Mycotoxins: Biotransformation and Bioavailability Assessment Using Caco-2 Cell Monolayer
Abstract
1. Introduction
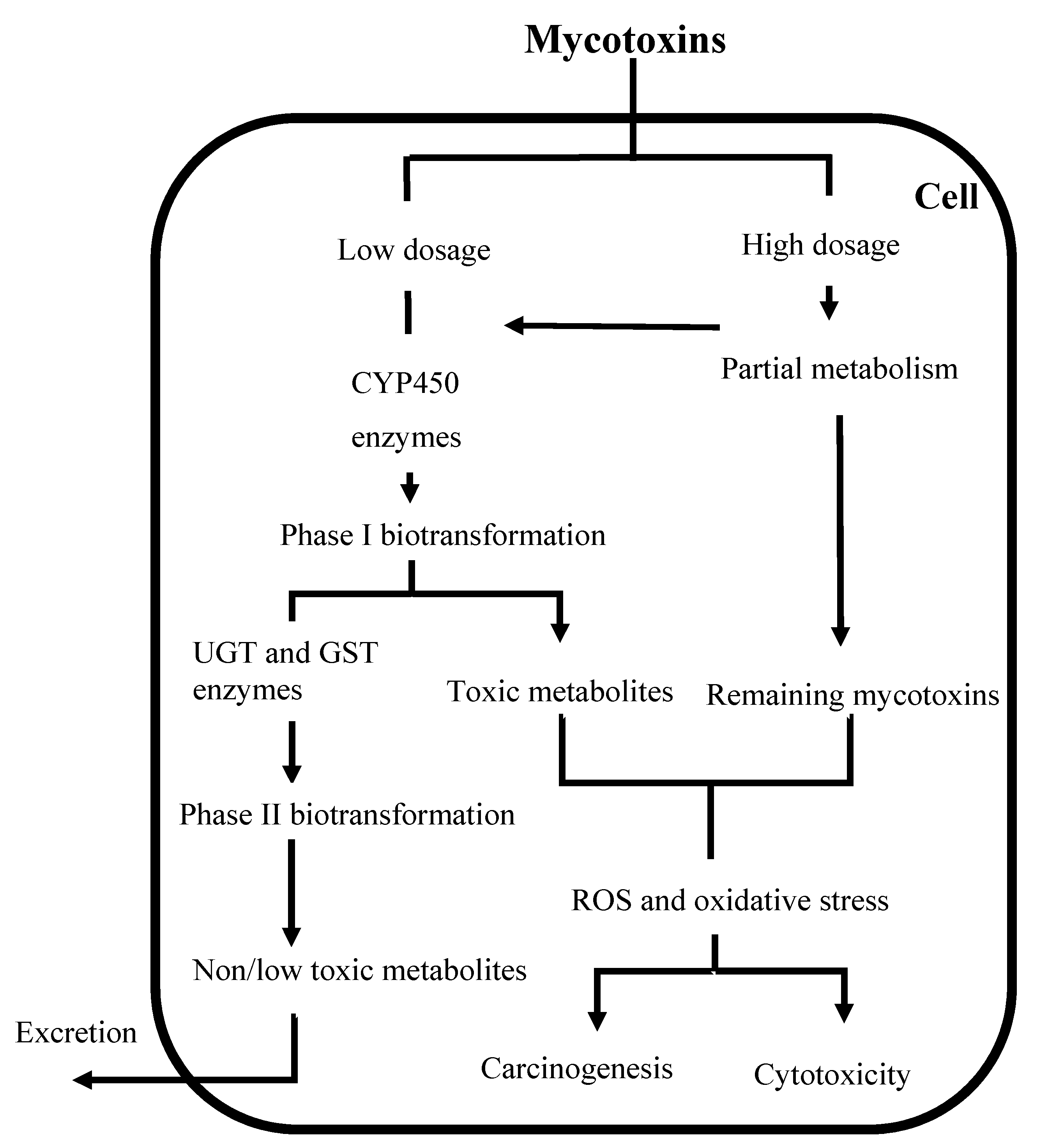
2. Biotransformation of Mycotoxins
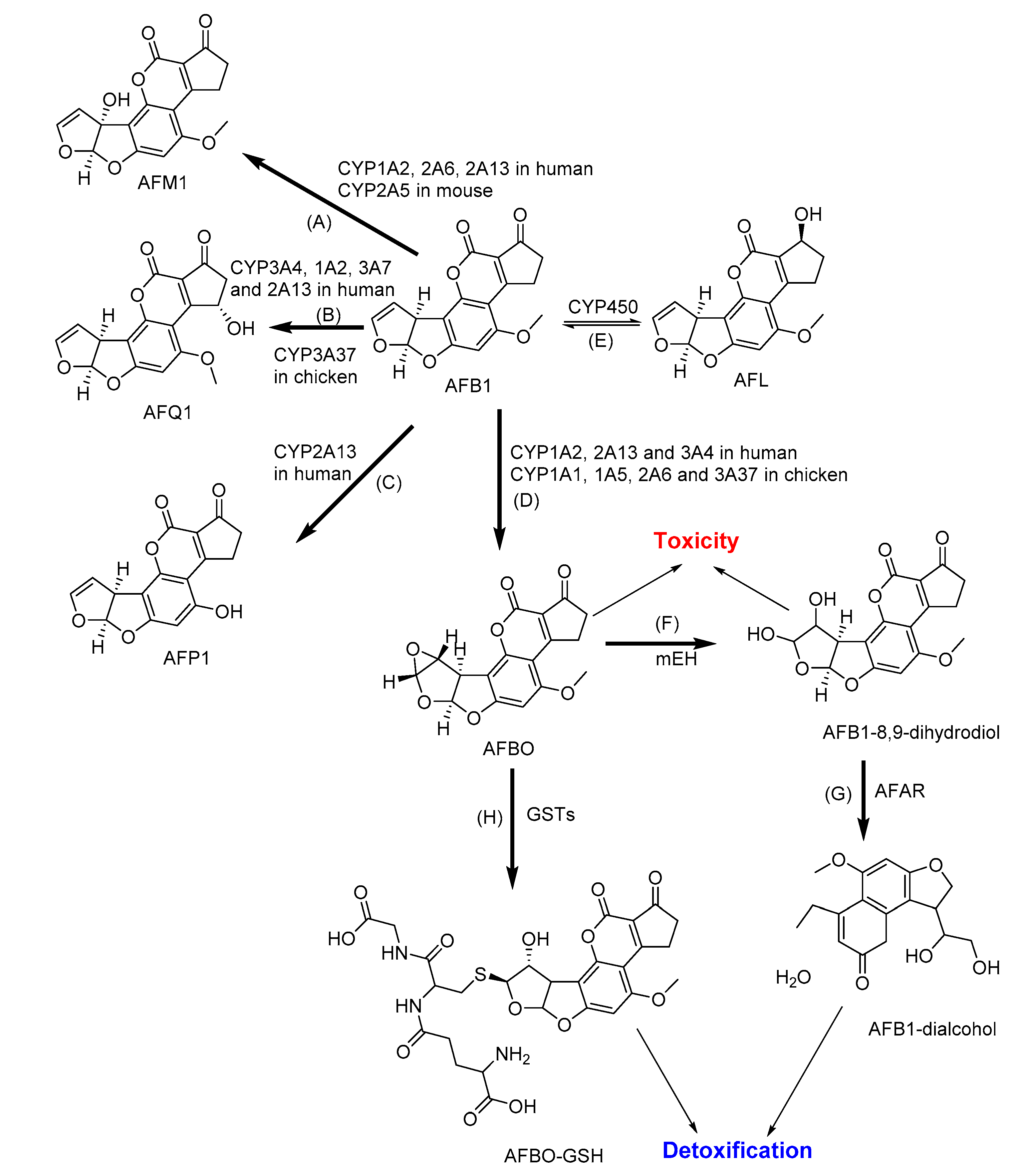
2.1. Biotransformation of Aflatoxins
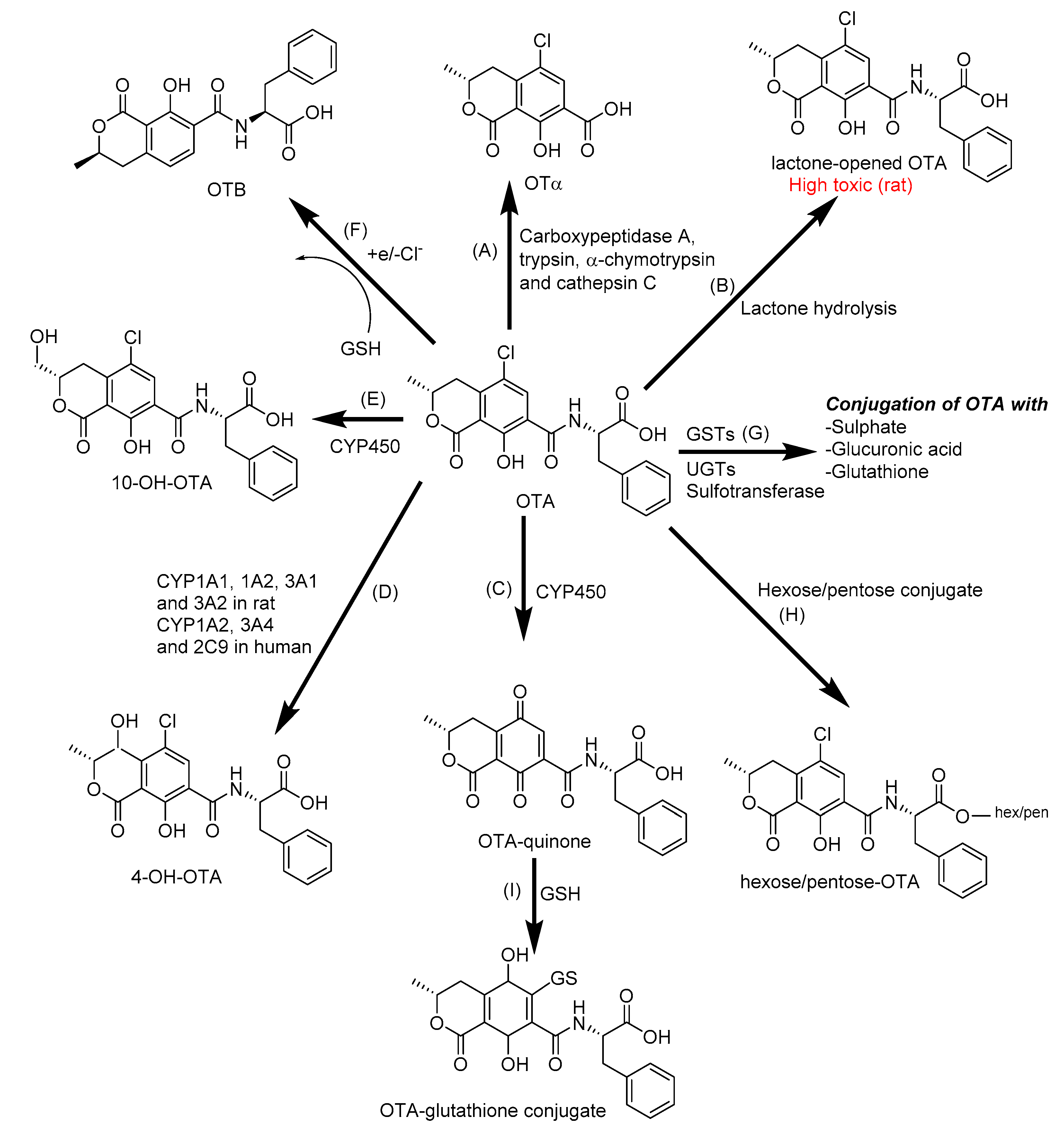
2.2. Biotransformation of Ochratoxin A
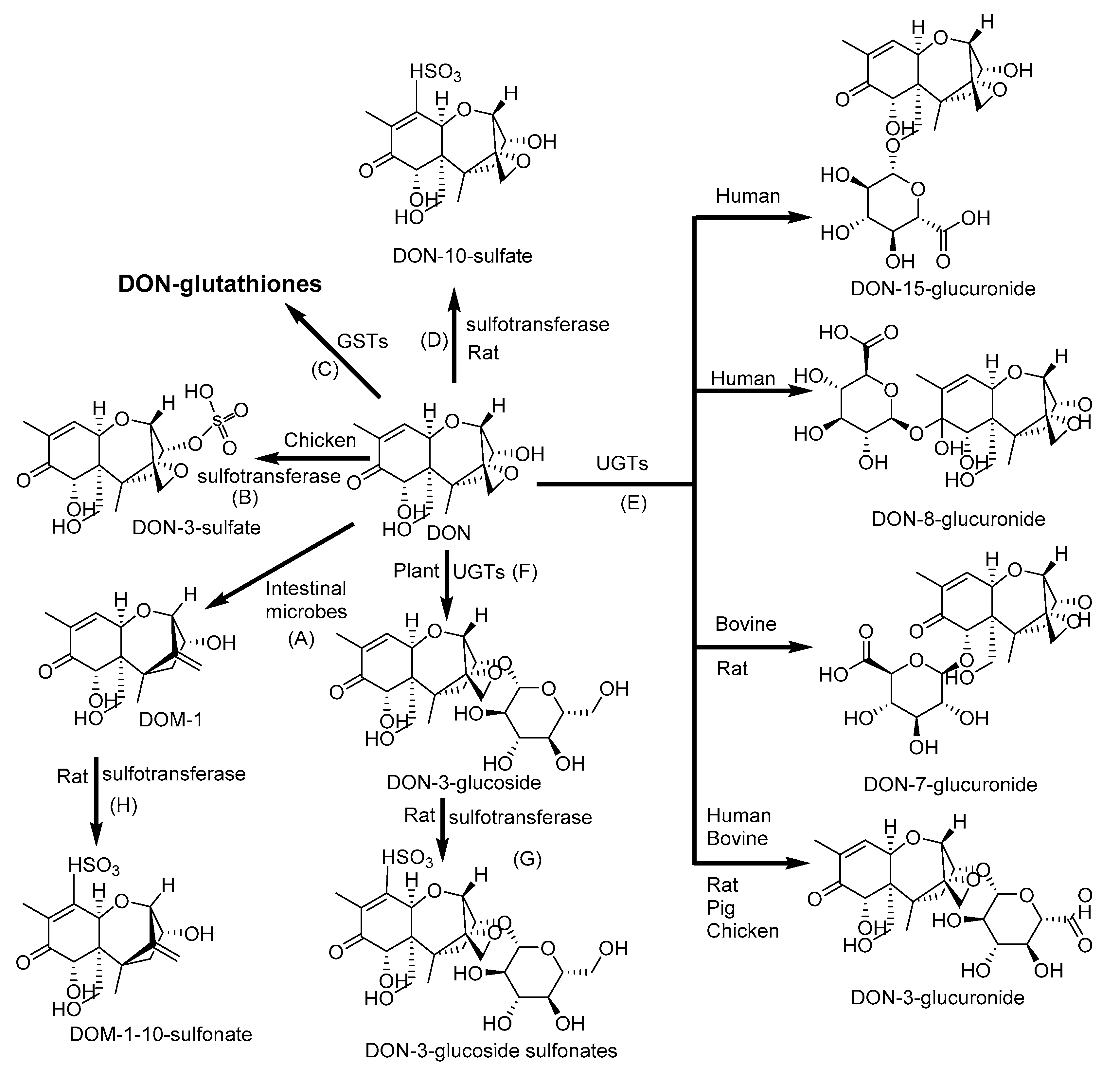
2.3. Biotransformation of Deoxynivalenol
2.4. Biotransformation of T-2 and HT-2
2.5. Biotransformation of Fumonisins
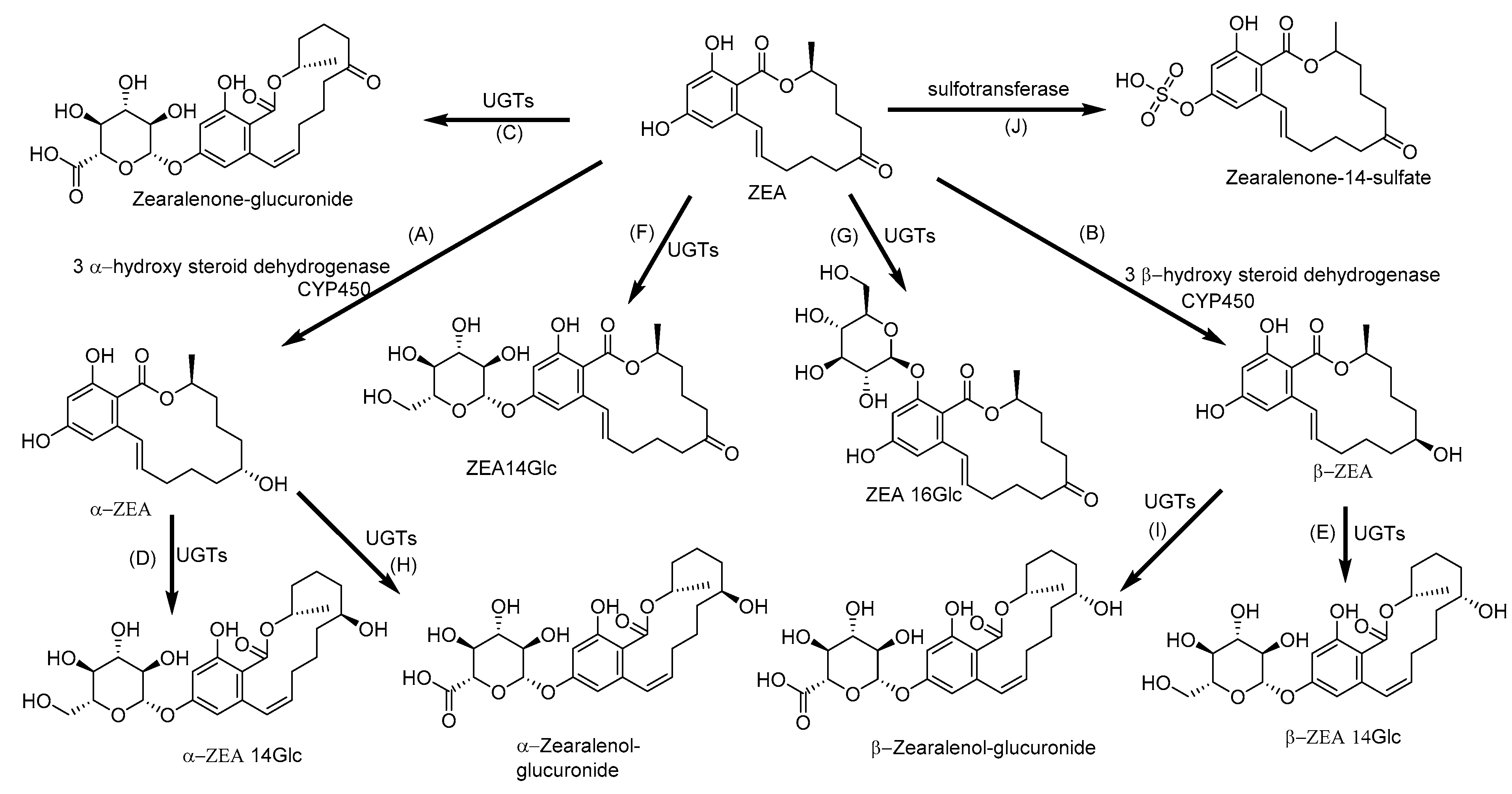
2.6. Biotransformation of Zearalenone
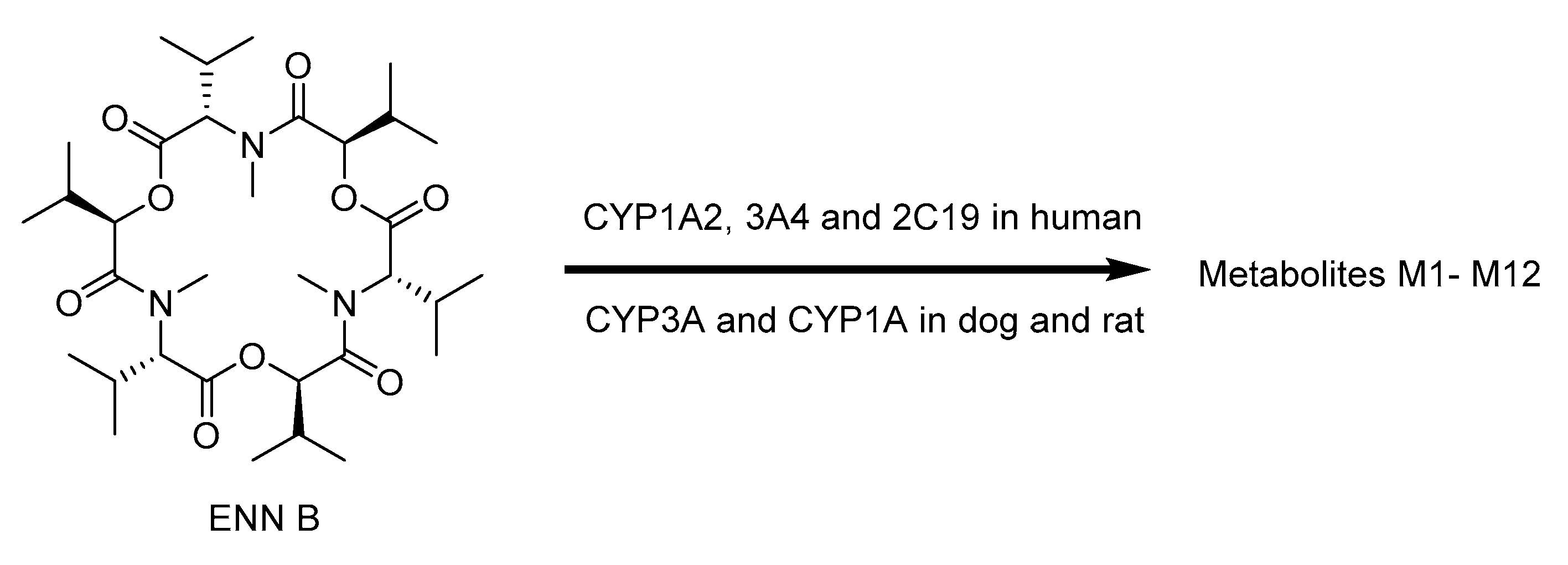
2.7. Biotransformation of Enniatins
2.8. Biotransformation of Beauvericin
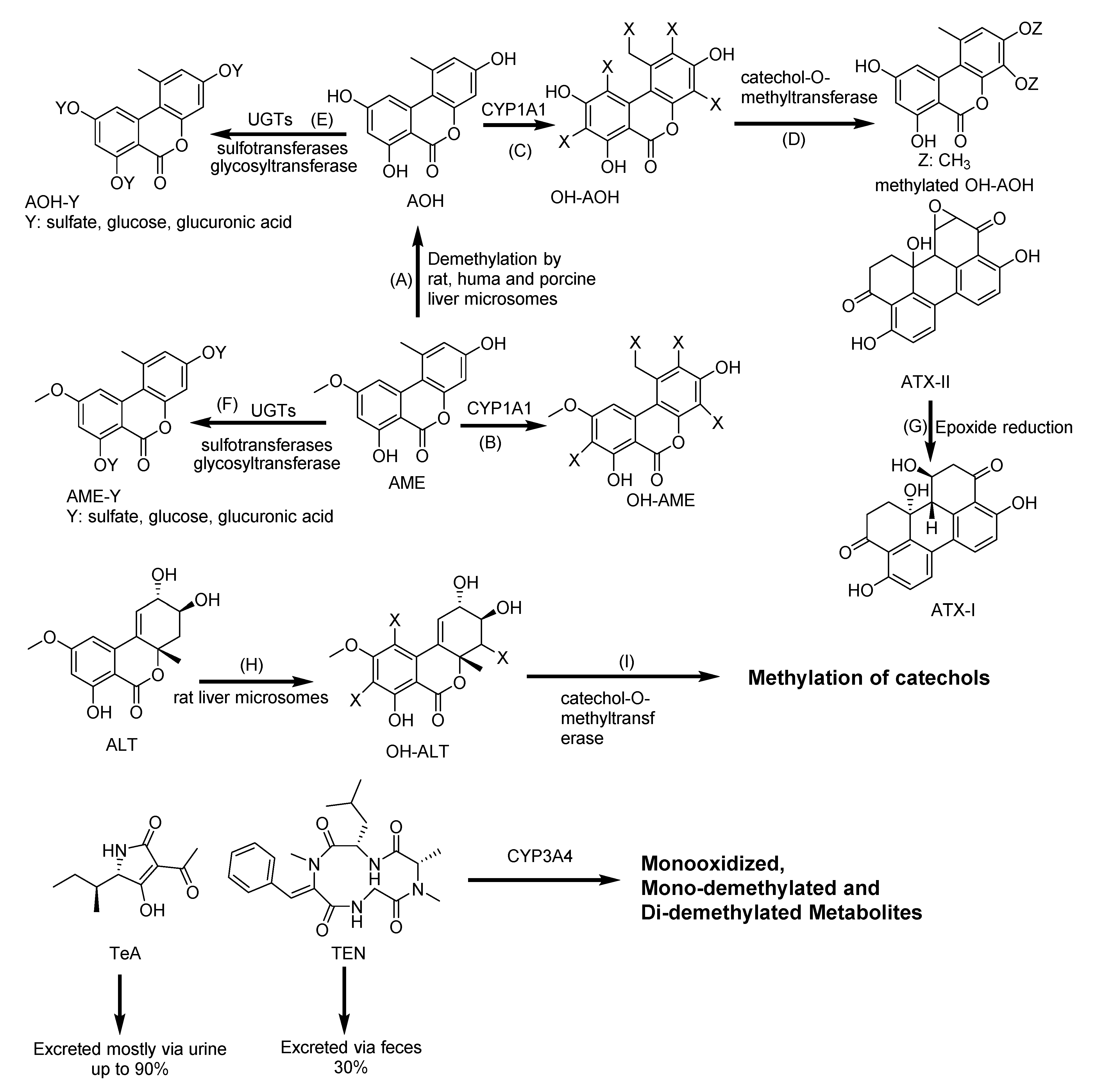
2.9. Biotransformation of Alternaria Mycotoxins
2.10. Biotransformation of Patulin
3. Assessment of Bioavailability of Mycotoxins Using Caco-2 Cell Monolayer
4. Conclusions
Funding
Conflicts of Interest
Abbreviations
| 3′-OH-HT-2 | 3′-hydroxy-HT-2 |
| 3′-OH-T-2 | 3′-hydroxy-T-2 |
| 4-OH-OTA | 4-hydroxy-ochratoxin A |
| 10-OH-OTA | 10-hydroxy-ochratoxin A |
| α-ZEA | α-zearalenone |
| β-ZEA | β-zearalenone |
| ABC | ATP–binding cassette |
| AFB1 | aflatoxin B1 |
| AFB2 | aflatoxin B2 |
| AFBO | Aflatoxin B1–8,9-epoxide |
| AFBO-GSH | Aflatoxin B1–8,9-epoxide-glutathiones |
| AFG1 | aflatoxin G1 |
| AFG2 | aflatoxin G2 |
| AFL | aflatoxicol |
| AFM1 | aflatoxin M1 |
| AFM2 | aflatoxin M2 |
| AFP1 | aflatoxin P1 |
| AFQ1 | aflatoxin Q1 |
| AhR | aryl the hydrocarbon receptor |
| AKR7 | aldo-keto reductase subfamily 7 |
| ALT | altenuene |
| AME | alternariol monomethyl ether |
| AOH | alternariol |
| AP | apical compartment |
| ATX I | altertoxin I |
| ATX II | altertoxin II |
| ATXs | altertoxins |
| BCRP | breast cancer resistance protein |
| BEA | beauvericin |
| BL | basolateral compartment |
| Caco-2 | caucasian colon adenocarcinoma |
| Caco-2/TC7 | TC7 clone was isolated from a late passage of the parental Caco-2 line |
| CAR | constitutive androstane receptor |
| CYP | cytochrome P |
| D3G | deoxynivalenol-3-glucoside |
| DNA | Deoxyribonucleic acid |
| DOM-1 | deepoxy-deoxynivalenol |
| DON | deoxynivalenol |
| ENN A | enniatin A |
| ENN A1 | enniatin A1 |
| ENN B | enniatin B |
| ENN B1 | enniatin B1 |
| ENNs | enniatins |
| ERK | extracellular signal regulated protein kinase |
| FB1 | fumonisin B1 |
| FBs | fumonisins |
| GI | gastrointestinal |
| GSH | glutathione |
| GST | glutathione S-transferase |
| HCT-16 | human colon carcinoma |
| HFB1 | aminopentol |
| HT-2 | HT-2 toxin |
| HT-29 | human colorectal adenocarcinoma |
| IL-8 | Interleukin-8 |
| JNK | c-Jun-N-terminal kinase |
| LD50 | median lethal dose |
| LOQ | limit of quantitation |
| MAPK | mitogen-activated protein kinase-dependent |
| mEH | microsomal epoxide hydrolase |
| MPA | mycophenolic acid |
| MRP | multidrug resistance protein |
| NAT | N-acetyltransferaseND (not detected) |
| NEO | neosolaniol |
| NF-κB | nuclear factor kappa–light–chain–enhancer of activated B cells |
| NIV | nivalenol |
| OH-ALT | hydroxy-altenuene |
| OH-AME | hydroxy-alternariol monomethyl ether |
| OH-AOH | hydroxyl-alternariol |
| OTα | ochratoxin α |
| OTA | ochratoxin A |
| OTB | ochratoxin B |
| Papp | apparent permeability |
| P-gp | P-glycoprotein |
| PAT | patulin |
| pHFB1 | partially hydrolyzed fumonisin B1 |
| PXR | pregnane X receptor |
| RNA | Ribonucleic acid |
| ROS | reactive oxygen species |
| SULT | sulfotransferase |
| SW480 | human colon adenocarcinoma |
| T-2 | T-2 toxin |
| TeA | tenuazonic acid |
| TEER | transepithelial electrical resistance |
| TEN | tentoxin |
| UGT: | uridine 5′-diphospho-glucuronosyltransferase |
| ZEA | zearalenone |
| ZEA14Glc | zearalenone-14-glucoside |
| ZEA16Glc | zearalenone-16-glucoside |
References
- De Boevre, M.; Graniczkowska, K.; Saeger, S. De Metabolism of modified mycotoxins studied through in vitro and in vivo models: An overview. Toxicol. Lett. 2015, 233, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.C.; Gheux, A.; Coton, M.; Madec, S.; Hymery, N.; Coton, E. In vitro co-culture models to evaluate acute cytotoxicity of individual and combined mycotoxin exposures on Caco-2, THP-1 and HepaRG human cell lines. Chem. Biol. Interact. 2018, 281, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Kebede, H.; Liu, X.; Jin, J.; Xing, F. Current status of major mycotoxins contamination in food and feed in Africa. Food Control 2020, 110, 106975. [Google Scholar] [CrossRef]
- Fernández-Blanco, C.; Elmo, L.; Waldner, T.; Ruiz, M.J. Cytotoxic effects induced by patulin, deoxynivalenol and toxin T2 individually and in combination in hepatic cells (HepG2). Food Chem. Toxicol. 2018, 120, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Pankaj, S.K.; Shi, H.; Keener, K.M. A review of novel physical and chemical decontamination technologies for aflatoxin in food. Trends Food Sci. Technol. 2018, 71, 73–83. [Google Scholar] [CrossRef]
- Seyed Toutounchi, N.; Hogenkamp, A.; Varasteh, S.; van’t Land, B.; Garssen, J.; Kraneveld, A.D.; Folkerts, G.; Braber, S. Fusarium Mycotoxins Disrupt the Barrier and Induce IL-6 Release in a Human Placental Epithelium Cell Line. Toxins 2019, 11, 665. [Google Scholar] [CrossRef]
- Stanciu, O.; Loghin, F.; Filip, L.; Cozma, A.; Miere, D.; Mañes, J.; Banc, R. Occurence of Fusarium Mycotoxins in Wheat from Europe—A Review. Acta Univ. Cibiniensis. Ser. E Food Technol. 2015, 19, 35–60. [Google Scholar] [CrossRef]
- Raghubeer, S.; Nagiah, S.; Chuturgoon, A. Ochratoxin A upregulates biomarkers associated with hypoxia and transformation in human kidney cells. Toxicol. In Vitro 2019, 57, 211–216. [Google Scholar] [CrossRef]
- Hymery, N.; Mounier, J.; Coton, E. Effect of Penicillium roqueforti mycotoxins on Caco-2 cells: Acute and chronic exposure. Toxicol. In Vitro 2018, 48, 188–194. [Google Scholar] [CrossRef]
- Assunção, R.; Ferreira, M.; Martins, C.; Diaz, I.; Padilla, B.; Dupont, D.; Bragança, M.; Alvito, P. Applicability of in vitro methods to study patulin bioaccessibility and its effects on intestinal membrane integrity. J. Toxicol. Environ. Heal. Part A Curr. Issues 2014, 77, 983–992. [Google Scholar] [CrossRef]
- Hussain, S.; Asi, M.R.; Iqbal, M.; Khalid, N.; Wajih-ul-Hassan, S.; Ariño, A. Patulin Mycotoxin in Mango and Orange Fruits, Juices, Pulps, and Jams Marketed in Pakistan. Toxins 2020, 12, 52. [Google Scholar] [CrossRef] [PubMed]
- Moss, M.O. Risk assessment for aflatoxins in foodstuffs. Int. Biodeterior. Biodegrad. 2002, 50, 137–142. [Google Scholar] [CrossRef]
- Rushing, B.R.; Selim, M.I. Aflatoxin B1: A review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem. Toxicol. 2019, 124, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Carrasco, Y.; Mañes, J.; Berrada, H.; Juan, C. Development and validation of a LC-ESI-MS/MS method for the determination of alternaria toxins alternariol, alternariol methyl-ether and tentoxin in tomato and tomato-based products. Toxins 2016, 8, 328. [Google Scholar] [CrossRef]
- Gotthardt, M.; Kanawati, B.; Schmidt, F.; Asam, S.; Hammerl, R.; Frank, O.; Hofmann, T.; Schmitt-Kopplin, P.; Rychlik, M. Comprehensive Analysis of the Alternaria Mycobolome Using Mass Spectrometry Based Metabolomics. Mol. Nutr. Food Res. 2020, 64, 1900558. [Google Scholar] [CrossRef]
- De Angelis, E.; Monaci, L.; Mackie, A.; Salt, L.; Visconti, A. Reprint of “bioaccessibility of T-2 and HT-2 toxins in mycotoxin contaminated bread models submitted to in vitro human digestion”. Innov. Food Sci. Emerg. Technol. 2013, 25, 88–96. [Google Scholar] [CrossRef]
- Ling, A.; Sun, L.; Guo, W.; Sun, S.; Yang, J.; Zhao, Z. Individual and combined cytotoxic effects of T-2 toxin and its four metabolites on porcine Leydig cells. Food Chem. Toxicol. 2020, 139, 111277. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, Y.; Qiu, M.; Sun, L.; Liao, J.; Wang, R.; Sun, X.; Bi, S.; Gooneratne, R. Effect of T-2 toxin-injected shrimp muscle extracts on mouse macrophage cells (RAW264.7). Drug Chem. Toxicol. 2018, 41, 16–21. [Google Scholar] [CrossRef]
- Kang, R.; Perveen, A.; Li, C. Effects of maternal T-2 toxin exposure on the hepatic glycolipid metabolism in young mice. Ecotoxicol. Environ. Saf. 2020, 196, 110530. [Google Scholar] [CrossRef]
- Kasimir, M.; Behrens, M.; Schulz, M.; Kuchenbuch, H.; Focke, C.; Humpf, H.-U. Intestinal Metabolism of α- and β-Glucosylated Modified Mycotoxins T-2 and HT-2 Toxin in the Pig Cecum Model. J. Agric. Food Chem. 2020. [Google Scholar] [CrossRef]
- Schuhmacher-Wolz, U.; Heine, K.; Schneider, K. Report on toxicity data on trichothecene mycotoxins HT-2 and T-2 toxins. EFSA Support. Publ. 2010, 7. [Google Scholar] [CrossRef]
- Zhou, H.; George, S.; Hay, C.; Lee, J.; Qian, H.; Sun, X. Individual and combined effects of Aflatoxin B1, Deoxynivalenol and Zearalenone on HepG2 and RAW 264.7 cell lines. Food Chem. Toxicol. 2017, 103, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Blanco, C.; Font, G.; Ruiz, M.J. Interaction effects of enniatin B, deoxinivalenol and alternariol in Caco-2 cells. Toxicol. Lett. 2016, 241, 38–48. [Google Scholar] [CrossRef] [PubMed]
- García, G.R.; Payros, D.; Pinton, P.; Dogi, C.A.; Laffitte, J.; Neves, M.; González Pereyra, M.L.; Cavaglieri, L.R.; Oswald, I.P. Intestinal toxicity of deoxynivalenol is limited by Lactobacillus rhamnosus RC007 in pig jejunum explants. Arch. Toxicol. 2018, 92, 983–993. [Google Scholar] [CrossRef]
