Active Vitamin D Ameliorates Arsenite-Induced Thyroid Dysfunction in Sprague–Dawley Rats by Inhibiting the Toll-like Receptor 4/NF-KappaB-Mediated Inflammatory Response
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Instruments
2.2. Animal Grouping and Sample Collection
2.3. Histopathological Analysis
2.4. TUNEL Analysis
2.5. Immunohistochemical Analysis
2.6. Enzyme-Linked Immunosorbent Assay
2.7. Statistic Analysis
3. Results
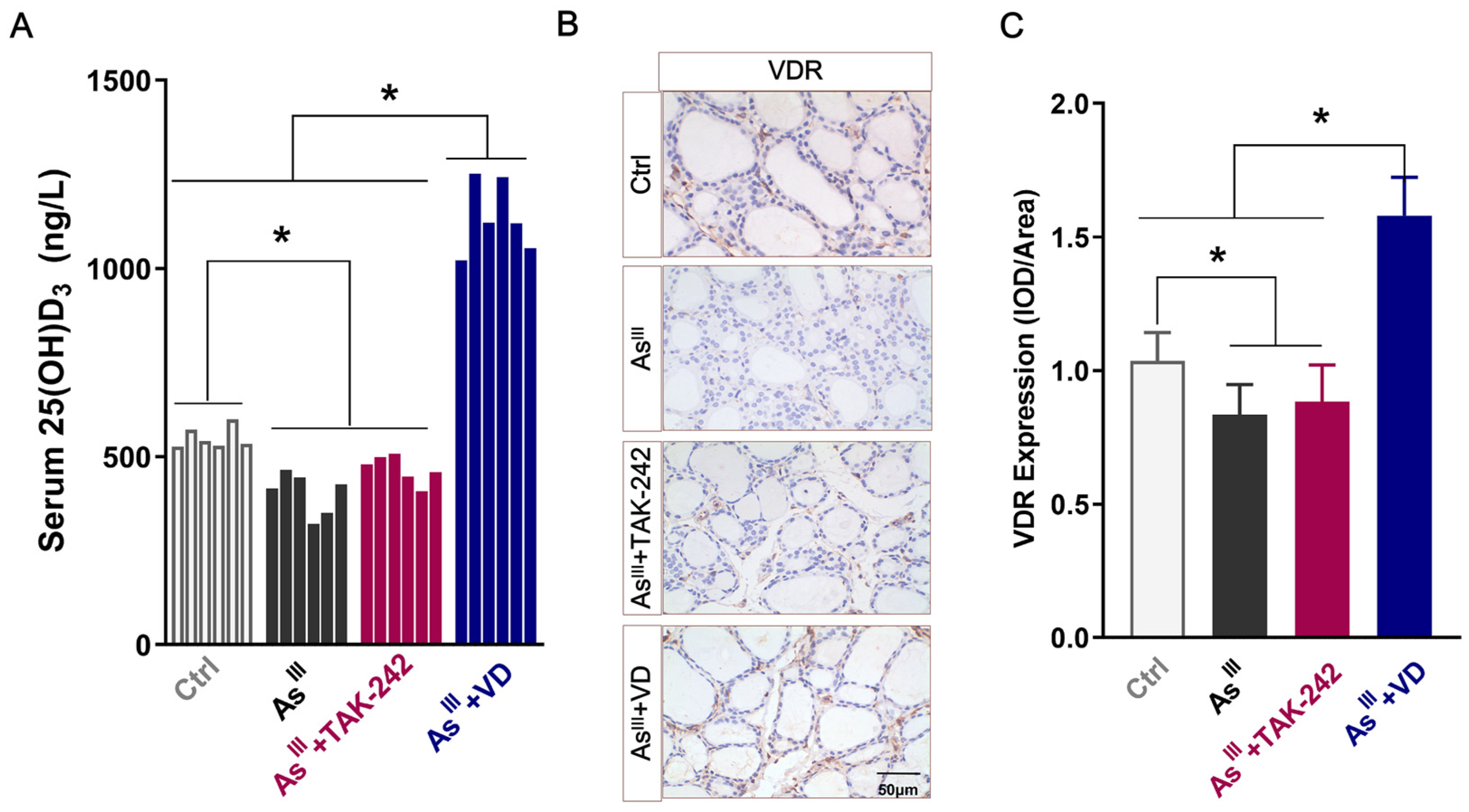
3.1. Serum 25(OH)D3 Levels and VDR Expression in Thyroid Tissue of Rats
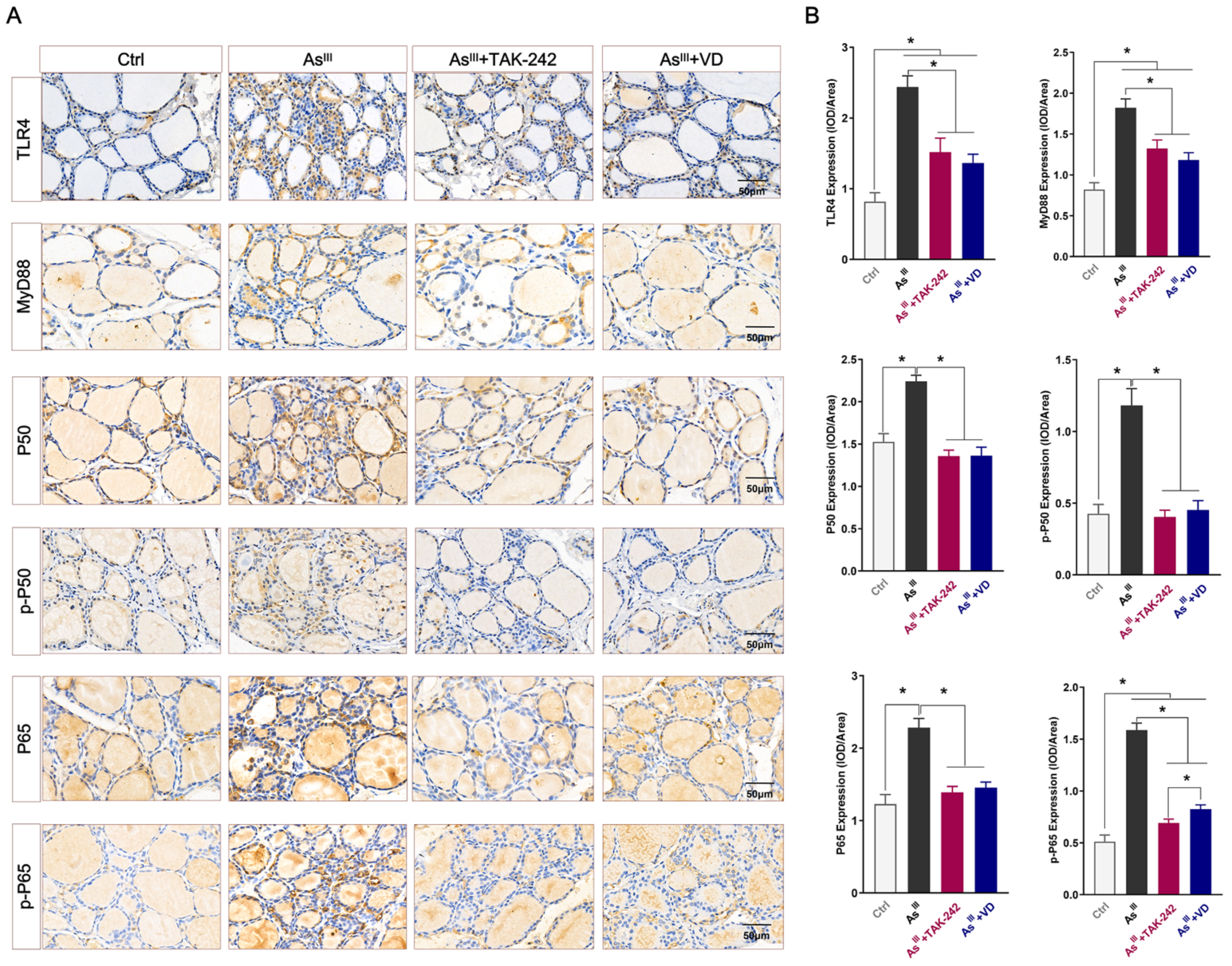
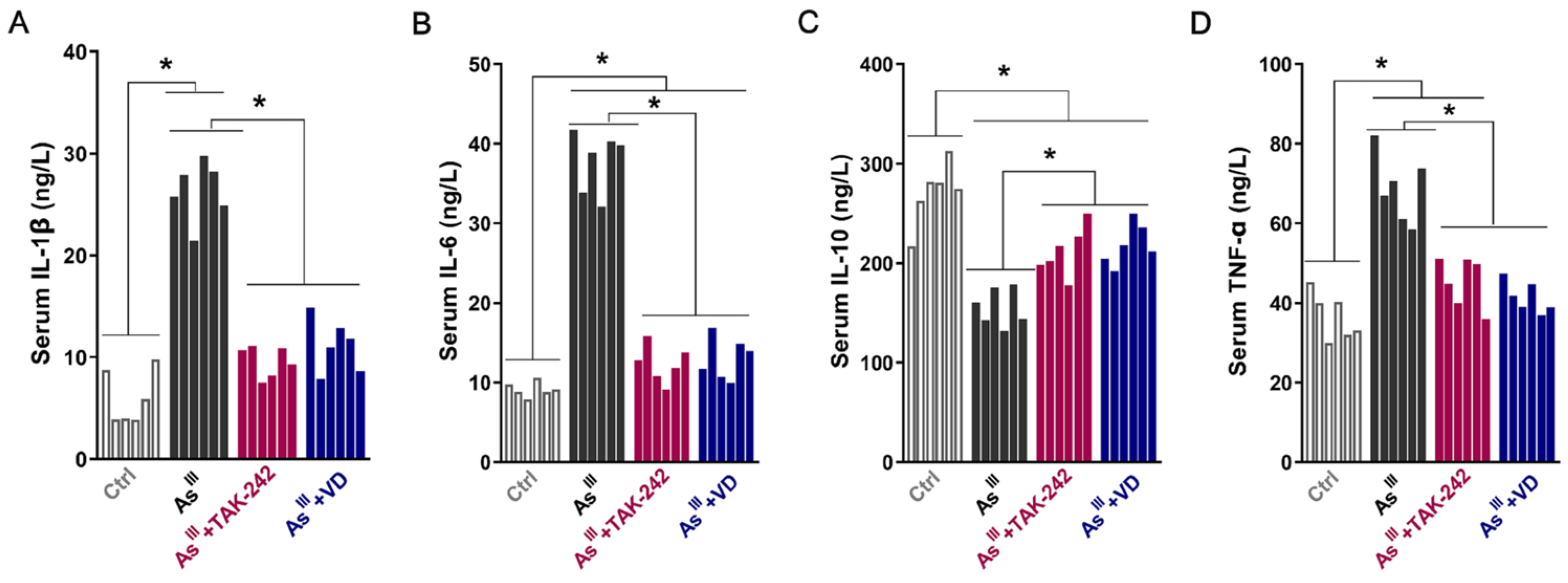
3.2. Active VD-Inhibited Arsenite Exposure Triggered the Activation of the TLR4/NF-κB Signaling Pathway in Rats’ Thyroid Tissue
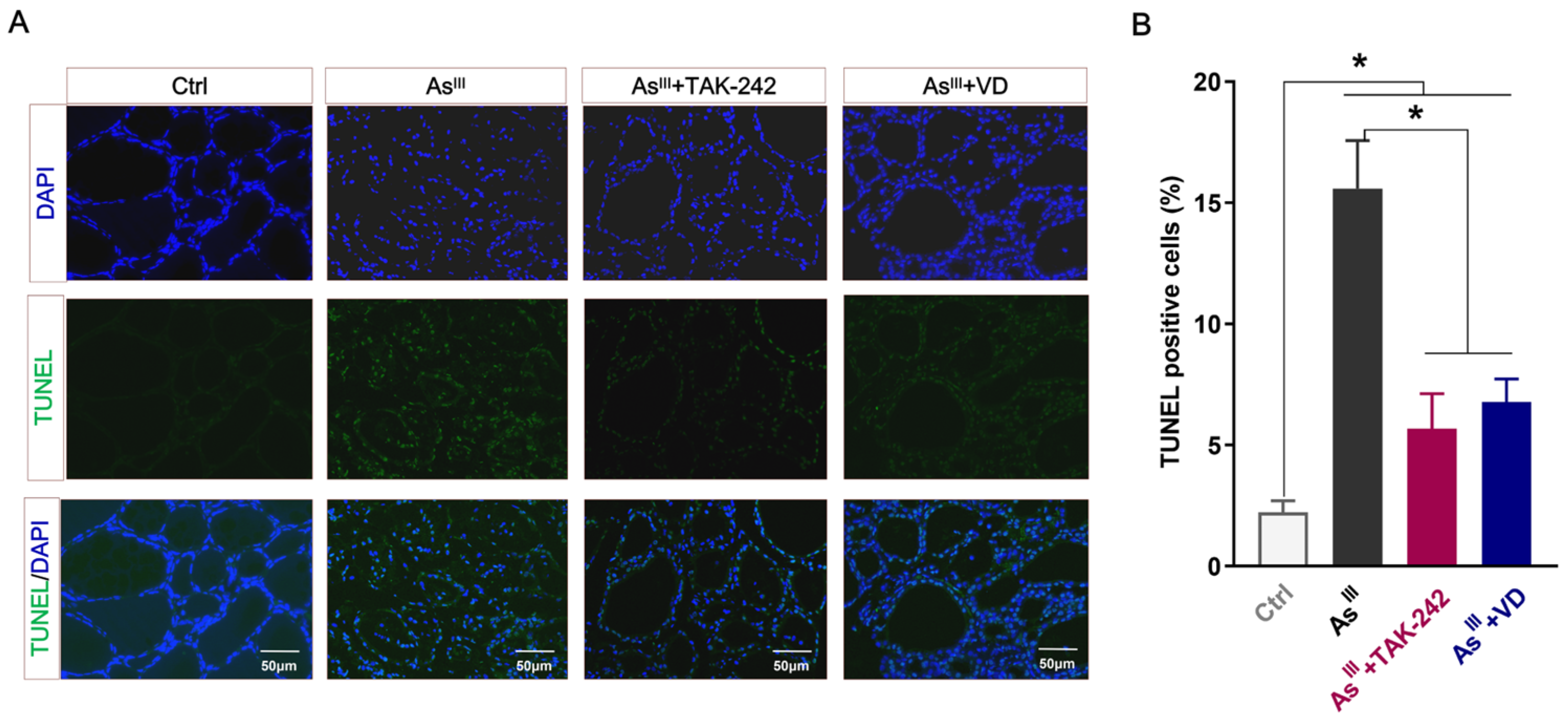
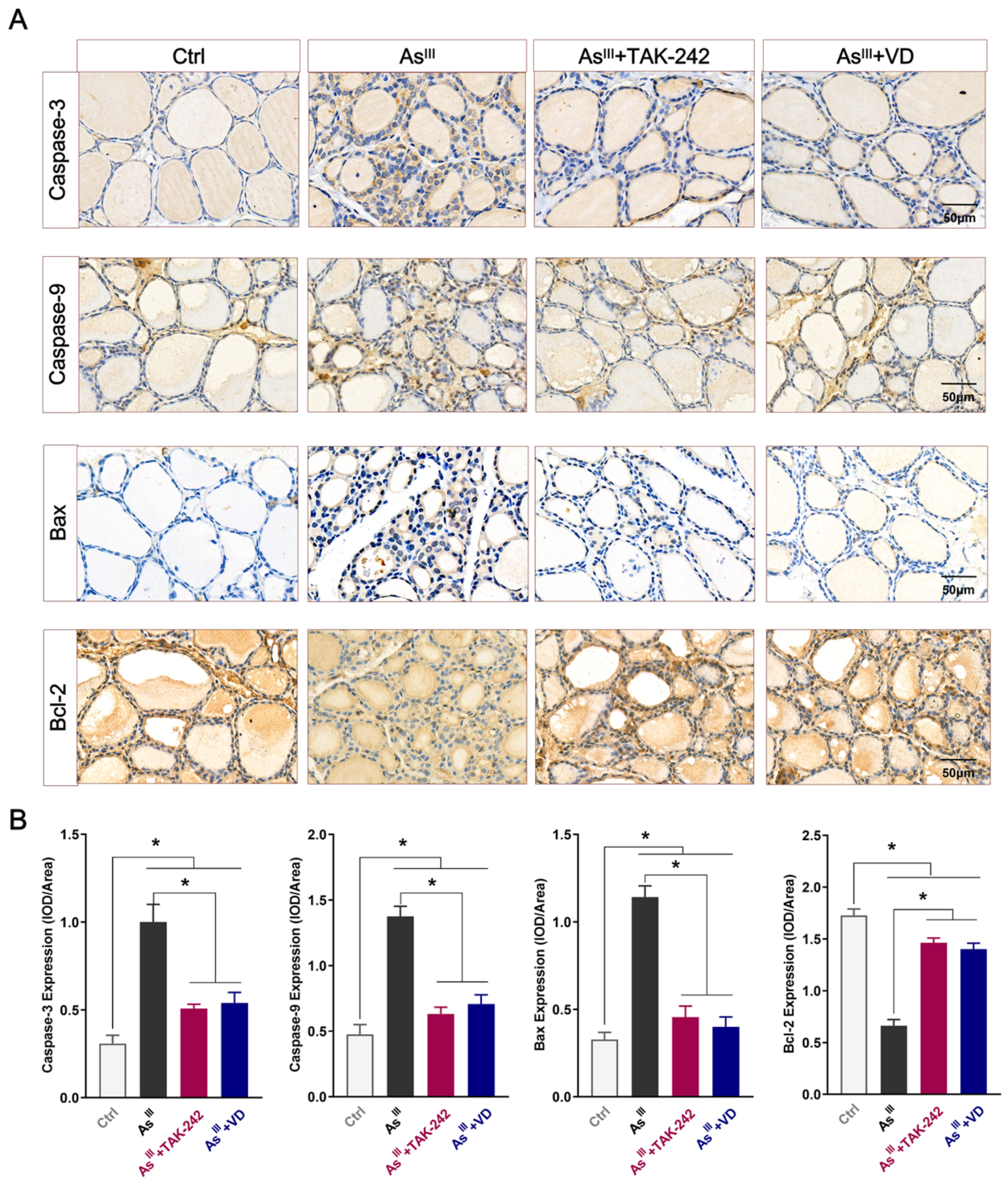
3.3. Active VD Can Reduce the Apoptosis of Thyroid Cells Induced by Arsenic Exposure in Rats
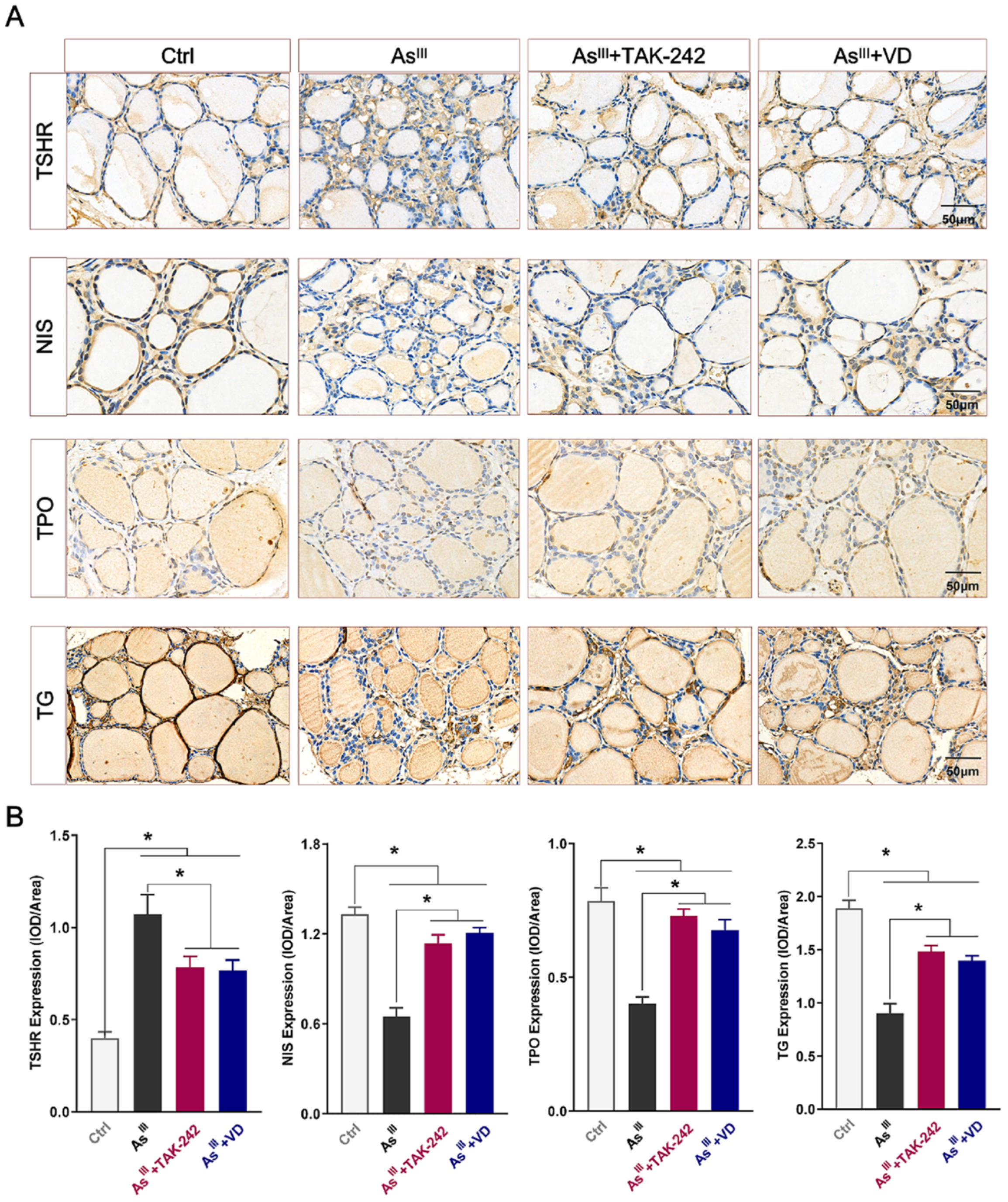
3.4. Active VD Reversed Arsenite Exposure Induced Abnormal Expression of THs Synthesis-Associated Proteins in the Thyroid Tissue of Rats
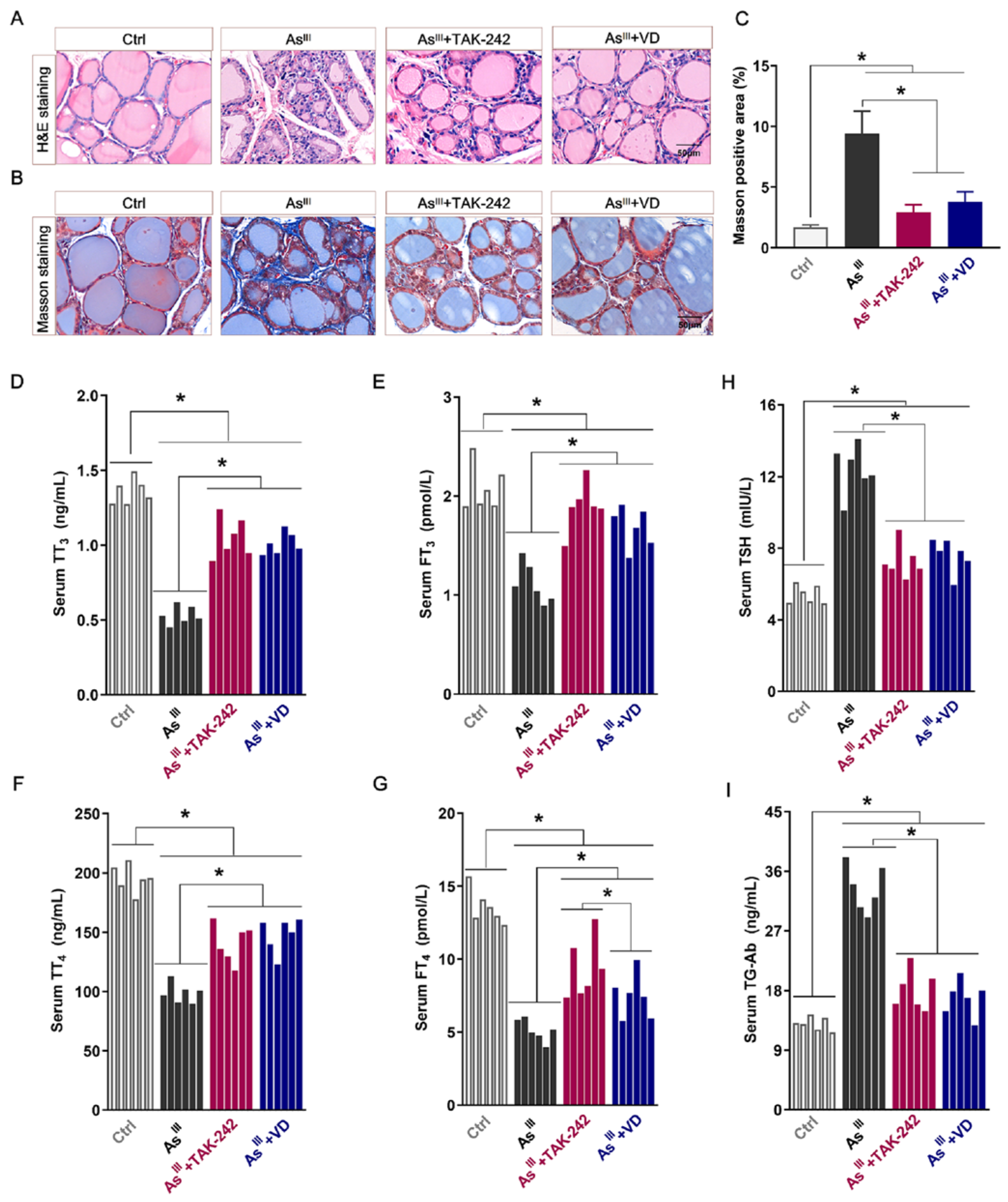
3.5. Active VD Ameliorated Arsenite Exposure Induced Thyroid Injury and Dysfunction in Rats
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaur, S.; Kamli, M.R.; Ali, A. Role of Arsenic and Its Resistance in Nature. Can. J. Microbiol. 2011, 57, 769–774. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy Metal Pollution in the Environment and Their Toxicological Effects on Humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Liu, Y.P.; Longfellow, D.; Aposhian, H.V.; Beck, B.; Fowler, B.; Rossman, R.M.T. Arsenic: Health Effects, Mechanisms of Actions, and Research Issues. Environ. Health Perspect. 1999, 107, 593–597. [Google Scholar]
