Harnessing Seed Endophytic Microbiomes: A Hidden Treasure for Enhancing Sustainable Agriculture
Abstract
1. Introduction
2. Why the Attention on Seed Endophytic Microbiomes?
3. Diversity and Community Assemblage of Seed Endophytic Microbiome
3.1. Bacteria
3.2. Fungi
3.3. Archaea
3.4. Endophyte–Pathogen Interactions
4. Mode of Transmission of Seed-Borne Endophytic Microbiomes
4.1. Horizontal Transmission
4.2. Vertical Transmission
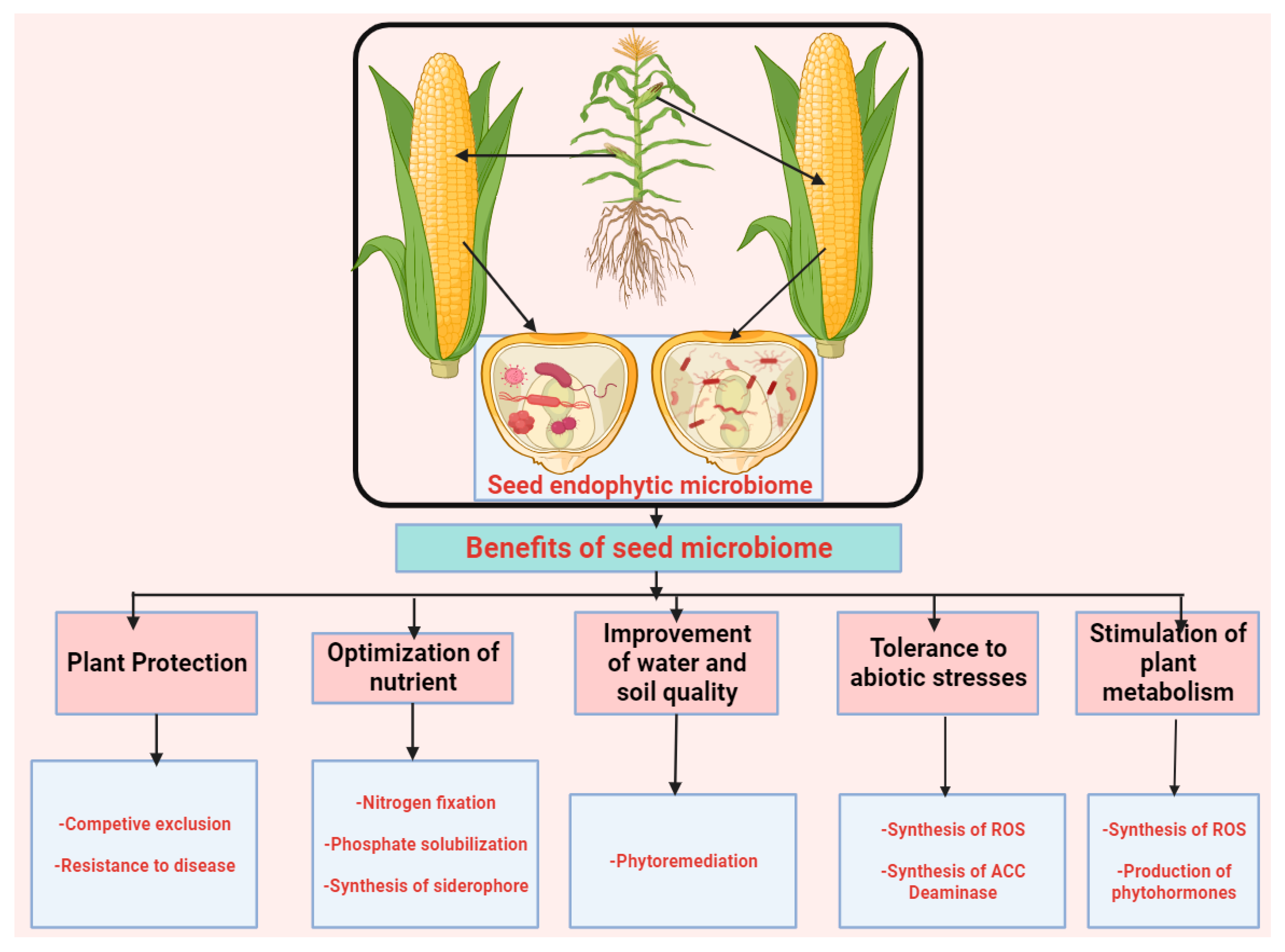
5. Potentials of Seed Endophytic Microbiome in Sustainable Agriculture
5.1. Direct Plant Growth Promotion
5.2. Biocontrol
5.3. Role of Seed Endophytes in Inducing Stress Tolerance
5.3.1. Tolerance to Heavy Metal
5.3.2. Tolerance to Drought
5.3.3. Tolerance to Salt Stress
6. Do Seed Endophytic Microbiomes Have Any Connection with the Quality of Plant Seeds?
7. Recent Advances in Seed Microbiome Identification and Their Role in Sustainable Agriculture
8. Future Prospects
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Malfanova, N.; Lugtenberg, B.J.; Berg, G. Bacterial endophytes, who and where and what are they doing there? In Molecular Microbial Ecology of the Rhizosphere; De Bruijn, F.J., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2013; pp. 391–403. [Google Scholar]
- Fadiji, A.E.; Babalola, O.O. Exploring the potentialities of beneficial endophytes for improved plant growth. Saudi J. Biol. Sci. 2020, 27, 3622–3633. [Google Scholar] [CrossRef] [PubMed]
- Truyens, S.; Weyens, N.; Cuypers, A.; Vangronsveld, J. Changes in the population of seed bacteria of transgenerationally Cd-exposed Arab. Thaliana. Plant Biol. 2013, 15, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Sahu, P.K. Vertical transmission of diverse cultivation-recalcitrant endophytic bacteria elucidated using watermelon seed embryos. Front. Microbiol. 2021, 12, 635810. [Google Scholar] [CrossRef]
- Nelson, E.B. Microbial dynamics and interactions in the spermosphere. Annu. Rev. Phytopathol. 2004, 42, 271–309. [Google Scholar] [CrossRef]
- Klupczyńska, E.A.; Pawłowski, T.A. Regulation of seed dormancy and germination mechanisms in a changing environment. Int. J. Mol. Sci. 2021, 22, 1357. [Google Scholar] [CrossRef] [PubMed]
- Chee-Sanford, J.C.; Williams, M.M.; Davis, A.S.; Sims, G.K. Do microorganisms influence seed-bank dynamics? Weed Sci. 2006, 54, 575–587. [Google Scholar] [CrossRef]
- Li, D.; Chen, W.; Luo, W.; Zhang, H.; Liu, Y.; Shu, D.; Wei, G. Seed microbiomes promote Astragalus mongholicus seed germination through pathogen suppression and cellulose degradation. Microbiome 2025, 13, 23. [Google Scholar]
- Mukhopadhyay, K.; Garrison, N.K.; Hinton, D.M.; Bacon, C.W.; Khush, G.S.; Peck, H.D.; Datta, N. Identification and characterization of bacterial endophytes of rice. Mycopathologia 1996, 134, 151–159. [Google Scholar] [CrossRef]
- Hallmann, J.; Quadt-Hallmann, A.; Mahaffee, W.; Kloepper, J. Bacterial endophytes in agricultural crops. Can. J. Microbiol. 1997, 43, 895–914. [Google Scholar] [CrossRef]
- Mundt, J.O.; Hinkle, N.F. Bacteria within ovules and seeds. Appl. Environ. Microbiol. 1976, 32, 694–698. [Google Scholar] [CrossRef]
- Nelson, E.B. The seed microbiome: Origins, interactions, and impacts. Plant Soil 2018, 422, 7–34. [Google Scholar] [CrossRef]
- Adams, D. Seed-borne bacterial endophytes in defferent cotton cultivars. Phytopathology 1996, 86, S97. [Google Scholar]
- Samreen, T.; Naveed, M.; Nazir, M.Z.; Asghar, H.N.; Khan, M.I.; Zahir, Z.A.; Kanwal, S.; Jeevan, B.; Sharma, D.; Meena, V.S. Seed associated bacterial and fungal endophytes: Diversity, life cycle, transmission, and application potential. Appl. Soil Ecol. 2021, 168, 104191. [Google Scholar] [CrossRef]
- Ugarte, R.M.; Martínez, M.H.; Díaz-Santiago, E.; Pugnaire, F.I. Microbial controls on seed germination. Soil Biol. Biochem. 2024, 199, 109576. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Srivastava, A.K.; Rajput, V.D.; Chauhan, P.K.; Bhojiya, A.A.; Jain, D.; Chaubey, G.; Dwivedi, P.; Sharma, B.; Minkina, T. Root exudates: Mechanistic insight of plant growth promoting rhizobacteria for sustainable crop production. Front. Microbiol. 2022, 13, 916488. [Google Scholar] [CrossRef]
- Rijavec, T.; Lapanje, A.; Dermastia, M.; Rupnik, M. Isolation of bacterial endophytes from germinated maize kernels. Can. J. Microbiol. 2007, 53, 802–808. [Google Scholar] [CrossRef]
- Sanz-Puente, I.; Redondo-Salvo, S.; Torres-Cortés, G.; de Toro, M.; Fernandes, S.; Börner, A.; Lorenzo, Ó.; de la Cruz, F.; Robledo, M. Vertical transmission of core endophytes through the seeds. bioRxiv 2025. [Google Scholar] [CrossRef]
- Mitter, B.; Pfaffenbichler, N.; Flavell, R.; Compant, S.; Antonielli, L.; Petric, A.; Berninger, T.; Naveed, M.; Sheibani-Tezerji, R.; Von Maltzahn, G. A new approach to modify plant microbiomes and traits by introducing beneficial bacteria at flowering into progeny seeds. Front. Microbiol. 2017, 8, 11. [Google Scholar] [CrossRef]
- Kim, H.; Jeon, J.; Lee, K.K.; Lee, Y.-H. Longitudinal transmission of bacterial and fungal communities from seed to seed in rice. Commun. Biol. 2022, 5, 772. [Google Scholar] [CrossRef]
- Hill, N.; Bouton, J.; Hiatt, E.; Kittle, B. Seed maturity, germination, and endophyte relationships in tall fescue. Crop Sci. 2005, 45, 859–863. [Google Scholar] [CrossRef]
- Bard, N.W.; Cronk, Q.C.; Davies, T.J. Fungal endophytes can modulate plant invasion. Biol. Rev. 2024, 99, 1652–1671. [Google Scholar] [CrossRef]
- Idbella, M.; Bonanomi, G.; De Filippis, F.; Amor, G.; Chouyia, F.E.; Fechtali, T.; Mazzoleni, S. Contrasting effects of Rhizophagus irregularis versus bacterial and fungal seed endophytes on Trifolium repens plant-soil feedback. Mycorrhiza 2021, 31, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Carrión, V.J.; Perez-Jaramillo, J.; Cordovez, V.; Tracanna, V.; De Hollander, M.; Ruiz-Buck, D.; Mendes, L.W.; van Ijcken, W.F.; Gomez-Exposito, R.; Elsayed, S.S. Pathogen-induced activation of disease-suppressive functions in the endophytic root microbiome. Science 2019, 366, 606–612. [Google Scholar] [CrossRef]
- Koskimäki, J.J.; Pohjanen, J.; Kvist, J.; Fester, T.; Härtig, C.; Podolich, O.; Fluch, S.; Edesi, J.; Häggman, H.; Pirttilä, A.M. The meristem-associated endosymbiont Methylorubrum extorquens DSM13060 reprograms development and stress responses of pine seedlings. Tree Physiol. 2022, 42, 391–410. [Google Scholar] [CrossRef]
- Mei, Y.-Z.; Zhu, Y.-L.; Huang, P.-W.; Yang, Q.; Dai, C.-C. Strategies for gene disruption and expression in filamentous fungi. Appl. Microbiol. Biotechnol. 2019, 103, 6041–6059. [Google Scholar] [CrossRef]