- Pestka, J.J. Mechanisms of deoxynivalenol-induced gene expression and apoptosis. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2008, 25, 1128–1140. [Google Scholar] [CrossRef]
- Pestka, J.J.; Smolinski, A.T. Deoxynivalenol: Toxicology and potential effects on humans. J. Toxicol. Environ. Heal. Part B Crit. Rev. 2005, 8, 39–69. [Google Scholar] [CrossRef]
- Wang, H.W.; Wang, J.Q.; Zheng, B.Q.; Li, S.L.; Zhang, Y.D.; Li, F.D.; Zheng, N. Cytotoxicity induced by ochratoxin A, zearalenone, and α-zearalenol: Effects of individual and combined treatment. Food Chem. Toxicol. 2014, 71, 217–224. [Google Scholar] [CrossRef]
- Yang, D.; Jiang, X.; Sun, J.; Li, X.; Li, X.; Jiao, R.; Peng, Z.; Li, Y.; Bai, W. Toxic effects of zearalenone on gametogenesis and embryonic development: A molecular point of review. Food Chem. Toxicol. 2018, 119, 24–30. [Google Scholar] [CrossRef]
- Zinedine, A.; Soriano, J.M.; Moltó, J.C.; Mañes, J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenic mycotoxin. Food Chem. Toxicol. 2007, 45, 1–18. [Google Scholar] [CrossRef]
- Ruiz, M.J.; Macáková, P.; Juan-García, A.; Font, G. Cytotoxic effects of mycotoxin combinations in mammalian kidney cells. Food Chem. Toxicol. 2011, 49, 2718–2724. [Google Scholar] [CrossRef]
- Mallebrera, B.; Prosperini, A.; Font, G.; Ruiz, M.J. In vitro mechanisms of Beauvericin toxicity: A review. Food Chem. Toxicol. 2018, 111, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Maranghi, F.; Tassinari, R.; Narciso, L.; Tait, S.; Rocca, C.L.; Felice, G.D.; Butteroni, C.; Corinti, S.; Barletta, B.; Cordelli, E.; et al. In vivo toxicity and genotoxicity of beauvericin and enniatins. Combined approach to study in vivo toxicity and genotoxicity of mycotoxins beauvericin (BEA) and enniatin B (ENNB). EFSA Support. Publ. 2018, 15, 1406E. [Google Scholar] [CrossRef]
- Prosperini, A.; Berrada, H.; Ruiz, M.J.; Caloni, F.; Coccini, T.; Spicer, L.J.; Perego, M.C.; Lafranconi, A. A Review of the Mycotoxin Enniatin B. Front. Public Health 2017, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Mu, P.; Deng, Y. Mycotoxins: Cytotoxicity and biotransformation in animal cells. Toxicol. Res. 2016, 5, 377–387. [Google Scholar] [CrossRef]
- Fernández-Blanco, C.; Juan-García, A.; Juan, C.; Font, G.; Ruiz, M.J. Alternariol induce toxicity via cell death and mitochondrial damage on Caco-2 cells. Food Chem. Toxicol. 2016, 88, 32–39. [Google Scholar] [CrossRef]
- Solhaug, A.; Eriksen, G.S.; Holme, J.A. Mechanisms of Action and Toxicity of the Mycotoxin Alternariol: A Review. Basic Clin. Pharmacol. Toxicol. 2016, 119, 533–539. [Google Scholar] [CrossRef]
- Nawaz, S.; Scudamore, K.A.; Rainbird, S.C. Mycotoxins in ingredients of animal feeding stuffs: I. determination of Alternaria mycotoxins in oilseed rape meal and sunflower seed meal. Food Addit. Contam. 1997, 14, 249–262. [Google Scholar] [CrossRef]
- Aichinger, G.; Puntscher, H.; Beisl, J.; Kütt, M.L.; Warth, B.; Marko, D. Delphinidin protects colon carcinoma cells against the genotoxic effects of the mycotoxin altertoxin II. Toxicol. Lett. 2018, 284, 136–142. [Google Scholar] [CrossRef]
- da Motta, S.; Valente Soares, L.M. Survey of Brazilian tomato products for alternariol, alternariol monomethyl ether, tenuazonic acid and cyclopiazonic acid. Food Addit. Contam. 2001, 18, 630–634. [Google Scholar] [CrossRef]
- Gruber-Dorninger, C.; Novak, B.; Nagl, V.; Berthiller, F. Emerging Mycotoxins: Beyond Traditionally Determined Food Contaminants. J. Agric. Food Chem. 2017, 65, 7052–7070. [Google Scholar] [CrossRef]
- Ge, N.; Xu, J.; Peng, B.; Pan, S. Adsorption mechanism of tenuazonic acid using inactivated lactic acid bacteria. Food Control 2017, 82, 274–282. [Google Scholar] [CrossRef]
- Kumari, A.; Tirkey, N.N. Tenuazonic Acid: A Potent Mycotoxin. In Recent Trends in Human and Animal Mycology; Springer: Singapore, 2019; pp. 203–211. [Google Scholar]
- Di Gregorio, M.C.; Bordin, K.; de Castro Souto, P.C.M.; Corassin, C.H.; Oliveira, C.A.F. Comparative biotransformation of aflatoxin B 1 in swine, domestic fowls, and humans. Toxin Rev. 2015, 34, 142–150. [Google Scholar] [CrossRef]
- Wu, T.Y.; Fridley, B.L.; Jenkins, G.D.; Batzler, A.; Wang, L.; Weinshilboum, R.M. Mycophenolic acid response biomarkers: A cell line model system-based genome-wide screen. Int. Immunopharmacol. 2011, 11, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef] [PubMed]
- Fraeyman, S.; Croubels, S.; Devreese, M.; Antonissen, G. Emerging fusarium and alternaria mycotoxins: Occurrence, toxicity and toxicokinetics. Toxins 2017, 9, 228. [Google Scholar] [CrossRef]
- González-Arias, C.A.; Crespo-Sempere, A.; Marín, S.; Sanchis, V.; Ramos, A.J. Modulation of the xenobiotic transformation system and inflammatory response by ochratoxin A exposure using a co-culture system of Caco-2 and HepG2 cells. Food Chem. Toxicol. 2015, 86, 245–252. [Google Scholar] [CrossRef]
- Bellí, N.; Marín, S.; Sanchis, V.; Ramos, A.J. Ochratoxin A (OTA) in Wines, Musts and Grape Juices: Occurrence, Regulations and Methods of Analysis. Food Sci. Technol. Int. 2002, 8, 325–335. [Google Scholar] [CrossRef]
- Fernández-Cruz, M.L.; Mansilla, M.L.; Tadeo, J.L. Mycotoxins in fruits and their processed products: Analysis, occurrence and health implications. J. Adv. Res. 2010, 1, 113–122. [Google Scholar] [CrossRef]
- Orlando, B.; Grignon, G.; Vitry, C.; Kashefifard, K.; Valade, R. Fusarium species and enniatin mycotoxins in wheat, durum wheat, triticale and barley harvested in France. Mycotoxin Res. 2019, 35, 369–380. [Google Scholar] [CrossRef]
- Tibola, C.S.; de Miranda, M.Z.; Paiva, F.F.; Fernandes, J.M.C.; Guarienti, E.M.; Nicolau, M. Effect of breadmaking process on mycotoxin content in white and whole wheat breads. Cereal Chem. 2018, 95, 660–665. [Google Scholar] [CrossRef]
- Mousavi Khaneghah, A.; Fakhri, Y.; Sant’Ana, A.S. Impact of unit operations during processing of cereal-based products on the levels of deoxynivalenol, total aflatoxin, ochratoxin A, and zearalenone: A systematic review and meta-analysis. Food Chem. 2018, 268, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Pascari, X.; Maul, R.; Kemmlein, S.; Marin, S.; Sanchis, V. The fate of several trichothecenes and zearalenone during roasting and enzymatic treatment of cereal flour applied in cereal-based infant food production. Food Control 2020, 114, 107245. [Google Scholar] [CrossRef]
- Generotti, S.; Cirlini, M.; Šarkanj, B.; Sulyok, M.; Berthiller, F.; Dall’Asta, C.; Suman, M. Formulation and processing factors affecting trichothecene mycotoxins within industrial biscuit-making. Food Chem. 2017, 229, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Kuchenbuch, H.S.; Becker, S.; Schulz, M.; Cramer, B.; Humpf, H.U. Thermal stability of t-2 and ht-2 toxins during biscuit-and crunchy muesli-making and roasting. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2018, 35, 2158–2167. [Google Scholar] [CrossRef]
- Serrano, A.B.; Font, G.; Mañes, J.; Ferrer, E. Effects of technological processes on enniatin levels in pasta. J. Sci. Food Agric. 2016, 96, 1756–1763. [Google Scholar] [CrossRef]
- Tolosa, J.; Font, G.; Mañes, J.; Ferrer, E. Mitigation of enniatins in edible fish tissues by thermal processes and identification of degradation products. Food Chem. Toxicol. 2017, 101, 67–74. [Google Scholar] [CrossRef]
- Estiarte, N.; Crespo-Sempere, A.; Marín, S.; Ramos, A.J.; Worobo, R.W. Stability of alternariol and alternariol monomethyl ether during food processing of tomato products. Food Chem. 2018, 245, 951–957. [Google Scholar] [CrossRef]
- Stadler, D.; Berthiller, F.; Suman, M.; Schuhmacher, R.; Krska, R. Novel analytical methods to study the fate of mycotoxins during thermal food processing. Anal. Bioanal. Chem. 2020, 412, 9–16. [Google Scholar] [CrossRef]
- Schaarschmidt, S.; Fauhl-Hassek, C. Mycotoxins during the processes of nixtamalization and tortilla production. Toxins 2019, 11, 227. [Google Scholar] [CrossRef]
- Morcia, C.; Tumino, G.; Ghizzoni, R.; Badeck, F.W.; Lattanzio, V.M.T.; Pascale, M.; Terzi, V. Occurrence of Fusarium langsethiae and T-2 and HT-2 toxins in Italian malting barley. Toxins 2016, 8, 247. [Google Scholar] [CrossRef]
- Agriopoulou, S.; Stamatelopoulou, E.; Varzakas, T. Control Strategies: Prevention and Detoxification in Foods. Foods 2020, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Abrunhosa, L.; Morales, H.; Soares, C.; Calado, T.; Vila-Chã, A.S.; Pereira, M.; Venâncio, A. A Review of Mycotoxins in Food and Feed Products in Portugal and Estimation of Probable Daily Intakes. Crit. Rev. Food Sci. Nutr. 2016, 56, 249–265. [Google Scholar] [CrossRef] [PubMed]
- Ayelign, A.; De Saeger, S. Mycotoxins in Ethiopia: Current status, implications to food safety and mitigation strategies. Food Control 2020, 113, 107163. [Google Scholar] [CrossRef]
- Balendres, M.A.O.; Karlovsky, P.; Cumagun, C.J.R. Mycotoxigenic fungi and mycotoxins in agricultural crop commodities in the Philippines: A review. Foods 2019, 8, 249. [Google Scholar] [CrossRef] [PubMed]
- Puntscher, H.; Cobankovic, I.; Marko, D.; Warth, B. Quantitation of free and modified Alternaria mycotoxins in European food products by LC-MS/MS. Food Control 2019, 102, 157–165. [Google Scholar] [CrossRef]
- Meca, G.; Mañes, J.; Font, G.; Ruiz, M.J. Study of the potential toxicity of enniatins A, A 1, B, B 1 by evaluation of duodenal and colonic bioavailability applying an invitro method by Caco-2 cells. Toxicon 2012, 59, 1–11. [Google Scholar] [CrossRef]