- Abdul, K.S.; Jayasinghe, S.S.; Chandana, E.P.S.; Jayasumana, C.; De Silva, P.M. Arsenic and Human Health Effects: A Review. Environ. Toxicol. Pharmacol. 2015, 40, 828–846. [Google Scholar] [CrossRef]
- Sun, H.; Xiang, P.; Luo, J.; Hong, H.; Lin, H.; Li, H.B.; Ma, L.Q. Mechanisms of Arsenic Disruption on Gonadal, Adrenal and Thyroid Endocrine Systems in Humans: A review. Environ. Int. 2016, 95, 61–68. [Google Scholar] [CrossRef]
- Babić Leko, M.; Gunjača, I.; Pleić, N.; Zemunik, T. Environmental Factors Affecting Thyroid-Stimulating Hormone and Thyroid Hormone Levels. Int. J. Mol. Sci. 2021, 22, 6521. [Google Scholar] [CrossRef]
- Jurdziak, M.; Gać, P.; Poręba, M.; Szymańska-Chabowska, A.; Mazur, G.; Poręba, R. Concentration of Thyrotropic Hormone in Persons Occupationally Exposed to Lead, Cadmium and Arsenic. Biol. Trace Elem. Res. 2018, 182, 196–203. [Google Scholar] [CrossRef]
- Ajjan, R.; Watson, P.; Findlay, C.; Metcalfe, R.; Crisp, M.; Ludgate, M.; Weetman, A. The sodium iodide symporter gene and its regulation by cytokines found in autoimmunity. J. Endocrinol. 1998, 158, 351–358. [Google Scholar] [CrossRef]
- Kim, K.; Argos, M.; Persky, V.W.; Freels, S.; Sargis, R.M.; Turyk, M.E. Associations of Exposure to Metal and Metal Mixtures with Thyroid Hormones: Results from the NHANES 2007–2012. Environ. Res. 2022, 212, 113413. [Google Scholar] [CrossRef]
- Khan, K.M.; Parvez, F.; Zoeller, R.T.; Hocevar, B.A.; Kamendulis, L.M.; Rohlman, D.; Eunus, M.; Graziano, J. Thyroid Hormones and Neurobehavioral Functions among Adolescents Chronically Exposed to Groundwater with Geogenic Arsenic in Bangladesh. Sci. Total Environ. 2019, 678, 278–287. [Google Scholar] [CrossRef]
