Automatic Stomatal Segmentation Based on Delaunay-Rayleigh Frequency Distance
Abstract
1. Introduction
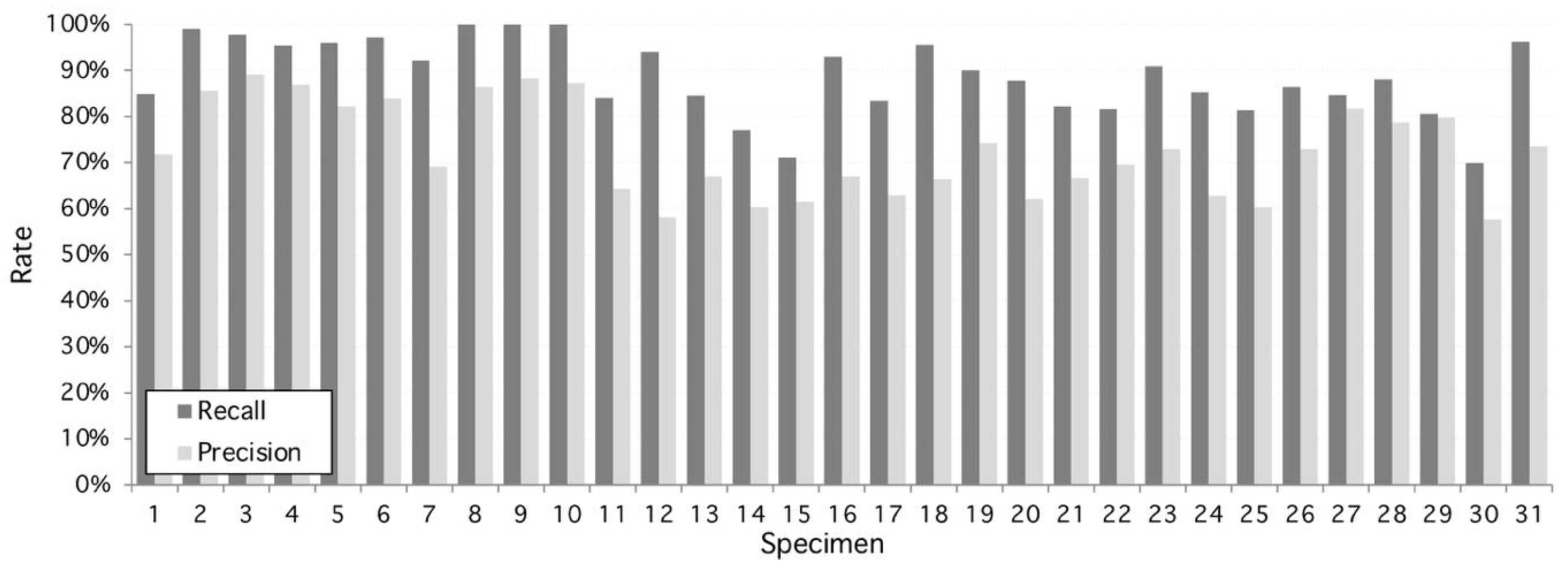
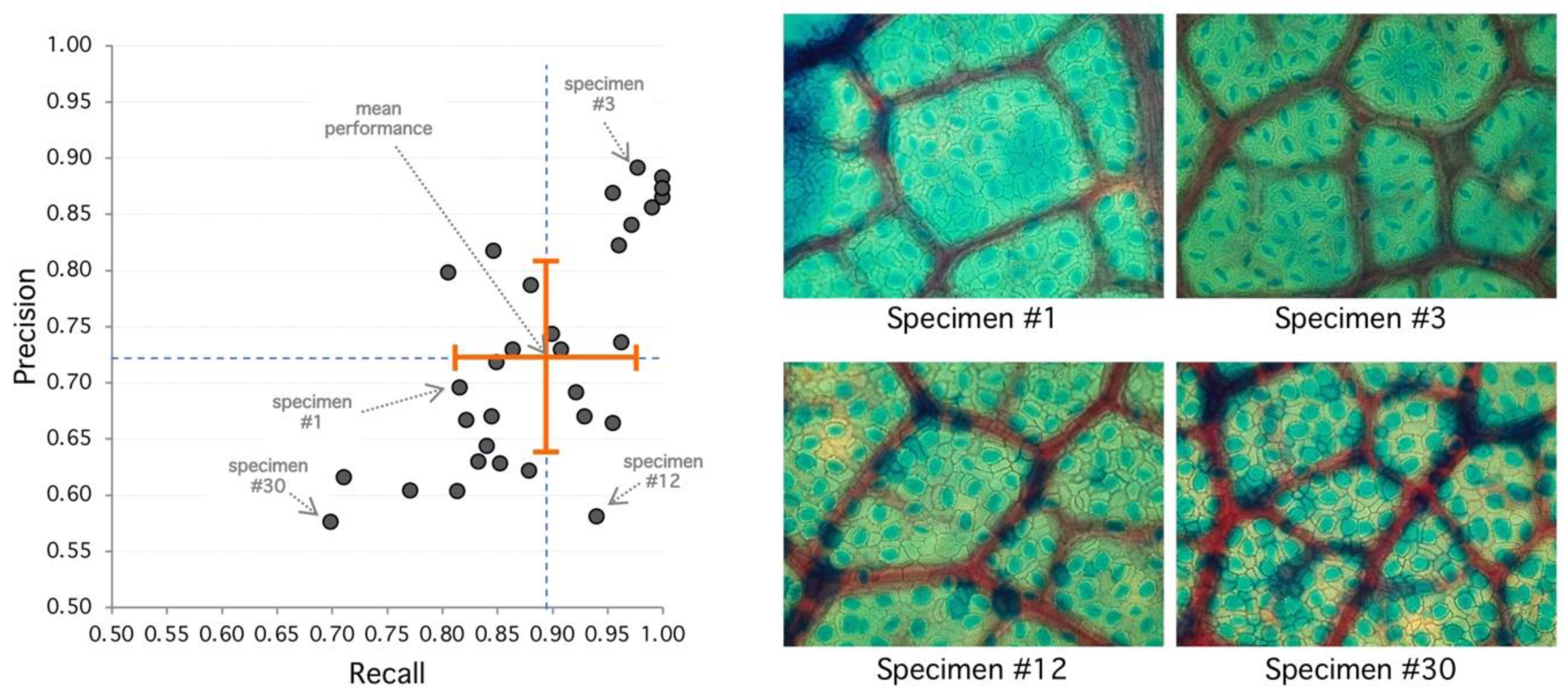
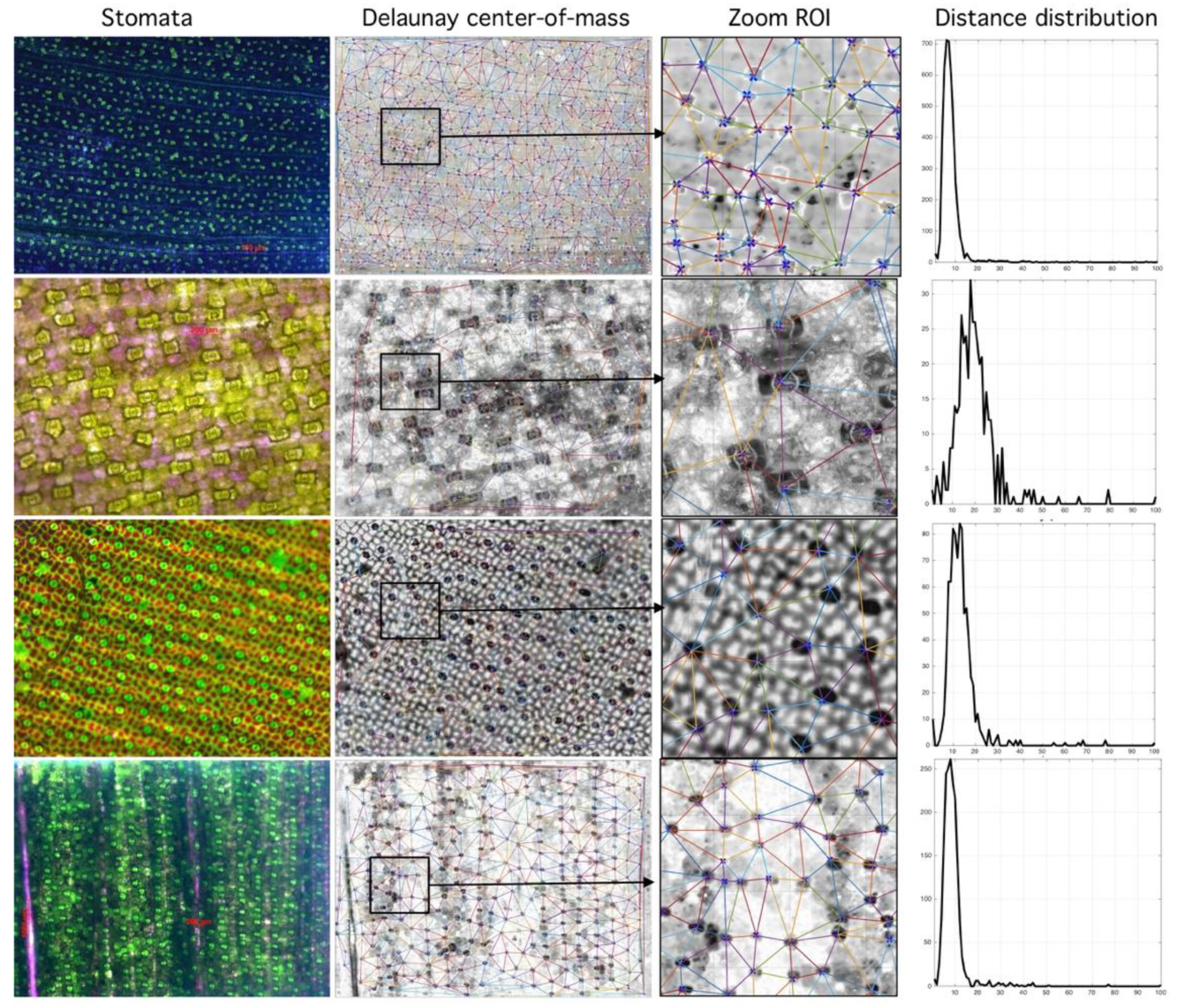
2. Results
3. Discussion
3.1. Manual Segmentation
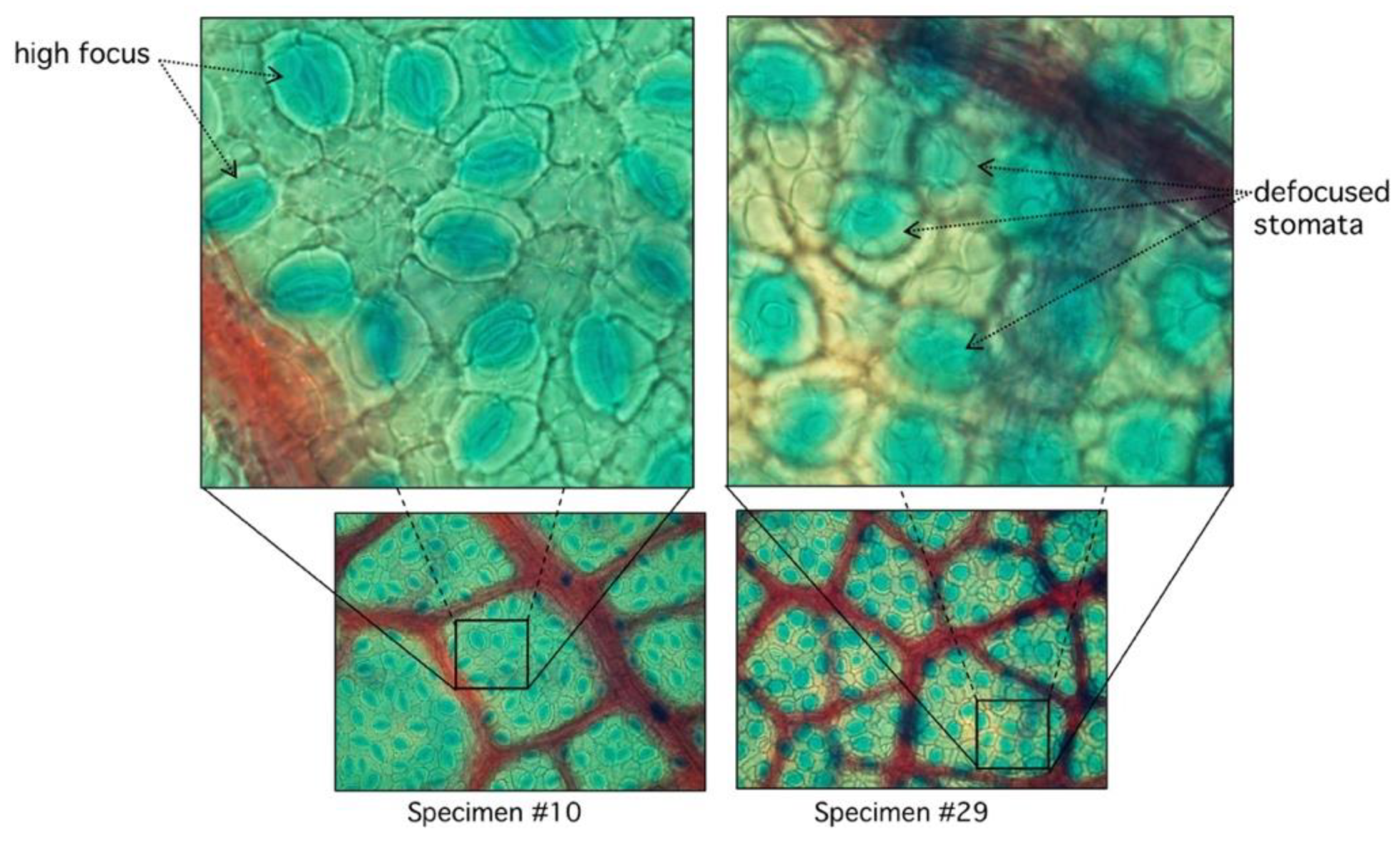
3.2. Defocus Stomata in Automatic Mode
3.3. Main Advantages and Disadvantages
4. Materials and Methods
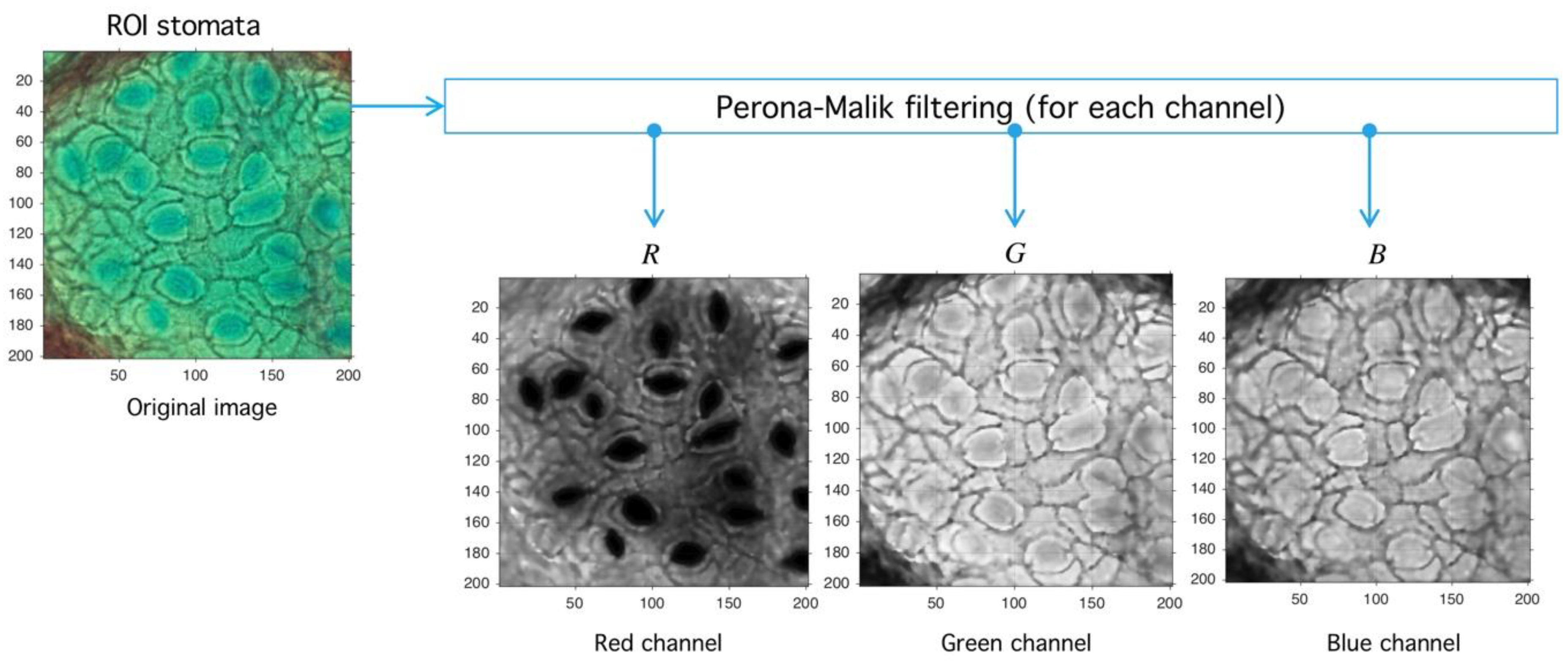
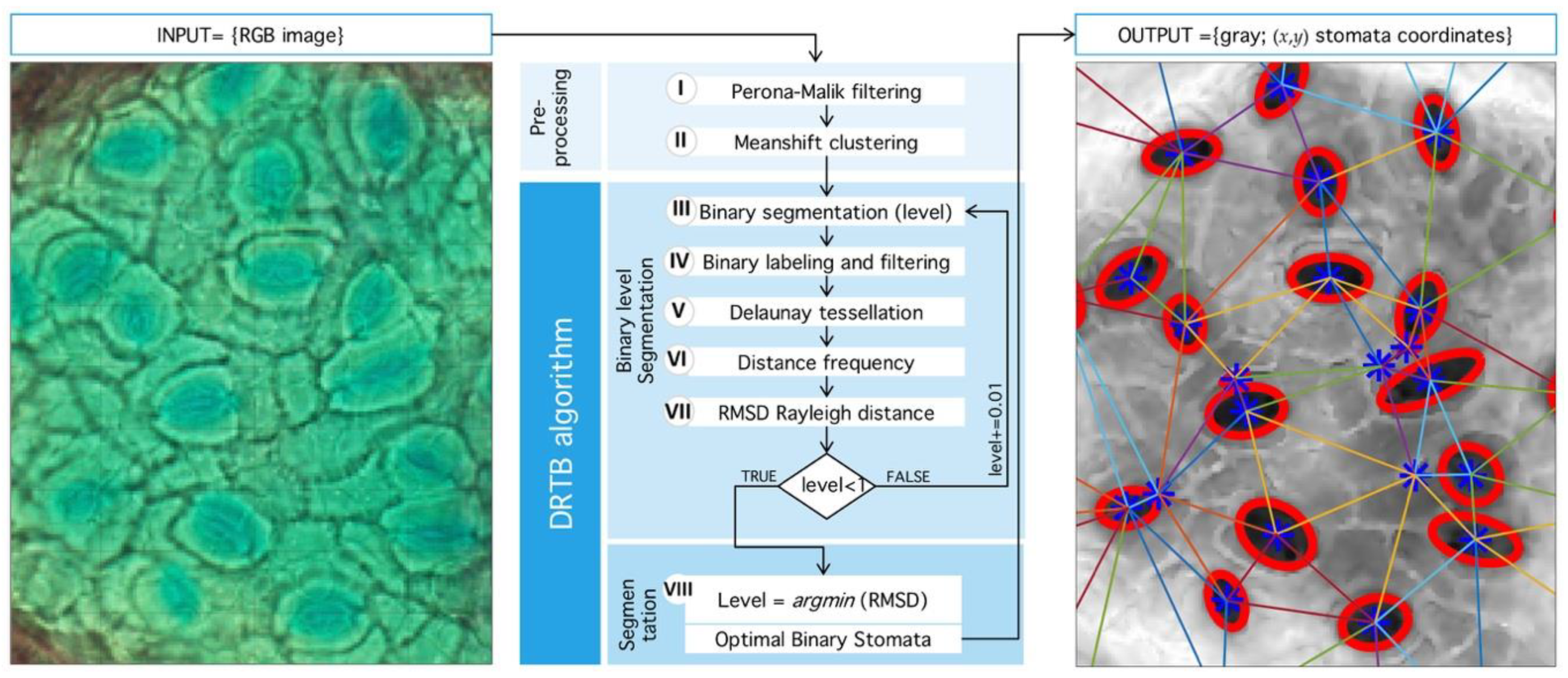
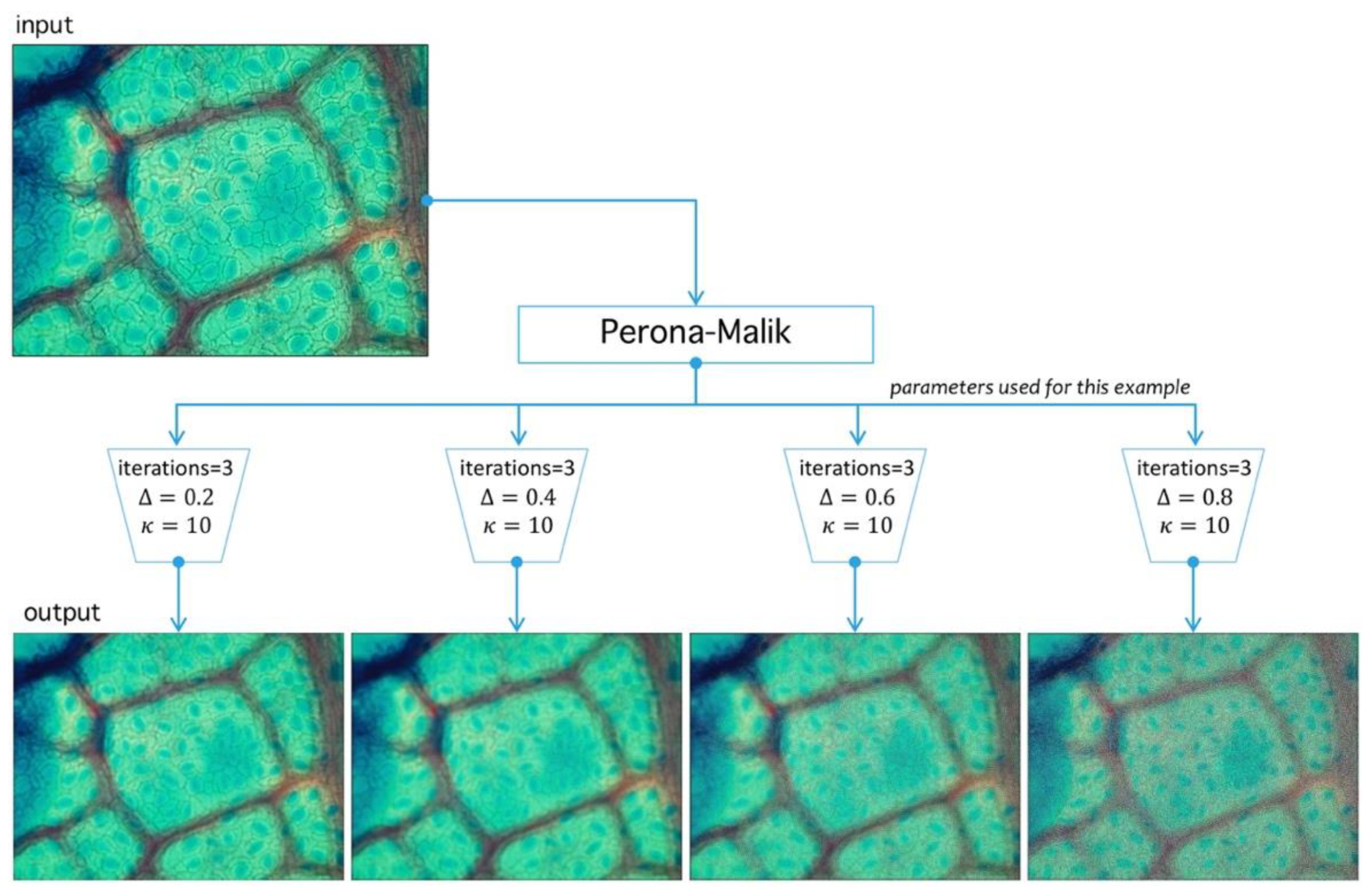
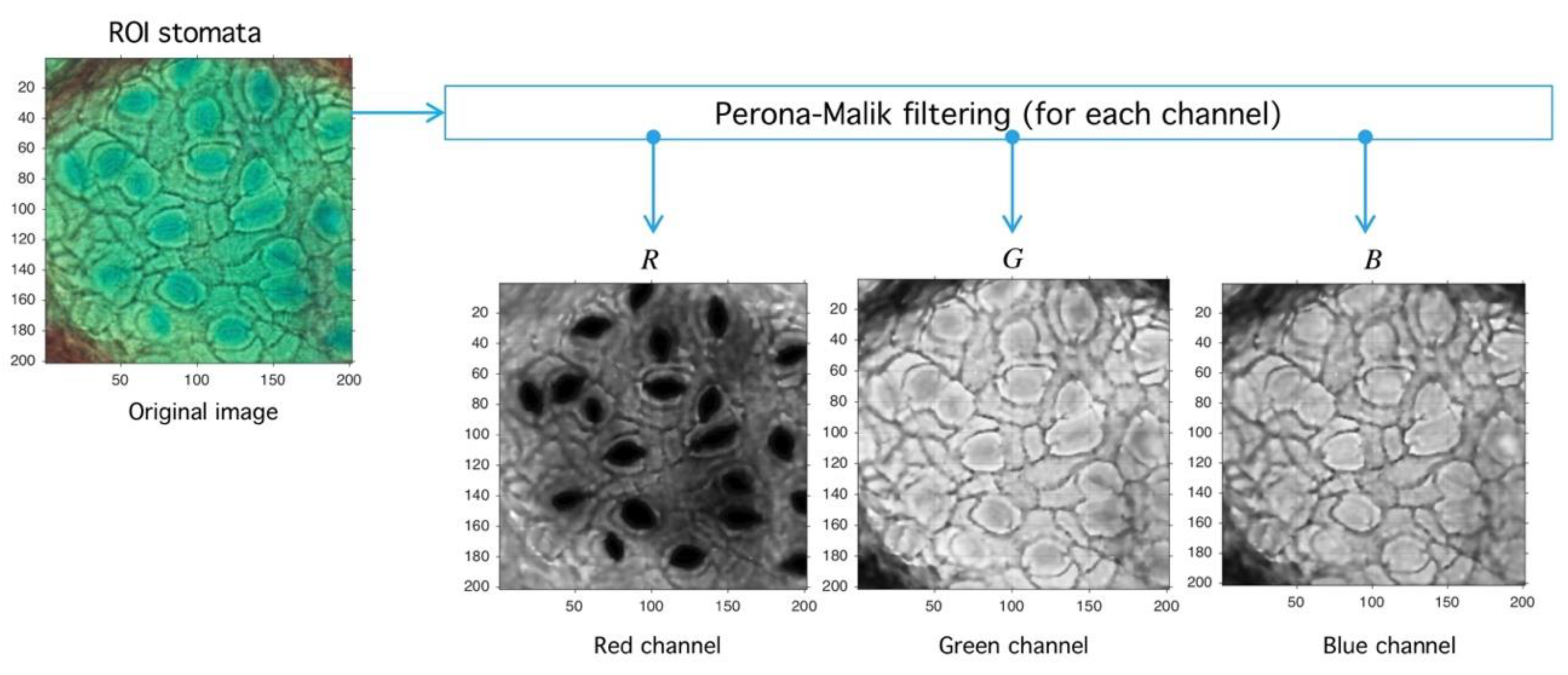
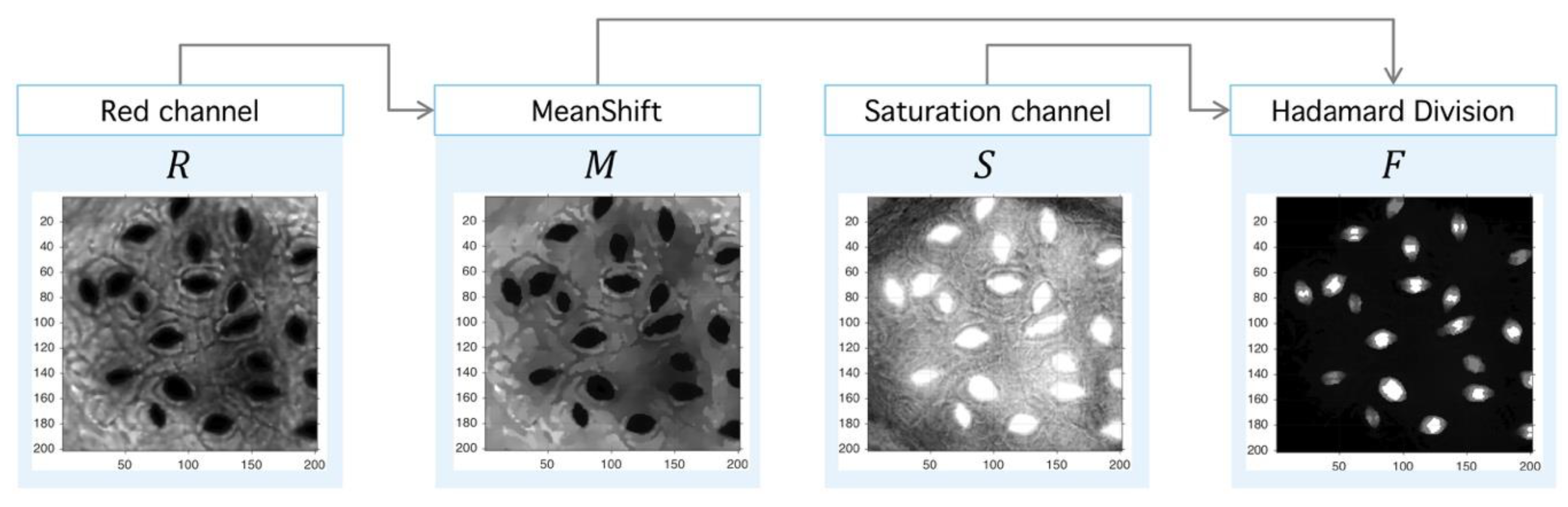
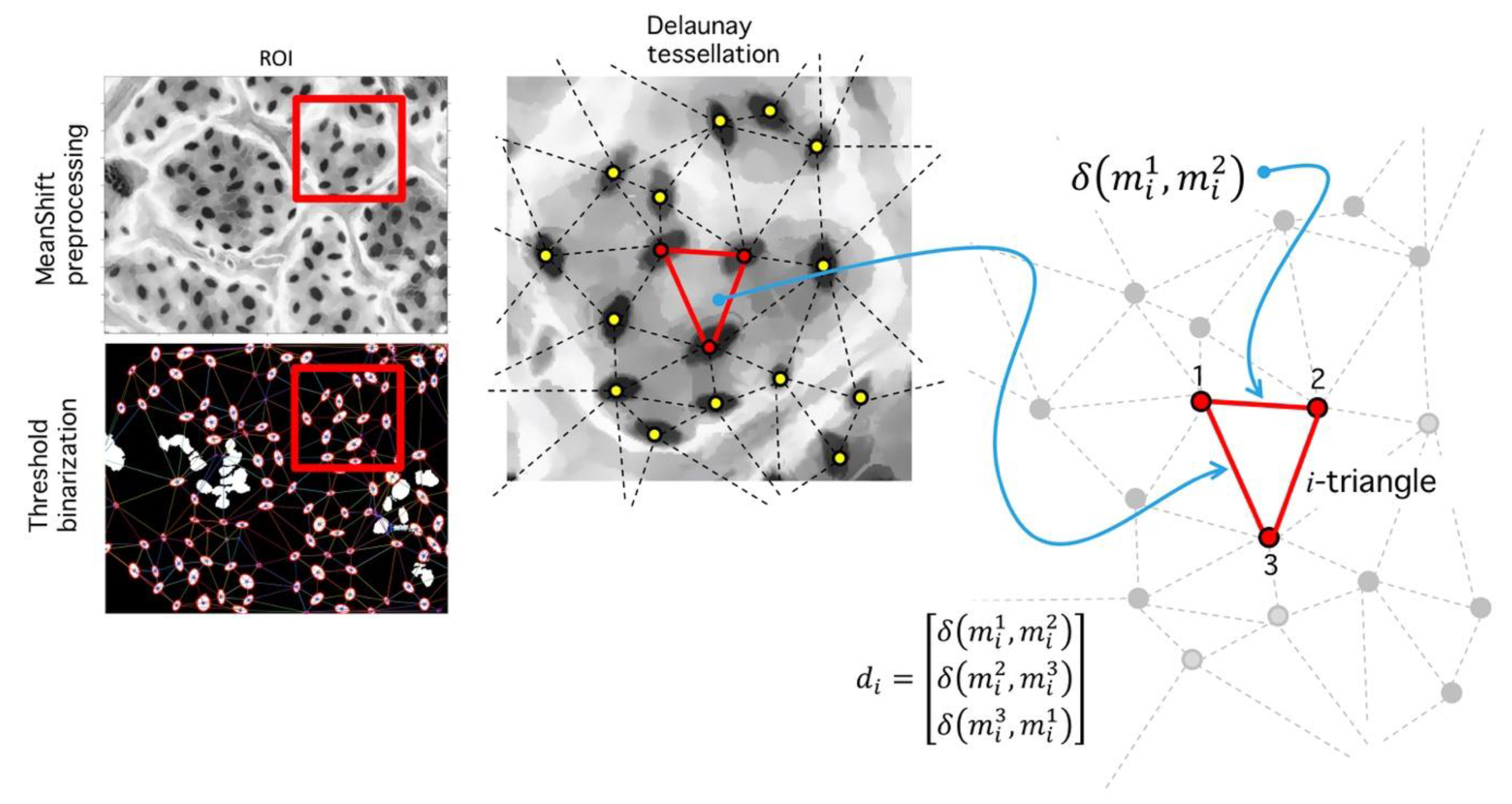
4.1. Preprocessing
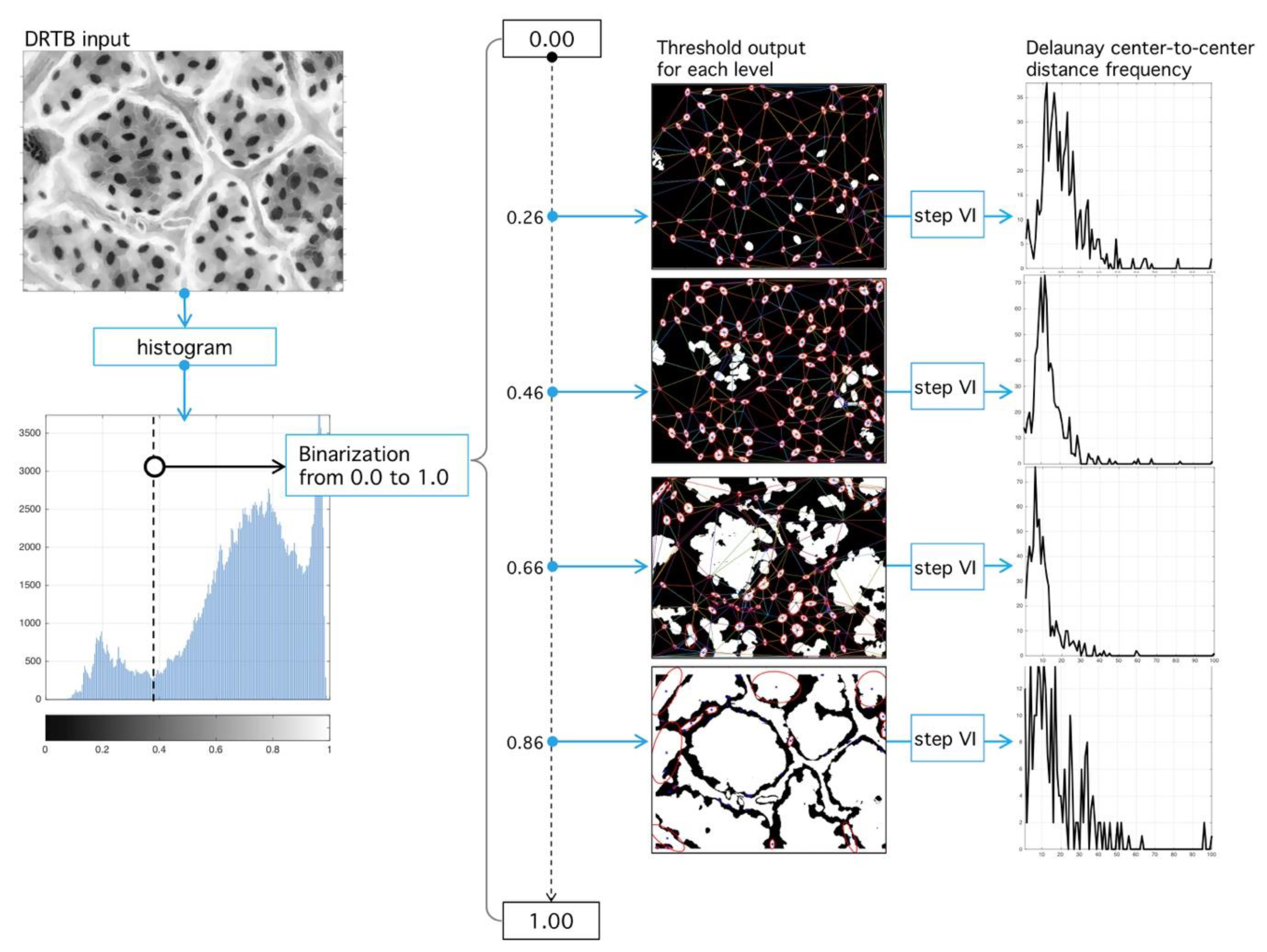
4.2. Delaunay-Rayleigh Threshold Binarization (DRTB Algorithm)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fanourakis, D.; Nikoloudakis, N.; Pappi, P.; Markakis, E.; Doupis, G.; Charova, S.; Delis, C.; Tsaniklidis, G. The Role of Proteases in Determining Stomatal Development and Tuning Pore Aperture: A Review. Plants 2020, 9, 340. [Google Scholar] [CrossRef] [PubMed]
- Fanourakis, D.; Aliniaeifard, S.; Sellin, A.; Giday, H.; Körner, O.; Nejad, A.R.; Delis, C.; Bouranis, D.; Koubouris, G.; Kambourakis, E.; et al. Stomatal behavior following mid- or long-term exposure to high relative air humidity: A review. Plant Physiol. Biochem. 2020, 153, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Pillitteri, L.J.; Torii, K.U. Mechanisms of Stomatal Development. Annu. Rev. Plant Biol. 2012, 63, 591–614. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Piao, S.; Myneni, R.B.; Huang, M.; Zeng, Z.; Canadell, J.G.; Ciais, P.; Sitch, S.; Friedlingstein, P.; Arneth, A.; et al. Greening of the Earth and its drivers. Nat. Clim. Chang. 2016, 6, 791–795. [Google Scholar] [CrossRef]