- Fernández-García, E.; Carvajal-Lérida, I.; Pérez-Gálvez, A. In vitro bioaccessibility assessment as a prediction tool of nutritional efficiency. Nutr. Res. 2009, 29, 751–760. [Google Scholar] [CrossRef]
- González-Arias, C.A.; Marín, S.; Sanchis, V.; Ramos, A.J. Mycotoxin bioaccessibility/absorption assessment using in vitro digestion models: A review. World Mycotoxin J. 2013, 6, 167–184. [Google Scholar] [CrossRef]
- Brandon, E.F.A.; Oomen, A.G.; Rompelberg, C.J.M.; Versantvoort, C.H.M.; Van Engelen, J.G.M.; Sips, A.J.A.M. Consumer product in vitro digestion model: Bioaccessibility of contaminants and its application in risk assessment. Regul. Toxicol. Pharmacol. 2006, 44, 161–171. [Google Scholar] [CrossRef]
- Tran, V.N.; Viktorova, J.; Augustynkova, K.; Jelenova, N.; Dobiasova, S.; Rehorova, K.; Fenclova, M.; Stranska-Zachariasova, M.; Vitek, L.; Hajslova, J.; et al. In silico and in vitro studies of mycotoxins and their cocktails; Their toxicity and its mitigation by silibinin pre-treatment. Toxins 2020, 12, 148. [Google Scholar] [CrossRef]
- Bordin, K.; Saladino, F.; Fernández-Blanco, C.; Ruiz, M.J.; Mañes, J.; Fernández-Franzón, M.; Meca, G.; Luciano, F.B. Reaction of zearalenone and α-zearalenol with allyl isothiocyanate, characterization of reaction products, their bioaccessibility and bioavailability in vitro. Food Chem. 2017, 217, 648–654. [Google Scholar] [CrossRef]
- Kabak, B.; Brandon, E.F.A.; Var, I.; Blokland, M.; Sips, A.J.A.M. Effects of probiotic bacteria on the bioaccessibility of aflatoxin B1 and ochratoxin A using an in vitro digestion model under fed conditions. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 2009, 44, 472–480. [Google Scholar] [CrossRef]
- CenciČ, A.; Langerholc, T. Functional cell models of the gut and their applications in food microbiology—A review. Int. J. Food Microbiol. 2010, 141, S4–S14. [Google Scholar] [CrossRef]
- Artursson, P.; Karlsson, J. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem. Biophys. Res. Commun. 1991, 175, 880–885. [Google Scholar] [CrossRef]
- Brandon, E.F.A.; Bosch, T.M.; Deenen, M.J.; Levink, R.; van der Wal, E.; van Meerveld, J.B.M.; Bijl, M.; Beijnen, J.H.; Schellens, J.H.M.; Meijerman, I. Validation of in vitro cell models used in drug metabolism and transport studies; genotyping of cytochrome P450, phase II enzymes and drug transporter polymorphisms in the human hepatoma (HepG2), ovarian carcinoma (IGROV-1) and colon carcinoma (CaCo-2, LS180) cell lines. Toxicol. Appl. Pharmacol. 2006, 211, 1–10. [Google Scholar]
- Lněničková, K.; Šadibolová, M.; Matoušková, P.; Szotáková, B.; Skálová, L.; Boušová, I. The Modulation of Phase II Drug-Metabolizing Enzymes in Proliferating and Differentiated CaCo-2 Cells by Hop-Derived Prenylflavonoids. Nutrients 2020, 12, 2138. [Google Scholar] [CrossRef]
- Schaut, A.; De Saeger, S.; Sergent, T.; Schneider, Y.-J.; Larondelle, Y.; Pussemier, L.; Van Peteghem, C. Study of the gastrointestinal biotransformation of zearalenone in a Caco-2 cell culture system with liquid chromatographic methods. J. Appl. Toxicol. 2008, 28, 966–973. [Google Scholar] [CrossRef]
- Cizkova, K. Expression of cytochrome P450 epoxygenases and soluble epoxide hydrolase is regulated by hypolipidemic drugs in dose-dependent manner. Toxicol. Appl. Pharmacol. 2018, 355, 156–163. [Google Scholar] [CrossRef]
- Šemeláková, M.; Jendželovský, R.; Fedoročko, P. Drug membrane transporters and CYP3A4 are affected by hypericin, hyperforin or aristoforin in colon adenocarcinoma cells. Biomed. Pharmacother. 2016, 81, 38–47. [Google Scholar] [CrossRef]
- Odenthal, J.; van Heumen, B.W.H.; Roelofs, H.M.J.; te Morsche, R.H.M.; Marian, B.; Nagengast, F.M.; Peters, W.H.M. The Influence of Curcumin, Quercetin, and Eicosapentaenoic Acid on the Expression of Phase II Detoxification Enzymes in the Intestinal Cell Lines HT-29, Caco-2, HuTu 80, and LT97. Nutr. Cancer 2012, 64, 856–863. [Google Scholar] [CrossRef]
- Langerholc, T.; Maragkoudakis, P.A.; Wollgast, J.; Gradisnik, L.; Cencic, A. Novel and established intestinal cell line models—An indispensable tool in food science and nutrition. Trends Food Sci. Technol. 2011, 22, S11–S20. [Google Scholar] [CrossRef]
- Haslam, I.S.; Jones, K.; Coleman, T.; Simmons, N.L. Induction of P-glycoprotein expression and function in human intestinal epithelial cells (T84). Biochem. Pharmacol. 2008, 76, 850–861. [Google Scholar] [CrossRef]
- Naruhashi, K.; Kurahashi, Y.; Fujita, Y.; Kawakita, E.; Yamasaki, Y.; Hattori, K.; Nishimura, A.; Shibata, N. Comparison of the Expression and Function of ATP Binding Cassette Transporters in Caco-2 and T84 cells on Stimulation by Selected Endogenous Compounds and Xenobiotics. Drug Metab. Pharmacokinet. 2011, 26, 145–153. [Google Scholar] [CrossRef]
- Theodoropoulos, C.; Demers, C.; Delvin, E.; Menard, D.; Gascon-Barre, M. Calcitriol regulates the expression of the genes encoding the three key vitamin D3 hydroxylases and the drug-metabolizing enzyme CYP3A4 in the human fetal intestine. Clin. Endocrinol. 2003, 58, 489–499. [Google Scholar] [CrossRef]
- Saaby, L.; Helms, H.C.C.; Brodin, B. IPEC-J2 MDR1, a Novel High-Resistance Cell Line with Functional Expression of Human P-glycoprotein (ABCB1) for Drug Screening Studies. Mol. Pharm. 2016, 13, 640–652. [Google Scholar] [CrossRef]
- Palócz, O.; Szita, G.; Csikó, G. Alteration in Inflammatory Responses and Cytochrome P450 Expression of Porcine Jejunal Cells by Drinking Water Supplements. Mediators Inflamm. 2019, 2019. [Google Scholar] [CrossRef]
- Loi, M.; Fanelli, F.; Liuzzi, V.C.; Logrieco, A.F.; Mulè, G. Mycotoxin biotransformation by native and commercial enzymes: Present and future perspectives. Toxins 2017, 9, 111. [Google Scholar] [CrossRef]
- Beyerle, J.; Frei, E.; Stiborova, M.; Habermann, N.; Ulrich, C.M. Biotransformation of xenobiotics in the human colon and rectum and its association with colorectal cancer. Drug Metab. Rev. 2015, 47, 199–221. [Google Scholar] [CrossRef]
- Gajecka, M.; Jakimiuk, E.; Zielonka, L.; Obremski, K.; Gajecka, M. The Biotransformation of Chosen Mycotoxins. Pol. J. Vet Sci. 2009, 12, 293–303. [Google Scholar]
- Galtier, P. Biotransformation and Fate of Mycotoxins. J. Toxicol. Toxin Rev. 1999, 18, 295–312. [Google Scholar]
- Sergent, T.; Ribonnet, L.; Kolosova, A.; Garsou, S.; Schaut, A.; De Saeger, S.; Van Peteghem, C.; Larondelle, Y.; Pussemier, L.; Schneider, Y.J. Molecular and cellular effects of food contaminants and secondary plant components and their plausible interactions at the intestinal level. Food Chem. Toxicol. 2008, 46, 813–841. [Google Scholar] [CrossRef]
- Lin, N.N.; Chen, J.; Xu, B.; Wei, X.; Guo, L.; Xie, J.W. The roles of carboxylesterase and CYP isozymes on the in vitro metabolism of T-2 toxin. Mil. Med. Res. 2015, 2, 13. [Google Scholar] [CrossRef][Green Version]
- Wang, J.; Jiang, J.; Zhang, H.; Wang, J.; Cai, H.; Li, C.; Li, K.; Liu, J.; Guo, X.; Zou, G.; et al. Integrated transcriptional and proteomic analysis with in vitro biochemical assay reveal the important role of CYP3A46 in T-2 toxin hydroxylation in porcine primary hepatocytes. Mol. Cell. Proteom. 2011, 10. [Google Scholar] [CrossRef]
- Wu, Q.; Huang, L.; Liu, Z.; Yao, M.; Wang, Y.; Dai, M.; Yuan, Z. A comparison of hepatic in vitro metabolism of T-2 toxin in rats, pigs, chickens, and carp. Xenobiotica 2011, 41, 863–873. [Google Scholar] [CrossRef]
- Ge, X.; Wang, J.; Liu, J.; Jiang, J.; Lin, H.; Wu, J.; Ouyang, M.; Tang, X.; Zheng, M.; Liao, M.; et al. The catalytic activity of cytochrome P450 3A22 is critical for the metabolism of T-2 toxin in porcine reservoirs. Catal. Commun. 2010, 12, 71–75. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhou, X.; Yang, J.; Li, M.; Qiu, X. T-2 toxin is hydroxylated by chicken CYP3A37. Food Chem. Toxicol. 2013, 62, 622–627. [Google Scholar] [CrossRef]
- Shang, S.; Jiang, J.; Deng, Y. Chicken cytochrome P450 1A5 is the key enzyme for metabolizing T-2 toxin to 3′OH-T-2. Int. J. Mol. Sci. 2013, 14, 10809–10818. [Google Scholar] [CrossRef]
- Dai, D.; Pan, Y.; Zeng, C.P.; Liu, S.; Yan, Y.; Wu, X.; Xu, Z.; Zhang, L. Activated FXR promotes xenobiotic metabolism of T-2 toxin and attenuates oxidative stress in broiler chicken liver. Chem. Biol. Interact. 2020, 316, 108912. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, Y.; Sun, L.; Lu, P.; Wang, R.; Ye, L.; Xu, D.; Ye, R.; Liu, Y.; Bi, S.; et al. Biotransformation enzyme activities and phase I metabolites analysis in Litopenaeus vannamei following intramuscular administration of T-2 toxin. Drug Chem. Toxicol. 2018, 41, 113–122. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, G.; Dai, Y.; Wang, Y.; Lee, Y.W.; Shi, J.; Xu, J. Biodegradation of Deoxynivalenol by a Novel Microbial Consortium. Front. Microbiol. 2020, 10, 2964. [Google Scholar] [CrossRef]
- Wu, Q.H.; Wang, X.; Yang, W.; Nüssler, A.K.; Xiong, L.Y.; Kuča, K.; Dohnal, V.; Zhang, X.J.; Yuan, Z.H. Oxidative stress-mediated cytotoxicity and metabolism of T-2 toxin and deoxynivalenol in animals and humans: An update. Arch. Toxicol. 2014, 88, 1309–1326. [Google Scholar] [CrossRef] [PubMed]
- Catteuw, A.; Broekaert, N.; De Baere, S.; Lauwers, M.; Gasthuys, E.; Huybrechts, B.; Callebaut, A.; Ivanova, L.; Uhlig, S.; De Boevre, M.; et al. Insights into in Vivo Absolute Oral Bioavailability, Biotransformation, and Toxicokinetics of Zearalenone, α-Zearalenol, β-Zearalenol, Zearalenone-14-glucoside, and Zearalenone-14-sulfate in Pigs. J. Agric. Food Chem. 2019, 67, 3448–3458. [Google Scholar] [CrossRef] [PubMed]
