Effects of Oat Beta-Glucan Intake on Lipid Profiles in Hypercholesterolemic Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search Strategy
2.2. Study Selection
2.3. Data Extraction and Quality Assessment
2.4. Quantitative Data Synthesis and Analysis
3. Results
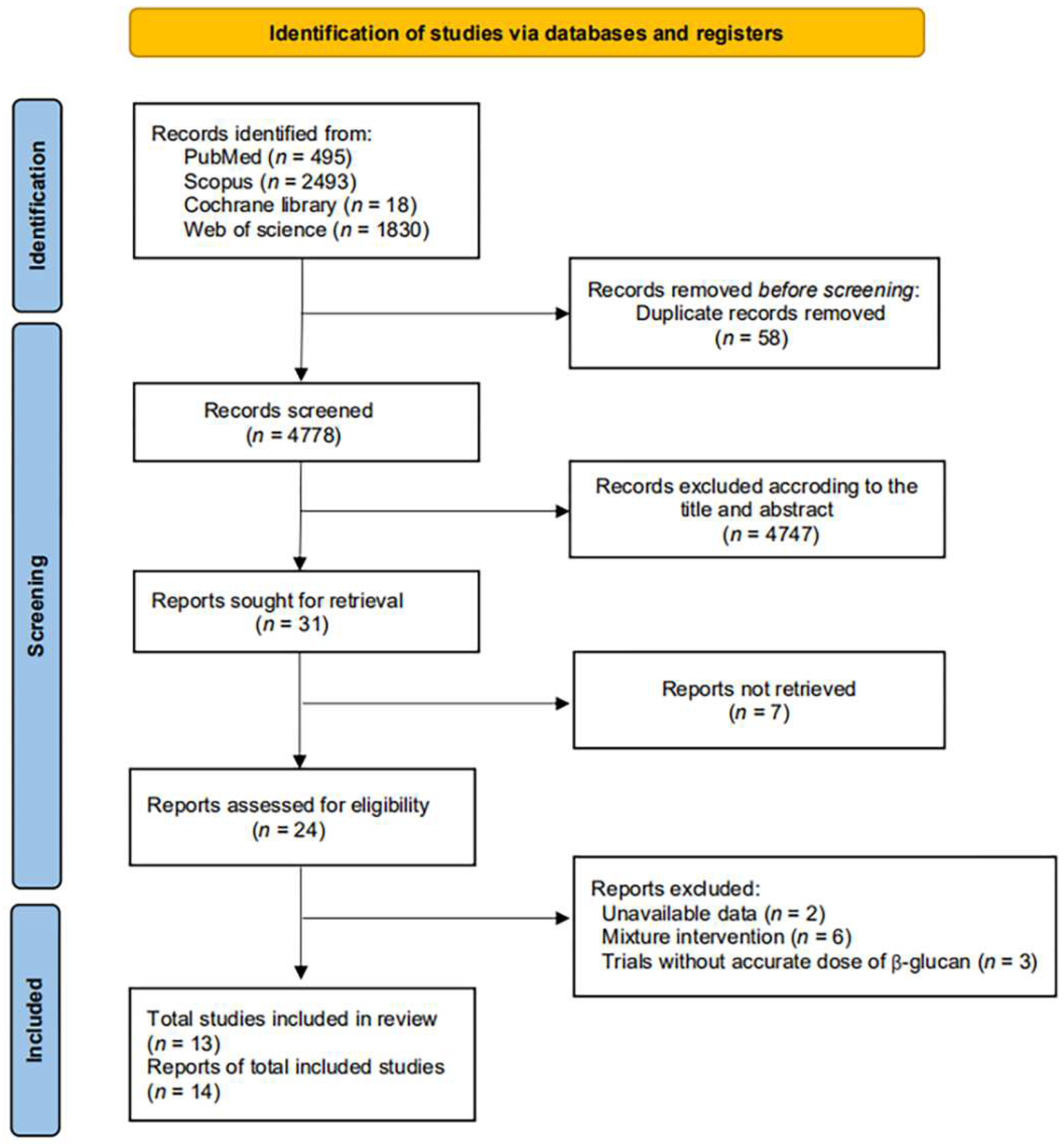
3.1. Search Results and Characteristics of Included Trials
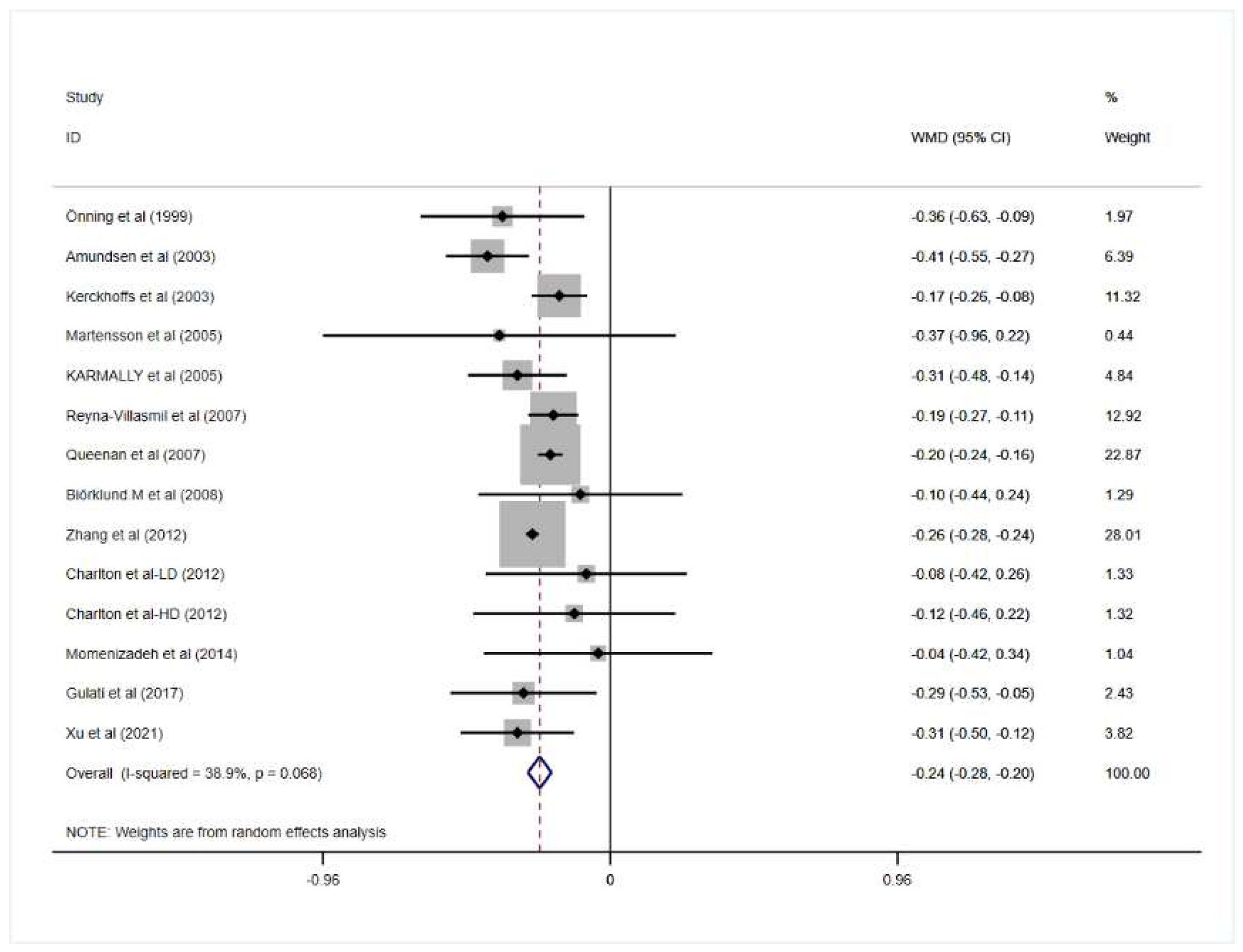
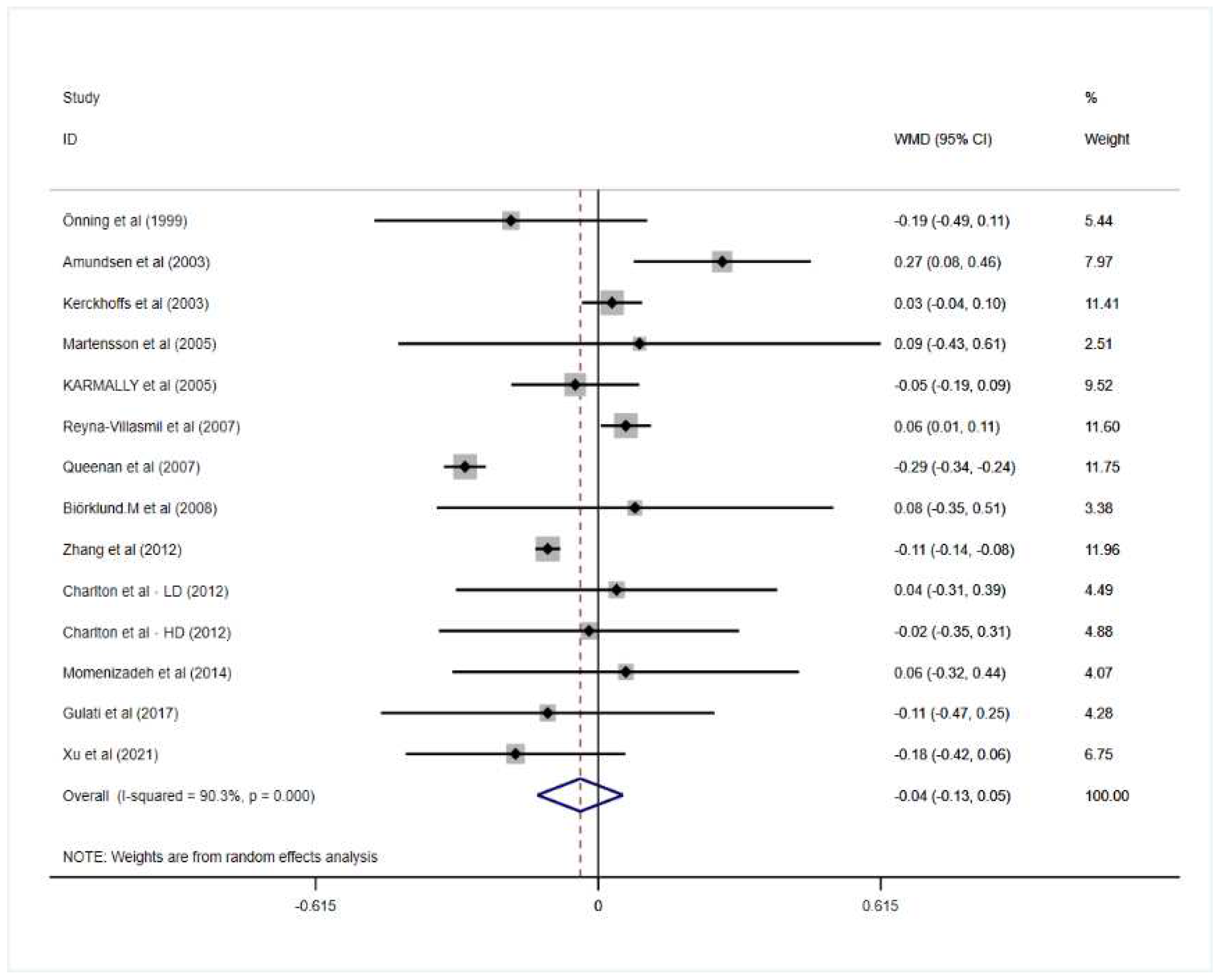
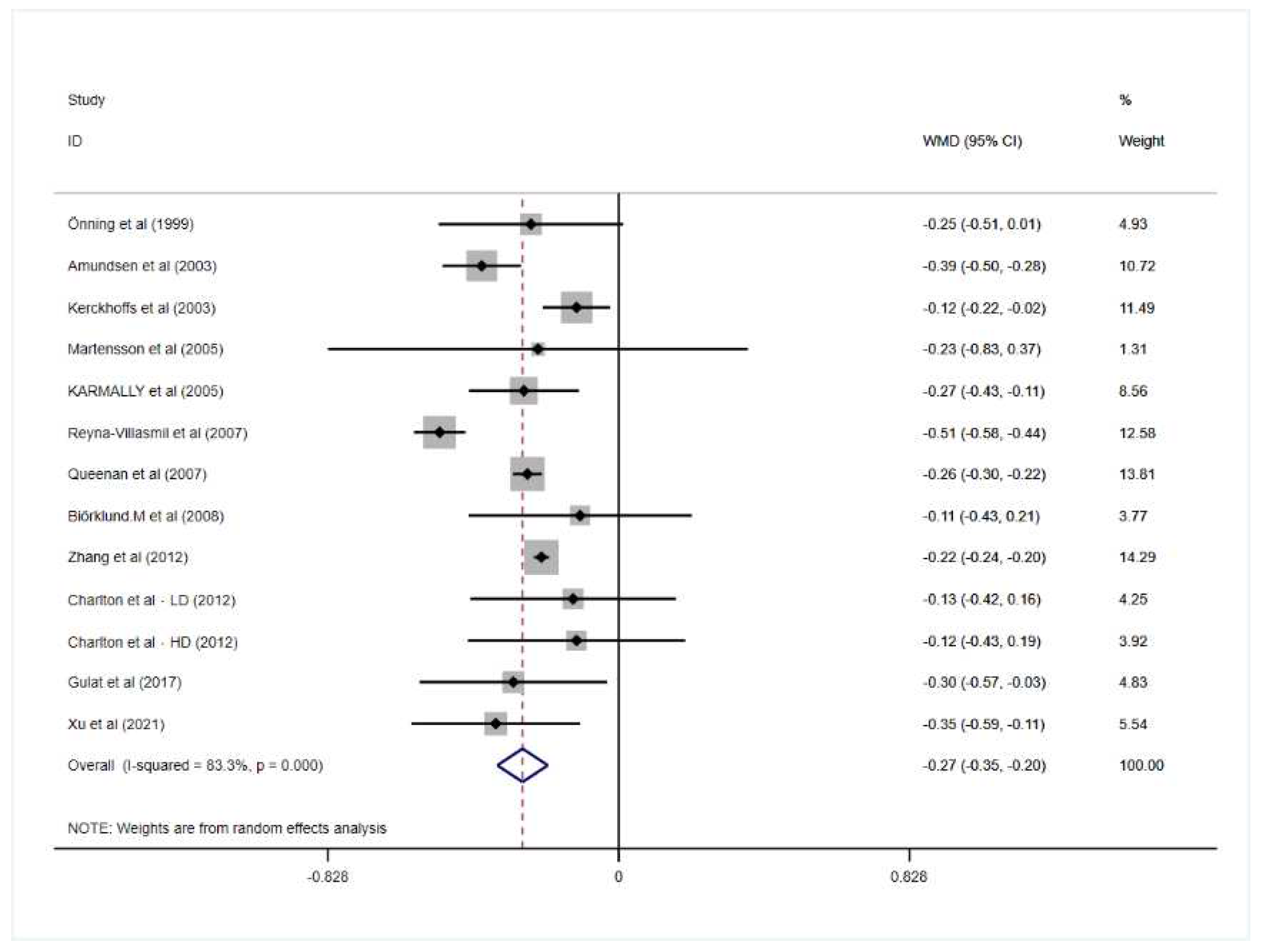
3.2. Effect of Oat Beta-Glucan on Lipid Profiles
3.3. Subgroup Analysis and Meta-Regression
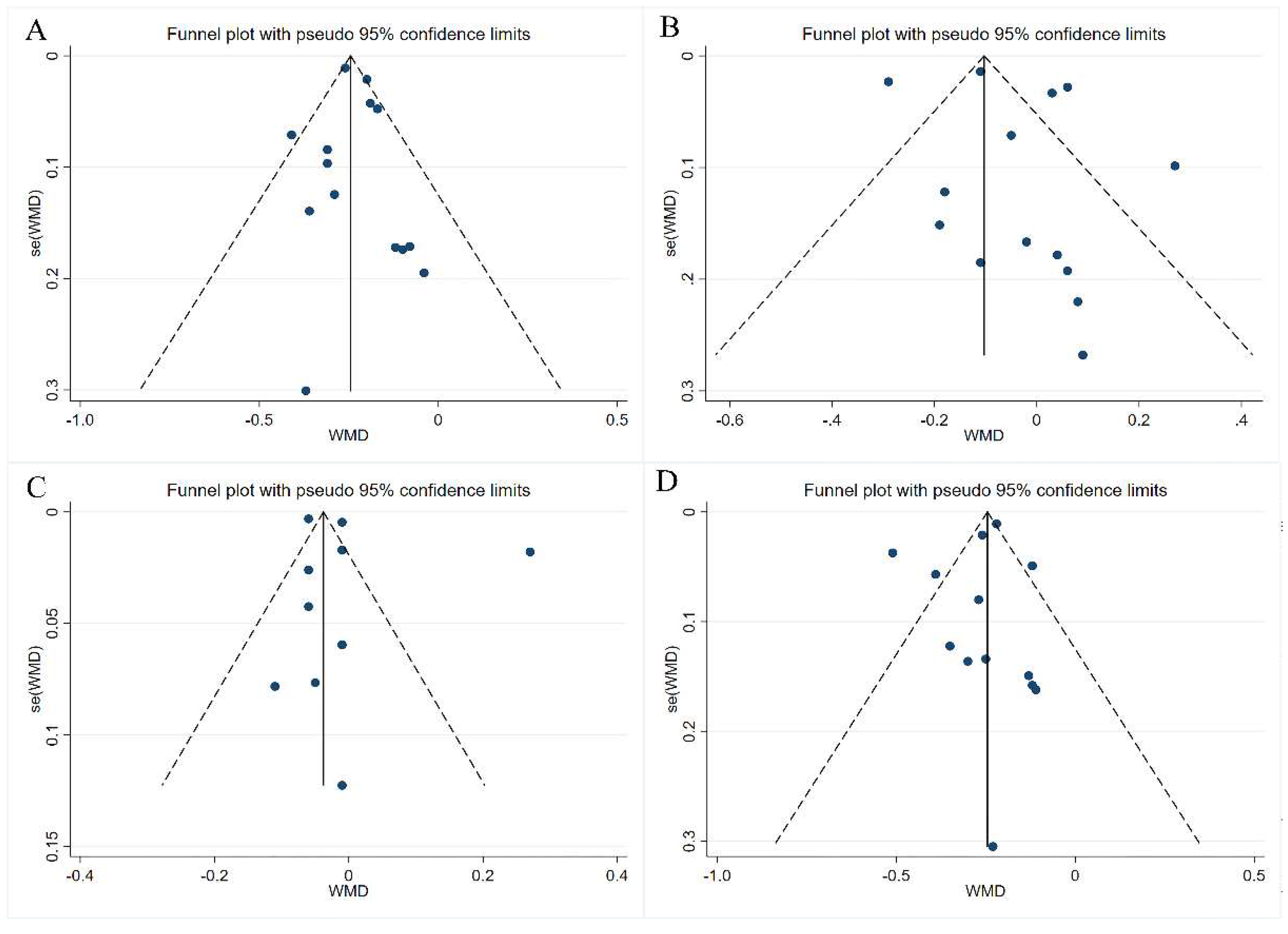
3.4. Publication Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2020 Update: A Report from the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef] [PubMed]
- Ballantyne, C.; Arroll, B.; Shepherd, J. Lipids and CVD management: Towards a global consensus. Eur. Heart J. 2005, 26, 2224–2231. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.L.; Lopez, A.D. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet 1997, 349, 1498–1504. [Google Scholar] [CrossRef]
- Yusuf, S.; Hawken, S.; Ôunpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Varigos, J.; et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet 2004, 364, 937–952. [Google Scholar] [CrossRef]
- Bruckert, E.; Hayem, G.; Dejager, S.; Yau, C.; Begaud, B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients—The PRIMO study. Cardiovasc. Drugs Ther. 2005, 19, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.D.; Brinton, E.A.; Ito, M.K.; Jacobson, T.A. Understanding Statin Use in America and Gaps in Patient Education (USAGE): An internet-based survey of 10,138 current and former statin users. J. Clin. Lipidol. 2012, 6, 208–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadiq Butt, M.; Tahir-Nadeem, M.; Khan, M.K.; Shabir, R.; Butt, M.S. Oat: Unique among the cereals. Eur. J. Nutr. 2008, 47, 68–79. [Google Scholar] [CrossRef]
- Du, B.; Lin, C.; Bian, Z.; Xu, B. An insight into anti-inflammatory effects of fungal beta-glucans. Trends Food Sci. Technol. 2015, 41, 49–59. [Google Scholar] [CrossRef]
- Izydorczyk, M.S.; Macri, L.J.; MacGregor, L.W. Structure and physicochemical properties of barley non-starch polysaccharides—I. Water extractable β-Glucans and arabinoxylans. Carbohydr. Polym. 1998, 35, 249–258. [Google Scholar] [CrossRef]
- Henry, R.J. Pentosan and (1 → 3),(1 → 4)-β-Glucan Concentrations in Endosperm and Whole grain of Wheat, Barley, Oats and Rye. J. Cereal Sci. 1987, 6, 253–258. [Google Scholar] [CrossRef]
- Liu, B.; Lin, Q.; Yang, T.; Zeng, L.; Shi, L.; Chen, Y.; Luo, F. Oat beta-glucan ameliorates dextran sulfate sodium (DSS)-induced ulcerative colitis in mice. Food Funct. 2015, 6, 3454–3463. [Google Scholar] [CrossRef] [PubMed]
