Liposome Formulation of Fullerene-Based Molecular Diagnostic and Therapeutic Agents
Abstract
:1. Introduction
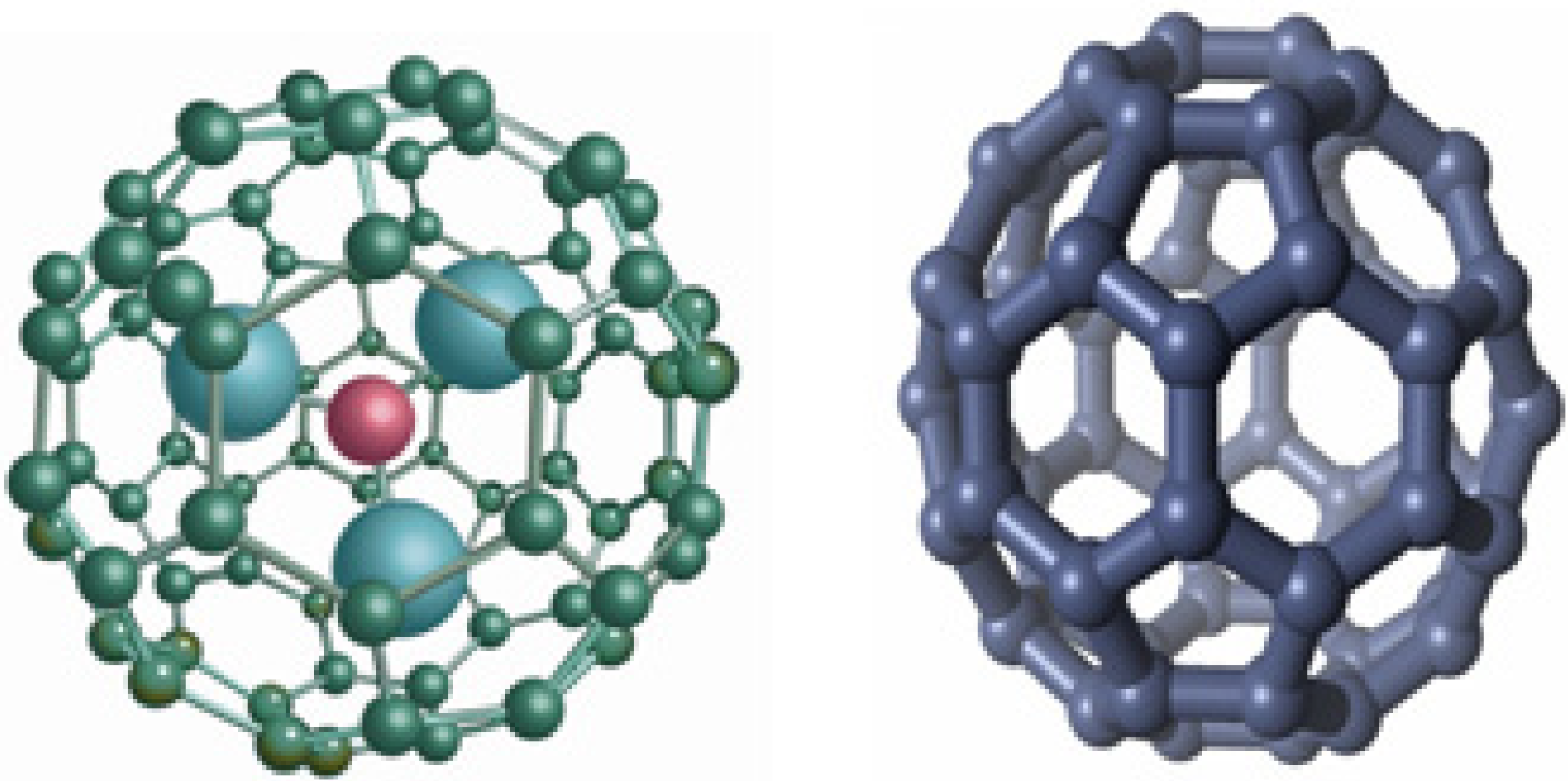
2. Biomedical Applications of Fullerenes
| Fullerene role | Applications | Commercialization leaders | |
|---|---|---|---|
| Antioxidant; Free radical scavenger | Anti-aging, | The Bronx Project Inc.; | |
| Neuroprotection | TDA Research Inc.; | ||
| Radioprotection | Vitamin C60 Bioresearch Corporation; | ||
| Cosmetics | Navya Biomedical Technologies LLC; | ||
| Encapsulating toxic or unstable species | MRI contrast agent, Radiopharmaceuticals | Kepley Biosystems Incorporated; | |
| Luna Innovations Incorporated (Luna acquired certain fullerene IPs originated from Tego Bioscience and C Sixty Inc.) | |||
| Three dimensional nanoscale building block | Gene delivery | ||
| Transfection vector | |||
| Enzyme inhibition | |||
| Photosensitizer | Photodynamic therapy, | ||
| Antimicrobials | |||
| Electron acceptor | Biosensors | ||
| Biofuel cells |
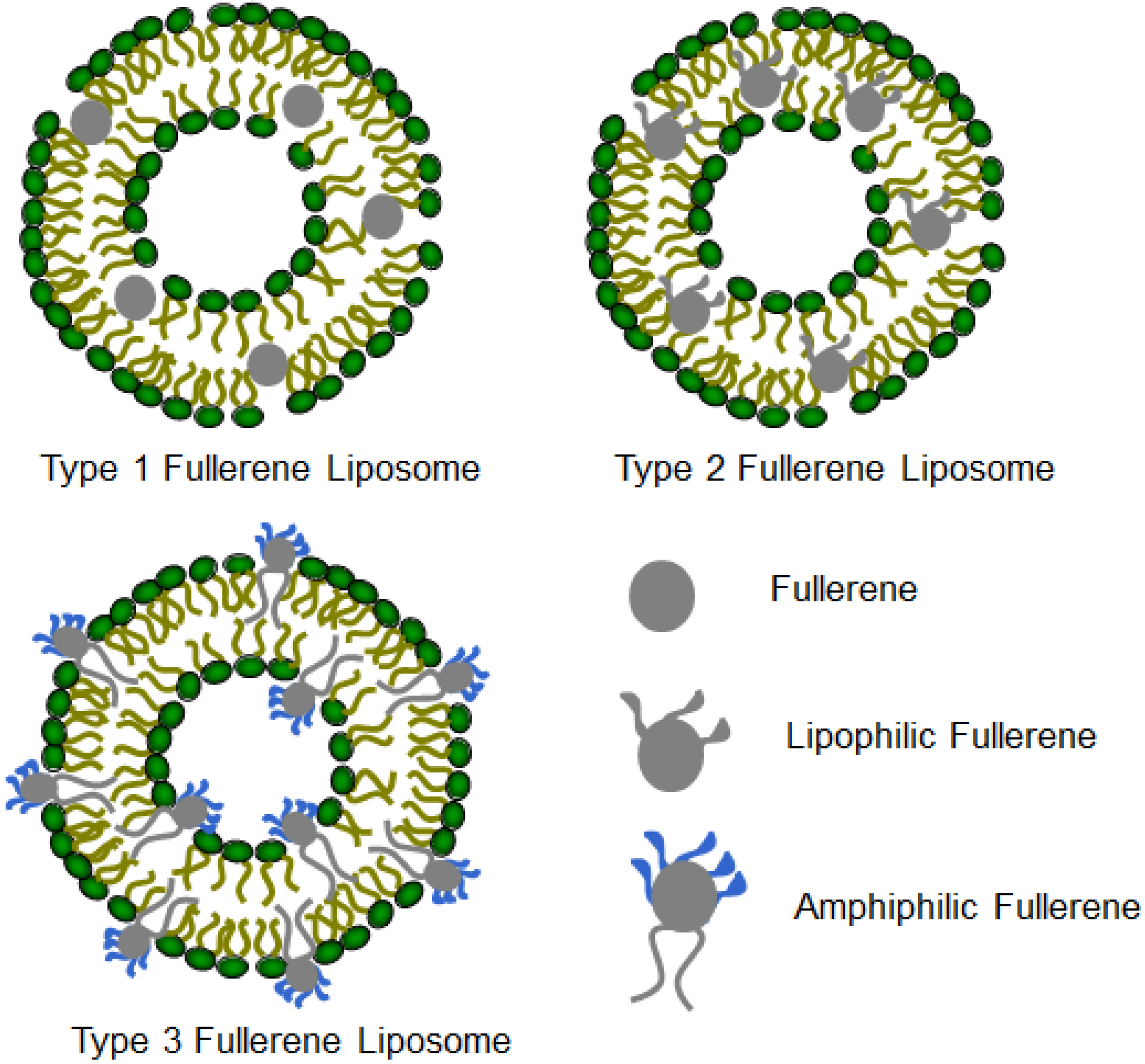
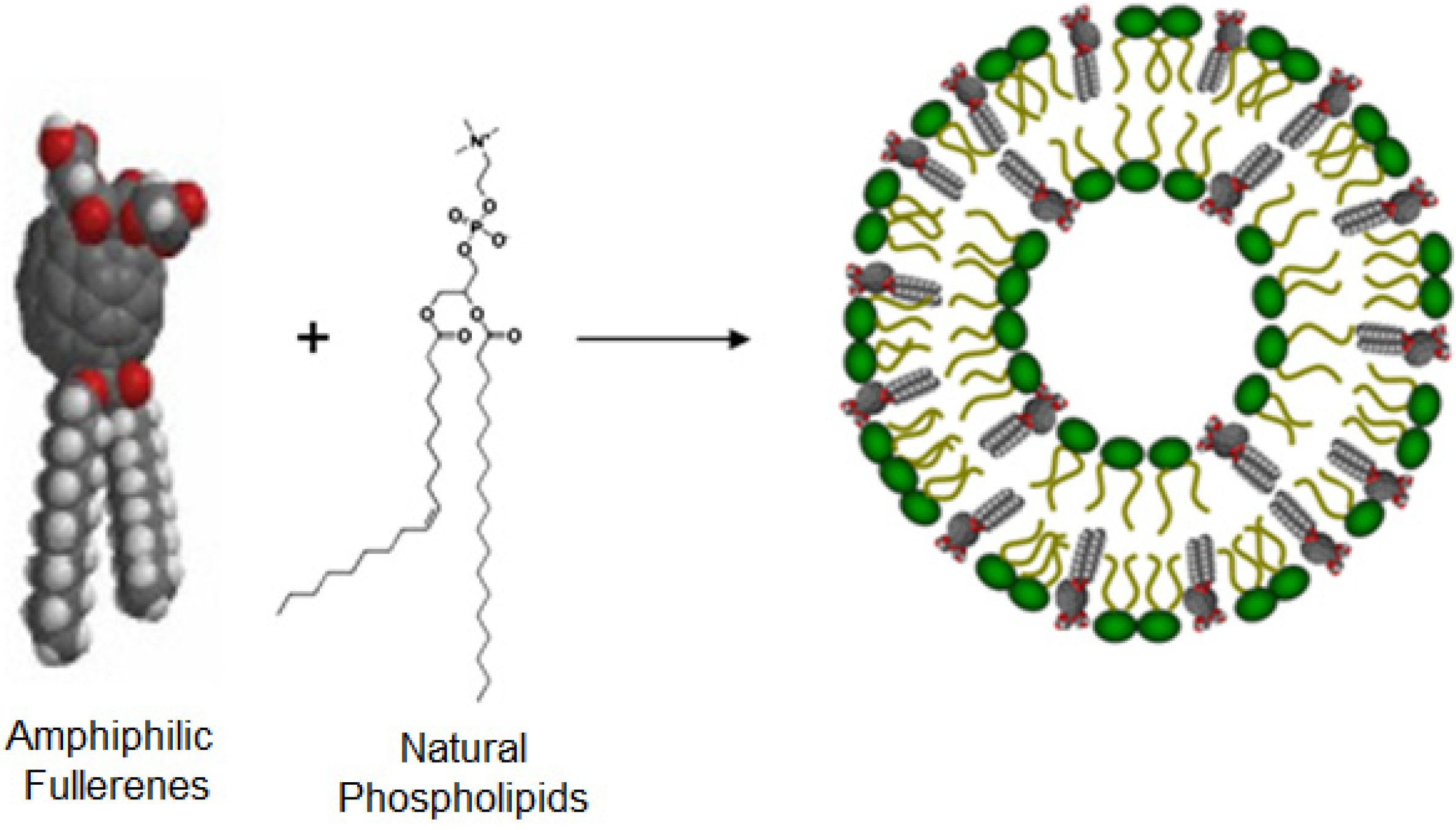
3. Structures and Compositions of Fullerene Liposome
4. Fullerene Interactions with Lipid Bilayers
5. Fullerene Liposome Antioxidants
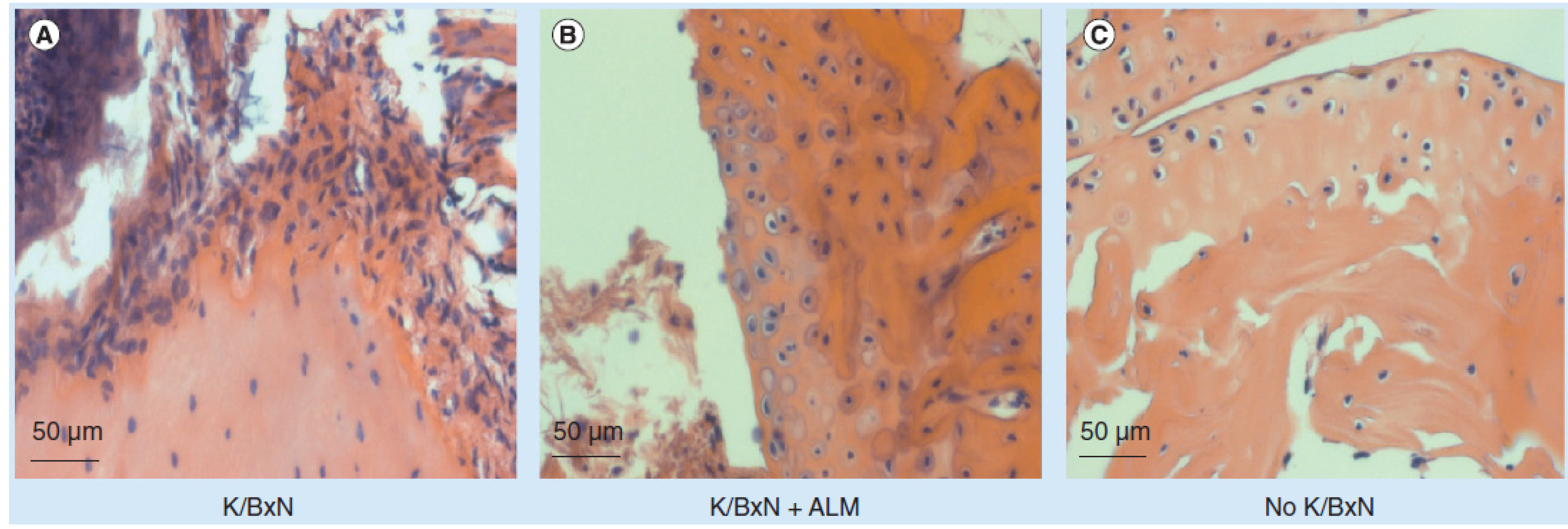
6. Fullerene Liposomes Inhibit Inflammation
7. Fullerene Liposome for Photodynamic Therapy
8. Metallofullerene Liposome for Contrast-Enhanced Molecular MR Imaging

9. Fullerene Liposome for Chemotherapy and Cancer Theranostics
10. Fullerene Liposomes for Cosmetics
11. Fullerene Buckysome
12. Summary and Perspectives
Acknowledgments
Conflicts of Interest
References
- Kroto, H.W.; Hearth, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. Buckminsterfullerenes. Nature 1985, 318, 162–163. [Google Scholar]
