A Recent Update on the Impact of Nano-Selenium on Plant Growth, Metabolism, and Stress Tolerance
Abstract
:1. Introduction
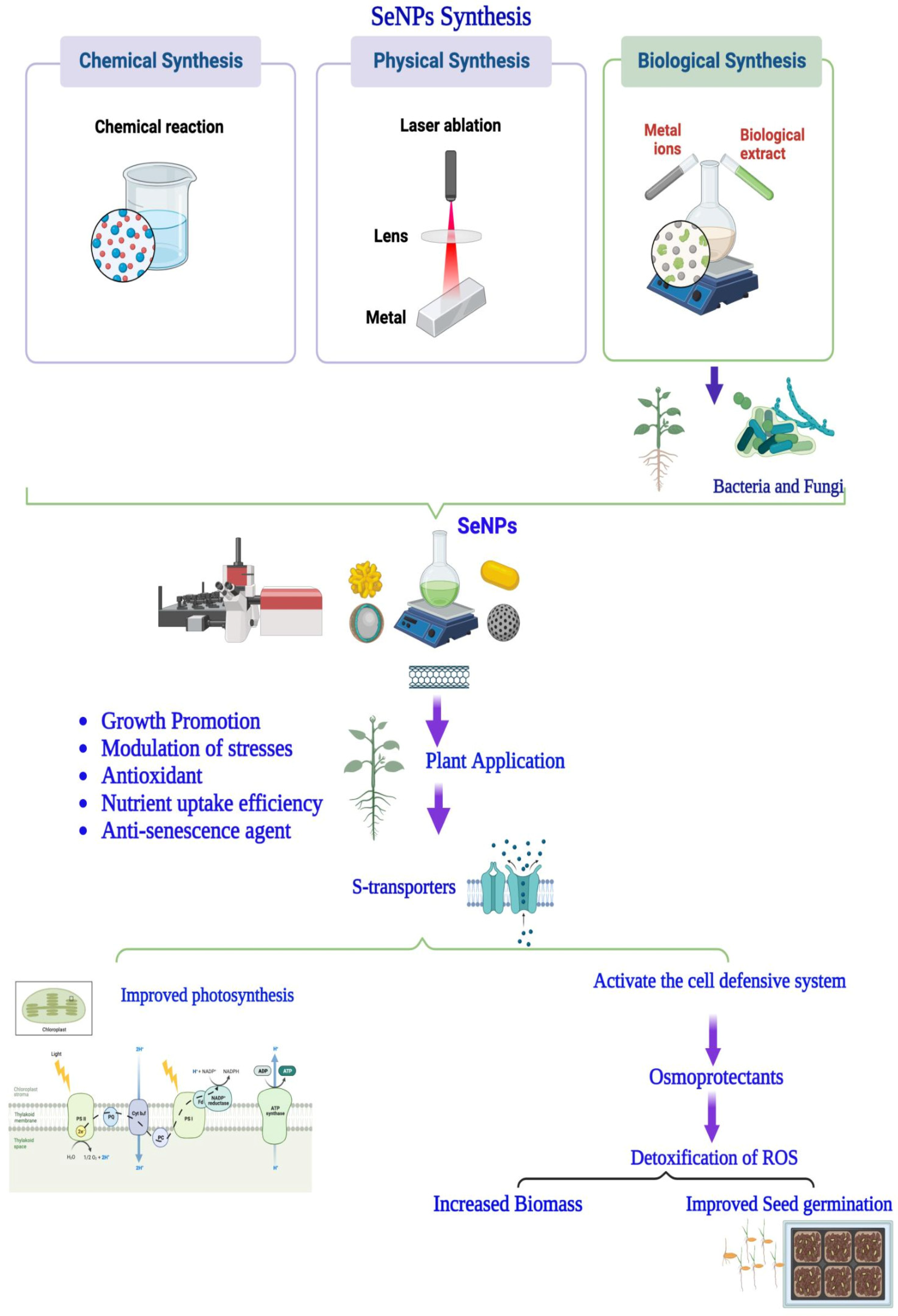
2. Modes of SeNP Synthesis
| Method of Synthesis | Name of Plant Source | Size | Shape | Operational Parameters | References |
|---|---|---|---|---|---|
| Green-synthesis | Vitis vinifera Extract | 3–18 nm | Spherical | 10 mL of Extract added with 4 × 10−5 M selenous acid for refluxed for 15 min and centrifuged at 15,000× g rpm | [29] |
| A. sativum | 48–87 nm | Spherical | Garlic extract (5 mL) mixed with 20 mM Na2SeO3 solution (50 mL) and stirred at 150 rpm at 60 °C. | [39] | |
| Clausena dentate leaf extracts | 46.32–78.88 nm | Spherical | 10 g of C. dentata leaf powder with 100 mL of DH2O boiled at 60 °C for 5 min. This broth (12 mL) was added to 1 mM aqueous selenium powder (88 mL) for the synthesis of Se-NPs | [40] | |
| Crataegus monogyna | 113 nm | Spherical | 0.01M Na2SeO3 was mixed with 2 mg mL−1 hawthorn fruits lyophilized powder for 12 hrs and dialyzed (MWCO 8000–14,000) for 48 hrs. | [41] | |
| Emblica officinalis | 20 to 60 nm | Spherical | 2 mL of aqueous fruit extract of E. officinalis was added with 10 mL of 10 mM Na2SeO3 under stirring. The mixture was allowed in dark conditions at 27 ± 2 °C and 120 rpm for 24 hrs. | [42] | |
| Catharanthus roseus and Peltophorumpterocarpum flowers | 23.2 nm and 30.44 nm | Spherical | 10 gm of the flowers was added with 90 mL of 10 mM aqueous solution of Na2SeO4 and incubated at 250 rpm at 36 °C for 7 days | [43] | |
| Withania somnifera leaves extract | 15 nm | Crystalline | One hundred milli liters of plant extract was mixed with 50 mM selenious acid and stirred and washed with distilled water and acetone at overnight | [44] | |
| Aloe vera leaf extract (ALE) Fabricated with Se-NPs | 50 nm | Spherical fabricated Se-NPs | 20 mL of culture filtrates, cell lysate, and crude cell wall from six Trichoderma spp was added with 70 mL of sterile distilled water containing 25 mM Na2SeO3 and stored 28 ± 1 °C on a shaker at 150 rpm for 6 days | [45] | |
| Zingiber officinale | 100 to 150 nm | Spherical | 1% ginger extract was added to 10 mM Na2SeO4 solution (9:1 ratio) with pH 9 at 37 °C for 75 hrs at 130 rpm | [46] | |
| Wheat (Triticum aestivum L.) | 140 ± 10 nm | Spherical | Biosynthesized SeNPs: Rahnella aquatilis HX2 cell broth filtered in Na2SeO3 at 5 mM for 48 hrs at 28 °C then centrifuged at 8000× g for 30 min and washed with 1 M NaOH for 20 min in a boiling water bath. | [47] | |
| Vigna unguiculata L. | 33.4 nm | Spherical | 10 mM of aq. solution of 10 mM Na2SeO4 added to the ascorbic acid powder 1.5% (w/v) under stirring at room temperature for 15 min. | [25] | |
| Cyamopsis tetragonoloba | 50–150 nm | Oval | 700 mg of Na2SeO3 in 50 mL of distilled water for 20 min, added with 50 mL of ascorbic acid solution | [22] | |
| Raphanus sativus var. sativus, Eruca sativa, Solanum melongena, Cucumis sativus and Capsicum annuum. | 100 nm | X-ray diffractograms | Laser ablation processes of solid Se then fiber ytterbium laser (1060 nm and 1070 nm), pulse rate 20 KHz in 80 nanoseconds in 20 W | [48] | |
| Brassica napus | 10–55 nm | Spherical | 1%ComamonastestosteroniS44 culture added with 10 mM Na2SeO3 for 72 hrs. Then, 2 M NaOH solution was added to this under stirring | [26] | |
| Allium sativum | 40 to 100 nm | Spherical | Cassia auriculata in fine powder added with 100 mL of 10 mM Na2SeO3 at a concentration (10 × 10−3 M) under magnetic stirring, then incubated at 37 °C | [49] |
3. Mode of Action
4. Impact of Se-NPs on Growth and Physiology
Influence on Primary and Secondary Metabolites
5. Influence of Se-NPs on Crop Yield
6. Impact of Se-NPs on Abiotic and Biotic Stress Tolerance
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ingle, A.P.; Duran, N.; Rai, M. Bioactivity, mechanism of action, and cytotoxicity of copper-based nanoparticles: A review. Appl. Microbiol. Biotechnol. 2014, 98, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Arshad, R.; Gulshad, L.; Haq, I.U.; Farooq, M.A.; Al-Farga, A.; Siddique, R.; Manzoor, M.F.; Karrar, E. Nanotechnology: A novel tool to enhance the bioavailability of micronutrients. Food Sci. Nutr. 2021, 9, 3354–3361. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Jia, Q.; Li, Y.; Zhang, T.; Chen, J.; Ren, Y.; Dong, K.; Xu, S.; Shi, N.N.; Fu, S. Effects of Arbuscular Mycorrhizal Fungi and Biochar on Growth, Nutrient Absorption, and Physiological Properties of Maize (Zea mays L.). J. Fungi 2022, 8, 1275. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Charles, L.S.; Yang, Z.; Du, G.; Fu, S. Differential mechanisms drive species loss under artificial shade and fertilization in the alpine meadow of the Tibetan Plateau. Front. Plant Sci. 2022, 13, 63. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, P.K.D.; Solanki, A.; Debnath, A.; Nayyar, A.; El-Sappagh, S.; Kwak, K.S. Advancing modern healthcare with nanotechnology, nanobiosensors, and internet of nano things: Taxonomies, applications, architecture, and challenges. IEEE Access 2020, 8, 65230–65266. [Google Scholar] [CrossRef]
- Wu, Z.; Li, T. Nanoparticle-mediated cytoplasmic delivery of messenger RNA vaccines: Challenges and future perspectives. Pharm. Res. 2021, 38, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Pulizzi, F. Nano in the future of crops. Nat. Nanotechnol. 2019, 14, 507. [Google Scholar] [CrossRef] [PubMed]
- Hamouda, R.A.; Hussein, M.H.; Abo-Elmagd, R.A.; Bawazir, S.S. Synthesis and biological characterization of silver nanoparticles derived from the cyanobacterium Oscillatoria limnetica. Sci. Rep. 2019, 9, 113071. [Google Scholar] [CrossRef]
- Dissanayake, C.B.; Chandrajith, R. Selenium-A New Entrant to Medical Geology. In Introduction to Medical Geology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 205–222. [Google Scholar]
- Winkel, L.H.E.; Vriens, B.; Jones, G.D.; Schneider, L.S.; Pilon-Smits, E.; Bañuelos, G.S. Selenium Cycling Across Soil-Plant-Atmosphere Interfaces: A Critical Review. Nutrients 2015, 7, 4199–4239. [Google Scholar] [CrossRef]
- RajaeeBehbahani, S.; Iranbakhsh, A.; Ebadi, M.; Majd, A.; Ardebili, Z.O. Red elemental selenium nanoparticles mediated substantial variations in growth, tissue differentiation, metabolism, gene transcription, epigenetic cytosine DNA methylation, and callogenesis in bittermelon (Momordica charantia); an in vitro experiment. PLoS ONE 2020, 15, e0235556. [Google Scholar]
