User Experiences of the Cue2walk Smart Cueing Device for Freezing of Gait in People with Parkinson’s Disease
Abstract
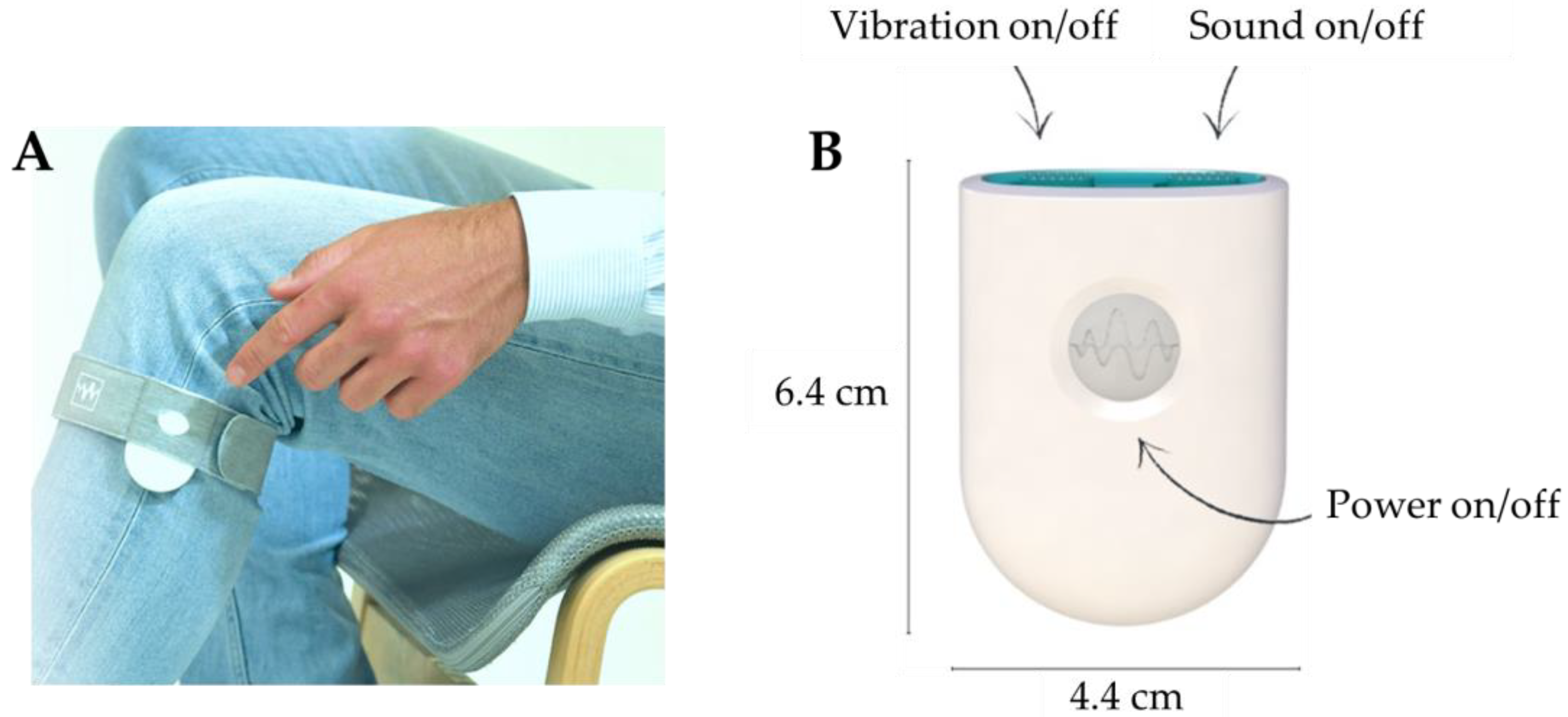
1. Introduction
2. Materials and Methods
2.1. Participant Selection
2.2. Online Survey
2.3. Data Analysis
3. Results
3.1. Participant Characteristics
3.2. Question Completion Rate
3.3. Possession of the Device and Device Usage
3.4. Modified EQ-5D-5L VAS Score
3.5. Modified EQ-5D-5L and FoG-Related Questions
3.6. Customer Satisfaction
3.7. Correlations Between Device Usage per Day and Modified EQ-5D-5L, FoG-Related Questions and Customer Satisfaction
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADL | Activities of daily living |
| FoG | Freezing of gait |
| EQ-5D-5L | EuroQol 5 dimensions 5 level survey |
| MCID | Minimal clinically important difference |
| PD | Parkinson’s disease |
| QoL | Quality of life |
| VAS | Visual analogue scale |
Appendix A. Online Survey
- Demographics
- -
- Are you: Male/Female
- -
- What is your age?