- Malekinejad, H.; Maas-Bakker, R.; Fink-Gremmels, J. Species differences in the hepatic biotransformation of zearalenone. Vet. J. 2006, 172, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Malekinejad, H.; Maas-Bakker, R.F.; Fink-Gremmels, J. Bioactivation of zearalenone by porcine hepatic biotransformation. Vet. Res. 2005, 36, 799–810. [Google Scholar] [CrossRef]
- Ayed-Boussema, I.; Pascussi, J.M.; Maurel, P.; Bacha, H.; Hassen, W. Zearalenone activates pregnane X receptor, constitutive androstane receptor and aryl hydrocarbon receptor and corresponding phase I target genes mRNA in primary cultures of human hepatocytes. Environ. Toxicol. Pharmacol. 2011, 31, 79–87. [Google Scholar] [CrossRef]
- Rodríguez-Carrasco, Y.; Heilos, D.; Richter, L.; Süssmuth, R.D.; Heffeter, P.; Sulyok, M.; Kenner, L.; Berger, W.; Dornetshuber-Fleiss, R. Mouse tissue distribution and persistence of the food-born fusariotoxins Enniatin B and Beauvericin. Toxicol. Lett. 2016, 247, 35–44. [Google Scholar] [CrossRef]
- Mei, L.; Zhang, L.; Dai, R. An inhibition study of beauvericin on human and rat cytochrome P450 enzymes and its pharmacokinetics in rats. J. Enzym. Inhib. Med. Chem. 2009, 24, 753–762. [Google Scholar] [CrossRef]
- Fæste, C.K.; Ivanova, L.; Uhlig, S. In vitro metabolism of the mycotoxin enniatin B in different species and cytochrome P450 enzyme phenotyping by chemical inhibitors. Drug Metab. Dispos. 2011, 39, 1768–1776. [Google Scholar] [CrossRef]
- Ivanova, L.; Fæste, C.K.; Uhlig, S. In vitro phase i metabolism of the depsipeptide enniatin B. Anal. Bioanal. Chem. 2011, 400, 2889–2901. [Google Scholar] [CrossRef]
- Ivanova, L.; Fæste, C.K.; Van Pamel, E.; Daeseleire, E.; Callebaut, A.; Uhlig, S. Presence of enniatin B and its hepatic metabolites in plasma and liver samples from broilers and eggs from laying hens. World Mycotoxin J. 2014, 7, 167–175. [Google Scholar] [CrossRef]
- Ivanova, L.; Denisov, I.G.; Grinkova, Y.V.; Sligar, S.G.; Fæste, C.K. Biotransformation of the Mycotoxin Enniatin B1 by CYP P450 3A4 and Potential for Drug-Drug Interactions. Metabolites 2019, 9, 158. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, L.; Uhlig, S.; Devreese, M.; Croubels, S.; Fæste, C.K. Biotransformation of the mycotoxin enniatin B1 in pigs: A comparative in vitro and in vivo approach. Food Chem. Toxicol. 2017, 105, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Debevere, S.; Cools, A.; De Baere, S.; Haesaert, G.; Rychlik, M.; Croubels, S.; Fievez, V. In Vitro Rumen Simulations Show a Reduced Disappearance of Deoxynivalenol, Nivalenol and Enniatin B at Conditions of Rumen Acidosis and Lower Microbial Activity. Toxins 2020, 12, 101. [Google Scholar] [CrossRef] [PubMed]
- Fraeyman, S.; Devreese, M.; Antonissen, G.; De Baere, S.; Rychlik, M.; Croubels, S. Comparative Oral Bioavailability, Toxicokinetics, and Biotransformation of Enniatin B1 and Enniatin B in Broiler Chickens. J. Agric. Food Chem. 2016, 64, 7259–7264. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.; Dekant, W.; Mally, A. Fumonisin B 1 and the kidney: Modes of action for renal tumor formation by fumonisin B 1 in rodents. Food Chem. Toxicol. 2012, 50, 3833–3846. [Google Scholar] [CrossRef]
- Schreck, I.; Deigendesch, U.; Burkhardt, B.; Marko, D.; Weiss, C. The Alternaria mycotoxins alternariol and alternariol methyl ether induce cytochrome P450 1A1 and apoptosis in murine hepatoma cells dependent on the aryl hydrocarbon receptor. Arch. Toxicol. 2012, 86, 625–632. [Google Scholar] [CrossRef]
- Aichinger, G.; Krüger, F.; Puntscher, H.; Preindl, K.; Warth, B.; Marko, D. Naturally occurring mixtures of Alternaria toxins: Anti-estrogenic and genotoxic effects in vitro. Arch. Toxicol. 2019, 93, 3021–3031. [Google Scholar] [CrossRef]
- Burkhardt, B.; Pfeiffer, E.; Metzler, M. Absorption and metabolism of the mycotoxins alternariol and alternariol-9-methyl ether in Caco-2 cells in vitro. Mycotoxin Res. 2009, 25, 149–157. [Google Scholar] [CrossRef]
- Scheibenzuber, S.; Hoffmann, T.; Effenberger, I.; Schwab, W.; Asam, S.; Rychlik, M. Enzymatic synthesis of modified alternaria mycotoxins using a whole-cell biotransformation system. Toxins 2020, 12, 264. [Google Scholar] [CrossRef]
- Puntscher, H.; Hankele, S.; Tillmann, K.; Attakpah, E.; Braun, D.; Kütt, M.L.; Del Favero, G.; Aichinger, G.; Pahlke, G.; Höger, H.; et al. First insights into Alternaria multi-toxin in vivo metabolism. Toxicol. Lett. 2019, 301, 168–178. [Google Scholar] [CrossRef]
- Yang, X.J.; Lu, H.Y.; Li, Z.Y.; Bian, Q.; Qiu, L.L.; Li, Z.; Liu, Q.; Li, J.; Wang, X.; Wang, S.L. Cytochrome P450 2A13 mediates aflatoxin B1-induced cytotoxicity and apoptosis in human bronchial epithelial cells. Toxicology 2012, 300, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Zhao, L.; Zhang, N.Y.; Karrow, N.A.; Krumm, C.S.; Qi, D.S.; Sun, L.H. Aflatoxin B 1 metabolism: Regulation by phase I and II metabolizing enzymes and chemoprotective agents. Mutat. Res. Rev. Mutat. Res. 2018, 778, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Ayed-Boussema, I.; Pascussi, J.M.; Zaied, C.; Maurel, P.; Bacha, H.; Hassen, W. Ochratoxin A induces CYP3A4, 2B6, 3A5, 2C9, 1A1, and CYP1A2 gene expression in primary cultured human hepatocytes: A possible activation of nuclear receptors. Drug Chem. Toxicol. 2012, 35, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.S.; Lee, H.J.; Pyo, M.C.; Ryu, D.; Lee, K.W. Ochratoxin a-induced hepatotoxicity through phase i and phase ii reactions regulated by ahr in liver cells. Toxins 2019, 11, 377. [Google Scholar] [CrossRef]
- Tao, Y.; Xie, S.; Xu, F.; Liu, A.; Wang, Y.; Chen, D.; Pan, Y.; Huang, L.; Peng, D.; Wang, X.; et al. Ochratoxin A: Toxicity, oxidative stress and metabolism. Food Chem. Toxicol. 2018, 112, 320–331. [Google Scholar] [CrossRef]
- Gross-Steinmeyer, K.; Weymann, J.; Hege, H.G.; Metzler, M. Metabolism and lack of DNA reactivity of the mycotoxin ochratoxin A in cultured rat and human primary hepatocytes. J. Agric. Food Chem. 2002, 50, 938–945. [Google Scholar] [CrossRef]
- Tozlovanu, M.; Canadas, D.; Pfohl-Leszkowicz, A.; Frenette, C.; Paugh, R.J.; Manderville, R.A. Glutathione conjugates of ochratoxin a as biomarkers of exposure. Arh. Hig. Rada Toksikol. 2012, 63, 417–425. [Google Scholar] [CrossRef]
- Kőszegi, T.; Poór, M. Ochratoxin a: Molecular interactions, mechanisms of toxicity and prevention at the molecular level. Toxins 2016, 8, 111. [Google Scholar] [CrossRef]
- Ayed-Boussema, I.; Pascussi, J.M.; Rjiba, K.; Maurel, P.; Bacha, H.; Hassen, W. The mycotoxin, patulin, increases the expression of PXR and AhR and their target cytochrome P450s in primary cultured human hepatocytes. Drug Chem. Toxicol. 2012, 35, 241–250. [Google Scholar] [CrossRef]
- Ji, C.; Fan, Y.; Zhao, L. Review on biological degradation of mycotoxins. Anim. Nutr. 2016, 2, 127–133. [Google Scholar] [CrossRef]
- Young, J.C.; Zhou, T.; Yu, H.; Zhu, H.; Gong, J. Degradation of trichothecene mycotoxins by chicken intestinal microbes. Food Chem. Toxicol. 2007, 45, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Pierron, A.; Mimoun, S.; Murate, L.S.; Loiseau, N.; Lippi, Y.; Bracarense, A.P.F.L.; Schatzmayr, G.; He, J.W.; Zhou, T.; Moll, W.D.; et al. Microbial biotransformation of DON: Molecular basis for reduced toxicity. Sci. Rep. 2016, 6, 29105. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Wang, C.; Liu, P.; Li, Y.; Ma, X. Effects of Dietary Mycotoxins on Gut Microbiome. Protein Pept. Lett. 2017, 24, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Antonissen, G.; Devreese, M.; De Baere, S.; Martel, A.; Van Immerseel, F.; Croubels, S. Impact of Fusarium mycotoxins on hepatic and intestinal mRNA expression of cytochrome P450 enzymes and drug transporters, and on the pharmacokinetics of oral enrofloxacin in broiler chickens. Food Chem. Toxicol. 2017, 101, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Murcia, H.W.; Diaz, G.J. In vitro hepatic aflatoxicol production is related to a higher resistance to aflatoxin B1 in poultry. Sci. Rep. 2020, 10, 5508. [Google Scholar] [CrossRef]
- Gross-Steinmeyer, K.; Eaton, D.L. Dietary modulation of the biotransformation and genotoxicity of aflatoxin B1. Toxicology 2012, 299, 69–79. [Google Scholar] [CrossRef]
- Peles, F.; Sipos, P.; Győri, Z.; Pfliegler, W.P.; Giacometti, F.; Serraino, A.; Pagliuca, G.; Gazzotti, T.; Pócsi, I. Adverse Effects, Transformation and Channeling of Aflatoxins Into Food Raw Materials in Livestock. Front. Microbiol. 2019, 10, 1–26. [Google Scholar] [CrossRef]
- Wu, J.; Xu, W.; Zhang, C.; Chang, Q.; Tang, X.; Li, K.; Deng, Y. Trp266 determines the binding specificity of a porcine aflatoxin B 1 aldehyde reductase for aflatoxin B1-dialdehyde. Biochem. Pharmacol. 2013, 86, 1357–1365. [Google Scholar] [CrossRef]
- Lyagin, I.; Efremenko, E. Enzymes for Detoxification of Various Mycotoxins: Origins and Mechanisms of Catalytic Action. Molecules 2019, 24, 2362. [Google Scholar] [CrossRef]
- Schrenk, D.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L. (Ron); Leblanc, J.; Nebbia, C.S.; Nielsen, E.; et al. Risk assessment of ochratoxin A in food. EFSA J. 2020, 18. [Google Scholar] [CrossRef]
