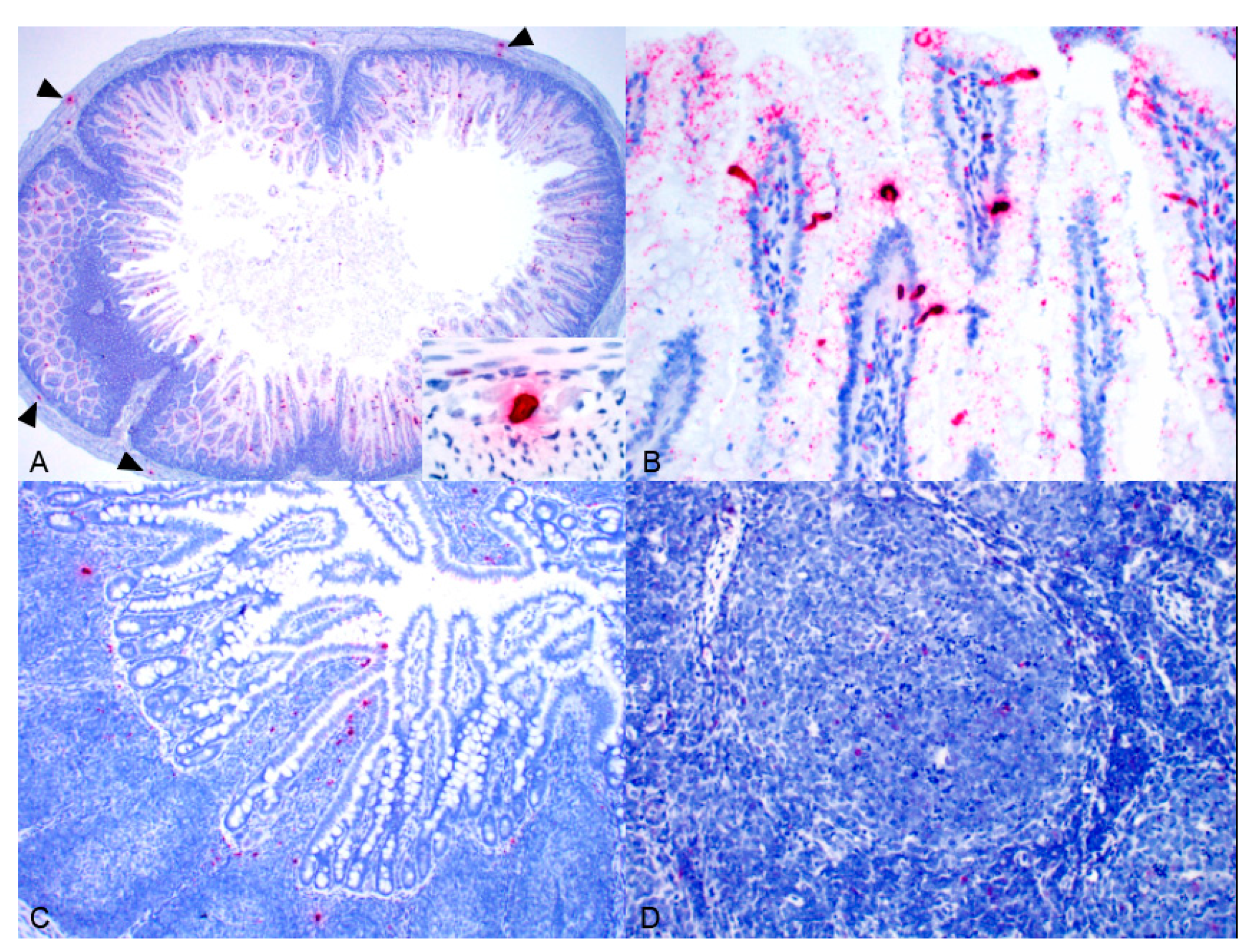
Pathology and ISH
3.3. Sow Farm 2
3.4. Sow Farm 3
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Monroe, S.S.; Carter, M.J.; Herrmann, J.; Mitchell, J.R.; Sanchez-Fauquier, A. Astroviridae. Virus Taxonomy. Eighth Report of the International Committee on Taxonomy of Viruses; Elsevier: Amsterdam, The Netherlands, 2005; pp. 859–864. [Google Scholar]
- Bosch, A.; King, A.M.Q.; Lefkowitz, E.; Adams, M.J.; Carstens, E.B. Family Astroviridae. Virus Taxonomy: Classification and Nomenclature of Viruses (Ninth Report of the International Committee on the Taxonomy of Viruses); Elsevier Academic Press: New York, NY, USA, 2011; pp. 953–959. [Google Scholar]
- Biđin, M.; Lojkić, I.; Tišliar, M.; Biđin, Z.; Majnarić, D. Astroviruses associated with stunting and pre-hatching mortality in duck and goose embryos. Avian Pathol. 2012, 41, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.K.; Poon, L.L.M.; Guan, Y.; Peiris, J.S.M. Novel astroviruses in insectivorous bats. J. Virol. 2008, 82, 9107–9114. [Google Scholar] [CrossRef] [PubMed]
- Englund, L.; Chriél, M.; Dietz, H.H.; Hedlund, K.O. Astrovirus epidemiologically linked to pre-weaning diarrhoea in mink. Vet. Microbiol. 2002, 85, 1–11. [Google Scholar] [CrossRef]
- Martella, V.; Moschidou, P.; Pinto, P.; Catella, C.; Desario, C.; Larocca, V.; Circella, E.; Bànyai, K.; Lavazza, A.; Magistrali, C.; et al. Astroviruses in rabbits. Emerg. Infect. Dis. 2011, 17, 2287–2293. [Google Scholar] [CrossRef] [PubMed]
- Mor, S.K.; Abin, M.; Costa, G.; Durrani, A.; Jindal, N.; Goyal, S.M.; Patnayak, D.P. The role of type-2 turkey astrovirus in poult enteritis syndrome. Poult. Sci. 2011, 90, 2747–2752. [Google Scholar] [CrossRef] [PubMed]
- Tse, H.; Chan, W.M.; Tsoi, H.W.; Fan, R.Y.; Lau, C.C.; Lau, S.K.; Woo, P.C.; Yuen, Y. Rediscovery and genomic characterization of bovine astroviruses. J. Gen. Virol. 2011, 92, 1888–1898. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.T.; Giménez-Lirola, L.G.; Gerber, P.F.; Jiang, Y.H.; Halbur, P.G.; Opriessnig, T. Identification and characterization of novel porcine astroviruses (PAstVs) with high prevalence and frequent co-infection of individual pigs with multiple PAstV types. J. Gen. Virol. 2013, 94, 570–582. [Google Scholar] [CrossRef] [PubMed]
- Benedictis, P.; Schultz-Cherry, S.; Burnham, A.; Cattoli, G. Astrovirus infections in humans and animals—Molecular biology, genetic diversity, and interspecies transmissions. Infect. Genet. Evol. 2011, 11, 1529–1544. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Roi, S.; Dastor, M.; Gallice, E.; Laurin, M.A.; L’Homme, Y. Multiple novel and prevalent astroviruses in pigs. Vet. Microbiol. 2011, 149, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Shan, T.; Li, L.; Simmonds, P.; Wang, C.; Moeser, A.; Delwart, E. The Fecal Virome of Pigs on a High-Density Farm. J. Virol. 2011, 85, 11697–11708. [Google Scholar] [CrossRef] [PubMed]
