Dietary Protein Requirements in Children: Methods for Consideration
Abstract
1. Introduction
2. Current Protein Intake Recommendations for Children
3. Physical Activity as a Modifier of Dietary Protein Requirements in Children
4. Methods to Assess Protein Metabolism in Children
4.1. Indicator Amino Acid Oxidation (IAAO) Method
4.2. 15N End-Product Method
4.3. Emerging D3-Creatine Method for Assessing Protein Metabolism
5. Further Considerations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Trumbo, P.; Schlicker, S.; Yates, A.A.; Poos, M.; Food and Nutrition Board of the Institute of Medicine; The National Academies. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J. Am. Diet Assoc. 2002, 102, 1621–1630. [Google Scholar] [CrossRef]
- Waterlow, J.C. The mysteries of nitrogen balance. Nutr. Res. Rev. 1999, 12, 25–54. [Google Scholar] [CrossRef]
- Sprinson, D.B.; Rittenberg, D. The rate of interaction of the amino acids of the diet with the tissue proteins. J. Biol. Chem. 1949, 180, 715–726. [Google Scholar] [CrossRef]
- Elango, R.; Ball, R.O.; Pencharz, P.B. Recent advances in determining protein and amino acid requirements in humans. Br. J. Nutr. 2012, 108, S22–S30. [Google Scholar] [CrossRef]
- Baxter-Jones, A.D.G.; Eisenmann, J.C.; Mirwald, R.L.; Faulkner, R.A.; Bailey, D.A. The influence of physical activity on lean mass accrual during adolescence: A longitudinal analysis. J. Appl. Physiol. 2008, 105, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Ramires, V.V.; Dumith, S.C.; Wehrmeister, F.C.; Hallal, P.C.; Menezes, A.M.B.; Gonçalves, H. Physical activity throughout adolescence and body composition at 18 years: 1993 Pelotas (Brazil) birth cohort study. Int. J. Behav. Nutr. Phys. Act. 2016, 13, 105. [Google Scholar] [CrossRef]
- Ness, A.R.; Leary, S.D.; Mattocks, C.; Blair, S.N.; Reilly, J.J.; Wells, J.; Ingle, S.; Tilling, K.; Smith, G.D.; Riddoch, C. Objectively Measured Physical Activity and Fat Mass in a Large Cohort of Children. PLoS Med. 2007, 4, e97. [Google Scholar] [CrossRef]
- Rennie, M.J.; Edwards, R.H.T.; Krywawych, S.; Davies, C.T.M.; Halliday, D.; Waterlow, J.C.; Millward, D.J. Effect of Exercise on Protein Turnover in Man. Clin. Sci. 1981, 61, 627–639. [Google Scholar] [CrossRef]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. Nutrition and Athletic Performance. Med. Sci. Sports Exerc. 2016, 48, 543–568. [Google Scholar] [CrossRef]
- Waterlow, J.C.; Garlick, P.J.; Ward, M.D. Protein Turnover in Mammalian Tissues and in the Whole Body; Elsevier/North-Holland Biomedical Press: Amsterdam, The Netherlands, 1978. [Google Scholar]
- Agricultural Research Service. What We Eat in America; NHANES 2017–2018; Food Surveys Research Group: Beltsville, MD, USA, 2020. [CrossRef]
- Scrimshaw, N.S. Criteria for valid nitrogen balance measurement of protein requirements. Eur. J. Clin. Nutr. 1996, 50, 196–197. [Google Scholar]
