Recent Advancements and Biotechnological Implications of Carotenoid Metabolism of Brassica
Abstract
1. Introduction
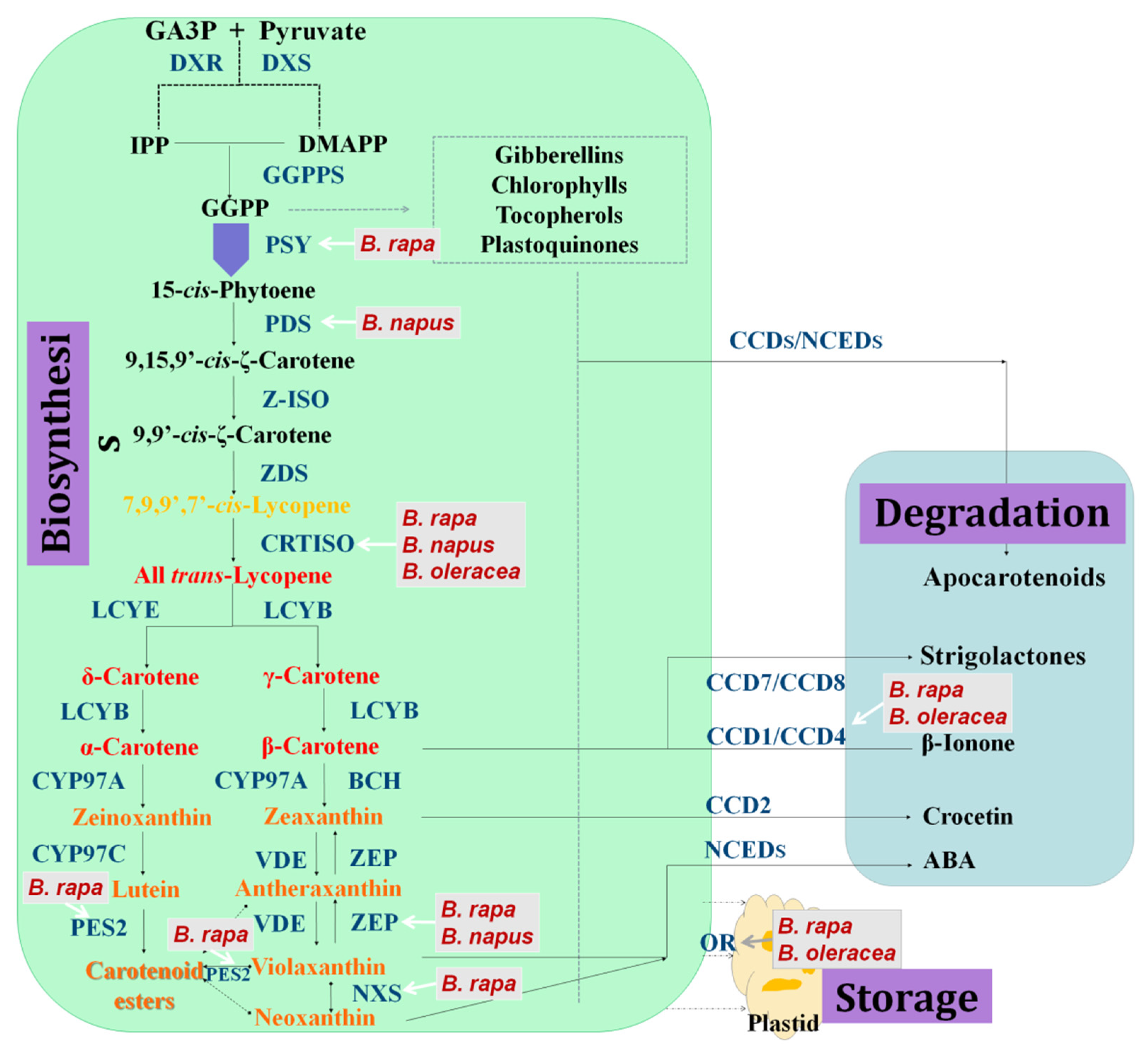
2. Overview of the Metabolic Pathway of Carotenoids
2.1. Carotenoid Biosynthesis
2.2. Degradation of Carotenoids
3. Genetic Study of Carotenoid Accumulation in Brassica Crops
4. Evolution of Carotenoid Biosynthesis and Some Key Carotenoid Genes in Brassica
5. Biotechnological Implications of Carotenoid Genes Identified by QTL-Mapping
6. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- George, B. Carotenoid research: History and new perspectives for chemistry in biological systems. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158699. [Google Scholar]
- Ho, J.; Kish, E.; Méndez-Hernández, D.D.; WongCarter, K.; Pillai, S.; Kodis, G.; Niklas, J.; Poluektov, O.G.; Gust, D.; Moore, T.A.; et al. Triplet-triplet energy transfer in artificial and natural photosynthetic antennas. Proc. Natl. Acad. Sci. USA 2017, 114, 5513–5521. [Google Scholar] [CrossRef]
- Cazzonelli, C.I. Carotenoids in nature: Insights from plants and beyond. Funct. Plant Biol. 2011, 38, 833–847. [Google Scholar] [CrossRef]
- Palmer, A.C.; Healy, K.; Barffour, M.A.; Siamusantu, W.; Chileshe, J.; Schulze, K.J.; West, K.P.; Labrique, A.B. Provitamin A Carotenoid-Biofortified Maize Consumption Increases Pupillary Responsiveness among Zambian Children in a Randomized Controlled Trial. J. Nutr. 2016, 146, 2551–2558. [Google Scholar] [CrossRef]
- Xiao, H.L.; Rong, B.Y.; Rong, L.; Zhen, X.H.; Cheng, C.H.; Zhong, H.Z.; Le, M. Association between Lutein and Zeaxanthin Status and the Risk of Cataract: A Meta-Analysis. Nutrients 2014, 6, 452–465. [Google Scholar] [CrossRef]
- Chang, M.X.; Xiong, F. Astaxanthin and Its Effects in Inflammatory Responses and Inflammation-Associated Diseases: Recent Advances and Future Directions. Molecules 2020, 25, 5342. [Google Scholar] [CrossRef]
- Alós, E.; Rodrigo, M.J.; Zacarias, L. Manipulation of Carotenoid Content in Plants to Improve Human Health. Sub-Cell. Biochem. 2016, 79, 311–343. [Google Scholar] [CrossRef]
- Carl, E.S.; David, E.K.; Dean, A.K.; Scott, M.J. Genetic variation in carotenoid concentrations among diploid and amphidiploid rapid-cycling Brassica species. HortScience 2007, 42, 461–465. [Google Scholar] [CrossRef]
- Cheng, L.; Charles, A.S.; Anthony, J.S. Modular engineering for microbial production of carotenoids. Metab. Eng. Commun. 2020, 10, e00118. [Google Scholar] [CrossRef]
- Bramley, P.M. Regulation of carotenoid formation during tomato fruit ripening and development. J. Exp. Bot. 2002, 53, 2107–2113. [Google Scholar] [CrossRef]