- Lachapelle, V.; Letellier, A.; Fravalo, P.; Brassard, J.; L’Homme, Y. Dynamics of virus distribution in a defined swine production network using enteric viruses as molecular markers. Appl. Environ. Microbiol. 2017, 83, e03187-16. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Yin, W.; Zhou, Y.; Li, B.; Ai, L.; Pan, M.; Guo, W. Molecular detection of Porcine astrovirus in Sichuan Province, China. Virol. J. 2016, 13, 6. [Google Scholar] [CrossRef] [PubMed]
- Li, J.S.; Li, M.Z.; Zheng, L.S.; Liu, N.; Li, D.D.; Duan, Z.J. Identification and genetic characterization of two porcine astroviruses from domestic piglets in China. Arch. Virol. 2015, 160, 3079–3084. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.T.; Luo, Z.; Lv, S.L.; Opriessnig, T.; Li, R.C.; Yu, X.L. Identification and characterization of multiple porcine astrovirus genotypes in Hunan province, China. Arch. Virol. 2017, 162, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Brnić, D.; Prpić, J.; Keros, T.; Roić, B.; Starešina, V.; Jemeršić, L. Porcine astrovirus viremia and high genetic variability in pigs on large holdings in Croatia. Infect. Genet. Evol. 2013, 14, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Dufkova, L.; Sčigalková, I.; Moutelíková, R.; Malenovská, H.; Prodelaova, J. Genetic diversity of porcine sapoviruses, kobuviruses, and astroviruses in asymptomatic pigs: An emerging new sapovirus GIII genotype. Arch. Virol. 2013, 158, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Amimo, J.O.; Okoth, E.; Junga, J.O.; Ogara, W.O.; Njahira, M.N.; Wang, Q.; Vlasova, A.N.; Saif, L.J.; Djikeng, A. Molecular detection and genetic characterization of kobuviruses and astroviruses in asymptomatic local pigs in East Africa. Arch. Virol. 2014, 159, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Goecke, N.B.; Hijulager, C.K.; Kongsted, H.; Boye, M.; Rasmussen, S.; Granberg, F.; Fischer, T.K.; Midgley, S.E.; Rasmussen, L.D.; Angen, Ø.; et al. No evidence of enteric viral involvement in the new neonatal porcine diarrhoea syndrome in Danish pigs. BMC Vet. Res. 2017, 13, 315. [Google Scholar] [CrossRef] [PubMed]
- Machnowska, P.; Ellerbroek, L.; Johne, R. Detection and characterization of potentially zoonotic viruses in faeces of pigs at slaughter in Germany. Vet. Microbiol. 2014, 168, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Sachsenröeder, J.; Twardziok, S.; Hammerl, J.A.; Janczyk, P.; Wrede, P.; Hertwig, S.; Johne, R. Simultaneous Identification of DNA and RNA Viruses Present in Pig Faeces Using Process-Controlled Deep Sequencing. PLoS ONE 2012, 7, e34631. [Google Scholar] [CrossRef] [PubMed]
- Boros, A.; Albert, M.; Pankovics, P.; Bíró, H.; Pesavento, P.A.; Phan, T.G.; Delwart, E.; Reuter, G. Outbreaks of Neuroinvasive Astrovirus Associated with Encephalomyelitis, Weakness, and Paralysis among Weaned Pigs, Hungary. Emerg. Infect. Dis. 2017, 23, 1999–2010. [Google Scholar] [CrossRef] [PubMed]