- Hodgson, S.; de Cates, C.; Hodgson, J.; Morley, N.J.; Sutton, B.C.; Gange, A.C. Vertical transmission of fungal endophytes is widespread in forbs. Ecol. Evol. 2014, 4, 1199–1208. [Google Scholar] [CrossRef]
- Zhang, W.; Mace, W.J.; Matthew, C.; Card, S.D. The impact of endophyte infection, seed aging, and imbibition on selected sugar metabolite concentrations in seed. J. Agric. Food Chem. 2019, 67, 6921–6929. [Google Scholar] [CrossRef]
- Geisen, S.; Kostenko, O.; Cnossen, M.C.; ten Hooven, F.C.; Vreš, B.; van Der Putten, W.H. Seed and root endophytic fungi in a range expanding and a related plant species. Front. Microbiol. 2017, 8, 1645. [Google Scholar] [CrossRef] [PubMed]
- Shearin, Z.R.; Filipek, M.; Desai, R.; Bickford, W.A.; Kowalski, K.P.; Clay, K. Fungal endophytes from seeds of invasive, non-native Phragmites australis and their potential role in germination and seedling growth. Plant Soil 2018, 422, 183–194. [Google Scholar] [CrossRef]
- Shade, A.; Jacques, M.-A.; Barret, M. Ecological patterns of seed microbiome diversity, transmission, and assembly. Curr. Opin. Microbiol. 2017, 37, 15–22. [Google Scholar] [CrossRef]
- Zhao, C.; Onyino, J.; Gao, X. Current advances in the functional diversity and mechanisms underlying endophyte–plant interactions. Microorganisms 2024, 12, 779. [Google Scholar] [CrossRef]
- Hardoim, P.R.; van Overbeek, L.S.; van Elsas, J.D. Properties of bacterial endophytes and their proposed role in plant growth. Trends Microbiol. 2008, 16, 463–471. [Google Scholar] [CrossRef]
- Hardoim, P.R.; Hardoim, C.C.; Van Overbeek, L.S.; Van Elsas, J.D. Dynamics of seed-borne rice endophytes on early plant growth stages. PLoS ONE 2012, 7, e30438. [Google Scholar] [CrossRef]
- Emitaro, W.O.; Kawaka, F.; Musyimi, D.M.; Adienge, A. Diversity of endophytic bacteria isolated from leguminous agroforestry trees in western Kenya. AMB Express 2024, 14, 18. [Google Scholar] [CrossRef]
- Lacava, P.T.; Bogas, A.C.; Cruz, F.d.P.N. Plant growth promotion and biocontrol by endophytic and rhizospheric microorganisms from the tropics: A review and perspectives. Front. Sustain. Food Syst. 2022, 6, 796113. [Google Scholar] [CrossRef]
- Guo, B.; Wang, Y.; Sun, X.; Tang, K. Bioactive natural products from endophytes: A review. Appl. Biochem. Microbiol. 2008, 44, 136–142. [Google Scholar] [CrossRef]
- Sun, N.; Gu, Y.; Jiang, G.; Wang, Y.; Wang, P.; Song, W.; Ma, P.; Duan, Y.; Jiao, Z. Bacterial communities in the endophyte and rhizosphere of white radish (Raphanus sativus) in different compartments and growth conditions. Front. Microbiol. 2022, 13, 900779. [Google Scholar] [CrossRef] [PubMed]
- Truyens, S.; Weyens, N.; Cuypers, A.; Vangronsveld, J. Bacterial seed endophytes: Genera, vertical transmission and interaction with plants. Environ. Microbiol. Rep. 2015, 7, 40–50. [Google Scholar] [CrossRef]
- Mahaffee, W.; Kloepper, J. Bacterial communities of the rhizosphere and endorhiza associated with field-grown cucumber plants inoculated with a plant growth-promoting rhizobacterium or its genetically modified derivative. Can. J. Microbiol. 1997, 43, 344–353. [Google Scholar] [CrossRef]
- Joubert, O.; Arnault, G.; Barret, M.; Simonin, M. Sowing success: Ecological insights into seedling microbial colonisation for robust plant microbiota engineering. Trends Plant Sci. 2024, 30, 21–34. [Google Scholar] [CrossRef]
- Pitzschke, A. Developmental peculiarities and seed-borne endophytes in quinoa: Omnipresent, robust bacilli contribute to plant fitness. Front. Microbiol. 2016, 7, 2. [Google Scholar] [CrossRef]
- Ge, M.; Wei, X. Spermosphere bacterial community at different germination stages of Ormosia henryi and its relationship with seed germination. Sci. Hortic. 2024, 324, 112608. [Google Scholar] [CrossRef]
- Hinton, D.M.; Bacon, C.W. Enterobacter cloacae is an endophytic symbiont of corn. Mycopathologia 1995, 129, 117–125. [Google Scholar] [CrossRef]
- Puente, M.E.; Li, C.Y.; Bashan, Y. Rock-degrading endophytic bacteria in cacti. Environ. Exp. Bot. 2009, 66, 389–401. [Google Scholar] [CrossRef]
- Abdelfattah, A.; Tack, A.J.; Lobato, C.; Wassermann, B.; Berg, G. From seed to seed: The role of microbial inheritance in the assembly of the plant microbiome. Trends Microbiol. 2023, 31, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Mano, H.; Tanaka, F.; Watanabe, A.; Kaga, H.; Okunishi, S.; Morisaki, H. Culturable surface and endophytic bacterial flora of the maturing seeds of rice plants (Oryza sativa) cultivated in a paddy field. Microbes Environ. 2006, 21, 86–100. [Google Scholar] [CrossRef]
- Shaik, S.P.; Thomas, P. In vitro activation of seed-transmitted cultivation-recalcitrant endophytic bacteria in tomato and host–endophyte mutualism. Microorganisms 2019, 7, 132. [Google Scholar] [CrossRef] [PubMed]
- Fadiji, A.E.; Babalola, O.O. Metagenomics methods for the study of plant-associated microbial communities: A review. J. Microbiol. Methods 2020, 170, 105860. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Ayangbenro, A.S.; Babalola, O.O. Metagenomic profiling of the community structure, diversity, and nutrient pathways of bacterial endophytes in maize plant. Antonie Van Leeuwenhoek 2020, 113, 1559–1571. [Google Scholar] [CrossRef]
- Walitang, D.I.; Kim, K.; Madhaiyan, M.; Kim, Y.K.; Kang, Y.; Sa, T. Characterizing endophytic competence and plant growth promotion of bacterial endophytes inhabiting the seed endosphere of Rice. BMC Microbiology 2017, 17, 209. [Google Scholar] [CrossRef]
- Petrosyan, K.; Thijs, S.; Piwowarczyk, R.; Ruraż, K.; Kaca, W.; Vangronsveld, J. Diversity and potential plant growth promoting capacity of seed endophytic bacteria of the holoparasite Cistanche phelypaea (Orobanchaceae). Sci. Rep. 2023, 13, 11835. [Google Scholar] [CrossRef]
- Faeth, S.H.; Fagan, W.F. Fungal endophytes: Common host plant symbionts but uncommon mutualists. Integr. Comp. Biol. 2002, 42, 360–368. [Google Scholar] [CrossRef]
- Freeman, E. The seed-fungus of Lolium temulentum, L., the darnel. Philos. Trans. R. Soc. London. Ser. B Contain. Pap. A Biol. Character 1904, 196, 27–30. [Google Scholar]
- Schardl, C.L. Epichloë festucae and related mutualistic symbionts of grasses. Fungal Genet. Biol. 2001, 33, 69–82. [Google Scholar] [CrossRef]
- Klaedtke, S.; Jacques, M.A.; Raggi, L.; Préveaux, A.; Bonneau, S.; Negri, V.; Chable, V.; Barret, M. Terroir is a key driver of seed-associated microbial assemblages. Environ. Microbiol. 2016, 18, 1792–1804. [Google Scholar] [CrossRef]
- Rodriguez, R.; White, J., Jr.; Arnold, A.E.; Redman, R. Fungal endophytes: Diversity and functional roles. New Phytol. 2009, 182, 314–330. [Google Scholar] [CrossRef]
