Tolerance to a Diet of Toxic Microcystis aeruginosa in Caenorhabditis elegans
Abstract
:1. Introduction
2. Results
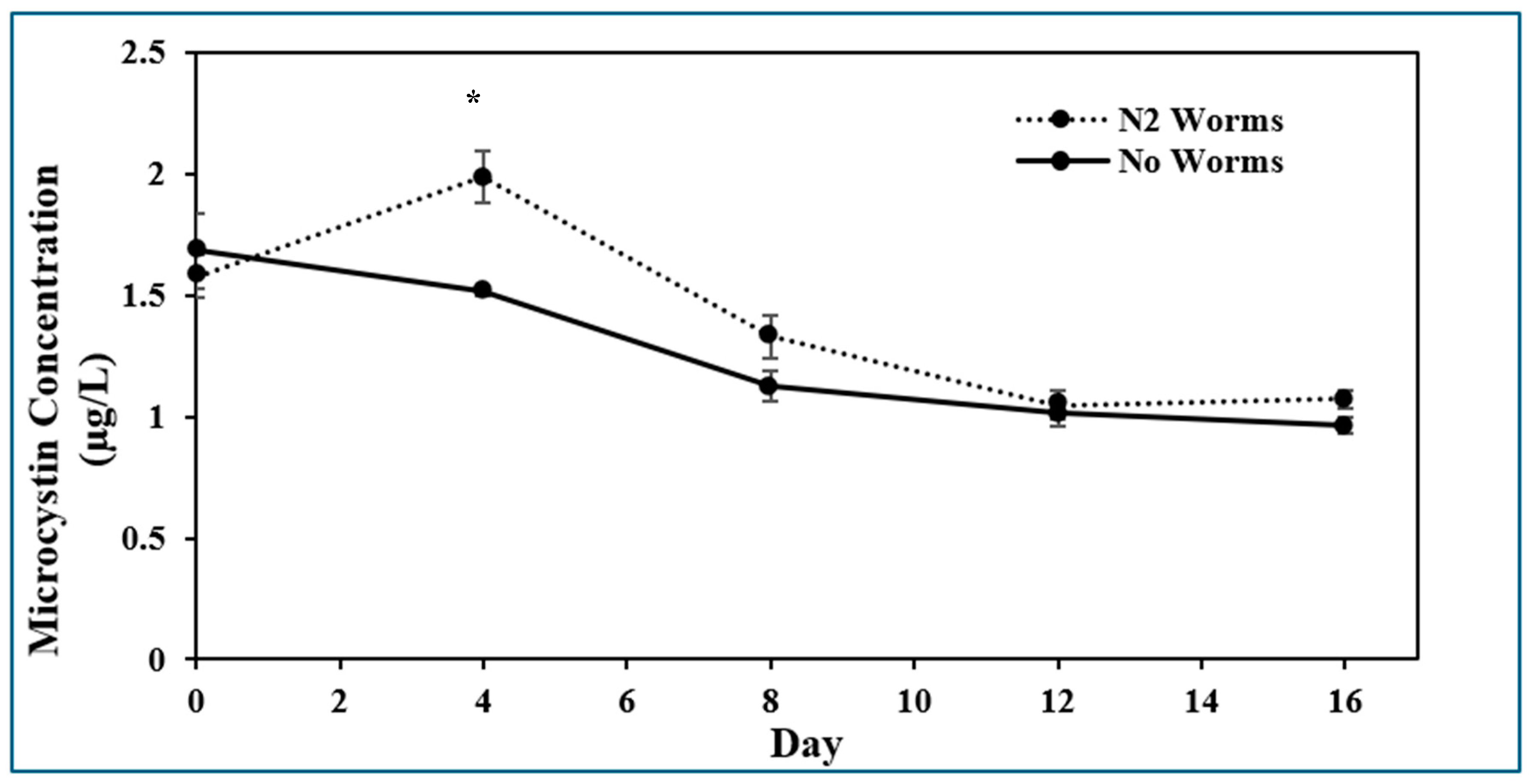
2.1. Presence of Microcystin-LR
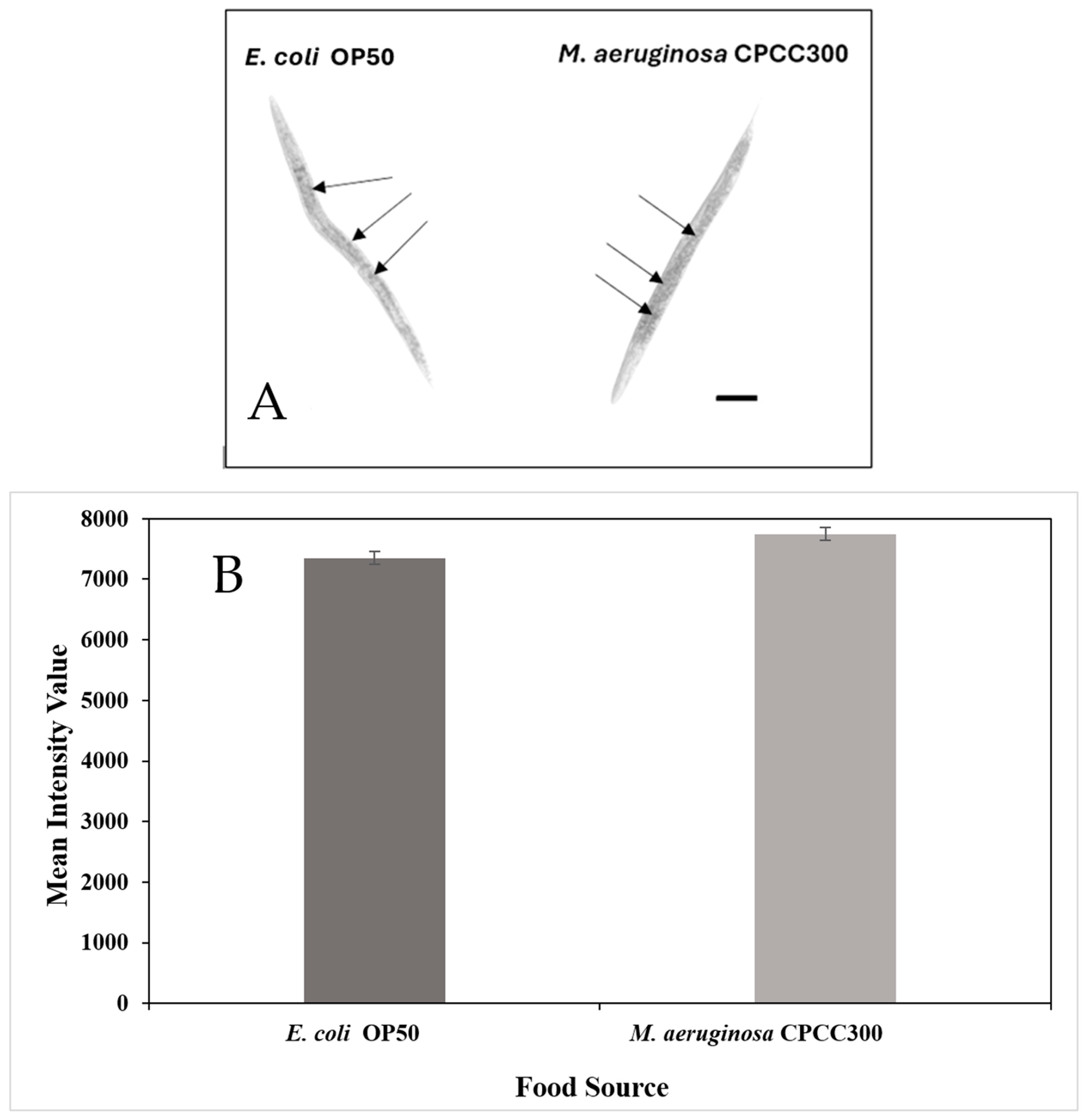
2.2. Fat Accumulation
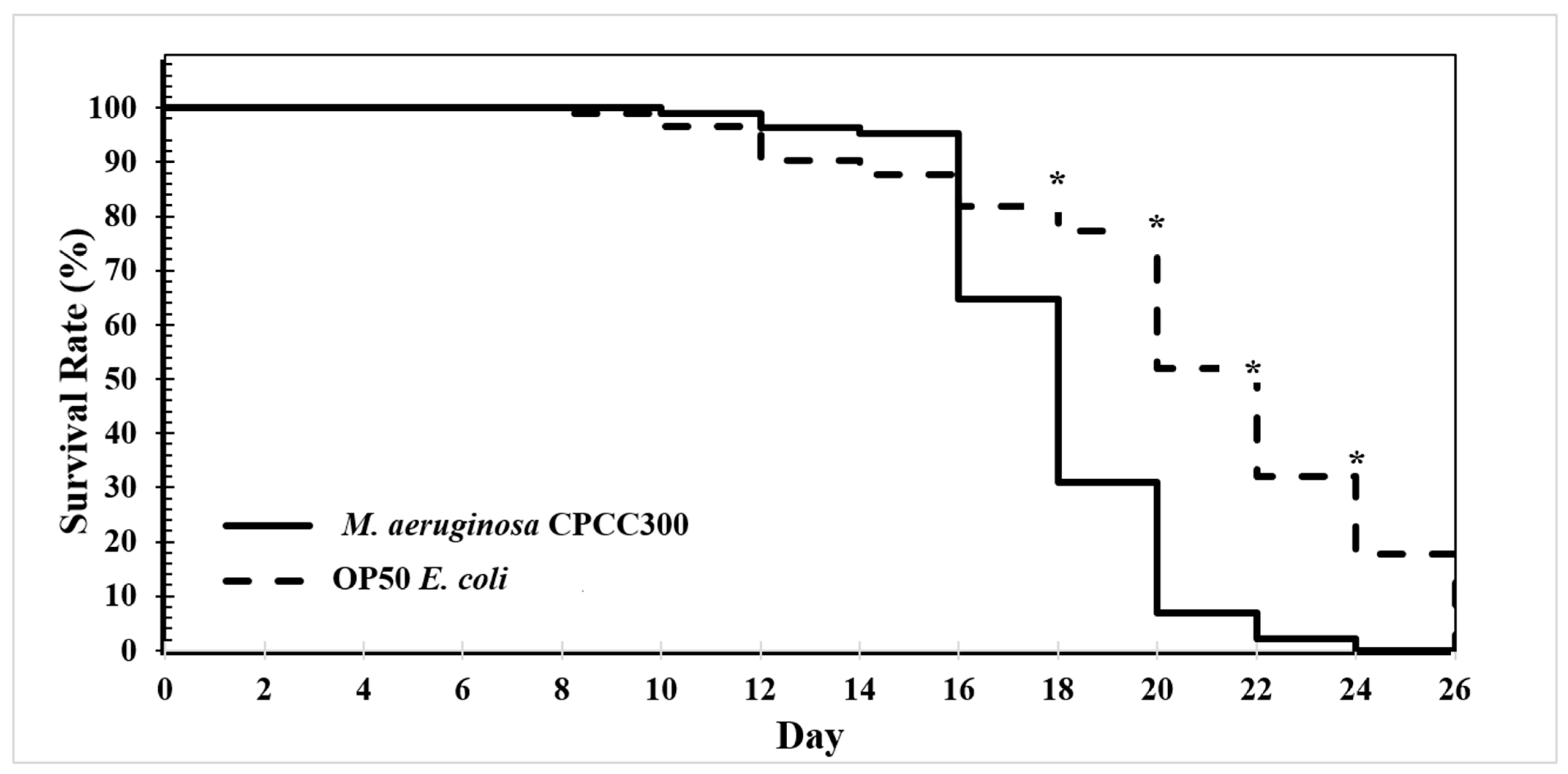
2.3. Longevity Assay
2.4. Stress Response Assay
2.5. SKN-1::GFP Expression
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Bacterial Cultures
5.2. Nematode Cultures
5.3. Microcystin-LR Assay
5.4. Fat Accumulation
5.5. Longevity Assay
5.6. Dauer Heat Stress Response Assay
5.7. Thrashing Assay
5.8. Pharyngeal Pumping Assay
5.9. SKN-1::GFP Expression
5.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The Rise of Harmful Cyanobacteria Blooms: The Potential Roles of Eutrophication and Climate Change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Paerl, H. Nutrient and Other Environmental Controls of Harmful Cyanobacterial Blooms along the Freshwater-Marine Continuum. Adv. Exp. Med. Biol. 2008, 619, 217–237. [Google Scholar] [CrossRef]
- Hense, I.; Beckmann, A. The Representation of Cyanobacteria Life Cycle Processes in Aquatic Ecosystem Models. Ecol. Model. 2010, 221, 2330–2338. [Google Scholar] [CrossRef]
- Carmichael, W.W.; Boyer, G.L. Health Impacts from Cyanobacteria Harmful Algae Blooms: Implications for the North American Great Lakes. Harmful Algae 2016, 54, 194–212. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, S.; Zeng, J.; Chabi, K.; Song, W.; Xian, X.; Yu, X. Impact of Chlorination on Cell Inactivation, Toxin Release and Degradation of Cyanobacteria of Development and Maintenance Stage. Chem. Eng. J. 2020, 397, 125378. [Google Scholar] [CrossRef]
- Hu, C.; Rzymski, P. Programmed Cell Death-Like and Accompanying Release of Microcystin in Freshwater Bloom-Forming Cyanobacterium Microcystis: From Identification to Ecological Relevance. Toxins 2019, 11, 706. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, S. A Web-Based Modeling Approach for Tracking Algal Blooms in Lower Great Lakes; State University of New York at Buffalo: Buffalo, NY, USA, 2008. [Google Scholar]
- Hudnell, H.K.; Dortch, Q. A Synopsis of Research Needs Identified at the Interagency, International Symposium on Cyanobacterial Harmful Algal Blooms (ISOC-HAB). Adv. Exp. Med. Biol. 2008, 619, 17–43. [Google Scholar] [CrossRef]
- Jančula, D.; Maršálek, B. Critical Review of Actually Available Chemical Compounds for Prevention and Management of Cyanobacterial Blooms. Chemosphere 2011, 85, 1415–1422. [Google Scholar] [CrossRef]
- Qu, M.; Lefebvre, D.D.; Wang, Y.; Qu, Y.; Zhu, D.; Ren, W. Algal Blooms: Proactive Strategy. Science 2014, 346, 175–176. [Google Scholar] [CrossRef]
- Canada, H. Guidelines for Canadian Drinking Water Quality: Guideline Technical Document—Cyanobacterial Toxins. Available online: https://www.canada.ca/en/health-canada/services/publications/healthy-living/guidelines-canadian-drinking-water-quality-guideline-technical-document-cyanobacterial-toxins-document.html (accessed on 6 December 2024).