- Jensen, A.W.; Wilson, S.R.; Schuster, D.I. Biological applications of fullerenes. Bioorg. Med. Chem. 1996, 4, 767–779. [Google Scholar]
- Ross, T.; Prato, M. Medicinal chemistry with fullerenes and fullerene derivatives. Chem. Commun. 1999, 8, 663–669. [Google Scholar]
- Nakamura, E.; Isobe, H. Functionalized fullerenes in water. The first 10 years of their chemistry, biology, and nanoscience. Acc. Chem. Res. 2003, 36, 807–815. [Google Scholar]
- Bosi, S.; da Ros, T.; Spalluto, G.; Prato, M. Fullerene derivatives: An attractive tool for biological applications. Eur. J. Med. Chem. 2003, 38, 913–923. [Google Scholar]
- Anilkumar, P.; Lu, F.; Cao, L.; Luo, P.G.; Liu, J.H.; Sahu, S.; Tackett, K.N.; Wang, Y.; Sun, Y.P. Fullerenes for applications in biology and medicine. Curr. Med. Chem. 2011, 18, 2045–2059. [Google Scholar]
- Bolskar, R.D. Gadofullerene MRI contrast agents. Nanomedicine 2008, 3, 201–213. [Google Scholar]
- Sinohara, H. Endohedral metallofullerenes. Rep. Prog. Phys. 2000, 63, 843–862. [Google Scholar]
- Krustic, P.J.; Wasserman, E.; Keizer, P.N.; Morton, J.R.; Preston, K.F. Radical reactions of C60. Science 1991, 254, 1183–1185. [Google Scholar]
- Partha, R.; Conyers, J.L. Biomedical applications of functionalized fullerene-based nanomaterials. Int. J. Nanomed. 2009, 4, 261–275. [Google Scholar] [Green Version]
- Tsai, M.C.; Chen, Y.H.; Chiang, L.Y. Polyhydroxylated C60, fullerenol, a novel free-radical trapper, prevented hydrogen peroxide- and cumene hydroperoxide-elicited changes in rat hippocampus in vitro. J. Pharm. Pharmacol. 1997, 49, 438–445. [Google Scholar] [Green Version]
- Husebo, L.O.; Sitharaman, B.; Furukawa, K.; Kato, T.; Wilson, L.J. Fullerenols revisited as stable radical anions. J. Am. Chem. Soc. 2004, 126, 12055–12064. [Google Scholar] [Green Version]
