Therapeutic Drug Monitoring in Children and Adolescents: Findings on Fluoxetine from the TDM-VIGIL Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Patient Assessment
2.3. Serum Concentration Analysis
2.4. Data Management and Statistical Analysis
3. Results
3.1. Descriptive Statistics
3.2. Dose and Metabolite Concentrations (Last Datapoints)
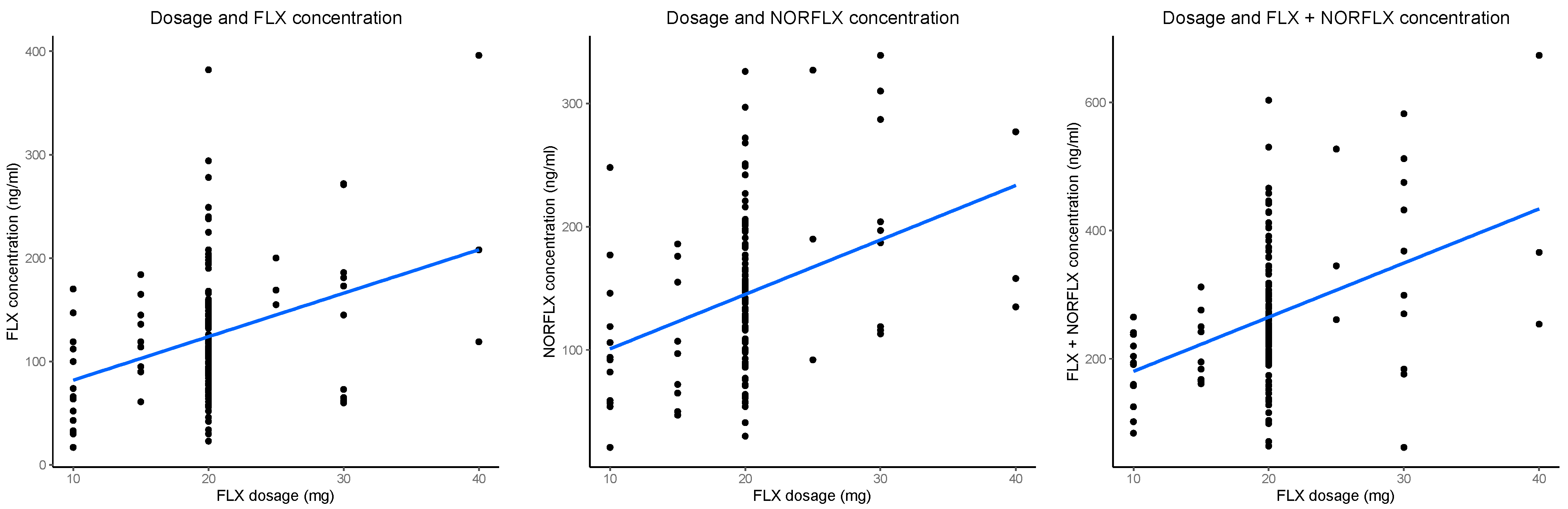
3.3. Dose and Metabolite Concentrations (All Datapoints)
3.4. Metabolite Concentrations and Clinical/Adverse Effects (Last Datapoints)
3.5. Metabolite Concentrations and Clinical/Adverse Effects (All Datapoints)
3.6. ROC Analysis and Effective Interquartile Ranges
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wong, D.T.; Horng, J.S.; Bymaster, F.P.; Hauser, K.L.; Molloy, B.B. A selective inhibitor of serotonin uptake: Lilly 110140, 3-(p-trifluoromethylphenoxy)-N-methyl-3-phenylpropylamine. Life Sci. 1974, 15, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Fuller, R.W.; Perry, K.W.; Molloy, B.B. Effect of an uptake inhibitor on serotonin metabolism in rat brain: Studies with 3-(p-trifluoromethylphenoxy)-N-methyl-3-phenylpropylamine (Lilly 110140). Life Sci. 1974, 15, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.T.; Perry, K.W.; Bymaster, F.P. The discovery of fluoxetine hydrochloride (Prozac). Nat. Rev. Drug Discov. 2005, 4, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Hetrick, S.E.; McKenzie, J.E.; Bailey, A.P.; Sharma, V.; Moller, C.I.; Badcock, P.B.; Cox, G.R.; Merry, S.N.; Meader, N. New generation antidepressants for depression in children and adolescents: A network meta-analysis. Cochrane Database Syst. Rev. 2021, 5, CD013674. [Google Scholar]
- Deodhar, M.; Rihani, S.B.A.; Darakjian, L.; Turgeon, J.; Michaud, V. Assessing the mechanism of fluoxetine-mediated CYP2D6 inhibition. Pharmaceutics 2021, 13, 148. [Google Scholar] [CrossRef]
- Zhou, X.; Teng, T.; Zhang, Y.; Del Giovane, C.; Furukawa, T.A.; Weisz, J.R.; Li, X.; Cuijpers, P.; Coghill, D.; Xiang, Y. Comparative efficacy and acceptability of antidepressants, psychotherapies, and their combination for acute treatment of children and adolescents with depressive disorder: A systematic review and network meta-analysis. Lancet Psychiatry 2020, 7, 581–601. [Google Scholar] [CrossRef]
- Cipriani, A.; Zhou, X.; Del Giovane, C.; Hetrick, S.E.; Qin, B.; Whittington, C.; Coghill, D.; Zhang, Y.; Hazell, P.; Leucht, S. Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: A network meta-analysis. Lancet 2016, 388, 881–890. [Google Scholar] [CrossRef]
- Eaton, L. European Agency Approves Use of Fluoxetine for Children and Teens. Available online: https://www-bmj-com.emedien.ub.uni-muenchen.de/content/332/7555/1407.2.short (accessed on 24 October 2022).