- Błaszczyk, K.; Gajewska, M.; Wilczak, J.; Kamola, D.; Majewska, A.; Harasym, J.; Gromadzka-Ostrowska, J. Oral administration of oat beta-glucan preparations of different molecular weight results in regulation of genes connected with immune response in peripheral blood of rats with LPS-induced enteritis. Eur. J. Nutr. 2018, 58, 2859–2873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henrion, M.; Francey, C.; Le, K.A.; Lamothe, L. Cereal B-Glucans: The Impact of Processing and How It Affects Physiological Responses. Nutrients 2019, 11, 1729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Sun, X.; Wang, M.; Zhang, C.; Cao, Y.; Mo, G.; Liang, J.; Zhu, S. Quantitative assessment of the effects of beta-glucan consumption on serum lipid profile and glucose level in hypercholesterolemic subjects. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [Green Version]
- Onning, G.; Wallmark, A.; Persson, M.; Akesson, B.; Elmstahl, S.; Oste, R. Consumption of oat milk for 5 weeks lowers serum cholesterol and LDL cholesterol in free-living men with moderate hypercholesterolemia. Ann. Nutr. Metab. 1999, 43, 301–309. [Google Scholar] [CrossRef]
- Amundsen, A.L.; Haugum, B.; Andersson, H. Changes in serum cholesterol and sterol metabolites after intake of products enriched with an oat bran concentrate within a controlled diet. Scand. J. Nutr. 2003, 47, 6–74. [Google Scholar] [CrossRef]
- Kerckhoffs, D.A.J.M.; Hornstra, G.; Mensink, R.P. Cholesterol-lowering effect of β-glucan from oat bran in mildly hypercholesterolemic subjects may decrease when β-glucan is incorporated into bread and cookies. Am. J. Clin. Nutr. 2003, 78, 221–227. [Google Scholar] [CrossRef] [Green Version]
- Martensson, O.; Biorklund, M.; Lambo, A.M.; Duenas-Chasco, M.; Irastorza, A.; Holst, O.; Norin, E.; Welling, G.; Oste, R.; Onning, G. Fermented, ropy, oat-based products reduce cholesterol levels and stimulate the bifidobacteria flora in humans. Nutr. Res. 2005, 25, 429–442. [Google Scholar] [CrossRef]
- Karmally, W.; Montez, M.G.; Palmas, W.; Martinez, W.; Branstetter, A.; Ramakrishnan, R.; Holleran, S.F.; Haffner, S.M.; Ginsberg, H.N. Cholesterol-lowering benefits of oat-containing cereal in Hispanic Americans. J. Am. Diet. Assoc. 2005, 105, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Reyna-Villasmil, N.; Bermudez, V.; Mengual-Moreno, E.; Arias, N.; Cano-Ponce, C.; Leal-Gonzalez, E.; Souki, A.; E Inglett, G.; Israili, Z.H.; Hernández-Hernández, R.; et al. Oat-derived beta-glucan significantly improves HDLC and diminishes LDLC and non-HDL cholesterol in overweight individuals with mild hypercholesterolemia. Am. J. Ther. 2007, 14, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Queenan, K.M.; Stewart, M.L.; Smith, K.N.; Thomas, W.; Fulcher, R.G.; Slavin, J.L. Concentrated oat β-glucan, a fermentable fiber, lowers serum cholesterol in hypercholesterolemic adults in a randomized controlled trial. Nutr. J. 2007, 6, 6–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biorklund, M.; Holm, J.; Onning, G. Serum lipids and postprandial glucose and insulin levels in hyperlipidemic subjects after consumption of an oat beta-glucan-containing ready meal. Ann. Nutr. Metab. 2008, 52, 83–90. [Google Scholar] [CrossRef]
- Charlton, K.E.; Tapsell, L.C.; Batterham, M.J.; O’Shea, J.; Thorne, R.; Beck, E.; Tosh, S.M. Effect of 6 weeks’ consumption of β-glucan-rich oat products on cholesterol levels in mildly hypercholesterolaemic overweight adults. Br. J. Nutr. 2012, 107, 1037–1047. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Li, L.; Song, P.; Wang, C.; Man, Q.; Meng, L.; Cai, J.; Kurilich, A. Randomized controlled trial of oatmeal consumption versus noodle consumption on blood lipids of urban Chinese adults with hypercholesterolemia. Nutr. J. 2012, 11, 54. [Google Scholar] [CrossRef] [Green Version]
- Momenizadeh, A.; Heidari, R.; Sadeghi, M.; Tabesh, F.; Ekramzadeh, M.; Haghighatian, Z.; Golshahi, J.; Baseri, M. Effects of oat and wheat bread consumption on lipid profile, Blood sugar, And endothelial function in hypercholesterolemic patients: A randomized controlled clinical trial. ARYA Atheroscler. 2014, 10, 259–265. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4251477/ (accessed on 11 April 2022).
- Gulati, S.; Misra, A.; Pandey, R.M. Effects of 3 g of soluble fiber from oats on lipid levels of Asian Indians—A randomized controlled, parallel arm study. Lipids Health Dis. 2017, 16, 71. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.F.; Wang, S.K.; Feng, M.Y.; Shete, V.; Chu, Y.F.; Kamil, A.; Yang, C.; Liu, H.C.; Xia, H.; Wang, X.; et al. Serum Metabolomics Reveals Underlying Mechanisms of Cholesterol-Lowering Effects of Oat Consumption: A Randomized Controlled Trial in a Mildly Hypercholesterolemic Population. Mol. Nutr. Food Res. 2021, 65, e2001059. [Google Scholar] [CrossRef]
- Duval, S. Trim and Fill: A Simple Funnel-Plot-Based Method of Testing and Adjusting for Publication Bias in Meta-Analysis. Biometrics 1999, 56, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Liu, H.; Yang, C.; Xia, H.; Pan, D.; Yang, X.; Yang, L.; Wang, S.; Sun, G. Effects of different delivering matrices of beta-glucan on lipids in mildly hypercholesterolaemic individuals: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2021, 125, 294–307. [Google Scholar] [CrossRef] [PubMed]