- Huang, S.S.; Tsai, S.K.; Chih, C.L.; Chiang, L.Y.; Hsieh, H.M.; Teng, C.M.; Tsai, M.C. Neuroprotective effect of hexasulfobutylated C660 on rats subjected to focal cerebral ischemia. Free Radic. Biol. Med. 2001, 30, 643–649. [Google Scholar] [Green Version]
- Dugan, L.L.; Turetsky, D.M.; Du, C.; Lobner, D.; Wheeler, M.; Almli, C.R.; Shen, C.K.F.; Luh, T.Y.; Choi, D.W.; Lin, T.S. Carboxyfullerenes as neuroprotective agents. Proc. Natl. Acad. Sci. USA 1997, 94, 9434–9439. [Google Scholar] [Green Version]
- Brettreich, M.; Hirsch, A. A highly water-soluble dendro[60]fullerene. Tetrahedron Lett. 1998, 39, 2731–2734. [Google Scholar] [Green Version]
- Johnston, H.J.; Hutchison, G.R.; Christensen, F.M.; Aschberger, K.; Stone, V. The biological mechanisms and physicochemical characteristics responsible for driving fullerene toxicity. Toxicol. Sci. 2010, 114, 162–182. [Google Scholar] [Green Version]
- Bensasson, R.V.; Bienvenue, E.; Dellinger, M.; Leach, S.; Seta, P.J. C60 in model biological systems. A visible-UV absorption study of solvent-dependent parameters and solute aggregation. J. Phys. Chem. 1994, 98, 3492–3496. [Google Scholar] [Green Version]
- Hungerbuhler, H.; Guldi, D.M.; Asmus, K.D. Incorporation of C60 into artificial lipid membranes. J. Am. Chem. Soc. 1993, 115, 3386–3390. [Google Scholar] [Green Version]
- Zhou, Z.; Schuster, D.I.; Wilson, S.R. Tether directed selective synthesis of fulleropyrrolidine bisadducts. J. Org. Chem. 2006, 71, 1545–1551. [Google Scholar] [Green Version]
- Zhou, Z.; Stephen, R.; Wilson, S.R. Tether-directed multiple functionalization of fullerene[60]. Curr. Org. Chem. 2005, 9, 789–811. [Google Scholar] [Green Version]