- Bhatia, P.; Aureli, F.; D’Amato, M.; Prakash, R.; Cameotra, S.S.; Nagaraja, T.P.; Cubadda, F. Selenium bioaccessibility and speciation in biofortified Pleurotus mushrooms grown on selenium-rich agricultural residues. Food Chem. 2013, 140, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Wadhwani, S.A.; Shedbalkar, U.U.; Singh, R.; Chopade, B.A. Biogenic selenium nanoparticles: Current status and future prospects. Appl. Microbiol. Biotechnol. 2016, 100, 2555–2566. [Google Scholar] [CrossRef] [PubMed]
- Hosnedlova, B.; Kepinska, M.; Skalickova, S.; Fernandez, C.; Ruttkay-Nedecky, B.; Peng, Q.; Baron, M.; Melcova, M.; Opatrilova, R.; Zidkova, J.; et al. Nano-selenium and its nanomedicine applications: A critical review. Int. J. Nanomed. 2018, 13, 2107–2128. [Google Scholar] [CrossRef] [PubMed]
- Malagoli, M.; Schiavon, M.; Dall’Acqua, S.; Pilon-Smits, E.A.H. Effects of selenium biofortification on crop nutritional quality. Front. Plant Sci. 2015, 6, 280. [Google Scholar] [CrossRef]
- Zohra, E.; Ikram, M.; Omar, A.A.; Hussain, M.; Satti, S.H.; Raja, N.I.; Ehsan, M. Potential applications of biogenic selenium nanoparticles in alleviating biotic and abiotic stresses in plants: A comprehensive insight on the mechanistic approach and future perspectives. Green Process. Synth. 2021, 10, 456–475. [Google Scholar] [CrossRef]
- Khurana, A.; Tekula, S.; Saifi, M.A.; Venkatesh, P.; Godugu, C. Therapeutic applications of selenium nanoparticles. Biomed. Pharmacother. 2019, 111, 802–812. [Google Scholar] [CrossRef]
- Ikram, M.; Javed, B.; Raja, N.I.; Mashwani, Z.-u.-R. Biomedical potential of plant-based selenium nanoparticles: A comprehensive review on therapeutic and mechanistic aspects. Int. J. Nanomed. 2021, 16, 249. [Google Scholar] [CrossRef]
- Khan, I.; Zaneb, H.; Masood, S.; Yousaf, M.S.; Rehman, H.F.; Rehman, H. Effect of Moringa oleifera leaf powder supplementation on growth performance and intestinal morphology in broiler chickens. J. Anim. Physiol. Anim. Nutr. 2017, 101, 114–121. [Google Scholar] [CrossRef]
- Pyrzynska, K.; Sentkowska, A. Biosynthesis of selenium nanoparticles using plant extracts. J. Nanostruct. Chem. 2021, 12, 467–480. [Google Scholar] [CrossRef]
- Panahi-Kalamuei, M.; Salavati-Niasari, M.; Hosseinpour-Mashkani, S.M. Facile microwave synthesis, characterization, and solar cell application of selenium nanoparticles. J. Alloys Compd. 2014, 617, 627–632. [Google Scholar] [CrossRef]
- Ragavan, P.; Ananth, A.; Rajan, M.R. Impact of selenium nanoparticles on growth, biochemical characteristics and yield of cluster bean Cyamopsis tetragonoloba. Int. J. Environ. Agric. Biotechnol. 2017, 2, 238983. [Google Scholar] [CrossRef]
- El-Ghazaly, M.A.; Fadel, N.; Rashed, E.; El-Batal, A.; Kenawy, S.A. Anti-inflammatory effect of selenium nanoparticles on the inflammation induced in irradiated rats. Can. J. Physiol. Pharmacol. 2017, 95, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Vahdati, M.; Tohidi Moghadam, T. Synthesis and characterization of selenium nanoparticles-lysozyme nanohybrid system with synergistic antibacterial properties. Sci. Rep. 2020, 10, 510. [Google Scholar] [CrossRef]
- El Lateef Gharib, F.A.; Zeid, I.M.; Ghazi, S.M.; Ahmed, E.Z. The response of cowpea (Vigna unguiculata L) plants to foliar application of sodium selenate and selenium nanoparticles (SeNPs). J. Nanomater. Mol. Nanotechnol. 2019, 8, 4. [Google Scholar]
- El-Badri, A.M.; Batool, M.; Wang, C.; Hashem, A.M.; Tabl, K.M.; Nishawy, E.; Kuai, J.; Zhou, G.; Wang, B. Selenium and zinc oxide nanoparticles modulate the molecular and morpho-physiological processes during seed germination of Brassica napus under salt stress. Ecotoxicol. Environ. Saf. 2021, 225, 112695. [Google Scholar] [CrossRef]
- Ikram, M.; Raja, N.I.; Mashwani, Z.U.R.; Omar, A.A.; Mohamed, A.H.; Satti, S.H.; Zohra, E. Phytogenic Selenium Nanoparticles Elicited the Physiological, Biochemical, and Antioxidant Defense System Amelioration of Huanglongbing-Infected ‘Kinnow’ Mandarin Plants. Nanomaterials 2022, 12, 356. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhou, H.; Bai, J.; Li, Y.; Yang, J.; Ma, Q.; Qu, Y. Biosynthesis of selenium nanoparticles mediated by fungus Mariannaea sp. HJ and their characterization. Colloids Surf. A Physicochem. Eng. Asp. 2019, 571, 9–16. [Google Scholar] [CrossRef]
- Sharma, G.; Sharma, A.R.; Bhavesh, R.; Park, J.; Ganbold, B.; Nam, J.S.; Lee, S.S. Biomolecule-mediated synthesis of selenium nanoparticles using dried Vitis vinifera (raisin) extract. Molecules 2014, 19, 2761–2770. [Google Scholar] [CrossRef]
- Anu, K.; Devanesan, S.; Prasanth, R.; AlSalhi, M.S.; Ajithkumar, S.; Singaravelu, G. Biogenesis of selenium nanoparticles and their anti-leukemia activity. J. King Saud. Univ. Sci. 2020, 32, 2520–2526. [Google Scholar] [CrossRef]
- Alam, H.; Khatoon, N.; Raza, M.; Ghosh, P.C.; Sardar, M. Synthesis and Characterization of Nano Selenium Using Plant Biomolecules and Their Potential Applications. Bio Nano Sci. 2019, 9, 96–104. [Google Scholar] [CrossRef]
- Joshi, S.M.; De Britto, S.; Jogaiah, S.; Ito, S.I. Mycogenic selenium nanoparticles as potential new generation broad spectrum antifungal molecules. Biomolecules 2019, 9, 419. [Google Scholar] [CrossRef]
- Seliem, M.K.; Hafez, Y.; El-Ramady, H. Using Nano-selenium in reducing the negative effects of high temperature stress on Chrysanthemum morifolium Ramat. J. Sustain. Agric. Sci. 2020, 46, 47–60. [Google Scholar] [CrossRef]
- Ismail, A.W.A.; Sidkey, N.M.; Arafa, R.A.; Fathy, R.M.; El-Batal, A.I. Evaluation of in vitro antifungal activity of silver and selenium nanoparticles against Alternaria solani caused early blight disease on potato. Br. Biotechnol. J. 2016, 12, 1–11. [Google Scholar] [CrossRef]
- Khalifa, H.M.S.; Sameer, W.M. Control of the green mold of orange using fungicides alone and in combination with antioxidants. Middle East J. Appl Sci. 2014, 4, 200–206. [Google Scholar]
- Nandini, B.; Hariprasad, P.; Prakash, H.S.; Shetty, H.S.; Geetha, N. Trichogenic-selenium nanoparticles enhance disease suppressive ability of Trichoderma against downy mildew disease caused by Sclerosporagraminicola in pearl millet. Sci. Rep. 2017, 7, 2612. [Google Scholar] [CrossRef]