- -
- How long ago (in years) were you diagnosed with Parkinson’s disease?
- -
- What medication do you use for your Parkinson’s disease and in what amount per day?
- Cue2walk device usage
- -
- How long (in months) have you been using the Cue2walk device?
- -
- How many hours per day do you wear the Cue2walk device?
- Modified EQ-5D-5L
- -
- Have you experienced any changes in your mobility since using the Cue2walk device?I experience
- ○
- much less walking problems;
- ○
- less walking problems;
- ○
- no more or less walking problems;
- ○
- more walking problems;
- ○
- much more walking problems.
- -
- Have you experienced any changes in your self-care since using the Cue2walk device?I experience
- ○
- much less problems with self-care;
- ○
- less problems with self-care;
- ○
- no more or less problems with self-care;
- ○
- more problems with self-care;
- ○
- much more problems with self-care.
- -
- Have you experienced any changes in the way you perform your usual activities since using the Cue2walk device?I experience
- ○
- much less problems with daily activities;
- ○
- less problems with daily activities;
- ○
- no more or less problems with daily activities;
- ○
- more problems with daily activities;
- ○
- much more problems with daily activities.
- -
- Have you experienced any changes in your pain and/or discomfort since using the Cue2walk device?I experience
- ○
- much less pain or discomfort;
- ○
- less pain or discomfort;
- ○
- no more or less pain or discomfort;
- ○
- more pain or discomfort;
- ○
- much more pain or discomfort.
- -
- Have you experienced any changes in your anxiety and/or depression since using the Cue2walk device?I feel
- ○
- much less anxious and/or depressed;
- ○
- less anxious and/or depressed;
- ○
- no more or less anxious and/or depressed;
- ○
- more anxious and/or depressed;
- ○
- much more anxious and/or depressed.
- -
- We would like to know how good or bad your health was BEFORE using the Cue2walk device. This scale is numbered from 0 to 10: 10 means the best health you can imagine; 0 means the worst health you can imagine.
- -
- We would like to know how good or bad your health is TODAY. This scale is numbered from 0 to 10: 10 means the best health you can imagine; 0 means the worst health you can imagine.
- Additional FoG-related questions to the modified EQ-5D-5L
- -
- To what extent does the use of the Cue2walk device influence the duration of a freezing of gait episode?The duration of a freezing of gait episode is
- ○
- much shorter;
- ○
- shorter;
- ○
- not shorter or longer;
- ○
- longer;
- ○
- much longer.
- -
- To what extent does the use of the Cue2walk device influence the number of falls?I experience
- ○
- much less falls;
- ○
- less falls;
- ○
- no more or less falls;
- ○
- more falls;
- ○
- much more falls.
- -
- To what extent does the use of the Cue2walk device influence your self-confidence?I feel
- ○
- much more confident;
- ○
- more confident;
- ○
- not more or less confident;
- ○
- less confident;
- ○
- much less confident.
- Customer satisfaction
- -
- How likely are you to recommend the Cue2walk device to someone else with Parkinson’s disease experiencing freezing of gait on a scale of 0 to 10?