- Elango, R.; Humayun, M.A.; Ball, R.O.; Pencharz, P.B. Protein requirement of healthy school-age children determined by the indicator amino acid oxidation method. Am. J. Clin. Nutr. 2011, 94, 1545–1552. [Google Scholar] [CrossRef]
- Wolfe, R.R. The underappreciated role of muscle in health and disease. Am. J. Clin. Nutr. 2006, 84, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.R.; Volterman, K.A.; Obeid, J.; Offord, E.A.; Timmons, B.W. Postexercise protein ingestion increases whole body net protein balance in healthy children. J. Appl. Physiol. 2014, 117, 1493–1501. [Google Scholar] [CrossRef]
- Volterman, K.A.; Obeid, J.; Wilk, B.; Timmons, B.W. Effects of postexercise milk consumption on whole body protein balance in youth. J. Appl. Physiol. 2014, 117, 1165–1169. [Google Scholar] [CrossRef]
- Volterman, K.A.; Moore, D.R.; Breithaupt, P.; Godin, J.-P.; Karagounis, L.G.; Offord, E.A.; Timmons, B.W. Postexercise Dietary Protein Ingestion Increases Whole-Body Leucine Balance in a Dose-Dependent Manner in Healthy Children. J. Nutr. 2017, 147, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Morton, R.W.; Murphy, K.T.; McKellar, S.R.; Schoenfeld, B.J.; Henselmans, M.; Helms, E.; Aragon, A.A.; Devries, M.C.; Banfield, L.; Krieger, J.W.; et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br. J. Sports Med. 2018, 52, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M.; Tipton, K.D.; Aarsland, A.; Wolf, S.E.; Wolfe, R.R. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am. J. Physiol. Metab. 1997, 273, E99–E107. [Google Scholar] [CrossRef]
- Bolster, D.R.; Pikosky, M.A.; McCarthy, L.M.; Rodriguez, N.R. Exercise Affects Protein Utilization in Healthy Children. J. Nutr. 2001, 131, 2659–2663. [Google Scholar] [CrossRef]
- Pikosky, M.; Faigenbaum, A.; Westcott, W.; Rodriguez, N. Effects of resistance training on protein utilization in healthy children. Med. Sci. Sports Exerc. 2002, 34, 820–827. [Google Scholar] [CrossRef]
- Moore, D.R. Protein Metabolism in Active Youth: Not Just Little Adults. Exerc. Sport Sci. Rev. 2019, 47, 29–36. [Google Scholar] [CrossRef]
- Boisseau, N.; Persaud, C.; Jackson, A.A.; Poortmans, J.R. Training does not affect protein turnover in pre- and early pubertal female gymnasts. Graefe’s Arch. Clin. Exp. Ophthalmol. 2005, 94, 262–267. [Google Scholar] [CrossRef]
- Boisseau, N.; Le Creff, C.; Loyens, M.; Poortmans, J. Protein intake and nitrogen balance in male non-active adolescents and soccer players. Graefe’s Arch. Clin. Exp. Ophthalmol. 2002, 88, 288–293. [Google Scholar] [CrossRef]
- Ball, R.O.; Bayley, H.S. Influence of dietary protein concentration on the oxidation of phenylalanine by the young pig. Br. J. Nutr. 1986, 55, 651–658. [Google Scholar] [CrossRef]
- Elango, R.; Ball, R.O.; Pencharz, P.B. Indicator Amino Acid Oxidation: Concept and Application. J. Nutr. 2008, 138, 243–246. [Google Scholar] [CrossRef]
- Kim, I.-Y.; Deutz, N.E.P.; Wolfe, R.R. Update on maximal anabolic response to dietary protein. Clin. Nutr. 2018, 37, 411–418. [Google Scholar] [CrossRef]