- El-Badri, A.M.; Hashem, A.M.; Batool, M.; Sherif, A.; Nishawy, E.; Ayaad, M.; Hassan, H.M.; Elrewainy, I.M.; Wang, J.; Kuai, J.; et al. Comparative efficacy of bio-selenium nanoparticles and sodium selenite on morpho-physiochemical attributes under normal and salt stress conditions, besides selenium detoxification pathways in Brassica napus L. J. Nanobiotechnol. 2022, 20, 163. [Google Scholar] [CrossRef]
- Korde, P.; Ghotekar, S.; Pagar, T.; Pansambal, S.; Oza, R.; Mane, D. Plant extract assisted eco-benevolent synthesis of selenium nanoparticles-a review on plant parts involved, characterization and their recent applications. J. Chem. Rev. 2020, 2, 157–168. [Google Scholar]
- Satgurunathan, T.; Bhavan, P.S.; Komathi, S. Green synthesis of selenium nanoparticles from sodium selenite using garlic extract and its enrichment on Artemia nauplii to feed the freshwater prawn Macrobrachiumrosenbergiipost-larvae. Res. J. Chem. Environ. 2017, 21, 1–12. [Google Scholar]
- Sowndarya, P.; Ramkumar, G.; Shivakumar, M.S. Green synthesis of selenium nanoparticles conjugated Clausena dentata plant leaf extract and their insecticidal potential against mosquito vectors. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1490–1495. [Google Scholar] [CrossRef]
- Cui, D.; Liang, T.; Sun, L.; Meng, L.; Yang, C.; Wang, L.; Liang, T.; Li, Q. Green synthesis of selenium nanoparticles with extract of hawthorn fruit induced HepG2 cells apoptosis. Pharm. Biol. 2018, 56, 528–534. [Google Scholar] [CrossRef]
- Gunti, L.; Dass, R.S.; Kalagatur, N.K. Phytofabrication of selenium nanoparticles from Emblica officinalis fruit extract and exploring its biopotential applications: Antioxidant, antimicrobial, and biocompatibility. Front. Microbiol. 2019, 10, 931. [Google Scholar] [CrossRef] [PubMed]
- Deepa, B.; Ganesan, V. Bioinspiredsynthesis of selenium nanoparticles using flowers of Catharanthus roseus (L.) G. Don.; Peltophorumpterocarpum (DC.) Backer ex Heyne–a comparison. Int. J. Chem. Technol. Res. 2015, 7, 725–733. [Google Scholar]
- Alagesan, V.; Venugopal, S. Green synthesis of selenium nanoparticle using leaves extract of Withaniasomnifera and its biological applications and photocatalytic activities. Bionanoscience 2019, 9, 105–116. [Google Scholar] [CrossRef]
- Fardsadegh, B.; Jafarizadeh-Malmiri, H. Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their in vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains. Green Process. Synth. 2019, 8, 399–407. [Google Scholar] [CrossRef]
- Menon, S.; KS, S.D.; Agarwal, H.; Shanmugam, V.K. Efficacy of biogenic selenium nanoparticles from an extract of ginger towards evaluation on anti-microbial and anti-oxidant activities. Colloid Interface Sci. Commun. 2019, 29, 1–8. [Google Scholar] [CrossRef]
- Hu, T.; Li, H.; Li, J.; Zhao, G.; Wu, W.; Liu, L.; Wang, Q.; Guo, Y. Absorption and bio-transformation of selenium nanoparticles by wheat seedlings (Triticum aestivum L.). Front. Plant Sci. 2018, 9, 597. [Google Scholar] [CrossRef] [Green Version]
- Gudkov, S.V.; Shafeev, G.A.; Glinushkin, A.P.; Shkirin, A.V.; Barmina, E.V.; Rakov, I.I.; Simakin, A.V.; Kislov, A.V.; Astashev, M.E.; Vodeneev, V.A.; et al. Production and use of selenium nanoparticles as fertilizers. ACS Omega 2020, 5, 17767–17774. [Google Scholar] [CrossRef]
- Anu, K.; Singaravelu, G.; Murugan, K.; Benelli, G. Green-synthesis of selenium nanoparticles using garlic cloves (Allium sativum): Biophysical characterization and cytotoxicity on vero cells. J. Clust. Sci. 2017, 28, 551–563. [Google Scholar] [CrossRef]
- El-Ramady, H.; Abdalla, N.; Taha, H.S.; Alshaal, T.; El-Henawy, A.; Faizy, S.E.D.A.; Shams, M.S.; Youssef, S.M.; Shalaby, T.; Bayoumi, Y.; et al. Selenium and nano-selenium in plant nutrition. Environ. Chem. Lett. 2016, 14, 123–147. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.B.; Raza, A.; Hawrylak-Nowak, B.; Matraszek-Gawron, R.; Nahar, K.; Fujita, M. Selenium toxicity in plants and environment: Biogeochemistry and remediation possibilities. Plants 2020, 9, 1711. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Hossain, M.A.; da Silva, J.A.T.; Fujita, M. Plant response and tolerance to abiotic oxidative stress: Antioxidant defense is a key factor. In Crop Stress and Its Management: Perspectives and Strategies; Springer: Dordrecht, The Netherlands, 2012; pp. 261–315. [Google Scholar]
- Malik, J.A.; Goel, S.; Kaur, N.; Sharma, S.; Singh, I.; Nayyar, H. Selenium antagonises the toxic effects of arsenic on mungbean (Phaseolus aureus Roxb.) plants by restricting its uptake and enhancing the antioxidative and detoxification mechanisms. Environ. Exp. Bot. 2012, 77, 242–248. [Google Scholar] [CrossRef]
- Shahbaz, M.; Fatima, N.; Mashwani, Z.U.R.; Akram, A.; Mehak, A.; Abasi, F.; Ajmal, M.; Yousaf, T.; Raja, N.I.; UlHassan, H.; et al. Effect of Phytosynthesized Selenium and Cerium Oxide Nanoparticles on Wheat (Triticum aestivum L.) against Stripe Rust Disease. Molecules 2022, 27, 8149. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, Y.; Li, K.; Wan, Y.; Wang, Q.; Zhuang, Z.; Guo, Y.; Li, H. Uptake, translocation and biotransformation of selenium nanoparticles in rice seedlings (Oryza sativa L.). J. Nanobiotechnol. 2020, 18, 103. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.G.; Huang, Y.; Hu, Y.; Liu, Y.; Christie, P. Interactions between selenium and iodine uptake by spinach (Spinacia oleracea L.) in solution culture. Plant Soil 2004, 261, 99–105. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in human diets–iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.; Varghese, S.H.; Nair, B.G.; Maekawa, T.; Yoshida, Y.; Kumar, D.S. Nanoparticulate material delivery to plants. Plant Sci. 2010, 179, 154–163. [Google Scholar] [CrossRef]
- Domokos-Szabolcsy, E.; Alshaal, T.; Elhawat, N.; Abdalla, N.; Reis, A.; El-Ramady, H. The interactions between selenium, nutrients and heavy metals in higher plants under abiotic stresses. Environ. Biodivers. Soil Secur. 2017, 1, 5–31. [Google Scholar]
- Schiavon, M.; Pilon-Smits, E.A. The fascinating facets of plant selenium accumulation–biochemistry, physiology, evolution and ecology. New Phytol. 2017, 213, 1582–1596. [Google Scholar] [CrossRef]
- Rastogi, A.; Tripathi, D.K.; Yadav, S.; Chauhan, D.K.; Živčák, M.; Ghorbanpour, M.; El-Sheery, N.I.; Brestic, M. Application of silicon nanoparticles in agriculture. 3 Biotech 2019, 9, 90. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Shah, T.; Ullah, R.; Zhou, P.; Guo, M.; Ovais, M.; Tan, Z.; Rui, Y. Review on recent progress in magnetic nanoparticles: Synthesis, characterization, and diverse applications. Front. Chem. 2021, 9, 629054. [Google Scholar] [CrossRef]