References
- Nutt, J.G.; Bloem, B.R.; Giladi, N.; Hallett, M.; Horak, F.B.; Nieuwboer, A. Freezing of gait: Moving forward on a mysterious clinical phenomenon. Lancet Neurol. 2011, 10, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Giladi, N.; Kao, R.; Fahn, S. Freezing phenomenon in patients with parkinsonian syndromes. Mov. Disord. 1997, 12, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.S.; Gao, C.; Tan, Y.Y.; Chen, S.D. Prevalence of freezing of gait in Parkinson’s disease: A systematic review and meta-analysis. J. Neurol. 2021, 268, 4138–4150. [Google Scholar] [CrossRef] [PubMed]
- Canning, C.G.; Paul, S.S.; Nieuwboer, A. Prevention of falls in Parkinson’s disease: A review of fall risk factors and the role of physical interventions. Neurodegener. Dis. Manag. 2014, 4, 203–221. [Google Scholar] [CrossRef]
- Cronin, P.; Collins, L.M.; Sullivan, A.M. Impacts of gait freeze on quality of life in Parkinson’s disease, from the perspectives of patients and their carers. Ir. J. Med. Sci. 2024, 193, 2041–2050. [Google Scholar] [CrossRef]
- Ghielen, I.; Koene, P.; Twisk, J.W.; Kwakkel, G.; van den Heuvel, O.A.; van Wegen, E.E. The association between freezing of gait, fear of falling and anxiety in Parkinson’s disease: A longitudinal analysis. Neurodegener. Dis. Manag. 2020, 10, 159–168. [Google Scholar] [CrossRef]
- Latt, M.D.; Lord, S.R.; Morris, J.G.; Fung, V.S. Clinical and physiological assessments for elucidating falls risk in Parkinson’s disease. Mov. Disord. 2009, 24, 1280–1289. [Google Scholar] [CrossRef]
- Walton, C.C.; Shine, J.M.; Hall, J.M.; O’Callaghan, C.; Mowszowski, L.; Gilat, M.; Szeto, J.Y.Y.; Naismith, S.L.; Lewis, S.J.G. The major impact of freezing of gait on quality of life in Parkinson’s disease. J. Neurol. 2015, 262, 108–115. [Google Scholar] [CrossRef]
- Bloem, B.R.; de Vries, N.M.; Ebersbach, G. Nonpharmacological treatments for patients with Parkinson’s disease. Mov. Disord. 2015, 30, 1504–1520. [Google Scholar] [CrossRef]
- Gámez-Leyva, G.; Cubo, E. Freezing of gait: Pharmacological and surgical options. Curr. Opin. Neurol. 2024, 37, 394–399. [Google Scholar] [CrossRef]
- Janssen Daalen, J.M.; Selvaraj, A.; Arnts, H.; Bloem, B.R.; Bartels, R.H.; Georgiev, D.; Esselink, R.A.J.; Vinke, R.S. Gait and balance worsening after bilateral deep brain stimulation of the subthalamic nucleus (STN-DBS) for Parkinson’s disease: A systematic review. BMJ Neurol. Open 2025, 7, e000898. [Google Scholar] [CrossRef]
- Nieuwboer, A.; Kwakkel, G.; Rochester, L.; Jones, D.; van Wegen, E.; Willems, A.M.; Chavret, F.; Hetherington, V.; Baker, K.; Lim, I. Cueing training in the home improves gait-related mobility in Parkinson’s disease: The RESCUE trial. J. Neurol. Neurosurg. Psychiatry 2007, 78, 134–140. [Google Scholar] [CrossRef]
- Ginis, P.; Nackaerts, E.; Nieuwboer, A.; Heremans, E. Cueing for people with Parkinson’s disease with freezing of gait: A narrative review of the state-of-the-art and novel perspectives. Ann. Phys. Rehabil. Med. 2018, 61, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Nonnekes, J.; Ružicka, E.; Nieuwboer, A.; Hallett, M.; Fasano, A.; Bloem, B.R. Compensation Strategies for Gait Impairments in Parkinson Disease: A Review. JAMA Neurol. 2019, 76, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Ahn, D.; Chung, H.; Lee, H.W.; Kang, K.; Ko, P.W.; Kim, N.S.; Park, T. Smart gait-aid glasses for Parkinson’s Disease patients. IEEE Trans. Biomed. Eng. 2017, 64, 2394–2402. [Google Scholar] [CrossRef] [PubMed]
