Multigenerational Consequences of Prenatal Exposure to Benzophenone-3 Demonstrate Sex- and Region-Dependent Neurotoxic and Pro-Apoptotic Effects in Mouse Brain
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals and Treatment
2.3. Assessment of Lactate Dehydrogenase (LDH) Level
2.4. Assessment of Hydrogen Peroxide (H2O2) Concentration
2.5. Assessment of Caspase-8 Activity
2.6. Assessment of Caspase-3 Activity
2.7. qPCR Analysis of the Apoptosis-Related Factors
2.8. Western Blot Analysis of the Apoptosis-Related Factors
2.9. Data Analysis
3. Results
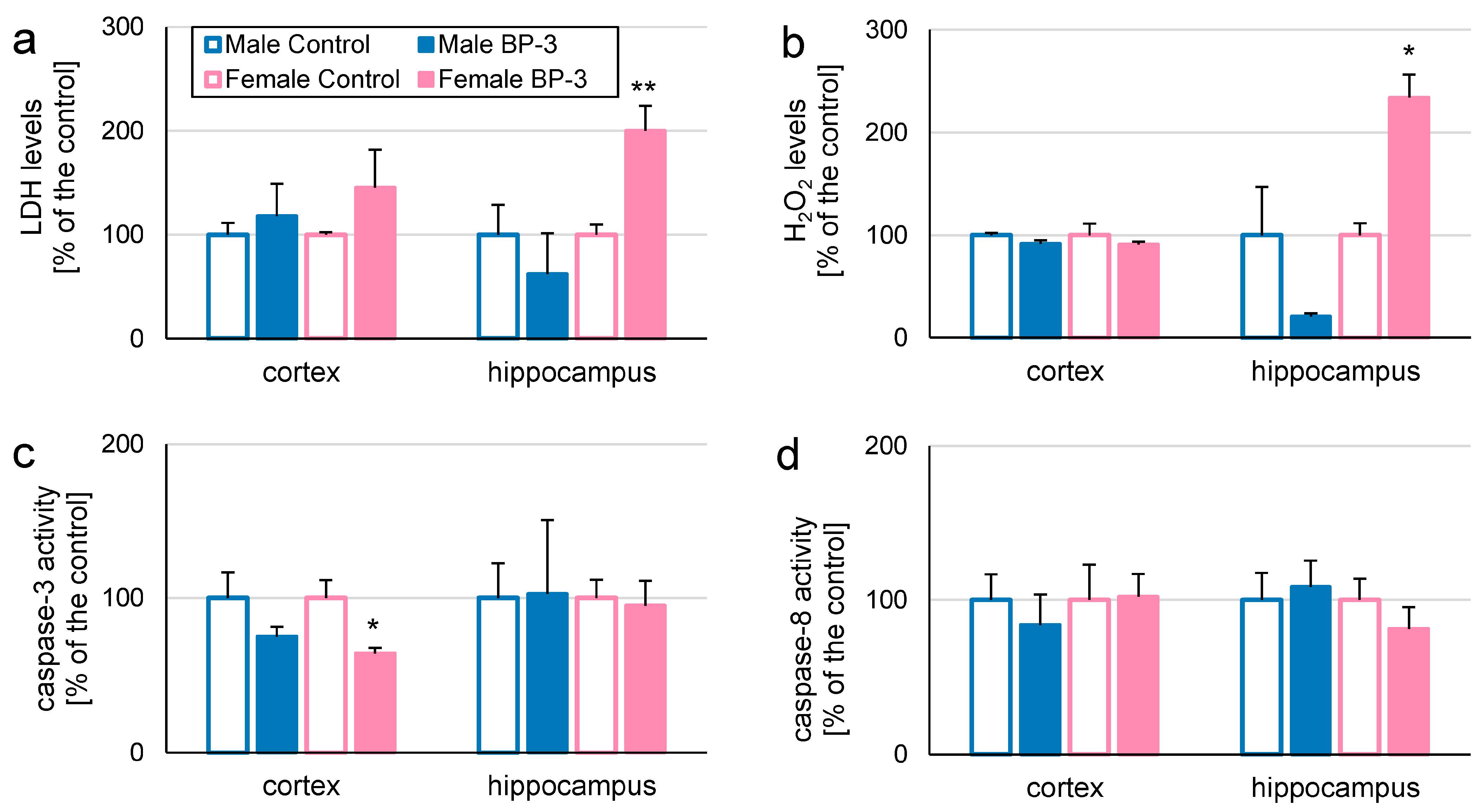
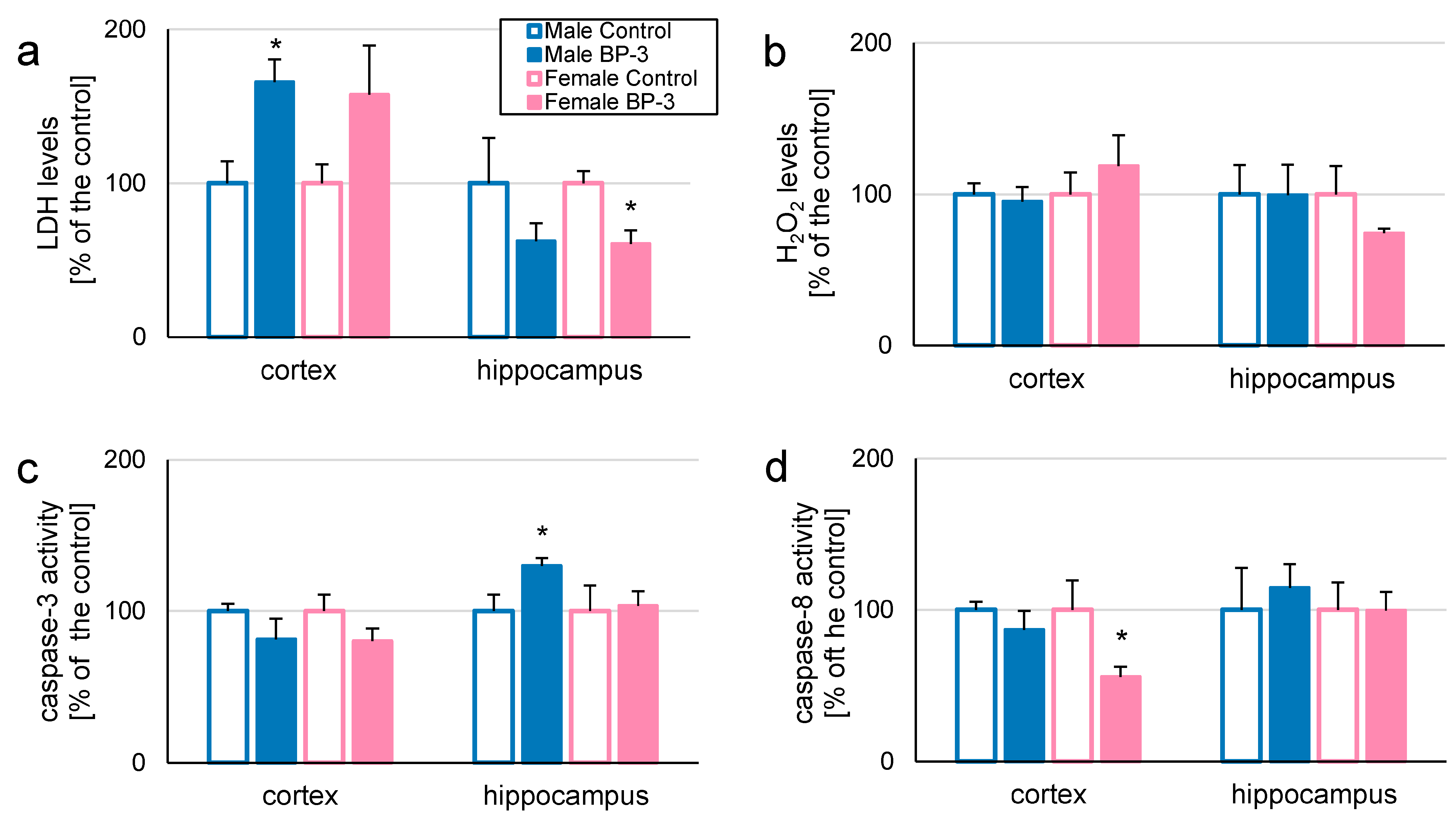
3.1. In 3-Month-Old Offspring, Prenatal Exposure to BP-3 Elevates LDH and H2O2 Levels in Female Hippocampi, but Decreases Caspase-3 Activity in Female Cerebral Cortices
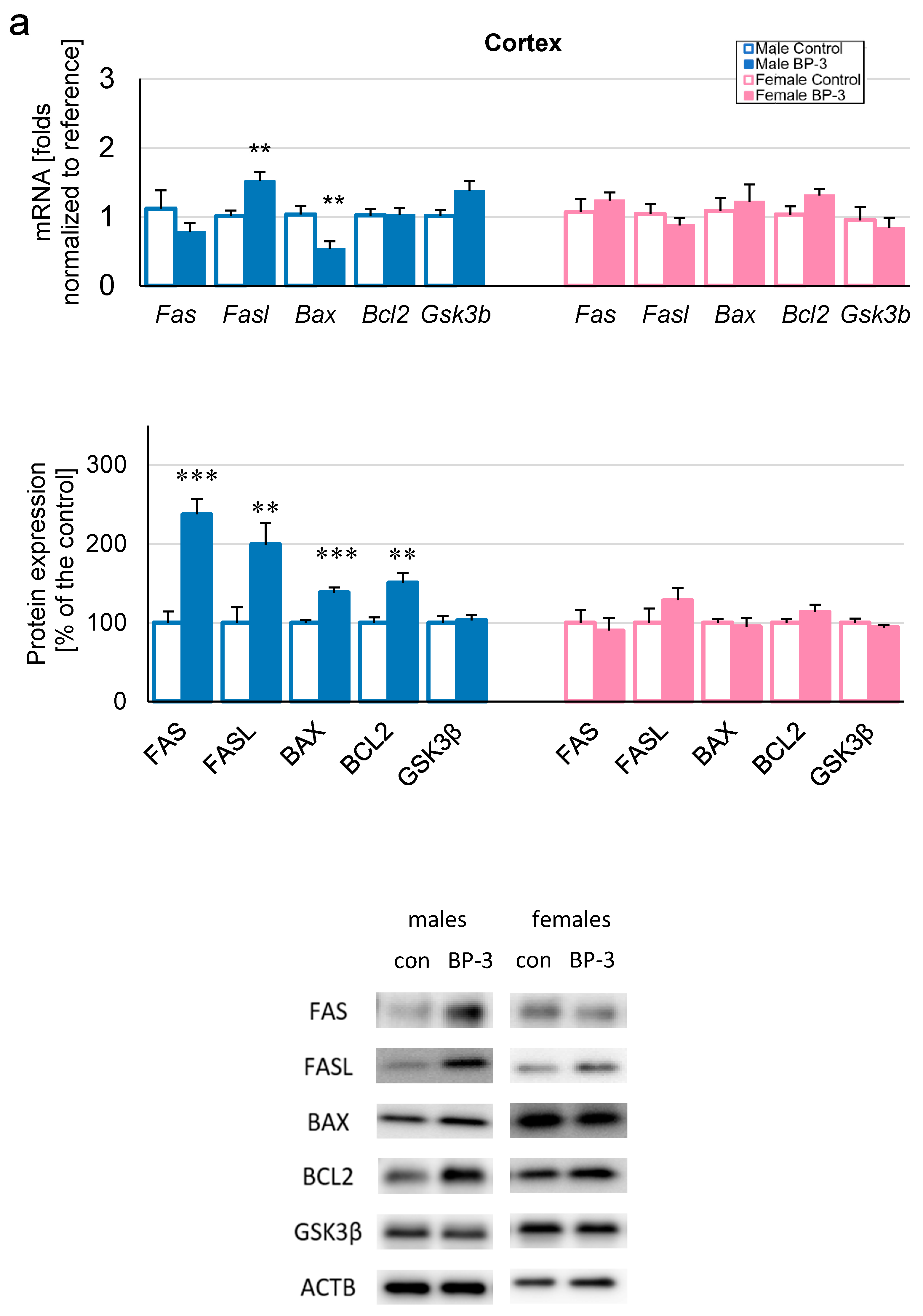
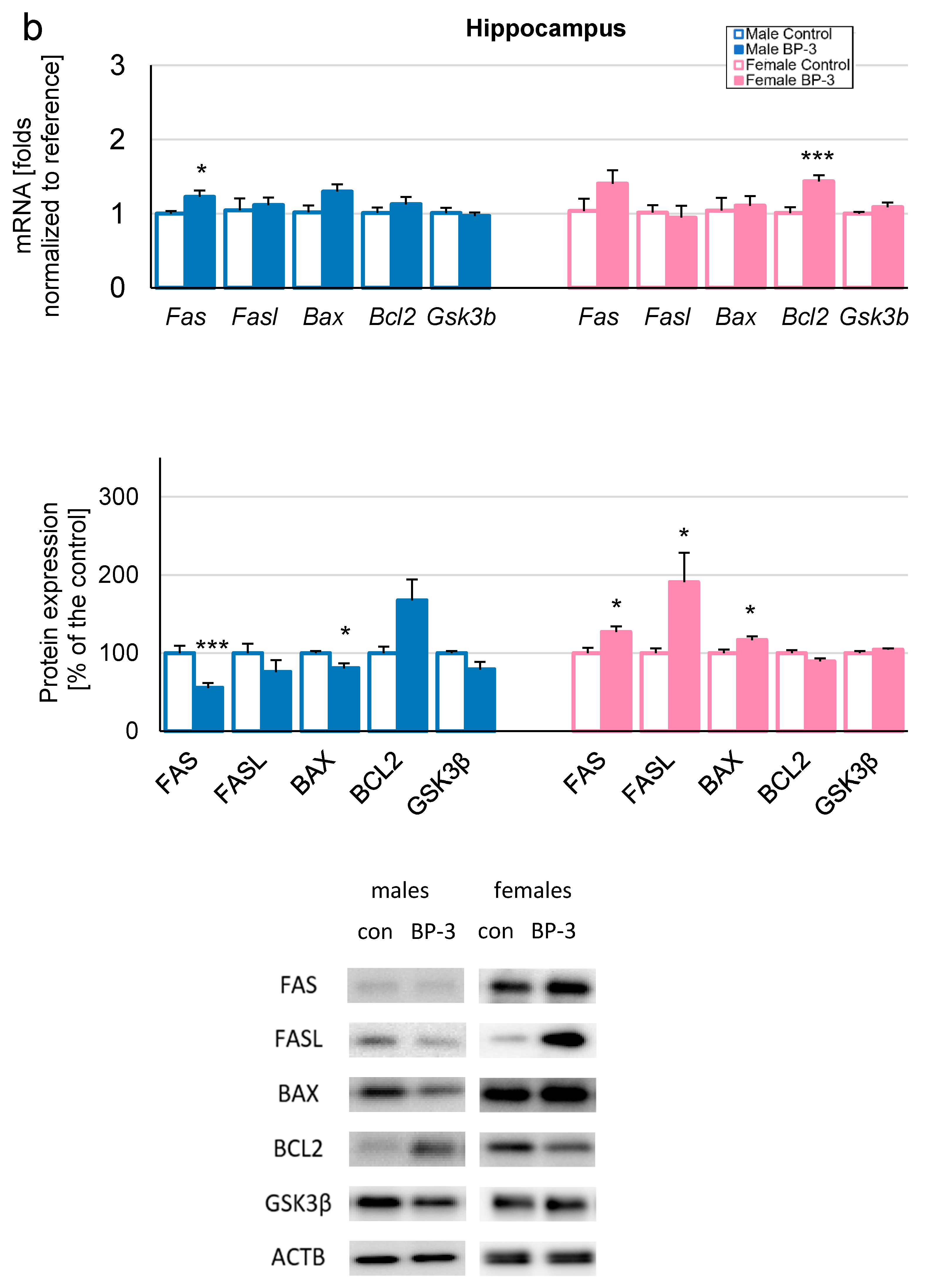
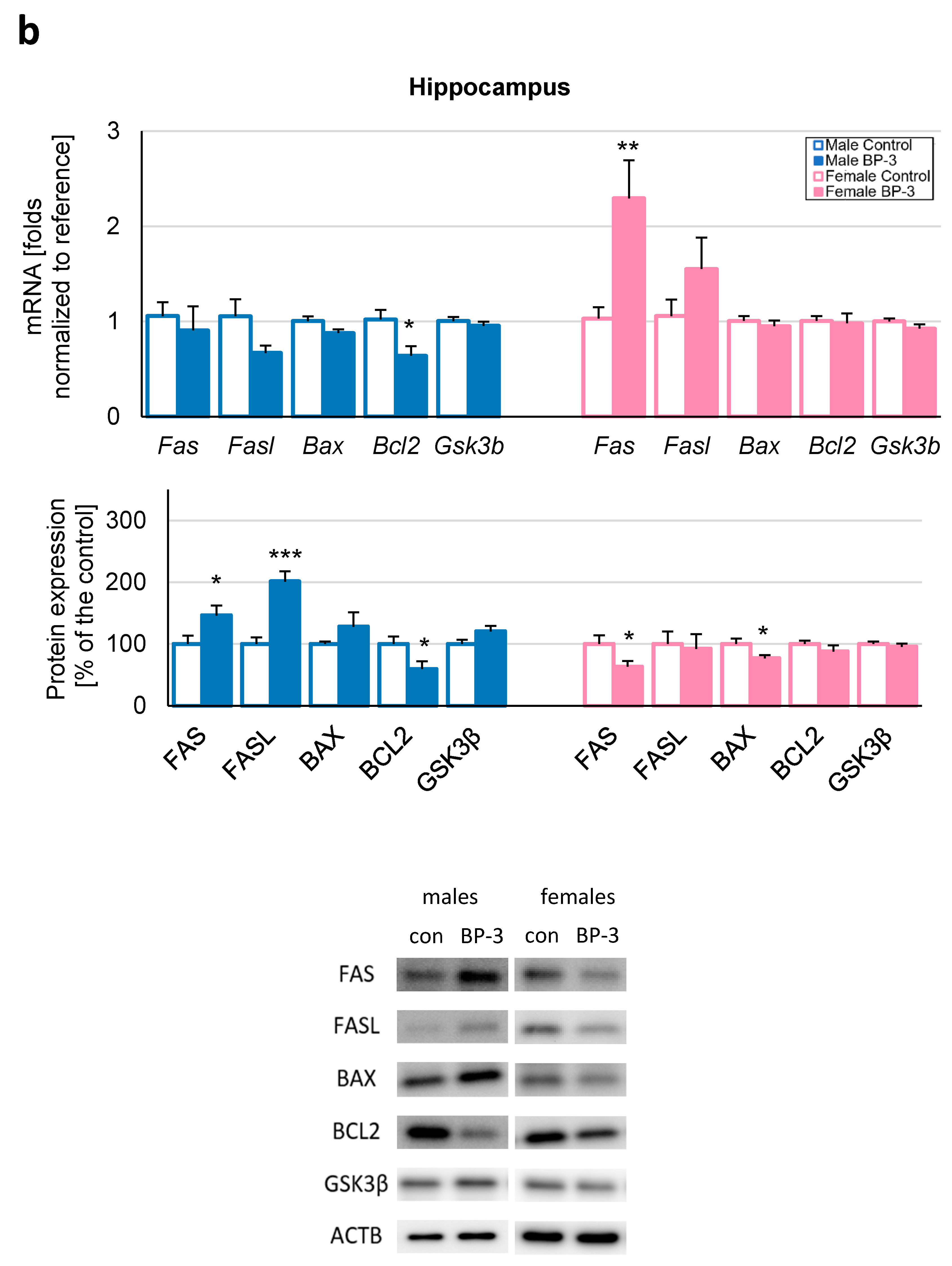
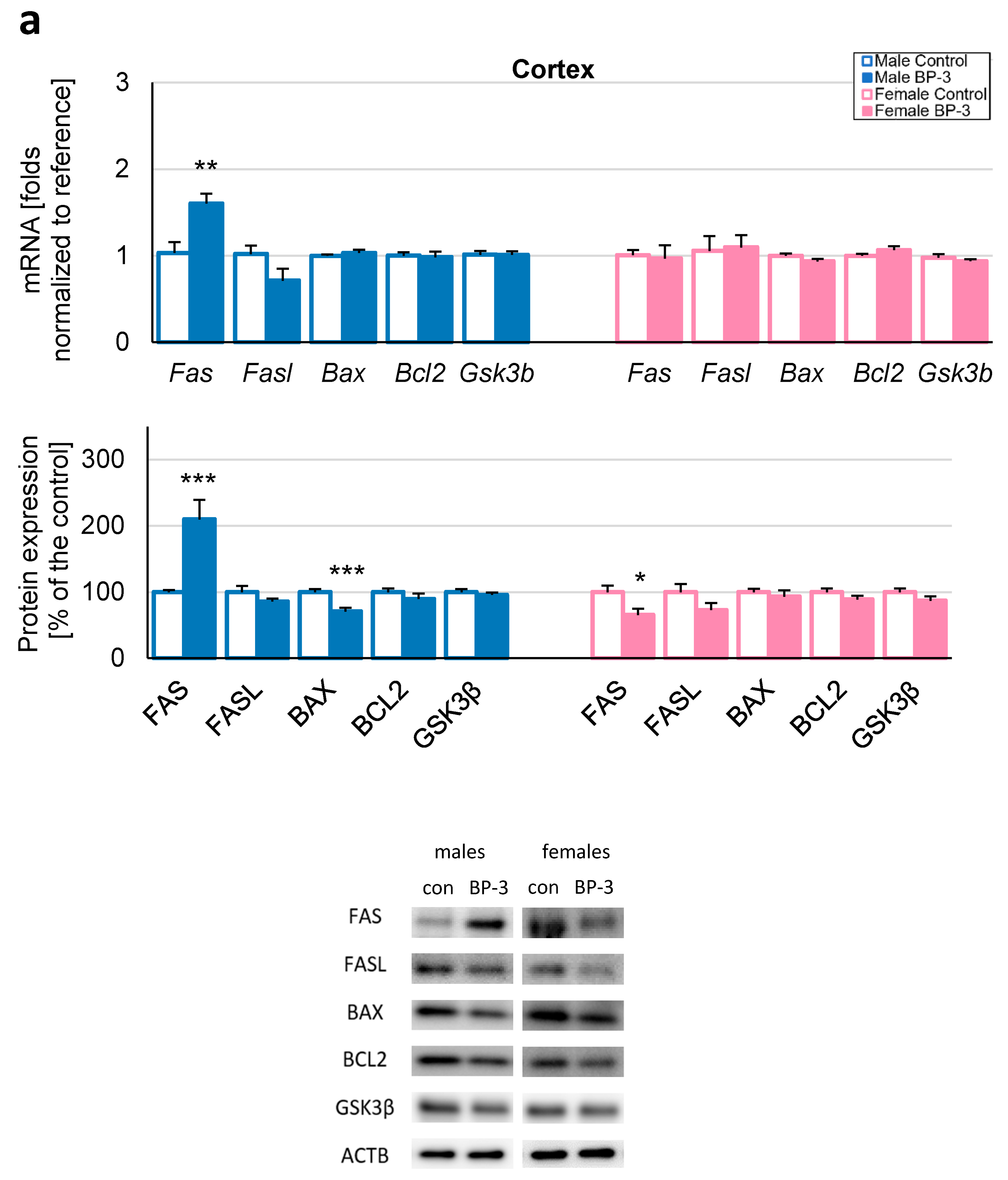