- Ruiz-Sola, M.Á.; Rodríguez-Concepción, M. Carotenoid Biosynthesis in Arabidopsis: A Colorful Pathway. Arab. Book 2012, 10, e0158. [Google Scholar] [CrossRef]
- Barj, M.V.; Ezquerro, M.; Beretta, S.; Diretto, G.; FlorezSarasa, I.; Feixes, E.; Fiore, A.; Karlova, R.; Fernie, A.R.; Beekwilder, J.; et al. Several geranylgeranyl diphosphate synthase isoforms supply metabolic substrates for carotenoid biosynthesis in tomato. New Phytol. 2021, 231, 255–272. [Google Scholar] [CrossRef]
- Hermanns, A.S.; Zhou, X.S.; Xu, Q.; Tadmor, Y.; Li, L. Carotenoid Pigment Accumulation in Horticultural Plants. Hortic. Plant J. 2020, 6, 343–360. [Google Scholar] [CrossRef]
- Breitenbach, J.; Sandmann, G. ζ-Carotene cis isomers as products and substrates in the plant poly-cis carotenoid biosynthetic pathway to lycopene. Planta 2005, 220, 785–793. [Google Scholar] [CrossRef]
- Francis, X.C. Regulation of carotenoid synthesis and accumulation in plants. Pure Appl. Chem. 2013, 74, 1409–1417. [Google Scholar] [CrossRef]
- Gupta, P.; Hirschberg, J. The Genetic Components of a Natural Color Palette: A Comprehensive List of Carotenoid Pathway Mutations in Plants. Front. Plant Sci. 2022, 12, 806184. [Google Scholar] [CrossRef]
- Luan, Y.T.; Fu, X.M.; Lu, P.J.; Grierson, D.; Xu, C.J. Molecular Mechanisms Determining the Differential Accumulation of Carotenoids in Plant Species and Varieties. Crit. Rev. Plant Sci. 2020, 39, 125–139. [Google Scholar] [CrossRef]
- Demmig, A.B.; Gilmore, A.M.; Adams, W.W. Carotenoids 3: In vivo function of carotenoids in higher plants. FASEB J. 1996, 10, 403–412. [Google Scholar] [CrossRef]
- Beatrycze, N.; Wojciech, S.; Kazimierz, S. New transgenic line of Arabidopsis thaliana with partly disabled zeaxanthin epoxidase activity displays changed carotenoid composition, xanthophyll cycle activity and non-photochemical quenching kinetics. J. Plant Physiol. 2009, 166, 1045–1056. [Google Scholar] [CrossRef]
- Wei, Z.; Arazi, T.; Hod, N.; Zohar, M.; Isaacson, T.; Doron-Faigenboim, A.; Reznik, N.; Yedidia, I. Transcriptome Profiling of Ornithogalum dubium Leaves and Flowers to Identify Key Carotenoid Genes for CRISPR Gene Editing. Plants 2020, 9, 540. [Google Scholar] [CrossRef]
- Zhao, J.; Li, J.; Zhang, J.; Chen, D.; Zhang, H.; Liu, C.; Qin, G. Genome-Wide Identification and Expression Analysis of the Carotenoid Cleavage Oxygenase Gene Family in Five Rosaceae Species. Plant Mol. Biol. Rep. 2021, 39, 739–751. [Google Scholar] [CrossRef]