- Elango, R.; Humayun, M.A.; Ball, R.O.; Pencharz, P.B. Indicator Amino Acid Oxidation Is Not Affected by Period of Adaptation to a Wide Range of Lysine Intake in Healthy Young Men. J. Nutr. 2009, 139, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- Fern, E.B.; Garlick, P.J.; Waterlow, J.C. Apparent compartmentation of body nitrogen in one human subject: Its consequences in measuring the rate of whole-body protein synthesis with 15N. Clin. Sci. 1985, 68, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Weiner, I.D.; Mitch, W.E.; Sands, J.M. Urea and Ammonia Metabolism and the Control of Renal Nitrogen Excretion. Clin. J. Am. Soc. Nephrol. 2014, 10, 1444–1458. [Google Scholar] [CrossRef] [PubMed]
- Abumrad, N.N.; Williams, P.; Frexes-Steed, M.; Geer, R.; Flakoll, P.; Cersosimo, E.; Brown, L.L.; Melki, I.; Bulus, N.; Hourani, H.; et al. Inter-organ metabolism of amino acids in vivo. Diabetes Metab. Rev. 1989, 5, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Felig, P. Amino Acid Metabolism in Man. Annu. Rev. Biochem. 1975, 44, 933–955. [Google Scholar] [CrossRef]
- Børsheim, E.; Chinkes, D.L.; McEntire, S.J.; Rodriguez, N.R.; Herndon, D.N.; Suman, O.E. Whole body protein kinetics measured with a non-invasive method in severely burned children. Burns 2010, 36, 1006–1012. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ferrando, A.A.; Lane, H.W.; Stuart, C.A.; Davis-Street, J.; Wolfe, R.R. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am. J. Physiol. Metab. 1996, 270, E627–E633. [Google Scholar] [CrossRef] [PubMed]
- Berryman, C.E.; Sepowitz, J.J.; McClung, H.L.; Lieberman, H.R.; Farina, E.K.; McClung, J.P.; Ferrando, A.A.; Pasiakos, S.M. Supplementing an energy adequate, higher protein diet with protein does not enhance fat-free mass restoration after short-term severe negative energy balance. J. Appl. Physiol. 2017, 122, 1485–1493. [Google Scholar] [CrossRef] [PubMed]
- Hellerstein, M.; Evans, W. Recent advances for measurement of protein synthesis rates, use of the ‘Virtual Biopsy’ approach, and measurement of muscle mass. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 191–200. [Google Scholar] [CrossRef]
- Shankaran, M.; King, C.L.; Angel, T.E.; Holmes, W.E.; Li, K.W.; Colangelo, M.; Price, J.C.; Turner, S.M.; Bell, C.; Hamilton, K.L.; et al. Circulating protein synthesis rates reveal skeletal muscle proteome dynamics. J. Clin. Investig. 2015, 126, 288–302. [Google Scholar] [CrossRef]
- Murphy, C.H.; Shankaran, M.; Churchward-Venne, T.A.; Mitchell, C.J.; Kolar, N.M.; Burke, L.M.; Hawley, J.A.; Kassis, A.; Karagounis, L.G.; Li, K.; et al. Effect of resistance training and protein intake pattern on myofibrillar protein synthesis and proteome kinetics in older men in energy restriction. J. Physiol. 2018, 596, 2091–2120. [Google Scholar] [CrossRef]
- Kuczmarski, R.J. CDC Growth Charts: United States; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention: Washington, DC, USA, 2000.