- Liang, C.; Han, Y.; Ma, L.; Wu, X.; Huang, K.; Yan, S.; Li, Z.; Xia, X.; Pan, W.; Sheng, J.; et al. Low Levels of Arsenic Exposure during Pregnancy and Maternal and Neonatal Thyroid Hormone Parameters: The Determinants for These Associations. Environ. Int. 2020, 145, 106114. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Li, S.; Li, C.; Wang, W.; Li, H.; Ma, L. Thyrotoxicity of Arsenate and Arsenite on Juvenile Mice at Organism, Subcellular, and Gene Levels under Low Exposure. Chemosphere 2017, 186, 580–587. [Google Scholar] [CrossRef]
- Ahangarpour, A.; Alboghobeish, S.; Oroojan, A.A.; Zeidooni, L.; Samimi, A.; Afshari, G. Effects of Combined Exposure to Chronic High-Fat Diet and Arsenic on Thyroid Function and Lipid Profile in Male Mouse. Biol. Trace Elem. Res. 2018, 182, 37–48. [Google Scholar] [CrossRef]
- K, N.; P, J.; Nalla, S.V.; Dubey, I.; Kushwaha, S. Arsenic-Induced Thyroid Hormonal Alterations and Their Putative Influence on Ovarian Follicles in Balb/c Mice. Biol. Trace Elem. Res. 2024, 202, 4087–4100. [Google Scholar] [CrossRef]
- Fan, L.; He, Z.; Wang, L.; Gaoyang, H.; Wang, D.; Luo, P. Alterations of Bax/Bcl-2 Ratio Contribute to NaAsO2 Induced Thyrotoxicity in Human Thyroid Follicular Epithelial Cells and SD Rats. Ecotoxicol. Environ. Saf. 2023, 264, 115449. [Google Scholar] [CrossRef]
- Fan, L.; Song, Q.; Jin, Y.; He, R.; Diao, H.; Luo, P.; Wang, D. Prolonged Exposure to NaAsO2 Induces Thyroid Dysfunction and Inflammatory Injury in Sprague‒Dawley Rats, Involvement of NLRP3 Inflammasome‒mediated Pyroptosis. Arch. Toxicol. 2024, 98, 3673–3687. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, X.; Bao, X.; Xiao, W.; Chen, G. Toll-like Receptor 4 (TLR4) Inhibitors: Current Research and Prospective. Eur. J. Med. Chem. 2022, 235, 114291. [Google Scholar] [CrossRef]