- Johnson, L.J.; de Bonth, A.C.; Briggs, L.R.; Caradus, J.R.; Finch, S.C.; Fleetwood, D.J.; Fletcher, L.R.; Hume, D.E.; Johnson, R.D.; Popay, A.J. The exploitation of epichloae endophytes for agricultural benefit. Fungal Divers. 2013, 60, 171–188. [Google Scholar] [CrossRef]
- Pérez-Jaramillo, J.E.; Mendes, R.; Raaijmakers, J.M. Impact of plant domestication on rhizosphere microbiome assembly and functions. Plant Mol. Biol. 2016, 90, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Links, M.G.; Demeke, T.; Gräfenhan, T.; Hill, J.E.; Hemmingsen, S.M.; Dumonceaux, T.J. Simultaneous profiling of seed-associated bacteria and fungi reveals antagonistic interactions between microorganisms within a shared epiphytic microbiome on Triticum and Brassica seeds. New Phytol. 2014, 202, 542–553. [Google Scholar] [CrossRef] [PubMed]
- Barret, M.; Briand, M.; Bonneau, S.; Préveaux, A.; Valière, S.; Bouchez, O.; Hunault, G.; Simoneau, P.; Jacques, M.-A. Emergence shapes the structure of the seed microbiota. Appl. Environ. Microbiol. 2015, 81, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Julian, T.; Armin, E.; Anastasia, B.; Berg, C.; Christine, M.-E.; Berg, G. What Is the Role of Archaea in Plants? New Insights from the Vegetation of Alpine Bogs. mSphere 2018, 3, e00122-18. [Google Scholar] [CrossRef]
- Bragina, A.; Oberauner-Wappis, L.; Zachow, C.; Halwachs, B.; Thallinger, G.G.; Müller, H.; Berg, G. The S phagnum microbiome supports bog ecosystem functioning under extreme conditions. Mol. Ecol. 2014, 23, 4498–4510. [Google Scholar] [CrossRef]
- Chaudhury, P.; Quax, T.E.; Albers, S.V. Versatile cell surface structures of archaea. Mol. Microbiol. 2018, 107, 298–311. [Google Scholar] [CrossRef]
- Boetius, A.; Ravenschlag, K.; Schubert, C.J.; Rickert, D.; Widdel, F.; Gieseke, A.; Amann, R.; Jørgensen, B.B.; Witte, U.; Pfannkuche, O. A marine microbial consortium apparently mediating anaerobic oxidation of methane. Nature 2000, 407, 623–626. [Google Scholar] [CrossRef]
- Moissl-Eichinger, C.; Pausan, M.; Taffner, J.; Berg, G.; Bang, C.; Schmitz, R. Archaea are interactive components of complex microbiomes. Trends Microbiol. 2017, 26, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Kim, J.-S.; Taffner, J.; Berg, G.; Ryu, C.-M. Archaea, tiny helpers of land plants. Comput. Struct. Biotechnol. J. 2020, 18, 2494–2500. [Google Scholar] [CrossRef] [PubMed]
- Amann, R.I.; Ludwig, W.; Schleifer, K.-H. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 1995, 59, 143–169. [Google Scholar] [CrossRef] [PubMed]
- Bates, S.T.; Berg-Lyons, D.; Caporaso, J.G.; Walters, W.A.; Knight, R.; Fierer, N. Examining the global distribution of dominant archaeal populations in soil. ISME J. 2011, 5, 908–917. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Ayangbenro, A.S.; Babalola, O.O. Organic Farming enhances the diversity and community structure of endophytic archaea and fungi in maize plant: A shotgun approach. J. Soil Sci. Plant Nutr. 2020, 20, 2587–2599. [Google Scholar] [CrossRef]
- Chow, C.; Padda, K.P.; Puri, A.; Chanway, C.P. An archaic approach to a modern issue: Endophytic archaea for sustainable agriculture. Curr. Microbiol. 2022, 79, 322. [Google Scholar] [CrossRef]
- Shi, Y.; TaPa, M.; Li, C.; Yang, H.; Zhang, T.; Gao, Y.; Sun, J.; Zeng, J.; Lin, Q.; Cao, Z. Diversity and space–time dynamics of endophytic archaea from sugar beet in the north slope of Tianshan Mountain revealed by 454 pyrosequencing and T-RFLP. World J. Microbiol. Biotechnol. 2015, 31, 1031–1039. [Google Scholar] [CrossRef]
- Bintarti, A.; Sulesky-Grieb, A.; Stopnisek, N.; Shade, A. Endophytic microbiome variation among single plant seeds. Phytobiomes J. 2022, 6, 45–55. [Google Scholar] [CrossRef]
- Liu, J.; Tang, Y.; Bao, J.; Wang, H.; Peng, F.; Tan, P.; Chu, G.; Liu, S. A stronger rhizosphere impact on the fungal communities compared to the bacterial communities in pecan plantations. Front. Microbiol. 2022, 13, 899801. [Google Scholar] [CrossRef]
- Benchlih, S.; Esmaeel, Q.; Aberkani, K.; Tahiri, A.; Belabess, Z.; Lahlali, R.; Barka, E.A. Modes of action of biocontrol agents and elicitors for sustainable protection against bacterial canker of tomato. Microorganisms 2023, 11, 726. [Google Scholar] [CrossRef] [PubMed]
- Tancos, M.A.; Chalupowicz, L.; Barash, I.; Manulis-Sasson, S.; Smart, C.D. Tomato fruit and seed colonization by Clavibacter michiganensis subsp. michiganensis through external and internal routes. Appl. Environ. Microbiol. 2013, 79, 6948–6957. [Google Scholar] [CrossRef]
- De León, L.; Siverio, F.; López, M.M.; Rodríguez, A. Clavibacter michiganesis subsp. michiganensis, a seedborne tomato pathogen: Healthy seeds are still the goal. Plant Dis. 2011, 95, 1328–1338. [Google Scholar] [CrossRef]
- Singh, H. Mycoremediation: Fungal Bioremediation; John Wiley and Sons Publication: Hoboken, NJ, USA, 2006; pp. 19–25. [Google Scholar]
- Khan, A.L.; Hussain, J.; Al-Harrasi, A.; Al-Rawahi, A.; Lee, I.-J. Endophytic fungi: Resource for gibberellins and crop abiotic stress resistance. Crit. Rev. Biotechnol. 2015, 35, 62–74. [Google Scholar] [CrossRef]
- Gundel, P.E.; Rudgers, J.A.; Whitney, K.D. Vertically transmitted symbionts as mechanisms of transgenerational effects. Am. J. Bot. 2017, 104, 787–792. [Google Scholar] [CrossRef]
- Madmony, A.; Chernin, L.; Pleban, S.; Peleg, E.; Riov, J. Enterobacter cloacae, an obligatory endophyte of pollen grains of Mediterranean pines. Folia Microbiol. 2005, 50, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Vandenkoornhuyse, P.; Quaiser, A.; Duhamel, M.; Le Van, A.; Dufresne, A. The importance of the microbiome of the plant holobiont. New Phytol. 2015, 206, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.L.; Hamayun, M.; Kang, S.-M.; Kim, Y.-H.; Jung, H.-Y.; Lee, J.-H.; Lee, I.-J. Endophytic fungal association via gibberellins and indole acetic acid can improve plant growth under abiotic stress: An example of Paecilomyces formosus LHL10. BMC Microbiol. 2012, 12, 3. [Google Scholar] [CrossRef]
- Fort, T.; Pauvert, C.; Zanne, A.E.; Ovaskainen, O.; Caignard, T.; Barret, M.; Compant, S.; Hampe, A.; Delzon, S.; Vacher, C. Maternal effects shape the seed mycobiome in Quercus petraea. New Phytol. 2021, 230, 1594–1608. [Google Scholar] [CrossRef] [PubMed]
- Maude, R.i. Seedborne Diseases and Their Control: Principles and Practice; CAB International: Wallingford, UK, 1996. [Google Scholar]
- Ferreira, A.; Quecine, M.C.; Lacava, P.T.; Oda, S.; Azevedo, J.L.; Araújo, W.L. Diversity of endophytic bacteria from Eucalyptus species seeds and colonization of seedlings by Pantoea agglomerans. FEMS Microbiol. Lett. 2008, 287, 8–14. [Google Scholar] [CrossRef]
- Johnston-Monje, D.; Raizada, M.N. Conservation and diversity of seed associated endophytes in Zea across boundaries of evolution, ethnography and ecology. PLoS ONE 2011, 6, e20396. [Google Scholar] [CrossRef]