- Franks, P.J.; Drake, P.L.; Beerling, D.J. Plasticity in maximum stomatal conductance constrained by negative correlation between stomatal size and density: An analysis usingEucalyptus globulus. Plant Cell Environ. 2009, 32, 1737–1748. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, L.; Zhao, H.; Jiang, Z.; Cai, J. Differences in Near Isohydric and Anisohydric Behavior of Contrasting Poplar Hybrids (I-101 (Populus alba L.) × 84K (Populus alba L. × Populus glandulosa Uyeki)) under Drought-Rehydration Treatments. Forests 2020, 11, 402. [Google Scholar] [CrossRef]
- Fanourakis, D.; Hyldgaard, B.; Giday, H.; Aulik, I.; Bouranis, D.; Körner, O.; Ottosen, C.-O. Stomatal anatomy and closing ability is affected by supplementary light intensity in rose (Rosa hybrida L.). Hortic. Sci. 2019, 46, 81–89. [Google Scholar] [CrossRef]
- Niu, Y.; Ahammed, G.J.; Tang, C.; Guo, L.; Yu, J. Physiological and Transcriptome Responses to Combinations of Elevated CO2 and Magnesium in Arabidopsis thaliana. PLoS ONE 2016, 11, e0149301. [Google Scholar] [CrossRef]
- Croxdale, J.L. Stomatal patterning in angiosperms. Am. J. Bot. 2000, 87, 1069–1080. [Google Scholar] [CrossRef]
- Franks, P.J.; Beerling, D.J. Maximum leaf conductance driven by CO2 effects on stomatal size and density over geologic time. Proc. Natl. Acad. Sci. USA 2009, 106, 10343–10347. [Google Scholar] [CrossRef]
- Dow, G.J.; Berry, J.A.; Bergmann, D.C. The physiological importance of developmental mechanisms that enforce proper stomatal spacing inArabidopsis thaliana. New Phytol. 2014, 201, 1205–1217. [Google Scholar] [CrossRef]