- Food and Drug Administration. Fluoxetine-Highlights of Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018936s108lbl.pdf (accessed on 3 April 2023).
- Correll, C.U.; Cortese, S.; Croatto, G.; Monaco, F.; Krinitski, D.; Arrondo, G.; Ostinelli, E.G.; Zangani, C.; Fornaro, M.; Estradé, A. Efficacy and acceptability of pharmacological, psychosocial, and brain stimulation interventions in children and adolescents with mental disorders: An umbrella review. World Psychiatry 2021, 20, 244–275. [Google Scholar] [CrossRef]
- Hagan, K.E.; Walsh, B.T. State of the art: The therapeutic approaches to Bulimia Nervosa. Clin. Ther. 2021, 43, 40–49. [Google Scholar] [CrossRef]
- Duan, H.; Zhu, L.; Li, M.; Zhang, X.; Zhang, B.; Fang, S. Comparative efficacy and acceptability of selective serotonin reuptake inhibitor antidepressants for binge eating disorder: A network meta-analysis. Front. Pharmacol. 2022, 13, 949823. [Google Scholar] [CrossRef]
- Cheer, S.M.; Goa, K.L. Fluoxetine: A review of its therapeutic potential in the treatment of depression associated with physical illness. Drugs 2001, 61, 81–110. [Google Scholar] [CrossRef] [PubMed]
- Hiemke, C.; Härtter, S. Pharmacokinetics of selective serotonin reuptake inhibitors. Pharmacol. Ther. 2000, 85, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Hiemke, C.; Bergemann, N.; Clement, H.; Conca, A.; Deckert, J.; Domschke, K.; Eckermann, G.; Egberts, K.; Gerlach, M.; Greiner, C. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: Update 2017. Pharmacopsychiatry 2018, 51, 9–62. [Google Scholar] [PubMed]
- Meyer, J.H.; Wilson, A.A.; Sagrati, S.; Hussey, D.; Carella, A.; Potter, W.Z.; Ginovart, N.; Spencer, E.P.; Cheok, A.; Houle, S. Serotonin transporter occupancy of five selective serotonin reuptake inhibitors at different doses: An [11C] DASB positron emission tomography study. Am. J. Psychiatry 2004, 161, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Luxton, R.; Kyriakopoulos, M. Depression in children and young people: Identification and management NICE guidelines. Arch. Dis. Child.-Educ. Pract. 2022, 107, 36–38. [Google Scholar] [CrossRef]
- Dolle, K.; Schulte-Körne, G. The treatment of depressive disorders in children and adolescents. Dtsch. Ärzteblatt Int. 2013, 110, 854. [Google Scholar] [CrossRef][Green Version]
- Jannini, T.B.; Lorenzo, G.D.; Bianciardi, E.; Niolu, C.; Toscano, M.; Ciocca, G.; Jannini, E.A.; Siracusano, A. Off-label uses of selective serotonin reuptake inhibitors (SSRIs). Curr. Neuropharmacol. 2022, 20, 693–712. [Google Scholar] [CrossRef]
- March, J.S.; Vitiello, B. Clinical messages from the treatment for adolescents with depression study (TADS). Am. J. Psychiatry 2009, 166, 1118–1123. [Google Scholar] [CrossRef]
- Hetrick, S.E.; McKenzie, J.E.; Merry, S.N. The use of SSRIs in children and adolescents. Curr. Opin. Psychiatry 2010, 23, 53–57. [Google Scholar] [CrossRef]
- Gassó, P.; Rodríguez, N.; Mas, S.; Pagerols, M.; Blázquez, A.; Plana, M.; Torra, M.; Lázaro, L.; Lafuente, A. Effect of CYP2D6, CYP2C9 and ABCB1 genotypes on fluoxetine plasma concentrations and clinical improvement in children and adolescent patients. Pharmacogenomics J. 2014, 14, 457–462. [Google Scholar] [CrossRef]
- Gaedigk, A.; Sangkuhl, K.; Whirl-Carrillo, M.; Klein, T.; Leeder, J.S. Prediction of CYP2D6 phenotype from genotype across world populations. Genet. Med. 2017, 19, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Bradford, L.D. CYP2D6 allele frequency in European Caucasians, Asians, Africans and their descendants. Pharmacogenomics 2002, 3, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Barbey, J.T.; Roose, S.P. SSRI safety in overdose. J. Clin. Psychiatry 1998, 59, 42. [Google Scholar] [PubMed]
