Functional Profile of Antigen Specific CD4+ T Cells in the Immune Response to Phospholipase A1 Allergen from Polybia paulista Venom
Abstract
:1. Introduction
2. Results
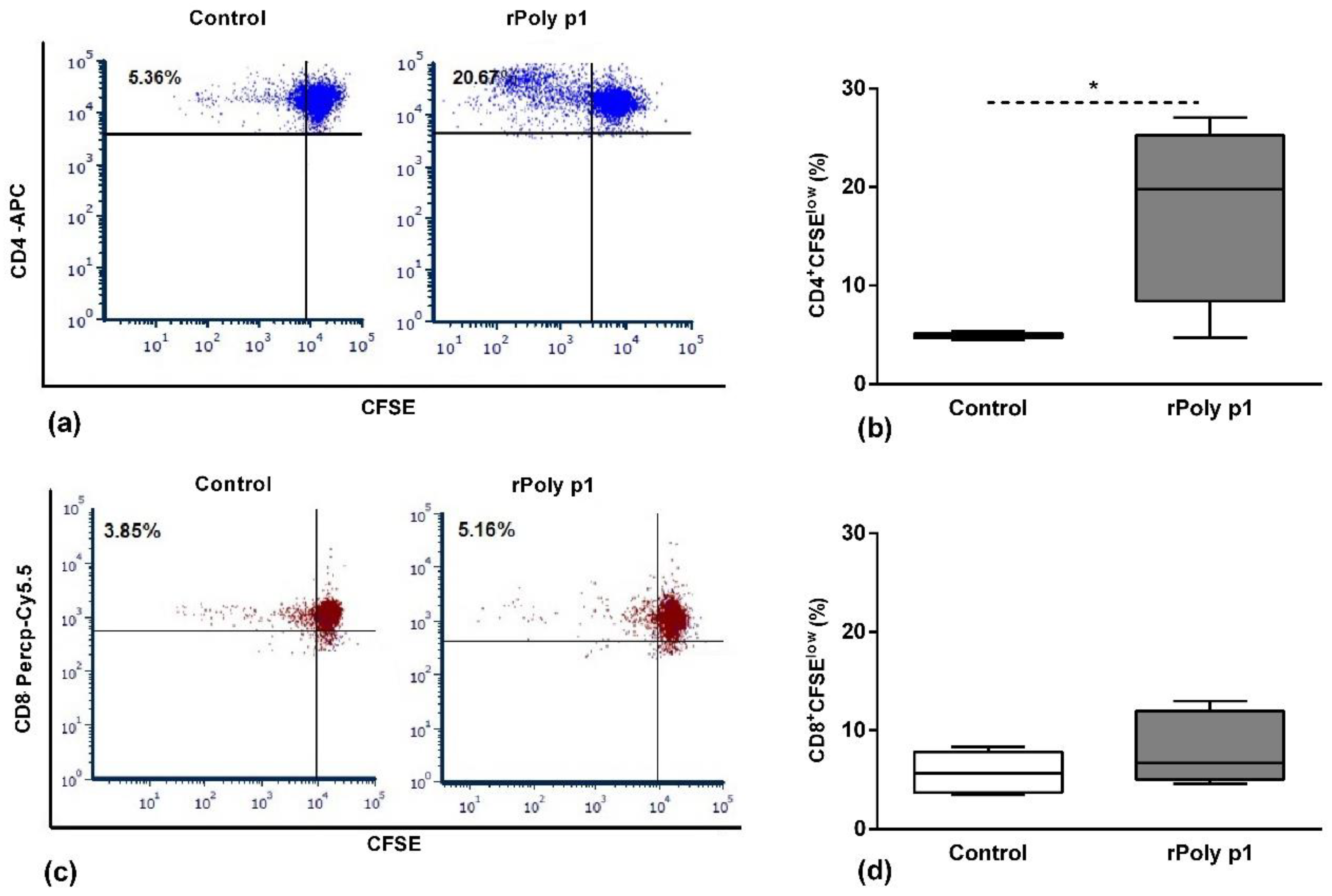
2.1. Antigen-Specific T Cell Proliferation
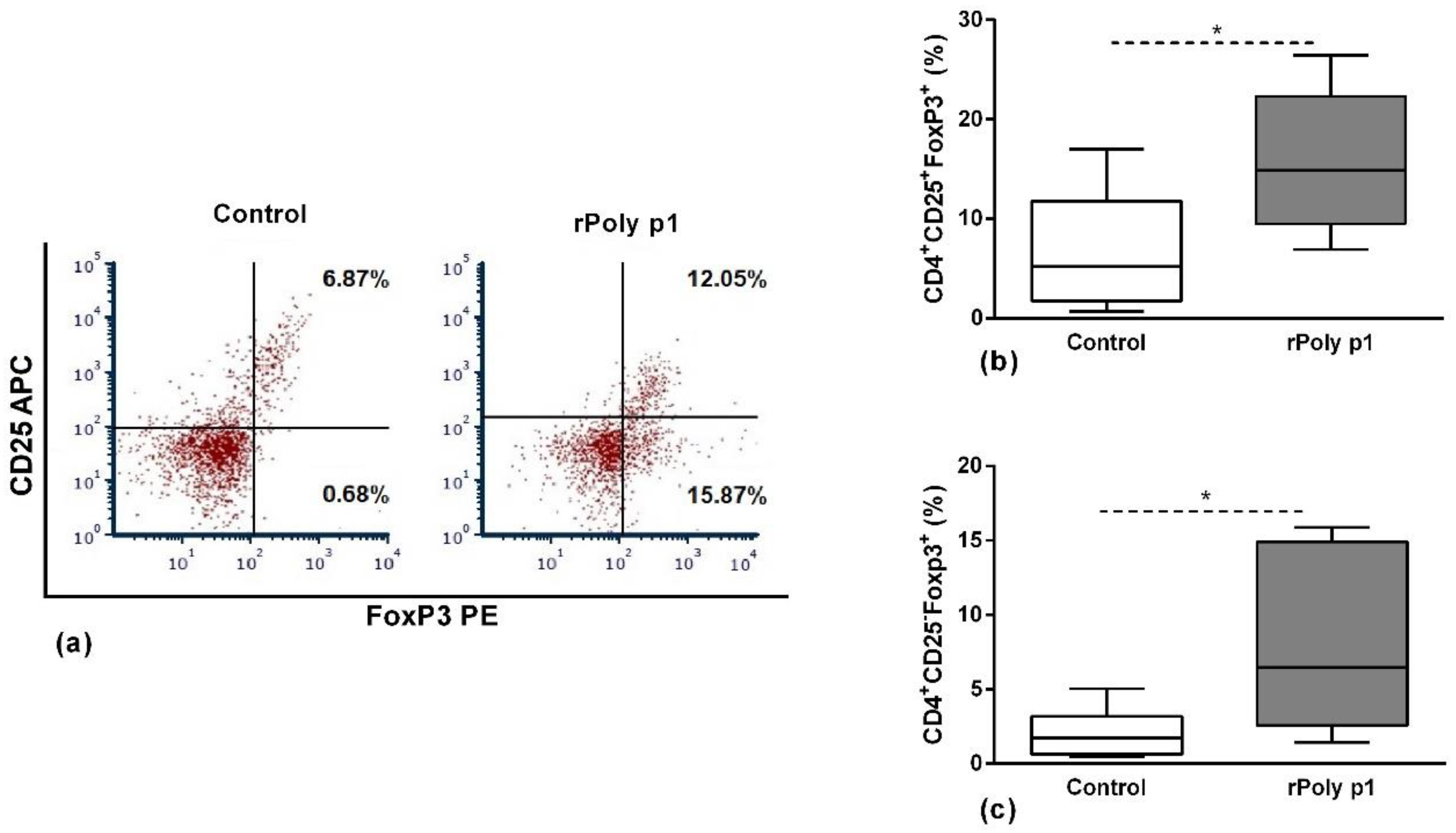
2.2. Antigen-Specific CD4+ T Cells Functional Profile
3. Discussion
4. Conclusions
5. Materials and Methods
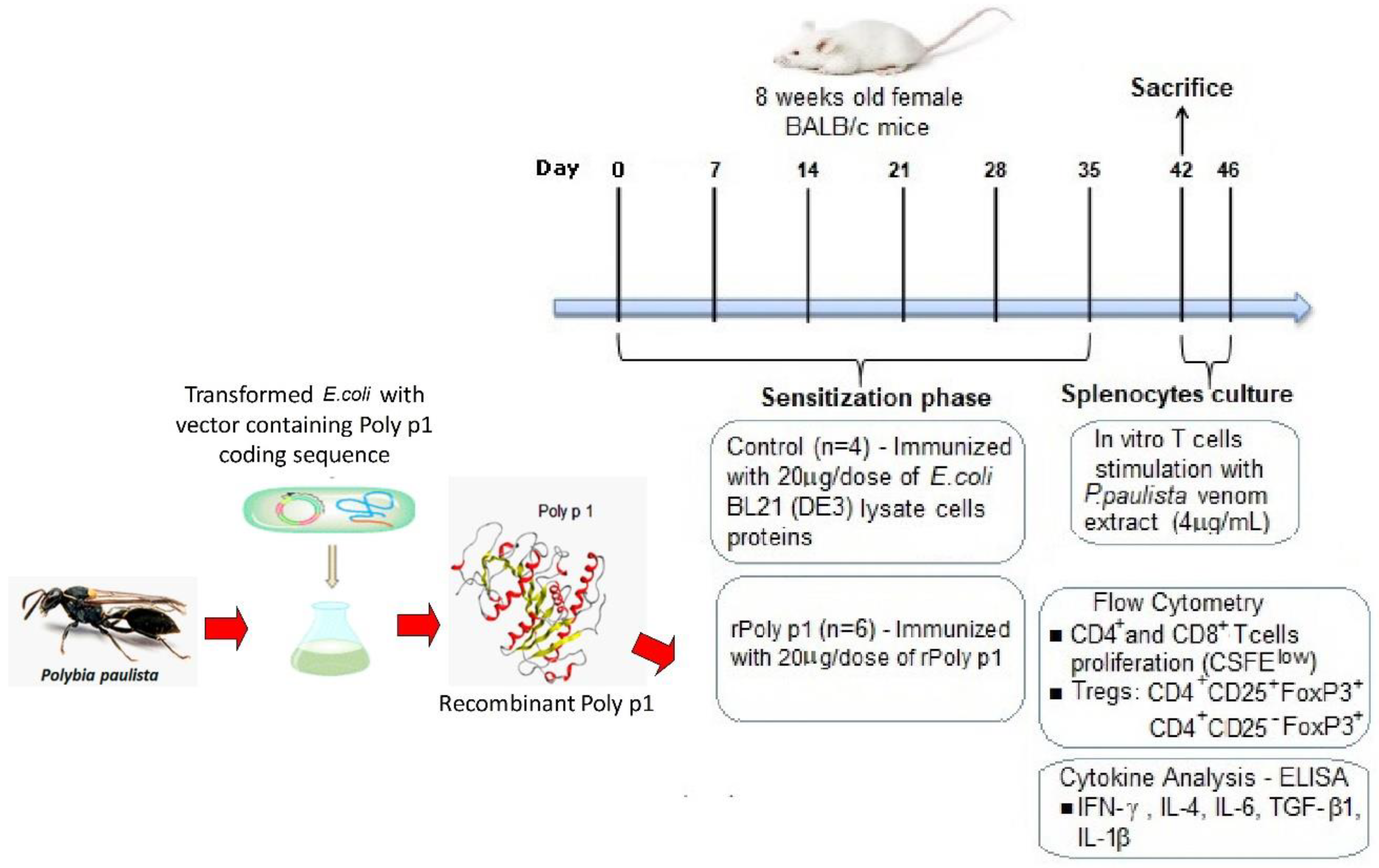
5.1. Animals
5.2. Polybia Paulista Venom Extracts and Recombinant PLA1
5.3. Mice Sensitization
5.4. Antigen-Specific T Cell Proliferation Assay
5.5. Measurement of Cytokines
5.6. Flow Cytometry
5.7. Statistical Analyses
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Supplementary Data


References
- Sole, D.; Ivancevich, J.C.; Borges, M.S.; Coelho, M.A.; Rosario, N.A.; Ardusso, L.R.F.; Bernd, L.A.G. Anaphylaxis in Latin America: A report of the online Latin American survey on anaphylaxis (OLASA). Clinics 2011, 66, 943–947. [Google Scholar] [CrossRef] [Green Version]
- Peters, R.S.; Krogmann, L.; Mayer, C.; Donath, A.; Gunkel, S.; Meusemann, K.; Kozlov, A.; Podsiadlowski, L.; Petersen, M.; Lanfear, R.; et al. Evolutionary History of the Hymenoptera. Curr. Biol. 2017, 27, 1013–1018. [Google Scholar] [CrossRef] [Green Version]
- Locher, G.A.; Togni, O.C.; Silveira, O.T.; Giannotti, E. The social wasp fauna of a riparian forest in southeastern Brazil (hymenoptera, vespidae). Sociobiology 2014, 61, 225–233. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos-Pinto, J.R.A.; Perez-Riverol, A.; Lasa, A.M.; Palma, M.S. Diversity of peptidic and proteinaceous toxins from social Hymenoptera venoms. Toxicon 2018, 148, 172–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larché, M.; Akdis, C.A.; Valenta, R. Immunological mechanisms of allergen-specific immunotherapy. Nat. Rev. Immunol. 2006, 6, 761–771. [Google Scholar] [CrossRef]