- Li, K.; Huang, J.; Song, W.; Wang, J.; Lv, S.; Wang, X. Automatic segmentation and measurement methods of living stomata of plants based on the CV model. Plant Methods 2019, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Li, J.; Li, K.; Chen, J.; Huang, J. An Automatic Method for Stomatal Pore Detection and Measurement in Microscope Images of Plant Leaf Based on a Convolutional Neural Network Model. Forests 2020, 11, 954. [Google Scholar] [CrossRef]
- Naulin, P.I.; Valenzuela, G.; Estay, S.A. Size matters: Point pattern analysis biases the estimation of spatial properties of stomata distribution. New Phytol. 2016, 213, 1956–1960. [Google Scholar] [CrossRef] [PubMed]
- Bhaiswar, N.; Dixit, D.V.V.; Student, P.G. A Review: Methods of Automatic Stomata Detection and Counting Through Microscopic Images of a Leaf. IJIRSET 2007, 5, 6. [Google Scholar]
- Da Silva, N.R.; Oliveira, M.W.D.S.; Filho, H.A.D.A.; Pinheiro, L.F.S.; Rossatto, D.R.; Kolb, R.M.; Bruno, O.M. Leaf epidermis images for robust identification of plants. Sci. Rep. 2016, 6, 25994. [Google Scholar] [CrossRef] [PubMed]
- Jayakody, H.; Liu, S.; Whitty, M.; Petrie, P. Microscope image based fully automated stomata detection and pore measurement method for grapevines. Plant Methods 2017, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Florindo, J.B.; Landini, G.; Filho, H.A.; Bruno, O.M. Analysis of Stomata Distribution Patterns for Quantification of the Foliar Plasticity of Tradescantia Zebrina. J. Phys. Conf. Ser. 2015, 633, 012113. [Google Scholar] [CrossRef]
- Zhu, J.; Yu, Q.; Xu, C.; Li, J.; Qin, G. Rapid Estimation of Stomatal Density and Stomatal Area of Plant Leaves Based on Object-Oriented Classification and Its Ecological Trade-Off Strategy Analysis. Forests 2018, 9, 616. [Google Scholar] [CrossRef]