- Rastogi, R.P.; Sinha, R.P.; Incharoensakdi, A. The Cyanotoxin-Microcystins: Current Overview. Rev. Environ. Sci. Biotechnol. 2014, 13, 215–249. [Google Scholar] [CrossRef]
- Ferrão-Filho, A.d.S.; Kozlowsky-Suzuki, B. Cyanotoxins: Bioaccumulation and Effects on Aquatic Animals. Mar. Drugs 2011, 9, 2729–2772. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, J.; Fan, H.; Xie, P.; He, J. A Review of Neurotoxicity of Microcystins. Environ. Sci. Pollut. Res. Int. 2016, 23, 7211–7219. [Google Scholar] [CrossRef] [PubMed]
- Chernoff, N.; Hill, D.; Lang, J.; Schmid, J.; Le, T.; Farthing, A.; Huang, H. The Comparative Toxicity of 10 Microcystin Congeners Administered Orally to Mice: Clinical Effects and Organ Toxicity. Toxins 2020, 12, 403. [Google Scholar] [CrossRef] [PubMed]
- Buckingham, S.D.; Sattelle, D.B. Fast, Automated Measurement of Nematode Swimming (Thrashing) without Morphometry. BMC Neurosci. 2009, 10, 84. [Google Scholar] [CrossRef]
- Lapierre, L.R.; Hansen, M. Lessons from C. elegans: Signaling Pathways for Longevity. Trends Endocrinol. Metab. 2012, 23, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Cypser, J.R.; Wu, D.; Park, S.-K.; Ishii, T.; Tedesco, P.M.; Mendenhall, A.R.; Johnson, T.E. Predicting Longevity in C. elegans: Fertility, Mobility and Gene Expression. Mech. Ageing Dev. 2013, 134, 291–297. [Google Scholar] [CrossRef]