- Black, R.E.; Allen, L.H.; Bhutta, Z.A.; Caulfield, L.E.; De Onis, M.; Ezzati, M.; Mathers, C.; Rivera, J. Maternal and child undernutrition: Global and regional exposures and health consequences. Lancet 2008, 371, 243–260. [Google Scholar] [CrossRef]
- Pařízková, J. Growth and Growth Velocity of Lean Body Mass and Fat in Adolescent Boys. Pediatr. Res. 1976, 10, 647–650. [Google Scholar] [CrossRef]
- Hall, K.D. Modeling Metabolic Adaptations and Energy Regulation in Humans. Annu. Rev. Nutr. 2012, 32, 35–54. [Google Scholar] [CrossRef]
- Consultation, F.E. Dietary protein quality evaluation in human nutrition. FAO Food Nutr. Pap. 2011, 92, 1–66. [Google Scholar]
- Tessema, M.; Gunaratna, N.S.; Brouwer, I.D.; Donato, K.; Cohen, J.L.; McConnell, M.; Belachew, T.; Belayneh, D.; De Groote, H. Associations among High-Quality Protein and Energy Intake, Serum Transthyretin, Serum Amino Acids and Linear Growth of Children in Ethiopia. Nutrients 2018, 10, 1776. [Google Scholar] [CrossRef] [PubMed]