- Wan, J.; Shan, Y.; Fan, Y.; Fan, C.; Chen, S.; Sun, J.; Zhu, L.; Qin, L.; Yu, M.; Lin, Z. NF-κB Inhibition Attenuates LPS-Induced TLR4 Activation in Monocyte Cells. Mol. Med. Rep. 2016, 14, 4505–4510. [Google Scholar] [CrossRef]
- Peng, S.; Li, C.; Wang, X.; Liu, X.; Han, C.; Jin, T.; Liu, S.; Zhang, X.; Zhang, H.; He, X.; et al. Increased Toll-Like Receptors Activity and TLR Ligands in Patients with Autoimmune Thyroid Diseases. Front. Immunol. 2016, 7, 578. [Google Scholar] [CrossRef]
- Liu, X.; Wei, W.; Wu, Y.; Wang, Y.; Zhang, W.; Wang, Y.; Dong, X.; Shi, Q. Emodin Treatment of Papillary Thyroid Cancer Cell Lines In Vitro Inhibits Proliferation and Enhances Apoptosis via Downregulation of NF-κB and Its Upstream TLR4 Signaling. Oncol. Lett. 2023, 26, 514. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Q.; Qin, L.; Yan, Z.; Wu, L.; Liu, T. Dioscin Ameliorates Experimental Autoimmune Thyroiditis via the mTOR and TLR4/NF-κB Signaling. Drug Des. Dev. Ther. 2023, 17, 2273–2285. [Google Scholar] [CrossRef] [PubMed]
- Aktaş, T.; Celik, S.K.; Genc, G.C.; Arpaci, D.; Can, M.; Dursun, A. Higher Levels of Serum TLR2 and TLR4 in Patients with Hashimoto’s Thyroiditis. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Sun, X.; Wang, X.; Wang, H.; Shan, Z.; Teng, W.; Li, C. Myeloid Related Proteins Are Up-Regulated in Autoimmune Thyroid Diseases and Activate Toll-like Receptor 4 and pro-Inflammatory Cytokines In Vitro. Int. Immunopharmacol. 2018, 59, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Lebiedziński, F.; Lisowska, K.A. Impact of Vitamin D on Immunopathology of Hashimoto’s Thyroiditis: From Theory to Practice. Nutrients 2023, 15, 3174. [Google Scholar] [CrossRef]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D Deficiency 2.0: An Update on the Current Status Worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef]
