Medical Device Development for Children and Young People—Reviewing the Challenges and Opportunities
Abstract
:1. Introduction
- diagnosis, prevention, monitoring, prediction, prognosis, treatment or alleviation of disease,
- diagnosis, monitoring, treatment, alleviation of, or compensation for, an injury or disability,
- investigation, replacement or modification of the anatomy or of a physiological or pathological process or state,
- providing information by means of in-vitro examination of specimens derived from the human body, including organ, blood and tissue donations,
- and which does not achieve its principal intended action by pharmacological, immunological or metabolic means, in or on the human body, but which may be assisted in its function by such means.
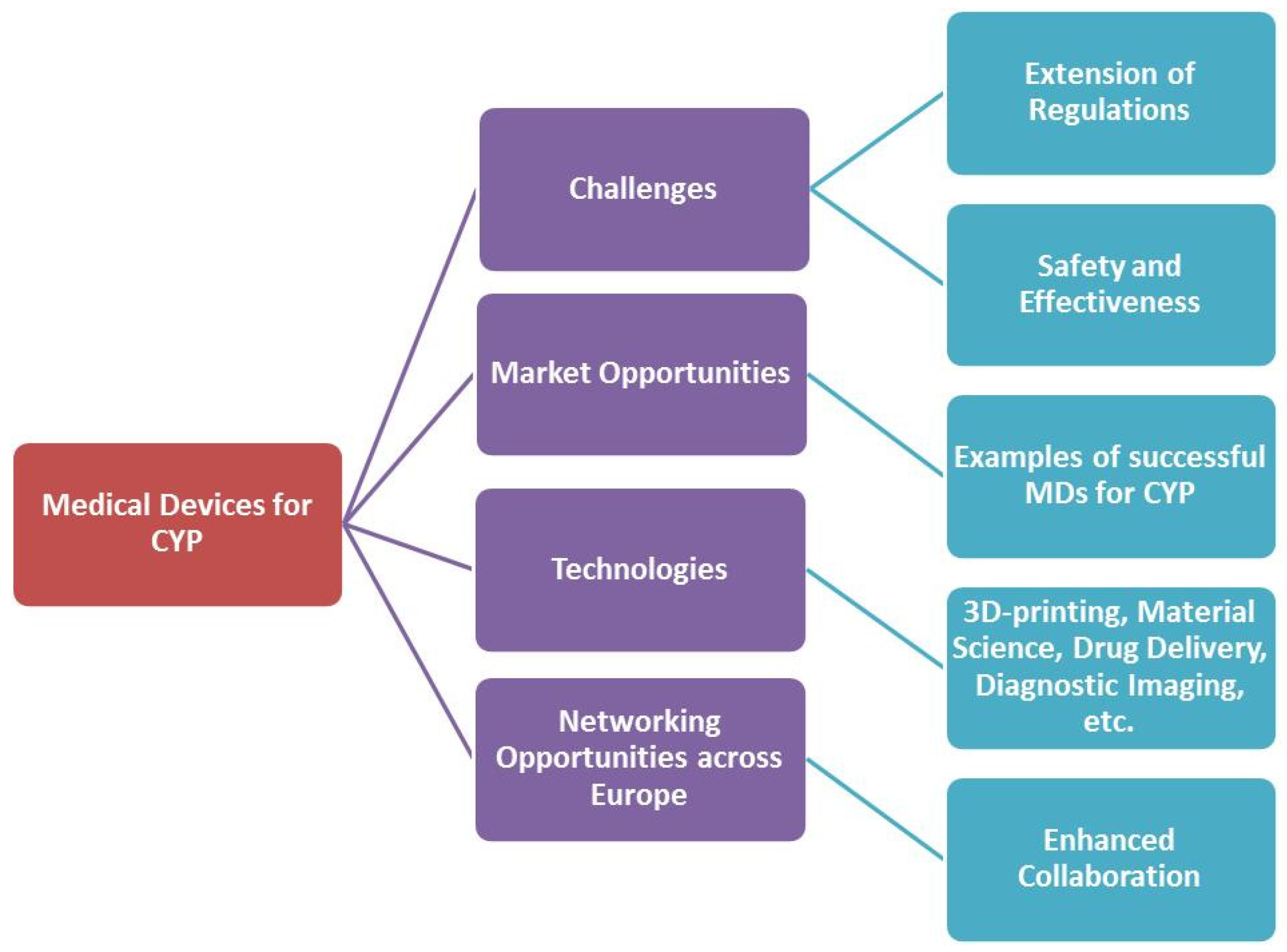
2. The Complexities in the Development of Medical Devices for Children
3. Addressing the Market Need for Pediatric Medical Devices
4. Addressing the Regulatory Needs for Pediatric MDs