- Monini, M.; Di Bartolo, I.; Ianiro, G.; Angeloni, G.; Magistrali, C.F.; Ostanello, F.; Ruggeri, F.M. Detection and molecular characterization of zoonotic viruses in swine fecal samples in Italian pig herds. Arch. Virol. 2015, 160, 2547–2556. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Kuroda, M.; Masuda, T.; Akagami, M.; Haga, K.; Tsuchiaka, S.; Kishimoto, M.; Naoi, Y.; Sano, K.; Omatsu, T.; et al. Whole genome analysis of porcine astroviruses detected in Japanese pigs reveals genetic diversity and possible intra-genotypic recombination. Infect. Genet. Evol. 2017, 50, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Salamunova, S.; Jackova, A.; Mandelik, R.; Novotny, J.; Vlasakova, M.; Vilcek, S. Molecular detection of enteric viruses and the genetic characterization of porcine astroviruses and sapoviruses in domestic pigs from Slovakian farms. BMC Vet. Res. 2018, 14, 313. [Google Scholar] [CrossRef] [PubMed]
- Blomstrom, A.L.; Cecilia, L.; Jacobson, M. Astrovirus as a possible cause of congenital tremor type AII in piglets? Acta Vet. Scand. 2014, 56, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karlsson, O.E.; Larsson, J.; Hayer, J.; Berg, M.; Jacobson, M. The Intestinal Eukaryotic Virome in Healthy and Diarrhoeic Neonatal Piglets. PLoS ONE 2016, 11, e0151481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.H.; Jeoung, H.Y.; Park, H.R.; Lim, J.A.; Song, J.Y.; An, D.J. Phylogenetic analysis of porcine astrovirus in domestic pigs and wild boars in South Korea. Virus Genes 2013, 46, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Kumthip, K.; Khamrin, P.; Saikruang, W.; Kongkaew, A.; Vachirachewin, R.; Ushijima, H.; Maneekarn, N. Detection and genetic characterization of porcine astroviruses in piglets with and without diarrhea in Thailand. Arch. Virol. 2018, 163, 1823–1829. [Google Scholar] [CrossRef] [PubMed]
- Arruda, B.; Arruda, P.; Hensch, M.; Chen, Q.; Zheng, Y.; Yang, C.; Gatto, I.R.H.; Matias, F.F.; Gauger, P.; Schwartz, K.; et al. Porcine Astrovirus Type 3 in Central Nervous System of Swine with Polioencephalomyelitis. Emerg. Infect. Dis. 2017, 23, 2097–2100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matias, F.F.; Arruda, P.; Hench, M.; Gatto, I.R.H.; Hause, B.; Li, G.; Chen, Q.; Zheng, Y.; Yang, C.; Harmon, K.; et al. Identification of a divergent porcine astrovirus type 3 in central nervous system tissue from swine with neurologic disease and encephalomyelitis: Diagnostic investigation, virus characterization and retrospective analysis of historic cases. In Proceedings of the American Association of Veterinary Laboratory Diagnosticians (AAVLD) Annual Conference Proceedings, San Diego, CA, USA, 12–18 October 2017. [Google Scholar]