- Marsh, R.; Cole, M.H.; Dissanayaka, N.N.W.; Au, T.R.; Clewett, S.; O’Sullivan, J.D.; Silburn, P.A. The CuePed Trial: How Does Environmental Complexity Impact Cue Effectiveness? A Comparison of Tonic and Phasic Visual Cueing in Simple and Complex Environments in a Parkinson’s Disease Population with Freezing of Gait. Park. Dis. 2019, 2019, 2478980. [Google Scholar] [CrossRef]
- Zoetewei, D.; Herman, T.; Ginis, P.; Palmerini, L.; Brozgol, M.; Thumm, P.C.; Ferrari, A.; Ceulemans, E.; Decaluwe, E.; Hausdorff, J.M.; et al. On-Demand Cueing for Freezing of Gait in Parkinson’s Disease: A Randomized Controlled Trial. Mov. Disord. 2024, 39, 876–886. [Google Scholar] [CrossRef]
- Bächlin, M.; Plotnik, M.; Roggen, D.; Giladi, N.; Hausdorff, J.M.; Tröster, G. A wearable system to assist walking of Parkinson’s Disease patients. Methods Inf. Med. 2010, 49, 88–95. [Google Scholar] [CrossRef]
- van der Laan, M.; van Wegen, E.E.H.; Keijser, J.A.N.; Minnoye, A.L.M.; van Zutven, R.; de Groot, V.; Rietberg, M.B.; Nonnekes, J. Performance of a Personalized Smart Cueing Device to Detect Freezing of Gait in Parkinson’s Disease. J. Park. Dis. 2025; submitted. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Bourke, S.; Bennett, B.; Oluboyede, Y.; Li, T.; Longworth, L.; O’Sullivan, S.B.; Braverman, J.; Soare, I.A.; Shaw, J.W. Estimating the minimally important difference for the EQ-5D-5L and EORTC QLQ-C30 in cancer. Health Qual. Life Outcomes 2024, 22, 81. [Google Scholar] [CrossRef]
- Chen, P.; Lin, K.C.; Liing, R.J.; Wu, C.Y.; Chen, C.L.; Chang, K.C. Validity, responsiveness, and minimal clinically important difference of EQ-5D-5L in stroke patients undergoing rehabilitation. Qual. Life Res. 2016, 25, 1585–1596. [Google Scholar] [CrossRef]
- Hu, X.; Jing, M.; Zhang, M.; Yang, P.; Yan, X. Responsiveness and minimal clinically important difference of the EQ-5D-5L in cervical intraepithelial neoplasia: A longitudinal study. Health Qual. Life Outcomes 2020, 18, 324. [Google Scholar] [CrossRef]
- Nolan, C.M.; Longworth, L.; Lord, J.; Canavan, J.L.; Jones, S.E.; Kon, S.S.C.; Man, W.D.-C. The EQ-5D-5L health status questionnaire in COPD: Validity, responsiveness and minimum important difference. Thorax 2016, 71, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Tsai, A.P.Y.; Hur, S.A.; Wong, A.; Safavi, M.; Assayag, D.; Johannson, K.A.; Morisset, J.; Fell, C.; Fisher, J.H.; Manganas, H.; et al. Minimum important difference of the EQ-5D-5L and EQ-VAS in fibrotic interstitial lung disease. Thorax 2021, 76, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Hulzinga, F.; Nieuwboer, A.; Dijkstra, B.W.; Mancini, M.; Strouwen, C.; Bloem, B.R.; Ginis, P. The New Freezing of Gait Questionnaire: Unsuitable as an Outcome in Clinical Trials? Mov. Disord. Clin. Pract. 2020, 7, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Papapetropoulos, S.S. Patient diaries as a clinical endpoint in Parkinson’s disease clinical trials. CNS Neurosci. Ther. 2012, 18, 380–387. [Google Scholar] [CrossRef]
- Punin, C.; Barzallo, B.; Clotet, R.; Bermeo, A.; Bravo, M.; Bermeo, J.P.; Llumiguano, C. A Non-Invasive Medical Device for Parkinson’s Patients with Episodes of Freezing of Gait. Sensors 2019, 19, 737. [Google Scholar] [CrossRef]
- Clinicaltrials.gov. Cue2Walk, Cost-Effectiveness of Automated Freezing Detection and Provision of External Cues in Comparison to Usual Care in People With Parkinson’s Disease; National Library of Medicine: Bethesda, MD, USA, 2024.