5. 3D Printing for Pediatric MD
6. New Materials for Pediatric Medical Devices
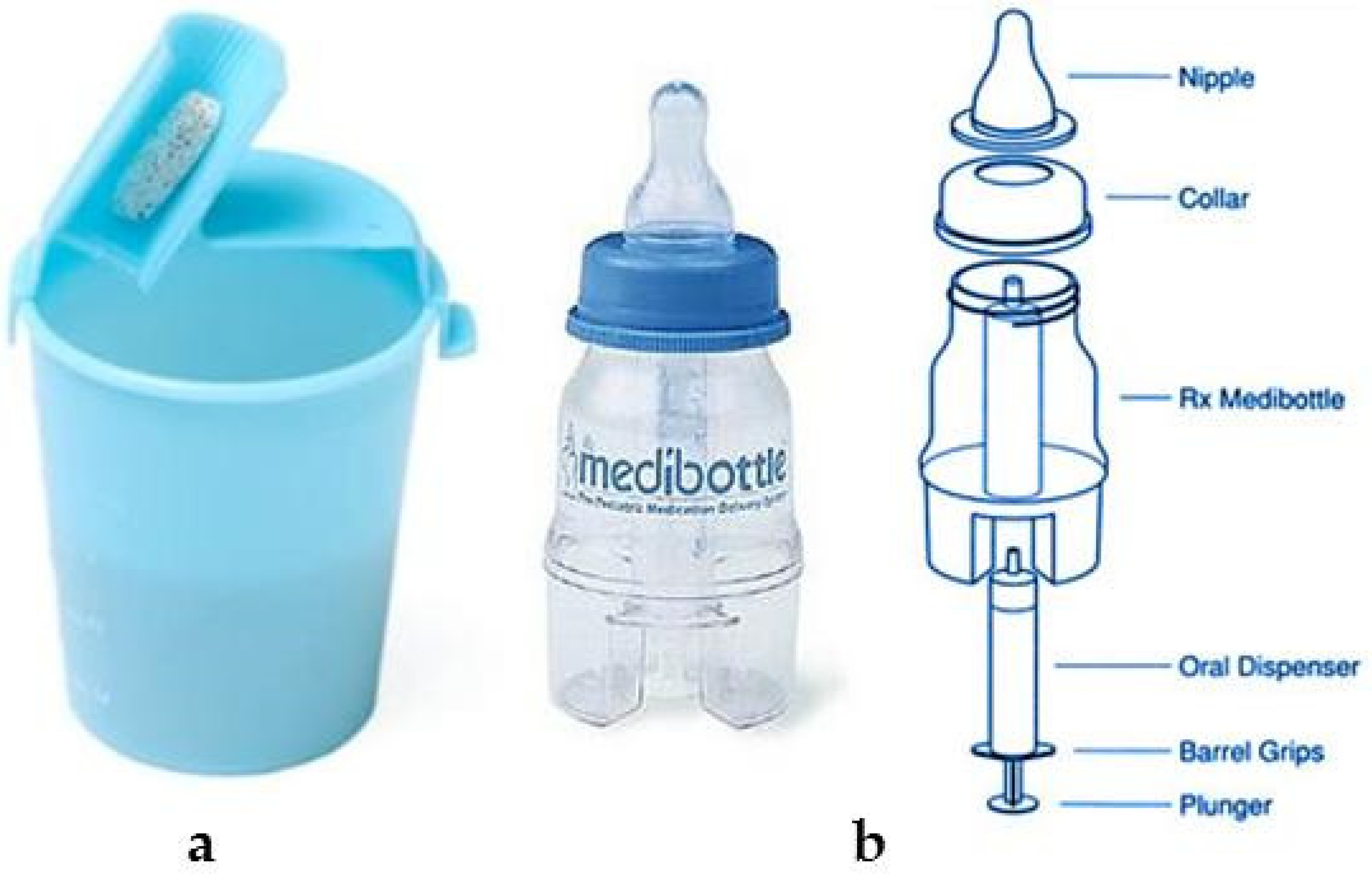
7. Delivery Devices for the Administration of Pediatric Formulations
8. Diagnostic Imaging Devices for CYP
9. Patient and Public Involvement in Medical Devices’ Research
10. Towards a European Infrastructure to Support Pediatric Medical Device Development
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Medical Device Market Size, Share &COVID-19 Impact Analysis, By Type (Orthopedic, Devices, Cardiovascular Devices, Diagnostic Imaging, In-Vitro Diagnostics, Minimally Invasive Surgery, Wound Management, Diabetes Care, Ophthalmic Devices, Dental Devices, Nephrology, General Surgery, and Others); By End User (Hospital & ASC’s, Clinical and Others), and Regional Forecast 2021–2028; Fortune Business Insights. Available online: https://www.fortunebusinessinsights.com/industry-reports/medical-devices-market-100085 (accessed on 30 July 2021).
