Delivery Systems in Ocular Retinopathies: The Promising Future of Intravitreal Hydrogels as Sustained-Release Scaffolds
Abstract
:1. Introduction to Retinal Diseases
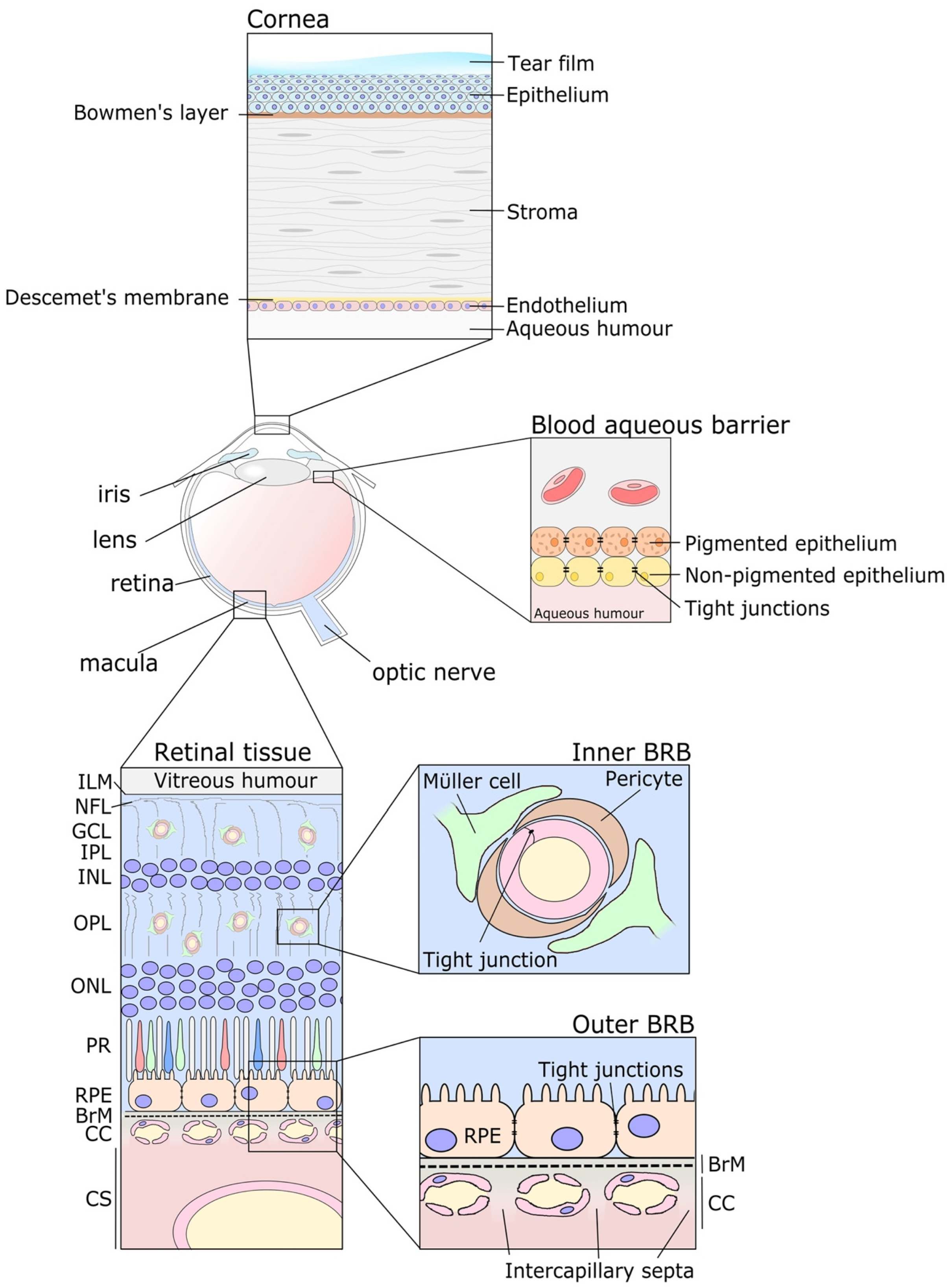
2. Ocular Barriers, a Hurdle to Overcome
3. Current Principal Administration Routes for Ocular Therapies
4. The Current Landscape of Approved Treatments for Retinal Degeneration
5. Facing the Drawbacks of Intravitreal Treatments
6. Understanding the Pharmacokinetic Challenges of Intravitreal Drug Delivery
7. Intravitreal Delivery Systems for Sustained Drug Release
7.1. Intraocular Implants
7.2. Hydrogels for Intravitreal Drug Delivery
7.3. Release Kinetics of Hydrogels at the Vitreal Cavity
7.4. Stimuli-Responsive Hydrogels in Retinopathies
7.5. Temperature-Responsive Hydrogels for Intraocular Delivery
7.6. Thermo-Responsive Hydrogels Containing Nanoformulations
8. In Silico Modeling to Optimize HG Design
9. Current Challenges and Future Perspectives: Facing the Clinical Development of Hydrogels
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adelson, J.D.; Bourne, R.R.A.; Briant, P.S.; Flaxman, S.R.; Taylor, H.R.B.; Jonas, J.B.; Abdoli, A.A.; Abrha, W.A.; Abualhasan, A.; Abu-Gharbieh, E.G.; et al. Blindness and Vision Impairment. Lancet Glob. Health 2021, 9, e144–e160. [Google Scholar]
- Heesterbeek, T.J.; Lorés-Motta, L.; Hoyng, C.B.; Lechanteur, Y.T.E.; den Hollander, A.I. Risk factors for progression of age-related macular degeneration. Ophthalmic Physiol. Opt. 2020, 40, 140–170. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.; Liew, G.; Gopinath, B.; Wong, T.Y. Age-related macular degeneration. Lancet 2018, 392, 1147–1159. [Google Scholar] [CrossRef]
- Wong, T.Y.; Cheung, C.M.G.; Larsen, M.; Sharma, S.; Simó, R. Diabetic retinopathy. Nat. Rev. Dis. Prim. 2016, 2, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.; Wong, T.Y.; Sabanayagam, C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis. 2015, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.J.; Bishop, P.N. The eye as a complement dysregulation hotspot. Semin. Immunopathol. 2018, 40, 65–74. [Google Scholar] [CrossRef]
- Xu, H.; Chen, M. Targeting the complement system for the management of retinal inflammatory and degenerative diseases. Eur. J. Pharmacol. 2016, 787, 94–104. [Google Scholar] [CrossRef]
- Georgiou, M.; Fujinami, K.; Michaelides, M. Inherited retinal diseases: Therapeutics, clinical trials and end points—A review. Clin. Exp. Ophthalmol. 2021, 49, 270–288. [Google Scholar] [CrossRef]
- Norman, R.E.; Flanagan, J.G.; Rausch, S.M.K.; Sigal, I.A.; Tertinegg, I.; Eilaghi, A.; Portnoy, S.; Sled, J.G.; Ethier, C.R. Dimensions of the human sclera: Thickness measurement and regional changes with axial length. Exp. Eye Res. 2010, 90, 277–284. [Google Scholar] [CrossRef]
- Kitazawa, K.; Inotmata, T.; Shih, K.; Hughes, J.W.B.; Bozza, N.; Tomioka, Y.; Numa, K.; Yokoi, N.; Campisi, J.; Dana, R.; et al. Impact of aging on the pathophysiology of dry eye disease: A systematic review and meta-analysis. Ocul. Surf. 2022, 25, 108–118. [Google Scholar] [CrossRef]
- Markoulli, M.; Flanagan, J.; Tummanapalli, S.S.; Wu, J.; Willcox, M. The impact of diabetes on corneal nerve morphology and ocular surface integrity. Ocul. Surf. 2018, 16, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Rudraraju, M.; Narayanan, S.P.; Somanath, P.R. Regulation of blood-retinal barrier cell-junctions in diabetic retinopathy. Pharmacol. Res. 2020, 161, 105115. [Google Scholar] [CrossRef] [PubMed]
- De Jong, S.; Tang, J.; Clark, S.J. Age-related macular degeneration: A disease of extracellular complement amplification. Immunol. Rev. 2022, 313, 279–297. [Google Scholar] [CrossRef]
- Peynshaert, K.; Devoldere, J.; Minnaert, A.K.; De Smedt, S.C.; Remaut, K. Morphology and Composition of the Inner Limiting Membrane: Species-Specific Variations and Relevance toward Drug Delivery Research. Curr. Eye Res. 2019, 44, 465–475. [Google Scholar] [CrossRef]
- Clark, S.; Keenan, T.D.L.; Fielder, H.L.; Collinson, L.J.; Holley, R.J.; Merry, C.; Van Kuppevelt, T.H.; Day, A.J.; Bishop, P.N. Mapping the differential distribution of glycosaminoglycans in the adult human retina, choroid, and sclera. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6511–6521. [Google Scholar] [CrossRef]
- Ashikari-Hada, S. Heparin Regulates Vascular Endothelial Growth Factor165-dependent Mitogenic Activity, Tube Formation, and Its Receptor Phosphorylation of Human Endothelial Cells: Comparison of the effects of heparin and modified heparins. J. Biol. Chem. 2005, 280, 31508–31515. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Fuster, M.; Sriramarao, P.; Esko, J.D. Endothelial heparan sulfate deficiency impairs L-selectin- and chemokine-mediated neutrophil trafficking during inflammatory responses. Nat. Immunol. 2005, 6, 902–910. [Google Scholar] [CrossRef]
- Dartt, D.A.; Willcox, M.D.P. Complexity of the tear film: Importance in homeostasis and dysfunction during disease. Exp. Eye Res. 2013, 117, 1–3. [Google Scholar] [CrossRef]
- Mofidfar, M.; Abdi, B.; Ahadian, S.; Mostafavi, E.; Desai, T.A.; Abbasi, F.; Sun, Y.; Manche, E.E.; Ta, C.N.; Flowers, C.W. Drug delivery to the anterior segment of the eye: A review of current and future treatment strategies. Int. J. Pharm. 2021, 607, 120924. [Google Scholar] [CrossRef]
- Argüeso, P.; Woodward, A.M.; AbuSamra, D.B. The Epithelial Cell Glycocalyx in Ocular Surface Infection. Front. Immunol. 2021, 12, 3443. [Google Scholar] [CrossRef]
- Fukuda, K.; Ishida, W.; Fukushima, A.; Nishida, T. Corneal fibroblasts as sentinel cells and local immune modulators in infectious keratitis. Int. J. Mol. Sci. 2017, 18, 1831. [Google Scholar] [CrossRef] [PubMed]
- Agrahari, V.; Mandal, A.; Agrahari, V.; Trinh, H.M.; Joseph, M.; Ray, A.; Hadji, H.; Mitra, R.; Pal, D.; Mitra, A.K. A comprehensive insight on ocular pharmacokinetics. Drug Deliv. Transl. Res. 2016, 6, 735–754. [Google Scholar]