3.2. Prenatal Exposure to BP-3 Causes Pronounced Changes in the Expression of Apoptosis-Related Factors in Male Cerebral Cortices and Hippocampi, and Female Hippocampi of 3-Month-Old Offspring
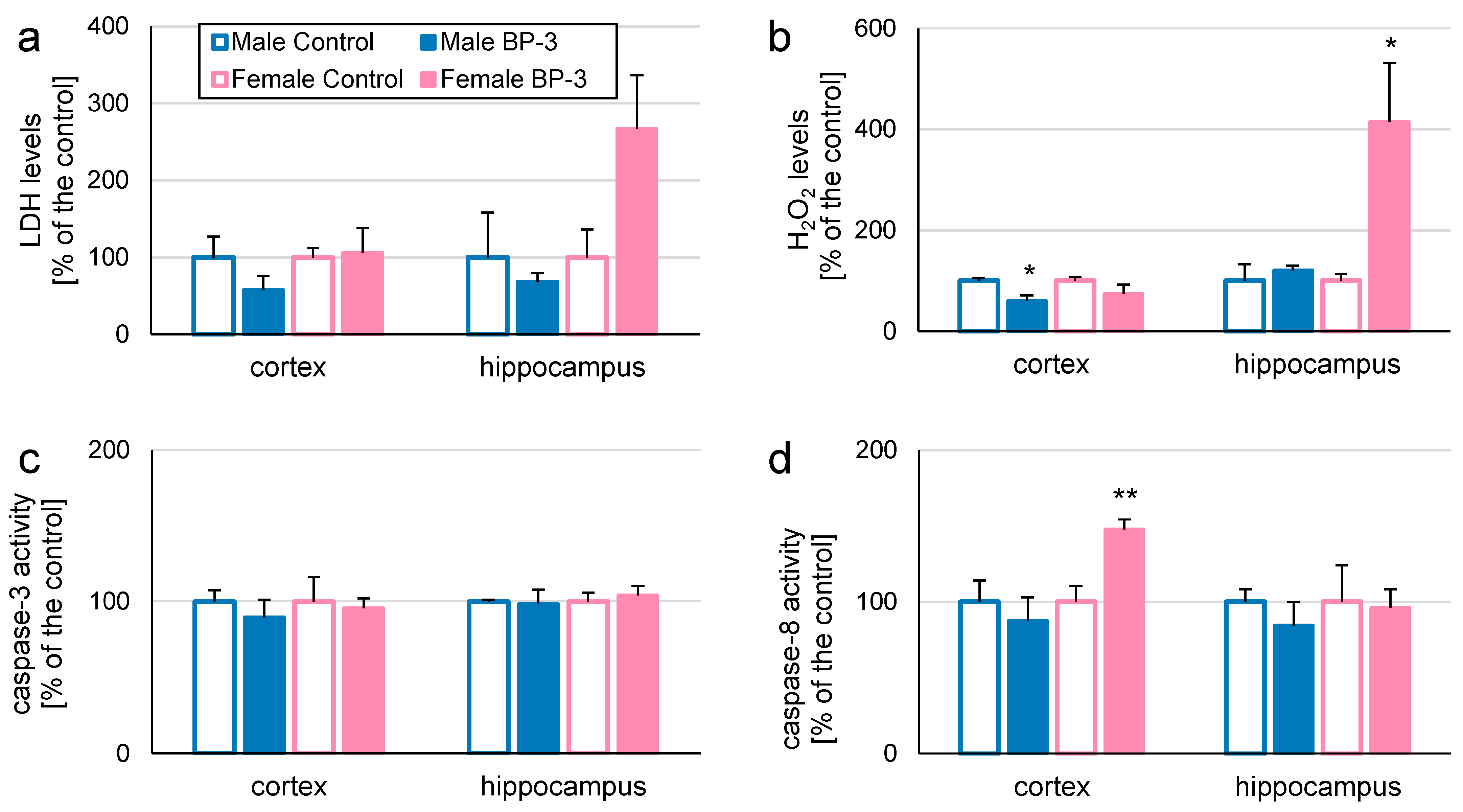
3.3. Prenatal Exposure to BP-3 Alters H2O2 Levels and Caspase-8 Activity in Cerebral Cortices and/or Hippocampi of 5-Month-Old Offspring
3.4. Prenatal Exposure to BP-3 Changes Expression of Apoptosis-Related Factors in Cerebral Cortices and/or Hippocampi of 5-Month-Old Offspring
3.5. Transgenerational Effects of Exposure to BP-3 Alters LDH, Caspase-3, and Caspase-8 Activity in Cerebral Cortices and/or Hippocampi of 1-Month-Old Mice
3.6. Transgenerational Effects of Exposure to BP-3 Changes Expression of Apoptosis-Related Factors in Cerebral Cortices and/or Hippocampi of 1-Month-Old Individuals
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Watson, M.; Holman, D.M.; Maguire-Eisen, M. Ultraviolet Radiation Exposure and Its Impact on Skin Cancer Risk. Seminars Oncol. Nurs. 2016, 32, 241–254. [Google Scholar] [CrossRef]