- European Parliament and Council. Regulation (EU) 2017/745 of 5 April 2017 on Medical Devices, Amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and Repealing Council Directives 90/385/EEC and 93/42/EEC. 2017. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32017R0745 (accessed on 30 July 2021).
- Takahashi, S.; Iwasaki, K.; Shirato, H.; Ho, M.; Umezu, M. Comparison of supportive regulatory measures for pediatric medical device development in Japan and the United States. J. Artif. Organs. 2021, 24, 90–101. [Google Scholar] [CrossRef]
- Humes, H.D.; Westover, A.J. Experience with Pediatric Medical Device Development. Front. Pediatr. 2020, 8, 79. [Google Scholar] [CrossRef] [Green Version]
- Samuels-Reid, J.H.; Cope, J.U. Medical devices and the paediatric population—A head-to-toe approach. Expert Rev. Med. Devices 2019, 16, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Chamley, C.; Carson, P.; Randall, D.; Sandwell, W. Developmental Anatomy and Physiology of Children—A Practical Approach, 1st ed.; Elsevier Churchill Livingstone: London, UK, 2005. [Google Scholar]
- The Royal Children’s Hospital Melbourne. Acceptable Ranges for Physiological Variables. Available online: https://www.rch.org.au/clinicalguide/guideline_index/Normal_Ranges_for_Physiological_Variables/ (accessed on 23 November 2021).
- Willox, M.; Metherall, P.; Jeays-Ward, K.; McCarthy, A.D.; Barker, N.; Reed, H.; Elphick, H.E. Custom-made 3D printed masks for children using non-invasive ventilation: A feasibility study of production method and testing of outcomes in adult volunteers. J. Med. Eng. Technol. 2020, 44, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Vijayavenkataraman, S.; Fuh, J.Y.H.; Lu, W.F. 3D Printing and 3D Bioprinting in Paediatrics. Bioengineering 2017, 4, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aimar, A.; Palermo, A.; Innocenti, B. The Role of 3D Printing in Medical Applications: A State of the Art. J. Healthc. Eng. 2019, 2019, 5340616. [Google Scholar] [CrossRef] [Green Version]
- European Union Agency for Fundamental Rights. UNICEF and Youth Policy Labs, Age Matters, Final Report. 2016. Available online: https://www.unicef.org/bulgaria/sites/unicef.org.bulgaria/files/2018-09/Age_Matters_Summary_ENG.pdf (accessed on 15 October 2021).
- World Health Organisation, Regional Office for Europe. Fact Sheets on Sustainable Development Goals: Health Targets. 2018. Available online: https://www.euro.who.int/__data/assets/pdf_file/0017/348011/Fact-sheet-SDG-Mental-health-UPDATE-02-05-2018.pdf (accessed on 15 October 2021).
- Vorhaus, J.; Duckworth, K.; Budge, D.; Feinstein, L. The Social and Personal Benefits of Learning: A Summary of Key Research Findings; Centre for Research on the Wider Benefits of Learning, Institute of Education, University of London: London, UK, 2008. [Google Scholar]
- Brooks, F.; Magnusson, J.; Klemera, E.; Chester, K.; Spencer, N. HBSC England National Report: World Health Organization Collaborative Cross National Study; CRIPACC: Hatfield, UK, 2011. [Google Scholar]
- Johnson, R.C.; Schoeni, R.F. Early-life origins of adult disease: National longitudinal population-based study of the United States. Am. J. Public Health 2011, 101, 2317–2324. [Google Scholar] [CrossRef] [PubMed]
- Hertzman, C. The Biological Embedding of Early Experience and Its Effects on Health in Adulthood. Ann. N. Y. Acad. Sci. 1999, 896, 85–95. [Google Scholar] [CrossRef]
- Forrest, C.B.; Riley, A.W. Childhood origins of adult health: A basis for life-course health policy. Health Aff. 2004, 23, 155–164. [Google Scholar] [CrossRef] [Green Version]
- Dimitri, P. Child health technology: Shaping the future of paediatrics and child health and improving NHS productivity. Arch. Dis. Child. 2019, 104, 184–188. [Google Scholar] [CrossRef]
- Vermeulen, E.; Karsenberg, K.; van der Lee, J.H.; de Wildt, S.N. Involve Children and Parents in Clinical Studies. Clin. Transl. Sci. 2020, 13, 11–13. [Google Scholar] [CrossRef]
- Paediatric Healthcare Market by Type (Chronic Illness and Acute Illness), by Indication (Asthma and Allergies, Metabolic Disorders, Cardiac Disorders, Genetic Disorders, and Others), and by Treatment (Immunotherapy, Medications, Surgeries, and Others): Global Industry Perspective, Comprehensive Analysis, and Forecast, 2018–2025; Zion Market Research. Available online: https://www.zionmarketresearch.com/report/paediatric-healthcare-market (accessed on 30 July 2021).