- West-Mays, J.A.; Dwivedi, D.J. The keratocyte: Corneal stromal cell with variable repair phenotypes. Int. J. Biochem. Cell Biol. 2006, 38, 1625–1631. [Google Scholar] [CrossRef]
- Alkanaan, A.; Barsotti, R.; Kirat, O.; Khan, A.; Almubrad, T.; Akhtar, S. Collagen fibrils and proteoglycans of peripheral and central stroma of the keratoconus cornea—Ultrastructure and 3D transmission electron tomography. Sci. Rep. 2019, 9, 19963. [Google Scholar] [CrossRef]
- Freddo, T.F. A contemporary concept of the blood-aqueous barrier. Prog. Retin. Eye Res. 2013, 32, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Pelis, R.M. Drug transport by the blood-aqueous humor barrier of the eye. Drug Metab. Dispos. 2016, 44, 1675–1681. [Google Scholar] [CrossRef]
- Arden, G.B.; Sidman, R.L.; Arap, W.; Schlingemann, R.O. Spare the rod and spoil the eye. Br. J. Ophthalmol. 2005, 89, 764–769. [Google Scholar] [CrossRef]
- Klaassen, I.; Van Noorden, C.J.F.; Schlingemann, R.O. Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Prog. Retin. Eye Res. 2013, 34, 19–48. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, M.; Grebe, R.; Bhutto, I.A.; Edwards, M.; Scott Mcleod, D.; Lutty, G.A. Albumen transport to bruch’s membrane and RPE by choriocapillaris caveolae. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2213–2224. [Google Scholar] [CrossRef]
- Molokhia, S.A.; Thomas, S.C.; Garff, K.J.; Mandell, K.J.; Wirostko, B.M. Anterior eye segment drug delivery systems: Current treatments and future challenges. J. Ocul. Pharmacol. Ther. 2013, 29, 92–105. [Google Scholar] [CrossRef]
- Holland, E.J.; Darvish, M.; Nichols, K.K.; Jones, L.; Karpecki, P.M. Efficacy of topical ophthalmic drugs in the treatment of dry eye disease: A systematic literature review. Ocul. Surf. 2019, 17, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Patton, T.F. Importance of the noncorneal absorption route in topical ophthalmic drug delivery. Investig. Ophthalmol. Vis. Sci. 1985, 26, 584–587. [Google Scholar]
- Ahmed, I.; Patton, T.F. Disposition of timolol and inulin in the rabbit eye following corneal versus non-corneal absorption. Int. J. Pharm. 1987, 38, 9–21. [Google Scholar] [CrossRef]
- Huang, A.J.W.; Tseng, S.C.G.; Kenyon, K.R. Paracellular permeability of corneal and conjunctival epithelia. Investig. Ophthalmol. Vis. Sci. 1989, 30, 684–689. [Google Scholar]
- Del Amo, E.M. Topical ophthalmic administration: Can a drug instilled onto the ocular surface exert an effect at the back of the eye? Front. Drug Deliv. 2022, 2, 26. [Google Scholar] [CrossRef]
- Löscher, M.; Seiz, C.; Hurst, J.; Schnichels, S. Topical Drug Delivery to the Posterior Segment of the Eye. Pharmaceutics 2022, 14, 134. [Google Scholar] [CrossRef]
- Qu, Y.; Liu, X.S.; Liang, A.Y.; Xiao, J.Y.; Zhao, C.; Gao, F.; Zhang, M.F. Subconjunctival injections of triamcinolone acetonide to treat uveitic macular edema. Int. J. Ophthalmol. 2020, 13, 1087–1096. [Google Scholar] [CrossRef]
- Fraunfelder, F.; Fritz, T.; Fraunfelder, F.; Rick, W. Hormones and Drugs Affecting Hormonal Mechanisms. In Drug-Induced Ocular Side Effects; Elsevier: Amsterdam, The Netherlands, 2021; pp. 241–264. [Google Scholar]
- Bakri, S.J.; Thorne, J.E.; Ho, A.C.; Ehlers, J.P.; Schoenberger, S.D.; Yeh, S.; Kim, S.J. Safety and Efficacy of Anti-Vascular Endothelial Growth Factor Therapies for Neovascular Age-Related Macular Degeneration: A Report by the American Academy of Ophthalmology. Ophthalmology 2019, 126, 55–63. [Google Scholar] [CrossRef]
- Teo, K.Y.C.; Nguyen, V.; O’Toole, L.; Daien, V.; Sanchez-Monroy, J.; Ricci, F.; Ponsioen, T.L.; Morros, H.B.; Cheung, C.M.G.; Arnold, J.J.; et al. Longer treatment intervals are associated with reduced treatment persistence in neovascular age related macular degeneration. Eye 2022, 37, 467–473. [Google Scholar] [CrossRef]
- Okada, M.; Mitchell, P.; Finger, R.P.; Eldem, B.; Talks, S.J.; Hirst, C.; Paladini, L.; Barratt, J.; Wong, T.Y.; Loewenstein, A. Nonadherence or Nonpersistence to intravitreal Injection Therapy for Neovascular Age-Related Macular Degeneration: A Mixed-Methods Systematic Review. Ophthalmology 2021, 128, 234–247. [Google Scholar] [CrossRef]
- Hurand, V.; Ducloyer, J.B.; Baudin, F.; Aho, S.; Weber, M.; Kodjikian, L.; Devin, F.; Gabrielle, P.H.; Creuzot-Garcher, C.; Massin, P. IMPACT study: Impact of adherence to anti-VEGF intravitreal injections for macular disease during COVID-19-related confinement in France. Acta Ophthalmol. 2022, 101, 91–99. [Google Scholar] [CrossRef]
- Liu, S.; Ng, J.K.Y.; Moon, E.H.; Morgan, D.; Woodhouse, N.; Agrawal, D.; Chan, L.; Chhabra, R. Impact of COVID-19-associated anxiety on the adherence to intravitreal injection in patients with macular diseases a year after the initial outbreak. Ther. Adv. Ophthalmol. 2022, 14, 251584142110708. [Google Scholar] [CrossRef]
- Wasser, L.M.; Weill, Y.; Brosh, K.; Magal, I.; Potter, M.; Strassman, I.; Gelman, E.; Koslowsky, M.; Zadok, D.; Hanhart, J. The Impact of COVID-19 on intravitreal Injection Compliance. SN Compr. Clin. Med. 2020, 2, 2546–2549. [Google Scholar] [CrossRef]
- Cao, Y.; Samy, K.E.; Bernards, D.A.; Desai, T.A. Recent advances in intraocular sustained-release drug delivery devices. Drug Discov. Today 2019, 24, 1694–1700. [Google Scholar] [CrossRef] [PubMed]
- Ranade, S.V.; Wieland, M.R.; Tam, T.; Rea, J.C.; Horvath, J.; Hieb, A.R.; Jia, W.; Grace, L.; Barteselli, G.; Stewart, J.M. The Port Delivery System with ranibizumab: A new paradigm for long-acting retinal drug delivery. Drug Deliv. 2022, 29, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Miraldi Utz, V.; Coussa, R.G.; Antaki, F.; Traboulsi, E.I. Gene therapy for RPE65-related retinal disease. Ophthalmic Genet. 2018, 39, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Xue, K.; Groppe, M.; Salvetti, A.P.; MacLaren, R.E. Technique of retinal gene therapy: Delivery of viral vector into the subretinal space. Eye 2017, 31, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Simunovic, M.P.; Xue, K.; Jolly, J.K.; MacLaren, R.E. Structural and functional recovery following limited iatrogenic macular detachment for retinal gene therapy. JAMA Ophthalmol. 2017, 135, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Ladha, R.; Caspers, L.E.; Willermain, F.; de Smet, M.D. Subretinal Therapy: Technological Solutions to Surgical and Immunological Challenges. Front. Med. 2022, 9, 661. [Google Scholar] [CrossRef]