- Jakob, T.; Müller, U.; Helbling, A.; Spillner, E. Component resolved diagnostics for hymenoptera venom allergy. Curr. Opin. Allergy Clin. Immunol. 2017, 17, 363–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Riverol, A.; dos Santos-Pinto, J.R.A.; Lasa, A.M.; Palma, M.S.; Brochetto-Braga, M.R. Wasp venomic: Unravelling the toxins arsenal of Polybia paulista venom and its potential pharmaceutical applications. J. Proteom. 2017, 161, 88–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, L.D.; Santos, K.S.; de Souza, B.M.; Arcuri, H.A.; Cunha-Neto, E.; Castro, F.M.; Kalil, J.E.; Palma, M.S. Purification, sequencing and structural characterization of the phospholipase A1 from the venom of the social wasp Polybia paulista (Hymenoptera, Vespidae). Toxicon 2007, 50, 923–937. [Google Scholar] [CrossRef]
- Roberto Aparecido dos Santos Pinto, J.; Delazari dos Santos, L.; Andrade Arcuri, H.; Baptista Dias, N.; Sergio Palma, M. Proteomic Characterization of the Hyaluronidase (E.C. 3.2.1.35) from the Venom of the Social Wasp Polybia paulista. Protein Pept. Lett. 2012, 19, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Justo Jacomini, D.L.; Campos Pereira, F.D.; Aparecido dos Santos Pinto, J.R.; dos Santos, L.D.; da Silva Neto, A.J.; Giratto, D.T.; Palma, M.S.; de Lima Zollner, R.; Brochetto Braga, M.R. Hyaluronidase from the venom of the social wasp Polybia paulista (Hymenoptera, Vespidae): Cloning, structural modeling, purification, and immunological analysis. Toxicon 2013, 64, 70–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dos Santos-Pinto, J.R.A.; Dos Santos, L.D.; Andrade Arcuri, H.; Castro, F.M.; Kalil, J.E.; Palma, M.S. Using