- Baldermann, S.; Kato, M.; Kurosawa, M.; Kurobayashi, Y.; Fujita, A.; Fleischmann, P.; Watanabe, N. Functional characterization of a carotenoid cleavage dioxygenase 1 and its relation to the carotenoid accumulation and volatile emission during the floral development of Osmanthus fragrans Lour. J. Exp. Bot. 2010, 61, 2967–2977. [Google Scholar] [CrossRef]
- Song, H.X.; Lu, Q.; Hou, L.P.; Li, M.L. The genes crucial to carotenoid metabolism under elevated CO2 levels in carrot (Daucus carota L.). Sci. Rep. 2021, 11, 12073. [Google Scholar] [CrossRef]
- Zhang, N.; Ma, X.M.; Li, R.; Xue, Y.H.; Sun, Y.S.; Nie, S.S.; Zhang, L.G. Transcriptome-based analysis of carotenoid accumulation-related gene expression in petals of Chinese cabbage (Brassica rapa L.). 3 Biotech 2019, 9, 274. [Google Scholar] [CrossRef]
- Jung, H.J.; Manoharan, R.K.; Park, J.I.; Chung, M.Y.; Lee, J.; Lim, Y.P.; Hur, Y.; Nou, I.S. Identification of Yellow Pigmentation Genes in Brassica rapa ssp. pekinensis Using Br300 Microarray. Int. J. Genom. 2014, 2014, 204969. [Google Scholar] [CrossRef]
- Etsuo, M.; Chika, Y.; Michio, O.; Motohisa, T. Linkage analysis of RFLP markers for clubroot resistance and pigmentation in Chinese cabbage (Brassica rapa ssp. pekinensis). Euphytica 1998, 104, 79–86. [Google Scholar] [CrossRef]
- Su, T.B.; Yu, S.C.; Wang, J.; Zhang, F.L.; Yu, Y.J.; Zhang, D.S.; Zhao, X.Y.; Wang, W.H. Loss of Function of the Carotenoid Isomerase Gene BrCRTISO Confers Orange Color to the Inner Leaves of Chinese Cabbage (Brassica rapa L. ssp. pekinensis). Plant Mol. Biol. Report. 2015, 33, 648–659. [Google Scholar] [CrossRef]
- Seohee, L.; Sangchoon, L.; Donghae, B.; Dongyoung, L.; Jeeyoung, P.; Jonghoon, L.; Hyunoh, L.; Sanghyun, S.; Taejin, Y. Association of molecular markers derived from the BrCRISTO1 gene with prolycopene-enriched orange-colored leaves in Brassica rapa. Theor. Appl. Genet. 2014, 127, 179–191. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, L.; Ma, S.; Wang, R.F.; He, Q.; Tian, M.; Zhang, L.G. Fine mapping and candidate gene analysis of the white flower gene Brwf in Chinese cabbage (Brassica rapa L.). Sci. Rep. 2020, 10, 6080. [Google Scholar] [CrossRef]
- Yang, S.J.; Tian, X.X.; Wang, Z.Y.; Wei, X.C.; Zhao, Y.Y.; Su, H.N.; Zhao, X.B.; Tian, B.M.; Yuan, Y.X.; Zhang, X.W. Fine Mapping and Candidate Gene Identification of a White Flower Gene BrWF3 in Chinese Cabbage (Brassica rapa L. ssp. pekinensis). Front. Plant Sci. 2021, 12, 646222. [Google Scholar] [CrossRef]