- Mor, S.K.; Chander, Y.; Marthaler, D.; Patnayak, D.P.; Goyal, S.M. Detection and molecular characterization of Porcine astrovirus strains associated with swine diarrhea. J. Vet. Diagn. Investig. 2012, 24, 1064–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Hog Farmer. Porcine Astrovirus Type 3 Emerging Cause of Neurologic Disease in Swine. Available online: https://www.nationalhogfarmer.com/animal-health/porcine-astrovirus-type-3-emerging-cause-neurologic-disease-swine (accessed on 15 August 2019).
- Rawal, G.; Matias, F.F.; Mueller, A.; Allison, G.; Hedberg, W.; Macedo, N.; Bradner, L.; Harmon, K.; Linhares, D.; Arruda, B. Porcine astrovirus type 3 is an emerging cause of atypical neurologic disease: Diagnostic cases and infection dynamics on affected flows. In Proceedings of the 50th Annual Meeting of the American Association of Swine Veterinarians (AASV), Orlando, FL, USA, 9–12 March 2019. [Google Scholar]
- Matias, F.F.; Bradner, L.K.; Burrough, E.R.; Cooper, V.L.; Derscheid, R.J.; Gauger, P.C.; Harmon, K.M.; Madson, D.; Piñeyro, P.E.; Schwartz, K.J.; et al. Polioencephalomyelitis in Domestic Swine Associated with Porcine Astrovirus Type 3. Vet. Pathol. 2019. [Google Scholar] [CrossRef]
- Schroeder, M.E.; Bounpheng, M.A.; Rodgers, S.; Baker, R.J.; Black, W.; Naikare, H.; Velayudhan, B.; Sneed, L.; Szonyi, B.; Clavijo, A. Development and performance evaluation of calf diarrhea pathogen nucleic acid purification and detection workflow. J. Vet. Diagn. Investig. 2012, 24, 945–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordey, S.; Vu, D.L.; Schibler, M.; L’Huilier, A.G.; Brito, F.; Docquier, M.; Posfay-Barbe, K.M.; Petty, T.J.; Turin, L.; Zdobnov, E.M.; et al. Astrovirus MLB2, a New Gastroenteric Virus Associated with Meningitis and Disseminated Infection. Emerg. Infect. Dis. 2016, 22, 846–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Diab, S.; McGraw, S.; Barr, B.; Traslavina, R.; Higgins, R.; Talbot, T.; Blanchard, P.; Rimoldi, G.; Fahsbender, E.; et al. Divergent astrovirus associated with neurologic disease in cattle. Emerg. Infect. Dis. 2013, 19, 1385–1392. [Google Scholar] [CrossRef] [PubMed]
- Pfaff, F.; Schlottau, K.; Scholes, S.; Courtnenay, A.; Hoffmann, B.; Höper, D.; Beer, M. A novel astrovirus associated with encephalitis and ganglionitis in domestic sheep. Transbound. Emerg. Dis. 2017, 64, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Quan, P.L.; Wagner, T.A.; Briese, T.; Torgerson, T.R.; Hornig, M.; Tashmukhamedova, A.; Firth, C.; Palacios, G.; Baisre-De-Leon, A.; Paddock, C.D.; et al. Astrovirus encephalitis in boy with X-linked agammaglobulinemia. Emerg. Infect. Dis. 2010, 16, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Virus Survival in the Environment with Special Attention to Survival in Sewage Droplets and other Environmental Media of Fecal or Respiratory Origin. Available online: http://www.oretek.com/drugs/WHO_VirusSurvivalReport_21Aug2003.pdf (accessed on 15 August 2019).