- Gagne-Bourgue, F.; Aliferis, K.; Seguin, P.; Rani, M.; Samson, R.; Jabaji, S. Isolation and characterization of indigenous endophytic bacteria associated with leaves of switchgrass (Panicum virgatum L.) cultivars. J. Appl. Microbiol. 2013, 114, 836–853. [Google Scholar] [CrossRef]
- Ngugi, H.K.; Scherm, H. Biology of flower-infecting fungi. Annu. Rev. Phytopathol. 2006, 44, 261–282. [Google Scholar] [CrossRef] [PubMed]
- Wiewióra, B.; Żurek, G.; Pańka, D. Is the vertical transmission of Neotyphodium lolii in perennial ryegrass the only possible way to the spread of endophytes? PLoS ONE 2015, 10, e0117231. [Google Scholar] [CrossRef]
- Chen, C.-X.; Guo, L.-R.; Wang, Y.-T.; Wen, Y.; Li, Y.; Lu, C.-X.; Zhou, P.; Huang, S.-Y.; Li, Y.-Q.; Pan, X.-X. Targeted Manipulation of Vertically Transmitted Endophytes to Confer Beneficial Traits in Grapevines. Horticulturae 2024, 10, 607. [Google Scholar] [CrossRef]
- Barret, M.; Guimbaud, J.F.; Darrasse, A.; Jacques, M.A. Plant microbiota affects seed transmission of phytopathogenic microorganisms. Mol. Plant Pathol. 2016, 17, 791. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, V.K.; Sinclair, J.B. Principles of Seed Pathology; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- Carroll, G. Fungal endophytes in stems and leaves: From latent pathogen to mutualistic symbiont. Ecology 1988, 69, 2–9. [Google Scholar] [CrossRef]
- Thomas, P.; Shaik, S.P. Molecular profiling on surface-disinfected tomato seeds reveals high diversity of cultivation-recalcitrant endophytic bacteria with low shares of spore-forming firmicutes. Microb. Ecol. 2020, 79, 910–924. [Google Scholar] [CrossRef]
- Liu, Y.; Zuo, S.; Xu, L.; Zou, Y.; Song, W. Study on diversity of endophytic bacterial communities in seeds of hybrid maize and their parental lines. Arch. Microbiol. 2012, 194, 1001–1012. [Google Scholar] [CrossRef]
- Croes, S.; Weyens, N.; Janssen, J.; Vercampt, H.; Colpaert, J.; Carleer, R.; Vangronsveld, J. Bacterial communities associated with Brassica napus L. grown on trace element-contaminated and non-contaminated fields: A genotypic and phenotypic comparison. Microb. Biotechnol. 2013, 6, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Okunishi, S.; Sako, K.; Mano, H.; Imamura, A.; Morisaki, H. Bacterial flora of endophytes in the maturing seed of cultivated rice (Oryza sativa). Microbes Environ. 2005, 20, 168–177. [Google Scholar] [CrossRef]
- Reiter, B.; Pfeifer, U.; Schwab, H.; Sessitsch, A. Response of endophytic bacterial communities in potato plants to infection with Erwinia carotovora subsp. atroseptica. Appl. Environ. Microbiol. 2002, 68, 2261–2268. [Google Scholar] [CrossRef]
- Delaux, P.-M.; Varala, K.; Edger, P.P.; Coruzzi, G.M.; Pires, J.C.; Ané, J.-M. Comparative phylogenomics uncovers the impact of symbiotic associations on host genome evolution. PLoS Genet. 2014, 10, e1004487. [Google Scholar] [CrossRef]
- Chimwamurombe, P.M.; Grönemeyer, J.L.; Reinhold-Hurek, B. Isolation and characterization of culturable seed-associated bacterial endophytes from gnotobiotically grown Marama bean seedlings. FEMS Microbiol. Ecol. 2016, 92, fiw083. [Google Scholar] [CrossRef]
- Dutta, D.; Puzari, K.C.; Gogoi, R.; Dutta, P. Endophytes: Exploitation as a tool in plant protection. Braz. Arch. Biol. 2014, 57, 621–629. [Google Scholar] [CrossRef]
- Dutta, B.; Ha, Y.; Lessl, J.; Avci, U.; Sparks, A.; Johnson, K.; Walcott, R. Pathways of bacterial invasion and watermelon seed infection by Acidovorax citrulli. Plant Pathol. 2015, 64, 537–544. [Google Scholar] [CrossRef]
- Dutta, B.; Schneider, R.W.; Robertson, C.L.; Walcott, R.R. Embryo localization enhances the survival of Acidovorax citrulli in watermelon seeds. Phytopathology 2016, 106, 330–338. [Google Scholar] [CrossRef]
- Lessl, J.; Fessehaie, A.; Walcott, R. Colonization of female watermelon blossoms by Acidovorax avenae ssp. citrulli and the relationship between blossom inoculum dosage and seed infestation. J. Phytopathol. 2007, 155, 114–121. [Google Scholar] [CrossRef]
- Rana, K.L.; Kour, D.; Yadav, N.; Yadav, A.N. Endophytic microbes in nanotechnology: Current development, and potential biotechnology applications. In Microbial Endophytes; Kumar, A., Kumar, V., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 231–262. [Google Scholar] [CrossRef]
- Jia, M.; Chen, L.; Xin, H.-L.; Zheng, C.-J.; Rahman, K.; Han, T.; Qin, L.-P. A friendly relationship between endophytic fungi and medicinal plants: A systematic review. Front. Microbiol. 2016, 7, 906. [Google Scholar] [CrossRef]
- Compant, S.; Clément, C.; Sessitsch, A. Plant growth-promoting bacteria in the rhizo-and endosphere of plants: Their role, colonization, mechanisms involved and prospects for utilization. Soil Biol. Biochem. 2010, 42, 669–678. [Google Scholar] [CrossRef]
- Glick, B.R.; Todorovic, B.; Czarny, J.; Cheng, Z.; Duan, J.; McConkey, B. Promotion of plant growth by bacterial ACC deaminase. Crit. Rev. Plant Sci. 2007, 26, 227–242. [Google Scholar] [CrossRef]
- Klironomos, J.N. Feedback with soil biota contributes to plant rarity and invasiveness in communities. Nature 2002, 417, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Puente, M.E.; Li, C.Y.; Bashan, Y. Endophytic bacteria in cacti seeds can improve the development of cactus seedlings. Environ. Exp. Bot. 2009, 66, 402–408. [Google Scholar] [CrossRef]
- Kholostiakov, V.; Burns, B.; Ridgway, H.; Padamsee, M. Variation in seed-borne microbial communities of Metrosideros excelsa Sol. ex Gaertn. with consequences for germination success. N. Z. J. Bot. 2024, 1–24. [Google Scholar] [CrossRef]
- Xu, M.; Sheng, J.; Chen, L.; Men, Y.; Gan, L.; Guo, S.; Shen, L. Bacterial community compositions of tomato (Lycopersicum esculentum Mill.) seeds and plant growth promoting activity of ACC deaminase producing Bacillus subtilis (HYT-12-1) on tomato seedlings. World J. Microbiol. Biotechnol. 2014, 30, 835–845. [Google Scholar] [CrossRef] [PubMed]
- López-López, A.; Rogel, M.A.; Ormeno-Orrillo, E.; Martínez-Romero, J.; Martínez-Romero, E. Phaseolus vulgaris seed-borne endophytic community with novel bacterial species such as Rhizobium endophyticum sp. nov. Syst. Appl. Microbiol. 2010, 33, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Omomowo, O.I.; Babalola, O.O. Bacterial and fungal endophytes: Tiny giants with immense beneficial potential for plant growth and sustainable agricultural productivity. Microorganisms 2019, 7, 481. [Google Scholar] [CrossRef]
- Nagabhyru, P.; Dinkins, R.D.; Schardl, C.L. Transcriptome analysis of Epichloë strains in tall fescue in response to drought stress. Mycologia 2022, 114, 697–712. [Google Scholar] [CrossRef] [PubMed]