- Carlberg, C.; Muñoz, A. An Update on Vitamin D Signaling and Cancer. Semin. Cancer Biol. 2022, 79, 217–230. [Google Scholar] [CrossRef]
- Mackawy, A.M.H.; Al-Ayed, B.M.; Al-Rashidi, B.M. Vitamin D Deficiency and Its Association with Thyroid Disease. Int. J. Health Sci. (Qassim) 2013, 7, 267–275. [Google Scholar] [CrossRef]
- Štefanić, M.; Tokić, S. Serum 25-Hydoxyvitamin D Concentrations in Relation to Hashimoto’s Thyroiditis: A Systematic Review, Meta-Analysis and Meta-Regression of Observational Studies. Eur. J. Nutr. 2020, 59, 859–872. [Google Scholar] [CrossRef]
- Durá-Travé, T.; Gallinas-Victoriano, F. Autoimmune Thyroiditis and Vitamin D. Int. J. Health Sci. (Qassim) 2024, 25, 3154. [Google Scholar] [CrossRef]
- Luo, M.; Xu, Y.; Li, J.; Luo, D.; Zhu, L.; Wu, Y.; Liu, X.; Wu, P. Vitamin D Protects Intestines from Liver Cirrhosis-Induced Inflammation and Oxidative Stress by Inhibiting the TLR4/MyD88/NF-κB Signaling Pathway. Open Med. (Wars) 2023, 18, 20230714. [Google Scholar] [CrossRef]
- Liu, P.; Li, F.; Xu, X.; Li, S.; Dong, X.; Chen, L.; Bai, B.; Wang, Y.; Qiu, M.; Dong, Y. 1,25(OH)2D3 Provides Protection against Diabetic Kidney Disease by Downregulating the TLR4-MyD88-NF-κB Pathway. Exp. Mol. Pathol. 2020, 114, 104434. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, Q.; Chai, Y.; Liu, Y.; Li, F.; Wang, B.; Zhu, C.; Cui, J.; Qu, H.; Zhu, M. 1,25(OH)2D3 Downregulates the Toll-like Receptor 4-Mediated Inflammatory Pathway and Ameliorates Liver Injury in Diabetic Rats. J. Endocrinol. Investig. 