- Tarrahi, R.; Mahjouri, S.; Khataee, A. A review on in vivo and in vitro nanotoxicological studies in plants: A headlight for future targets. Ecotoxicol. Environ. Saf. 2021, 208, 111697. [Google Scholar] [CrossRef] [PubMed]
- Mozafariyan, M.; Shekari, L.; Hawrylak-Nowak, B.; Kamelmanesh, M.M. Protective role of selenium on pepper exposed to cadmium stress during reproductive stage. Biol. Trace Elem. Res. 2014, 160, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hu, C.; Wang, X.; Qing, X.; Wang, P.; Zhang, Y.; Zhang, X.; Zhao, X. Selenium alleviated chromium stress in Chinese cabbage (Brassica campestris L. ssp. Pekinensis) by regulating root morphology and metal element uptake. Ecotoxicol. Environ. Saf. 2019, 173, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Djanaguiraman, M.; Belliraj, N.; Bossmann, S.H.; Prasad, P.V. High-temperature stress alleviation by selenium nanoparticle treatment in grain sorghum. ACS Omega 2018, 3, 2479–2491. [Google Scholar] [CrossRef]
- Al-Deriny, S.H.; Dawood, M.A.; Elbialy, Z.I.; El-Tras, W.F.; Mohamed, R.A. Selenium nanoparticles and spirulina alleviate growth performance, hemato-biochemical, immune-related genes, and heat shock protein in Nile tilapia (Oreochromis niloticus). Biol. Trace Elem. Res. 2020, 19, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.A.; Blinov, A.V.; Serov, A.V.; Gvozdenko, A.A.; Kravtsov, A.A.; Nagdalian, A.A.; Raffa, V.V.; Maglakelidze, D.G.; Blinova, A.A.; Kobina, A.V.; et al. Effect of selenium nanoparticles on germination of Hordéumvulgáre barley seeds. Coatings 2021, 11, 862. [Google Scholar] [CrossRef]
- Bideshki, A.; Arvin, M.J.; Aien, A.; Hasandokht, M.R.; Khalighi, A. Interactive effects of Foliar 24-Epibrassinolide and selenium applications on yield, reduce nitrate accumulation and selenium enrichment in potato tuber in field. Cogent Food Agric. 2019, 5, 1690315. [Google Scholar] [CrossRef]
- Dai, Z.; Imtiaz, M.; Rizwan, M.; Yuan, Y.; Huang, H.; Tu, S. Dynamics of selenium uptake, speciation, and antioxidant response in rice at different panicle initiation stages. Sci. Total Environ. 2019, 691, 827–834. [Google Scholar] [CrossRef] [PubMed]
- El-Kinany, R.G.; Brengi, S.H.; Nassar, A.K.; El-Batal, A. Enhancement of Plant Growth, Chemical Composition and Secondary Metabolites of Essential Oil of Salt-Stressed Coriander (Coriandrum sativum L.) Plants Using Selenium, Nano-Selenium, and Glycine Betaine. Sci. J. Flowers Ornam. Plants 2019, 6, 151–173. [Google Scholar] [CrossRef]
- Alves, L.R.; Rossatto, D.R.; Rossi, M.L.; Martinelli, A.P.; Gratão, P.L. Selenium improves photosynthesis and induces ultrastructural changes but does not alleviate cadmium-stress damages in tomato plants. Protoplasma 2020, 257, 597–605. [Google Scholar] [CrossRef]
- Lyu, L.; Wang, H.; Liu, R.; Xing, W.; Li, J.; Man, Y.B.; Wu, F. Size-dependent transformation, uptake, and transportation of SeNPs in a wheat–soil system. J. Hazard. Mater. 2022, 424, 127323. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yan, J.; Qin, Y.; Xu, J.; Shohag, M.J.I.; Wei, Y.; Gu, M. Effect of different forms of selenium on the physiological response and the cadmium uptake by rice under cadmium stress. Int. J. Environ. Res. Public Health 2020, 17, 6991. [Google Scholar] [CrossRef]
- Rady, M.M.; Belal, H.E.; Gadallah, F.M.; Semida, W.M. Selenium application in two methods promotes drought tolerance in Solanum lycopersicum plant by inducing the antioxidant defense system. Sci. Hortic. 2020, 266, 109290. [Google Scholar] [CrossRef]
- Xu, J.; Gong, Y.; Sun, Y.; Cai, J.; Liu, Q.; Bao, J.; Yang, J.; Zhang, Z. Impact of selenium deficiency on inflammation, oxidative stress, and phagocytosis in mouse macrophages. Biol. Trace Elem. Res. 2020, 194, 237–243. [Google Scholar] [CrossRef]
- Mroczek-Zdyrska, M.; Wójcik, M. The influence of selenium on root growth and oxidative stress induced by lead in Vicia faba L. minor plants. Biol. Trace Elem. Res. 2012, 147, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Molnár, Á.; Kolbert, Z.; Kéri, K.; Feigl, G.; Ördög, A.; Szőllősi, R.; Erdei, L. Selenite-induced nitro-oxidative stress processes in Arabidopsis thaliana and Brassica juncea. Ecotoxicol. Environ. Saf. 2018, 148, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Skalickova, S.; Milosavljevic, V.; Cihalova, K.; Horky, P.; Richtera, L.; Adam, V. Selenium nanoparticles as a nutritional supplement. Nutrition 2017, 33, 83–90. [Google Scholar] [CrossRef]
- Yu, Y.; Fu, P.; Huang, Q.; Zhang, J.; Li, H. Accumulation, subcellular distribution, and oxidative stress of cadmium in Brassica chinensis supplied with selenite and selenate at different growth stages. Chemosphere 2019, 216, 331–340. [Google Scholar] [CrossRef]
- Qi, W.Y.; Li, Q.; Chen, H.; Liu, J.; Xing, S.F.; Xu, M.; Yan, Z.; Song, C.; Wang, S.G. Selenium nanoparticles ameliorate Brassica napus L. cadmium toxicity by inhibiting the respiratory burst and scavenging reactive oxygen species. J. Hazard. Mater. 2021, 417, 125900. [Google Scholar] [CrossRef]
- Pereira, A.G.; Gerolis, L.G.L.; Gonçalves, L.S.; Moreira, L.M.C.; Gastelois, P.L.; Neves, M.J. Radiolytic synthesis and characterization of selenium nanoparticles: Comparative biosafety evaluation with selenite and ionizing radiation. World J. Microbiol. Biotechnol. 2022, 38, 33. [Google Scholar] [CrossRef]
- Wang, K.; Wang, Y.; Wan, Y.; Mi, Z.; Wang, Q.; Wang, Q.; Li, H. The fate of arsenic in rice plants (Oryza sativa L.): Influence of different forms of selenium. Chemosphere 2021, 264, 128417. [Google Scholar] [CrossRef] [PubMed]
- Azimi, F.; Oraei, M.; Gohari, G.; Panahirad, S.; Farmarzi, A. Chitosan-selenium nanoparticles (Cs–Se NPs) modulate the photosynthesis parameters, antioxidant enzymes activities and essential oils in Dracocephalummoldavica L. under cadmium toxicity stress. Plant Physiol. Biochem. 2021, 167, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Zhang, E.; Zhang, X.; Wang, Q. Silicon alleviates salinity stress in licorice (Glycyrrhiza uralensis) by regulating carbon and nitrogen metabolism. Sci. Rep. 2021, 11, 1115. [Google Scholar] [CrossRef]