- Li, P.; Lv, S.; Zhang, D.; Su, T.; Xin, X.; Wang, W.; Zhao, X.; Yu, Y.; Zhang, Y.; Yu, S.; et al. The Carotenoid Esterification Gene BrPYP Controls Pale-Yellow Petal Color in Flowering Chinese Cabbage (Brassica rapa L. subsp. parachinensis). Front. Plant Sci. 2022, 13, 844140. [Google Scholar] [CrossRef]
- Li, H.L.; Yu, K.D.; Amoo, O.; Yu, Y.L.; Guo, M.X.; Deng, S.Y.; Li, M.T.; Hu, L.M.; Wang, J.Z.; Fan, C.C.; et al. Site-Directed Mutagenesis of the Carotenoid Isomerase Gene BnaCRTISO Alters the Color of Petals and Leaves in Brassica napus L. Front. Plant Sci. 2022, 13, 801456. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, F.; Yuan, Q.; Lin, P.X.; Zheng, H.; Liang, S.; Jian, Y.; Miao, H.Y.; Li, H.X.; Wang, Q.M.; et al. Characterization of BoaCRTISO Reveals Its Role in Carotenoid Biosynthesis in Chinese Kale. Front. Plant Sci. 2021, 12, 662684. [Google Scholar] [CrossRef] [PubMed]
- Bo, S.; Min, J.; Hao, Z.; Yue, J.; Wen, L.H.; Qiao, Y.; Ai, H.Z.; Qing, C.; Yun, T.Z.; Yuan, X.L.; et al. Color-related chlorophyll and carotenoid concentrations of Chinese kale can be altered through CRISPR/Cas9 targeted editing of the carotenoid isomerase gene BoaCRTISO. Hortic. Res. 2020, 7, 94–102. [Google Scholar] [CrossRef]
- Yang, S.J.; Liu, H.L.; Zhao, Y.Y.; Su, H.N.; Wei, X.C.; Wang, Z.Y.; Zhao, X.B.; Zhang, X.W.; Yuan, Y.X. Map-Based Cloning and Characterization of Br-dyp1, a Gene Conferring Dark Yellow Petal Color Trait in Chinese Cabbage (Brassica rapa L. ssp. pekinensis). Front. Plant Sci. 2022, 13, 841328. [Google Scholar] [CrossRef]
- Liu, Y.J.; Ye, S.H.; Yuan, G.G.; Ma, X.W.; Heng, S.P.; Yi, B.; Ma, C.Z.; Shen, J.X.; Tu, J.X.; Fu, T.D.; et al. Gene silencing of BnaA09.ZEP and BnaC09.ZEP confers orange color in Brassica napus flowers. Plant J. 2020, 104, 932–949. [Google Scholar] [CrossRef]
- Gonzalez, J.S.; Ha, S.H.; Magallanes, L.M.; Gilliland, L.U.; Zhou, A.; Lipka, A.E.; Nguyen, Y.N.; Angelovici, R.; Lin, H.N.; Cepela, J.; et al. Carotenoid cleavage dioxygenase4 is a negative regulator of β-carotene content in Arabidopsis seeds. Plant Cell 2013, 25, 4812–4826. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, C.; Wang, Y.Q.; Yao, X.; Wang, F.; Wu, J.S.; King, G.J.; Liu, K. Disruption of a CAROTENOID CLEAVAGE DIOXYGENASE 4 gene converts flower colour from white to yellow in Brassica species. New Phytol. 2015, 206, 1513–1526. [Google Scholar] [CrossRef]
- Han, F.; Cui, H.; Zhang, B.; Liu, X.; Yang, L.; Zhuang, M.; Lv, H.; Li, Z.; Wang, Y.; Fang, Z.; et al. Map-based cloning and characterization of BoCCD4, a gene responsible for white/yellow petal color in B. oleracea. BMC Genom. 2019, 20, 242. [Google Scholar] [CrossRef]