- Leroy, B.P.; Fischer, M.D.; Flannery, J.G.; MacLaren, R.E.; Dalkara, D.; Scholl, H.P.N.; Chung, D.C.; Spera, C.; Viriato, D.; Banhazi, J. Gene therapy for inherited retinal disease: Long-term durability of effect. Ophthalmic Res. 2022, 66, 179–196. [Google Scholar] [CrossRef]
- Prado, D.A.; Acosta-Acero, M.; Maldonado, R.S. Gene therapy beyond luxturna: A new horizon of the treatment for inherited retinal disease. Curr. Opin. Ophthalmol. 2020, 31, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Bill, A.; Sperber, G.O. Control of retinal and choroidal blood flow. Eye 1990, 4, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Zouache, M.A.; Eames, I.; Luthert, P.J. Blood flow in the choriocapillaris. J. Fluid Mech. 2015, 774, 37–66. [Google Scholar] [CrossRef]
- Weber, T. Anti-AAV Antibodies in AAV Gene Therapy: Current Challenges and Possible Solutions. Front. Immunol. 2021, 12, 702. [Google Scholar] [CrossRef]
- Darrow, J.J. Luxturna: FDA documents reveal the value of a costly gene therapy. Drug Discov. Today 2019, 24, 949–954. [Google Scholar] [CrossRef]
- Ronco, V.; Dilecce, M.; Lanati, E.; Canonico, P.L.; Jommi, C. Price and reimbursement of advanced therapeutic medicinal products in Europe: Are assessment and appraisal diverging from expert recommendations? J. Pharm. Policy Pract. 2021, 14, 30. [Google Scholar] [CrossRef] [PubMed]
- Everett, L.A.; Paulus, Y.M. Laser Therapy in the Treatment of Diabetic Retinopathy and Diabetic Macular Edema. Curr. Diab. Rep. 2021, 21, 35. [Google Scholar] [CrossRef] [PubMed]
- Al-Hussainy, S.; Dodson, P.M.; Gibson, J.M. Pain response and follow-up of patients undergoing panretinal laser photocoagulation with reduced exposure times. Eye 2008, 22, 96–99. [Google Scholar] [CrossRef]
- Dobler, E.; Mohammed, B.R.; Chavan, R.; Lip, P.L.; Mitra, A.; Mushtaq, B. Clinical efficacy and safety of intravitreal fluocinolone acetonide implant for the treatment of chronic diabetic macular oedema: Five-year real-world results. Eye 2022, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Castro-Navarro, V.; Cervera-Taulet, E.; Navarro-Palop, C.; Monferrer-Adsuara, C.; Hernández-Bel, L.; Montero-Hernández, J. Intravitreal dexamethasone implant Ozurdex® in naïve and refractory patients with different subtypes of diabetic macular edema 11 Medical and Health Sciences 1103 Clinical Sciences. BMC Ophthalmol. 2019, 19, 15. [Google Scholar]
- Nomoto, H.; Shiraga, F.; Kuno, N.; Kimura, E.; Fujii, S.; Shinomiya, K.; Nugent, A.K.; Hirooka, K.; Baba, T. Pharmacokinetics of bevacizumab after topical, subconjunctival, and intravitreal administration in rabbits, Invest. Ophthalmol. Vis. Sci. 2009, 50, 4807–4813. [Google Scholar] [CrossRef]
- Kang-Mieler, J.J.; Osswald, C.R.; Mieler, W.F. Advances in ocular drug delivery: Emphasis on the posterior segment. Expert Opin. Drug Deliv. 2014, 11, 1647–1660. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Kim, H.; Woo, S.J.; Park, J.H.; Park, S.; Hwang, D.J.; Park, K.H. Pharmacokinetics of intravitreally injected bevacizumab in vitrectomized eyes. J. Ocul. Pharmacol. Ther. 2013, 29, 612–618. [Google Scholar] [CrossRef]
- Moisseiev, E.; Waisbourd, M.; Ben-Artsi, E.; Levinger, E.; Barak, A.; Daniels, T.; Csaky, K.; Loewenstein, A.; Barequet, I.S. Pharmacokinetics of bevacizumab after topical and intravitreal administration in human eyes. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Del Amo, E.M.; Rimpela, A.K.; Heikkinen, E.; Kari, O.K.; Ramsay, E.; Lajunen, T.; Schmitt, M.; Pelkonen, L.; Bhattacharya, M.; Richardson, D.; et al. Pharmacokinetic aspects of retinal drug delivery. Prog. Retin. Eye Res. 2017, 57, 134–185. [Google Scholar] [CrossRef]
- Min Kim, H.; Woo, S.J. Ocular Drug Delivery to the Retina: Current Innovations and Future Perspectives. Pharmaceutics 2021, 13, 108. [Google Scholar] [CrossRef] [PubMed]
- Gaudreault, J.; Fei, D.; Beyer, J.C.; Ryan, A.; Rangell, L.; Shiu, V.; Damico, L.A. Pharmacokinetics and retinal distribution of ranibizumab, a humanized antibody fragment directed against VEGF-A, following intravitreal administration in rabbits. Retina 2007, 27, 1260–1266. [Google Scholar] [CrossRef]
- Ahn, S.J.; Ahn, J.; Park, S.; Kim, H.; Hwang, D.J.; Park, J.H.; Park, J.Y.; Chung, J.Y.; Park, K.H.; Woo, S.J. Intraocular pharmacokinetics of ranibizumab in vitrectomized versus nonvitrectomized eyes. Investig. Ophthalmol. Vis. Sci. 2014, 55, 567–573. [Google Scholar] [CrossRef]
- Park, S.J.; Oh, J.; Kim, Y.K.; Park, J.H.; Park, J.Y.; Hong, H.K.; Park, K.H.; Lee, J.E.; Kim, H.M.; Chung, J.Y.; et al. Intraocular pharmacokinetics of intravitreal vascular endothelial growth factor-Trap in a rabbit model. Eye 2015, 29, 561–568. [Google Scholar] [CrossRef]
- Ahn, S.J.; Hong, H.K.; Na, Y.M.; Park, S.J.; Ahn, J.; Oh, J.; Chung, J.Y.; Park, K.H.; Woo, S.J. Use of Rabbit Eyes in Pharmacokinetic Studies of Intraocular Drugs. J. Vis. Exp. 2016, 113, e53878. [Google Scholar]
- Park, S.J.; Choi, Y.; Na, Y.M.; Hong, H.K.; Park, J.Y.; Park, K.H.; Chung, J.Y.; Woo, S.J. Intraocular Pharmacokinetics of intravitreal Aflibercept (Eylea) in a Rabbit Model. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2612–2617. [Google Scholar] [CrossRef] [PubMed]
- Center for Drug Evaluation and Research Application Number 761125Orig1s000, Clinical Pharmacology Reviews. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/761125Orig1s000ClinPharmR.pdf (accessed on 13 October 2020).