- Reis, M.; Aamo, T.; Ahlner, J.; Druid, H. Reference concentrations of antidepressants. A compilation of postmortem and therapeutic levels. J. Anal. Toxicol. 2007, 31, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.; Brent, J.; Kulig, K.; Heiligenstein, J.; Birkett, M.; Group, A.S. Fluoxetine versus tricyclic antidepressants: A prospective multicenter study of antidepressant drug overdoses. J. Emerg. Med. 1997, 15, 439–445. [Google Scholar] [CrossRef]
- Borys, D.J.; Setzer, S.C.; Ling, L.J.; Reisdorf, J.J.; Day, L.C.; Krenzelok, E.P. The effects of fluoxetine in the overdose patient. J. Toxicol. Clin. Toxicol. 1990, 28, 331–340. [Google Scholar] [CrossRef]
- Borys, D.J.; Setzer, S.C.; Ling, L.J.; Reisdorf, J.J.; Day, L.C.; Krenzelok, E.P. Acute fluoxetine overdose: A report of 234 cases. Am. J. Emerg. Med. 1992, 10, 115–120. [Google Scholar] [CrossRef]
- Barthez, S.; Revet, A.; Chouchana, L.; Jonville-Bera, A.-P.; Pizzoglio, V.; Raynaud, J.-P.; Chebane, L.; Lapeyre-Mestre, M.; Montastruc, F. Adverse drug reactions in infants, children and adolescents exposed to antidepressants: A French pharmacovigilance study. Eur. J. Clin. Pharmacol. 2020, 76, 1591–1599. [Google Scholar] [CrossRef]
- Gerlach, M.; Egberts, K.; Dang, S.-Y.; Plener, P.; Taurines, R.; Mehler-Wex, C.; Romanos, M. Therapeutic drug monitoring as a measure of proactive pharmacovigilance in child and adolescent psychiatry. Expert Opin. Drug Saf. 2016, 15, 1477–1482. [Google Scholar] [CrossRef]
- Mandrioli, R.; Mercolini, L.; Saracino, M.A.; Raggi, M.A. Selective serotonin reuptake inhibitors (SSRIs): Therapeutic drug monitoring and pharmacological interactions. Curr. Med. Chem. 2012, 19, 1846–1863. [Google Scholar] [CrossRef]
- Koelch, M.; Pfalzer, A.-K.; Kliegl, K.; Rothenhöfer, S.; Ludolph, A.; Fegert, J.M.; Burger, R.; Mehler-Wex, C.; Stingl, J.; Taurines, R. Therapeutic drug monitoring of children and adolescents treated with fluoxetine. Pharmacopsychiatry 2012, 45, 72–76. [Google Scholar] [CrossRef]
- Blázquez, A.; Mas, S.; Plana, M.T.; Gassó, P.; Méndez, I.; Torra, M.; Arnaiz, J.A.; Lafuente, A.; Lázaro, L. Plasma fluoxetine concentrations and clinical improvement in an adolescent sample diagnosed with major depressive disorder, obsessive-compulsive disorder, or generalized anxiety disorder. J. Clin. Psychopharmacol. 2014, 34, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Sakolsky, D.J.; Perel, J.M.; Emslie, G.J.; Clarke, G.N.; Wagner, K.D.; Vitiello, B.; Keller, M.B.; Birmaher, B.; Asarnow, J.R.; Ryan, N.D. Antidepressant exposure as a predictor of clinical outcomes in the Treatment of Resistant Depression in Adolescents (TORDIA) study. J. Clin. Psychopharmacol. 2011, 31, 92. [Google Scholar] [CrossRef] [PubMed]
- Egberts, K.; Plener, P.; Malzhan, U.; Taurines, R.; Reuter-Dang, S.; Gerlach, M. Sicherheit von psychopharmaka bei kindern und jugendlichen in der klinischen praxis–Erkenntnisse einer prospektiven studie. Bull. Zur Arzneimittelsicherheit 2020, 3, 4–10. [Google Scholar]
- March, J.; Karayal, O.; Chrisman, A. CAPTN: The pediatric adverse event rating scale. In Proceedings of the The Scientific Proceedings of the 2007 Annual Meeting of the American Academy of Child and Adolescent Psychiatry, Boston, MA, USA, 23–28 October 2007; pp. 23–28. [Google Scholar]