- Fetter, K.C.; Eberhardt, S.; Barclay, R.S.; Wing, S.; Keller, S.R. StomataCounter: A neural network for automatic stomata identification and counting. New Phytol. 2019, 223, 1671–1681. [Google Scholar] [CrossRef]
- Toda, Y.; Toh, S.; Bourdais, G.; Robatzek, S.; MacLean, D.; Kinoshita, T. DeepStomata: Facial Recognition Technology for Automated Stomatal Aperture Measurement. Bioinformatics 2018, 365098. [Google Scholar] [CrossRef]
- Casado-García, A.; Del-Canto, A.; Sanz-Saez, A.; Pérez-López, U.; Bilbao-Kareaga, A.; Fritschi, F.B.; Miranda-Apodaca, J.; Muñoz-Rueda, A.; Sillero-Martínez, A.; Yoldi-Achalandabaso, A.; et al. LabelStoma: A tool for stomata detection based on the YOLO algorithm. Comput. Electron. Agric. 2020, 178, 105751. [Google Scholar] [CrossRef]
- García Álvarez, S.; Morla Juaristi, C.; Solana Gutiérrez, J.; García-Amorena, I. Taxonomic differences between Pinus sylvestris and P. uncinata revealed in the stomata and cuticle characters for use in the study of fossil material. Rev. Palaeobot. Palynol. 2009, 155, 61–68. [Google Scholar] [CrossRef]
- Sweeney, C.A. A key for the identification of stomata of the native conifers of Scandinavia. Rev. Palaeobot. Palynol. 2004, 128, 281–290. [Google Scholar] [CrossRef]
- Zhang, K.; Zhao, Y.; Guo, X. Conifer stomata analysis in paleoecological studies on the Loess Plateau: An example from Tianchi Lake, Liupan Mountains. J. Arid. Environ. 2011, 75, 1209–1213. [Google Scholar] [CrossRef]
- Camargo, M.A.B.; Marenco, R.A. Density, size and distribution of stomata in 35 rainforest tree species in Central Amazonia. Acta Amaz. 2011, 41, 205–212. [Google Scholar] [CrossRef]
- Eisele, J.F.; Fäßler, F.; Bürgel, P.F.; Chaban, C. A Rapid and Simple Method for Microscopy-Based Stomata Analyses. PLoS ONE 2016, 11, e0164576. [Google Scholar] [CrossRef]