- Li, P.; Su, R.; Yin, R.; Lai, D.; Wang, M.; Liu, Y.; Zhou, L. Detoxification of mycotoxins through biotransformation. Toxins 2020, 12, 121. [Google Scholar] [CrossRef] [PubMed]
- Ringot, D.; Chango, A.; Schneider, Y.-J.; Larondelle, Y. Toxicokinetics and toxicodynamics of ochratoxin A, an update. Chem. Biol. Interact. 2006, 159, 18–46. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Dohnal, V.; Huang, L.; Kuca, K.; Wang, X.; Chen, G.; Yuan, Z. Metabolic Pathways of Ochratoxin A. Curr. Drug Metab. 2011, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Schelstraete, W.; Devreese, M.; Croubels, S. Impact of subacute exposure to T-2 toxin and zearalenone on the pharmacokinetics of midazolam as CYP3A probe drug in a porcine animal model: A pilot study. Front. Pharmacol. 2019, 10, 399. [Google Scholar] [CrossRef] [PubMed]
- Payros, D.; Alassane-Kpembi, I.; Pierron, A.; Loiseau, N.; Pinton, P.; Oswald, I.P. Toxicology of deoxynivalenol and its acetylated and modified forms. Arch. Toxicol. 2016, 90, 2931–2957. [Google Scholar] [CrossRef] [PubMed]
- Springler, A.; Hessenberger, S.; Reisinger, N.; Kern, C.; Nagl, V.; Schatzmayr, G.; Mayer, E. Deoxynivalenol and its metabolite deepoxy-deoxynivalenol: Multi-parameter analysis for the evaluation of cytotoxicity and cellular effects. Mycotoxin Res. 2017, 33, 25–37. [Google Scholar] [CrossRef]
- Nagl, V.; Woechtl, B.; Schwartz-Zimmermann, H.E.; Hennig-Pauka, I.; Moll, W.D.; Adam, G.; Berthiller, F. Metabolism of the masked mycotoxin deoxynivalenol-3-glucoside in pigs. Toxicol. Lett. 2014, 229, 190–197. [Google Scholar] [CrossRef]
- Schwartz, H.E.; Hametner, C.; Slavik, V.; Greitbauer, O.; Bichl, G.; Kunz-Vekiru, E.; Schatzmayr, D.; Berthiller, F. Characterization of three deoxynivalenol sulfonates formed by reaction of deoxynivalenol with sulfur reagents. J. Agric. Food Chem. 2013, 61, 8941–8948. [Google Scholar] [CrossRef]
- Gerding, J.; Cramer, B.; Humpf, H.U. Determination of mycotoxin exposure in Germany using an LC-MS/MS multibiomarker approach. Mol. Nutr. Food Res. 2014, 58, 2358–2368. [Google Scholar] [CrossRef]
- Pestka, J.J. Deoxynivalenol: Toxicity, mechanisms and animal health risks. Anim. Feed Sci. Technol. 2007, 137, 283–298. [Google Scholar] [CrossRef]
- Gao, X.; Mu, P.; Zhu, X.; Chen, X.; Tang, S.; Wu, Y.; Miao, X.; Wang, X.; Wen, J.; Deng, Y. Dual function of a novel bacterium, slackia sp. D-G6: Detoxifying deoxynivalenol and producing the Natural Estrogen Analogue, Equol. Toxins 2020, 12, 85. [Google Scholar] [CrossRef] [PubMed]
- Mackei, M.; Orbán, K.; Molnár, A.; Pál, L.; Dublecz, K.; Husvéth, F.; Neogrády, Z.; Mátis, G. Cellular Effects of T-2 Toxin on Primary Hepatic Cell Culture Models of Chickens. Toxins 2020, 12, 46. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Z.; Beier, R.C.; Shen, J.; De Smet, D.; De Saeger, S.; Zhang, S. T-2 toxin, a trichothecene mycotoxin: Review of toxicity, metabolism, and analytical methods. J. Agric. Food Chem. 2011, 59, 3441–3453. [Google Scholar] [CrossRef] [PubMed]
- Welsch, T.; Humpf, H.U. HT-2 toxin 4-glucuronide as new T-2 toxin metabolite: Enzymatic synthesis, analysis, and species specific formation of T-2 and HT-2 toxin glucuronides by rat, mouse, pig, and human liver microsomes. J. Agric. Food Chem. 2012, 60, 10170–10178. [Google Scholar] [CrossRef]
- Masching, S.; Naehrer, K.; Schwartz-Zimmermann, H.E.; Sărăndan, M.; Schaumberger, S.; Dohnal, I.; Nagl, V.; Schatzmayr, D. Gastrointestinal degradation of fumonisin B1 by carboxylesterase FumD prevents fumonisin induced alteration of sphingolipid metabolism in Turkey and swine. Toxins 2016, 8, 84. [Google Scholar] [CrossRef]
- Daud, N.; Currie, V.; Duncan, G.; Busman, M.; Gratz, S.W. Intestinal hydrolysis and microbial biotransformation of diacetoxyscirpenol-α-glucoside, HT-2-β-glucoside and N-(1-deoxy-d-fructos-1-yl) fumonisin B1 by human gut microbiota in vitro. Int. J. Food Sci. Nutr. 2019, 71, 540–548. [Google Scholar] [CrossRef]
- Merrill, A.H.; Morgan, E.T.; Nikolova-Karakashian, M.; Stewart, J. Sphingomyelin hydrolysis and regulation of the expression of the gene for cytochrome P450. Biochem. Soc. Trans. 1999, 27, 383–387. [Google Scholar] [CrossRef]
- Spotti, M.; Maas, R.F.M.; De Nijs, C.M.; Fink-Gremmels, J. Effect of fumonisin B1 on rat hepatic P450 system. Environ. Toxicol. Pharmacol. 2000, 8, 197–204. [Google Scholar] [CrossRef]
- Riley, R.T.; Merrill, A.H. Ceramide synthase inhibition by fumonisins: A perfect storm of perturbed sphingolipid metabolism, signaling, and disease. J. Lipid Res. 2019, 60, 1183–1189. [Google Scholar] [CrossRef]
- Harrer, H.; Laviad, E.L.; Humpf, H.U.; Futerman, A.H. Identification of N-acyl-fumonisin B1 as new cytotoxic metabolites of fumonisin mycotoxins. Mol. Nutr. Food Res. 2013, 57, 516–522. [Google Scholar] [CrossRef]
- Dellafiora, L.; Galaverna, G.; Dall’Asta, C. Mechanisms of Fumonisin B1 Toxicity: A Computational Perspective beyond the Ceramide Synthases Inhibition. Chem. Res. Toxicol. 2018, 31, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; et al. Risks for animal health related to the presence of fumonisins, their modified forms and hidden forms in feed. EFSA J. 2018, 16, e05242. [Google Scholar] [PubMed]
- Ieko, T.; Inoue, S.; Inomata, Y.; Inoue, H.; Fujiki, J.; Iwano, H. Glucuronidation as a metabolic barrier against zearalenone in rat everted intestine. J. Vet. Med. Sci. 2020, 82, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Binder, S.B.; Schwartz-Zimmermann, H.E.; Varga, E.; Bichl, G.; Michlmayr, H.; Adam, G.; Berthiller, F. Metabolism of zearalenone and its major modified forms in pigs. Toxins 2017, 9, 56. [Google Scholar] [CrossRef]
- Videmann, B.; Mazallon, M.; Tep, J.; Lecoeur, S. Metabolism and transfer of the mycotoxin zearalenone in human intestinal Caco-2 cells. Food Chem. Toxicol. 2008, 46, 3279–3286. [Google Scholar] [CrossRef]
- Dellafiora, L.; Galaverna, G.; Righi, F.; Cozzini, P.; Dall’Asta, C. Assessing the hydrolytic fate of the masked mycotoxin zearalenone-14-glucoside—A warning light for the need to look at the “maskedome”. Food Chem. Toxicol. 2017, 99, 9–16. [Google Scholar] [CrossRef]
- Keller, L.; Abrunhosa, L.; Keller, K.; Rosa, C.; Cavaglieri, L.; Venâncio, A. Zearalenone and Its Derivatives α-Zearalenol and β-Zearalenol Decontamination by Saccharomyces cerevisiae Strains Isolated from Bovine Forage. Toxins 2015, 7, 3297–3308. [Google Scholar] [CrossRef]
- Rogowska, A.; Pomastowski, P.; Walczak, J.; Railean-Plugaru, V.; Rudnicka, J.; Buszewski, B. Investigation of zearalenone adsorption and biotransformation by microorganisms cultured under cellular stress conditions. Toxins 2019, 11, 463. [Google Scholar] [CrossRef]
- Rogowska, A.; Pomastowski, P.; Rafińska, K.; Railean-Plugaru, V.; Złoch, M.; Walczak, J.; Buszewski, B. A study of zearalenone biosorption and metabolisation by prokaryotic and eukaryotic cells. Toxicon 2019, 169, 81–90. [Google Scholar] [CrossRef]
- Chlebicz, A.; Śliżewska, K. In Vitro Detoxification of Aflatoxin B1, Deoxynivalenol, Fumonisins, T-2 Toxin and Zearalenone by Probiotic Bacteria from Genus Lactobacillus and Saccharomyces cerevisiae Yeast. Probiotics Antimicrob. Proteins 2020, 12, 289–301. [Google Scholar] [CrossRef]
- Pfeiffer, E.; Schebb, N.H.; Podlech, J.; Metzler, M. Novel oxidative in vitro metabolites of the mycotoxins alternariol and alternariol methyl ether. Mol. Nutr. Food Res. 2007, 51, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, E.; Burkhardt, B.; Altemöller, M.; Podlech, J.; Metzler, M. Activities of human recombinant cytochrome P450 isoforms and human hepatic microsomes for the hydroxylation ofAlternaria toxins. Mycotoxin Res. 2008, 24, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Tiessen, C.; Ellmer, D.; Mikula, H.; Pahlke, G.; Warth, B.; Gehrke, H.; Zimmermann, K.; Heiss, E.; Fröhlich, J.; Marko, D. Impact of phase I metabolism on uptake, oxidative stress and genotoxicity of the emerging mycotoxin alternariol and its monomethyl ether in esophageal cells. Arch. Toxicol. 2017, 91, 1213–1226. [Google Scholar] [CrossRef] [PubMed]
- Lemke, A.; Burkhardt, B.; Bunzel, D.; Pfeiffer, E.; Metzler, M.; Huch, M.; Kulling, S.E.; Franz, C.M.A.P. Alternaria toxins of the alternariol type are not metabolised by human faecal microbiota. World Mycotoxin J. 2016, 9, 41–49. [Google Scholar] [CrossRef]
- Fleck, S.C.; Pfeiffer, E.; Podlech, J.; Metzler, M. Epoxide Reduction to an Alcohol: A Novel Metabolic Pathway for Perylene Quinone-Type Alternaria Mycotoxins in Mammalian Cells. Chem. Res. Toxicol. 2014, 27, 247–253. [Google Scholar] [CrossRef]
- Burkhardt, B.; Wittenauer, J.; Pfeiffer, E.; Schauer, U.M.D.; Metzler, M. Oxidative metabolism of the mycotoxins alternariol and alternariol-9-methyl ether in precision-cut rat liver slices in vitro. Mol. Nutr. Food Res. 2011, 55, 1079–1086. [Google Scholar] [CrossRef]
- Pfeiffer, E.; Schmit, C.; Burkhardt, B.; Altemöller, M.; Podlech, J.; Metzler, M. Glucuronidation of the mycotoxins alternariol and alternariol-9-methyl ether in vitro: Chemical structures of glucuronides and activities of human UDP-glucuronosyltransferase isoforms. Mycotoxin Res. 2009, 25, 3–10. [Google Scholar] [CrossRef]
- Soukup, S.T.; Kohn, B.N.; Pfeiffer, E.; Geisen, R.; Metzler, M.; Bunzel, M.; Kulling, S.E. Sulfoglucosides as Novel Modified Forms of the Mycotoxins Alternariol and Alternariol Monomethyl Ether. J. Agric. Food Chem. 2016, 64, 8892–8901. [Google Scholar] [CrossRef]