- Neysanian, M.; Iranbakhsh, A.; Ahmadvand, R.; OraghiArdebili, Z.; Ebadi, M. Comparative efficacy of selenate and selenium nanoparticles for improving growth, productivity, fruit quality, and postharvest longevity through modifying nutrition, metabolism, and gene expression in tomato; potential benefits and risk assessment. PLoS ONE 2020, 15, e0244207. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.H.; Alnusairi, G.S.; Khan, A.A.; Alnusaire, T.S.; Fakhr, M.A.; Abdulmajeed, A.M.; Aldesuquy, H.S.; Yahya, M.; Najeeb, U. Biochar and Selenium Nanoparticles Induce Water Transporter Genes for Sustaining Carbon Assimilation and Grain Production in Salt-Stressed Wheat. J. Plant Growth Regul. 2022, 1–22. [Google Scholar] [CrossRef]
- Marslin, G.; Sheeba, C.J.; Franklin, G. Nanoparticles alter secondary metabolism in plants via ROS burst. Front. Plant Sci. 2017, 8, 832. [Google Scholar] [CrossRef]
- Battin, E.E.; Brumaghim, J.L. Antioxidant activity of sulfur and selenium: A review of reactive oxygen species scavenging, glutathione peroxidase, and metal-binding antioxidant mechanisms. Cell Biochem. Biophys. 2009, 55, 1–23. [Google Scholar] [CrossRef]
- Schiavon, M.; Lima, L.W.; Jiang, Y.; Hawkesford, M.J. Effects of selenium on plant metabolism and implications for crops and consumers. In Selenium in Plants; Springer: Cham, Switzerland, 2017; pp. 257–275. [Google Scholar]
- Yarizade, K.; Hosseini, R. Expression analysis of ADS, DBR2, ALDH1 and SQS genes in Artemisia vulgaris hairy root culture under nano cobalt and nano zinc elicitation. Ext. J. App. Sci. 2015, 3, 69–76. [Google Scholar]
- Zhai, X.; Jia, M.; Chen, L.; Zheng, C.J.; Rahman, K.; Han, T.; Qin, L.P. The regulatory mechanism of fungal elicitor-induced secondary metabolite biosynthesis in medical plants. Crit. Rev. Microbiol. 2017, 43, 238–261. [Google Scholar] [CrossRef]
- Hussain, S.M.; Khalid, A.; Shahzad, M.M.; Rasul, A.; Akram, A.M.; Ahmad, N.; Khalid, F. Effect of dietary supplementation of selenium nanoparticles on growth performance and nutrient digestibility of common carp (Cyprinus carpio Linnaeus, 1758) fingerlings fed sunflower meal-based diet. Indian J. Fish. 2019, 66, 55–61. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Abdelrahman, M.; Hosseini, M.S.; Hoveizeh, N.F.; Tran, L.S.P. Alleviation of the effect of salinity on growth and yield of strawberry by foliar spray of selenium-nanoparticles. Environ. Pollut. 2019, 253, 246–258. [Google Scholar] [CrossRef]
- Ciccolini, V.; Pellegrino, E.; Coccina, A.; Fiaschi, A.I.; Cerretani, D.; Sgherri, C. Biofortification with Iron and Zinc Improves Nutritional and Nutraceutical Properties of Common Wheat Flour and Bread. J. Agric. Food Chem. 2017, 65, 5443–5452. [Google Scholar] [CrossRef]
- Li, D.; An, Q.; Wu, Y.; Li, J.Q.; Pan, C. Foliar application of selenium nanoparticles on celery stimulates several nutrient component levels by regulating the α-linolenic acid pathway. ACS Sustain. Chem. Eng. 2020, 8, 10502–10510. [Google Scholar] [CrossRef]
- Tian, M.; Xu, X.; Liu, F.; Fan, X.; Pan, S. Untargeted metabolomics reveals predominant alterations in primary metabolites of broccoli sprouts in response to pre-harvest selenium treatment. Food Res. Int. 2018, 111, 205–211. [Google Scholar] [CrossRef]
- Garza-García, J.J.; Hernández-Díaz, J.A.; Zamudio-Ojeda, A.; León-Morales, J.M.; Guerrero-Guzmán, A.; Sánchez-Chiprés, D.R.; López-Velázquez, J.C.; García-Morales, S. The role of selenium nanoparticles in agriculture and food technology. Biol. Trace Elem. Res. 2021, 200, 2528–2548. [Google Scholar] [CrossRef]
- García Márquez, V.; Morelos Moreno, Á.; Benavides Mendoza, A.; Medrano Macías, J. Ionic selenium and nanoselenium as biofortifiers and stimulators of plant metabolism. Agronomy 2020, 10, 1399. [Google Scholar] [CrossRef]
- Paciolla, C.; de Leonardis, S.; Dipierro, S. Effects of selenite and selenate on the antioxidant systems in Senecio scandens L. Plant Biosyst. 2011, 145, 253–259. [Google Scholar] [CrossRef]
- Hashem, H.A.; Hassanein, R.A.; Bekheta, M.A.; El-Kady, F.A. Protective role of selenium in canola (Brassica napus L.) plant subjected to salt stress. Egypt. J. Exp. Biol. 2013, 9, 199–211. [Google Scholar]
- Golubkina, N.; Moldovan, A.; Fedotov, M.; Kekina, H.; Kharchenko, V.; Folmanis, G.; Alpatov, A.; Caruso, G. Iodine and Selenium Biofortification of Chervil Plants Treated with Silicon Nanoparticles. Plants 2021, 10, 2528. [Google Scholar] [CrossRef]
- Zhu, L.; Peng, X.; Li, H.; Zhang, Y.; Yao, S. On–off–on fluorescent silicon nanoparticles for recognition of chromium (VI) and hydrogen sulfide based on the inner filter effect. Sens. Actuators B Chem. 2017, 238, 196–203. [Google Scholar] [CrossRef]
- Silva, V.M.; Boleta, E.H.M.; Lanza, M.G.D.B.; Lavres, J.; Martins, J.T.; Santos, E.F.; dos Santos, F.L.M.; Putti, F.F.; Junior, E.F.; White, P.J.; et al. Physiological, biochemical, and ultrastructural characterization of selenium toxicity in cowpea plants. Environ. Exp. Bot. 2018, 150, 172–182. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, S.; Zhao, J.; Wang, F.; Du, Y.; Zou, S.; Li, H.; Wen, D.; Huang, Y. Comparative responses to silicon and selenium in relation to antioxidant enzyme system and the glutathione-ascorbate cycle in flowering Chinese cabbage (Brassica campestris L. ssp. chinensis var. utilis) under cadmium stress. Environ. Exp. Bot. 2017, 133, 1–11. [Google Scholar] [CrossRef]
- Bian, Z.-H.; Lei, B.; Cheng, R.-F.; Wang, Y.; Li, T.; Yang, Q.-C. Selenium distribution and nitrate metabolism in hydroponic lettuce (Lactuca sativa L.): Effects of selenium forms and light spectra. J. Integr. Agric. 2020, 19, 133–144. [Google Scholar] [CrossRef]
- Padervand, M. Reusable Porous Na (SiAl) O6. xH2O/NiFe2O4 Structure for Selective Removal of Heavy Metals from Waste Waters. U.S. Patent 11,014,082, 25 May 2021. [Google Scholar]
- Duma, M.; Alsina, I.; Dubova, L.; Stroksa, L.; Smiltina, Z. The effect of sodium selenite and selenate on the quality of lettuce. In Proceedings of the 6th Baltic Conference on Food Science and Technology Foodbalt, Jelgava, Latvia, 5–6 May 2011. [Google Scholar]
- Haghighi, M.; Abolghasemi, R.; da Silva, J.A.T. Low and high temperature stress affect the growth characteristics of tomato in hydroponic culture with Se and nano-Se amendment. Sci. Hortic. 2014, 178, 231–240. [Google Scholar] [CrossRef]
- Nawaz, F.; Ashraf, M.Y.; Ahmad, R.; Waraich, E.A.; Shabbir, R.N.; Hussain, R.A. Selenium supply methods and time of application influence spring wheat (Triticum aestivum L.) yield under water deficit conditions. J. Agric. Sci. 2017, 155, 643–656. [Google Scholar] [CrossRef]