- Kumar, A.; Baruah, A.; Tomioka, M.; Iino, Y.; Kalita, M.C.; Khan, M. Caenorhabditis elegans: A Model to Understand Host-Microbe Interactions. Cell. Mol. Life Sci. 2020, 77, 1229–1249. [Google Scholar] [CrossRef] [PubMed]
- Mullaney, B.C.; Ashrafi, K.C. C. elegans Fat Storage and Metabolic Regulation. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2009, 1791, 474–478. [Google Scholar] [CrossRef]
- Trojanowski, N.F.; Raizen, D.M.; Fang-Yen, C. Pharyngeal Pumping in Caenorhabditis elegans Depends on Tonic and Phasic Signaling from the Nervous System. Sci. Rep. 2016, 6, 22940. [Google Scholar] [CrossRef]
- Avery, L.; You, Y.-J.C. elegans Feeding. In WormBook: The Online Review of C. elegans Biology [Internet]; WormBook: Pasadena, CA, USA, 2012. [Google Scholar]
- Bretscher, A.J.; Kodama-Namba, E.; Busch, K.E.; Murphy, R.J.; Soltesz, Z.; Laurent, P.; de Bono, M. Temperature, Oxygen, and Salt-Sensing Neurons in C. elegans Are Carbon Dioxide Sensors That Control Avoidance Behavior. Neuron 2011, 69, 1099–1113. [Google Scholar] [CrossRef] [PubMed]
- Stiernagle, T. Maintenance of C. elegans. In WormBook: The Online Review of C. elegans Biology [Internet]; WormBook: Pasadena, CA, USA, 2006. [Google Scholar]
- Fielenbach, N.; Antebi, A.C. C. elegans Dauer Formation and the Molecular Basis of Plasticity. Genes Dev. 2008, 22, 2149–2165. [Google Scholar] [CrossRef]