- Hasbani, D.J.; Ghaoui, N.; Haddad, F.; Shahla, W.A.; Saade, S.; Bandali, T.; Menhem, Z.; Saade, D. Sun Protection Knowledge, Attitudes and Practices: A Lebanese-Based Survey. JEADV Clin. Pract. 2024, 3, 565–574. [Google Scholar] [CrossRef]
- Afiouni, R.; Helou, J.; Bou-Orm, I. Knowledge of the Risks of Ultraviolet Radiation, Sun Exposure Attitudes and Practices among Lebanese University Students. Prev. Med. Rep. 2024, 47, 102900. [Google Scholar] [CrossRef]
- Tang, X.; Yang, T.; Yu, D.; Xiong, H.; Zhang, S. Current Insights and Future Perspectives of Ultraviolet Radiation (UV) Exposure: Friends and Foes to the Skin and Beyond the Skin. Environ. Int. 2024, 185, 108535. [Google Scholar] [CrossRef] [PubMed]
- Sani, M.A.; Khezerlou, A.; Tavassoli, M.; Abedini, A.H.; McClements, D.J. Development of Sustainable UV-Screening Food Packaging Materials: A Review of Recent Advances. Trends Food Sci. Technol. 2024, 145, 104366. [Google Scholar] [CrossRef]
- Yousif, E.; Haddad, R. Photodegradation and Photostabilization of Polymers, Especially Polystyrene: Review. SpringerPlus 2013, 2, 398. [Google Scholar] [CrossRef] [PubMed]
- Wnuk, A.; Kajta, M. Is the Commonly Used UV Filter Benzophenone-3 a Risk Factor for the Nervous System? Acta Biochim. Pol. 2021, 68, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Choi, K. Occurrences, Toxicities, and Ecological Risks of Benzophenone-3: A Common Component of Organic Sunscreen Products: A Mini-Review. Environ. Int. 2014, 70, 143–157. [Google Scholar] [CrossRef]
- Mustieles, V.; Balogh, R.K.; Axelstad, M.; Montazeri, P.; Márquez, S.; Vrijheid, M.; Draskau, M.K.; Taxvig, C.; Peinado, F.M.; Berman, T.; et al. Benzophenone-3: Comprehensive Review of the Toxicological and Human Evidence with Meta-Analysis of Human Biomonitoring Studies. Environ. Int. 2023, 173, 107739. [Google Scholar] [CrossRef]
- Commission Regulation (EU) 2017/238 of 10 February 2017 Amending Annex VI to Regulation (EC) No 1223/2009 of the European Parliament and of the Council on Cosmetic Products. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32017R0238 (accessed on 8 December 2024).