- Shimizu, M.; Shirai, J.; Narita, M.; Yamane, T. Cytopathic astrovirus isolated from porcine acute gastroenteritis in an established cell line derived from porcine embryonic kidney. J. Clin. Microbiol. 1990, 28, 201–206. [Google Scholar] [PubMed]
- Abad, F.X.; Pintó, R.M.; Villena, C.; Gajardo, R.; Bosch, A. Astrovirus survival in drinking water. Appl. Environ. Microbiol. 1997, 63, 3119–3122. [Google Scholar] [PubMed]
- Banos-Lara, M.R.; Mèndez, E. Role of individual caspases induced by astrovirus on the processing of its structural protein and its release from the cell through a non-lytic mechanism. Virol. J. 2010, 401, 322–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arias, C.F.; DuBois, M. The Astrovirus Capsid: A Review. Viruses 2017, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Curtis, R.; Adryan, K.M.; Zhu, Y.; Harkness, P.J.; Lindsay, R.M.; DiStefano, P.S. Retrograde axonal transport of ciliary neurotrophic factor is increased by peripheral nerve injury. Nature 1993, 365, 253–255. [Google Scholar] [CrossRef] [PubMed]
- Gromeier, M.; Wimmer, E. Mechanism of injury-provoked poliomyelitis. J. Virol. 1998, 72, 5056–5060. [Google Scholar] [PubMed]
- Ren, R.; Racaniello, V.R. Poliovirus spreads from muscle to the central nervous system by neural pathways. J. Infect. Dis. 1992, 166, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, K.Z.; Pfeiffer, J.K. Limited Trafficking of a Neurotropic Virus Through Inefficient Retrograde Axonal Transport and the Type I Interferon Response. PLoS Pathog. 2010, 6, e1000791. [Google Scholar] [CrossRef] [PubMed]

| Age Group | Sow Farm 1 (Phase-I) | Sow Farm 1 (Phase-II) | Sow Farm 2 | Sow Farm 3 | ||||
|---|---|---|---|---|---|---|---|---|
| % Positive (Sample n) | Mean (Cq Range) | % Positive (Sample n) | Mean (Cq Range) | % Positive (Sample n) | Mean (Cq Range) | % Positive (Sample n) | Mean (Cq Range) | |
| Sows | 90% a (9 of 10) | 35.17 a (32.47– 39.43) | 75% a (15 of 20) | 34.23 a (31.05–38.05) | 52% a (16 of 31) | 35.89 a (30.99–38.02) | ||
| Gilts | 20% b (2 of 10) | 37.01 a (35.71– 38.32) | 29% a (23 of 80) | 32.00 a (28.16– 39.09) | ||||
| Piglets | 86% a (43 of 50) | 31.82 b (22.19–38.96) | 86% a (192 of 224) | 26.86 b (14.60–39.15) | 97% b (30 of 31) | 26.50 b (14.69–38.66) | 100% b (90 of 90) | 31.70 a (30.74–32.68) |
| Parity | % Positive (Sampling n) | Mean (Cq Range) |
|---|---|---|
| 1 | 88% (7 of 8) | 33.72 (31.05–36.93) |
| 4 | 67% (4 of 6) | 34.17 (32.68–37.54) |
| 5 | 100% (2 of 2) | 36.58 (35.11–38.04) |
| 7 | 100% (2 of 2) | 33.75 (33.05–34.45) |
| 10 | 0% (0 of 2) | - |