- Bruneel, J.; D’Este, P.; Salter, A. Investigating the factors that diminish the barriers to university—Industry collaboration. Res. Policy 2010, 39, 858–868. [Google Scholar] [CrossRef]
- Tartari, V.; Salter, A.; D’Este, P. Crossing the Rubicon: Exploring the factors that shape academics’ perceptions of the barriers to working with industry. Camb. J. Econ. 2012, 36, 655–677. [Google Scholar] [CrossRef] [Green Version]
- Dias, C.; Escoval, A. The open nature of innovation in the hospital sector: The role of external collaboration networks. Health Policy Technol. 2012, 1, 181–186. [Google Scholar] [CrossRef]
- European Council. Directive 93/42/EEC of 14 June 1993 concerning Medical Device. 1993. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex:31993L0042 (accessed on 30 July 2021).
- European Council. Directive 90/385/EEC of 20 June 1990 on the Approximation of the Laws of the Member States Relating to Active Implantable Medical Devices. 1990. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex:31990L0385 (accessed on 30 July 2021).
- European Parliament and Council. Directive 98/79/EC of 27 October 1998 on In Vitro Diagnostic Medical Devices. 1998. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A31998L0079 (accessed on 30 July 2021).
- FDA. Premarket Assessment of Paediatric Medical Devices. Guidance for Industry and Food and Drug Administration Staff. 2014. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/premarket-assessment-paediatric-medical-devices (accessed on 30 July 2021).
- European Commission. Guidance MEDDEVs. 2020. Available online: https://ec.europa.eu/health/sites/health/files/md_sector/docs/md_guidance_meddevs.pdf (accessed on 30 July 2021).
- European Commission. MDCG Endorsed Documents. 2020. Available online: https://ec.europa.eu/health/md_sector/new_regulations/guidance_en (accessed on 30 July 2021).
- European Commission. Commission Implementing Decision (EU) 2020/437 of 24 March 2020 on the Harmonised Standards for Medical Devices Drafted in Support of Council Directive 93/42/EEC. 2020. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32020D0437&from=EN (accessed on 30 July 2021).
- Redaelli, D.F.; Abbate, V.; Storm, F.A.; Ronca, A.; Sorrentino, A.; De Capitani, C.; Biffi, E.; Ambrosio, L.; Colombo, G.; Fraschini, P. 3D printing orthopedic scoliosis braces: A test comparing FDM with thermoforming. Int. J. Adv. Manuf. Technol. 2020, 111, 1707–1720. [Google Scholar] [CrossRef]
- Redaelli, D.F.; Biffi, E.; Colombo, G.; Fraschini, P.; Reni, G. Current and Future Manufacturing of Chest Orthoses, Considering the Case of Osteogenesis Imperfecta. In Proceedings of the ASME 2018 38th International Design Engineering Technical Conferences and Computers and Information in Engineering Conference, Quebec City, QC, Canada, 26–29 August 2018; Volume 1B. [Google Scholar]
- Sukanya, V.S.; Panigrahy, N.; Rath, S.N. Recent approaches in clinical applications of 3D printing in neonates and paediatrics. Eur. J. Pediatr. 2021, 180, 323–332. [Google Scholar]
- Williams, L.; Fan, K.; Bentley, R. Titanium cranioplasty in children and adolescents. J. Cranio-Maxillofac. Surg. 2016, 44, 789–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazy, D.; Elbaum, R.; Beckers, G.; Matriche, C.; Vannieuwenhove, O. Orthopaedic support with 3D printing in children: Marketing effect or solution of the future? Acta Orthop. Belg. 2020, 86, 378–382. [Google Scholar] [PubMed]
- Banks, J. Adding Value in Additive Manufacturing: Researchers in the United Kingdom and Europe Look to 3D Printing for Customization. IEEE Pulse 2013, 4, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Printing—Prosthetic Kids Hand Challenge. Available online: http://www.handchallenge.com/printing.html (accessed on 30 July 2021).