- Zhou, Z.; Magriotis, P.A. A new method for the functionalization of [60]fullerene: An unusual 1,3-dipolar cycloaddition pathway leading to a C60 housane derivative. Org. Lett. 2005, 7, 5849–5851. [Google Scholar] [Green Version]
- Zhou, Z.; David, I.; Schuster, D.I.; Wilson, S.R. Selective syntheses of polyether fullerene multiple adducts. J. Org. Chem. 2003, 68, 7612–7617. [Google Scholar] [Green Version]
- Zhou, Z.; Lenk, R.P.; Dellinger, A.; Kepley, C.; Wilson, S.R.; Sadler, R. Liposomal formulation of amphiphilic fullerene antioxidants. Bioconjug. Chem. 2010, 21, 1656–1661. [Google Scholar] [Green Version]
- Rossi, G. Partitioning and solubility of C60 fullerene in lipid membranes. Phys. Scr. 2013, 87, 058503. [Google Scholar] [Green Version]
- Zupanc, J.; Drobne, D.; Drasler, B.; Valant, J.; Iglic, A.; Kralj-Iglic, V.; Makovec, D.; Rappolt, M.; Sartori, B.; Kogej, K. Experimental evidence for the interaction of C-60 fullerene with lipid vesicle membranes. Carbon 2012, 50, 1170–1178. [Google Scholar] [Green Version]
- Bouropoulos, N.; Katsamenis, O.L.; Cox, P.A.; Norman, S.; Kallinteri, P.; Favretto, M.E.; Yannopoulos, S.N.; Bakandritsos, A.; Fatouros, D.G. Probing the perturbation of lecithin bilayers by unmodified C60 fullerenes using experimental methods and computational simulations. J. Phys. Chem. 2012, 116, 3867–3874. [Google Scholar] [Green Version]
- Zhan, W.; Jiang, K. A modular photocurrent generation system based on phospholipid-assembled fullerenes. Langmuir 2008, 24, 13258–13261. [Google Scholar] [Green Version]
- Gan, L.; Huang, S.; Zhang, X.; Zhang, A.; Cheng, B.; Cheng, H.; Li, X.; Shang, G. Fullerenes as a tert-butylperoxy radical trap, metal catalyzed reaction of tert-butyl hydroperoxide with fullerenes, and formation of the first fullerene mixed peroxides. J. Am. Chem. Soc. 2002, 124, 13384–13385. [Google Scholar] [Green Version]