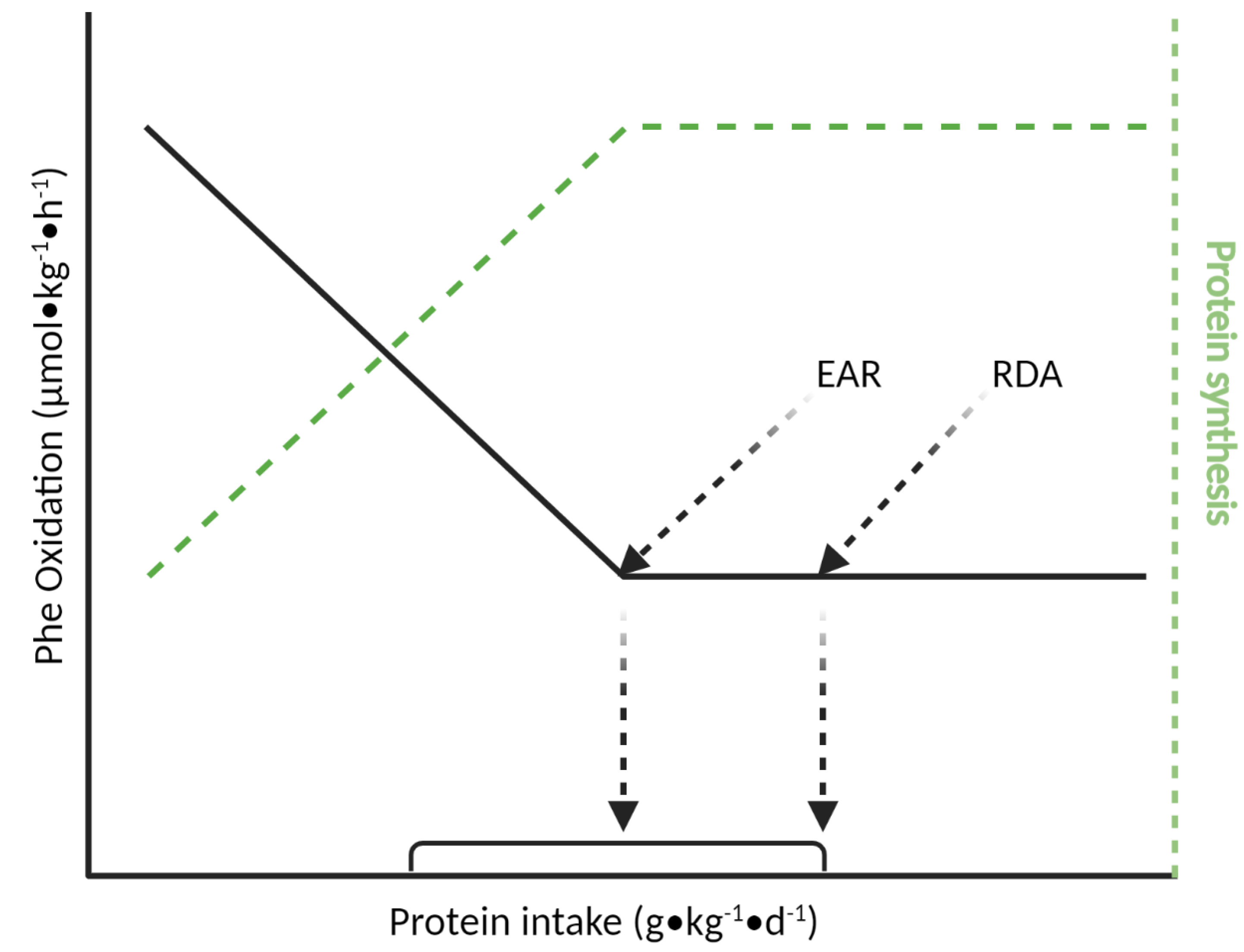
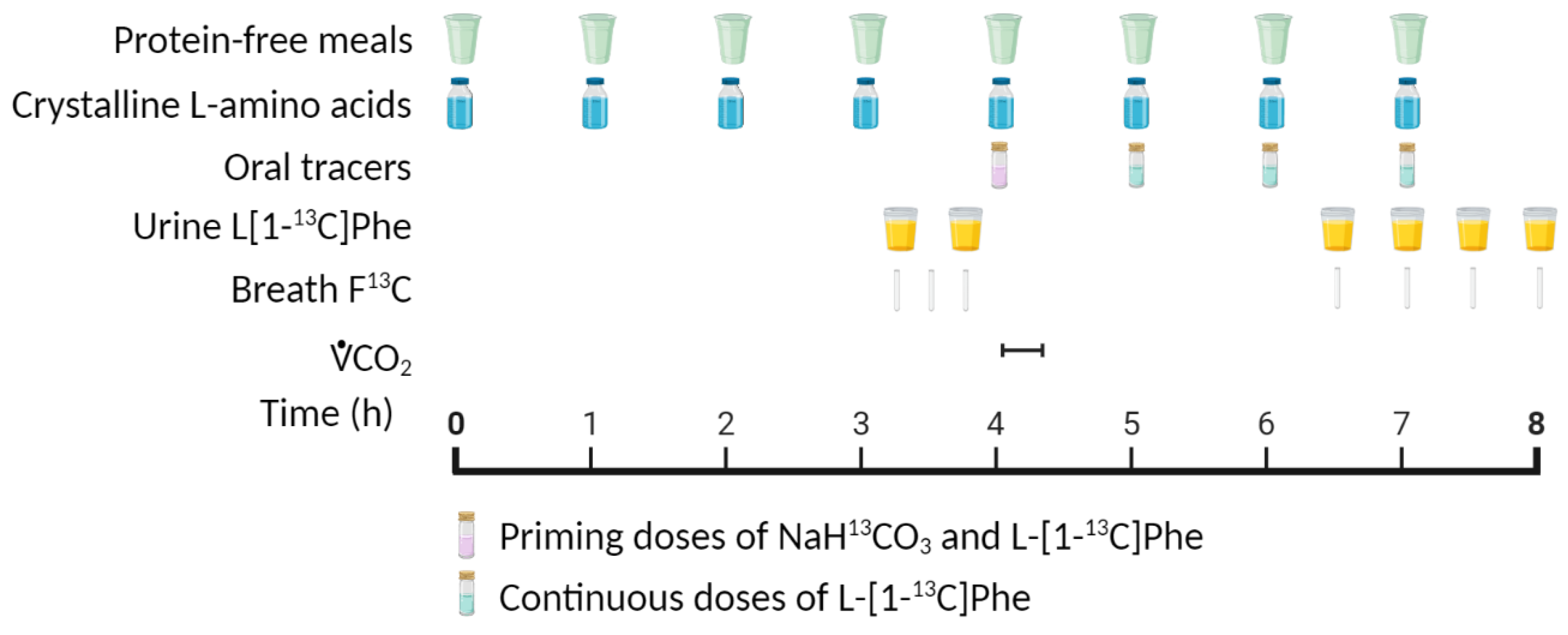
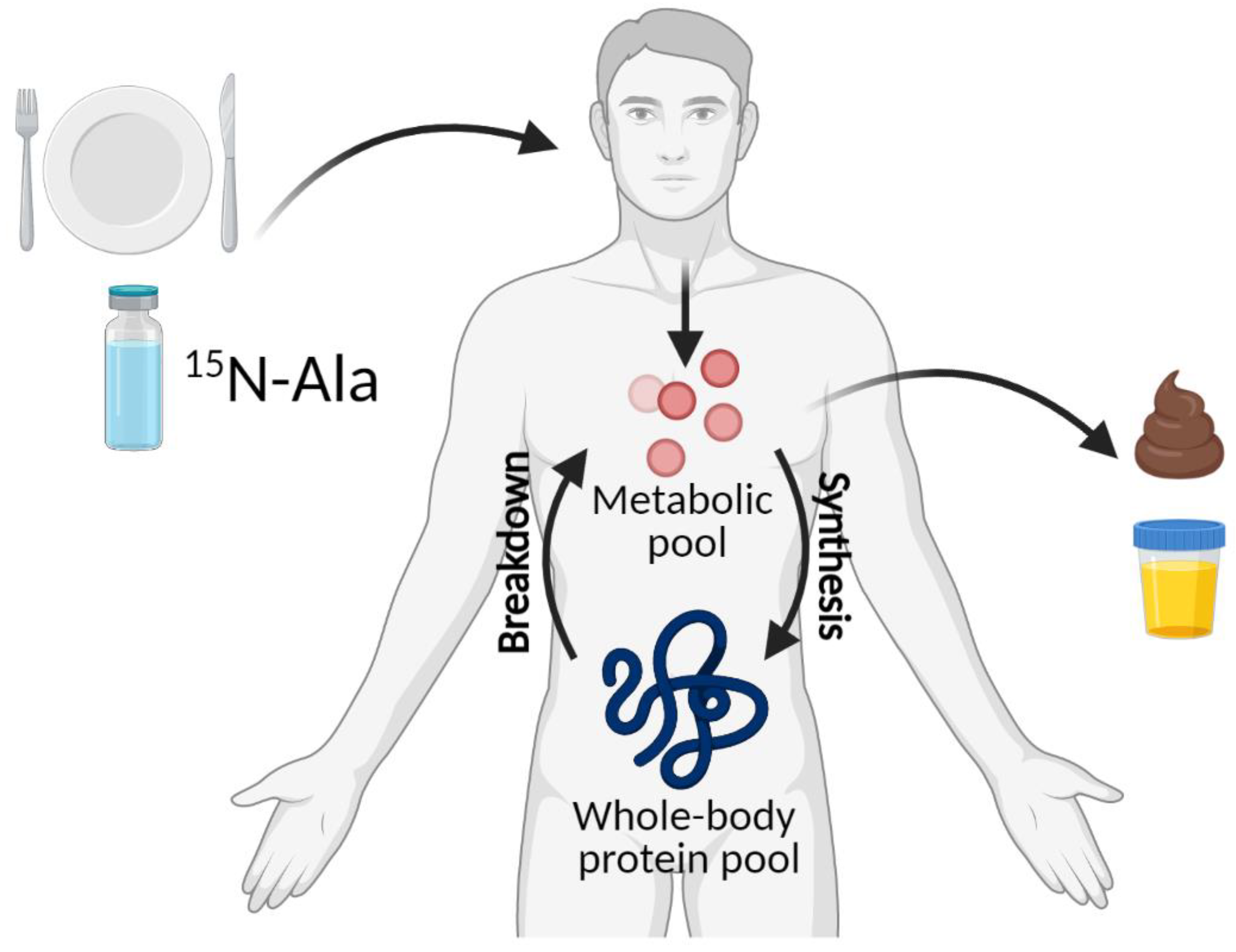
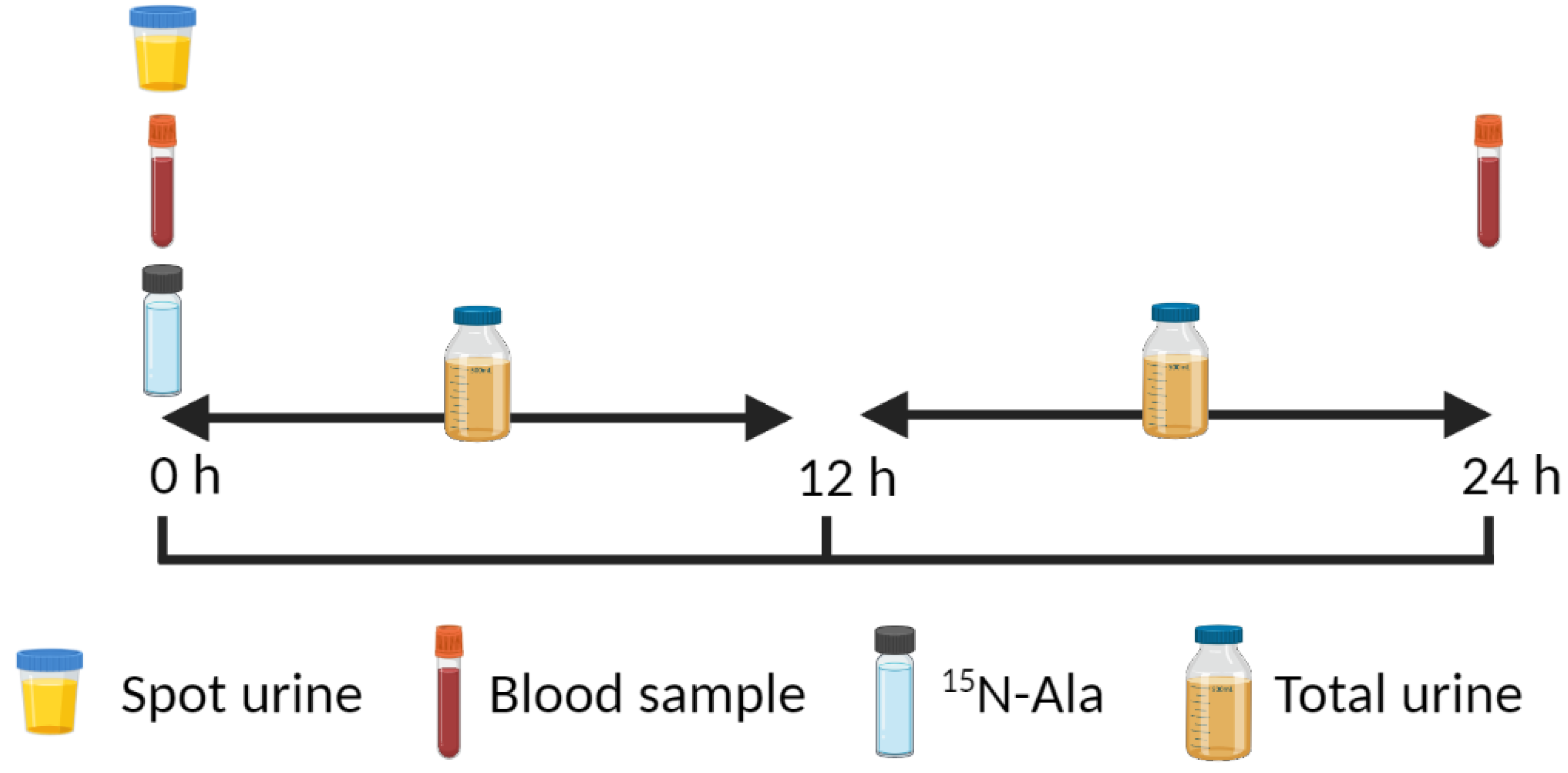
| Age Group | Nitrogen Balance 1 | IAAO 2 | ||
|---|---|---|---|---|
| EAR, g·kg−1·day−1 | RDA, g·kg−1·day−1 | EAR, g·kg−1·day−1 | RDA, g·kg−1·day−1 | |
| 7–12 months | 1.0 | 1.2 | ||
| 1–3 years | 0.87 | 1.05 | ||
| 4–8 years | 0.76 | 0.95 | 1.30 | 1.55 |
| 9–13 years | 0.76 | 0.95 | ||
| 14–18 years, boys | 0.73 | 0.85 | ||
| 14–18 years, girls | 0.71 | 0.85 | ||
| The Authors | M/F | Age, Year | Initial Training Status | Training During Measurement? | Duration | Method | Protein, g·kg−1⋅Period−1 | Outcome | |
|---|---|---|---|---|---|---|---|---|---|
| Bolster et al., 2001 [20] | 5/2 | 8–10 | One group | No | 6 weeks. aerobic training | 15N-Gly: 10 h overnight | >2.0 | Flux, synthesis and breakdown decreased from pre to post. Net balance tended to decrease (p = 0.36). | |