- Shatz, W.; Hass, P.E.; Mathieu, M.; Kim, H.S.; Leach, K.; Zhou, M.; Crawford, Y.; Shen, A.; Wang, K.; Chang, D.P.; et al. Contribution of Antibody Hydrodynamic Size to Vitreal Clearance Revealed through Rabbit Studies Using a Species-Matched Fab. Mol. Pharm. 2016, 13, 2996–3003. [Google Scholar] [CrossRef] [PubMed]
- Sinapis, C.I.; Routsias, J.G.; Sinapis, A.I.; Sinapis, D.I.; Agrogiannis, G.D.; Pantopoulou, A.; Theocharis, S.E.; Baltatzis, S.; Patsouris, E.; Perrea, D. Pharmacokinetics of intravitreal bevacizumab (Avastin(R)) in rabbits. Clin. Ophthalmol. 2011, 5, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lei, N.; Zhang, M.; Li, Y.; Xiao, H.; Hao, X. Pharmacokinetics of a long-lasting anti-VEGF fusion protein in rabbit. Exp. Eye Res. 2012, 97, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Bakri, S.J.; Snyder, M.R.; Reid, J.M.; Pulido, J.S.; Ezzat, M.K.; Singh, R.J. Pharmacokinetics of intravitreal ranibizumab (Lucentis). Ophthalmology 2007, 114, 2179–2182. [Google Scholar] [CrossRef]
- Bakri, S.J.; Snyder, M.R.; Reid, J.M.; Pulido, J.S.; Singh, R.J. Pharmacokinetics of intravitreal bevacizumab (Avastin). Ophthalmology 2007, 114, 855–859. [Google Scholar] [CrossRef]
- Christoforidis, J.B.; Carlton, M.M.; Knopp, M.V.; Hinkle, G.H. PET/CT imaging of I-124-radiolabeled bevacizumab and ranibizumab after intravitreal injection in a rabbit model. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5899–5903. [Google Scholar] [CrossRef]
- Niwa, Y.; Kakinoki, M.; Sawada, T.; Wang, X.; Ohji, M. Ranibizumab and Aflibercept: Intraocular Pharmacokinetics and Their Effects on Aqueous VEGF Level in Vitrectomized and Nonvitrectomized Macaque Eyes. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6501–6505. [Google Scholar] [CrossRef]
- Regula, J.T.; von Leithner, P.L.; Foxton, R.; Barathi, V.A.; Cheung, C.M.; Bo Tun, S.B.; Wey, Y.S.; Iwata, D.; Dostalek, M.; Moelleken, J.; et al. Targeting key angiogenic pathways with a bispecific CrossMAb optimized for neovascular eye diseases. EMBO Mol. Med. 2016, 8, 1265–1288. [Google Scholar] [CrossRef]
- Gaudreault, J.; Fei, D.; Rusit, J.; Suboc, P.; Shiu, V. Preclinical pharmacokinetics of Ranibizumab (rhuFabV2) after a single intravitreal administration. Investig. Ophthalmol. Vis. Sci. 2005, 46, 726–733. [Google Scholar] [CrossRef]
- Drolet, D.W.; Nelson, J.; Tucker, C.E.; Zack, P.M.; Nixon, K.; Bolin, R.; Judkins, M.B.; Farmer, J.A.; Wolf, J.L.; Gill, S.C.; et al. Pharmacokinetics and safety of an anti-vascular endothelial growth factor aptamer (NX1838) following injection into the vitreous humor of rhesus monkeys. Pharm. Res. 2000, 17, 1503–1510. [Google Scholar] [CrossRef] [PubMed]
- Christoforidis, J.B.; Briley, K.; Binzel, K.; Bhatia, P.; Wei, L.; Kumar, K.; Knopp, M.V. Systemic Biodistribution and intravitreal Pharmacokinetic Properties of Bevacizumab, Ranibizumab, and Aflibercept in a Nonhuman Primate Model. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5636–5645. [Google Scholar] [CrossRef] [PubMed]
- Krohne, T.U.; Eter, N.; Holz, F.G.; Meyer, C.H. Intraocular pharmacokinetics of bevacizumab after a single intravitreal injection in humans. Am. J. Ophthalmol. 2008, 146, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Krohne, T.U.; Liu, Z.; Holz, F.G.; Meyer, C.H. Intraocular pharmacokinetics of ranibizumab following a single intravitreal injection in humans. Am. J. Ophthalmol. 2012, 154, 682–686.e682. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.H.; Krohne, T.U.; Holz, F.G. Intraocular pharmacokinetics after a single intravitreal injection of 1.5 mg versus 3.0 mg of bevacizumab in humans. Retina 2011, 31, 1877–1884. [Google Scholar] [CrossRef]
- Shastri, D.H.; Silva, A.C.; Almeida, H. Ocular Delivery of Therapeutic Proteins: A Review. Pharmaceutics 2023, 15, 205. [Google Scholar] [CrossRef]
- Ilochonwu, B.C.; Urtti, A.; Hennink, W.E.; Vermonden, T. Intravitreal hydrogels for sustained release of therapeutic proteins. J. Control. Release 2020, 326, 419–441. [Google Scholar] [CrossRef]
- Kim, H.M.; Park, K.H.; Chung, J.Y.; Woo, S.J. A Prediction Model for the Intraocular Pharmacokinetics of intravitreally Injected Drugs Based on Molecular Physicochemical Properties. Ophthalmic. Res. 2020, 63, 41–49. [Google Scholar] [CrossRef]
- Mandal, A.; Pal, D.; Agrahari, V.; Trinh, H.M.; Joseph, M.; Mitra, A.K. Ocular delivery of proteins and peptides: Challenges and novelformulation approaches. Adv. Drug Deliv. Rev. 2018, 126, 67–95. [Google Scholar] [CrossRef]
- Radhakrishnan, K.; Sonali, N.; Moreno, M.; Nirmal, J.; Fernandez, A.A.; Venkatraman, S.; Agrawal, R. Protein delivery to the back of the eye: Barriers, carriers and stability of anti-VEGF proteins. Drug Discov. Today 2017, 22, 416–423. [Google Scholar] [CrossRef]
- Xu, Q.; Boylan, N.J.; Suk, J.S.; Wang, Y.Y.; Nance, E.A.; Yang, J.C.; McDonnell, P.J.; Cone, R.A.; Duh, E.J.; Hanes, J. Nanoparticle diffusion in and microrheology of the bovine vitreous ex vivo. J. Control. Release 2013, 167, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Peeters, L.; Sanders, N.N.; Braeckmans, K.; Boussery, K.; Van de Voorde, J.; De Smedt, S.C.; Demeester, J. Vitreous: A barrier to nonviral ocular gene therapy. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3553–3561. [Google Scholar] [CrossRef] [PubMed]
- Maurice, D.M.; Mishima, S. Ocular Pharmacokinetics. In Pharmacology of the Eye; Springer: Berlin/Heidelberg, Germany, 1984; pp. 16–119. [Google Scholar]
- Dias, C.; Mitra, A. Vitreal elimination kinetics of large molecular weight FITC-labeled dextrans in albino rabbits using a novel microsampling technique. J. Pharm. Sci. 2000, 89, 572–578. [Google Scholar] [CrossRef]
- Kasdorf, B.T.; Arends, F.; Lieleg, O. Diffusion regulation in the vitreous humor. Biophys. J. 2015, 109, 2171–2181. [Google Scholar] [CrossRef]
- Del Amo, E.M.; Vellonen, K.S.; Kidron, H.; Urtti, A. Intravitreal clearance and volume of distribution of compounds in rabbits: In silico prediction and pharmacokinetic simulations for drug development. Eur. J. Pharm. Biopharm. 2015, 95, 215–226. [Google Scholar] [CrossRef]
- Crowell, S.R.; Wang, K.; Famili, A.; Shatz, W.; Loyet, K.M.; Chang, V.; Liu, Y.; Prabhu, S.; Kamath, A.V.; Kelley, R.F. Influence of Charge, Hydrophobicity, and Size on Vitreous Pharmacokinetics of Large Molecules. Transl. Vis. Sci. Technol. 2019, 8, 1. [Google Scholar] [CrossRef]
- Shan, B.H.; Wu, F.G. Hydrogel-Based Growth Factor Delivery Platforms: Strategies and Recent Advances. Adv. Mater. 2023, 2210707. [Google Scholar] [CrossRef]
- Huang, S.; Hong, X.; Zhao, M.; Liu, N.; Liu, H.; Zhao, J.; Shao, L.; Xue, W.; Zhang, H.; Zhu, P.; et al. Nanocomposite hydrogels for biomedical applications. Bioeng. Transl. Med. 2022, 7, e10315. [Google Scholar] [CrossRef]
- Del Amo, E.M.; Urtti, A. Rabbit as an animal model for intravitreal pharmacokinetics: Clinical predictability and quality of the published data. Exp. Eye Res. 2015, 137, 111–124. [Google Scholar] [CrossRef]
- Patel, S.S.; Janer, D.; Miller, B.; Ehrlich, J.S.; Perlroth, V.; Valazquez-Martin, J.P. Updated Results of Phase 1b Study of KSI-301, an Anti-VEGF Antibody Biopolymer Conjugate with Extended Durability, in wAMD, DME, and RVO. Investig. Ophthalmol. Vis. Sci. 2020, 61, 4286. Available online: https://iovs.arvojournals.org/article.aspx?articleid=2769586 (accessed on 29 September 2020).