- Birkett, P.R.; Avent, A.G.; Darwish, A.D.; Kroto, H.W.; Taylor, R.; Walton, D.R.M. Formation and characterization of C70Cl10. J. Chem. Soc. Chem. Commun. 1995, 683–684. [Google Scholar]
- Scuseria, G.E. The equilibrium structure of C70. An ab initio Hartree-Fock study. Chem. Phys. Lett. 1991, 180, 451–469. [Google Scholar] [Green Version]
- Lens, M.; Medenica, L.; Citernesi, U. Antioxidative capacity of C60 (buckminsterfullerene) and newly synthesized fulleropyrrolidine derivatives encapsulated in liposomes. Biotechnol. Appl. Biochem. 2008, 51, 135–140. [Google Scholar] [Green Version]
- Williams, R.M.; Crielaard, W.; Hellingwerf, K.J.; Verhoeven, J.W. Incorporaiton of fullerene-C60 and C60 adducts in micellar and vesicular supermolecular assemblies: Introductory flash photolysis and photoredox experiments in micelles. Recl. Trav. Chim. Pays-Bas 1996, 115, 72–76. [Google Scholar] [Green Version]
- Dellinger, A.; Zhou, Z.; Lenk, R.; McFarland, D.; Kepley, C.L. Fullerene nanomaterials inhibit phorbol myristate acetate-induced inflammation. Exp. Dermatol. 2009, 18, 1079–1081. [Google Scholar] [Green Version]
- Norton, S.; Wijesinghe, D.; Dellinger, A.; Sturgill, J.; Zhou, Z.; Barbour, S.; Chalfant, C.; Conrad, D.; Kepley, C.L. Epoxyeicosatrienoic acids are involved in the C70 fullerene derivative-induced control of allergic asthma. J. Allergy Clin. Immunol. 2012, 130, 761–769. [Google Scholar] [Green Version]
- Dellinger, A.; Zhou, Z.; Connor, J.; Madhankumar, A.B.; Pamujula, S.; Sayes, C.M.; Kepley, C.L. Application of fullerenes in nanomedicine: An update. Nanomedicine 2013, 8, 1191–1208. [Google Scholar] [Green Version]
- Yamakoshi, Y. Active oxygen species generated from photoexcited fullerene (C60) as potential medicines: O2−• versus 1O2. J. Am. Chem. Soc. 2003, 125, 12803–12809. [Google Scholar] [Green Version]