- Lant, B.; Storey, K.B. An Overview of Stress Response and Hypometabolic Strategies in Caenorhabditis elegans: Conserved and Contrasting Signals with the Mammalian System. Int. J. Biol. Sci. 2010, 6, 9–50. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liang, X.-F.; Liao, W.-Q.; Lei, L.-M.; Han, B.-P. Structural and Functional Characterization of Microcystin Detoxification-Related Liver Genes in a Phytoplanktivorous Fish, Nile Tilapia (Oreochromis niloticus). Comp. Biochem. Physiol. Part. C Toxicol. Pharmacol. 2006, 144, 216–227. [Google Scholar] [CrossRef]
- Choe, K.P.; Przybysz, A.J.; Strange, K. The WD40 Repeat Protein WDR-23 Functions with the CUL4/DDB1 Ubiquitin Ligase To Regulate Nuclear Abundance and Activity of SKN-1 in Caenorhabditis elegans. Mol. Cell. Biol. 2009, 29, 2704–2715. [Google Scholar] [CrossRef]
- Fukushige, T.; Smith, H.E.; Miwa, J.; Krause, M.W.; Hanover, J.A. A Genetic Analysis of the Caenorhabditis elegans Detoxification Response. Genetics 2017, 206, 939–952. [Google Scholar] [CrossRef]
- Anabtawi, H.M.; Lee, W.H.; Al-Anazi, A.; Mohamed, M.M.; Aly Hassan, A. Advancements in Biological Strategies for Controlling Harmful Algal Blooms (HABs). Water 2024, 16, 224. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Additional Information About Cyanotoxins in Drinking Water. Available online: https://www.epa.gov/ground-water-and-drinking-water/additional-information-about-cyanotoxins-drinking-water (accessed on 6 December 2024).