- Shapiro, B.E.; Jönsson, H.; Sahlin, P.; Heisler, M.; Roeder, A.; Burl, M.; Meyerowitz, E.M.; Mjolsness, E.D. Tessellations and Pattern Formation in Plant Growth and Development. In Tessellations in the Sciences: Virtues, Techniques and Applications of Geometric Tilings; van de Weijgaert, R., Vegter, G., Ritzerveld, J., Icke, V., Eds.; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Karabourniotis, G.; Tzobanoglou, D.; Nikolopoulos, D.; Liakopoulos, G. Epicuticular Phenolics Over Guard Cells: Exploitation for in situ Stomatal Counting by Fluorescence Microscopy and Combined Image Analysis. Ann. Bot. 2001, 87, 631–639. [Google Scholar] [CrossRef]
- Cao, L.; Bala, G.; Caldeira, K.; Nemani, R.; Ban-Weiss, G. Importance of carbon dioxide physiological forcing to future climate change. Proc. Natl. Acad. Sci. USA 2010, 107, 9513–9518. [Google Scholar] [CrossRef]
- Konrad, W.; Roth-Nebelsick, A.; Grein, M. Modelling of stomatal density response to atmospheric. J. Theor. Biol. 2008, 253, 638–658. [Google Scholar] [CrossRef] [PubMed]
- Chater, C.; Peng, K.; Movahedi, M.; Dunn, J.A.; Walker, H.J.; Liang, Y.-K.; McLachlan, D.H.; Casson, S.A.; Isner, J.C.; Wilson, I.D.; et al. Elevated CO2 -Induced Responses in Stomata Require ABA and ABA Signaling. Curr. Biol. 2015, 25, 2709–2716. [Google Scholar] [CrossRef] [PubMed]
- Tomimatsu, H.; Tang, Y. Effects of high CO2 levels on dynamic photosynthesis: Carbon gain, mechanisms, and environmental interactions. J. Plant Res. 2016, 129, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Franklin, G.L. Preparation of Thin Sections of Synthetic Resins and Wood-Resin Composites, and a New Macerating Method for Wood. Nat. Cell Biol. 1945, 155, 51. [Google Scholar] [CrossRef]