- KODIAK. A Study to Evaluate the Efficacy and Safety of KSI-301, an Anti-VEGF Antibody Biopolymer Conjugate, versus Aflibercept in Patients with Neovascular (Wet) Age-Related Macular Degeneration. Available online: https://kodiak.com/ourpipeline/ (accessed on 29 September 2020).
- Rodrigues, G.A.; Mason, M.; Christie, L.-A.; Hansen, C.; Hernandez, L.M.; Burke, J.; Luhrs, K.A.; Hohman, T.C. Functional characterization of abicipar-pegol, an anti-VEGF DARPin therapeutic that potently inhibits angiogenesis and vascular permeability. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5836–5846. [Google Scholar] [CrossRef] [PubMed]
- Campochiaro, P.A.; Channa, R.; Berger, B.B.; Heier, J.S.; Brown, D.M.; Fiedler, U.; Hepp, J.; Stumpp, M.T. Treatment of diabetic macular edema with a designed ankyrin repeat protein that binds vascular endothelial growth factor: A phase I/II study. Am. J. Ophthalmol. 2013, 155, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Kunimoto, D.; Yoon, Y.H.; Wykoff, C.C.; Chang, A.; Khurana, R.N.; Maturi, R.K.; Agostini, H.; Souied, E.; Chow, D.R.; Lotery, A.J.; et al. Efficacy and Safety of Abicipar in Neovascular Age-Related Macular Degeneration. Ophthalmology 2020, 127, 1331–1344. [Google Scholar] [CrossRef]
- Bourges, J.L.; Bloquel, C.; Thomas, A.; Froussart, F.; Bochot, A.; Azan, F.; Gurny, R.; BenEzra, D.; Behar-Cohen, F. Intraocular implants for extended drug delivery: Therapeutic applications. Adv. Drug Deliv. Rev. 2006, 58, 1182–1202. [Google Scholar] [CrossRef] [PubMed]
- Haller, J.A.; Dugel, P.; Weinberg, D.V.; Chou, C.; Whitcup, S.M. Evaluation of the safety and performance of an applicator for a novel IVT dexamethasone drug delivery system for the treatment of macular edema. Retina 2009, 29, 46–51. [Google Scholar] [CrossRef]
- Lee, S.S.; Hughes, P.; Ross, A.D.; Robinson, M.R. Biodegradable implants for sustained drug release in the eye. Pharm. Res. 2010, 27, 2043–2053. [Google Scholar] [CrossRef]
- Haghjou, N.; Soheilian, M.; Abdekhodaie, M.J. Sustained release intraocular drug delivery devices for treatment of uveitis. J. Ophthal. Vis. Res. 2011, 6, 317–329. [Google Scholar]
- Chan, A.; Leung, L.-S. Critical appraisal of the clinical utility of the dexamethasone intravitreal implant (Ozurdex-R) for the treatment of macular edema related to branch retinal vein occlusion or central retinal vein occlusion. Clin. Ophthalmol. 2011, 5, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Kuppermann, B.D.; Patel, S.S.; Boyer, D.S.; Augustin, A.J.; Freeman, W.R.; Kerr, K.J.; Guo, Q.; Schneider, S.; López, F.J. Phase 2 study of the safety and efficacy of brimonidine drug delivery system (brimo dds) generation 1 in patients with geographic atrophy secondary to age-related macular degeneration. Retina 2021, 41, 144–155. [Google Scholar] [CrossRef]
- Freeman, W.R.; Bandello, F.; Souied, E.; Guymer, R.H.; Garg, S.J.; Chen, F.K.; Rich, R.; Holz, F.G.; Patel, S.S.; Kim, K.; et al. Randomized Phase IIb Study of Brimonidine Drug Delivery System Generation 2 for Geographic Atrophy in Age-Related Macular Degeneration. Ophthalmol. Retin. 2023, in press. [Google Scholar] [CrossRef]
- Jaffe, G.J.; McCallum, R.M.; Branchaud, B.; Skalak, C.; Butuner, Z.; Ashton, P. Long-term follow-up results of a pilot trial of a fluocinolone acetonide implant to treat posterior uveitis. Ophthalmology 2005, 112, 1192–1198. [Google Scholar] [CrossRef] [PubMed]
- Kuppermann, B.D.; Blumenkranz, M.S.; Haller, J.A.; Williams, G.A.; Weinberg, D.; Chou, C.; Whitcup, S.M. Randomized controlled study of an intravitreous dexamethasone drug delivery system in patients with persistent macular edema. Arch. Ophthalmol. 2007, 125, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Kane, F.E.; Burdan, J.; Cutino, A.; Green, K.E. Iluvien: A new sustained delivery technology for posterior eye disease. Expert Opin. Drug. Deliv. 2008, 5, 1039–1046. [Google Scholar] [CrossRef]
- Frokjaer, S.; Otzen, D.E. Protein drug stability: A formulation challenge. Nat. Rev. Drug Discov. 2005, 4, 298. [Google Scholar] [CrossRef] [PubMed]
- Vermonden, T.; Censi, R.; Hennink, W.E. Hydrogels for protein delivery. Chem. Rev. 2012, 112, 2853–2888. [Google Scholar] [CrossRef]
- Kirchhof, S.; Abrami, M.; Messmann, V.; Hammer, N.; Goepferich, A.M.; Grassi, M.; Brandl, F.P. Diels-alder hydrogels for controlled antibody release: Correlation between mesh size and release rate. Mol. Pharm. 2015, 12, 3358–3368. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Metters, A.T. hydrogels in controlled release formulations: Network design and mathematical modeling. Adv. Drug Deliv. Rev. 2006, 58, 1379–1408. [Google Scholar] [CrossRef] [PubMed]
- Lovett, M.L.; Wang, X.; Yucel, T.; York, L.; Keirstead, M.; Haggerty, L.; Kaplan, D.L. Silk hydrogels for sustained ocular delivery of anti-vascular endothelial growth factor (anti-VEGF) therapeutics. Eur. J. Pharm. Biopharm. 2015, 95, 271–278. [Google Scholar] [CrossRef]
- Yu, Y.; Lau, L.C.; Lo, A.C.; Chau, Y. Injectable chemically crosslinked hydrogel for the controlled release of bevacizumab in vitreous: A 6-month in vivo study. Transl. Vis. Sci. Technol. 2015, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Seah, I.; Zhao, X.; Lin, Q.; Liu, Z.; Su, S.Z.Z.; Yuen, Y.S.; Hunziker, W.; Lingam, G.; Loh, X.J.; Su, X. Use of biomaterials for sustained delivery of anti-VEGF to treat retinal diseases. Eye 2020, 34, 1341–1356. [Google Scholar] [CrossRef] [PubMed]
- Franssen, O.; Vandervennet, L.; Roders, P.; Hennink, W.E. Degradable dextran hydrogels: Controlled release of a model protein from cylinders and microspheres. J. Control. Release 1999, 60, 211–221. [Google Scholar] [CrossRef]
- Buwalda, S.J.; Bethry, A.; Hunger, S.; Kandoussi, S.; Coudane, J.; Nottelet, B. Ultrafast in situ forming poly(ethylene glycol)-poly(amido amine) hydrogels with tunable drug release properties via controllable degradation rates. Eur. J. Pharm. Biopharm. 2019, 139, 232–239. [Google Scholar] [CrossRef]