- Puntscher, H.; Marko, D.; Warth, B. The fate of altertoxin ii during tomato processing steps at a laboratory scale. Front. Nutr. 2019, 6, 92. [Google Scholar] [CrossRef]
- Pfeiffer, E.; Herrmann, C.; Altemöller, M.; Podlech, J.; Metzler, M. Oxidative in vitro metabolism of the Alternaria toxins altenuene and isoaltenuene. Mol. Nutr. Food Res. 2009, 53, 452–459. [Google Scholar] [CrossRef]
- Rychlik, M.; Kircher, F.; Schusdziarra, V.; Lippl, F. Absorption of the mycotoxin patulin from the rat stomach. Food Chem. Toxicol. 2004, 42, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Rychlik, M. Rapid degradation of the mycotoxin patulin in man quantified by stable isotope dilution assays. Food Addit. Contam. 2003, 20, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wei, W.; Rao, S.; Gao, L.; Li, H.; Yang, Z. Degradation of patulin in fruit juice by a lactic acid bacteria strain Lactobacillus casei YZU01. Food Control 2020, 112, 107147. [Google Scholar] [CrossRef]
- Tannous, J.; Snini, S.P.; El Khoury, R.; Canlet, C.; Pinton, P.; Lippi, Y.; Alassane-Kpembi, I.; Gauthier, T.; El Khoury, A.; Atoui, A.; et al. Patulin transformation products and last intermediates in its biosynthetic pathway, E- and Z-ascladiol, are not toxic to human cells. Arch. Toxicol. 2017, 91, 2455–2467. [Google Scholar] [CrossRef]
- Ianiri, G.; Idnurm, A.; Wright, S.A.I.; Durán-Patrón, R.; Mannina, L.; Ferracane, R.; Ritieni, A.; Castoria, R. Searching for genes responsible for patulin degradation in a biocontrol yeast provides insight into the basis for resistance to this mycotoxin. Appl. Environ. Microbiol. 2013, 79, 3101–3115. [Google Scholar] [CrossRef]
- Xu, R.; Zhu, H.; Hu, L.; Yu, B.; Zhan, X.; Yuan, Y.; Zhou, P. Characterization of the intestinal absorption of morroniside from Cornus officinalis Sieb. et Zucc via a Caco-2 cell monolayer model. PLoS ONE 2020, 15, e0227844. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, N.; Liu, J.; Li, F.D.; Li, S.L.; Wang, J.Q. Aflatoxin B1 and aflatoxin M1 induced cytotoxicity and DNA damage in differentiated and undifferentiated Caco-2 cells. Food Chem. Toxicol. 2015, 83, 54–60. [Google Scholar] [CrossRef]
- Fogh, J.; Fogh, J.M.; Orfeo, T. One Hundred and Twenty-Seven Cultured Human Tumor Cell Lines Producing Tumors in Nude Mice23. JNCI J. Natl. Cancer Inst. 1977, 59, 221–226. [Google Scholar] [CrossRef]
- Iftikhar, M.; Iftikhar, A.; Zhang, H.; Gong, L.; Wang, J. Transport, metabolism and remedial potential of functional food extracts (FFEs) in Caco-2 cells monolayer: A Review. Food Res. Int. 2020, 136, 109240. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Capanoglu, E.; Grootaert, C.; Van Camp, J. Anthocyanin Absorption and Metabolism by Human Intestinal Caco-2 Cells—A Review. Int. J. Mol. Sci. 2015, 16, 21555–21574. [Google Scholar] [CrossRef]
- Awortwe, C.; Fasinu, P.S.; Rosenkranz, B. Application of Caco-2 cell line in herb-drug interaction studies: Current approaches and challenges. J. Pharm. Pharm. Sci. Publ. Can. Soc. Pharm. Sci. Société Can. Sci. Pharm. 2014, 17, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, L.; Zhang, G. Impact of deoxynivalenol and kaempferol on expression of tight junction proteins at different stages of Caco-2 cell proliferation and differentiation. RSC Adv. 2019, 9, 34607–34616. [Google Scholar] [CrossRef]
- Wang, X.; Li, L.; Zhang, G. A proteomic study on the protective effect of kaempferol pretreatment against deoxynivalenol-induced intestinal barrier dysfunction in a Caco-2 cell model. Food Funct. 2020, 11, 7266–7279. [Google Scholar] [CrossRef]
- Akbari, P.; Braber, S.; Varasteh, S.; Alizadeh, A.; Garssen, J.; Fink-Gremmels, J. The intestinal barrier as an emerging target in the toxicological assessment of mycotoxins. Arch. Toxicol. 2017, 91, 1007–1029. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.; Akbari, P.; Varasteh, S.; Braber, S.; Malekinejad, H.; Fink-Gremmels, J. Ochratoxin A challenges the intestinal epithelial cell integrity: Results obtained in model experiments with Caco-2 cells. World Mycotoxin J. 2019, 12, 399–407. [Google Scholar] [CrossRef]
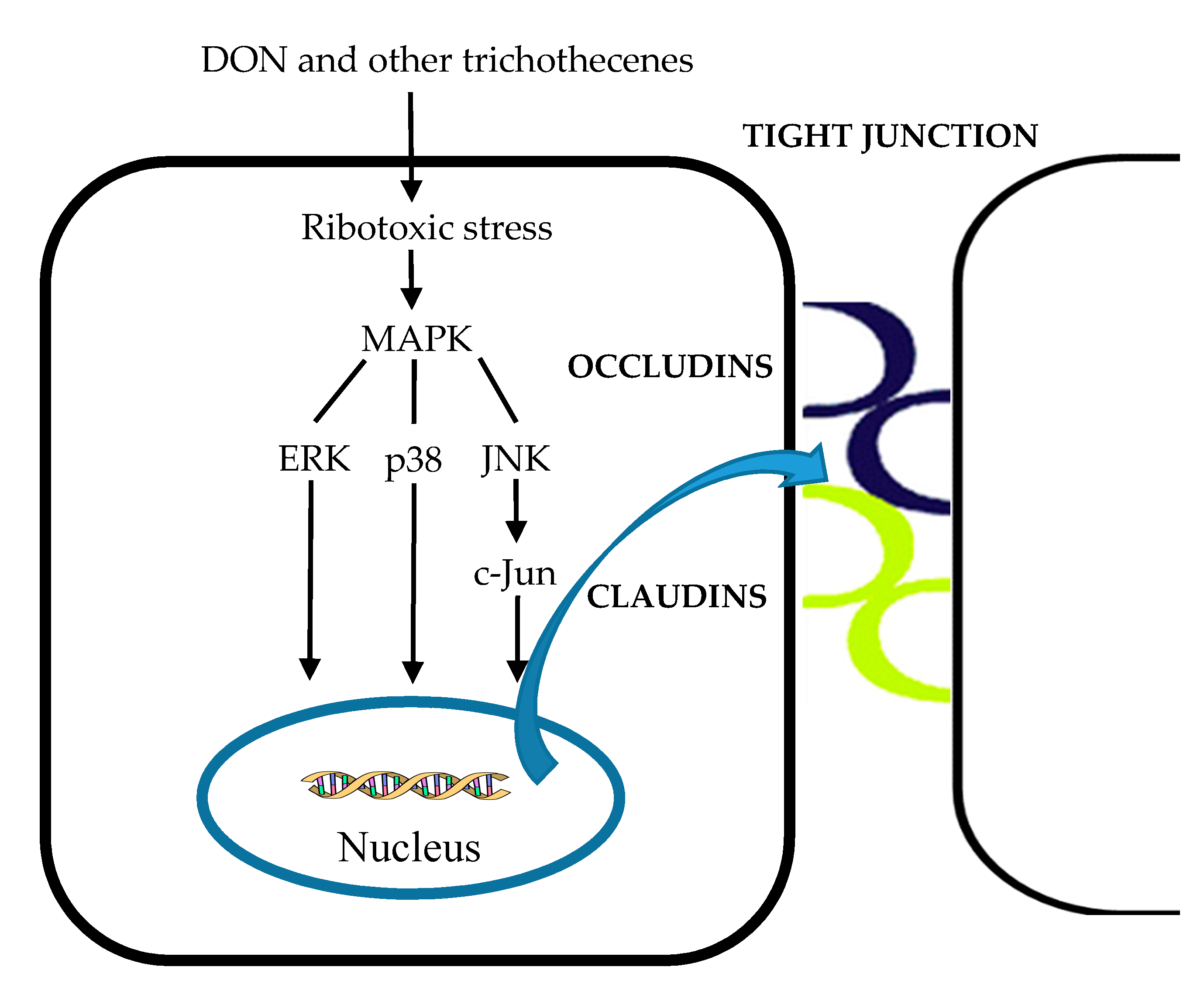
- Pinton, P.; Nougayrède, J.P.; Del Rio, J.C.; Moreno, C.; Marin, D.E.; Ferrier, L.; Bracarense, A.P.; Kolf-Clauw, M.; Oswald, I.P. The food contaminant deoxynivalenol, decreases intestinal barrier permeability and reduces claudin expression. Toxicol. Appl. Pharmacol. 2009, 237, 41–48. [Google Scholar] [CrossRef]
- Romero, A.; Ares, I.; Ramos, E.; Castellano, V.; Martínez, M.M.A.; Martínez-Larrañaga, M.R.; Anadón, A.; Martínez, M.M.A. Mycotoxins modify the barrier function of Caco-2 cells through differential gene expression of specific claudin isoforms: Protective effect of illite mineral clay. Toxicology 2016, 353–354, 21–33. [Google Scholar] [CrossRef]
- Sun, H.; Chow, E.C.Y.; Liu, S.; Du, Y.; Pang, K.S. The Caco-2 cell monolayer: Usefulness and limitations. Expert Opin. Drug Metab. Toxicol. 2008, 4, 395–411. [Google Scholar] [CrossRef]
- Ferruzza, S.; Scarino, M.L.; Gambling, L.; Natella, F.; Sambuy, Y. Biphasic effect of iron on human intestinal Caco-2 cells: Early effect on tight junction permeability with delayed onset of oxidative cytotoxic damage. Cell. Mol. Biol. 2003, 49, 89–99. [Google Scholar]
- Videmann, B.; Tep, J.; Cavret, S.; Lecoeur, S. Epithelial transport of deoxynivalenol: Involvement of human P-glycoprotein (ABCB1) and multidrug resistance-associated protein 2 (ABCC2). Food Chem. Toxicol. 2007, 45, 1938–1947. [Google Scholar] [CrossRef]
- Sergent, T.; Parys, M.; Garsou, S.; Pussemier, L.; Schneider, Y.J.; Larondelle, Y. Deoxynivalenol transport across human intestinal Caco-2 cells and its effects on cellular metabolism at realistic intestinal concentrations. Toxicol. Lett. 2006, 164, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Artursson, P.; Palm, K.; Luthman, K. Caco-2 monolayers in experimental and theoretical predictions of drug transport. Adv. Drug Deliv. Rev. 2012, 22, 280–289. [Google Scholar] [CrossRef]
- Kadota, T.; Furusawa, H.; Hirano, S.; Tajima, O.; Kamata, Y.; Sugita-Konishi, Y. Comparative study of deoxynivalenol, 3-acetyldeoxynivalenol, and 15-acetyldeoxynivalenol on intestinal transport and IL-8 secretion in the human cell line Caco-2. Toxicol. In Vitro 2013, 27, 1888–1895. [Google Scholar] [CrossRef]
- Sobral, M.M.C.; Faria, M.A.; Cunha, S.C.; Miladinovic, B.; Ferreira, I.M. Transport of mycotoxins across human gastric NCI–N87 and intestinal Caco-2 cell models. Food Chem. Toxicol. 2019, 131, 110595. [Google Scholar] [CrossRef] [PubMed]
- Tep, J.; Videmann, B.; Mazallon, M.; Balleydier, S.; Cavret, S.; Lecoeur, S. Transepithelial transport of fusariotoxin nivalenol: Mediation of secretion by ABC transporters. Toxicol. Lett. 2007, 170, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, E.; Kommer, A.; Dempe, J.S.; Hildebrand, A.A.; Metzler, M. Absorption and metabolism of the mycotoxin zearalenone and the growth promotor zeranol in Caco-2 cells in vitro. Mol. Nutr. Food Res. 2011, 55, 560–567. [Google Scholar] [CrossRef]
- Cirlini, M.; Barilli, A.; Galaverna, G.; Michlmayr, H.; Adam, G.; Berthiller, F.; Dall’Asta, C. Study on the uptake and deglycosylation of the masked forms of zearalenone in human intestinal Caco-2 cells. Food Chem. Toxicol. 2016, 98, 232–239. [Google Scholar] [CrossRef]
- Prosperini, A.; Meca, G.; Font, G.; Ruiz, M.J. Study of the cytotoxic activity of beauvericin and fusaproliferin and bioavailability in vitro on Caco-2 cells. Food Chem. Toxicol. 2012, 50, 2356–2361. [Google Scholar] [CrossRef]
- De Angelis, I.; Friggè, G.; Raimondi, F.; Stammati, A.; Zucco, F.; Caloni, F. Absorption of Fumonisin B1 and aminopentol on an in vitro model of intestinal epithelium; the role of P-glycoprotein. Toxicon 2005, 45, 285–291. [Google Scholar] [CrossRef]
- Berger, V.; Gabriel, A.F.; Sergent, T.; Trouet, A.; Larondelle, Y.; Schneider, Y.J. Interaction of ochratoxin A with human intestinal Caco-2 cells: Possible implication of a multidrug resistance-associated protein (MRP2). Toxicol. Lett. 2003, 140–141, 465–476. [Google Scholar] [CrossRef]
- De Walle, J.V.; Sergent, T.; Piront, N.; Toussaint, O.; Schneider, Y.-J.; Larondelle, Y. Deoxynivalenol affects in vitro intestinal epithelial cell barrier integrity through inhibition of protein synthesis. Toxicol. Appl. Pharmacol. 2010, 245, 291–298. [Google Scholar] [CrossRef] [PubMed]
- De Nijs, M.; Van Den Top, H.J.; Portier, L.; Oegema, G.; Kramer, E.; Van Egmond, H.P.; Hoogenboom, L.A.P. Digestibility and absorption of deoxynivalenol-3-ß-glucoside in in vitro models. World Mycotoxin J. 2012, 5, 319–324. [Google Scholar] [CrossRef]
- Schrickx, J.; Lektarau, Y.; Fink-Gremmels, J. Ochratoxin A secretion by ATP-dependent membrane transporters in Caco-2 cells. Arch. Toxicol. 2006, 80, 243–249. [Google Scholar] [CrossRef]
- Tuntiteerawit, P.; Jarukamjorn, K.; Porasuphatana, S. The effect of green tea catechins on breast cancer resistance protein activity and intestinal efflux of aflatoxin B1 via breast cancer resistance protein in Caco-2 cells. Toxicol. Res. 2020, 36, 293–300. [Google Scholar] [CrossRef]
- Li, X.; Mu, P.; Wen, J.; Deng, Y. Carrier-Mediated and Energy-Dependent Uptake and Efflux of Deoxynivalenol in Mammalian Cells. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Mu, P.; Qiao, H.; Wen, J.; Deng, Y. JNK-AKT-NF-κB controls P-glycoprotein expression to attenuate the cytotoxicity of deoxynivalenol in mammalian cells. Biochem. Pharmacol. 2018, 156, 120–134. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, L.; Fæste, C.K.; Solhaug, A. Role of P-glycoprotein in deoxynivalenol-mediated in vitro toxicity. Toxicol. Lett. 2018, 284, 21–28. [Google Scholar] [CrossRef]