| Pikosky et al., 2002 [21] | 7/4 | 7–10 | One group | No | 6 weeks. resistance training | 15N-Gly: 10 h overnight | >1.5 | Flux, synthesis and breakdown decreased from pre to post. Net balance tended to decrease (p = 0.07). | |
| Boisseau et al., 2002 [24] | 8/0, | 15 | Control | No | Cross-sectional | Nitrogen balance: 24 h | 1.5 | The control group tended to have a negative nitrogen balance compared to the athlete group, p > 0.05. | |
| 11/0 | Soccer players | Yes, aerobic | 1.68 | ||||||
| Boisseau et al., 2005 [23] | 0/10 | 7–12 | Controls | No | Cross-sectional | 15N-Gly: 24 h | 1.60 | Flux, synthesis and breakdown were not different between groups. Net balance was greater in the controls than in the gymnasts. | |
| 0/10 | Gymnasts | No | 1.79 | ||||||
| Moore et al., 2014 [15] | 7/6 | ~12 | Active | Yes, aerobic | Cross-over | 15N-Gly: 9 and 24 h | 0.69 | 1.16 | Over 9 h, net balance was greater in high protein (p < 0.05) than low and control, respectively, with a trend (p = 0.075) toward low protein being greater than control. Net balance was positive over 9 h for all conditions but only over 24 h for high protein. |
| 0.87 | 1.35 | ||||||||
| 1.02 | 1.49 | ||||||||
| Volterman et al., 2014 [16] | 13/15 | 7–17 | Active | No | Cross-over | 15N-Gly: 16 h | 0.83 | Over 16 h, flux and synthesis were greater with skim milk than control and carbohydrate, respectively. Net balance was more negative with control and carbohydrate than with skim milk. | |
| 0.82 | |||||||||
| 1.24 | |||||||||
| Volterman et al., 2017 [17] | 7/3 | 9–13 | Active | Yes, aerobic | Cross-sectional | 13C-Leu: 3 h | 0 g protein | Dose response increase in net protein balance. | |
| 6/2 | 5 g protein | ||||||||
| 7/2 | 10 g protein | ||||||||
| 6/2 | 15 g protein | ||||||||
| IAAO Method | 15N End-Product Method | D3-Creatine Method |
|---|---|---|
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hudson, J.L.; Baum, J.I.; Diaz, E.C.; Børsheim, E. Dietary Protein Requirements in Children: Methods for Consideration. Nutrients 2021, 13, 1554. https://doi.org/10.3390/nu13051554
Hudson JL, Baum JI, Diaz EC, Børsheim E. Dietary Protein Requirements in Children: Methods for Consideration. Nutrients. 2021; 13(5):1554. https://doi.org/10.3390/nu13051554
Chicago/Turabian StyleHudson, Joshua L., Jamie I. Baum, Eva C. Diaz, and Elisabet Børsheim. 2021. "Dietary Protein Requirements in Children: Methods for Consideration" Nutrients 13, no. 5: 1554. https://doi.org/10.3390/nu13051554
APA StyleHudson, J. L., Baum, J. I., Diaz, E. C., & Børsheim, E. (2021). Dietary Protein Requirements in Children: Methods for Consideration. Nutrients, 13(5), 1554. https://doi.org/10.3390/nu13051554