- Wehrli, F.; Taneri, P.E.; Bano, A.; Bally, L.; Blekkenhorst, L.C.; Bussler, W.; Metzger, B.; Minder, B.; Glisic, M.; Muka, T.; et al. Oat Intake and Risk of Type 2 Diabetes, Cardiovascular Disease and All-Cause Mortality: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 2560. [Google Scholar] [CrossRef] [PubMed]
- Rebello, C.J.; Chu, Y.F.; Johnson, W.D.; Martin, C.K.; Han, H.; Bordenave, N.; Shi, Y.; O’Shea, M.; Greenway, F.L. The role of meal viscosity and oat β-glucan characteristics in human appetite control: A randomized crossover trial. Nutr. J. 2014, 13, 49. [Google Scholar] [CrossRef] [PubMed]
- Ellegard, L.; Andersson, H. Oat bran rapidly increases bile acid excretion and bile acid synthesis: An ileostomy study. Eur. J. Clin. Nutr. 2007, 61, 938–945. [Google Scholar] [CrossRef] [Green Version]
- Smith, K.N.; Queenan, K.M.; Fulcher, R.G.; Slavin, J.L.; Thomas, W. Physiological Effects of Concentrated Barley β-Glucan in Mildly Hypercholesterolemic Adults. J. Am. Coll. Nutr. 2008, 27, 434–440. [Google Scholar] [CrossRef]
- Tosh, S.M.; Brummer, Y.; Miller, S.S.; Regand, A.; Defelice, C.; Duss, R.; Wolever, T.M.; Wood, P.J. Processing affects the physicochemical properties of beta-glucan in oat bran cereal. J. Agric. Food Chem. 2010, 58, 7723–7730. [Google Scholar] [CrossRef]
- Wang, Q.; Ellis, P.R. Oat beta-glucan: Physico-chemical characteristics in relation to its blood-glucose and cholesterol-lowering properties. Br. J. Nutr. 2014, 112 (Suppl. S2), S4–S13. [Google Scholar] [CrossRef] [Green Version]
- Liao, M.Y.; Shen, Y.C.; Chiu, H.F.; Ten, S.M.; Lu, Y.Y.; Han, Y.C.; Venkatakrishnan, K.; Yang, S.F.; Wang, C.K. Down-regulation of partial substitution for staple food by oat noodles on blood lipid levels: A randomized, double-blind, clinical trial. J. Food Drug Anal. 2019, 27, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Theuwissen, E.; Mensink, R.P. Simultaneous Intake of b-Glucan and Plant Stanol Esters Affects Lipid Metabolism in Slightly Hy percholesterolemic Subjects. J. Nutr. 2006, 137, 583–588. [Google Scholar] [CrossRef] [Green Version]

| Number | First Author | Year | Study Region | Sample Size (Male/Female) | Age (Year) | Sources of Oatβ-Glucan | Amounts of Oatβ-Glucan (g/d) | Duration (Week) | Comparison Group | Study Design | Baseline of TC (mmol/L) (CG) | Baseline of TG (mmol/L) (CG) | Baseline of HDL-C (mmol/L) (CG) | Baseline of LDL-C (mmol/L) (CG) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Gunilla Önning | 1999 | Sweden | 52 (52/0) | 62.6 ± 5.6 | oat milk | 3.9 | 5 | milk without oatβ-glucan | randomized controlled, double-blind | 6.39 ± 0.68 | 1.56 ± 0.69 | 1.34 ± 0.33 | 4.34 ± 0.66 |