- Naz, F.S.; Yusuf, M.; Khan, T.A.; Fariduddin, Q.; Ahmad, A. Low level of selenium increases the efficacy of 24-epibrassinolide through altered physiological and biochemical traits of Brassica juncea plants. Food Chem. 2015, 185, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Zu, C.; Shen, J.; Shao, F.; Li, T. Effects of selenium on the growth and photosynthetic characteristics of flue-cured tobacco (Nicotiana tabacum L.). Acta Soc. Bot. Pol. 2015, 84, 71–77. [Google Scholar] [CrossRef]
- Pezzarossa, B.; Rosellini, I.; Borghesi, E.; Tonutti, P.; Malorgio, F. Effects of Se-enrichment on yield, fruit composition and ripening of tomato (Solanum lycopersicum) plants grown in hydroponics. Sci. Hortic. 2014, 165, 106–110. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, Y.; Zhang, X.; Li, M. Effect of Foliar Treatment of Sodium Selenate on Postharvest Decay and Quality of Tomato Fruits. Sci. Hortic. 2016, 198, 304–310. [Google Scholar] [CrossRef]
- Mimmo, T.; Tiziani, R.; Valentinuzzi, F.; Lucini, L.; Nicoletto, C.; Sambo, P.; Scampicchio, M.; Pii, Y.; Cesco, S. Selenium biofortification in Fragaria × ananassa: Implications on strawberry fruits quality, content of bioactive health beneficial compounds and metabolomic profile. Front. Plant Sci. 2017, 8, 1887. [Google Scholar] [CrossRef]
- Puccinelli, M.; Pezzarossa, B.; Pintimalli, L.; Malorgio, F. Selenium biofortification of three wild species, Rumex acetosa L.; Plantago coronopus L.; and Portulaca oleracea L.; grown as microgreens. Agronomy 2021, 11, 1155. [Google Scholar] [CrossRef]
- Lidon, F.; Oliveira, K.; Galhano, C.; Guerra, M.; Ribeiro, M.; Pelica, J.; Pataco, I.; Ramalho, J.; Leitão, A.; Almeida, A. Selenium biofortification of rice through foliar application with selenite and selenate. Exp. Agric. 2018, 55, 528–542. [Google Scholar] [CrossRef]
- Leija-Martínez, P.; Benavides-Mendoza, A.; Cabrera-De La Fuente, M.; Robledo-Olivo, A.; Ortega-Ortíz, H.; Sandoval-Rangel, A.; González-Morales, S. Lettuce biofortification with selenium in chitosan-polyacrylic acid complexes. Agronomy 2018, 8, 275. [Google Scholar] [CrossRef]
- Ahmed, H.S.; Ahmed, M.F.; Shoala, T.; Salah, M. Impact of Single or Fractionated Radiation and Selenium Nano-particles on Acid Lime (Citrus aurantifolia L.) Seed Germination Ability and Seedlings Growth. Adv. Agric. Environ. Sci. Open Access 2018, 1, 91–100. [Google Scholar] [CrossRef]
- De Oliveira, V.C.; Faquin, V.; Guimarães, K.C.; Andrade, F.R.; Pereira, J.; Guilherme, L.R.G. Agronomic biofortification of carrot with selenium. Cienc. Agrotecnol. 2018, 42, 138–147. [Google Scholar] [CrossRef]
- Puccinelli, M.; Malorgio, F.; Terry, L.A.; Tosetti, R.; Rosellini, I.; Pezzarossa, B. Effect of selenium enrichment on metabolism of tomato (Solanum lycopersicum) fruit during postharvest ripening. J. Sci. Food Agric. 2019, 99, 2463–2472. [Google Scholar] [CrossRef] [PubMed]
- Ramkissoon, C.; Degryse, F.; da Silva, R.C.; Baird, R.; Young, S.D.; Bailey, E.H.; McLaughlin, M.J. Improving the efficacy of selenium fertilizers for wheat biofortification. Sci. Rep. 2019, 9, 19520. [Google Scholar] [CrossRef]
- Lara, T.S.; de Lima Lessa, J.H.; de Souza, K.R.D.; Corguinha, A.P.B.; Martins, F.A.D.; Lopes, G.; Guilherme, L.R.G. Selenium biofortification of wheat grain via foliar application and its effect on plant metabolism. J. Food Compos. Anal. 2019, 81, 10–18. [Google Scholar] [CrossRef]
- Morales-Espinoza, M.C.; Cadenas-Pliego, G.; Pérez-Alvarez, M.; Hernández-Fuentes, A.D.; De La Fuente, M.C.; Benavides-Mendoza, A.; Valdés-Reyna, J.; Juárez-Maldonado, A. Se nanoparticles induce changes in the growth, antioxidant responses, and fruit quality of tomato developed under NaCl stress. Molecules 2019, 24, 3030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeid, I.M.; Gharib, Z.F.A.E.; Ghazi, S.M.; Ahmed, E.Z. Promotive effect of ascorbic acid, gallic acid, selenium and nano-selenium on seed germination, seedling growth and some hydrolytic enzymes activity of cowpea (Vigna unguiculata) seedling. J. Plant Physiol. Pathol. 2019, 7, 1000193. [Google Scholar]
- Amina, Z.; Samar, O. Nano Selenium: Reduction of severe hazards of Atrazine and promotion of changes in growth and gene expression patterns on Vicia faba seedlings. Afr. J. Biotechnol. 2019, 18, 502–510. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Saad, A.M.; Najjar, A.A.; Alzahrani, S.O.; Alkhatib, F.M.; Shafi, M.E.; Selem, E.; Desoky, E.S.M.; Fouda, S.E.; El-Tahan, A.M.; et al. The use of biological selenium nanoparticles to suppress Triticum aestivum L. crown and root rot diseases induced by Fusarium species and improve yield under drought and heat stress. Saudi J. Biol. Sci. 2021, 28, 4461–4471. [Google Scholar] [CrossRef] [PubMed]
- Sheikhalipour, M.; Esmaielpour, B.; Behnamian, M.; Gohari, G.; Giglou, M.T.; Vachova, P.; Rastogi, A.; Brestic, M.; Skalicky, M. Chitosan–selenium nanoparticle (Cs–Se NP) foliar spray alleviates salt stress in bitter melon. Nanomaterials 2021, 11, 684. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Tan, J.; Cui, L.; Zhou, Z.; Zhou, S.; Zhang, Z.; Zheng, R.; Xue, Y.; Zhang, M.; Li, S.; et al. Graphene and Au NPs co-mediated enzymatic silver deposition for the ultrasensitive electrochemical detection of cholesterol. Biosens. Bioelectron. 2018, 102, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, S.M.; Moharrami, F.; Sarikhani, S.; Padervand, M. Selenium and silica nanostructure-based recovery of strawberry plants subjected to drought stress. Sci. Rep. 2020, 10, 17672. [Google Scholar] [CrossRef]
- Hajiboland, R. Selenium supplementation stimulates vegetative and reproductive growth in canola (Brassica napus L.) plants. Acta Agric. Slov. 2012, 99, 13. [Google Scholar] [CrossRef]
- Dong, J.Z.; Wang, Y.; Wang, S.H.; Yin, L.P.; Xu, G.J.; Zheng, C.; Lei, C.; Zhang, M.Z. Selenium increases chlorogenic acid, chlorophyll and carotenoids of Lyciumchinense leaves. J. Sci. Food Agric. 2013, 93, 310–315. [Google Scholar] [CrossRef]
- Le, V.N.; Rui, Y.; Gui, X.; Li, X.; Liu, S.; Han, Y. Uptake, Transport, distribution and bio-effects of SiO2 nanoparticles in Bt-transgenic cotton. J. Nanobiotechnol. 2014, 12, 50. [Google Scholar] [CrossRef]
- Sarraf, M.; Vishwakarma, K.; Kumar, V.; Arif, N.; Das, S.; Johnson, R.; Janeeshma, E.; Puthur, J.T.; Aliniaeifard, S.; Chauhan, D.K.; et al. Metal/metalloid-based nanomaterials for plant abiotic stress tolerance: An overview of the mechanisms. Plants 2022, 11, 316. [Google Scholar] [CrossRef]