- Ikeda, A.; Doi, Y.; Hashizume, M.; Kikuchi, J.; Konishi, T. An extremely effective DNA photocleavage utilizing functionalized liposomes with a fullerene-enriched lipid bilayer. J. Am. Chem. Soc. 2007, 129, 4140–4141. [Google Scholar] [Green Version]
- Ikeda, A. Efficient photoclevage of DNA utilising water-soluble lipid membrane-incorporated [60]fullerenes prepared using a [60]fullerene exchange method. Org. Biomol. Chem. 2005, 3, 2907–2909. [Google Scholar] [Green Version]
- Ikeda, A.; Kawai, Y.; Kikuchi, J.; Akiyama, M.; Nakata, E.; Uto, Y.; Hori, H. Formation and regulation of fullerene-incorporation in liposomes under the phase transition temperature. Org. Biomol. Chem. 2011, 9, 2622–2627. [Google Scholar] [Green Version]
- Du, C.; Xiong, H.; Ji, H.; Liu, Q.; Xiao, H.; Yang, Z. The antiviral effect of fullerene-liposome complex against influenza virus (H1N1) in vivo. Sci. Res. Essays 2012, 7, 705–711. [Google Scholar] [Green Version]
- Dellinger, A.; Olson, J.; Link, K.; Vance, S.; Sandros, M.G.; Yang, J.; Zhou, Z. Functionalization of gadolinium metallofullerenes for detecting atherosclerotic plaque lesions by cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2013, 15, 7. [Google Scholar] [Green Version]
- McFarland, D.K.; Walker, K.L.; Lenk, R.P.; Wilson, S.R.; Kumar, K.; Kepley, C.L.; Garbow, J.R. Hydrochalarones: A novel endohedral metallofullerene platform for enhancing magnetic resonance imaging contrast. J. Med. Chem. 2008, 51, 3681–3683. [Google Scholar] [Green Version]
- Podrez, E.A.; Poliakov, E.; Shen, Z. Identification of a novel family of oxidized phospholipids that serve as ligands for the macrophage scavenger receptor CD36. J. Biol. Chem. 2002, 277, 38503–38518. [Google Scholar] [Green Version]