- Commission Regulation (EU) 2022/1176 of 7 July 2022 Amending Regulation (EC) No 1223/2009 of the European Parliament and of the Council as Regards the Use of Certain UV Filters in Cosmetic Products. Available online: https://eur-lex.europa.eu/eli/reg/2022/1176 (accessed on 8 December 2024).
- Suh, S.; Pham, C.; Smith, J.; Mesinkovska, N.A. The Banned Sunscreen Ingredients and Their Impact on Human Health: A Systematic Review. Int. J. Dermatol. 2020, 59, 1033–1042. [Google Scholar] [CrossRef]
- Du, Y.; Wang, W.-Q.; Pei, Z.-T.; Ahmad, F.; Xu, R.-R.; Zhang, Y.-M.; Sun, L.-W. Acute Toxicity and Ecological Risk Assessment of Benzophenone-3 (BP-3) and Benzophenone-4 (BP-4) in Ultraviolet (UV)-Filters. Int. J. Environ. Res. Public Health 2017, 14, 1414. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Román, M.I.; Casiano-Muñiz, I.M.; Román-Velázquez, F.R. Toxicity of UV Filter Benzophenone-3 in Brine Shrimp Nauplii (Artemia salina) and Zebrafish (Danio rerio) Embryos. J. Xenobiotics 2024, 14, 537–553. [Google Scholar] [CrossRef]
- Wnuk, W.; Michalska, K.; Krupa, A.; Pawlak, K. Benzophenone-3, a Chemical UV-Filter in Cosmetics: Is It Really Safe for Children and Pregnant Women? Postepy Dermatol. Alergol. 2022, 39, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lu, G.; Zhang, P.; Ling, X.; Zhou, R.; Yan, Z.; Liu, J. Influence of Organic Colloids on the Uptake, Accumulation and Effects of Benzophenone-3 in Aquatic Animals. Environ. Sci. Nano 2021, 8, 1366–1378. [Google Scholar] [CrossRef]
- European Chemicals Agency. 2020 Substance Information: Oxybenzone; Annankatu 18; European Chemicals Agency: Helsinki, Finland, 2020. [Google Scholar]
- CDC (Centers for Disease Control and Prevention). 2017 Fourth National Report on Human Exposure to Environmental Chemicals; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2017. Available online: https://stacks.cdc.gov/view/cdc/21813 (accessed on 8 December 2024).
- SCCS (Scientific Committee on Consumer Safety). Opinion on Benzophenone-3 (CAS No 131-57-7, EC No 205-031-5), Preliminary Version of 15 December 2020, Final Version of 30–31 March 2021, SCCS/1625/20. Available online: https://health.ec.europa.eu/system/files/2022-08/sccs_o_247.pdf (accessed on 8 December 2024).
- Ruszkiewicz, J.A.; Pinkas, A.; Ferrer, B.; Peres, T.V.; Tsatsakis, A.; Aschner, M. Neurotoxic Effect of Active Ingredients in Sunscreen Products: A Contemporary Review. Toxicol. Rep. 2017, 4, 245–259. [Google Scholar] [CrossRef]
- Mao, J.F.; Li, W.; Ong, C.N.; He, Y.; Jong, M.C.; Gin, K.Y. Assessment of Human Exposure to Benzophenone-Type UV Filters: A Review. Environ. Int. 2022, 167, 107405. [Google Scholar] [CrossRef]
- Tibbetts, J. Shining a Light on BP-3 Exposure. Environ. Health Perspect. 2008, 116, A306. [Google Scholar] [CrossRef]
- Gustavsson Gonzalez, H.; Farbrot, A.; Larkö, O. Percutaneous Absorption of Benzophenone-3: A Common Component of Topical Sunscreens. Clin. Exp. Dermatol. 2002, 27, 691–694. [Google Scholar] [CrossRef]
- Janjua, N.R.; Kongshoj, B.; Andersson, A.M.; Wulf, H.C. Sunscreens in Human Plasma and Urine after Repeated Whole-body Topical Application. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Parks, C.G.; Meier, H.C.S.; Jusko, T.A.; Wilkerson, J.; Miller, F.W.; Sandler, D.P. Benzophenone-3 and Antinuclear Antibodies in U.S. Adolescents and Adults Ages 12–39 Years. Front. Immunol. 2022, 13, 958527. [Google Scholar] [CrossRef] [PubMed]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Some Chemicals Present in Industrial and Consumer Products, Food and Drinking-Water. IARC Monogr. Eval. Carcinog. Risks Hum. 2013, 101, 9–549. [Google Scholar]
- Yao, Y.N.; Wang, Y.; Zhang, H.; Gao, Y.; Zhang, T.; Kannan, K. A Review of Sources, Pathways, and Toxic Effects of Human Exposure to Benzophenone Ultraviolet Light Filters. Eco-Environ. Health 2024, 3, 30–44. [Google Scholar] [CrossRef]
- Gavrila, A.A.; Dasteridis, I.S.; Tzimas, A.A.; Chatzimitakos, T.G.; Stalikas, C.D. Benzophenones in the Environment: Occurrence, Fate and Sample Preparation in the Analysis. Molecules 2023, 28, 1229. [Google Scholar] [CrossRef]
- Wan, Y.; Xue, J.; Kannan, K. Occurrence of Benzophenone-3 in Indoor Air from Albany, New York, USA, and Its Implications for Inhalation Exposure. Sci. Total Environ. 2015, 537, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Asimakopoulos, A.G.; Kannan, K. Accumulation of 19 Environmental Phenolic and Xenobiotic Heterocyclic Aromatic Compounds in Human Adipose Tissue. Environ. Int. 2015, 78, 45–50. [Google Scholar] [CrossRef]
- Van der Meer, T.P.; Artacho-Cordón, F.; Swaab, D.F.; Struik, D.; Makris, K.C.; Wolffenbuttel, B.H.R.; Frederiksen, H.; Van Vliet-Ostaptchouk, J.V. Distribution of Non-Persistent Endocrine Disruptors in Two Different Regions of the Human Brain. Int. J. Environ. Res. Public Health 2017, 14, 1059. [Google Scholar] [CrossRef] [PubMed]
- Wnuk, A.; Rzemieniec, J.; Staroń, J.; Litwa, E.; Lasoń, W.; Bojarski, A.; Kajta, M. Prenatal Exposure to Benzophenone-3 Impairs Autophagy, Disrupts RXRs/PPARγ Signaling, and Alters Epigenetic and Post-Translational Statuses in Brain Neurons. Mol. Neurobiol. 2019, 56, 4820–4837. [Google Scholar] [CrossRef]