- El-Ramady, H.; Abdalla, N.; Alshaal, T.; El-Henawy, A.; Elmahrouk, M.; Bayoumi, Y.; Shalaby, T.; Amer, M.; Shehata, S.; Fári, M.; et al. Plant nano-nutrition: Perspectives and challenges. In Nanotechnology, Food Security and Water Treatment; Gothandam, K., Ranjan, S., Dasgupta, N., Ramalingam, C., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2018; pp. 129–161. [Google Scholar]
- Hawrylak-Nowak, B.; Hasanuzzaman, M.; Matraszek-Gawron, R. Mechanisms of selenium-induced enhancement of abiotic stress tolerance in plants. In Plant Nutrients and Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Oku, H., Nahar, K., Hawrylak-Nowak, B., Eds.; Springer: Singapore, 2018; pp. 269–295. [Google Scholar]
- Gao, M.; Zhou, J.; Liu, H.; Zhang, W.; Hu, Y.; Liang, J.; Zhou, J. Foliar spraying with silicon and selenium reduces cadmium uptake and mitigates cadmium toxicity in rice. Sci. Total Environ. 2018, 631, 1100–1108. [Google Scholar] [CrossRef]
- Babajani, A.; Iranbakhsh, A.; OraghiArdebili, Z.; Eslami, B. Differential growth, nutrition, physiology, and gene expression in Melissa officinalis mediated by zinc oxide and elemental selenium nanoparticles. Environ. Sci. Pollut. Res. 2019, 26, 24430–24444. [Google Scholar] [CrossRef] [PubMed]
- Soleymanzadeh, R.; Iranbakhsh, A.; Habibi, G.; Ardebili, Z.O. Selenium nanoparticle protected strawberry against salt stress through modifications in salicylic acid, ion homeostasis, antioxidant machinery, and photosynthesis performance. Acta Biol. Crac. S. Bot. 2020, 62, 33–42. [Google Scholar]
- Zsiros, O.; Nagy, V.; Párducz, Á.; Nagy, G.; Ünnep, R.; El-Ramady, H.; Prokisch, J.; Lisztes-Szabó, Z.; Fári, M.; Csajbók, J.; et al. Effects of selenate and red Se-nanoparticles on the photosynthetic apparatus of Nicotiana tabacum. Photosynth. Res. 2019, 139, 449–460. [Google Scholar] [CrossRef] [PubMed]
- IranBakhsh, A.; Soleymanzadeh, R.; Habibi, G.; OraghiArdebili, Z. Soil supplementation with silicon nanoparticles to alleviate toxicity signs of salinity in strawberry. Iran. J. Plant Physiol. 2022, 12, 4099–4109. [Google Scholar]
- Matraszek-Gawron, R.; Hawrylak-Nowak, B. Micronutrient status and selected physiological parameters of roots in nickel-exposed Sinapis alba L. affected by different sulphur levels. Plants 2019, 8, 440. [Google Scholar] [CrossRef]
- Feng, R.; Wang, L.; Yang, J.; Zhao, P.; Zhu, Y.; Li, Y.; Yu, Y.; Liu, H.; Rensing, C.; Wu, Z. Underlying mechanisms responsible for restriction of uptake and translocation of heavy metals (metalloids) by selenium via root application in plants. J. Hazard. Mater. 2021, 402, 123570. [Google Scholar] [CrossRef]
- Mohammadhassan, R.; Ferdosi, A.; Seifalian, A.M.; Seifalian, M.; Malmir, S. Nanoelicitors Application Promote Antioxidant Capacity of Asparagus officinalis (In Vitro). J. Trop. Life Sci. 2021, 11, 259–265. [Google Scholar] [CrossRef]
- Sahay, S.; Khan, E.; Praveen, A.; Panthri, M.; Mirza, Z.; Gupta, M. Sulphur potentiates selenium to alleviate arsenic-induced stress by modulating oxidative stress, accumulation and thiol-ascorbate metabolism in Brassica juncea L. Environ. Sci. Pollut. Res. 2020, 27, 11697–11713. [Google Scholar] [CrossRef]
- Desoky, E.S.M.; Merwad, A.R.M.; Abo El-Maati, M.F.; Mansour, E.; Arnaout, S.M.; Awad, M.F.; Ramadan, M.F.; Ibrahim, S.A. Physiological and biochemical mechanisms of exogenously applied selenium for alleviating destructive impacts induced by salinity stress in bread wheat. Agronomy 2021, 11, 926. [Google Scholar] [CrossRef]
- Khalofah, A.; Migdadi, H.; El-Harty, E. Antioxidant enzymatic activities and growth response of quinoa (Chenopodium quinoa willd.) to exogenous selenium application. Plants 2021, 10, 719. [Google Scholar] [CrossRef]
- Sardar, R.; Ahmed, S.; Shah, A.A.; Yasin, N.A. Selenium nanoparticles reduced cadmium uptake, regulated nutritional homeostasis and antioxidative system in Coriandrum sativum grown in cadmium toxic conditions. Chemosphere 2022, 287, 132332. [Google Scholar] [CrossRef] [PubMed]
- Nasibi, F.; Aminian, F.; Mohammadinejad, G.; Hassanshahian, M. Seed priming with selenium nanoparticle and plant growth promoting rhizobacteria improve seedling development of foxtail millet (Setaria italica) under salinity stress. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Schiavon, M.; dall’Acqua, S.; Mietto, A.; Pilon-Smits, E.A.; Sambo, P.; Masi, A.; Malagoli, M. Selenium fertilization alters the chemical composition and antioxidant constituents of tomato (Solanum lycopersicon L.). J. Agric. Food Chem. 2013, 61, 10542–10554. [Google Scholar] [CrossRef] [PubMed]
- Samadi, S.; Lajayer, B.A.; Moghiseh, E.; Rodríguez-Couto, S. Effect of carbon nanomaterials on cell toxicity, biomass production, nutritional and active compound accumulation in plants. Environ. Technol. Innov. 2021, 21, 101323. [Google Scholar] [CrossRef]
- Qasim, U.; Osman, A.I.; Al-Muhtaseb, A.A.H.; Farrell, C.; Al-Abri, M.; Ali, M.; Vo, D.V.N.; Jamil, F.; Rooney, D.W. Renewable cellulosic nanocomposites for food packaging to avoid fossil fuel plastic pollution: A review. Environ. Chem. Lett. 2021, 19, 613–641. [Google Scholar] [CrossRef]
| Plant Species | Se-NPsSource (Selenium and Their Combination) | Dosage/Size | Mode of Application | Impact of Se-NPs | Biochemical/ Molecular Function | References |
|---|---|---|---|---|---|---|
| Lactuca sativa | Na2SeO3 and SeO3 | 50 mg L−1 | Foliar spray | Plant growth | 1. Chlorophyll content was elevated at the early stages of plant germination. 2. Senescence prevention | [108] |
| Brassica napus | Na2SeO4 | 2.5 and 5.0 mg L−1 | Foliar spray | Plant growth and yield | Increased in yield, shoot length, and number of leaves plant−1 under salt stress conditions | [101] |
| Solanum lycopersicum | N-Se, Na2SeO4 | 1 µM and 2.5 µM | Foliar spray | Plant growth at low and high-temperature conditions | Improved 27.5% green pigments content in hydroponic culture | [109] |
| Triticum aestivum | Se | 7.06 μM | Foliar spray | Plant growth | Maintaining higher growth, fresh and dry matter content | [110] |
| Brassica juncea | Na2SeO4 | 10 µM | Foliar spray | Plant growth improvement | Enhancing growth and photosynthesis efficacy | [111] |
| Nicotiana tabacum | Na2SeO3 | 6 mg kg−1 | Foliar spray | Plant growth improvement | Improved plant growth by uplifting plant photosynthesis | [112] |
| S. lycopersicum | Na2SeO4 | 10 to 20 mg kg−1 | Directly to peat | Plant growth improvement | Increased vitamin A content in tomato fruit | [113] |