- Yan, C.; Huang, Y.; Liu, Z.; Guo, F.; Jiao, Z.; Yang, W.; Zhu, F.; Qiu, Z. Rapid identification of yellow-flowered gene Bofc in cauliflower (Brassica oleracea var. botrytis) by bulked segregant analysis and whole-genome resequencing. Euphytica 2020, 216, 1348–1351. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, J.; Chen, L.; Ren, W.J.; Han, F.Q.; Fang, Z.Y.; Yang, L.M.; Zhuang, M.; Lv, H.H.; Wang, Y.; et al. Transcriptome Analysis Reveals Key Genes and Pathways Associated with the Petal Color Formation in Cabbage (Brassica oleracea L. var. capitata). Int. J. Mol. Sci. 2022, 23, 6656. [Google Scholar] [CrossRef]
- Lu, S.; Van, E.J.; Zhou, X.J.; Lopez, A.B.; O’Halloran, D.M.; Cosman, K.M.; Conlin, B.J.; Paolillo, D.J.; Garvin, D.F.; Vrebalov, J.; et al. The cauliflower Or gene encodes a DnaJ cysteine-rich domain-containing protein that mediates high levels of beta-carotene accumulation. Plant Cell 2006, 18, 3594–3605. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, S.F.; Dai, Y.; Wang, S.X.; Wang, C.G.; Li, F.; Zhang, H.; Chen, G.H.; Yuan, L.Y.; Hou, J.F.; et al. Mapping and Validation of BrGOLDEN: A Dominant Gene Regulating Carotenoid Accumulation in Brassica rapa. Int. J. Mol. Sci. 2022, 23, 12442. [Google Scholar] [CrossRef]
- Yuto, O.; Miho, T.; Atsushi, S.; Norihiko, M.; Hiroshi, S. Orange protein, phytoene synthase regulator, has protein disulfide reductase activity. Plant Signal. Behav. 2022, 17, 2072094. [Google Scholar] [CrossRef]
- Zhao, C.J.; Bin, S.L.; Xie, M.L.; Shi, M.J.; Dong, Z.X.; Yang, L.; Cheng, X.H.; Liu, Y.Y.; Bai, Z.T.; Xiang, Y.; et al. Mutation of the PHYTOENE DESATURASE 3 gene causes yellowish-white petals in Brassica napus. Crop J. 2021, 9, 1124–1134. [Google Scholar] [CrossRef]
- Jian, Y.; Zhang, C.L.; Wang, Y.T.; Li, Z.Q.; Chen, J.; Zhou, W.T.; Huang, W.L.; Jiang, M.; Zheng, H.; Li, M.Y.; et al. Characterization of the Role of the Neoxanthin Synthase Gene BoaNXS in Carotenoid Biosynthesis in Chinese Kale. Genes 2021, 12, 1122. [Google Scholar] [CrossRef]
- Elvis, K.; Daniela, Q.M.; Elizabeth, I.K.; Paula, V.T.; Annaliese, S.M. Interspecific Hybridization for Brassica Crop Improvement. Crop Breed. Genet. Genom. 2019, 1, e190007. [Google Scholar] [CrossRef]
- Wang, X.W.; Wang, H.Z.; Wang, J.; Sun, R.F.; Wu, J.; Liu, S.Y.; Bai, Y.Q.; Mun, J.H.; Bancroft, I.; Cheng, F.; et al. The genome of the mesopolyploid crop species Brassica rapa. Nat. Genet. 2011, 43, 1035–1039. [Google Scholar] [CrossRef]