- Pomierny, B.; Krzyżanowska, W.; Broniowska, Ż.; Strach, B.; Bystrowska, B.; Starek-Świechowicz, B.; Maciejska, A.; Skórkowska, A.; Wesołowska, J.; Walczak, M.; et al. Benzophenone-3 Passes Through the Blood-Brain Barrier, Increases the Level of Extracellular Glutamate, and Induces Apoptotic Processes in the Hippocampus and Frontal Cortex of Rats. Toxicol. Sci. 2019, 171, 485–500. [Google Scholar] [CrossRef] [PubMed]
- Maciejska, A.; Pomierny, B.; Krzyżanowska, W.; Starek-Świechowicz, B.; Skórkowska, A.; Budziszewska, B. Mechanism of Microglial Cell Activation in the Benzophenone-3 Exposure Model. Neuroscience 2023, 533, 63–76. [Google Scholar] [CrossRef]
- Vela-Soria, F.; Jiménez-Díaz, I.; Rodríguez-Gómez, R.; Zafra-Gómez, A.; Ballesteros, O.; Navalón, A.; Vílchez, J.; Fernández, M.; Olea, N. Determination of Benzophenones in Human Placental Tissue Samples by Liquid Chromatography-Tandem Mass Spectrometry. Talanta 2011, 85, 1848–1855. [Google Scholar] [CrossRef] [PubMed]
- Philippat, C.; Wolff, M.S.; Calafat, A.M.; Ye, X.; Bausell, R.; Meadows, M.; Stone, J.; Slama, R.; Engel, S.M. Prenatal Exposure to Environmental Phenols: Concentrations in Amniotic Fluidand Variability in Urinary Concentrations During Pregnancy. Environ. Health Perspect. 2013, 121, 1225–1231. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.; Frederiksen, H.; Sundberg, K.; Jørgensen, F.; Jensen, L.; Nørgaard, P.; Jørgensen, C.; Ertberg, P.; Juul, A.; Drzewiecki, K.; et al. Presence of Benzophenones Commonly Used as UV Filtersand Absorbers in Paired Maternal and Fetal Samples. Environ. Int. 2018, 110, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Schlumpf, M.; Kypke, K.; Wittassek, M.; Angerer, J.; Mascher, H.; Mascher, D.; Vökt, C.; Birchler, M.; Lichtensteiger, W. Exposure Patterns of UV Filters, Fragrances, Parabens, Phthalates, Organochlor Pesticides, PBDEs, and PCBs in Human Milk: Correlation of UV Filters with Use of Cosmetics. Chemosphere 2010, 81, 1171–1183. [Google Scholar] [CrossRef] [PubMed]
- Molins-Delgado, D.; Olmo-Campos, M.D.M.; Valeta-Juan, G.; Pleguezuelos-Hernández, V.; Barceló, D.; Díaz-Cruz, M.S. Determination of UV Filters in Human Breast Milk Using Turbulent Flow Chromatography and Babies’ Daily Intake Estimation. Environ. Res. 2018, 161, 532–539. [Google Scholar] [CrossRef]
- Iribarne-Durán, L.M.; Serrano, L.; Peinado, F.M.; Peña-Caballero, M.; Hurtado, J.A.; Vela-Soria, F.; Fernández, M.F.; Freire, C.; Artacho-Cordón, F.; Olea, N. Biomonitoring Bisphenols, Parabens, and Benzophenones in Breast Milk from a Human Milk Bank in Southern Spain. Sci. Tot. Environ. 2022, 830, 154737. [Google Scholar] [CrossRef]
- Wang, L.; Kannan, K. Characteristic Profiles of Benzophenone-3 and Its Derivatives in Urine of Children and Adults from the United States and China. Environ. Sci. Technol. 2013, 47, 12532–12538. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Sun, H.; Qin, X.; Wu, Q.; Zhang, Y.; Ma, J.; Kannan, K. Benzophenone-Type UV Filters in Urine and Blood from Children, Adults, and Pregnant Women in China: Partitioning Between Blood and Urine as Well as Maternal and Fetal Cord Blood. Sci. Total Environ. 2013, 461–462, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Holmboe, S.A.; Henriksen, L.S.; Frederiksen, H.; Andersson, A.-M.; Priskorn, L.; Jørgensen, N.; Juul, A.; Toppari, J.; Skakkebæk, N.E.; Main, K.M. Prenatal Exposure to Phenols and Benzophenones in Relation to Markers of Male Reproductive Function in Adulthood. Front. Endocrinol. 2022, 13, 1071761. [Google Scholar] [CrossRef] [PubMed]
- Wnuk, A.; Kajta, M. Steroid and Xenobiotic Receptor Signalling in Apoptosis and Autophagy of the Nervous System. Int. J. Mol. Sci. 2017, 18, 2394. [Google Scholar] [CrossRef]
- Hollville, E.; Romero, S.E.; Deshmukh, M. Apoptotic Cell Death Regulation in Neurons. FEBS J. 2019, 286, 3276–3298. [Google Scholar] [CrossRef]
- Wnuk, A.; Rzemieniec, J.; Lasoń, W.; Krzeptowski, W.; Kajta, M. Apoptosis Induced by the UV Filter Benzophenone-3 in Mouse Neuronal Cells Is Mediated via Attenuation of Erα/Pparγ and Stimulation of Erβ/Gpr30 Signaling. Mol. Neurobiol. 2018, 55, 2362–2383. [Google Scholar] [CrossRef] [PubMed]
- Wnuk, A.; Rzemieniec, J.; Lasoń, W.; Krzeptowski, W.; Kajta, M. Benzophenone-3 Impairs Autophagy, Alters Epigenetic Status, and Disrupts Retinoid X Receptor Signaling in Apoptotic Neuronal Cells. Mol. Neurobiol. 2018, 55, 5059–5074. [Google Scholar] [CrossRef]
- Wnuk, A.; Rzemieniec, J.; Litwa, E.; Lasoń, W.; Kajta, M. Prenatal Exposure to Benzophenone-3 (BP-3) Induces Apoptosis, Disrupts Estrogen Receptor Expression and Alters the Epigenetic Status of Mouse Neurons. J. Steroid Biochem. Mol. Biol. 2018, 182, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Rudin, C.M.; Thompson, C.B. Apoptosis and Disease: Regulation and Clinical Relevance of Programmed Cell Death. Annu. Rev. Med. 1997, 48, 267–281. [Google Scholar] [CrossRef] [PubMed]
- Margolis, R.L.; Chuang, D.M.; Post, R.M. Programmed Cell Death: Implications for Neuropsychiatric Disorders. Biol. Psychiatry 1994, 35, 946–956. [Google Scholar] [CrossRef]
- Jarskog, L.F.; Glantz, L.A.; Gilmore, J.H.; Lieberman, J.A. Apoptotic Mechanisms in the Pathophysiology of Schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2005, 29, 846–858. [Google Scholar] [CrossRef]