proteomic strategies for sequencing and post-translational modifications assignment of antigen-5, a major allergen from the venom of the social wasp Polybia paulista. J. Proteome Res. 2014, 13, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Perez-Riverol, A.; Campos Pereira, F.D.; Musacchio Lasa, A.; Romani Fernandes, L.G.; dos Santos-Pinto, J.R.A.; Justo-Jacomini, D.L.; Oliveira de Azevedo, G.; Bazon, M.L.; Palma, M.S.; de Lima Zollner, R.; et al. Molecular cloning, expression and IgE-immunoreactivity of phospholipase A1, a major allergen from Polybia paulista (Hymenoptera: Vespidae) venom. Toxicon 2016, 124, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Jakob, T.; Rafei-Shamsabadi, D.; Spillner, E.; Müller, S. Diagnostics in Hymenoptera venom allergy: Current concepts and developments with special focus on molecular allergy diagnostics. Allergo J. Int. 2017, 26, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Perez-Riverol, A.; Justo-Jacomini, D.L.; de Lima Zollner, R.; Brochetto-Braga, M.R. Facing hymenoptera venom allergy: From natural to recombinant allergens. Toxins 2015, 7, 2551–2570. [Google Scholar] [CrossRef] [PubMed]
- Perez-Riverol, A.; Fernandes, L.G.R.; Musacchio Lasa, A.; dos Santos-Pinto, J.R.A.; Moitinho Abram, D.; Izuka Moraes, G.H.; Jabs, F.; Miehe, M.; Seismman, H.; Palma, M.S.; et al. Phospholipase A1-based cross-reactivity among venoms of clinically relevant Hymenoptera from Neotropical and temperate regions. Mol. Immunol. 2018, 93, 87–93. [Google Scholar] [CrossRef]
- Akdis, C.A.; Akdis, M. Mechanisms of allergen-specific immunotherapy and immune tolerance to allergens. World Allergy Organ. J. 2015, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Steinke, J.W.; Lawrence, M.G. T-cell biology in immunotherapy. Ann. Allergy Asthma Immunol. 2014, 112, 195–199. [Google Scholar] [CrossRef] [Green Version]