- Amirbekian, V.; Lipinski, M.J.; Briley-Saebo, K.C. Detecting and assessing macrophages in vivo to evaluate atherosclerosis noninvasively using molecular MRI. Proc. Natl. Acad. Sci. USA 2007, 104, 961–966. [Google Scholar] [Green Version]
- Nunn, A.D.; Linder, K.E.; Tweedle, M.F. Can receptors be imaged with MRI agents? Q. J. Nucl. Med. 1997, 41, 155–162. [Google Scholar] [Green Version]
- Zakharian, T.Y.; Seryshev, A.; Sitharaman, B.; Gilbert, B.E.; Knight, V.; Wilson, L.J. A fullerene-paclitaxel chemotherapeutic: Synthesis, characterization, and study of biological activity in tissue culture. J. Am. Chem. Soc. 2005, 127, 12508–12516. [Google Scholar] [Green Version]
- Shinya, K.; Hisae, A.; Yasukazu, S.; Nobuhiko, M. Fullerene-C60 incorporated in liposome exerts persistent hydroxyl radical-scavenging activity and cytoprotection in UVA/B-irradiated keratinocytes. J. Nanosci. Nanotechnol. 2011, 11, 3814–3823. [Google Scholar] [Green Version]
- Kato, S.; Kikuchi, R.; Aoshima, H.; Saitoh, Y.; Miwa, N. Defensive effects of fullerene-C60/liposome complex against UVA-induced intracellular reactive oxygen species generation and cell death in human skin keratinocytes HaCaT, associated with intracellular uptake and extracellular excretion of fullerene-C60. J. Photochem. Photobiol. B 2010, 98, 144–151. [Google Scholar] [Green Version]
- Kato, S.; Aoshima, H.; Saitoh, Y.; Miwa, N. Fullerene-C60/liposome complex: Defensive effects against UVA-induced damages in skin structure, nucleus and collagen type I/IV fibrils, and the permeability into human skin tissue. J. Photochem. Photobiol. B 2010, 98, 99–105. [Google Scholar] [Green Version]
- Brettreich, M.; Burghardt, S.; Bottcher, C.; Bayerl, T.; Bayerl, S.; Hirsch, A. Globular amphiphiles: Membraneforming hexaadducts of C60. Angew. Chem. Int. Ed. 2000, 39, 1845–1848. [Google Scholar] [Green Version]