- Peat, H.J.; Fitter, A.H. A comparative study of the distribution and density of stomata in the British flora. Biol. J. Linn. Soc. 1994, 52, 377–393. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Xu, M.; Hou, R.; Shen, R.; Qiu, S.; Ouyang, Z. Effects of experimental warming on stomatal traits in leaves of maize (Zea may L.). Ecol. Evol. 2013, 3, 3095–3111. [Google Scholar] [CrossRef] [PubMed]
- Fanourakis, D.; Heuvelink, E.; Carvalho, S.M.P. Spatial heterogeneity in stomatal features during leaf elongation: An analysis using Rosa hybrida. Funct. Plant Biol. 2015, 42, 737. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Zhou, L.; Shen, Z.-J.; Shen, Z.-X.; Zhang, Y.-Q.; Wang, G.-X. Stomatal clustering, a new marker for environmental perception and adaptation in terrestrial plants. Bot. Stud. 2010, 51, 12. [Google Scholar]
- Weickert, J. Anisotropic Diffusion in Image Processing; Teubner, B.G., Ed.; European Consortium for Mathematics in Industry: Stuttgart, Germany, 1998. [Google Scholar]
- Buades, A.; Coll, B.; Morel, J.M. A Review of Image Denoising Algorithms, with a New One. Multiscale Model. Simul. 2005, 4, 490–530. [Google Scholar] [CrossRef]
- Liu, K.; Tan, J.; Ai, L. Hybrid regularizers-based adaptive anisotropic diffusion for image denoising. SpringerPlus 2016, 5, 404. [Google Scholar] [CrossRef][Green Version]
- Staff, L.; Hurd, P.; Reale, L.; Seoighe, C.; Rockwood, A.; Gehring, C. The Hidden Geometries of the Arabidopsis thaliana Epidermis. PLoS ONE 2012, 7, e43546. [Google Scholar] [CrossRef]
- Chen, D.; Aw, W.Y.; Devenport, D.; Torquato, S. Structural Characterization and Statistical-Mechanical Model of Epidermal Patterns. Biophys. J. 2016, 111, 2534–2545. [Google Scholar] [CrossRef] [PubMed]