- Egberts, K.M.; Gerlach, M.; Correll, C.U.; Plener, P.L.; Malzahn, U.; Heuschmann, P.; Unterecker, S.; Scherf-Clavel, M.; Rock, H.; Antony, G. Serious adverse drug reactions in children and adolescents treated on-and off-label with antidepressants and antipsychotics in clinical practice. Pharmacopsychiatry 2022, 55, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Tini, E.; Smigielski, L.; Romanos, M.; Wewetzer, C.; Karwautz, A.; Reitzle, K.; Correll, C.U.; Plener, P.L.; Malzahn, U.; Heuschmann, P. Therapeutic drug monitoring of sertraline in children and adolescents: A naturalistic study with insights into the clinical response and treatment of obsessive-compulsive disorder. Compr. Psychiatry 2022, 115, 152301. [Google Scholar] [CrossRef]
- Peña, E.A.; Slate, E.H. Global validation of linear model assumptions. J. Am. Stat. Assoc. 2006, 101, 341–354. [Google Scholar] [CrossRef]
- Hiemke, C. Concentration-Effect Relationships of Psychoactive Drugs and the Problem to Calculate Therapeutic Reference Ranges. Ther. Drug Monit. 2019, 41, 174–179. [Google Scholar] [CrossRef]
- Lundmark, J.; Reis, M.; Bengtsson, F. Serum concentrations of fluoxetine in the clinical treatment setting. Ther. Drug Monit. 2001, 23, 139–147. [Google Scholar] [CrossRef]
- Reis, M.; Aamo, T.; Spigset, O.; Ahlner, J. Serum concentrations of antidepressant drugs in a naturalistic setting: Compilation based on a large therapeutic drug monitoring database. Ther. Drug Monit. 2009, 31, 42–56. [Google Scholar] [CrossRef]
- Shah, R.R.; Smith, R.L. Addressing phenoconversion: The Achilles’ heel of personalized medicine. Br. J. Clin. Pharmacol. 2015, 79, 222–240. [Google Scholar] [CrossRef] [PubMed]
- Lam, Y.F.; Gaedigk, A.; Ereshefsky, L.; Alfaro, C.L.; Simpson, J. CYP2D6 inhibition by selective serotonin reuptake inhibitors: Analysis of achievable steady-state plasma concentrations and the effect of ultrarapid metabolism at CYP2D6. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2002, 22, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Cicali, E.J.; Wiisanen, K. The importance of phenoconversion when using the CYP2D6 genotype in clinical practice. Pharmacogenomics 2022, 23, 749–752. [Google Scholar] [CrossRef] [PubMed]
- Sychev, D.A.; Ashraf, G.M.; Svistunov, A.A.; Maksimov, M.L.; Tarasov, V.V.; Chubarev, V.N.; Otdelenov, V.A.; Denisenko, N.j.P.; Barreto, G.E.; Aliev, G. The cytochrome P450 isoenzyme and some new opportunities for the prediction of negative drug interaction in vivo. Drug Des. Dev. Ther. 2018, 12, 1147–1156. [Google Scholar] [CrossRef]
- Miksys, S.; Tyndale, R. Nicotine induces brain CYP enzymes: Relevance to Parkinson’s disease. Park. Dis. Relat. Disord. 2006, 70, 177–180. [Google Scholar]
- van den Anker, J.; Reed, M.D.; Allegaert, K.; Kearns, G.L. Developmental changes in pharmacokinetics and pharmacodynamics. J. Clin. Pharmacol. 2018, 58, S10–S25. [Google Scholar] [CrossRef]
- Leeder, J.S.; Gaedigk, A.; Wright, K.J.; Staggs, V.S.; Soden, S.E.; Lin, Y.S.; Pearce, R.E. A longitudinal study of cytochrome P450 2D6 (CYP2D6) activity during adolescence. Clin. Transl. Sci. 2022, 15, 2514–2527. [Google Scholar] [CrossRef]
- Soldin, O.P.; Mattison, D.R. Sex differences in pharmacokinetics and pharmacodynamics. Clin. Pharmacokinet. 2009, 48, 143–157. [Google Scholar] [CrossRef]
- Amsterdam, J.; Fawcett, J.; Quitkin, F.; Reimherr, F.; Rosenbaum, J.; Michelson, D.; Hornig-Rohan, M.; Beasley, C. Fluoxetine and norfluoxetine plasma concentrations in major depression: A multicenter study. Am. J. Psychiatry 1997, 154, 963–969. [Google Scholar]