- Anderle, P.; Niederer, E.; Rubas, W.; Hilgendorf, C.; Spahn-Langguth, H.; Wunderli-Allenspach, H.; Merkle, H.P.; Langguth, P. P-glycoprotein (P-gp) mediated efflux in Caco-2 cell monolayers: The influence of culturing conditions and drug exposure on P-gp expression levels. J. Pharm. Sci. 1998, 87, 757–762. [Google Scholar] [CrossRef]
- Videmann, B.; Mazallon, M.; Prouillac, C.; Delaforge, M.; Lecoeur, S. ABCC1, ABCC2 and ABCC3 are implicated in the transepithelial transport of the myco-estrogen zearalenone and its major metabolites. Toxicol. Lett. 2009, 190, 215–223. [Google Scholar] [CrossRef]
- Xu, R.; Karrow, N.A.; Shandilya, U.K.; Sun, L.H.; Kitazawa, H. In-vitro cell culture for efficient assessment of mycotoxin exposure, toxicity and risk mitigation. Toxins 2020, 12, 146. [Google Scholar] [CrossRef]
- Gao, Y.; Li, S.; Wang, J.; Luo, C.; Zhao, S.; Zheng, N. Modulation of intestinal epithelial permeability in differentiated caco-2 cells exposed to aflatoxin M1 and ochratoxin a individually or collectively. Toxins 2018, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Marin, D.; Motiu, M.; Taranu, I. Food Contaminant Zearalenone and Its Metabolites Affect Cytokine Synthesis and Intestinal Epithelial Integrity of Porcine Cells. Toxins 2015, 7, 1979–1988. [Google Scholar] [CrossRef] [PubMed]
- Bouhet, S.; Hourcade, E.; Loiseau, N.; Fikry, A.; Roselli, M.; Galtier, P.; Mengheri, E.; Oswald, I.P.; Martinez, S.; Roselli, M.; et al. The mycotoxin fumonisin B1 alters the proliferation and the barrier function of porcine intestinal epithelial cells. Toxicol. Sci. 2004, 77, 165–171. [Google Scholar] [CrossRef]
- Pinton, P.; Braicu, C.; Nougayrede, J.-P.; Laffitte, J.; Taranu, I.; Oswald, I.P. Deoxynivalenol Impairs Porcine Intestinal Barrier Function and Decreases the Protein Expression of Claudin-4 through a Mitogen-Activated Protein Kinase-Dependent Mechanism. J. Nutr. 2010, 140, 1956–1962. [Google Scholar] [CrossRef] [PubMed]
- Madara, J.L. Regulation of the Movement of Solutes Across Tight Junctions. Annu. Rev. Physiol. 1998, 60, 143–159. [Google Scholar] [CrossRef]
- Gao, Y.N.; Wang, J.Q.; Li, S.L.; Zhang, Y.D.; Zheng, N. Aflatoxin M1 cytotoxicity against human intestinal Caco-2 cells is enhanced in the presence of other mycotoxins. Food Chem. Toxicol. 2016, 96, 79–89. [Google Scholar] [CrossRef]
- Fleck, S.C.; Pfeiffer, E.; Metzler, M. Permeation and metabolism of Alternaria mycotoxins with perylene quinone structure in cultured Caco-2 cells. Mycotoxin Res. 2014, 30, 17–23. [Google Scholar] [CrossRef]
- González-Arias, C.A.; Marín, S.; Rojas-García, A.E.; Sanchis, V.; Ramos, A.J. UPLC-MS/MS analysis of ochratoxin A metabolites produced by Caco-2 and HepG2 cells in a co-culture system. Food Chem. Toxicol. 2017, 109, 333–340. [Google Scholar] [CrossRef]
- Matter, K.; Balda, M.S. Signalling to and from tight junctions. Nat. Rev. Mol. Cell Biol. 2003, 4, 225–237. [Google Scholar] [CrossRef]
- Moon, Y. Vomitoxin-Induced Cyclooxygenase-2 Gene Expression in Macrophages Mediated by Activation of ERK and p38 but Not JNK Mitogen-Activated Protein Kinases. Toxicol. Sci. 2002, 69, 373–382. [Google Scholar] [CrossRef]
- Zhou, H.-R.; Jia, Q.; Pestka, J.J. Ribotoxic Stress Response to the Trichothecene Deoxynivalenol in the Macrophage Involves the Src Family Kinase Hck. Toxicol. Sci. 2005, 85, 916–926. [Google Scholar] [CrossRef]
- Pinton, P.; Graziani, F.; Pujol, A.; Nicoletti, C.; Paris, O.; Ernouf, P.; Di Pasquale, E.; Perrier, J.; Oswald, I.P.; Maresca, M. Deoxynivalenol inhibits the expression by goblet cells of intestinal mucins through a PKR and MAP kinase dependent repression of the resistin-like molecule β. Mol. Nutr. Food Res. 2015, 59, 1076–1087. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.K. MAP Kinase Pathways. Cold Spring Harb. Perspect. Biol. 2012, 4, a011254. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Lim, W.; Park, S.; Kim, J.; You, S.; Song, G. Deoxynivalenol induces apoptosis and disrupts cellular homeostasis through MAPK signaling pathways in bovine mammary epithelial cells. Environ. Pollut. 2019, 252, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Bouhet, S.; Le, E.; Peres, S.; Fairbrother, J.M.; Oswald, I.P.; Hyacinthe, S. Mycotoxin fumonisin B 1 selectively down-regulates the basal IL-8 expression in pig intestine: In vivo and in vitro studies. Food Chem. Toxicol. 2006, 44, 1768–1773. [Google Scholar] [CrossRef]
- Beisl, J.; Pahlke, G.; Abeln, H.; Ehling-Schulz, M.; Del Favero, G.; Varga, E.; Warth, B.; Sulyok, M.; Abia, W.; Ezekiel, C.N.; et al. Combinatory effects of cereulide and deoxynivalenol on in vitro cell viability and inflammation of human Caco-2 cells. Arch. Toxicol. 2020, 94, 833–844. [Google Scholar] [CrossRef]
- Pellegrina, C.D.; Perbellini, O.; Scupoli, M.T.; Tomelleri, C.; Zanetti, C.; Zoccatelli, G.; Fusi, M.; Peruffo, A.; Rizzi, C.; Chignola, R. Effects of wheat germ agglutinin on human gastrointestinal epithelium: Insights from an experimental model of immune/epithelial cell interaction. Toxicol. Appl. Pharmacol. 2009, 237, 146–153. [Google Scholar] [CrossRef]
- Gao, Y.; Ye, Q.; Bao, X.; Huang, X.; Wang, J.; Zheng, N. Transcriptomic and proteomic profiling reveals the intestinal immunotoxicity induced by aflatoxin M1 and ochratoxin A. Toxicon 2020, 180, 49–61. [Google Scholar] [CrossRef]
- Trapecar, M.; Cencic, A. Application of Gut Cell Models for Toxicological and Bioactivity Studies of Functional and Novel Foods. Foods 2014, 1, 40–51. [Google Scholar] [CrossRef]
- Fu, J.; Cui, Y. In vitro digestion/Caco-2 cell model to estimate cadmium and lead bioaccessibility/bioavailability in two vegetables: The influence of cooking and additives. Food Chem. Toxicol. 2013, 59, 215–221. [Google Scholar] [CrossRef]
- Ekmekcioglu, C. A physiological approach for preparing and conducting intestinal bioavailability studies using experimental systems. Food Chem. 2002, 76, 225–230. [Google Scholar] [CrossRef]
- Seithel, A.; Karlsson, J.; Hilgendorf, C.; Bj, A.; Ungell, A.-L.; Björquist, A.; Ungell, A.-L. Variability in mRNA expression of ABC- and SLC-transporters in human intestinal cells: Comparison between human segments and Caco-2 cells. Eur. J. Pharm. Sci. 2006, 28, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Taipalensuu, J.; Törnblom, H.; Lindberg, G.; Einarsson, C.; Sjöqvist, F.; Melhus, H.; Garberg, P.; Sjöström, B.; Lundgren, B.; Artursson, P. Correlation of Gene Expression of Ten Drug Efflux Proteins of the ATP-Binding Cassette Transporter Family in Normal Human Jejunum and in Human Intestinal Epithelial Caco-2 Cell Monolayers. J. Pharmacol. Exp. Ther. 2001, 299, 164–170. [Google Scholar] [PubMed]
- Lampen, A.; Bader, A.; Bestmann, T.; Winkler, M.; Witte, L.; Borlak, J.T. Catalytic activities, protein- and mRNA-expression of cytochrome P450 isoenzymes in intestinal cell lines. Xenobiotica 1998, 28, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Caloni, F.; Cortinovis, C.; Pizzo, F.; De Angelis, I. Transport of aflatoxin M1 in human intestinal Caco-2/TC7 cells. Front. Pharmacol. 2012, 3, 111. [Google Scholar] [CrossRef]
- Wu, C.; Gao, Y.; Li, S.; Huang, X.; Bao, X.; Wang, J.; Zheng, N. Modulation of intestinal epithelial permeability and mucin mRNA (MUC2, MUC5AC, and MUC5B) expression and protein secretion in Caco-2/HT29-MTX co-cultures exposed to aflatoxin M1, ochratoxin A, and zearalenone individually or collectively. Toxicol. Lett. 2019, 309, 1–9. [Google Scholar] [CrossRef]





| Mycotoxins | Effects | LD50 (mg/kg) | References |
|---|---|---|---|
| T-2 and HT-2 | Inhibition of DNA, RNA and protein synthesis. Induction of mutations and apoptosis. | T-2 Rodents: 5–10 Pig: 5 Chicken: 2–6 Shrimp: 30 Mice: 2–5 HT-2 Rodents: 5–10 | [16,17,18,19,20,21] |
| DON | Inhibition of DNA, RNA and protein synthesis. Decrease of the cell proliferation. | Mice: 46–78 Duck: 27 Chicken: 140 | [22,23,24,25,26] |
| ZEA | Activation of the estrogen receptor. Inhibition of DNA and protein synthesis. Triggering lipid peroxidation and cell death. | Mice: 2000–20,000 Rat: 4000–10,000 Pig: 5000 | [27,28,29] |
| BEA | Increase of the biological membrane permeability. Loss of ionic homeostasis. Induction of lipid peroxidation. | Mice: 100 | [30,31,32] |
| ENNs | Increase of the membrane permeability for cations. | No acute in vivo toxicity data | [32,33] |
| FB1 | Inhibition the activity of ceramide synthase. | >1000 | [34] |
| AOH and AME | Single and double strand DNA breaks. Decrease of the cell proliferation. | Mice: 400 for AOH and AME | [35,36,37] |
| ATXs | DNA strand breaks. | Mice: 0.2 | [37,38] |
| TeA | Inhibition of protein synthesis. Inhibition of photosynthetic activity. | Mice: 81(female), 186–225 (male) Rat: 168 (female), 180 (male) | [39,40,41,42] |
| AFB1 | Damage of DNA Inhibition of protein synthesis through interfering with RNA transcription and translation. Induction of oxidative stress. | Swine: 0.62 Duck: 0.37 Turkey: 0.5–1 Chicken: 6.5–12.5 Quail: 19.5 | [22,43] |
| MPA | Inhibition of inosine 5′-monophosphate dehydrogenase. Blocking of the DNA synthesis and proliferation of both T and B lymphocytes. | Rat: 450 Mice: 1900 | [40,44] |