2015, 38, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Molin, M.; Ulven, S.M.; Dahl, L.; Lundebye, A.; Holck, M.; Alexander, J.; Meltzer, H.M.; Ydersbond, T.A. Arsenic in Seafood Is Associated with Increased Thyroid-Stimulating Hormone (TSH) in Healthy Volunteers–A Randomized Controlled Trial. J. Trace Elem. Med. Biol. 2017, 44, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Aboh, N.A. Pattern of Thyroid Autoantibodies, Essential and Toxic Trace Elements in Various Thyroid Disorders in Nigeria. Ph.D. Thesis, University of Ibadan, Ibadan, Nigeria, 2018. [Google Scholar]
- Czabotar, P.E.; Garcia-Saez, A.J. Mechanisms of BCL-2 Family Proteins in Mitochondrial Apoptosis. Nat. Rev. Mol. Cell Biol. 2023, 24, 732–748. [Google Scholar] [CrossRef] [PubMed]
- Van Opdenbosch, N.; Lamkanfi, M. Caspases in Cell Death, Inflammation, and Disease. Immunity 2019, 50, 1352–1364. [Google Scholar] [CrossRef]
- Moulana, F.I.; Priyani, A.A.H.; De Silva, M.V.C.; Dassanayake, R.S. BRAF-Oncogene-Induced Senescence and the Role of Thyroid-Stimulating Hormone Signaling in the Progression of Papillary Thyroid Carcinoma. Horm. Cancer 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Płóciennikowska, A.; Hromada-Judycka, A.; Borzęcka, K.; Kwiatkowska, K. Co-operation of TLR4 and Raft Proteins in LPS-Induced Pro-inflammatory Signaling. Cell. Mol. Life Sci. 2015, 72, 557–581. [Google Scholar] [CrossRef]
- Duan, T.; Du, Y.; Xing, C.; Wang, H.Y.; Wang, R.-F. Toll-like Receptor Signaling and Its Role in Cell-Mediated Immunity. Front. Immunol. 2022, 13, 812774. [Google Scholar] [CrossRef]
- Matsunaga, N.; Tsuchimori, N.; Matsumoto, T.; Ii, M. TAK-242 (Resatorvid), a Small-Molecule Inhibitor of Toll-Like Receptor (TLR) 4 Signaling, Binds Selectively to TLR4 and Interferes with Interactions between TLR4 and Its Adaptor Molecules. Mol. Pharmacol. 2011, 79, 34–41. [Google Scholar] [CrossRef]