- Mehner, T.; Benndorf, J.; Kasprzak, P.; Koschel, R. Biomanipulation of Lake Ecosystems: Successful Applications and Expanding Complexity in the Underlying Science. Freshw. Biol. 2002, 47, 2453–2465. [Google Scholar] [CrossRef]
- Alonge, O.O.; Famakinwa, T.O.; Matouke, M.M. Perspectives of Bioremediation as a Panacea for Ecological Pollution. Glob. J. Pure Appl. Sci. 2017, 23, 377–381. [Google Scholar] [CrossRef]
- Liu, Y.; Benitez, M.G.; Chen, J.; Harrison, E.; Khusnutdinova, A.N.; Mahadevan, R. Opportunities and Challenges for Microbial Synthesis of Fatty Acid-Derived Chemicals (FACs). Front. Bioeng. Biotechnol. 2021, 9, 613322. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Brookes, J.D.; Qin, B.; Paerl, H.W.; Gao, G.; Wu, P.; Zhang, W.; Deng, J.; Zhu, G.; Zhang, Y.; et al. Environmental Factors Controlling Colony Formation in Blooms of the Cyanobacteria Microcystis spp. in Lake Taihu, China. Harmful Algae 2014, 31, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Kehr, J.-C.; Dittmann, E. Biosynthesis and Function of Extracellular Glycans in Cyanobacteria. Life 2015, 5, 164–180. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Rao, L.; Chiu, Y.-T.; Lin, T.-F. Impact of Chlorine on the Cell Integrity and Toxin Release and Degradation of Colonial Microcystis. Water Res. 2016, 102, 394–404. [Google Scholar] [CrossRef]
- Augusti, P.R.; Brasil, A.V.S.; Souto, C.; Göethel, G.; de Oliveira Rios, A.; Emanuelli, T.; Bürger, M.E.; Garcia, S.C. Microcystin-LR Exposure Induces Oxidative Damage in Caenorhabditis elegans: Protective Effect of Lutein Extracted from Marigold Flowers. Food Chem. Toxicol. 2017, 109, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Zhan, C.; Gong, J. Mutations at Two Key Sites in PP2A Safeguard Caenorhabditis elegans Neurons from Microcystin-LR Toxicity. Toxins 2024, 16, 145. [Google Scholar] [CrossRef]
- Pickett, C.L.; Kornfeld, K. Age-Related Degeneration of the Egg-Laying System Promotes Matricidal Hatching in Caenorhabditis elegans. Aging Cell 2013, 12, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Ruan, Q.; Li, X.; Liu, R.; Li, Y.; Pu, Y.; Yin, L.; Wang, D. Neurotoxicological Evaluation of Microcystin-LR Exposure at Environmental Relevant Concentrations on Nematode Caenorhabditis elegans. Environ. Sci. Pollut. Res. 2013, 20, 1823–1830. [Google Scholar] [CrossRef] [PubMed]
- Saul, N.; Chakrabarti, S.; Stürzenbaum, S.R.; Menzel, R.; Steinberg, C.E.W. Neurotoxic Action of Microcystin-LR Is Reflected in the Transcriptional Stress Response of Caenorhabditis elegans. Chem. Biol. Interact. 2014, 223, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Hoagland, P.; Anderson, D.M.; Kaoru, Y.; White, A.W. The Economic Effects of Harmful Algal Blooms in the United States: Estimates, Assessment Issues, and Information Needs. Estuaries 2002, 25, 819–837. [Google Scholar] [CrossRef]
- Ichimura, T. Sexual Cell Division and Conjugation—Papilla Formation in Sexual Reproduction of Closterium strigosum. In Proceedings of the 7th International Seaweed Symposium, Sapporo, Japan, 8–12 August 1971. [Google Scholar]
- Arata, Y.; Oshima, T.; Ikeda, Y.; Kimura, H.; Sako, Y. OP50, a Bacterial Strain Conventionally Used as Food for Laboratory Maintenance of C. elegans, Is a Biofilm Formation Defective Mutant. MicroPublication Biol. 2020, 2020. [Google Scholar] [CrossRef]
- Landberg, J.; Mundhada, H.; Nielsen, A.T. An Autoinducible Trp-T7 Expression System for Production of Proteins and Biochemicals in Escherichia coli. Biotechnol. Bioeng. 2020, 117, 1513–1524. [Google Scholar] [CrossRef]
- Prochazka, G.J.; Payne, W.J.; Mayberry, W.R. Calorific Content of Certain Bacteria and Fungi. J. Bacteriol. 1970, 104, 646–649. [Google Scholar] [CrossRef] [PubMed]
- Brenner, S. The Genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef] [PubMed]
- Basic Caenorhabditis elegans Methods: Synchronization and Observation. Available online: https://app.jove.com/t/4019/basic-caenorhabditis-elegans-methods-synchronization-and-observation (accessed on 6 December 2024).