- Censi, R.; Vermonden, T.; van Steenbergen, M.J.; Deschout, H.; Braeckmans, K.; De Smedt, S.C.; van Nostrum, C.F.; di Martino, P.; Hennink, W.E. Photopolymerized thermosensitive hydrogels for tailorable diffusion-controlled protein delivery. J. Control. Release 2009, 140, 230–236. [Google Scholar] [CrossRef]
- Huang, X.; Brazel, C.S. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J. Control. Release 2001, 73, 121–136. [Google Scholar] [CrossRef]
- Yu, J.; Xu, X.; Yao, F.; Luo, Z.; Jin, L.; Xie, B.; Shi, S.; Ma, H.; Li, X.; Chen, H. In situ covalently cross-linked PEG hydrogel for ocular drug delivery applications. Int. J. Pharm. 2014, 470, 151–157. [Google Scholar] [CrossRef]
- Weber, L.M.; Lopez, C.G.; Anseth, K.S. Effects of PEG hydrogel crosslinking density on protein diffusion and encapsulated islet survival and function. J. Biomed. Mater. Res. Part A 2009, 90, 720–729. [Google Scholar] [CrossRef]
- Kirchhof, S.; Gregoritza, M.; Messmann, V.; Hammer, N.; Goepferich, A.M.; Brandl, F.P. Diels-Alder hydrogels with enhanced stability: First step toward controlled release of bevacizumab. Eur. J. Pharm. Biopharm. 2015, 96, 217–225. [Google Scholar] [CrossRef]
- Ikada, Y.; Tabata, Y. Protein release from gelatin matrices. Adv. Drug Deliv. Rev. 1998, 31, 287–301. [Google Scholar]
- Mellott, M.B.; Searcy, K.; Pishko, M.V. Release of protein from highly cross-linked hydrogels of poly(ethylene glycol) diacrylate fabricated by UV polymerization. Biomaterials 2001, 22, 929–941. [Google Scholar] [CrossRef]
- Oo, C.; Kalbag, S.S. Leveraging the attributes of biologics and small molecules, and releasing the bottlenecks: A new wave of revolution in drug development. Expert Rev. Clin. Pharmacol. 2016, 9, 747–749. [Google Scholar] [CrossRef]
- Zhang, J.; Muirhead, B.; Dodd, M.; Liu, L.; Xu, F.; Mangiacotte, N. An injectable hydrogel prepared using a PEG/Vitamin E copolymer facilitating aqueous-driven gelation. Biomacromolecules 2016, 17, 3648–3658. [Google Scholar] [CrossRef]
- Elhayek, R.F.; Jarrett, T.; Lattrell, Z.; Takach, S.; Jarrett, P.K.; McGrath, M.; Talamo, J.H.; Sawhney, A. Efficacy of a 6 month sustained hydrogel delivery system for Tyrosine kinase inhibitors in a VEGF induced retinal leakage model. ARVO Abstract. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1968. [Google Scholar]
- Lee, S.C.; Kwon, I.K.; Park, K. Hydrogels for delivery of bioactive agents: A historical perspective. Adv. Drug Deliv. Rev. 2013, 65, 17–20. [Google Scholar] [CrossRef]
- Turturro, S.B.; Guthrie, M.J.; Appel, A.A.; Drapala, P.W.; Brey, E.M.; Pérez-Luna, V.H.; Mieler, W.F.; Kang-Mieler, J.J. The effects of cross-linked thermo-responsive PNIPAAm-based hydrogel injection on retinal function. Biomaterials 2011, 32, 3620–3626. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Liang, Y.; Zhang, T.; Ma, P.X.; Guo, B. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials 2018, 183, 185–199. [Google Scholar] [CrossRef]
- Kushwaha, S.K.; Saxena, P.; Rai, A. Stimuli sensitive hydrogels for ophthalmic drug delivery: A review. Int. J. Pharm. Investig. 2012, 2, 54. [Google Scholar] [CrossRef]
- Thrimawithana, T.; Rupenthal, I.; Young, S.; Alany, R. Environment-sensitive polymers for ophthalmic drug delivery. J. Drug Deliv. Sci. Technol. 2012, 22, 117–124. [Google Scholar] [CrossRef]
- Tanihara, M.; Suzuki, Y.; Nishimura, Y.; Suzuki, K.; Kakimaru, Y. Thrombin-sensitive peptide linkers for biological signal-responsive drug release systems. Peptides 1998, 19, 421–425. [Google Scholar] [CrossRef]
- Shigemitsu, H.; Fujisaku, T.; Onogi, S.; Yoshii, T.; Ikeda, M.; Hamachi, I. Preparation of supramolecular hydrogel-enzyme hybrids exhibiting biomolecule-responsive gel degradation. Nat. Protoc. 2016, 11, 1744–1756. [Google Scholar] [CrossRef]
- Miyata, T.; Uragami, T.; Nakamae, K. Biomolecule-sensitive hydrogels. Adv. Drug Del. Rev. 2002, 54, 79–98. [Google Scholar] [CrossRef]
- Agrawal, A.K.; Das, M.; Jain, S. In situ gel systems as ‘smart’ carriers for sustained ocular drug delivery. Expert Opin. Drug Deliv. 2012, 9, 383–402. [Google Scholar] [CrossRef]
- Ruel-Gariépy, E.; Leroux, J.-C. In situ-forming hydrogels—Review of temperature-sensitive systems. Eur. J. Pharm. Biopharm. 2004, 58, 409–426. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, C.; Shen, W.; Cheng, Z.; Yu, L.; Ping, Q. Poly(N-isopropylacrylamide)–chitosan as thermosensitive In Situ gel-forming system for ocular drug delivery. J. Control. Release 2007, 120, 186–194. [Google Scholar] [CrossRef]
- Klouda, L. Thermoresponsive hydrogels in biomedical applications a seven-year update. Eur. J. Pharm. Biopharm. 2015, 97, 338–349. [Google Scholar] [CrossRef]
- Liu, Z.; Liow, S.S.; Lai, S.L.; Alli-Shaik, A.; Holder, G.E.; Parikh, B.H.; Krishnakumar, S.; Li, Z.; Tan, M.J.; Gunaratne, J.; et al. Retinal-detachment repair and vitreous-like-body reformation via a thermogelling polymer endotamponade. Nat. Biomed. Eng. 2019, 3, 598–610. [Google Scholar] [CrossRef]
- Drapala, P.W.; Brey, E.M.; Mieler, W.F.; Venerus, D.C.; Derwent, J.J.K.; Pérez-Luna, V.H. Role of thermo-responsiveness and poly (ethylene glycol) diacrylate cross-link density on protein release from poly(N-isopropylacrylamide) hydrogels. J. Biomater. Sci. Polym. Ed. 2011, 22, 59–75. [Google Scholar] [CrossRef]
- Drapala, P.W.; Jiang, B.; Chiu, Y.-C.; Mieler, W.F.; Brey, E.M.; Kang-Mieler, J.J.; Pérez-Luna, V.H. The effect of glutathione as chain transfer agent in PNIPAAm-based thermo-responsive hydrogels for controlled release of proteins. Pharm. Res. 2014, 31, 742–753. [Google Scholar] [CrossRef]
- Wang, C.H.; Hwang, Y.S.; Chiang, P.R.; Shen, C.R.; Hong, W.H.; Hsiue, G.H. Extended release of bevacizumab by thermosensitive biodegradable and biocompatible HG. Biomacromolecules 2012, 13, 40–48. [Google Scholar] [CrossRef]
- Park, D.; Shah, V.; Rauck, B.M.; Friberg, T.R.; Wang, Y. An antiangiogenic reverse thermal gel as a drug-delivery system for agerelated wet macular degeneration. Macromol. Biosci. 2013, 13, 464–469. [Google Scholar] [CrossRef]