- Unterecker, S.; Riederer, P.; Proft, F.; Maloney, J.; Deckert, J.; Pfuhlmann, B. Effects of gender and age on serum concentrations of antidepressants under naturalistic conditions. J. Neural Transm. 2013, 120, 1237–1246. [Google Scholar] [CrossRef]
- Braun, C.; Adams, A.; Rink, L.; Bschor, T.; Kuhr, K.; Baethge, C. In search of a dose–response relationship in SSRIs—A systematic review, meta-analysis, and network meta-analysis. Acta Psychiatr. Scand. 2020, 142, 430–442. [Google Scholar] [CrossRef] [PubMed]
- Blazquez, A.; Mas, S.; Plana, M.T.; Lafuente, A.; Lázaro, L. Fluoxetine pharmacogenetics in child and adult populations. Eur. Child Adolesc. Psychiatry 2012, 21, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Fratelli, C.; Siqueira, J.; Silva, C.; Ferreira, E.; Silva, I. 5HTTLPR genetic variant and major depressive disorder: A review. Genes 2020, 11, 1260. [Google Scholar] [CrossRef] [PubMed]
- Mas, S.; Blázquez, A.; Rodríguez, N.; Boloc, D.; Lafuente, A.; Arnaiz, J.A.; Lázaro, L.; Gassó, P. Pharmacogenetic study focused on fluoxetine pharmacodynamics in children and adolescent patients: Impact of the serotonin pathway. Pharmacogenetics Genom. 2016, 26, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, P.; Alves, G.; Fortuna, A.; Llerena, A.; Falcão, A. Pharmacogenetics and therapeutic drug monitoring of fluoxetine in a real-world setting: A PK/PD analysis of the influence of (non-) genetic factors. Exp. Clin. Psychopharmacol. 2020, 28, 589. [Google Scholar] [CrossRef]
- Tsapakis, E.M.; Soldani, F.; Tondo, L.; Baldessarini, R.J. Efficacy of antidepressants in juvenile depression: Meta-analysis. Br. J. Psychiatry 2008, 193, 10–17. [Google Scholar] [CrossRef]
- Romanescu, M.; Buda, V.; Lombrea, A.; Andor, M.; Ledeti, I.; Suciu, M.; Danciu, C.; Dehelean, C.A.; Dehelean, L. Sex-related differences in pharmacological response to CNS drugs: A narrative review. J. Pers. Med. 2022, 12, 907. [Google Scholar] [CrossRef]
- Sramek, J.J.; Murphy, M.F.; Cutler, N.R. Sex differences in the psychopharmacological treatment of depression. Dialogues Clin. Neurosci. 2022, 18, 447–457. [Google Scholar] [CrossRef]
- Altemus, M.; Sarvaiya, N.; Epperson, C.N. Sex differences in anxiety and depression clinical perspectives. Front. Neuroendocrinol. 2014, 35, 320–330. [Google Scholar] [CrossRef]
- Keers, R.; Aitchison, K.J. Gender differences in antidepressant drug response. Int. Rev. Psychiatry 2010, 22, 485–500. [Google Scholar] [CrossRef]
- Jannuzzi, G.; Gatti, G.; Magni, P.; Spina, E.; Pacifici, R.; Zuccaro, P.; Torta, R.; Guarneri, L.; Perucca, E. Plasma concentrations of the enantiomers of fluoxetine and norfluoxetine: Sources of variability and preliminary observations on relations with clinical response. Ther. Drug Monit. 2002, 24, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Tsujii, T.; Sakurai, H.; Takeuchi, H.; Suzuki, T.; Mimura, M.; Uchida, H. Predictors of response to pharmacotherapy in children and adolescents with psychiatric disorders: A combined post hoc analysis of four clinical trial data. Neuropsychopharmacol. Rep. 2022, 42, 516–520. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Overall (n = 138) |
|---|---|
| Sex | |
| male | 34 (24.6%) |
| female | 104 (75.4%) |
| Age * | |
| Mean (SD) | 15 |
| Range | 7–18 |
| Age group | |
| children (7–12 years) | 7 (5.1%) |
| adolescents (13–18 years) | 131 (94.9%) |
| Medication dosage | |
| Mean (SD) | 19.93 (5.30) |
| Range | 10.00–40.00 |
| FLX concentration | |
| Mean (SD) | 123 (65.17) |
| Range | 17–396 |
| NORFLX concentration | |
| N-Miss | 4 |
| Mean (SD) | 144 (65.93) |
| Range | 21–339 |
| FLX+NORFLX concentration | |