- Soldan, R.; Mapelli, F.; Crotti, E.; Schnell, S.; Daffonchio, D.; Marasco, R.; Fusi, M.; Borin, S.; Cardinale, M. Bacterial endophytes of mangrove propagules elicit early establishment of the natural host and promote growth of cereal crops under salt stress. Microbiol. Res. 2019, 223, 33–43. [Google Scholar] [CrossRef]
- Jeong, S.; Kim, T.-M.; Choi, B.; Kim, Y.; Kim, H.; Kim, E. Genotype-Specific Plastic Responses to Seed Bacteria under Drought Stress in Lactuca serriola. Microorganisms 2022, 10, 1604. [Google Scholar] [CrossRef] [PubMed]
- Abideen, Z.; Cardinale, M.; Zulfiqar, F.; Koyro, H.-W.; Rasool, S.G.; Hessini, K.; Darbali, W.; Zhao, F.; Siddique, K.H. Seed endophyte bacteria enhance drought stress tolerance in Hordeum vulgare by regulating, physiological characteristics, antioxidants and minerals uptake. Front. Plant Sci. 2022, 13, 980046. [Google Scholar] [CrossRef]
- Shahzad, R.; Waqas, M.; Khan, A.L.; Asaf, S.; Khan, M.A.; Kang, S.-M.; Yun, B.-W.; Lee, I.-J. Seed-borne endophytic Bacillus amyloliquefaciens RWL-1 produces gibberellins and regulates endogenous phytohormones of Oryza sativa. Plant Physiol. Biochem. 2016, 106, 236–243. [Google Scholar] [CrossRef]
- Liu, Y.; Bai, F.; Li, N.; Wang, W.; Cheng, C. Identification of endophytic bacterial strain RSE1 from seeds of super hybrid rice Shenliangyou 5814 (Oryza sativa L.,) and evaluation of its antagonistic activity. Plant Growth Regul. 2017, 82, 403–408. [Google Scholar] [CrossRef]
- Shahzad, R.; Waqas, M.; Khan, A.L.; Al-Hosni, K.; Kang, S.-M.; Seo, C.-W.; Lee, I.-J. Indoleacetic acid production and plant growth promoting potential of bacterial endophytes isolated from rice (Oryza sativa L.) seeds. Acta Biol. Hung. 2017, 68, 175–186. [Google Scholar] [CrossRef]
- Ruiza, D.; Agaras, B.; de Werrab, P.; Wall, L.G.; Valverde, C. Characterization and screening of plant probiotic traits of bacteria isolated from rice seeds cultivated in Argentina. J. Microbiol. 2011, 49, 902–912. [Google Scholar] [CrossRef]
- Verma, S.K.; Kingsley, K.; Irizarry, I.; Bergen, M.; Kharwar, R.; White, J., Jr. Seed-vectored endophytic bacteria modulate development of rice seedlings. J. Appl. Microbiol. 2017, 122, 1680–1691. [Google Scholar] [CrossRef]
- Kaga, H.; Mano, H.; Tanaka, F.; Watanabe, A.; Kaneko, S.; Morisaki, H. Rice seeds as sources of endophytic bacteria. Microbes Environ. 2009, 24, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Pennell, C.; Rolston, M.; De Bonth, A.; Simpson, W.; Hume, D. Development of a bird-deterrent fungal endophyte in turf tall fescue. N. Z. J. Agric. Res. 2010, 53, 145–150. [Google Scholar] [CrossRef]
- Young, C.; Hume, D.; McCulley, R. Forages and pastures symposium: Fungal endophytes of tall fescue and perennial ryegrass: Pasture friend or foe? J. Anim. Sci. 2013, 91, 2379–2394. [Google Scholar] [CrossRef]
- Mastretta, C.; Taghavi, S.; Van Der Lelie, D.; Mengoni, A.; Galardi, F.; Gonnelli, C.; Barac, T.; Boulet, J.; Weyens, N.; Vangronsveld, J. Endophytic bacteria from seeds of Nicotiana tabacum can reduce cadmium phytotoxicity. Int. J. Phytoremediation 2009, 11, 251–267. [Google Scholar] [CrossRef]
- Rozpądek, P.; Wężowicz, K.; Nosek, M.; Ważny, R.; Tokarz, K.; Lembicz, M.; Miszalski, Z.; Turnau, K. The fungal endophyte Epichloë typhina improves photosynthesis efficiency of its host orchard grass (Dactylis glomerata). Planta 2015, 242, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Frommel, M.; Nowak, J.; Lazarovits, G.J.P. Treatment of potato tubers with a growth promoting Pseudomonas sp.: Plant growth responses and bacterium distribution in the rhizosphere. Plant Soil 1993, 150, 51–60. [Google Scholar] [CrossRef]
- Roberts, D.P.; McKenna, L.F.; Lohrke, S.M.; Rehner, S.; de Souza, J.T. Pyruvate dehydrogenase activity is important for colonization of seeds and roots by Enterobacter cloacae. Soil Biol. Biochem. 2007, 39, 2150–2159. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Babalola, O.O. Elucidating mechanisms of endophytes used in plant protection and other bioactivities with multifunctional prospects. Front. Bioeng. Biotechnol. 2020, 8, 467. [Google Scholar] [CrossRef]
- Kumar, S.; Kaushik, N. Batch culture fermentation of endophytic fungi and extraction of their metabolites. Bio-Protocol 2013, 3, e926. [Google Scholar] [CrossRef]
- Roy, S.; Mili, C.; Talukdar, R.; Wary, S.; Tayung, K. Seed borne endophytic fungiassociated with some indigenous rice varieties of North East India and their growth promotion and antifungal potential. Indian J. Agric. Res. 2021, 55, 603–608. [Google Scholar]
- Cottyn, B.; Regalado, E.; Lanoot, B.; De Cleene, M.; Mew, T.; Swings, J. Bacterial populations associated with rice seed in the tropical environment. Phytopathology 2001, 91, 282–292. [Google Scholar] [CrossRef]
- Matsumoto, H.; Fan, X.; Wang, Y.; Kusstatscher, P.; Duan, J.; Wu, S.; Chen, S.; Qiao, K.; Wang, Y.; Ma, B. Bacterial seed endophyte shapes disease resistance in rice. Nat. Plants 2021, 7, 60–72. [Google Scholar] [CrossRef]
- Sessitsch, A.; Hardoim, P.; Döring, J.; Weilharter, A.; Krause, A.; Woyke, T.; Mitter, B.; Hauberg-Lotte, L.; Friedrich, F.; Rahalkar, M. Functional characteristics of an endophyte community colonizing rice roots as revealed by metagenomic analysis. Mol. Plant-Microbe Interact. 2012, 25, 28–36. [Google Scholar] [CrossRef]
- Loper, J.E.; Henkels, M.D. Utilization of heterologous siderophores enhances levels of iron available to Pseudomonas putida in the rhizosphere. Appl. Environ. Microbiol. 1999, 65, 5357–5363. [Google Scholar] [CrossRef]
- Mathesius, U.; Mulders, S.; Gao, M.; Teplitski, M.; Caetano-Anollés, G.; Rolfe, B.G.; Bauer, W.D. Extensive and specific responses of a eukaryote to bacterial quorum-sensing signals. Proc. Natl. Acad. Sci.USA 2003, 100, 1444–1449. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, E.M.; Raizada, M.N. Bacterial seed endophytes of domesticated cucurbits antagonize fungal and oomycete pathogens including powdery mildew. Front. Microbiol. 2018, 9, 42. [Google Scholar]
- Cheplick, G.P.; Faeth, S.H. Ecology and Evolution of the Grass-Endophyte Symbiosis; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Saikkonen, K.; Wäli, P.R.; Helander, M. Genetic compatibility determines endophyte-grass combinations. PLoS ONE 2010, 5, e11395. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.; Abdellaoui, N.; Lim, J.Y.; Seo, J.-A. The presence of a significant endophytic fungus in mycobiome of rice seed compartments. Sci. Rep. 2024, 14, 23367. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.-L.; Huang, S.-Y.; Ma, C.-Y.; Zhang, X.-Y.; Sun, K.; Zhang, W.; Dai, C.-C. Seed-borne bacterial synthetic community resists seed pathogenic fungi and promotes plant growth. J. Appl. Microbiol. 2024, 135, lxae073. [Google Scholar] [CrossRef]