- Yang, B.; Li, Y.; Wang, F.; Auyeung, S.; Leung, M.; Mak, M.; Tao, X. Intelligent wearable system with accurate detection of abnormal gait and timely cueing for mobility enhancement of people with Parkinson’s disease. Wearable Technol. 2022, 3, e12. [Google Scholar] [CrossRef]
- Donovan, S.; Lim, C.; Diaz, N.; Browner, N.; Rose, P.; Sudarsky, L.R.; Tarsy, D.; Fahn, S.; Simon, D.K. Laserlight cues for gait freezing in Parkinson’s disease: An open-label study. Park. Relat. Disord. 2011, 17, 240–245. [Google Scholar] [CrossRef]
- Thaut, M.H.; Rice, R.R.; Braun Janzen, T.; Hurt-Thaut, C.P.; McIntosh, G.C. Rhythmic auditory stimulation for reduction of falls in Parkinson’s disease: A randomized controlled study. Clin. Rehabil. 2019, 33, 34–43. [Google Scholar] [CrossRef]
- Cassimatis, C.; Liu, K.P.; Fahey, P.; Bissett, M. The effectiveness of external sensory cues in improving functional performance in individuals with Parkinson’s disease: A systematic review with meta-analysis. Int. J. Rehabil. Res. 2016, 39, 211–218. [Google Scholar] [CrossRef]
- Huang, X.; Dong, K.; Gan, C.; Xu, Z.; Lei, D.; Dong, X.; Liu, H.; Chen, X. Effect of Rhythmically Cued Exercise Interventions on Functions in Patients With Parkinson Disease: A Meta-Analysis. Phys. Ther. 2024, 104, pzad158. [Google Scholar] [CrossRef]
- Klaver, E.C.; van Vugt, J.P.P.; Bloem, B.R.; van Wezel, R.J.A.; Nonnekes, J.; Tjepkema-Cloostermans, M.C. Good vibrations: Tactile cueing for freezing of gait in Parkinson’s disease. J. Neurol. 2023, 270, 3424–3432. [Google Scholar] [CrossRef]
- Tosserams, A.; Keijsers, N.; Kapelle, W.; Kessels, R.P.C.; Weerdesteyn, V.; Bloem, B.R.; Nonnekes, J. Evaluation of Compensation Strategies for Gait Impairment in Patients With Parkinson Disease. Neurology 2022, 99, e2253–e2263. [Google Scholar] [CrossRef]
- Suteerawattananon, M.; Morris, G.S.; Etnyre, B.R.; Jankovic, J.; Protas, E.J. Effects of visual and auditory cues on gait in individuals with Parkinson’s disease. J. Neurol. Sci. 2004, 219, 63–69. [Google Scholar] [CrossRef]


| Participant Characteristic | Mean (Standard Deviation) or n |
|---|---|
| Age (years) | 74 (7) |
| Sex (M/F) | 12/4 |
| Disease duration (years) | 10 (7) |
| Device usage total (months) | 6 (4) |
| Device usage per day (h/day) | 9 (4) (n = 14) As needed (n = 2) |
| Domain | Device Usage per Day | |
|---|---|---|
| r | p | |
| Mobility | 0.320 | 0.257 |
| Self-care | 0.129 | 0.660 |
| ADL | 0.452 | 0.108 |
| Pain/discomfort | 0.329 | 0.272 |
| Anxious/depressed | 0.089 | 0.778 |
| Duration of FoG episode | 0.358 | 0.217 |
| Falls | 0.274 | 0.346 |
| Self-confidence | 0.188 | 0.520 |
| Customer satisfaction | 0.331 | 0.245 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van der Laan, M.; Rietberg, M.B.; van der Ent, M.; Waardenburg, F.; de Groot, V.; Nonnekes, J.; van Wegen, E.E.H. User Experiences of the Cue2walk Smart Cueing Device for Freezing of Gait in People with Parkinson’s Disease. Sensors 2025, 25, 4702. https://doi.org/10.3390/s25154702
van der Laan M, Rietberg MB, van der Ent M, Waardenburg F, de Groot V, Nonnekes J, van Wegen EEH. User Experiences of the Cue2walk Smart Cueing Device for Freezing of Gait in People with Parkinson’s Disease. Sensors. 2025; 25(15):4702. https://doi.org/10.3390/s25154702
Chicago/Turabian Stylevan der Laan, Matthijs, Marc B. Rietberg, Martijn van der Ent, Floor Waardenburg, Vincent de Groot, Jorik Nonnekes, and Erwin E. H. van Wegen. 2025. "User Experiences of the Cue2walk Smart Cueing Device for Freezing of Gait in People with Parkinson’s Disease" Sensors 25, no. 15: 4702. https://doi.org/10.3390/s25154702
APA Stylevan der Laan, M., Rietberg, M. B., van der Ent, M., Waardenburg, F., de Groot, V., Nonnekes, J., & van Wegen, E. E. H. (2025). User Experiences of the Cue2walk Smart Cueing Device for Freezing of Gait in People with Parkinson’s Disease. Sensors, 25(15), 4702. https://doi.org/10.3390/s25154702