| Mean (SD) | 264 (110.85) |
| Range | 62–673 |
| Body weight | |
| Mean (SD) | 59.67 (16.99) |
| Range | 24.40–124.70 |
| Body mass index | |
| Mean (SD) | 21.81 (4.94) |
| Range | 13.88–43.66 |
| Smoking | |
| 11–20 cigarettes/day | 2 (1.4%) |
| 6–10 cigarettes/day | 1 (0.7%) |
| Up to 5 cigarettes/day | 5 (3.6%) |
| Occasionally | 9 (6.5%) |
| No | 121 (87.7%) |
| Antipsychotic co-medication | |
| No | 128 (92.8%) |
| Yes | 10 (7.2%) |
| Other antidepressant co-medication | |
| No | 134 (97.1%) |
| Yes | 4 (2.9%) |
| Treatment modality | |
| outpatient | 8 (5.8%) |
| inpatient | 101 (73.2%) |
| day care treatment | 29 (21.0%) |
| Clinical Global Impression—Severity | |
| normal, not at all ill | 2 (1.4%) |
| borderline mentally ill | 4 (2.9%) |
| mildly ill | 21 (15.2%) |
| moderately ill | 61 (44.2%) |
| markedly ill | 37 (26.8%) |
| severely ill | 13 (9.4%) |
| among the most extremely ill patients | 0 (0%) |
| Clinical Global Impression—Improvement | |
| no assessment possible | 2 (1.4%) |
| very much improved | 10 (7.2%) |
| much improved | 53 (38.4%) |
| minimally improved | 60 (43.5%) |
| no change | 8 (5.8%) |
| minimally worse | 3 (2.2%) |
| much worse | 2 (1.4%) |
| very much worse | 0 (0%) |
| Pediatric Adverse Events Rating Scale (Max. Severity) | |
| N-Miss | 4 |
| none | 87 (64.9%) |
| slight | 18 (13.4%) |
| moderate | 26 (19.4%) |
| severe | 2 (1.5%) |
| extremely severe | 1 (0.7%) |
| Main diagnosis (ICD-10) Major depressive disorders (MDD) | |
| F32.0 MDD, mild episode | 2 (1.4%) |
| F32.1 MDD, moderate episode | 63 (45.7%) |
| F32.2 MDD, severe episode | 19 (13.8%) |
| F32.3 MDD, severe episode with psychotic symptoms | 2 (1.4%) |
| F33.1 recurrent MDD, moderate episode | 6 (4.3%) |
| F33.2 recurrent MDD, severe episode | 1 (0.7%) |
| Anxiety disorders | |
| F40.1 social phobias | 5 (3.6%) |
| F40.2 specific phobias | 1 (0.7%) |
| F41.2 mixed anxiety and depressive disorder Obsessive-compulsive disorders | 3 (2.2%) |
| F42.2 mixed obsessional thoughts and acts Reaction to severe stress, and adjustment disorders | 1 (0.7%) |
| F43.1 posttraumatic stress disorder (PTSD) | 1 (0.7%) |
| F43.2 adjustment disorders Somatoform disorders | 1 (0.7%) |
| F45.1 undifferentiated somatoform disorder Eating disorders | 1 (0.7%) |
| F50.0 anorexia nervosa | 11 (7.9%) |
| F50.1 atypical anorexia nervosa | 2 (1.4%) |
| F50.2 bulimia nervosa | 4 (2.9%) |
| F50.3 atypical bulimia nervosa Personality disorders | 2 (1.4%) |
| F60.31emotionally unstable, borderline Pervasive developmental disorders | 4 (2.9%) |
| F84.1 atypical autism Behavioral and emotional disorders | 1 (0.7%) |
| F91.- conduct disorder | 1 (0.7%) |
| F92.0 depressive conduct disorder | 5 (3.6%) |
| F94.0 elective mutism | 2 (1.4%) |
| Diagnostic groups by sex | |
| Affective disorders | |
| male | 23 (67.6%) |
| female | 70 (67.3%) |
| Anxiety disorders | |
| male | 4 (11.8%) |
| female | 5 (4.8%) |
| Obsessive-compulsive disorders | |
| male | 0 |
| female | 1 (1.0%) |
| Eating disorders | |
| male | 0 |
| female | 19 (18.3%) |
| Other disorders | |
| male | 7 (20.6%) |
| female | 9 (8.7%) |
| Co-morbidity | |
| 1 diagnosis | 70 (50.7%) |
| ≥2 diagnoses | 68 (49.3%) |
| A. Model 1 (Last Datapoint) | Multiple Linear Regressions | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| FLX(1/2) | NORFLX(1/2) | FLX+NORFLX(1/2) | |||||||
| Predictors | Est. | CI | p | Est. | CI | p | Est. | CI | p |
| (Intercept) | 9.99 | 6.49–13.48 | <0.001 | 8.82 | 5.19–12.45 | <0.001 | 13.42 | 9.38–17.46 | <0.001 |
| Dose | 0.24 | 0.15–0.32 | <0.001 | 0.21 | 0.13–0.30 | <0.001 | 0.30 | 0.20–0.40 | <0.001 |