- Kamalaveni, V.; Rajalakshmi, R.A.; Narayanankutty, K. Image Denoising Using Variations of Perona-Malik Model with Different Edge Stopping Functions. Procedia Comput. Sci. 2015, 58, 673–682. [Google Scholar] [CrossRef]
- Tschumperlé, D.; Deriche, R. Anisotropic Diffusion Partial Differential Equations in MultiChannel Image Processing: Framework and Applications; Advances in Imaging and Electron Physics (AIEP); Academic Press: Cambridge, MA, USA, 2007; pp. 145–209. [Google Scholar]
- Perona, P.; Malik, J. Scale-space and edge detection using anisotropic diffusion. IEEE Trans. Pattern Anal. Mach. Intell. 1990, 12, 629–639. [Google Scholar] [CrossRef]
- Fashing, M.; Tomasi, C. Mean shift is a bound optimization. IEEE Trans. Pattern Anal. Mach. Intell. 2005, 27, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Reams, R. Hadamard inverses, square roots and products of almost semidefinite matrices. Linear Algebra Appl. 1999, 288, 35–43. [Google Scholar] [CrossRef]
- Leys, C.; Klein, O.; Bernard, P.; Licata, L. Detecting outliers: Do not use standard deviation around the mean, use absolute deviation around the median. J. Exp. Soc. Psychol. 2013, 49, 764–766. [Google Scholar] [CrossRef]
- Grimmett, G.; Stirzaker, D. Probability and Random Processes; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Vanselow, R. About Delaunay Triangulations and Discrete Maximum Principles for the Linear Conforming FEM Applied to the Poisson Equation. Appl. Math. 2001, 46, 13–28. [Google Scholar] [CrossRef]

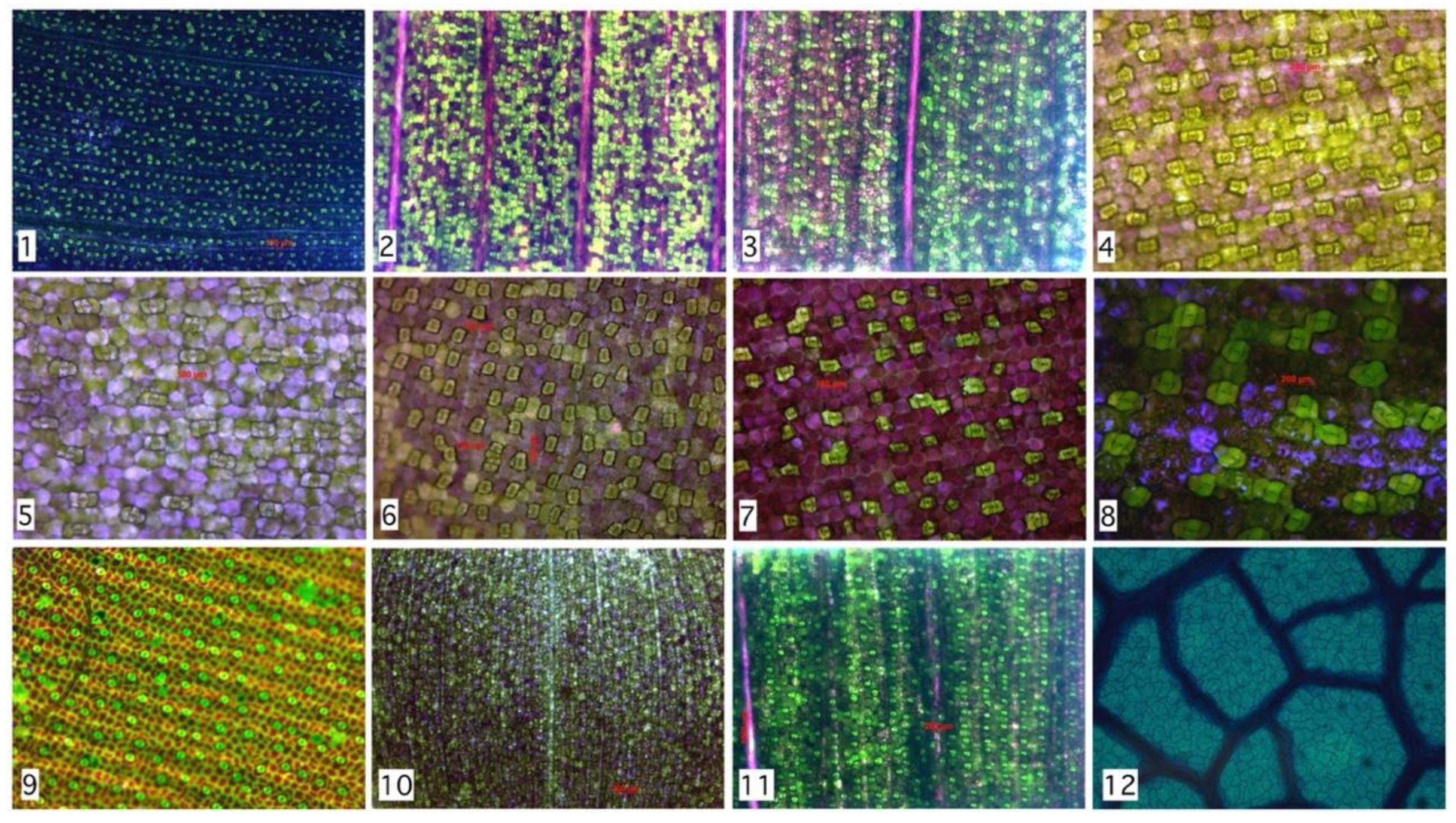
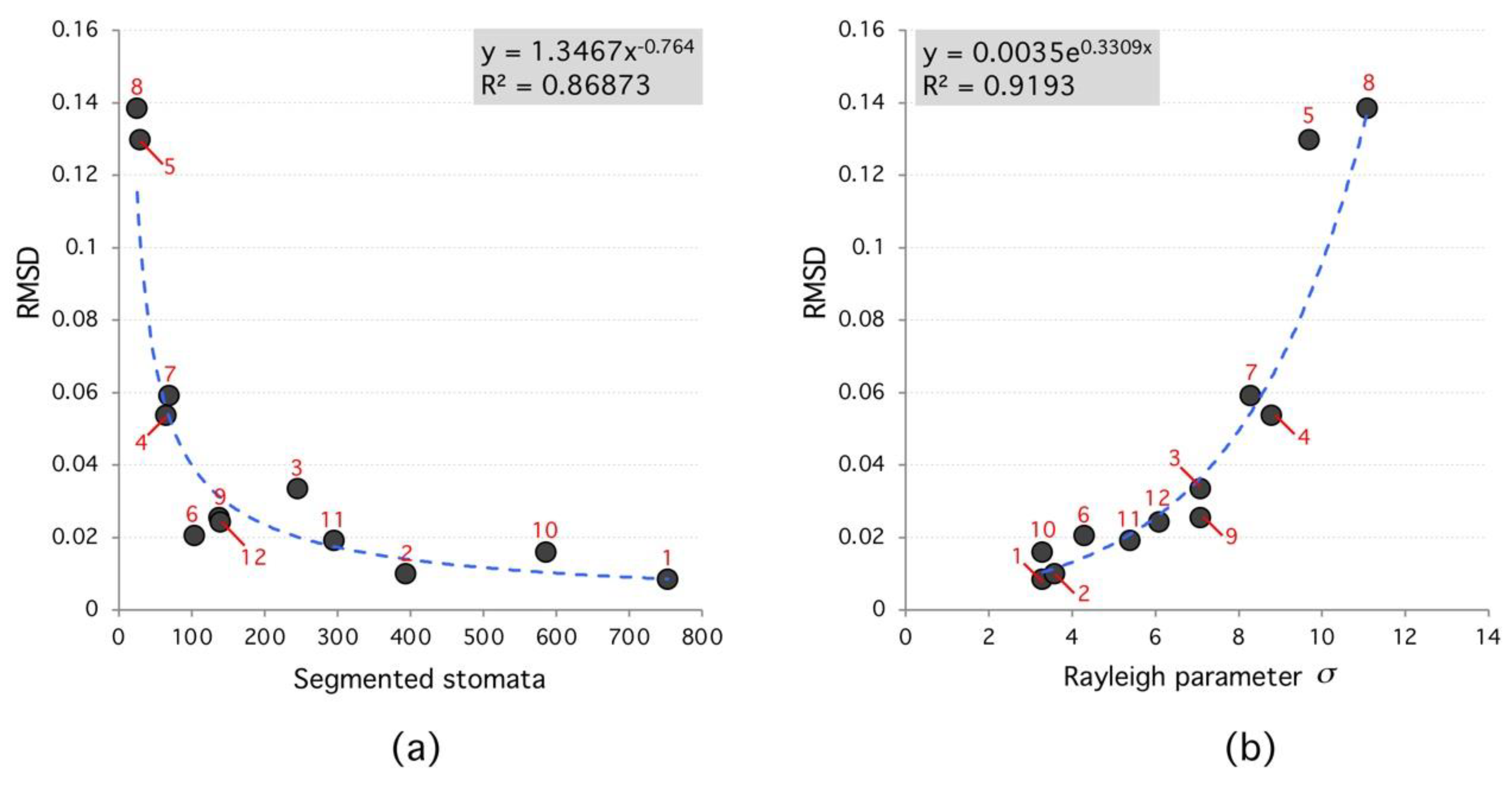

| # | Species Analyzed | Stomata’s Number | RMSD | Rayleigh Parameter | Group | Family |
|---|---|---|---|---|---|---|