- Norman, J.; Madurawe, R.D.; Moore, C.M.V.; Khan, M.A.; Khairuzzaman, A. A new chapter in pharmaceutical manufacturing: 3D-printed drug products. Adv. Drug Deliv. Rev. 2017, 108, 39–50. [Google Scholar] [CrossRef]
- Xu, X.; Awad, A.; Robles-Martinez, P.; Gaisford, S.; Goyanes, A.; Basit, A.W. Vat photopolymerization 3D printing for advanced drug delivery and medical device applications. J. Control. Release 2021, 329, 743–757. [Google Scholar] [CrossRef]
- Degerli, Y.I.; Dogu, F.; Oksuz, C. Manufacturing an assistive device with 3D printing technology—A case report. Assist. Technol. 2020, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.F. A model of biocompatibility and its evaluation. J. Biomed. Eng. 1989, 11, 185–191. [Google Scholar] [CrossRef]
- Morrison, R.; Kashlan, K.N.; Flanangan, C.L.; Wright, J.K.; Green, G.E.; Hollister, S.; Weatherwax, K.J. Regulatory Considerations in the Design and Manufacturing of Implantable 3D-Printed Medical Devices. Clin. Transl. Sci. 2015, 8, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Michanetzis, G.P.A.K.; Missirlis, Y.F.; Antimisiaris, S.G. Haemocompatibility of Nanosized Drug Delivery Systems: Has It Been Adequately Considered? J. Biomed. Nanotechnol. 2008, 4, 218–233. [Google Scholar] [CrossRef]
- Wang, C.; Hefflin, B.; Cope, J.U.; Gross, T.P.; Ritchie, M.B.; Qi, Y.; Chu, J. Emergency Department Visits for Medical Device-Associated Adverse Events Among Children. Pediatrics 2010, 126, 247–259. [Google Scholar] [CrossRef] [Green Version]
- Beekman, R.H., III; Duncan, B.W.; Hagler, D.J.; Jones, T.K.; Kugler, J.D.; Moore, J.W.; Jenkins, K.J. Workgroup on Paediatric Cardiac Devices, Section on Cardiology and Cardiac Surgery, American Academy of Paediatrics. Pathways to approval of paediatric cardiac devices in the United States: Challenges and solutions. Pediatrics 2009, 124, e155–e162. [Google Scholar] [CrossRef] [PubMed]
- Grun, N.G.; Holweg, P.L.; Donohue, N.; Klestil, T.; Weinberg, A.M. Resorbable implants in paediatric fracture. Treat. Innov. Surg. Sci. 2018, 3, 119–125. [Google Scholar]
- Caffarel-Salvador, E.; Tuan-Mahmood, T.-M.; McElnay, J.C.; McCarthy, H.O.; Mooney, K.; Woolfson, A.; Donnelly, R.F. Potential of hydrogel-forming and dissolving microneedles for use in paediatric populations. Int. J. Pharm. 2015, 489, 158–169. [Google Scholar] [CrossRef]
- Committee for Medicinal Products for Human Use (CHMP). Reflection Paper: Formulations of Choice for the Paediatric Population. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-formulations-choice-paediatric-population_en.pdf (accessed on 30 July 2021).
- Thomson, S.A.; Tuleu, C.; Wong, I.C.K.; Keady, S.; Pitt, K.G.; Sutcliffe, A. Minitablets: New Modality to Deliver Medicines to Preschool-Aged Children. Pediatrics 2009, 123, e235–e238. [Google Scholar] [CrossRef]
- OralfloTM. The Pill Swallowing Cup. Available online: https://oralflo.com/ (accessed on 30 July 2021).
- XStraw by DS Technology. Available online: https://d-s.technology/xstraw/ (accessed on 30 July 2021).
- Walsh, J.; Bickmann, D.; Breitkreutz, J.; Chariot-Goulet, M.; the European Paediatric Formulation Initiative (EuPFI). Delivery devices for the administration of paediatric formulations: Overview of current practice, challenges and recent developments. Int. J. Pharm. 2011, 415, 221–231. [Google Scholar] [CrossRef]
- Medibottle. Available online: http://www.medibottle.com/home.html (accessed on 30 July 2021).
- Easypod® Features. Available online: https://www.easypod.ca/en/index.html (accessed on 30 July 2021).
- PharmaJet. Available online: https://pharmajet.com/ (accessed on 30 July 2021).
- Freitag, F.G. Sumatriptan needle-free subcutaneous (Sumavel® DosePro™) approved for the acute treatment of migraine, with or without aura, and cluster headaches. Expert Rev. Neurother. 2011, 11, 481–490. [Google Scholar] [CrossRef]
- Kumar, R.B.; Rahman, Z.U. Needle free injection systems. IJPSR 2013, 4, 132–147. [Google Scholar]
- Maahs, D.M.; Horton, L.A.; Chase, H.P. The Use of Insulin Pumps in Youth with Type 1 Diabetes. Diabetes Technol. Ther. 2010, 12 (Suppl. S1), 59–65. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, J.; Hovorka, R. Benefits and Challenges of Current Closed-Loop Technologies in Children and Young People with Type 1 Diabetes. Front. Pediatr. 2021, 9, 679484. [Google Scholar] [CrossRef]
- Kwok, P.C.L.; Chan, H.-K. Delivery of inhalation drugs to children for asthma and other respiratory diseases. Adv. Drug Deliv. Rev. 2014, 73, 83–88. [Google Scholar] [CrossRef] [Green Version]
- Kamin, W.; Kreplin, A. Teaching the inhalation manoeuvre to asthmatic children by means of visual feedback. Pneumologie 2007, 61, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Smaldone, G.C.; Berg, E.; Nikander, K. Variation in paediatric aerosol delivery: Importance of facemask. J. Aerosol Med. 2005, 18, 354–363. [Google Scholar] [CrossRef]
- PARI. eFlow Rapid Nebuliser System. Available online: https://www.pari.com/int/products/inhalation-devices-for-the-lungs/eflow-rapid-nebuliser-system-3/ (accessed on 13 October 2021).