| OTA | Inhibition the activity of many enzymes which use phenylalanine as a substrate. Disruption of phenylalanine metabolism. Production of reactive oxygen species Lipid peroxidation, cell membranes and DNA damage | Dog: 0.2 Pig: 1 Chicken: 3.3 Rat and mouse: 20–50 | [34,45] |
| Mycotoxins | Commodity | Concentration Range (µg/kg) | Country | References |
|---|---|---|---|---|
| T-2 and HT-2 | Barley grain | 26–787 | Italy | [61,62] |
| Maize | 146 | Hungary | ||
| Cereal-based products | <LOD-209 | Tunisia | ||
| Wheat | 6.7–15.2 | Spain | ||
| DON | Cereal and corn | 96–1790 | Portugal | [63,64] |
| Wheat-based product | 333–1821 | Portugal | ||
| Maize grain | ND-700 | Ethiopia | ||
| Sorghum grain | 40–112 | Ethiopia | ||
| ZEA | Corn | 59–505 | Philippines | [63,64,65] |
| Cereal and corn | 5–930 | Portugal | ||
| Sorghum grain | 7.2–382 | Ethiopia | ||
| BEA | Rice | 3800–26,300 | Morocco | [46] |
| Cereal | 0.1–10,600 | Morocco | ||
| ENN A | Rice | 8400–119,500 | Morocco | [46] |
| ENN A1 | Rice | 56,200–448,700 | Morocco | |
| ENN B | Rice | 4400–26,200 | Morocco | |
| ENN B1 | Rice | 3600–23,700 | Morocco | |
| FB1 | Maize | ND-1106 | Zimbabwe | [3,63] |
| Industrial processed food | 43–836 | Nigeria | ||
| Dried sweet potato chips | 29.34–628.78 | Tanzania | ||
| Corn | 113–1162 | Portugal | ||
| Corn products | 183–2026 | Portugal | ||
| AOH | Tomato sauce | 1.2–20.8 | Europe | [46,64,66] |
| Sunflower oil | 0.7–2.9 | Europe | ||
| Sorghum grain | 75–1090 | Ethiopia | ||
| Cereal | 0.75–832 | Germany | ||
| Fruit juices | 15–100 | Germany | ||
| AME | Tomato sauce | <LOQ-4.7 | Europe | [46,64,66] |
| Sunflower oil | <LOQ-7.1 | Europe | ||
| Sorghum grain | 13–257 | Ethiopia | ||
| Cereal | 0.3–905 | Germany | ||
| Fruit juices | 0.13–4.9 | Germany | ||
| ALT | Tomato products | 6.1–62 | Belgium | [46] |
| Fruit juices | 1.18–18.4 | Germany | ||
| ATXs | Tomato sauce | 0.5–3.7 | Europe | [66] |
| Sunflower oil | 2–4.7 | Europe | ||
| TeA | Tomato sauce | <LOQ-691 | Europe | [46,66] |
| Sunflower oil | 24–458 | Europe | ||
| Fruit juices | 1.1–250 | Germany | ||
| Infant food | 0.8–1200 | Germany | ||
| TEN | Tomato sauce | 0.2–1.2 | Europe | [46,66] |
| Sunflower oil | <LOQ-21.8 | Europe | ||
| Fruit juices | 0.5–10.7 | Germany | ||
| AFB1 | Polished rice | 1–2546 | Philippines | [64,65] |
| Sorghum grain | <7.5–359 | Ethiopia | ||
| PAT | Apples | 3.2–1500 | Portugal | [63] |
| Quince jam | 9.7–28.7 | Portugal | ||
| OTA | Cereals | 0.27–7.97 | Portugal | [63,64,65] |
| Coffee beans | 8-36,561 | Philippines | ||
| Sorghum grain | 3.7–163 | Ethiopia |
| Models | Advantages | Disadvantages |
|---|---|---|
| In vitro models | ||
| Simulation of gastrointestinal transformation | Similar to the physiological processes in the human body Suitable for high-throughput format Ability of testing a specific mechanisms of action Focus on small number of components Validation with reference material | No hormonal and nervous control Lack of feedback mechanisms Absence of mucosal cell activity Deficiency of complexity of peristaltic movements, and involvement of the local immune system Homeostatic mechanisms are not present Difficult to achieve the anaerobic assay conditions |
| Caco-2 cells | Reproducibility of results Provides information about efficiency of digestion, absorption Ability of studying transport mechanisms Phenotypically similar to absorptive epithelial cells Suitable for high-throughput format | Human colonic adenocarcinoma origin Higher TEER value than human intestine Lack of mucin, microflora, biofilms, and epithelial cell types Variation of efflux transporters expression levels Incapability of simulating the changes of pH |
| In vivo models | In vivo condition Well-known biology Selection of specific subjects Better-understanding kinetic of mycotoxins | High-throughput limitation Extremely complex functional systems Influence of different factors-phenotypic variation Lack of certified reference standards Ethical issues and high cost Time consuming and labor intensive |
| Cell line | Origin | Transporters, Enzymes and Other Relevant Proteins | References |
|---|---|---|---|
| Caco-2 | Human colon adenocarcinoma | CYP1A1, 1A2 GST, UGT, SULT, NAT P-gp, MRP-2, BCRP | [76,77,78] |
| HT-29 | Human colon adenocarcinoma | CYP2C8, CYP2J2, CYP3A4 GST, UGT MRP1, MRP2, p-gp, BCRP | [79,80,81] |
| TC-7 | Caco-2 subclones | Similar to Caco-2 | [82] |
| T84 | Human colonic carcinoma | P-gp, MRP2, MRP3 | [83,84] |
| H4 | Human small foetal intestine | CYP3A4 | [85] |
| IPEC-J2 | Neonatal pig small intestine | CYP1A1, 1A2, 3A29 P-gp, MRP1, BCRP | [86,87] |
| Mycotoxins | Induced CYP450 | Phase I Biotransformation | Phase II Biotransformation | References |
|---|---|---|---|---|
| T-2 and HT-2 | CYP3A46, 3A29 and 3A22 in pig CYP1A5, 3A37 in chicken CYP1A1 in human | NEO, 3′-OH-T-2, 3′-OH-HT-2, T-2 triol, T-2 tetraol, and some C12,13-deepoxy products | T-2 glucuronides HT-2 glucuronides | [34,93,94,95,96,97,98,99,100,101] |
| DON | CYP2B1 and 2B2 | DOM-1 | DON-3-gluccoside, DON-, DOM- and DON -3-Glucoside-sulfonates, DON-3-, DON-7-, DON-8- and DON-15- glucuronides | [34,102] |
| ZEA | CYP1A1, 1A2, 2B6, 2C9, 3A4 and 3A5 in human CYP2C7, 2E1, 3A1 and 3A2 in rat | α-ZEA and β-ZEA | ZEA, α-ZEA and β-ZEA-glucuronides ZEA-14-Glucoside, α-ZEA-14-Glucoside, β-ZEA-14-Glucoside, ZEA-14-Sulfate and ZEA-16-Glucoside | [34,103,104,105,106] |
| BEA | CYP3A4/5 and CYP2C19 in human CYP3A1/2 in rat | No metabolites detected | No metabolites detected | [107,108] |
| ENNs | CYP3A4, 2C9, 1A2 in human CYP3A and 1A in rat and dog | M1–M12 with rat, dog and human liver microsomes M1–M5, M9–M13 in chicken | No sulfated or glucuronidated of ENN B and B1 detected | [109,110,111,112,113,114,115] |
| FB1 | CYP 1A1 and 4A1 in rat CYP1B1 in human | HFB1 and pHFB1 | Unknown | [34,116] |
| AOH and AME | CYP1A1 | OH-AOH and OH-AME | AOH-3-glucoside, AOH-9-glucoside and AME-3-glucoside | [117,118,119,120] |
| ATXs | CYP1A1 | ATX I | No metabolites detected | [121] |
| ALT | Unknown | OH-ALT | ALT-glucuronide | [121] |
| TeA | Unknown | No metabolites detected | No metabolites detected | [121] |
| TEN | CYP3A4 | Monooxidized, mono-methylated and di-methylated metabolites | Unknown | [121] |
| AFB1 | CYP1A1, 1A2, 2B6, 2C9, 3A4 and 3A5 in human liver | AFBO, AFM1, AFL, AFQ1 and AFP1 | AFB1-glutathiones, glucuronides and sulfates | [34,122,123] |
| OTA | CYP1A1, 1A2, 2B6, 2C9, 3A4 and 3A5 in human liver | Lactone-open OTA, OTα, OTB, 4-OH-OTA and 10-OH-OTA | OTA-glutathiones, OTA-hexose/pentose, OTA-sulfates | [34,124,125,126,127,128,129] |
| PAT | CYP1A1, 1A2, 2B6, 2C9, 3A4 and 3A5 in human hepatocytes | E-ascladiol, Z-ascladiol, hydroascladiol and deosypatulinic acid | PAT-glutathiones | [34,130] |
| Mycotoxins | Concentration (µM) | Incubation Time (h) | Major Findings | References |
|---|---|---|---|---|
| AOH and AME | 20 | 1–3 | 22.7–25.8% and 3–7.1% applied AOH and AME reached the basolateral compartment (including their metabolites). | [119] |
| ATXs | 10 | 0.5 | 6% and 0.3% applied ATX I and ATX II found in basolateral compartment. ATX I were not metabolized. 13 and 4% metabolites of ATX II found in apical and basolateral compartments. | [201] |
| AFB1 | 1–25 | 24–48 | CYP1A2 and 3A4 were the main CYP450 isoforms for AFB1 activation into the genotoxic metabolite aflatoxin-exo-7-8-epoxyde. | [9] |
| AFB1, FB1, OTA and T-2 | 100 | 24 | AFB1, FB1, T2 and OTA disrupted the intestinal barrier permeability. | [198] |
| BEA | 1.5–3 | 4 | Bioavailability was from 50.1–54.3 for BEA | |
| DON | 5-30 | 24 | DON transcellular passage was either by passive/facilitated diffusion or by active transport. DON was a substrate for both P-gp and MRP2. | [202] |
| ENNs | 1.5–3 | 4 48 | Duodenal bioavailability: 57.7–76.8% for ENN A, 68.8–70.2% for ENN A1, 65.0–67.0% for ENN B, and 62.2–65.1% for ENN B1. Colonic bioavailability: 17.3–33.3% for ENN A, 40.8–50.0% for ENN A1, 47.7–55.0% for ENN B, and 52.4–57.4% for ENN B1 | [67] |
| MPA | 0–780 | - | Decrease in the barrier function of Caco-2 cell monolayer. | [9] |
| NIV | 5 | 6 | Bioavailability: 32.6% NIV would not be metabolized in Caco-2 cells. NIV was a substrate for P-gp and MRP2. | [203] |
| OTA | 1–100 5–45 | 1 3–24 | OTA was a substrate for MRP2 and BCRP Metabolites were OTB, OTA methyl ester, OTA ethyl ester and the OTA glutathione conjugate. | [204] [205] |
| ZEA | 25 | 4 | ZEA was substrates for ABCC1, ABCC2 and metabolites into α- and β-zearalenol and glucuronides. | [206] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, V.N.; Viktorová, J.; Ruml, T. Mycotoxins: Biotransformation and Bioavailability Assessment Using Caco-2 Cell Monolayer. Toxins 2020, 12, 628. https://doi.org/10.3390/toxins12100628
Tran VN, Viktorová J, Ruml T. Mycotoxins: Biotransformation and Bioavailability Assessment Using Caco-2 Cell Monolayer. Toxins. 2020; 12(10):628. https://doi.org/10.3390/toxins12100628
Chicago/Turabian StyleTran, Van Nguyen, Jitka Viktorová, and Tomáš Ruml. 2020. "Mycotoxins: Biotransformation and Bioavailability Assessment Using Caco-2 Cell Monolayer" Toxins 12, no. 10: 628. https://doi.org/10.3390/toxins12100628
APA StyleTran, V. N., Viktorová, J., & Ruml, T. (2020). Mycotoxins: Biotransformation and Bioavailability Assessment Using Caco-2 Cell Monolayer. Toxins, 12(10), 628. https://doi.org/10.3390/toxins12100628