| 2 | Ågot Lia Amundsen | 2003 | Sweden | 16 (9/7) | 57 ± 7.9 | diet with oatβ-glucan | 5 | 3 | diet without oatβ-glucan | single-blind, randomized, cross-over | 7.64 ± 0.24 | 2.15 ± 0.37 | 1.68 ± 0.13 | 5.12 ± 0.20 |
| 3 | Daniëlle AJM Kerckhoffs | 2003 | Netherland | 48 (21/27) | 51.3 ± 2 | bread and cookies with oatβ-glucan | 5 | 4 | bread and cookies without oatβ-glucan | parallel, randomized controlled | 6.00 ± 0.16 | 1.15 ± 0.09 | 1.38 ± 0.09 | 4.09 ± 0.17 |
| 4 | Olof Martensson | 2005 | Sweden | 56 (24/32) | 55.0 ± 9.0 | Oat-based product | 3.25 | 5 | dairy-based product | randomized, double blind, parallel | 6.04 ± 1.02 | 1.51 ± 0.44 | 1.21 ± 0.32 | 4.09 ± 1.19 |
| 5 | WAHIDA KARMALLY | 2005 | US | 152 (49/103) | 49.1 ± 10.3 | oat-containing cereal | 3 | 6 | corn | randomized, controlled trial | 5.15 ± 0.72 | 1.64 ± 0.73 | 0.96 ± 0.23 | 3.45 ± 0.63 |
| 6 | Nadia Reyna-Villasmil | 2007 | Venezuela | 38 (38/0) | 59.8 ± 0.6 | oat bread | 6 | 8 | whole wheat bread | randomized, controlled trial | 6.02 ± 0.07 | 1.44 ± 0.11 | 1.09 ± 0.07 | 4.15 ± 0.07 |
| 7 | Katie M Queenan | 2007 | US | 75 (25/50) | 45.0 ± 2.1 | concentrated oatβ-glucan | 6 | 6 | dextrose monohydrate | randomized, double-blind parallel | 6.20 ± 0.10 | 1.90 ± 0.20 | 1.30 ± 0.05 | 4.20 ± 0.10 |
| 8 | M. Biörklund | 2008 | Sweden | 43 (19/24) | 58.0 ± 8.2 | soup with oatβ-glucan | 4 | 5 | soup without oatβ-glucan | parallel, placebo-controlled | 6.66 ± 0.52 | 1.54 ± 0.66 | NR | 4.06 ± 0.52 |
| 9 | Karen E. Charlton | 2012 | Australia | 90 (43/47) | 51.3 ± 10.22 | oat porridge, oat-based cereal bars | 1.5 (LD), 3.2 (HD) | 6 | diet without oatβ-glucan | parallel, randomized, controlled, single-blind | 6.03 ± 0.58 | 1.56 ± 0.60 | NR | 3.86 ± 0.55 |
| 10 | Jian Zhang | 2012 | China | 166 (65/101) | 53.19 ± 0.87 | diet with oatβ-glucan | 3 | 6 | diet without oatβ-glucan | randomized, controlled | 6.09 ± 0.08 | 1.89 ± 0.11 | 1.51 ± 0.03 | 4.17 ± 0.08 |
| 11 | Amir Momenizadeh | 2014 | Iran | 60 (21/39) | 51.12 ± 9.31 | oat bread | 6 | 6 | wheat bread oatβ-glucan | randomized, controlled | 5.80 ± 0.45 | 1.95 ± 0.67 | NR | NR |
| 12 | Seema Gulati | 2017 | US | 69 | 31.2 ± 6.54 | porridge and upma with oatβ-glucan | 3 | 4 | diet without oatβ-glucan | prospective, randomized, parallel, controlled | 5.62 ± 0.29 | 1.78 ± 0.63 | 1.01 ± 0.21 | 3.76 ± 0.46 |
| 13 | Dengfeng Xu | 2021 | China | 62 (22/40) | 44.69 ± 11.42 | oats | 3 | 6 | rice without oatβ-glucan | randomized, controlled | 5.55 ± 0.29 | 1.22 ± 0.43 | 1.53 ± 0.30 | 3.30 ± 0.41 |
| Number | First Author | Year | Endpoint of TC (mmol/L) (CG) | Endpoint of TG (mmol/L) (CG) | Endpoint of HDL-C (mmol/L) (CG) | Endpoint of LDL-C (mmol/L) (CG) | Baseline of TC (mmol/L) (IG) | Baseline of TG (mmol/L) (IG) | Baseline of HDL-C (mmol/L) (IG) | Baseline of LDL-C (mmol/L) (IG) | Endpoint of TC(mmol/L)(IG) | Endpoint of TG (mmol/L) (IG) | Endpoint of HDL-C (mmol/L) (IG) | Endpoint of LDL-C (mmol/L) (IG) |