- Samarpita, S.; Kim, J.Y.; Rasool, M.K.; Kim, K.S. Investigation of Toll-like Receptor (TLR) 4 Inhibitor TAK-242 as a New Potential Anti-Rheumatoid Arthritis Drug. Arthritis Res. Ther. 2020, 22, 16. [Google Scholar] [CrossRef]
- Feng, Y.; Ju, Y.; Wu, Q.; Sun, G.; Yan, Z. TAK-242, a Toll-like Receptor 4 Antagonist, against Brain Injury by Alleviates Autophagy and Inflammation in Rats. Open Life Sci. 2023, 18, 20220662. [Google Scholar] [CrossRef] [PubMed]
- Rice, T.W.; Wheeler, A.P.; Bernard, G.R.; Vincent, J.L.; Angus, D.C.; Aikawa, N.; Demeyer, I.; Sainati, S.; Amlot, N.; Cao, C.; et al. A Randomized, Double-Blind, Placebo-Controlled Trial of TAK-242 for the Treatment of Severe Sepsis. Crit. Care Med. 2010, 38, 1685–1694. [Google Scholar] [CrossRef] [PubMed]
- Baeke, F.; Takiishi, T.; Korf, H.; Gysemans, C.; Mathieu, C. Vitamin D: Modulator of the Immune System. Curr. Opin. Pharmacol. 2010, 10, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Šošić-Jurjević, B.; Trifunović, S.; Živanović, J.; Ajdžanović, V.; Miler, M.; Ristić, N.; Filipović, B. Vitamin D3 Treatment Alters Thyroid Functional Morphology in Orchidectomized Rat Model of Osteoporosis. Int. J. Health Sci. (Qassim) 2022, 23, 791. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xiong, F.; Liu, E.M.; Zhu, M.; Lei, P.Y. Effects of 1,25-dihydroxyvitamin D3 in rats with experimental autoimmune thyroiditis. J. South Med. Univ. 2010, 30, 1573–1576. [Google Scholar]
- Tang, J.; Shan, S.; Li, F.; Yun, P. Effects of Vitamin D Supplementation on Autoantibodies and Thyroid Function in Patients with Hashimoto’s Thyroiditis: A Systematic Review and Meta-Analysis. Medicine 2023, 102, e36759. [Google Scholar] [CrossRef]