- Raheeba, T.N.; Shaheen, K.J.; Farooq, A.B.; Tariq, R.R.; Asha, N.; Altaf, A.W.; Rehana, A.; Nahida, A.; Moneesa, B.; Ishfaq, M.S.; et al. Review on “Crispr-CAS9—A Genome editing tools for plant disease management”. Plant Cell Biotechnol. Mol. Biol. 2022, 23, 1–14. [Google Scholar]
- Fu, H.; Chao, H.B.; Zhao, X.J.; Wang, H.Y.; Li, H.X.; Zhao, W.G.; Sun, T.; Li, M.T.; Huang, J.Y. Anthocyanins identification and transcriptional regulation of anthocyanin biosynthesis in purple Brassica napus. Plant Mol. Biol. 2022, 110, 53–68. [Google Scholar] [CrossRef]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, pharmacology and treatment. Br. J. Pharmacol. 2017, 174, 1290–1324. [Google Scholar] [CrossRef]
- Johnson, E.J. A possible role for lutein and zeaxanthin in cognitive function in the elderly. Am. J. Clin. Nutr. 2012, 96, 1161S–1165S. [Google Scholar] [CrossRef]
- Cao, W.X.; Wang, P.; Yang, L.M.; Fang, Z.Y.; Zhang, Y.Y.; Zhuang, M.; Lv, H.H.; Wang, Y.; Ji, J.L. Carotenoid Biosynthetic Genes in Cabbage: Genome-Wide Identification, Evolution, and Expression Analysis. Genes 2021, 12, 2027. [Google Scholar] [CrossRef]
- Pham, A.T.; Jae, K.K.; Jeongyeo, L.; Woo, T.P.; Do, Y.K.; Yeon, B.K.; Haeng, H.K.; Hye, R.K. Analysis of carotenoid accumulation and expression of carotenoid biosynthesis genes in different organs of Chinese cabbage (Brassica rapa subsp. pekinensis). EXCLI J. 2012, 11, 508–516. [Google Scholar]
| Regulated Genes | Species | Major Changes | Genes (Gene Accession) | Color Change/Tissue | Reference |
|---|---|---|---|---|---|
| CRTISO | B. rapa | The mutant Br-oy protein cannot convert prolycopene to all- trans -lycopene. | BrOy (Bra031539) | white/yellow → orange inner leaf | [26,27] |
| The loss of BrCRTISO function leads to the accumulation of prolycopene. | Br-oy or BrCRTISO (Bra031539) | ||||
| Loss of BrWF3 function interferes with plastoglobules assembly and decreases expression levels of genes associated with carotenoid metabolism. | Brwf3 (Bra032957) | yellow → white/flower petal | [30] | ||
| The key factor for the pale-yellow color of petals was the decrease in esterified carotenoid content due to the loss of PYP function. | BrPYP (BraA02g037170.3C) | yellow → pale-yellow/flower petal | [31] | ||
| B. napus | The contents of carotenoids in petals and leaves of BnaCRTISO double mutant were reduced. In petals, the content of chalcone decreased, the content of some carotene (lycopene, α-carotene, γ-carotene) increased. | BnaA09. CRTISO (BnaA09g49740D) BnaC08. CRTISO (BnaC08g44970D) | yellow → milky white flower petals yellow → pale yellow leaves | [32] | |