- Golden, D.B.K. Anaphylaxis to insect stings. Immunol. Allergy Clin. N. Am. 2015, 35, 287–302. [Google Scholar] [CrossRef]
- Antonicelli, L.; Bilò, M.B.; Bonifazi, F. Epidemiology of hymenoptera allergy. Curr. Opin. Allergy Clin. Immunol. 2002, 2, 341–346. [Google Scholar] [CrossRef]
- Akdis, M.; Akdis, C.A. Mechanisms of allergen-specific immunotherapy: Multiple suppressor factors at work in immune tolerance to allergens. J. Allergy Clin. Immunol. 2014, 133, 621–631. [Google Scholar] [CrossRef]
- Palomares, O.; Akdis, M.; Martín-Fontecha, M.; Akdis, C.A. Mechanisms of immune regulation in allergic diseases: The role of regulatory T and B cells. Immunol. Rev. 2017, 278, 219–236. [Google Scholar] [CrossRef] [PubMed]
- Calzada, D.; Baos, S.; Cremades-Jimeno, L.; Cárdaba, B. Immunological mechanisms in allergic diseases and allergen tolerance: The role of Treg cells. J. Immunol. Res. 2018, 2018, 6012053. [Google Scholar] [CrossRef]
- Sahiner, U.M.; Durham, S.R. Hymenoptera venom allergy: How does venom immunotherapy prevent anaphylaxis from bee and wasp stings? Front. Immunol. 2019, 10, 01959. [Google Scholar] [CrossRef]
- Dhami, S.; Zaman, H.; Varga, E.M.; Sturm, G.J.; Muraro, A.; Akdis, C.A.; Antolín-Amérigo, D.; Bilò, M.B.; Bokanovic, D.; Calderon, M.A.; et al. Allergen immunotherapy for insect venom allergy: A systematic review and meta-analysis. Allergy Eur. J. Allergy Clin. Immunol. 2017, 72, 342–365. [Google Scholar] [CrossRef] [Green Version]
- Mosbech, H.; Muller, U. Side-effects of insect venom immunotherapy: Results from an EAACI multicenter study. Allergy Eur. J. Allergy Clin. Immunol. 2000, 55, 1005–1010. [Google Scholar] [CrossRef] [PubMed]