- Rauck, B.M.; Friberg, T.R.; Medina Mendez, C.A.; Park, D.; Shah, V.; Bilonick, R.A.; Wang, Y. Biocompatible reverse thermal gel sustains the release of intravitreal bevacizumab in vivo. Investig. Ophthalmol. Vis. Sci. 2014, 55, 469–476. [Google Scholar] [CrossRef]
- Xie, B.; Jin, L.; Luo, Z.; Yu, J.; Shi, S.; Zhang, Z.; Shen, M.; Chen, H.; Li, X.; Song, Z. An injectable thermosensitive polymeric hydrogel for sustained release of Avastin(R) to treat posterior segment disease. Int. J. Pharm. 2015, 490, 375–383. [Google Scholar] [CrossRef]
- López-Cano, J.J.; Sigen, A.; Andrés-Guerrero, V.; Tai, H.; Bravo-Osuna, I.; Molina-Martínez, I.T.; Wang, W.; Herrero-Vanrell, R. Thermo-responsive PLGA-PEG-PLGA hydrogels as novel injectable platforms for neuroprotective combined therapies in the treatment of retinal degenerative diseases. Pharmaceutics 2021, 13, 234. [Google Scholar] [CrossRef]
- Xue, K.; Zhao, X.; Zhang, Z.; Qiu, B.; Tan, Q.S.W.; Ong, K.H.; Liu, Z.; Parikh, B.H.; Barathi, V.A.; Yu, W.; et al. Sustained delivery of anti-VEGFs from thermogel depots inhibits angiogenesis without the need for multiple injections. Biomater. Sci. 2019, 7, 4603–4614. [Google Scholar] [CrossRef]
- Moritera, T.; Ogura, Y.; Hondo, Y.; Wadaf, R.; Hyoaf, S.-H.; Ikadaf, Y. Microspheres of biodegradable polymers as a drug-delivery system in the vitreous. Investig. Ophthalmol. Vis. Sci. 1991, 32, 1785–1790. [Google Scholar]
- Buwalda, S.J.; Vermonden, T.; Hennink, W.E. Hydrogels for therapeutic delivery: Current developments and future directions. Biomacromolecules 2017, 18, 316–330. [Google Scholar] [CrossRef]
- Kang-Mieler, J.J.; Dosmar, E.; Liu, W.; Mieler, W.F. Extended ocular drug delivery systems for the anterior and posterior segments: Biomaterial options and applications. Expert Opin. Drug Deliv. 2017, 14, 611–620. [Google Scholar] [CrossRef]
- Kompella, U.B.; Amrite, A.C.; Ravi, R.P.; Durazo, S.A. Nanomedicines for back of the eye drug delivery, gene delivery, and imaging. Prog. Retin. Eye Res. 2013, 36, 172–198. [Google Scholar] [CrossRef]
- Sepahvandi, A.; Eskandari, M.; Moztarzadeh, F. Drug Delivery Systems to the Posterior Segment of the Eye: Implants and Nanoparticles. BioNanoScience 2016, 6, 276–283. [Google Scholar] [CrossRef]
- Li, F.; Hurley, B.; Liu, Y.; Leonard, B.; Griffith, M. Controlled Release of Bevacizumab through Nanospheres for Extended Treatment of Age-Related Macular Degeneration. Open Ophthalmol. J. 2012, 6, 54–58. [Google Scholar] [CrossRef]
- Osswald, C.R.; Kang-Mieler, J.J. Controlled and extended release of a model protein from a microsphere-hydrogel drug delivery system. Ann. Biomed. Eng. 2015, 43, 2609–2617. [Google Scholar] [CrossRef]
- Osswald, C.R.; Kang-Mieler, J.J. Controlled and extended in vitro release of bioactive anti-vascular endothelial growth factors from a microsphere-hydrogel drug delivery system. Curr. Eye Res. 2016, 41, 1216–1222. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Lee, B.S.; Mieler, W.F.; Kang-Mieler, J.J. Biodegradable microsphere-hydrogel ocular drug delivery system for controlled and extended release of bioactive aflibercept in vitro. Curr. Eye Res. 2019, 44, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Borrell, M.A.; Venerus, D.C.; Mieler, W.F.; Kang-Mieler, J.J. Characterization of biodegradable microsphere-hydrogel ocular drug delivery system for controlled and extended release of ranibizumab. Transl. Vis. Sci. Technol. 2019, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Osswald, C.R.; Guthrie, M.J.; Avila, A.; Valio, J.A., Jr.; Mieler, W.F.; Kang-Mieler, J.J. In vivo efficacy of an injectable microsphere hydrogel ocular drug delivery system. Curr. Eye Res. 2017, 42, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.C.; Chaw, J.R.; Chen, C.F.; Liu, H.W. Controlled release bevacizumab in thermoresponsive hydrogel found to in-hibit angiogenesis. Bio-Med. Mater. Eng. 2014, 24, 1941–1950. [Google Scholar] [CrossRef]
- Hu, C.C.; Chaw, J.R.; Chen, Y.C.; Chen, C.F.; Liu, H.W. A novel thermo-responsive nanogel for intraocular drug delivery. J. Comput. Theory Nanosci. 2015, 12, 762–768. [Google Scholar] [CrossRef]
- Hu, C.C.; Chiu, Y.C.; Chaw, J.R.; Chen, C.F.; Liu, H.W. Thermo-responsive hydrogel as an anti-VEGF drug delivery system to inhibit retinal angiogenesis in Rex rabbits. Technol. Health Care 2019, 27, 153–163. [Google Scholar] [CrossRef]
- Pachis, K.; Blazaki, S.; Tzatzarakis, M.; Klepetsanis, P.; Naoumidi, E.; Tsilimbaris, M.; Antimisiaris, S.G. Sus-tained release of intravitreal flurbiprofen from a novel drug-in-liposome-in-hydrogel formulation. Eur. J. Pharm. Sci. 2017, 109, 324–333. [Google Scholar] [CrossRef]
- Sapino, S.; Peira, E.; Chirio, D.; Chindamo, G.; Guglielmo, S.; Oliaro-Bosso, S.; Barbero, R.; Vercelli, C.; Re, G.; Brunella, V.; et al. Thermosensitive Nanocomposite hydrogels for intravitreal Delivery of Cefuroxime. Nanomaterials 2019, 9, 1461. [Google Scholar] [CrossRef]
- Bardini, R.; Di Carlo, S. Computational modeling and optimization of biofabrication in Tissue Engineering and Regenerative Medicine—A literature review. bioRxiv 2023. [Google Scholar] [CrossRef]
- Forero-Doria, O.; Polo, E.; Marican, A.; Guzmán, L.; Venegas, B.; Vijayakumar, S.; Wehinger, S.; Guerrero, M.; Gallego, J.; Durán-Lara, E.F. Supramolecular hydrogels based on cellulose for sustained release of therapeutic substances with antimicrobial and wound healing properties. Carbohydr. Polym. 2020, 242, 116383. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Salas, F.; Marican, A.; Pinochet, S.; Carreño, G.; Valdés, O.; Venegas, B.; Donoso, W.; Cabrera-Barjas, G.; Vijayakumar, S.; Durán-Lara, E.F. Film dressings based on hydrogels: Simultaneous and sustained-release of bioactive compounds with wound healing properties. Pharmaceutics 2019, 11, 447. [Google Scholar] [CrossRef] [PubMed]
- Valdes, O.; Ávila-Salas, F.; Marican, A.; Fuentealba, N.; Villasenor, J.; Arenas-Salinas, M.; Argandoña, Y.; Durán-Lara, E.F. Methamidophos removal from aqueous solutions using a super adsorbent based on crosslinked poly (vinyl alcohol) hydrogel. J. Appl. Polym. Sci. 2018, 135, 45964. [Google Scholar] [CrossRef]