| Age | 0.02 | −0.23–0.28 | 0.862 | 0.12 | −0.15–0.38 | 0.385 | 0.11 | −0.19–0.41 | 0.474 |
| Sex [male] | −0.08 | −1.09–0.92 | 0.867 | −0.97 | −2.00–0.06 | 0.064 | −0.79 | −1.95–0.37 | 0.178 |
| Body weight | −0.07 | −0.10–−0.04 | <0.001 | −0.05 | −0.08–−0.02 | 0.001 | −0.08 | −0.11–−0.05 | <0.001 |
| Smoking [yes] | −0.02 | −1.81–1.77 | 0.981 | −0.13 | −1.94–1.68 | 0.890 | −0.04 | −2.10–2.03 | 0.971 |
| Observations | 138 | 134 | 138 | ||||||
| R2/R2 adjusted | 0.284/0.257 | 0.241/0.212 | 0.320/0.294 | ||||||
| B. Model 2 (All Datapoints) | Linear Mixed Effect Models | ||||||||
| FLX(1/2) | NORFLX(1/2) | FLX+NORFLX(1/2) | |||||||
| Predictors | Est. | CI | p | Est. | CI | p | Est. | CI | p |
| (Intercept) | 9.40 | 6.69–12.12 | <0.001 | 8.19 | 5.23–11.16 | <0.001 | 12.55 | 9.43–15.68 | <0.001 |
| Dose | 0.34 | 0.29–0.40 | <0.001 | 0.26 | 0.20–0.31 | <0.001 | 0.41 | 0.34–0.47 | <0.001 |
| Age | −0.09 | −0.28–0.10 | 0.369 | 0.10 | −0.10–0.31 | 0.328 | 0.02 | −0.20–0.24 | 0.886 |
| Sex [male] | −0.29 | −1.06–0.48 | 0.463 | −0.85 | −1.70–−0.01 | 0.047 | −0.80 | −1.69–0.08 | 0.074 |
| Body weight | −0.07 | −0.09–−0.05 | <0.001 | −0.05 | −0.08–−0.03 | <0.001 | −0.09 | −0.11–−0.06 | <0.001 |
| Smoking [yes] | −0.02 | −1.39–1.36 | 0.982 | 0.04 | −1.24–1.31 | 0.953 | 0.12 | −1.46–1.71 | 0.878 |
| Time | 0.004 | 0.001–0.01 | 0.019 | 0.00 | −0.00–0.00 | 0.390 | 0.00 | −0.00–0.01 | 0.061 |
| Random Effects | |||||||||
| σ2 | 4.03 | 2.68 | 5.39 | ||||||
| τ00 ID | 1.72 | 3.47 | 2.24 | ||||||
| ICC | 0.30 | 0.56 | 0.29 | ||||||
| Observations | 287 | 281 | 287 | ||||||
| R2/R2 adjusted | 0.407/0.584 | 0.282/0.687 | 0.425/0.594 | ||||||
| A. CGI-I Model 1 (Last Datapoints) | Cumulative Link Models (Ordinal Regressions) | ||||||||
| CGI-I (FLX) | CGI-I (NORFLX) | CGI-I (FLX+NORFLX) | |||||||
| Predictors | Est. | CI | p | Est. | CI | p | Est. | CI | p |
| FLX/NORFLX/FLX+NORFLX | 1.00 | 0.99–1.00 | 0.732 | 1.00 | 1.00–1.01 | 0.089 | 1.00 | 1.00–1.01 | 0.168 |
| Age | 1.13 | 0.94–1.35 | 0.196 | 1.10 | 0.91–1.32 | 0.334 | 1.11 | 0.93–1.33 | 0.238 |
| Sex [male] | 2.66 | 1.23–5.74 | 0.013 | 3.05 | 1.37–6.80 | 0.006 | 2.89 | 1.33–6.27 | 0.007 |
| Co-medication [no] | 0.52 | 0.18–1.49 | 0.226 | 0.43 | 0.14–1.30 | 0.136 | 0.51 | 0.18–1.46 | 0.211 |
| Comorbidity [1 diagn.] | 0.79 | 0.41–1.54 | 0.493 | 0.85 | 0.43–1.68 | 0.633 | 0.81 | 0.41–1.57 | 0.529 |
| Observations | 136 | 132 | 136 | ||||||
| R2 Nagelkerke | 0.079 | 0.208 | 0.093 | ||||||
| B. PAERS Model 2 (Last Datapoints) | Cumulative Link Models (Ordinal Regressions) | ||||||||
| PAERS (FLX) | PAERS (NORFLX) | PAERS (FLX+NORFLX) | |||||||
| Predictors | Est. | CI | p | Est. | CI | p | Est. | CI | p |
| FLX/NORFLX/FLX+NORFLX | 1.00 | 0.99–1.00 | 0.437 | 1.00 | 0.99–1.00 | 0.753 | 1.00 | 1.00–1.00 | 0.513 |
| Age | 0.99 | 0.82–1.20 | 0.934 | 0.98 | 0.80–1.19 | 0.837 | 1.00 | 0.82–1.21 | 0.990 |
| Sex [male] | 0.92 | 0.39–2.16 | 0.851 | 0.94 | 0.39–2.23 | 0.883 | 0.90 | 0.38–2.11 | 0.808 |
| Co-medication [no] | 0.51 | 0.17–1.48 | 0.214 | 0.69 | 0.23–2.08 | 0.516 | 0.52 | 0.18–1.51 | 0.232 |
| Observations | 134 | 130 | 134 | ||||||
| R2 Nagelkerke | 0.018 | 0.095 | 0.016 | ||||||
| C. CGI-I Model 2 (All Datapoints) | Cumulative Link Mixed Models | ||||||||
| CGI-I | CGI-I | CGI-I | |||||||
| Predictors | Est. | CI | p | Est. | CI | p | Est. | CI | p |
| FLX/NORFLX/FLX+NORFLX | 1.00 | 0.99–1.01 | 0.842 | 1.01 | 1.00–1.01 | 0.160 | 1.00 | 1.00–1.01 | 0.245 |
| Age | 1.06 | 0.81–1.39 | 0.676 | 1.03 | 0.77–1.38 | 0.845 | 1.06 | 0.79–1.42 | 0.690 |