- Roy, M.; Kang, B.; Yang, S.; Choi, H.; Choi, K. Characterization of Tomato Seed Endophytic Bacteria as Growth Promoters and Potential Biocontrol Agents. Plant Pathol. J. 2024, 40, 578. [Google Scholar] [CrossRef]
- Bing, H.; Qi, C.; Gu, J.; Zhao, T.; Yu, X.; Cai, Y.; Zhang, Y.; Li, A.; Wang, X.; Zhao, J. Isolation and identification of NEAU-CP5: A seed-endophytic strain of B. velezensis that controls tomato bacterial wilt. Microb. Pathog. 2024, 192, 106707. [Google Scholar] [CrossRef]
- Popović Milovanović, T.; Iličić, R.; Bagi, F.; Aleksić, G.; Trkulja, N.; Trkulja, V.; Jelušić, A. Biocontrol of Seedborne Fungi on Small-Grained Cereals Using Bacillus halotolerans Strain B33. J. Fungi 2025, 11, 144. [Google Scholar] [CrossRef]
- Shahzad, R.; Khan, A.L.; Bilal, S.; Asaf, S.; Lee, I.-J. Plant growth-promoting endophytic bacteria versus pathogenic infections: An example of Bacillus amyloliquefaciens RWL-1 and Fusarium oxysporum f. sp. lycopersici in tomato. PeerJ 2017, 5, e3107. [Google Scholar] [CrossRef]
- Diabankana, R.G.C.; Afordoanyi, D.M.; Safin, R.I.; Nizamov, R.M.; Karimova, L.Z.; Validov, S.Z. Antifungal properties, abiotic stress resistance, and biocontrol ability of Bacillus mojavensis PS17. Curr. Microbiol. 2021, 78, 3124–3132. [Google Scholar] [CrossRef]
- Pal, G.; Kumar, K.; Verma, A.; Verma, S.K. Seed inhabiting bacterial endophytes of maize promote seedling establishment and provide protection against fungal disease. Microbiol. Res. 2022, 255, 126926. [Google Scholar] [CrossRef]
- Mukherjee, A.; Singh, B.K.; Verma, J.P. Harnessing chickpea (Cicer arietinum L.) seed endophytes for enhancing plant growth attributes and bio-controlling against Fusarium sp. Microbiol. Res. 2020, 237, 126469. [Google Scholar]
- Tyc, O.; Putra, R.; Gols, R.; Harvey, J.A.; Garbeva, P. The ecological role of bacterial seed endophytes associated with wild cabbage in the United Kingdom. Microbiologyopen 2020, 9, e00954. [Google Scholar] [CrossRef]
- Kouzai, Y.; Akimoto-Tomiyama, C. A seed-borne bacterium of rice, Pantoea dispersa BB1, protects rice from the seedling rot caused by the bacterial pathogen Burkholderia glumae. Life 2022, 12, 791. [Google Scholar] [CrossRef]
- Herrera, S.D.; Grossi, C.; Zawoznik, M.; Groppa, M.D. Wheat seeds harbour bacterial endophytes with potential as plant growth promoters and biocontrol agents of Fusarium graminearum. Microbiol. Res. 2016, 186, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Daisley, B.A.; Monachese, M.; Trinder, M.; Bisanz, J.E.; Chmiel, J.A.; Burton, J.P.; Reid, G. Immobilization of cadmium and lead by Lactobacillus rhamnosus GR-1 mitigates apical-to-basolateral heavy metal translocation in a Caco-2 model of the intestinal epithelium. Gut Microbes 2019, 10, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Janeeshma, E.; Puthur, J.T. Potential role of microbial endophytes in xenobiotic stress management. In Sustainable Environmental Clean-Up; Mishra, V.K., Kumar, A., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2021; pp. 165–185. [Google Scholar]
- Paithankar, J.G.; Saini, S.; Dwivedi, S.; Sharma, A.; Chowdhuri, D.K. Heavy metal associated health hazards: An interplay of oxidative stress and signal transduction. Chemosphere 2021, 262, 128350. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Ramírez, G.; Flores-Vallejo, R.d.C.; Rivera-Leyva, J.C.; Tovar-Sánchez, E.; Sánchez-Reyes, A.; Mena-Portales, J.; Sánchez-Carbente, M.d.R.; Gaitán-Rodríguez, M.F.; Batista-García, R.A.; Villarreal, M.L. Characterization of fungal endophytes isolated from the metal hyperaccumulator plant Vachellia farnesiana growing in mine tailings. Microorganisms 2020, 8, 226. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Parmar, S.; Sharma, V.K.; White, J.F. Seed endophytes and their potential applications. In Seed Endophytes; Verma, S., White, J., Jr., Eds.; Springer: Cham, Switzerland, 2019; pp. 35–54. [Google Scholar] [CrossRef]
- Zhou, J.; Li, P.; Meng, D.; Gu, Y.; Zheng, Z.; Yin, H.; Zhou, Q.; Li, J. Isolation, characterization and inoculation of Cd tolerant rice endophytes and their impacts on rice under Cd contaminated environment. Environ. Pollut. 2020, 260, 113990. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Wang, R.; Sun, L.; He, L.; Sheng, X. Cadmium-resistant and arginine decarboxylase-producing endophytic Sphingomonas sp. C40 decreases cadmium accumulation in host rice (Oryza sativa Cliangyou 513). Chemosphere 2021, 275, 130109. [Google Scholar] [CrossRef]
- Shahzad, R.; Bilal, S.; Imran, M.; Khan, A.L.; Alosaimi, A.A.; Al-Shwyeh, H.A.; Almahasheer, H.; Rehman, S.; Lee, I.-J. Amelioration of heavy metal stress by endophytic Bacillus amyloliquefaciens RWL-1 in rice by regulating metabolic changes: Potential for bacterial bioremediation. Biochem. J. 2019, 476, 3385–3400. [Google Scholar] [CrossRef]
- Chu, L.; Li, W.; Li, X.; Xiong, Z.; Li, H. Diversity and heavy metal resistance of endophytic fungi from seeds of hyperaccumulators. Jiangsu Acad. Agric. Sci. 2017, 33, 43–49. [Google Scholar]
- Truyens, S.; Beckers, B.; Thijs, S.; Weyens, N.; Cuypers, A.; Vangronsveld, J. Cadmium-induced and trans-generational changes in the cultivable and total seed endophytic community of Arabidopsis thaliana. Plant Biol. 2016, 18, 376–381. [Google Scholar] [CrossRef]
- Wang, J.; Hou, W.; Christensen, M.J.; Li, X.; Xia, C.; Li, C.; Nan, Z. Role of Epichloë endophytes in improving host grass resistance ability and soil properties. J. Agric. Food Chem. 2020, 68, 6944–6955. [Google Scholar] [CrossRef]
- Truyens, S.; Jambon, I.; Croes, S.; Janssen, J.; Weyens, N.; Mench, M.; Carleer, R.; Cuypers, A.; Vangronsveld, J. The effect of long-term Cd and Ni exposure on seed endophytes of Agrostis capillaris and their potential application in phytoremediation of metal-contaminated soils. Int. J. Phytoremediation 2014, 16, 643–659. [Google Scholar] [CrossRef]
- Sánchez-López, A.S.; González-Chávez, M.; del Carmen, A.; Solís-Domínguez, F.A.; Carrillo-González, R.; Rosas-Saito, G.H. Leaf epiphytic bacteria of plants colonizing mine residues: Possible exploitation for remediation of air pollutants. Front. Microbiol. 2018, 9, 3028. [Google Scholar] [CrossRef]
- Zhang, K. Isolation of Endophytes from Sesbania cannabina and Plant Growth-Promoting Characteristics of Endophyte. Master’s Thesis, Shanxi Agricultural University, Taiyuan, China, 2020. [Google Scholar] [CrossRef]