- Maierhofer, A.P.; Brettreich, M.; Burghardt, S.; Vostrowsky, O.; Hirsch, A.; Langridge, S.; Bayerl, T.M. Structure and electrostatic interaction properties of monolayers of amphiphilic molecules derived from C60-fullerenes: A film balance, neutron, and infrared reflection study. Langmuir 2000, 16, 8884–8891. [Google Scholar] [Green Version]
- Partha, R.; Lackey, M.; Hirsch, A.; Casscells, S.W.; Conyers, J.L. Self-assembly of amphiphilic C60 fullerene derivatives into nanoscale supramolecular structures. J. Nanobiotechnol. 2007, 5, 6. [Google Scholar] [Green Version]
- Partha, R.; Mitchell, L.R.; Lyon, J.L.; Joshi, P.P.; Conyers, J.L. Buckysomes: Fullerene-based nanocarriers for hydrophobic molecule delivery. ACS Nano 2008, 2, 1950–1957. [Google Scholar] [Green Version]
- Danila, D.; Golunski, E.; Partha, R.; McManus, M.; Little, T.; Conyers, J. Buckysomes: New nanocarriers for anticancer drugs. J. Pharm. 2013, 2013. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhou, Z. Liposome Formulation of Fullerene-Based Molecular Diagnostic and Therapeutic Agents. Pharmaceutics 2013, 5, 525-541. https://doi.org/10.3390/pharmaceutics5040525
Zhou Z. Liposome Formulation of Fullerene-Based Molecular Diagnostic and Therapeutic Agents. Pharmaceutics. 2013; 5(4):525-541. https://doi.org/10.3390/pharmaceutics5040525
Chicago/Turabian StyleZhou, Zhiguo. 2013. "Liposome Formulation of Fullerene-Based Molecular Diagnostic and Therapeutic Agents" Pharmaceutics 5, no. 4: 525-541. https://doi.org/10.3390/pharmaceutics5040525
APA StyleZhou, Z. (2013). Liposome Formulation of Fullerene-Based Molecular Diagnostic and Therapeutic Agents. Pharmaceutics, 5(4), 525-541. https://doi.org/10.3390/pharmaceutics5040525