- Kraemer, R. Babyhaler—A new paediatric aerosol device. J. Aerosol Med. 1995, 8, S19–S26. [Google Scholar] [CrossRef]
- PARI. VORTEX Holding Chamber. Available online: https://www.pari.com/int/products/vortex-holding-chamber/ (accessed on 30 July 2021).
- Luessen, H.L. Pulmonary Drug Delivery: Achievements, Trends and Opportunities; OnDrugDelivery Ltd.: East Sussex, UK, 2007; pp. 3–5. [Google Scholar]
- Watt, P.M.; Clements, B.; Devadason, S.G.; Chaney, G.M. Funhaler spacer: Improving adherence without compromising delivery. Arch. Dis. Child. 2003, 88, 579–581. [Google Scholar] [CrossRef]
- Blake, K.V. Improving adherence to asthma medications: Current knowledge and future perspectives. Curr. Opin. Pulm. Med. 2017, 23, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Smith-Bindman, R.; Kwan, M.L.; Marlow, E.; Theis, M.K.; Bolch, W.; Cheng, S.Y.; Bowles, E.J.A.; Duncan, J.R.; Greenlee, R.T.; Kushi, L.H.; et al. Trends in Use of Medical Imaging in US Health Care Systems and in Ontario, Canada, 2000–2016. JAMA 2019, 322, 843–856. [Google Scholar] [CrossRef]
- Miglioretti, D.L.; Johnson, E.; Williams, A.; Greenlee, R.T.; Weinmann, S.; Solberg, L.I.; Feigelson, H.S.; Roblin, D.; Flynn, M.J.; Vanneman, N.; et al. The Use of Computed Tomography in Paediatrics and the Associated Radiation Exposure and Estimated Cancer Risk. JAMA Paediatr. 2013, 167, 700–707. [Google Scholar] [CrossRef]
- Shah, D.J.; Sachs, R.K.; Wilson, D. Radiation-induced cancer: A modern view. Br. J. Radiol. 2012, 85, e1166–e1173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Law, M.; Ma, W.K.; Chan, E.; Mui, C.; Ma, V.; Ho, W.Y.; Yip, L.; Lam, W. Cumulative Effective Dose and Cancer Risk of Paediatric Population in Repetitive Whole-Body Scan Using Dual-Energy X-ray Absorptiometry. J. Clin. Densitom. 2017, 22, 52–58. [Google Scholar] [CrossRef]
- FDA. Paediatric Information for X-ray Imaging Device Premarket Notifications. Guidance for Industry and Food and Drug Administration Staff. 2017. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/pediatric-information-x-ray-imaging-device-premarket-notifications (accessed on 30 July 2021).
- Strauss, J.K.; Frush, D.P.; Goske, M.J. Image Gently Campaign: Making a world of difference. Med. Phys. Int. 2015, 3, 94–108. [Google Scholar]
- Zhang, M.; Young, G.S.; Chen, H.; Li, J.; Qin, L.; McFaline-Figueroa, J.R.; Reardon, D.A.; Cao, X.; Wu, X.; Xu, X. Deep-Learning Detection of Cancer Metastases to the Brain on MRI. J. Magn. Reson. Imaging 2020, 52, 1227–1236. [Google Scholar] [CrossRef]
- Mandavia, D. The Future of Paediatric Imaging. Clin. Serv. J. 2019. Available online: https://www.clinicalservicesjournal.com/story/30547/the-future-of-paediatric-imaging (accessed on 15 October 2021).
- Hwang, M.; Piskunowicz, M.; Darge, K. Advanced Ultrasound Techniques for Paediatric Imaging. Paediatric 2019, 143, e20182609. [Google Scholar] [CrossRef] [Green Version]
- Ashmore, J.; Di Pietro, J.; Williams, K.; Stokes, E.; Symons, A.; Smith, M.; Clegg, L.; McGrath, C. A Free Virtual Reality Experience to Prepare Paediatric Patients for Magnetic Resonance Imaging: Cross-Sectional Questionnaire Study. JMIR Pediatr. Parent. 2019, 18, e11684. [Google Scholar] [CrossRef] [Green Version]
- Bray, L.; Sharpe, A.; Gichuru, P.; Fortune, P.-M.; Blake, L.; Appleton, V. The Acceptability and Impact of the Xploro Digital Therapeutic Platform to Inform and Prepare Children for Planned Procedures in a Hospital: Before and after Evaluation Study. J. Med. Internet Res. 2020, 22, e17367. [Google Scholar] [CrossRef] [PubMed]
- The United Nations Convention on the Rights of the Child. Available online: https://downloads.unicef.org.uk/wp-content/uploads/2010/05/UNCRC_united_nations_convention_on_the_rights_of_the_child.pdf?_adal_sd=www.unicef.org.uk.1627645039158&_adal_ca=so%3DGoogle%26me%3Dorganic%26ca%3D(not%2520set)%26co%3D(not%2520set)%26ke%3D(not%2520set).1627645039158&_adal_cw=1627645038454.1627645039158&_adal_id=fa26031a-d1df-4f7d-ac75-0c4bc8716fca.1627645038.2.1627645038.1627645038.334d2e4e-fe61-4c4c-9546-f6258ecabfe4.1627645039158&_ga=2.76008726.288082667.1627645037-494189370.1627645037 (accessed on 30 July 2021).