| 1 | Gunilla Önning | 1999 | 6.58 ± 0.80 | 1.85 ± 0.92 | 1.39 ± 0.34 | 4.38 ± 0.82 | 6.42 ± 0.66 | 1.57 ± 0.74 | 1.43 ± 0.51 | 4.35 ± 0.65 | 6.25 ± 0.67 | 1.67 ± 0.67 | 1.37 ± 0.33 | 4.14 ± 0.56 |
| 2 | Ågot Lia Amundsen | 2003 | 7.34 ± 0.18 | 1.68 ± 0.21 | 1.53 ± 0.11 | 5.11 ± 0.15 | 7.66 ± 0.19 | 1.77 ± 0.25 | 1.74 ± 0.13 | 5.19 ± 0.14 | 6.95 ± 0.16 | 1.57 ± 0.20 | 1.53 ± 0.11 | 4.79 ± 0.14 |
| 3 | Daniëlle AJM Kerckhoffs | 2003 | 6.04 ± 0.15 | 1.12 ± 0.10 | 1.41 ± 0.09 | 4.11 ± 0.16 | 5.98 ± 0.16 | 1.13 ± 0.12 | 1.50 ± 0.09 | 3.96 ± 0.16 | 5.85 ± 0.18 | 1.13 ± 0.14 | 1.47 ± 0.08 | 3.86 ± 0.17 |
| 4 | Olof Martensson | 2005 | 6.15 ± 1.00 | 1.46 ± 0.52 | 1.29 ± 0.32 | 4.11 ± 1.12 | 6.08 ± 0.75 | 1.52 ± 1.09 | 1.36 ± 0.41 | 3.78 ± 0.73 | 5.82 ± 0.80 | 1.56 ± 0.96 | 1.43 ± 0.40 | 3.57 ± 0.80 |
| 5 | WAHIDA KARMALLY | 2005 | 5.18 ± 0.70 | 1.63 ± 0.75 | 0.97 ± 0.23 | 3.48 ± 0.58 | 5.41 ± 0.77 | 1.79 ± 0.73 | 0.93 ± 0.25 | 3.66 ± 0.68 | 5.12 ± 0.65 | 1.71 ± 0.71 | 0.93 ± 0.26 | 3.41 ± 0.56 |
| 6 | Nadia Reyna-Villasmil | 2007 | 5.24 ± 0.17 | 1.35 ± 0.08 | 1.08 ± 0.06 | 3.44 ± 0.14 | 6.00 ± 0.11 | 1.27 ± 0.05 | 1.02 ± 0.05 | 4.34 ± 0.11 | 5.02 ± 0.11 | 1.24 ± 0.08 | 1.28 ± 0.05 | 3.12 ± 0.11 |
| 7 | Katie M Queenan | 2007 | 6.10 ± 0.10 | 1.70 ± 0.15 | 1.29 ± 0.05 | 4.2 ± 0.07 | 6.20 ± 0.10 | 1.90 ± 0.10 | 1.40 ± 0.06 | 4.10 ± 0.10 | 5.90 ± 0.10 | 1.99 ± 0.10 | 1.38 ± 0.06 | 3.80 ± 0.10 |
| 8 | M. Biörklund | 2008 | 6.57 ± 0.60 | 1.51 ± 0.64 | NR | 4.01 ± 0.60 | 6.62 ± 0.51 | 1.58 ± 0.81 | NR | 4.29 ± 0.50 | 6.43 ± 0.63 | 1.63 ± 0.76 | NR | 4.13 ± 0.50 |
| 9 | Karen E. Charlton | 2012 | 5.67 ± 0.68 | 1.55 ± 0.78 | NR | 3.60 ± 0.53 | 6.12 ± 0.54 (LD) 5.97 ± 0.55 (HD) | 1.53 ± 0.73 (LD) 1.37 ± 0.59 (HD) | NR | 3.84 ± 0.67 (LD) 3.82 ± 0.56 (HD) | 5.68 ± 0.77 (LD) 5.49 ± 0.80 (HD) | 1.56 ± 0.58 (LD) 1.34 ± 0.60 (HD) | NR | 3.49 ± 0.70 (LD) 3.46 ± 0.69 (HD) |
| 10 | Jian Zhang | 2012 | 5.94 ± 0.09 | 1.85 ± 0.11 | 1.41 ± 0.03 | 4.00 ± 0.08 | 6.26 ± 0.07 | 2.06 ± 0.10 | 1.47 ± 0.03 | 1.47 ± 0.03 | 5.85 ± 0.09 | 1.91 ± 0.11 | 1.43 ± 0.03 | 3.91 ± 0.08 |
| 11 | Amir Momenizadeh | 2014 | 5.67 ± 0.88 | 1.87 ± 0.84 | NR | NR | 6.08 ± 0.70 | 2.03 ± 0.71 | NR | NR | 5.89 ± 0.80 | 2.00 ± 0.72 | NR | NR |
| 12 | Seema Gulati | 2017 | 5.40 ± 0.63 | 1.92 ± 0.91 | 0.96 ± 0.19 | 3.56 ± 0.74 | 5.62 ± 0.28 | 1.83 ± 0.67 | 1.08 ± 0.33 | 3.73 ± 0.41 | 5.11 ± 0.56 | 1.85 ± 0.76 | 1.02 ± 0.25 | 3.23 ± 0.52 |
| 13 | Dengfeng Xu | 2021 | 5.39 ± 0.46 | 1.24 ± 0.51 | 1.56 ± 0.35 | 3.20 ± 0.59 | 5.59 ± 0.30 | 1.34 ± 0.50 | 1.49 0.28 | 3.48 ± 0.44 | 5.12 ± 0.40 | 1.18 ± 0.45 | 1.47 ± 0.26 | 3.03 ± 0.45 |