| Sex [male] | 3.55 | 1.09–11.48 | 0.035 | 4.06 | 1.06–15.50 | 0.040 | 4.20 | 1.05–16.84 | 0.043 |
| Co-medication [no] | 0.76 | 0.17–3.33 | 0.719 | 0.68 | 0.14–3.27 | 0.633 | 0.78 | 0.16–3.73 | 0.751 |
| Comorbidity [1 diagn.] | 0.44 | 0.17–1.15 | 0.093 | 0.49 | 0.16–1.48 | 0.207 | 0.42 | 0.13–1.33 | 0.138 |
| Time | 1.00 | 1.00–1.01 | 0.086 | 1.00 | 1.00–1.01 | 0.096 | 1.00 | 1.00–1.01 | 0.145 |
| Random Effects | |||||||||
| σ2 | 3.29 | 3.29 | 3.29 | ||||||
| τ00 ID | 5.07 | 5.91 | 6.18 | ||||||
| ICC | 0.61 | 0.64 | 0.65 | ||||||
| Observations | 213 | 208 | 213 | ||||||
| Marginal R2/Conditional R2 | 0.066/0.633 | 0.073/0.669 | 0.073/0.678 | ||||||
| D. PAERS Model 2 (All Datapoints) | Cumulative Link Mixed Models | ||||||||
| PAERS | PAERS | PAERS | |||||||
| Predictors | Est. | CI | p | Est. | CI | p | Est. | CI | p |
| FLX/NORFLX/FLX+NORFLX | 1.00 | 0.99–1.00 | 0.548 | 1.00 | 1.00–1.01 | 0.570 | 1.00 | 1.00–1.00 | 0.937 |
| Age | 1.07 | 0.87–1.32 | 0.536 | 1.05 | 0.86–1.27 | 0.658 | 1.07 | 0.87–1.32 | 0.527 |
| Sex [male] | 0.95 | 0.38–2.37 | 0.911 | 0.98 | 0.42–2.31 | 0.966 | 0.95 | 0.38–2.36 | 0.908 |
| Co-medication [no] | 0.62 | 0.19–1.97 | 0.414 | 0.79 | 0.26–2.37 | 0.676 | 0.63 | 0.20–2.00 | 0.434 |
| Time | 1.00 | 0.99–1.00 | 0.536 | 1.05 | 0.99–1.00 | 0.463 | 1.00 | 0.99–1.00 | 0.451 |
| Random Effects | |||||||||
| σ2 | 3.29 | 3.29 | 3.29 | ||||||
| τ00 ID | 1.36 | 0.89 | 1.34 | ||||||
| ICC | 0.29 | 0.21 | 0.29 | ||||||
| Observations | 210 | 205 | 210 | ||||||
| Marginal R2/Conditional R2 | 0.015/0.303 | 0.009/0.220 | 0.013/0.299 | ||||||
| Responders Transdiagnostic n = 64 | Responders with Depression n = 44 | |||||
|---|---|---|---|---|---|---|
| FLX | NORFLX | FLX+NORFLX | FLX | NORFLX | FLX+NORFLX | |
| Q1 | 73 | 102.5 | 208 | 69.5 | 98.75 | 201.5 |
| Q3 | 155 | 186 | 328 | 156.25 | 184.25 | 306 |
| M-1SD | 57.97 | 85.28 | 167.49 | 62.51 | 78.88 | 160.67 |
| M+1SD | 191.29 | 218.24 | 385.31 | 181.19 | 223.57 | 385.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frey, M.; Smigielski, L.; Tini, E.; Fekete, S.; Fleischhaker, C.; Wewetzer, C.; Karwautz, A.; Correll, C.U.; Gerlach, M.; Taurines, R.; et al. Therapeutic Drug Monitoring in Children and Adolescents: Findings on Fluoxetine from the TDM-VIGIL Trial. Pharmaceutics 2023, 15, 2202. https://doi.org/10.3390/pharmaceutics15092202
Frey M, Smigielski L, Tini E, Fekete S, Fleischhaker C, Wewetzer C, Karwautz A, Correll CU, Gerlach M, Taurines R, et al. Therapeutic Drug Monitoring in Children and Adolescents: Findings on Fluoxetine from the TDM-VIGIL Trial. Pharmaceutics. 2023; 15(9):2202. https://doi.org/10.3390/pharmaceutics15092202
Chicago/Turabian StyleFrey, Michael, Lukasz Smigielski, Elvira Tini, Stefanie Fekete, Christian Fleischhaker, Christoph Wewetzer, Andreas Karwautz, Christoph U. Correll, Manfred Gerlach, Regina Taurines, and et al. 2023. "Therapeutic Drug Monitoring in Children and Adolescents: Findings on Fluoxetine from the TDM-VIGIL Trial" Pharmaceutics 15, no. 9: 2202. https://doi.org/10.3390/pharmaceutics15092202
APA StyleFrey, M., Smigielski, L., Tini, E., Fekete, S., Fleischhaker, C., Wewetzer, C., Karwautz, A., Correll, C. U., Gerlach, M., Taurines, R., Plener, P. L., Malzahn, U., Kornbichler, S., Weninger, L., Brockhaus, M., Reuter-Dang, S.-Y., Reitzle, K., Rock, H., Imgart, H., ... Egberts, K. M. (2023). Therapeutic Drug Monitoring in Children and Adolescents: Findings on Fluoxetine from the TDM-VIGIL Trial. Pharmaceutics, 15(9), 2202. https://doi.org/10.3390/pharmaceutics15092202