- Winkler, B.; Bolwig, C.; Seppälä, U.; Spangfort, M.D.; Ebner, C.; Wiedermann, U. Allergen-specific immunosuppression by mucosal treatment with recombinant Ves v 5, a major allergen of Vespula vulgaris venom, in a murine model of wasp venom allergy. Immunology 2003, 110, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Kochoumian, L.; King, T.P. Sequence identity and antigenic cross-reactivity of white face hornet venom allergen, also a hyaluronidase, with other proteins. J. Biol. Chem. 1995, 270, 4457–4465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kochoumian, L.; Lu, G. Murine T and B cell responses to natural and recombinant hornet venom allergen Dol m 5.02 and its recombinant fragments. J. Immunol. 1995, 154, 577–584. [Google Scholar]
- Bohle, B.; Zwölfer, B.; Fischer, G.F.; Seppälä, U.; Kinaciyan, T.; Bolwig, C.; Spangfort, M.D.; Ebner, C. Characterization of the human T cell response to antigen 5 from Vespula vulgaris (Ves v 5). Clin. Exp. Allergy 2005, 35, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Kerstan, A.; Albert, C.; Klein, D.; Bröcker, E.B.; Trautmann, A. Wasp venom immunotherapy induces activation and homing of CD4 +CD25+ forkhead box protein 3-positive regulatory T cells controlling TH1 responses. J. Allergy Clin. Immunol. 2011, 127, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Coleman, M.M.; Finlay, C.M.; Moran, B.; Keane, J.; Dunne, P.J.; Mills, K.H.G. The immunoregulatory role of CD4 +FoxP3 +CD25-regulatory T cells in lungs of mice infected with Bordetella pertussis. FEMS Immunol. Med. Microbiol. 2012, 64, 413–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishioka, T.; Shimizu, J.; Iida, R.; Yamazaki, S.; Sakaguchi, S. CD4 + CD25 + Foxp3 + T Cells and CD4 + CD25 − Foxp3 + T Cells in Aged Mice. J. Immunol. 