- Kolbas, A.; Kidd, P.; Guinberteau, J.; Jaunatre, R.; Herzig, R.; Mench, M. Endophytic bacteria take the challenge to improve Cu phytoextraction by sunflower. Environ. Sci. Pollut. Res. 2015, 22, 5370–5382. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jia, X.; Chen, S.; Wang, J.; Ji, R.; Zhao, L. Response of soil microbial communities to engineered nanomaterials in presence of maize (Zea mays L.) plants. Environ. Pollut. 2020, 267, 115608. [Google Scholar] [CrossRef]
- Chakrabarti, M.; Nagabhyru, P.; Schardl, C.L.; Dinkins, R.D. Differential gene expression in tall fescue tissues in response to water deficit. Plant Genome 2022, 15, e20199. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Kim, T.-M.; Choi, B.; Kim, Y.; Kim, E. Invasive Lactuca serriola seeds contain endophytic bacteria that contribute to drought tolerance. Sci. Rep. 2021, 11, 13307. [Google Scholar] [CrossRef] [PubMed]
- Kane, K.H. Effects of endophyte infection on drought stress tolerance of Lolium perenne accessions from the Mediterranean region. Environ. Exp. Bot. 2011, 71, 337–344. [Google Scholar] [CrossRef]
- Dai, Y.; Li, X.Y.; Wang, Y.; Li, C.X.; He, Y.; Lin, H.H.; Wang, T.; Ma, X.R. The differences and overlaps in the seed-resident microbiome of four Leguminous and three Gramineous forages. Microb. Biotechnol. 2020, 13, 1461–1476. [Google Scholar] [CrossRef]
- Shah, D.; Khan, M.S.; Aziz, S.; Ali, H.; Pecoraro, L. Molecular and biochemical characterization, antimicrobial activity, stress tolerance, and plant growth-promoting effect of endophytic bacteria isolated from wheat varieties. Microorganisms 2021, 10, 21. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Xu, G.; Zhou, L.; Li, Y. Arbuscular mycorrhizal fungi improve the growth and drought tolerance of Zenia insignis seedlings under drought stress. New For. 2019, 50, 593–604. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Santoyo, G.; Yadav, A.N.; Babalola, O.O. Efforts toward overcoming drought stress in crops: Revisiting the mechanisms employed by plant growth-promoting bacteria. Front. Microbiol. 2022, 13, 962427. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhu, Y.; Jing, R.; Wu, X.; Li, N.; Liu, H.; Zhang, X.; Wang, W.; Liu, Y. High-throughput sequencing-based analysis of the composition and diversity of endophytic bacterial community in seeds of upland rice. Arch. Microbiol. 2021, 203, 609–620. [Google Scholar] [CrossRef]
- Verma, H.; Kumar, D.; Kumar, V.; Kumari, M.; Singh, S.K.; Sharma, V.K.; Droby, S.; Santoyo, G.; White, J.F.; Kumar, A. The potential application of endophytes in management of stress from drought and salinity in crop plants. Microorganisms 2021, 9, 1729. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Yadav, A.N.; Santoyo, G.; Babalola, O.O. Understanding the plant-microbe interactions in environments exposed to abiotic stresses: An overview. Microbiol. Res. 2023, 271, 127368. [Google Scholar] [CrossRef]
- Walitang, D.I.; Kim, C.-G.; Kim, K.; Kang, Y.; Kim, Y.K.; Sa, T. The influence of host genotype and salt stress on the seed endophytic community of salt-sensitive and salt-tolerant rice cultivars. BMC Plant Biol. 2018, 18, 51. [Google Scholar] [CrossRef] [PubMed]
- Dent, K.C.; Stephen, J.R.; Finch-Savage, W.E. Molecular profiling of microbial communities associated with seeds of Beta vulgaris subsp. vulgaris (sugar beet). J. Microbiol. Methods 2004, 56, 17–26. [Google Scholar] [CrossRef]
- Hill, N.; Roach, P. Endophyte survival during seed storage: Endophyte–host interactions and heritability. Crop Sci. 2009, 49, 1425–1430. [Google Scholar] [CrossRef]
- Holland, M.A.; Polacco, J.C. PPFMs and other covert contaminants: Is there more to plant physiology than just plant? Annu. Rev. Plant Biol. 1994, 45, 197–209. [Google Scholar] [CrossRef]
- Bordenstein, S.R.; Theis, K.R. Host biology in light of the microbiome: Ten principles of holobionts and hologenomes. PLoS Biol. 2015, 13, e1002226. [Google Scholar] [CrossRef] [PubMed]
- Mitter, B.; Pfaffenbichler, N.; Sessitsch, A. Plant–microbe partnerships in 2020. Microb. Biotechnol. 2016, 9, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Wassermann, B.; Cernava, T.; Müller, H.; Berg, C.; Berg, G. Seeds of native alpine plants host unique microbial communities embedded in cross-kingdom networks. Microbiome 2019, 7, 108. [Google Scholar] [CrossRef]
- Simonin, M.; Briand, M.; Chesneau, G.; Rochefort, A.; Marais, C.; Sarniguet, A.; Barret, M. Seed microbiota revealed by a large-scale meta-analysis including 50 plant species. New Phytol. 2022, 234, 1448–1463. [Google Scholar] [CrossRef]
- Bauermeister, A.; Mannochio-Russo, H.; Costa-Lotufo, L.V.; Jarmusch, A.K.; Dorrestein, P.C. Mass spectrometry-based metabolomics in microbiome investigations. Nat. Rev. Microbiol. 2022, 20, 143–160. [Google Scholar] [CrossRef]
- Uehling, J.; Gryganskyi, A.; Hameed, K.; Tschaplinski, T.; Misztal, P.; Wu, S.; Desirò, A.; Vande Pol, N.; Du, Z.; Zienkiewicz, A. Comparative genomics of Mortierella elongata and its bacterial endosymbiont Mycoavidus cysteinexigens. Environ. Microbiol. 2017, 19, 2964–2983. [Google Scholar] [CrossRef]
- Williams, A.; Langridge, H.; Straathof, A.L.; Fox, G.; Muhammadali, H.; Hollywood, K.A.; Xu, Y.; Goodacre, R.; de Vries, F.T. Comparing root exudate collection techniques: An improved hybrid method. Soil Biol. Biochem. 2021, 161, 108391. [Google Scholar] [CrossRef]
- Torres-Cruz, T.J.; Billingsley Tobias, T.L.; Almatruk, M.; Hesse, C.N.; Kuske, C.R.; Desirò, A.; Benucci, G.M.N.; Bonito, G.; Stajich, J.E.; Dunlap, C. Bifiguratus adelaidae, gen. et sp. nov., a new member of Mucoromycotina in endophytic and soil-dwelling habitats. Mycologia 2017, 109, 363–378. [Google Scholar] [CrossRef] [PubMed]
- Finkel, O.M.; Salas-González, I.; Castrillo, G.; Conway, J.M.; Law, T.F.; Teixeira, P.J.P.L.; Wilson, E.D.; Fitzpatrick, C.R.; Jones, C.D.; Dangl, J.L. A single bacterial genus maintains root growth in a complex microbiome. Nature 2020, 587, 103–108. [Google Scholar] [CrossRef]
- Liu, H.; Qiu, Z.; Ye, J.; Verma, J.P.; Li, J.; Singh, B.K. Effective colonisation by a bacterial synthetic community promotes plant growth and alters soil microbial community. J. Sustain. Agric. Environ. 2022, 1, 30–42. [Google Scholar] [CrossRef]
- Sessitsch, A.; Pfaffenbichler, N.; Mitter, B. Microbiome applications from Lab to Field: Facing complexity. Trends Plant Sci. 2019, 24, 194–198. [Google Scholar] [CrossRef]
- Ke, J.; Wang, B.; Yoshikuni, Y. Microbiome engineering: Synthetic biology of plant-associated microbiomes in sustainable agriculture. Trends Biotechnol. 2021, 39, 244–261. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Adeleke, B.S.; Shi, Y.; Li, C. The seed microbiomes of staple food crops. Microb. Biotechnol. 2023, 16, 2236–2249. [Google Scholar] [CrossRef] [PubMed]