| B.oleracea | Carotenoid and chlorophyll levels were reduced in the mutant of BoaCRTISO. | BoaCRTISO (GenBank accession MN810158) | green → yellowing leaves | [33,34] | |
| ZEP | B. rapa | The loss of function of ZEP disrupts the metabolism of carotenoids and leads to the increase in total carotenoid accumulation. | Br-dyp1 (Bra037130) | yellow → dark yellow flower petal | [35] |
| B. napus | The abolishment of both genes led to a substantial increase in lutein content and a sharp decline in violaxanthin content in petals. | BnaA09. ZEP (BnaA09g07610D) BnaC09. ZEP (BnaC07g16350D) | yellow → orange flower petal | [36] | |
| CCD4 | B. napus | In yellow petals, a large amount of α-carotene, α-cryptoxanthin, β-cryptoxanthin, violaxanthin, 9-cis-violaxanthin, lutein, and cis-neoxanthinwere accumulated. | BnaC3.CCD4 (Bol029878) | white → yellow flower petal | [38] |
| B.oleracea | Not available. | BoCCD4 (Bol029878) | white/pale yellow → yellow flower petal | [39] | |
| These key genes may interact with BoCCD4 to jointly regulate carotenoid biosynthesis in petals. | WRKY (Bo2g151880) SBP (Bo3g024180) | [41] | |||
| OR | B.oleracea | The OR gene mutation confers the accumulation of high levels of β-carotene in various tissues normally devoid of carotenoids. | OR (GenBank accession DQ482460) | white → orange | [42] |
| B. rapa | The BrGOLDEN lines are rich in β-carotene and lutein. | BrGOLDEN (BraA09g007080.3C) | golden → light yellow inner leaf | [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, L.; Chang, L.; Yu, Y.; Zhang, D.; Zhao, X.; Wang, W.; Li, P.; Xin, X.; Zhang, F.; Yu, S.; et al. Recent Advancements and Biotechnological Implications of Carotenoid Metabolism of Brassica. Plants 2023, 12, 1117. https://doi.org/10.3390/plants12051117
Shi L, Chang L, Yu Y, Zhang D, Zhao X, Wang W, Li P, Xin X, Zhang F, Yu S, et al. Recent Advancements and Biotechnological Implications of Carotenoid Metabolism of Brassica. Plants. 2023; 12(5):1117. https://doi.org/10.3390/plants12051117
Chicago/Turabian StyleShi, Lichun, Lin Chang, Yangjun Yu, Deshuang Zhang, Xiuyun Zhao, Weihong Wang, Peirong Li, Xiaoyun Xin, Fenglan Zhang, Shuancang Yu, and et al. 2023. "Recent Advancements and Biotechnological Implications of Carotenoid Metabolism of Brassica" Plants 12, no. 5: 1117. https://doi.org/10.3390/plants12051117
APA StyleShi, L., Chang, L., Yu, Y., Zhang, D., Zhao, X., Wang, W., Li, P., Xin, X., Zhang, F., Yu, S., Su, T., Dong, Y., & Shi, F. (2023). Recent Advancements and Biotechnological Implications of Carotenoid Metabolism of Brassica. Plants, 12(5), 1117. https://doi.org/10.3390/plants12051117