- Mayall, B. Children’s Rights and the Sociology of Childhood. In The Routledge International Handbook of Children’s Rights Studies, 1st ed.; Vandenhole, W., Desmet, E., Reynaert, D., Lembrechts, S., Eds.; Routledge: London, UK, 2015. [Google Scholar]
- Winch, R.; McColgan, M.P.; Sparrow, E.; Modi, N.; Greenough, A. Public and patient involvement in child health research and service improvements: A survey of hospital doctors. BMJ Paediatr. Open 2018, 2, e000206. [Google Scholar] [CrossRef] [Green Version]
- UNICEF. Every Child’s Right to Be Heard. 2011. Available online: https://www.unicef.org/files/Every_Childs_Right_to_be_Heard.pdf (accessed on 13 October 2021).
- Lupo, M.; Intini, A.; Filannino, D. Informed participation and patient empowerment: A patient-centered approach to improve paediatric clinical research. In The Management of Clinical Trials; IntechOpen: London, UK, 2018. [Google Scholar]
- Fleming, J.; Boeck, T. Involving Children and Young People in Health and Social Care Research, 1st ed.; Fleming, J., Boeck, T., Eds.; Routledge: London, UK, 2012. [Google Scholar]
- Brady, L.M.; Graham, B. Social Research with Children and Young People. A Practical Guide, 1st ed.; Policy Press Shorts: Bristol, UK, 2019. [Google Scholar]
- Abrehart, N.; Frost, K.; Young Persons Advisory Group; Harris, R.; Wragg, A.; Stewart, D.; Sharif, H.; Matthews, R.; Marciani, L. “A little [PPI] MAGIC can take you a long way”: Involving children and young people in research from inception of a novel medical device to multi-centre clinical trial. Res. Involv. Engagem. 2021, 7, 2. [Google Scholar] [CrossRef]
- Technology Innovation Transforming Child Health (TITCH) Network. Available online: https://www.titch.org.uk (accessed on 30 July 2021).
- STRATOS_INNOVATION_GROUP. Co-Design: A Powerful Force for Creativity and Collaboration. 2016. Available online: https://medium.com/@thestratosgroup/co-design-a-powerful-force-for-creativity-and-collaboration-bed1e0f13d46 (accessed on 30 July 2021).
- DESIGN_COUNCIL. The Design Process: What Is the Double Diamond? 2005. Available online: https://www.designcouncil.org.uk/news-opinion/design-process-what-double-diamond (accessed on 30 July 2021).
- Lang, A.R.; Martin, J.L.; Sharples, S.; Crowe, J.A. The effect of design on the usability and real world effectiveness of medical devices: A case study with adolescent users. Appl. Ergon. 2013, 44, 799–810. [Google Scholar] [CrossRef]
- Blower, S.; Swallow, V.; Maturana, C.; Stones, S.; Phillips, R.; Dimitri, P.; Marshman, Z.; Knapp, P.; Dean, A.; Higgins, S.; et al. Children and young people’s concerns and needs relating to their use of health technology to self-manage long-term conditions: A scoping review. Arch. Dis. Child. 2020, 105, 1093–1104. [Google Scholar] [CrossRef]
- Donzelli, S.; Zaina, F.; Martinez, G.; Di Felice, F.; Negrini, A.; Negrini, S. Adolescents with idiopathic scoliosis and their parents have a positive attitude towards the Thermobrace monitor: Results from a survey. Scoliosis Spinal Disord. 2017, 12, 12. [Google Scholar] [CrossRef] [Green Version]
- Howard, S.; Lang, A.; Sharples, S.; Shaw, D. See I told you I was taking it!—Attitudes of adolescents with asthma towards a device monitoring their inhaler use: Implications for future design. Appl. Ergon. 2017, 58, 224–237. [Google Scholar] [CrossRef]
- Stewart, A.C.; Gannon, K.N.; Beresford, F.; Fleming, L. Adolescent and caregivers’ experiences of electronic adherence assessment in paediatric problematic severe asthma. J. Child Health Care 2018, 22, 238–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majeed-Ariss, R.; Baildam, E.; Campbell, M.; Chieng, A.; Fallon, D.; Hall, A.; McDonagh, J.E.; Stones, S.R.; Thomson, W.; Swallow, V. Apps and Adolescents: A Systematic Review of Adolescents’ Use of Mobile Phone and Tablet Apps That Support Personal Management of Their Chronic or Long-Term Physical Conditions. J. Med. Internet Res. 2015, 17, e287. [Google Scholar] [CrossRef] [Green Version]
- Aldiss, S.; Baggott, C.; Gibson, F.; Mobbs, S.; Taylor, R.M. A Critical Review of the Use of Technology to Provide Psychosocial Support for Children and Young People with Long-Term Conditions. J. Pediatr. Nurs. 2015, 30, 87–101. [Google Scholar] [CrossRef]
- Waite-Jones, J.; Swallow, V.; Smith, J.; Stones, S.R.; Majeed-Ariss, R.; Van Rooyen, V. Developing a mobile-app to aid young people’s self-management of chronic rheumatic disease: A qualitative study. Rheumatology 2017, 56. [Google Scholar] [CrossRef] [Green Version]
- Children and Young People MedTech Co-Operative. Available online: https://cypmedtech.nihr.ac.uk/ (accessed on 30 July 2021).