- Johnson, B.H.; Hecht, M.H. Recombinant Proteins Can Be Isolated from E. coli Cells by Repeated Cycles of Freezing and Thawing. Nat. Biotechnol. 1994, 12, 1357–1360. [Google Scholar] [CrossRef]
- O’Rourke, E.J.; Soukas, A.A.; Carr, C.E.; Ruvkun, G.C. C. elegans Major Fats Are Stored in Vesicles Distinct from Lysosome-Related Organelles. Cell Metab. 2009, 10, 430–435. [Google Scholar] [CrossRef]
- M9 Minimal Medium (Standard). Cold Spring Harb. Protoc. 2010, 2010, pdb.rec12295. [CrossRef]
- Park, H.-E.H.; Jung, Y.; Lee, S.-J.V. Survival Assays Using Caenorhabditis elegans. Mol. Cells 2017, 40, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Chiu, H.; Boudreau, J.; Papanicolaou, T.; Bendena, W.; Chin-Sang, I. A Functional Study of All 40 Caenorhabditis elegans Insulin-like Peptides. J. Biol. Chem. 2018, 293, 16912–16922. [Google Scholar] [CrossRef]
- Nawa, M.; Masaaki, M. The Method of the Body Bending Assay Using Caenorhabditis elegans. Available online: https://bio-protocol.org/en/bpdetail?id=253&type=0 (accessed on 31 January 2025).
- Raizen, D.; Song, B.; Trojanowski, N.; You, Y.-J. Methods for Measuring Pharyngeal Behaviors. In WormBook: The Online Review of C. elegans Biology [Internet]; WormBook: Pasadena, CA, USA, 2018. [Google Scholar]
- Wang, Z.; Ma, X.; Li, J.; Cui, X. Peptides from Sesame Cake Extend Healthspan of Caenorhabditis elegans via Upregulation of Skn-1 and Inhibition of Intracellular ROS Levels. Exp. Gerontol. 2016, 82, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Zaiontz, C. Studentized Range q Table. Available online: https://real-statistics.com/statistics-tables/studentized-range-q-table/ (accessed on 17 October 2024).

| Physiological Parameter | M. aeruginosa X̄ ± SE | E. coli X̄ ± SE | n | p-Value |
|---|---|---|---|---|
| Dauer under Chronic Heat Stress (% in dauer) | 86.27 ± 1.0 | 83.65 ± 1.0 | 8 | 0.10 |
| Thrashing (thrashes/min) | 209.25 ± 7.0 | 210.15 ± 4.4 | 40 | 0.91 |
| Pharyngeal Pumping (pumps/min) | 304.2 ± 9.3 | 330.0 ± 10.4 | 10 | 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balson, J.; Boudreau, J.R.; Chin-Sang, I.D.; Wang, Y.; Lefebvre, D.D. Tolerance to a Diet of Toxic Microcystis aeruginosa in Caenorhabditis elegans. Toxins 2025, 17, 109. https://doi.org/10.3390/toxins17030109
Balson J, Boudreau JR, Chin-Sang ID, Wang Y, Lefebvre DD. Tolerance to a Diet of Toxic Microcystis aeruginosa in Caenorhabditis elegans. Toxins. 2025; 17(3):109. https://doi.org/10.3390/toxins17030109
Chicago/Turabian StyleBalson, Jordan, Jeffrey R. Boudreau, Ian D. Chin-Sang, Yuxiang Wang, and Daniel D. Lefebvre. 2025. "Tolerance to a Diet of Toxic Microcystis aeruginosa in Caenorhabditis elegans" Toxins 17, no. 3: 109. https://doi.org/10.3390/toxins17030109
APA StyleBalson, J., Boudreau, J. R., Chin-Sang, I. D., Wang, Y., & Lefebvre, D. D. (2025). Tolerance to a Diet of Toxic Microcystis aeruginosa in Caenorhabditis elegans. Toxins, 17(3), 109. https://doi.org/10.3390/toxins17030109