| S. lycopersicum | Na2SeO4 | 1 mg L−1 | Foliar | Improved plant growth and disease management | 1. Increased antioxidant activity. 2. Control of gray mold rot infection | [114] |
| Raphanus sativus cv. Saxa | Se | 5 mg | Foliar spray | Improved plant growth | Increasing the polyphenol content (flavanols, kaempferol derivative, and hydroxycinnamic acids) up to 10% higher than the control. | [90] |
| Se | 10 or 20µM | Foliar spray | Improved plant growth | 1. Enhanced in Glucosinolates, 2. Dimeric-4 mercaptobutyl (DMB-GLS) concentration | ||
| Se | 40 µM | Foliar spray | Improved plant growth | In plant root systems, the stimulation of high-affinity sulfate transporter genes (Sultr1;1 and Sultr1;2) | ||
| S. lycopersicum | Na2SeO4 | 1 mg L−1 | Foliar pre-treatment | Improved plant growth | 1. Delay in tomato fruit 2. Inhibition of ethylene biosynthetic genes—ACC synthase | [103] |
| Fragaria × ananassa | Na2SeO4 | 1.9 and 19 mg L−1 | Nutrient solution | Plant growth and fruit yield | Growth regulator’s upregulation, biomass, and nutraceutical quality | [115] |
| Ocimum basilicum | Na2SeO4 | 4, 8, and 12 mg L−1 | Nutrient solution | Plant growth | Biofortification (Se enrichment in the leaves) | [116] |
| Oryza sativa | Na2SeO3 and Na2SeO4 | 120–300 g ha−1 | Foliar | Improved yield | Enhanced Se content in the rice grains | [117] |
| Cyamopsis tetragonoloba | Se-NPs | 400 mg | Foliar spray | Enhancing the growth yield | Enhancing the biochemical activity | [22] |
| Fragaria × ananassa | Se | 100 µM | Nutrient solution | Enhancing the growth | Enhanced the accumulation of anthocyanins | [115] |
| L. sativa | SeO2 | 5 mg L−1 | Nutrient solution | Enhancing the growth and biomass | Accumulation of 24 mg Kg−1 of Se in leaves (Dry Weight) | [118] |
| Citrus aurantifolia | Se-NPs | 50 mg L−1 | Imbibition of seeds | Plant growth improvement | Plant growth was improved | [119] |
| T. aestivum | Se-NPs | 5 µM | Imbibition | Plant growth improvement | Enhance the root aquaporins | [47] |
| Daucus carota | Na2SeO4 | 1 mg L−1 | Foliar apply | Enhancing the yield | Increased yield, decreased fruit ripening | [120] |
| S. lycopersicum | Na2SeO4 | 1 and 1.5 L−1 | Hydroponics | Yield improvement | Delayed postharvest ripening | [121] |
| T. aestivum | Na2SeO4 | 10 g ha−1 | Soil application | Yield improvement | 50% accumulation of Se in grains | [122] |
| T. aestivum | Na2SeO4 + surfactant | 120 g ha−1 | Foliar apply | Enhancing the growth and biomass | 1. Increased production by 48% and biomass by 30% 2. Increasing the grain weight (DW) | [123] |
| Arachis hypogaea | nSe | 40 mg L−1 | Foliar | Enhanced plant growth | Improved antioxidant potential | [93] |
| S. lycopersicum | nSe | 10 mg L−1 | Foliar | Plant growth improvement | 1. Induce the salinity tolerance of growth 2. Enhanced antioxidant enzyme activity | [124] |
| Lubia | nSe | 1.18 mg L−1 | Imbibition of seeds | Plant growth improvement | The total proteins, sugars, and increased seedling enzyme activity (α, β amylase, and protease) | [125] |
| Coriandrum sativum | nSe | 25 and 50 mg L−1 | Foliar + surfactant tween 80–0.005 | Plant growth improvement | Ascorbic acid content was improved. | [71] |
| Vicia faba | nSe | 10 and 20 mg L−1 | Imbibition of seeds | Yield improvement | The cytotoxicity activity | [126] |
| (Fragaria × ananassa) | Se-NPs | 10 and 20 mg L−1 | Foliar spray | Yield improvement | Enhanced organic acids and sugars content | [94] |
| Brassica chinensis | SeO32− | 10 μM | Hydroponic | Plant growth improvement | Enhanced antioxidant activity | [80] |
| Vigna unguiculata | Se-NPs | 6.25 µM | Foliar applications | Plant growth improvement | Increased the level of Indole Acetic Acid (IAA), Gibberellic Acid, and Cytokinins | [25] |
| Apium graveolens | SeNPs | 5 mg L−1, 50–78 nm | Foliar spray (Once in 10 days, 3 times application | Plant growth improvement | Increased primary and secondary metabolites | [96] |
| T. aestivum | Bio Se-NPs | 100 µg mL−1 | Mixture with the soil at the rate of 5% (v/w).) | Plant germination and yield improvement | Improvement of plant growth and 5–40% enhancement of the grain quantity and quality | [127] |
| Dracocephalum moldavicum | Cs–Se NPs | 5 mg L−1 | Exogenously applied | Plant growth and yield improvement | Enhance the improvement of the agronomic traits | [84] |
| Momordica charantia | Cs–Se NPs | 10, and 20 mg L−1 | Foliar spray | Plant growth enhancement | Increasing antioxidant enzyme activity, proline, and relative water content. | [128] |
| Hordeum vulgare | Se-NPs | 100 mg L−1 | Foliar mode | Enhancing plant growth | 1. Increase in phenolic composites under saline conditions. 2. Decrease in ROS-mediated cellular membrane harm markers | [98] |
| H. vulgare | Se-NPs | 4.65 g mL−1 | Dosage | Improvement of seedling growth | Greatest seed germination percentage | [68] |
| Citrus nobilis × Citrus deliciosa | Se-NPs | 25, 50, 75, and 100 mg L−1 | Bio-Fabrication | Improvement of yield | Improving the content of carotenoids, chlorophyll, flavonoid, and soluble sugar) | [27] |
| Brassica napus | Bio Se-NPs | 150 µmol L−1 | Exogenously applied | Improvement in growth | Increased the shoot and root length under salt stress conditions. | [37] |
| T. aestivum | Se-NPs | 30 ppm (once a week) | Soybean straw biochar mixed with soil media | Improved growth | Significantly increased PSII efficiency under salt treatments | [87] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samynathan, R.; Venkidasamy, B.; Ramya, K.; Muthuramalingam, P.; Shin, H.; Kumari, P.S.; Thangavel, S.; Sivanesan, I. A Recent Update on the Impact of Nano-Selenium on Plant Growth, Metabolism, and Stress Tolerance. Plants 2023, 12, 853. https://doi.org/10.3390/plants12040853
Samynathan R, Venkidasamy B, Ramya K, Muthuramalingam P, Shin H, Kumari PS, Thangavel S, Sivanesan I. A Recent Update on the Impact of Nano-Selenium on Plant Growth, Metabolism, and Stress Tolerance. Plants. 2023; 12(4):853. https://doi.org/10.3390/plants12040853
Chicago/Turabian StyleSamynathan, Ramkumar, Baskar Venkidasamy, Karthikeyan Ramya, Pandiyan Muthuramalingam, Hyunsuk Shin, Pandy Saravana Kumari, Sivakumar Thangavel, and Iyyakkannu Sivanesan. 2023. "A Recent Update on the Impact of Nano-Selenium on Plant Growth, Metabolism, and Stress Tolerance" Plants 12, no. 4: 853. https://doi.org/10.3390/plants12040853