- Tripathi, V.K.; Dasgupta, B.; Deb, K. A computational method for viewing molecular interactions in docking. Lect. Notes Comput. Sci. 2007, 4370, 152. [Google Scholar]
- Rezac, J.; Hobza, P. Advanced corrections of hydrogen bonding and dispersion for semiempirical quantum mechanical methods. J. Chem. Theory Comput. 2012, 8, 141–151. [Google Scholar] [CrossRef]
- Carvalho Martins, L.; Cino, E.A.; Ferreira, R.S. PyAutoFEP: An automated free energy perturbation workflow for GROMACS integrating enhanced sampling methods. J. Chem. Theory Comput. 2021, 17, 4262–4273. [Google Scholar] [CrossRef]
- Rafael, D.; Montero, S.; Carcavilla, P.; Andrade, F.; German-Cortés, J.; Diaz-Riascos, Z.V.; Seras-Franzoso, J.; Llaguno, M.; Fernández, B.; Pereira, A.; et al. Intracellular Delivery of Anti-Kirsten Rat Sarcoma Antibodies Mediated by Polymeric Micelles Exerts Strong In Vitro and In Vivo Anti-Tumorigenic Activity in Kirsten Rat Sarcoma-Mutated Cancers. ACS Appl. Mater. Interfaces 2023, 15, 10398–10413. [Google Scholar] [CrossRef]
- Marican, A.; Avila-Salas, F.; Valdés, O.; Wehinger, S.; Villaseñor, J.; Fuentealba, N.; Arenas-Salinas, M.; Argandoña, Y.; Carrasco-Sánchez, V.; Durán-Lara, E.F. Rational design, synthesis and evaluation of γ-CD-containing cross-linked polyvinyl alcohol hydrogel as a prednisone delivery platform. Pharmaceutics 2018, 10, 30. [Google Scholar] [CrossRef]
- Avila-Salas, F.; Rodriguez Nuñez, Y.A.; Marican, A.; Castro, R.I.; Villaseñor, J.; Santos, L.S.; Wehinger, S.; Durán-Lara, E.F. Rational development of a novel hydrogel as a pH-sensitive controlled release system for nifedipine. Polymers 2018, 10, 806. [Google Scholar] [CrossRef]
- Pereira, A.; Valdés-Muñoz, E.; Marican, A.; Cabrera-Barjas, G.; Vijayakumar, S.; Valdés, O.; Rafael, D.; Andrade, F.; Abaca, P.; Bustos, D.; et al. Rational Design of hydrogels for Cationic Antimicrobial Peptide Delivery: A Molecular Modeling Approach. Pharmaceutics 2023, 15, 474. [Google Scholar] [CrossRef]
- Carreño, G.; Pereira, A.; Ávila-Salas, F.; Marican, A.; Andrade, F.; Roca-Melendres, M.M.; Valdés, O.; Vijayakumar, S.; Schwartz, S., Jr.; Abasolo, I.; et al. Development of “on-demand” thermo-responsive hydrogels for anti-cancer drugs sustained release: Rational design, in silico prediction and in vitro validation in colon cancer models. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 131, 112483. [Google Scholar] [CrossRef] [PubMed]
- Avila-Salas, F.; Marican, A.; Villaseñor, J.; Arenas-Salinas, M.; Argandoña, Y.; Caballero, J.; Durán-Lara, E.F. In-Silico Design, Synthesis and Evaluation of a Nanostructured hydrogel as a Dimethoate Removal Agent. Nanomaterials 2018, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.; Avila-Salas, F.; Santos, L.S.; Iturmendi, N.; Moine, V.; Cheynier, V.; Saucier, C. Rosé wine fining using polyvinylpolypyrrolidone: Colorimetry, targeted polyphenomics, and molecular dynamics simulations. J. Agric. Food Chem. 2017, 65, 10591–10597. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services. Final Guidance on “Use of International Standard ISO 10993-1, Biological Evaluation of Medical Devices—Part 1: Evaluation and Testing within a Risk Management Process; Food & Drug Administration: Silver Spring, MD, USA, 2016. [Google Scholar]
- Abud, M.; Baranov, P.; Hicks, C.; Patel, S.; Lieppman, B.; Regatieri, C.; Sinden, J.; Isaac, D.; Avila, M.; Young, M. The Effect of Transient Local Anti-inflammatory Treatment on the Survival of Pig Retinal Progenitor Cell Allotransplants. Transl. Vis. Sci. Technol. 2015, 4, 6. [Google Scholar] [CrossRef] [PubMed]
- Rong, X.; Ji, Y.; Zhu, X.; Yang, J.; Qian, D.; Mo, X.; Lu, Y. Neuroprotective effect of insulin-loaded chitosan nanoparticles/PLGA-PEG-PLGA hydrogel on diabetic retinopathy rats. Int. J. Nanomed. 2019, 14, 45. [Google Scholar] [CrossRef] [PubMed]

| Disease Indication | Drug/Brand Name | Drug Type | Mode of Action | Administration Route |
|---|---|---|---|---|
| AMD (wet) Diabetic retinopathy | Aflibercept Eylea | Fusion protein | Anti-VEGF | Monthly IVT injection |
| Bevacizumab * Avastin | Monoclonal antibody | |||
| Brolucizumab Beovu | Single chain humanized antibody fragment (scFV) | |||
| Faricimab Faricimab-svoa | Bispecific IgG1 antibody | |||
| Pegaptanib Macugen | Pegylated aptamer | |||
| Ranibizumab Lucentis | Monoclonal antibody fragment (Fab) | |||
| Laser photocoagulation | - | Destruction of abnormal blood vessels | Laser surgery | |
| Diabetic macula edema Posterior uveitis | Dexamethasone Ozurdex | Corticosteroid | Anti-inflammatory | Slow-release implant |
| Fluocinolone acetonide Iluvien | ||||
| Fluocinolone acetonide Retisert | ||||
| AMD (dry) | Pegcetacoplan SYFOVRE | Pegylated peptide | Complement inhibitor (C3) | Monthly IVT injection |
| Avacincaptad pegol ** Zimura | Pegylated aptamer | Complement inhibitor (C5) | ||
| IRD (biallelic RPE65) | Voretigene neparvovec Luxterna | Gene replacement therapy | RPE65 replacement | Subretinal injection AAV2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rafael, D.; Guerrero, M.; Marican, A.; Arango, D.; Sarmento, B.; Ferrer, R.; Durán-Lara, E.F.; Clark, S.J.; Schwartz, S., Jr. Delivery Systems in Ocular Retinopathies: The Promising Future of Intravitreal Hydrogels as Sustained-Release Scaffolds. Pharmaceutics 2023, 15, 1484. https://doi.org/10.3390/pharmaceutics15051484
Rafael D, Guerrero M, Marican A, Arango D, Sarmento B, Ferrer R, Durán-Lara EF, Clark SJ, Schwartz S Jr. Delivery Systems in Ocular Retinopathies: The Promising Future of Intravitreal Hydrogels as Sustained-Release Scaffolds. Pharmaceutics. 2023; 15(5):1484. https://doi.org/10.3390/pharmaceutics15051484
Chicago/Turabian StyleRafael, Diana, Marcelo Guerrero, Adolfo Marican, Diego Arango, Bruno Sarmento, Roser Ferrer, Esteban F. Durán-Lara, Simon J. Clark, and Simo Schwartz, Jr. 2023. "Delivery Systems in Ocular Retinopathies: The Promising Future of Intravitreal Hydrogels as Sustained-Release Scaffolds" Pharmaceutics 15, no. 5: 1484. https://doi.org/10.3390/pharmaceutics15051484