- Misharin, A.; Hewison, M.; Chen, C.R.; Lagishetty, V.; Aliesky, H.A.; Mizutori, Y.; Rapoport, B.; McLachlan, S.M. Vitamin D Deficiency Modulates Graves’ Hyperthyroidism Induced in BALB/c Mice by Thyrotropin Receptor Immunization. J. Endocrinol. 2009, 150, 1051–1060. [Google Scholar] [CrossRef]
- Zhang, Y.; Leung, D.Y.M.; Richers, B.N.; Liu, Y.; Remigio, L.K.; Riches, D.W.; Goleva, E. Vitamin D Inhibits Monocyte/Macrophage Proinflammatory Cytokine Production by Targeting MAPK Phosphatase-1. J. Immunol. 2012, 188, 2127–2135. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Xiang, J.; Song, Q.; Jin, Y.; Zhou, M.; Fan, L.; Wang, D. Active Vitamin D Ameliorates Arsenite-Induced Thyroid Dysfunction in Sprague–Dawley Rats by Inhibiting the Toll-like Receptor 4/NF-KappaB-Mediated Inflammatory Response. Toxics 2024, 12, 887. https://doi.org/10.3390/toxics12120887
Li H, Xiang J, Song Q, Jin Y, Zhou M, Fan L, Wang D. Active Vitamin D Ameliorates Arsenite-Induced Thyroid Dysfunction in Sprague–Dawley Rats by Inhibiting the Toll-like Receptor 4/NF-KappaB-Mediated Inflammatory Response. Toxics. 2024; 12(12):887. https://doi.org/10.3390/toxics12120887
Chicago/Turabian StyleLi, Hui, Jie Xiang, Qian Song, Ying Jin, Meitong Zhou, Lili Fan, and Dapeng Wang. 2024. "Active Vitamin D Ameliorates Arsenite-Induced Thyroid Dysfunction in Sprague–Dawley Rats by Inhibiting the Toll-like Receptor 4/NF-KappaB-Mediated Inflammatory Response" Toxics 12, no. 12: 887. https://doi.org/10.3390/toxics12120887
APA StyleLi, H., Xiang, J., Song, Q., Jin, Y., Zhou, M., Fan, L., & Wang, D. (2024). Active Vitamin D Ameliorates Arsenite-Induced Thyroid Dysfunction in Sprague–Dawley Rats by Inhibiting the Toll-like Receptor 4/NF-KappaB-Mediated Inflammatory Response. Toxics, 12(12), 887. https://doi.org/10.3390/toxics12120887