| Endophytic Microbes | Host Plant | Functions | References |
|---|---|---|---|
| Epichloë coenophiala | Festuca arundinacea | Activate stress response mechanisms for protection | [116] |
| Gordonia terrae KMP456-M40 | Mangrove propagules | Promotes mangrove root growth | [117] |
| Kosakonia cowanii | Lactuca serriola | Drought tolerance in invasive lettuce | [118] |
| Pseudomonas sp., Pantoea sp. | Hordeum vulgare L. | Improved biomass, mineral balance and antioxidant capacity under drought | [119] |
| Bacillus amyloliquefaciens | Oryza sativa | Production of Phytohormone | [120] |
| Paenibacillus polymyxa | Oryza sativa | Resistant to pathogens and production of glucanase | [121] |
| Microbacterium yunnanensis, Exiguobacterium soli, Micrococcus luteus, Leclercia adecarboxylata, Staphylococcus epidermidis, Pantoea dispersa | Oryza sativa | IAA production and enhancement of plant growth | [122] |
| Acinetobacter sp., Curtobacterium citreum, Microbacterium sp., Pantoea ananatis, Pseudomonas sp., Paenibacillus sp., Pantoea agglomerans, Pantoea sp., Staphylococcus cohnii, Microbacterium sp., Rathayibacter larrymoorei, Sphingomonas sp., Curtobacterium sp. | Oryza sativa | Phytohormone and metabolite production, phosphate-solubilizing, antifungal, plant growth promotion | [123] |
| Enterobacter asburiae, Pseudomonas putida, Pantoea dispersa | Oryza sativa | IAA production, antifungal, phosphate-solubilizing and promotion of plant growth | [124] |
| Bacillus, Nocardioides, Acinetobacter, Paracoccus, Enterococcus, Sphingomonas and Phyllobacterium | Glycine max | Phytate-solubilizing | [114] |
| Bacillus subtilis | Lycopersicon esculentum | Plant growth promotion, phytohormone and production of metabolite | [113] |
| Kosakonia, Massilia, Pantoea, Sphingomonas, Burkholderia, Pseudorhodoferax, Caulobacter, Bacillus sp., Methylobacterium, Microbacterium, Curtobacterium, Chitinophaga and Mucilaginibacter | Triticum esculentum | Plant growth promotion, production of metabolite and phytohormone | [101] |
| Klebsiella palustris, Bacillus pumilus, Microbacterium fujisawaense, Pantoea ananatis, Microbacterium radiotolerans | Oryza sativa | Enzyme production, osmotic stress tolerance | [125] |
| Neotyphodium oenophialum | Festuca arundinacea | Ergovaline and loline alkaloid production and improved protection against herbivores | [126] |
| Alternaria sp., Penicillium corylophilum and Phoma sp. | Invasive Phragmites | Improvement of seedling growth and seed germination | [30] |
| Epichloë ceonophiala | Salvadora phoenix | Improved resistance against herbivores and environmental stresses | [127] |
| Diaporthe sp. | Citrus ledgeriana | Production of alkaloid | [128] |
| Epichloë typhina | Dactylis glomerata | Improvement of photosynthesis and growth of host plant | [129] |
| Endophytes | Pathogens | Plant Host of the Endophytes | References |
|---|---|---|---|
| Moesziomyces spp. | Alternaria sp., Fusarium sp. | Oryza sativa | [143] |
| Synthetic bacterial community | Aspergillus flavus, Fusarium oxysporum | Arachis hypogaea | [144] |
| Bacillus subtilis BHN1, Bacillus stercoris BHR2, Paenibacillus peoriae YHR2-1 | Fusarium oxysporum (races 1 and 2), Botrytis cinerea | Solanum lycopersicum | [145] |
| Bacillus velezensis NEAU-CP5 | Ralstonia solanacearum | Solanum lycopersicum | [146] |
| Bacillus halotolerans strain B33 | Fusarium graminearum, Alternaria alternata, Aspergillus flavus | Small-grained cereals (wheat, barley, oats) | [147] |
| Bacillus amyloliquefaciens RWL-1 | Fusarium oxysporum | Oryza sativa | [148] |
| Bacillus mojavensis PS17 | Fusarium oxysporum ZUM2407 | Triticum aestivum | [149] |
| Bacillus velezensis ZMW8 | Fusarium verticillioides | Zea mays | [150] |
| Pseudomonas aeruginosa BHUJPCS-7 | Fusarium oxysporum | Cicer arietinum | [151] |
| Pseudomonas marginalis B1 | Fusarium culmorum | Brassica oleracea | [152] |
| Pantoea dispersa BB1 | Burkholderia glumae | Oryza sativa | [153] |
| Lactococcus and Pantoea | Oomycete pathogens | Cucurbits | [140] |
| Paenibacillus | Fusarium graminearum | Triticum aestivum | [154] |
| Seed Endophytes | Stress | Host | References |
|---|---|---|---|
| Heavy Metal Tolerance | |||
| Pantoea and Bacillus | Cadmium (Cd) | Agrostis capillaris | [166] |
| Bacillus amyloliquefaciens | Copper (Cu) | Oryza sativa | [162] |
| Epichloë | Cadmium (Cd) | Lolium perenne | [165] |
| Pseudomonas | Lead (Pb) | Nicotiana tabacum | [159] |
| Methylobacterium | Cadmium (Cd) | Carex pumila | [167] |
| Cellulosimicrobium cellulans | Copper (Cu) | Sesbania cannabina | [168] |
| Sphingomonas | Cadmium (Cd) | Oryza sativa | [161] |
| Rhodococcus and Bacillus | Copper (Cu) | Agrostis capillaris | [169] |
| Cellulosimicrobium cellulans | Copper (Cu) | Sesbania cannabina | [170] |
| Drought Tolerance | |||
| Epichloë coenophiala | Drought | Festuca arundinacea Schreb | [171] |
| Pantoea and Pseudomonas | Drought | Hordeum vulgare | [119] |
| K. cowanii | Drought | Lactuca serriola | [172] |
| Epichloë festucae var. lolii | Drought | Lolium perenne | [173] |
| Salt Tolerance | |||
| Bacillus mojavensis PS17 | Salt | Triticum aestivum | [149] |
| Gordonia terrae KMP456-M40 | Salt | Mangroves | [117] |
| Pantoea agglomerans Ed-3 and Bacillus subtilis Es-1 | Salt | Elymus | [174] |
| Bacillus aryabhattai, Bacillus altitudinis, Bacillus gladioli, Bacillus wiedmannii and Pseudomonas aeruginosa, | Salt | Triticum aestivum | [175] |
| Xanthomonas, Flavobacterium, and Microbacterium, | Salt | Oryza sativa | [51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fadiji, A.E.; Lanrewaju, A.A.; Omomowo, I.O.; Parra-Cota, F.I.; de los Santos-Villalobos, S. Harnessing Seed Endophytic Microbiomes: A Hidden Treasure for Enhancing Sustainable Agriculture. Plants 2025, 14, 2421. https://doi.org/10.3390/plants14152421
Fadiji AE, Lanrewaju AA, Omomowo IO, Parra-Cota FI, de los Santos-Villalobos S. Harnessing Seed Endophytic Microbiomes: A Hidden Treasure for Enhancing Sustainable Agriculture. Plants. 2025; 14(15):2421. https://doi.org/10.3390/plants14152421
Chicago/Turabian StyleFadiji, Ayomide Emmanuel, Adedayo Ayodeji Lanrewaju, Iyabo Olunike Omomowo, Fannie Isela Parra-Cota, and Sergio de los Santos-Villalobos. 2025. "Harnessing Seed Endophytic Microbiomes: A Hidden Treasure for Enhancing Sustainable Agriculture" Plants 14, no. 15: 2421. https://doi.org/10.3390/plants14152421
APA StyleFadiji, A. E., Lanrewaju, A. A., Omomowo, I. O., Parra-Cota, F. I., & de los Santos-Villalobos, S. (2025). Harnessing Seed Endophytic Microbiomes: A Hidden Treasure for Enhancing Sustainable Agriculture. Plants, 14(15), 2421. https://doi.org/10.3390/plants14152421