| 1 | Tradescantia Zebrina | 753 | 0.00832 | 3.3 | Monocot | C |
| 2 | Tradescantia Pallida—under 24 h of light | 394 | 0.00983 | 3.6 | Monocot | C |
| 3 | Tradescantia Pallida—in natural condition | 245 | 0.03327 | 7.1 | Monocot | C |
| 4 | Callisia reppens | 65 | 0.05358 | 8.8 | Monocot | C |
| 5 | Callisia reppens | 29 | 0.12969 | 9.7 | Monocot | C |
| 6 | Callisia reppens | 104 | 0.02037 | 4.3 | Monocot | C |
| 7 | Tradescantia Zebrina | 69 | 0.05905 | 8.3 | Monocot | C |
| 8 | Tradescantia Pallid | 25 | 0.13832 | 11.1 | Monocot | C |
| 9 | Ctenanthe Oppenheimiana | 138 | 0.02531 | 7.1 | Monocot | M |
| 10 | Calisia reppens—using stereoscope 15× | 586 | 0.01578 | 3.3 | Monocot | C |
| 11 | Tradescantia Pallida using stereoscope 15× | 295 | 0.01902 | 5.4 | Monocot | C |
| 12 | Hymenaea Courbaril | 139 | 0.02420 | 6.1 | Dicot | F |
| Total Regions | 2842 | μ = 0.04473 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrasco, M.; Toledo, P.A.; Velázquez, R.; Bruno, O.M. Automatic Stomatal Segmentation Based on Delaunay-Rayleigh Frequency Distance. Plants 2020, 9, 1613. https://doi.org/10.3390/plants9111613
Carrasco M, Toledo PA, Velázquez R, Bruno OM. Automatic Stomatal Segmentation Based on Delaunay-Rayleigh Frequency Distance. Plants. 2020; 9(11):1613. https://doi.org/10.3390/plants9111613
Chicago/Turabian StyleCarrasco, Miguel, Patricio A. Toledo, Ramiro Velázquez, and Odemir M. Bruno. 2020. "Automatic Stomatal Segmentation Based on Delaunay-Rayleigh Frequency Distance" Plants 9, no. 11: 1613. https://doi.org/10.3390/plants9111613
APA StyleCarrasco, M., Toledo, P. A., Velázquez, R., & Bruno, O. M. (2020). Automatic Stomatal Segmentation Based on Delaunay-Rayleigh Frequency Distance. Plants, 9(11), 1613. https://doi.org/10.3390/plants9111613