- Bolislis, W.R.; Corriol-Rohou, S.; Hill-Venning, C.; Hoogland, H.; Joos, A.; King, D.; Kitcatt, V.; Le Visage, G.; Kühler, T.C. Orphan Medicines for Pediatric Use: A Focus on the European Union. Clin. Ther. 2019, 41, 2630–2642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Paediatric Translational Research Infrastructure. Available online: https://www.eptri.eu (accessed on 30 July 2021).


| Age | Approximate Weight (kg) | Systolic Blood Pressure (mmHg) | Heart Rate (Beats per Minute) | Respiratory Rate (Breath per Minute) |
|---|---|---|---|---|
| Term | 3.5 | 60–105 | 110–170 | 25–60 |
| 3 months | 6 | 65–115 | 105–165 | 25–55 |
| 6 months | 8 | 65–115 | 105–165 | 25–55 |
| 1 year | 10 | 70–120 | 85–150 | 20–40 |
| 2 years | 13 | 70–120 | 85–150 | 20–40 |
| 4 years | 15 | 70–120 | 85–150 | 20–40 |
| 6 years | 20 | 80–130 | 70–135 | 16–34 |
| 8 years | 25 | 80–130 | 70–135 | 16–34 |
| 10 years | 30 | 80–130 | 70–135 | 16–34 |
| 12 years | 40 | 95–140 | 60–120 | 14–26 |
| 14 years | 50 | 95–140 | 60–120 | 14–26 |
| 17 years + | 70 | 95–140 | 60–120 | 14–26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimitri, P.; Pignataro, V.; Lupo, M.; Bonifazi, D.; Henke, M.; Musazzi, U.M.; Ernst, F.; Minghetti, P.; Redaelli, D.F.; Antimisiaris, S.G.; et al. Medical Device Development for Children and Young People—Reviewing the Challenges and Opportunities. Pharmaceutics 2021, 13, 2178. https://doi.org/10.3390/pharmaceutics13122178
Dimitri P, Pignataro V, Lupo M, Bonifazi D, Henke M, Musazzi UM, Ernst F, Minghetti P, Redaelli DF, Antimisiaris SG, et al. Medical Device Development for Children and Young People—Reviewing the Challenges and Opportunities. Pharmaceutics. 2021; 13(12):2178. https://doi.org/10.3390/pharmaceutics13122178
Chicago/Turabian StyleDimitri, Paul, Valeria Pignataro, Mariangela Lupo, Donato Bonifazi, Maria Henke, Umberto M. Musazzi, Floris Ernst, Paola Minghetti, Davide F. Redaelli, Sophia G. Antimisiaris, and et al. 2021. "Medical Device Development for Children and Young People—Reviewing the Challenges and Opportunities" Pharmaceutics 13, no. 12: 2178. https://doi.org/10.3390/pharmaceutics13122178
APA StyleDimitri, P., Pignataro, V., Lupo, M., Bonifazi, D., Henke, M., Musazzi, U. M., Ernst, F., Minghetti, P., Redaelli, D. F., Antimisiaris, S. G., Migliaccio, G., Bonifazi, F., Marciani, L., Courtenay, A. J., Denora, N., & Lopedota, A. (2021). Medical Device Development for Children and Young People—Reviewing the Challenges and Opportunities. Pharmaceutics, 13(12), 2178. https://doi.org/10.3390/pharmaceutics13122178