| Study | Year | Risk of Bias Assessment | ||||||
|---|---|---|---|---|---|---|---|---|
| Random Sequence Generation | Allocation Concealment | Blinding of Participants and Personnel | Blinding of Outcome Assessment | Incomplete Outcome Data | Selective Reporting | Other Bias | ||
| Önning et al. | 1999 | High | Low | Low | Low | Low | Low | Low |
| Amundsen et al. | 2003 | Unclear | Unclear | Low | Low | Low | Low | Low |
| Kerckhoffs et al. | 2003 | High | Low | High | Low | Low | Low | Unclear |
| Martensson et al. | 2005 | Unclear | Low | Low | Low | Low | Low | Unclear |
| Karmally et al. | 2005 | Unclear | Low | Low | Low | Low | Low | Unclear |
| Reyna-Villasmil et al. | 2007 | Unclear | Low | Low | Low | Low | Low | Low |
| Queenan et al. | 2007 | Low | Low | Low | Low | Low | Low | Low |
| Biörklund et al. | 2008 | Low | Low | Low | Low | Low | Low | Low |
| Zhang et al. | 2012 | Low | Low | Low | Low | Low | Low | Low |
| Charlton et al. | 2012 | Low | Low | Low | Low | Low | Low | Low |
| Momenizadeh et al. | 2014 | Low | Low | Low | Low | Low | Low | Low |
| Gulat et al. | 2017 | Unclear | High | High | Low | Low | Low | Unclear |
| Xu et al. | 2021 | Low | Low | Low | Low | Low | Low | Low |
| Lipid Profiles | Subgroup Analyses | |||||
|---|---|---|---|---|---|---|
| No. of Trials | WMD | p | I2 (%) | p Value of Heterogeneity | ||
| Mean | 95% CI | |||||
| TC | ||||||
| Overall | 14 | −0.24 | −0.28, −0.20 | 0.000 | 38.9 | 0.068 |
| Disease severity | ||||||
| mild | 12 | −0.22 | −0.27, −0.17 | 0.000 | 22.8 | 0.219 |
| moderate | 2 | −0.26 | −0.28, −0.24 | 0.000 | 0.0 | 0.475 |
| Daily intervention dose (g) | ||||||
| ≤3 | 5 | −0.26 | −0.28, −0.24 | 0.000 | 0.0 | 0.777 |
| >3 | 9 | −0.22 | −0.27, −0.16 | 0.000 | 32.8 | 0.156 |
| Source of oat β-glucan | ||||||
| oats and oat-based products | 8 | −0.22 | −0.29, −0.16 | 0.000 | 0.0 | 0.585 |
| food or diet added with oat β-glucan | 6 | −0.24 | −0.29, −0.19 | 0.000 | 66.9 | 0.010 |
| Duration | ||||||
| ≤5 weeks | 6 | −0.28 | −0.40, −0.16 | 0.000 | 47.5 | 0.090 |
| >5 weeks | 8 | −0.23 | −0.27, −0.19 | 0.000 | 40.3 | 0.110 |
| TG | ||||||
| Overall | 14 | −0.04 | −0.13, 0.05 | 0.413 | 90.3 | 0.000 |
| Disease severity | ||||||
| mild | 12 | −0.01 | −0.15, 0.12 | 0.846 | 91.7 | 0.000 |
| moderate | 2 | −0.11 | −0.14, −0.08 | 0.000 | 0.0 | 0.599 |
| Daily intervention dose (g) | ||||||
| ≤3 | 5 | −0.11 | −0.13, −0.08 | 0.000 | 0.0 | 0.787 |
| >3 | 9 | 0.00 | −0.16, 0.16 | 0.983 | 93.9 | 0.000 |
| Source of oat β-glucan | ||||||
| oats and oat-based products | 8 | 0.01 | −0.05, 0.07 | 0.732 | 8.6 | 0.364 |
| food or diet added with oat β-glucan | 6 | −0.05 | −0.18, 0.09 | 0.498 | 94.5 | 0.000 |