| Pig ID | Group | Age (days) | Litter ID | GL | PoAstV3 Cq | GS | AE | CL | In Situ Hybridization | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GM | Duo | Jej | Ile | CM | SC | MSLN | MP | |||||||||
| 1 | Control | 11 | A | DU | 17.96 | 0 | 1 | 0 | NA | NA | NA | NA | NA | NA | NA | NA |
| 2 | Control | 11 | A | FF | 25.42 | 0 | 1 | 0 | ND | ND | ND | OE | ND | OC | GC;PLS;M | ND |
| 3 | Control | 11 | B | DU | 21.10 | 1 | 1 | 1 | NA | NA | NA | NA | NA | NA | NA | NA |
| 4 | Control | 11 | C | DU | 20.83 | 0 | 1 | 1 | OGP | ND | RE-NE | ND | ND | RC;L;CL | GC;PLS;M | I;J |
| 5 | Enteric | 11 | A | DU | UD | 1 | 0 | 0 | NA | NA | NA | NA | NA | NA | NA | NA |
| 6 | Enteric | 15 | A | FF | 32.25 | 1 | 0 | 1 | NA | NA | NA | NA | NA | NA | NA | NA |
| 7 | Enteric | 11 | B | FF | 31.66 | 0 | 0 | 0 | NA | NA | NA | NA | NA | NA | NA | NA |
| 8 | Enteric | 15 | B | FF | UD | 1 | 0 | 0 | NA | NA | NA | NA | NA | NA | NA | NA |
| 9 | Enteric | 15 | C | FF | UD | 0 | 1 | 1 | NA | NA | NA | NA | NA | NA | NA | NA |
| 10 | Enteric | 11 | C | DU | 23.50 | 1 | 0 | 0 | RGP | ND | RE | OE;LP;PP | ND | ND | GC;PLS;M | I |
| 11 | Enteric | 11 | D | FF | UD | 0 | 1 | 0 | NA | NA | NA | NA | NA | NA | NA | NA |
| 12 | Enteric | 19 | D | DU | 35.71 | 0 | 1 | 1 | NA | NA | NA | NA | NA | NA | NA | NA |
| 13 | Enteric | 15 | E | FF | 25.80 | 1 | 0 | 0 | RGP | ND | ME;LP | RE;PP | ND | ND | PLS | I |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rawal, G.; Matias Ferreyra, F.; R. Macedo, N.; K. Bradner, L.; M. Harmon, K.; Mueller, A.; Allison, G.; C. L. Linhares, D.; L. Arruda, B. Detection and Cellular Tropism of Porcine Astrovirus Type 3 on Breeding Farms. Viruses 2019, 11, 1051. https://doi.org/10.3390/v11111051
Rawal G, Matias Ferreyra F, R. Macedo N, K. Bradner L, M. Harmon K, Mueller A, Allison G, C. L. Linhares D, L. Arruda B. Detection and Cellular Tropism of Porcine Astrovirus Type 3 on Breeding Farms. Viruses. 2019; 11(11):1051. https://doi.org/10.3390/v11111051
Chicago/Turabian StyleRawal, Gaurav, Franco Matias Ferreyra, Nubia R. Macedo, Laura K. Bradner, Karen M. Harmon, Adam Mueller, Grant Allison, Daniel C. L. Linhares, and Bailey L. Arruda. 2019. "Detection and Cellular Tropism of Porcine Astrovirus Type 3 on Breeding Farms" Viruses 11, no. 11: 1051. https://doi.org/10.3390/v11111051
APA StyleRawal, G., Matias Ferreyra, F., R. Macedo, N., K. Bradner, L., M. Harmon, K., Mueller, A., Allison, G., C. L. Linhares, D., & L. Arruda, B. (2019). Detection and Cellular Tropism of Porcine Astrovirus Type 3 on Breeding Farms. Viruses, 11(11), 1051. https://doi.org/10.3390/v11111051