2006, 176, 6586–6593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujimoto, M.; Nakano, M.; Terabe, F.; Kawahata, H.; Ohkawara, T.; Han, Y.; Ripley, B.; Serada, S.; Nishikawa, T.; Kimura, A.; et al. The Influence of Excessive IL-6 Production In Vivo on the Development and Function of Foxp3 + Regulatory T Cells. J. Immunol. 2011, 186, 32–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Cytokine (pg/mL) | Control 1 (n = 4) | rPoly p1 1 (n = 6) | Statistical Significance 2 |
|---|---|---|---|
| IFN-γ | 135.30 ± 74.67 | 1040 ± 172.50 | p = 0.0006 * |
| IL-4 | 4.36 ± 0.44 | 4.99 ± 0.37 | p = 0.28 |
| IL-6 | 37.22 ± 19.25 | 265.10 ± 57.48 | p = 0.0048 * |
| IL-1β | 5.94 ± 0.48 | 6.35 ± 1.06 | p = 0.91 |
| TGF-β1 | 643.8 ± 140.4 | 488.3 ± 145.2 | p = 0.474 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, L.G.R.; Perez-Riverol, A.; Bazon, M.L.; Abram, D.M.; Brochetto-Braga, M.R.; Zollner, R.d.L. Functional Profile of Antigen Specific CD4+ T Cells in the Immune Response to Phospholipase A1 Allergen from Polybia paulista Venom. Toxins 2020, 12, 379. https://doi.org/10.3390/toxins12060379
Fernandes LGR, Perez-Riverol A, Bazon ML, Abram DM, Brochetto-Braga MR, Zollner RdL. Functional Profile of Antigen Specific CD4+ T Cells in the Immune Response to Phospholipase A1 Allergen from Polybia paulista Venom. Toxins. 2020; 12(6):379. https://doi.org/10.3390/toxins12060379
Chicago/Turabian StyleFernandes, Luís Gustavo Romani, Amilcar Perez-Riverol, Murilo Luiz Bazon, Débora Moitinho Abram, Márcia Regina Brochetto-Braga, and Ricardo de Lima Zollner. 2020. "Functional Profile of Antigen Specific CD4+ T Cells in the Immune Response to Phospholipase A1 Allergen from Polybia paulista Venom" Toxins 12, no. 6: 379. https://doi.org/10.3390/toxins12060379