| Duration | ||||||
| ≤5 weeks | 6 | 0.05 | −0.08, 0.18 | 0.471 | 41.6 | 0.128 |
| >5 weeks | 8 | −0.08 | −0.20, 0.03 | 0.157 | 92.9 | 0.000 |
| HDL-c | ||||||
| Overall | 10 | 0.00 | −0.05, 0.05 | 0.993 | 97.7 | 0.000 |
| Disease severity | ||||||
| mild | 8 | 0.01 | −0.08, 0.10 | 0.784 | 97.1 | 0.000 |
| moderate | 2 | −0.06 | −0.07, −0.05 | 0.000 | 0.0 | 0.523 |
| Daily intervention dose (g) | ||||||
| ≤3 | 4 | −0.04 | −0.08, 0.00 | 0.063 | 66.5 | 0.030 |
| >3 | 6 | 0.01 | −0.12, 0.14 | 0.890 | 97.9 | 0.000 |
| Source of oat β-glucan | ||||||
| oats and oat-based products | 5 | 0.03 | −0.15, 0.20 | 0.775 | 97.2 | 0.000 |
| food or diet added with oat β-glucan | 5 | −0.04 | −0.08, 0.00 | 0.029 | 95.1 | 0.000 |
| Duration | ||||||
| ≤5 weeks | 5 | −0.06 | −0.10, −0.02 | 0.004 | 0.0 | 0.870 |
| >5 weeks | 5 | 0.03 | −0.03, 0.10 | 0.309 | 99.0 | 0.000 |
| LDL-c | ||||||
| Overall | 13 | −0.27 | −0.35, −0.20 | 0.000 | 83.3 | 0.000 |
| Disease severity | ||||||
| mild | 11 | −0.28 | −0.38, −0.18 | 0.000 | 82.0 | 0.000 |
| moderate | 2 | −0.22 | −0.24, −0.20 | 0.000 | 0.0 | 0.824 |
| Daily intervention dose (g) | ||||||
| ≤3 | 5 | −0.22 | −0.24, −0.20 | 0.000 | 0.0 | 0.700 |
| >3 | 8 | −0.28 | −0.40, −0.15 | 0.000 | 87.0 | 0.000 |
| Source of oat β-glucan | ||||||
| oats and oat-based products | 7 | −0.30 | −0.44, −0.15 | 0.001 | 68.5 | 0.004 |
| food or diet added with oat β-glucan | 6 | −0.24 | −0.30, −0.18 | 0.000 | 69.8 | 0.005 |
| Duration | ||||||
| ≤5 weeks | 6 | −0.24 | −0.38, −0.10 | 0.001 | 63.5 | 0.018 |
| >5 weeks | 7 | −0.29 | −0.39, −0.20 | 0.000 | 89.7 | 0.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Xia, J.; Yang, C.; Pan, D.; Xu, D.; Sun, G.; Xia, H. Effects of Oat Beta-Glucan Intake on Lipid Profiles in Hypercholesterolemic Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2022, 14, 2043. https://doi.org/10.3390/nu14102043
Yu J, Xia J, Yang C, Pan D, Xu D, Sun G, Xia H. Effects of Oat Beta-Glucan Intake on Lipid Profiles in Hypercholesterolemic Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients. 2022; 14(10):2043. https://doi.org/10.3390/nu14102043
Chicago/Turabian StyleYu, Junhui, Jiayue Xia, Chao Yang, Da Pan, Dengfeng Xu, Guiju Sun, and Hui Xia. 2022. "Effects of Oat Beta-Glucan Intake on Lipid Profiles in Hypercholesterolemic Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Nutrients 14, no. 10: 2043. https://doi.org/10.3390/nu14102043
APA StyleYu, J., Xia, J., Yang, C., Pan, D., Xu, D., Sun, G., & Xia, H. (2022). Effects of Oat Beta-Glucan Intake on Lipid Profiles in Hypercholesterolemic Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients, 14(10), 2043. https://doi.org/10.3390/nu14102043