- Dong, D.; Zielke, H.R.; Yeh, D.; Yang, P. Cellular Stress and Apoptosis Contribute to the Pathogenesis of Autism Spectrum Disorder. Autism Res. 2018, 11, 1076–1090. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, D.A.; Genuis, S.J.; Frye, R.E. Environmental Toxicants and Autism Spectrum Disorders: A Systematic Review. Transl. Psychiatry 2014, 4, e360. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.S.; Leung, A.O.W.; Wong, M.H. The Association of Environmental Toxicants and Autism Spectrum Disorders in Children. Environ. Pollut. 2017, 227, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Plana-Ripoll, O.; Antonsen, S.; Brandt, J.; Geels, C.; Landecker, H.; Sullivan, P.F.; Pedersen, C.B.; Rzhetsky, A. Environmental Pollution is Associated with Increased Risk of Psychiatric Disorders in the US and Denmark. PLoS Biol. 2019, 17, e3000353. [Google Scholar] [CrossRef] [PubMed]
- Robinson, N.; Bergen, S.E. Environmental Risk Factors for Schizophrenia and Bipolar Disorder and Their Relationship to Genetic Risk: Current Knowledge and Future Directions. Front. Genet. 2021, 12, 686666. [Google Scholar] [CrossRef] [PubMed]
- Attademo, L.; Bernardini, F.; Garinella, R.; Compton, M.T. Environmental Pollution and Risk of Psychotic Disorders: A Review of the Science to Date. Schizophr. Res. 2017, 181, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhao, H.; Xia, W.; Li, Y.; Liu, H.; Hao, K.; Chen, J.; Sun, X.; Liu, W.; Li, J.; et al. Prenatal Exposure to Benzophenones, Parabens and Triclosan and Neurocognitive Development at 2 Years. Environ. Int. 2019, 126, 413–421. [Google Scholar] [CrossRef]
- Lopez-Torres, B.; Ares, I.; Martínez, M.; Maximiliano, J.E.; Martínez-Larrañaga, M.R.; Anadón, A.; Martínez, M.A. Neurotoxicity Induced by the Pyrethroid Lambda-Cyhalothrin: Alterations in Monoaminergic Systems and Dopaminergic and Serotoninergic Pathways in the Rat Brain. Food Chem. Toxicol. 2022, 169, 113434. [Google Scholar] [CrossRef] [PubMed]
- Sotelo-Orozco, J.; Calafat, A.M.; Cook Botelho, J.; Schmidt, R.J.; Hertz-Picciotto, I.; Bennett, D.H. Exposure to Endocrine Disrupting Chemicals Including Phthalates, Phenols, and Parabens in Infancy: Associations with Neurodevelopmental Outcomes in the MARBLES Study. Int. J. Hyg. Environ. Health 2024, 261, 114425. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, J.L. Annual Research Review: Development of the Cerebral Cortex: Implications for Neurodevelopmental Disorders. J. Child. Psychol. Psychiatry 2011, 52, 339–355. [Google Scholar] [CrossRef]
- Li, Y.; Shen, M.; Stockton, M.E.; Zhao, X. Hippocampal Deficits in Neurodevelopmental Disorders. Neurobiol. Learn. Mem. 2019, 165, 106945. [Google Scholar] [CrossRef] [PubMed]
- Schug, T.T.; Blawas, A.M.; Gray, K.; Heindel, J.J.; Lawler, C.P. Elucidating the Links Between Endocrine Disruptors and Neurodevelopment. Endocrinology 2015, 156, 1941–1951. [Google Scholar] [CrossRef] [PubMed]
- Doi, M.; Usui, N.; Shimada, S. Prenatal Environment and Neurodevelopmental Disorders. Front. Endocrinol. 2022, 13, 860110. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in underweight and obesity from 1990 to 2022: A pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet 2024, 403, 1027–1050. [Google Scholar] [CrossRef]
- Janjua, N.R.; Mogensen, B.; Andersson, A.-M.; Petersen, J.H.; Henriksen, M.; Skakkebæk, N.E.; Wulf, H.C. Systemic Absorption of the Sunscreens Benzophenone-3, Octyl-Methoxycinnamate, and 3-(4-Methyl-Benzylidene) Camphor After Whole-Body Topical Application and Reproductive Hormone Levels in Humans. J. Investig. Dermatol. 2004, 123, 57–61. [Google Scholar] [CrossRef]
- Kajta, M.; Wnuk, A.; Rzemieniec, J.; Litwa, E.; Lason, W.; Zelek-Molik, A.; Nalepa, I.; Rogóż, Z.; Grochowalski, A.; Wojtowicz, A.K. Depressive-Like Effect of Prenatal Exposure to DDT Involves Global DNA Hypomethylation and Impairment of GPER1/ESR1 Protein Levels but Not ESR2 and AHR/ARNT Signaling. J. Steroid Biochem. Mol. Biol. 2017, 171, 94–109. [Google Scholar] [CrossRef] [PubMed]
- Wnuk, A.; Rzemieniec, J.; Przepiórska, K.; Pietrzak, B.A.; Maćkowiak, M.; Kajta, M. Prenatal Exposure to Triclocarban Impairs ESR1 Signaling and Disrupts Epigenetic Status in Sex-Specific Ways as Well as Dysregulates Expression of Neurogenesis-and Neurotransmitter-Related Genes in the Postnatal Mouse Brain. Int. J. Mol. Sci. 2021, 22, 13121. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ganley, C.J. Safety Threshold Considerations for Sunscreen Systemic Exposure: A Simulation Study. Clin. Pharmacol. Ther. 2019, 105, 161–167. [Google Scholar] [CrossRef]
- Halliwell, B. Biochemistry of Oxidative Stress. Biochem. Soc. Trans. 2007, 35(Pt5), 1147–1150. [Google Scholar] [CrossRef]
- Xing, B.; Chen, H.; Zhang, M.; Zhao, D.; Jiang, R.; Liu, X.; Zhang, S. Ischemic Postconditioning Inhibits Apoptosis After Focal Cerebral Ischemia/Reperfusion Injury in the Rat. Stroke 2008, 39, 2362–2369. [Google Scholar] [CrossRef] [PubMed]
- Khemakhem, A.M.; Frye, R.E.; El-Ansary, A.; Al-Ayadhi, L.; Bacha, A.B. Novel Biomarkers of Metabolic Dysfunctionis Autism Spectrum Disorder: Potential for Biological Diagnostic Markers. Metab. Brain Dis. 2017, 32, 1983–1997. [Google Scholar] [CrossRef]
- Giné-Servén, E.; Martinez-Ramirez, M.; Boix-Quintana, E.; Davi-Loscos, E.; Guanyabens, N.; Casado, V.; Muriana, D.; Torres-Rivas, C.; Crespo-Facorro, B.; Labad, J. Routine Cerebrospinal Fluid Parameters as Biomarkers in First-Episode Psychosis: A Prospective Observational Study. Prog. Neuropsychopharmacol. Biol. Psychiatry 2022, 112, 110424. [Google Scholar] [CrossRef]
- Broniowska, Ż.; Pomierny, B.; Smaga, I.; Filip, M.; Budziszewska, B. The Effect of UV-Filters on the Viability of Neuroblastoma (SH-SY5Y) Cell Line. Neurotoxicology 2016, 54, 44–52. [Google Scholar] [CrossRef]
- Long, D.M.; Frame, A.K.; Reardon, P.N.; Cumming, R.C.; Hendrix, D.A.; Kretzschmar, D.; Giebultowicz, J.M. Lactate Dehydrogenase Expression Modulates Longevity and Neurodegeneration in Drosophila melanogaster. Aging 2020, 12, 10041–10058. [Google Scholar] [CrossRef]
- de la Monte, S.M.; Sohn, Y.K.; Wands, J.R. Correlates of p53- and Fas (CD95)-Mediated Apoptosis in Alzheimer’s Disease. J. Neurol. Sci. 1997, 152, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Ethell, D.W.; Buhler, L.A. Fas Ligand-Mediated Apoptosis in Degenerative Disorders of the Brain. J. Clin. Immunol. 2003, 23, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhang, Y.; Zhao, S.; Chen, J.; Yang, J.; Wang, T.; Zou, H.; Wang, Y.; Gu, J.; Liu, X.; et al. Cadmium-Induced Apoptosis in Neuronal Cells is mediated by Fas/FasL-Mediated Mitochondrial Apoptotic Signaling Pathway. Sci. Rep. 2018, 8, 8837. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Zhang, L. Upregulation of Bax and Bcl-2 following Prenatal Cocaine Exposure Induces Apoptosis in Fetal Rat Brain. Int. J. Med. Sci. 2008, 5, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Satou, T.; Cummings, B.J.; Cotman, C.W. Immunoreactivity for Bcl-2 Protein Within Neurons in the Alzheimer’s Disease Brain Increases with Disease Severity. Brain Res. 1995, 697, 35–43. [Google Scholar] [CrossRef]
- O’Barr, S.; Schultz, J.; Rogers, J. Expression of the Protooncogene Bcl-2 in Alzheimer’s Disease Brain. Neurobiol. Aging 1996, 17, 131–136. [Google Scholar] [CrossRef]
- Kitamura, Y.; Shimohama, S.; Kamoshima, W.; Ota, T.; Matsuoka, Y.; Nomura, Y.; A Smith, M.; Perry, G.; Whitehouse, P.J.; Taniguchi, T. Alteration of Proteins Regulating Apoptosis, Bcl-2, Bcl-x, Bax, Bak, Bad, ICH-1 and CPP32, in Alzheimer’s Disease. Brain Res. 1998, 780, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Wnuk, A.; Przepiórska, K.; Rzemieniec, J.; Pietrzak, B.; Kajta, M. Selective Targeting of Non-Nuclear Estrogen Receptors with PaPE-1 as a New Treatment Strategy for Alzheimer’s Disease. Neurotox. Res. 2020, 38, 957–966. [Google Scholar] [CrossRef] [PubMed]
- Przepiórska, K.; Wnuk, A.; Beyer, C.; Kajta, M. Amorfrutin B Protects Mouse Brain Neurons from Hypoxia/Ischemia By Inhibiting Apoptosis and Autophagy Processes Through Gene Methylation- and miRNA-Dependent Regulation. Mol. Neurobiol. 2023, 60, 576–595. [Google Scholar] [CrossRef] [PubMed]
- Zárate, S.; Stevnsner, T.; Gredilla, R. Role of Estrogen and Other Sex Hormones in Brain Aging. Neuroprotection and DNA Repair. Front. Aging Neurosci. 2017, 9, 430. [Google Scholar] [CrossRef]
- Guerrero-Bosagna, C.; Settles, M.; Lucker, B.; Skinner, M.K. Epigenetic Transgenerational Actions of Vinclozolin on Promoter Regions of the Sperm Epigenome. PLoS ONE 2010, 5, e13100. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, E.; King, S.E.; McBirney, M.; Kubsad, D.; Pappalardo, M.; Beck, D.; Sadler-Riggleman, I.; Skinner, M.K. Vinclozolin Induced Epigenetic Transgenerational Inheritance of Pathologies and Sperm Epimutation Biomarkers for Specific Diseases. PLoS ONE 2018, 13, e0202662. [Google Scholar] [CrossRef] [PubMed]



| Gene Name | Catalog Number of TaqMan Probe |
|---|---|
| Fas | Mm00433237_m1 |
| Fasl | Mm00438864_m1 |
| Bax | Mm00432051_m1 |
| Bcl2 | Mm00477631_m1 |
| Gsk3b | Mm00444911_m1 |
| Hprt | Mm01318741_m1 |
| Actb | Mm00607939_s1 |
| Gapdh | Mm05724508_g1 |
| Protein Name | Catalog Number of an Anti-Body |
|---|---|
| FAS | sc-74540 |
| FASL | sc-19681 |
| BAX | sc-7480 |
| BCL2 | sc-7382 |
| GSK3β | sc-9166 |
| ACTB | sc-47778 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Przepiórska-Drońska, K.; Łach, A.; Pietrzak-Wawrzyńska, B.A.; Rzemieniec, J.; Kajta, M.; Wawrzczak-Bargieła, A.; Bilecki, W.; Noworyta, K.; Wnuk, A. Multigenerational Consequences of Prenatal Exposure to Benzophenone-3 Demonstrate Sex- and Region-Dependent Neurotoxic and Pro-Apoptotic Effects in Mouse Brain. Toxics 2024, 12, 906. https://doi.org/10.3390/toxics12120906
Przepiórska-Drońska K, Łach A, Pietrzak-Wawrzyńska BA, Rzemieniec J, Kajta M, Wawrzczak-Bargieła A, Bilecki W, Noworyta K, Wnuk A. Multigenerational Consequences of Prenatal Exposure to Benzophenone-3 Demonstrate Sex- and Region-Dependent Neurotoxic and Pro-Apoptotic Effects in Mouse Brain. Toxics. 2024; 12(12):906. https://doi.org/10.3390/toxics12120906
Chicago/Turabian StylePrzepiórska-Drońska, Karolina, Andrzej Łach, Bernadeta Angelika Pietrzak-Wawrzyńska, Joanna Rzemieniec, Małgorzata Kajta, Agnieszka Wawrzczak-Bargieła, Wiktor Bilecki, Karolina Noworyta, and Agnieszka Wnuk. 2024. "Multigenerational Consequences of Prenatal Exposure to Benzophenone-3 Demonstrate Sex- and Region-Dependent Neurotoxic and Pro-Apoptotic Effects in Mouse Brain" Toxics 12, no. 12: 906. https://doi.org/10.3390/toxics12120906
APA StylePrzepiórska-Drońska, K., Łach, A., Pietrzak-Wawrzyńska, B. A., Rzemieniec, J., Kajta, M., Wawrzczak-Bargieła, A., Bilecki, W., Noworyta, K., & Wnuk, A. (2024). Multigenerational Consequences of Prenatal Exposure to Benzophenone-3 Demonstrate Sex- and Region-Dependent Neurotoxic and Pro-Apoptotic Effects in Mouse Brain. Toxics, 12(12), 906. https://doi.org/10.3390/toxics12120906







