Response to Malnutrition Treatment in Low Weight-for-Age Children: Secondary Analyses of Children 6–59 Months in the ComPAS Cluster Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
- Group 1: MUAC < 11.5 cm and WAZ ≥ −3;
- Group 2: MUAC between 11.5 cm and <12.5 cm and WAZ < −3;
- Group 3: MUAC < 11.5 cm and WAZ < −3;
- Group 4: MUAC between 11.5 cm and <12.5 cm and WAZ ≥ −3.0.
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Black, R.E.; Victora, C.G.; Walker, S.P.; Bhutta, Z.A.; Christian, P.; de Onis, M.; Ezzati, M.; Grantham-McGregor, S.; Katz, J.; Martorell, R.; et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013, 382, 427–451. [Google Scholar] [CrossRef]
- UNICEF; World Health Organization; World Bank Group. Levels and Trends in Child Malnutrition: Key Findings of the 2019 Edition of the Joint Child Malnutrition Estimates. 2019. Available online: https://www.unicef.org/reports/joint-child-malnutrition-estimates-levels-and-trends-child-malnutrition-2019 (accessed on 22 March 2021).
- Isanaka, S.; Boundy, E.O.; Grais, R.F.; Myatt, M.; Briend, A. Improving Estimates of Numbers of Children With Severe Acute Malnutrition Using Cohort and Survey Data. Am. J. Epidemiol. 2016, 184, 861–869. [Google Scholar] [CrossRef]
- Hure, A.; Oldmeadow, C.; Attia, J. Invited Commentary: Improving Estimates of Severe Acute Malnutrition Requires More Data. Am. J. Epidemiol. 2016, 184, 870–872. [Google Scholar] [CrossRef]
- Briend, A.; Collins, S.; Golden, M.; Manary, M.; Myatt, M. Maternal and child nutrition. Lancet 2013, 382, 1549. [Google Scholar] [CrossRef]
- Roberton, T.; Carter, E.D.; Chou, V.B.; Stegmuller, A.R.; Jackson, B.D.; Tam, Y.; Sawadogo-Lewis, T.; Walker, N. Early estimates of the indirect effects of the COVID-19 pandemic on maternal and child mortality in low-income and middle-income countries: A modelling study. Lancet Glob. Health 2020, 8, e901–e908. [Google Scholar] [CrossRef]
- Shoham, J.; Dolan, C.; Gostelow, L. Managing Acute Malnutrition at Scale: A Review of Donor and Government Financing Arrangements; Overseas Development Institute: London, UK, 2013. [Google Scholar]
- Menon, P.; Stoltzfus, R.J. Building convergence in science, programs, and policy actions on child undernutrition: Symposium rationale and overview. Adv. Nutr. 2012, 3, 224–226. [Google Scholar] [CrossRef]
- Bergeron, G.; Castleman, T. Program responses to acute and chronic malnutrition: Divergences and convergences. Adv. Nutr. 2012, 3, 242–249. [Google Scholar] [CrossRef]
- Khara, T.; Dolan, C. The Relationship between Wasting and Stunting, Policy, Programming and Research Implications; Emergency Nutrition Network: Oxford, UK, 2014; Available online: https://www.ennonline.net/attachments/1862/WAST_140714.pdf (accessed on 22 March 2021).
- Reinhardt, K.; Fanzo, J. Addressing Chronic Malnutrition through Multi-Sectoral, Sustainable Approaches: A Review of the Causes and Consequences. Front. Nutr. 2014, 1, 13. [Google Scholar] [CrossRef]
- Martorell, R.; Young, M.F. Patterns of stunting and wasting: Potential explanatory factors. Adv. Nutr. 2012, 3, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Victora, C.G. The association between wasting and stunting: An international perspective. J. Nutr. 1992, 122, 1105–1110. [Google Scholar] [CrossRef]
- Khara, T.; Mwangome, M.; Ngari, M.; Dolan, C. Children concurrently wasted and stunted: A meta-analysis of prevalence data of children 6–59 months from 84 countries. Matern. Child. Nutr. 2018, 14, e12516. [Google Scholar] [CrossRef]
- WFP; UNICEF. Updated Guidance on Mutual Areas of Responsibility and Collaboration for Nutrition: An Update to the UNICEF, WFP 2005 MOU; World Food Program: Rome, Italy, 2011. [Google Scholar]
- McDonald, C.M.; Olofin, I.; Flaxman, S.; Fawzi, W.W.; Spiegelman, D.; Caulfield, L.E.; Black, R.E.; Ezzati, M.; Danaei, G.; Nutrition Impact Model Study. The effect of multiple anthropometric deficits on child mortality: Meta-analysis of individual data in 10 prospective studies from developing countries. Am. J. Clin. Nutr. 2013, 97, 896–901. [Google Scholar] [CrossRef]
- Myatt, M.; Khara, T.; Schoenbuchner, S.; Pietzsch, S.; Dolan, C.; Lelijveld, N.; Briend, A. Children who are both wasted and stunted are also underweight and have a high risk of death: A descriptive epidemiology of multiple anthropometric deficits using data from 51 countries. Arch. Public Health 2018, 76, 28. [Google Scholar] [CrossRef]
- Victora, C.G.; Christian, P.; Vidaletti, L.P.; Gatica-Dominguez, G.; Menon, P.; Black, R.E. Revisiting maternal and child undernutrition in low-income and middle-income countries: Variable progress towards an unfinished agenda. Lancet 2021. [Google Scholar] [CrossRef]
- Myatt, M.; Khara, T.; Dolan, C.; Garenne, M.; Briend, A. Improving screening for malnourished children at high risk of death: A study of children aged 6-59 months in rural Senegal. Public Health Nutr. 2019, 22, 862–871. [Google Scholar] [CrossRef]
- Myatt, M. Multiple Cohort Mortality Analysis. Wasting Stunting Technical Interest Group Meeting—Revisiting Mortality and Anthropometric Deficits and Reconsidering ‘Risk’; Emergency Nutrition Network: London, UK, 9 December 2019; Available online: https://www.ennonline.net/wastswgmortalitymeetingreportdec2019 (accessed on 22 March 2021).
- Briend, A.; Alvarez, J.L.; Avril, N.; Bahwere, P.; Bailey, J.; Berkley, J.A.; Binns, P.; Blackwell, N.; Dale, N.; Deconinck, H.; et al. Low mid-upper arm circumference identifies children with a high risk of death who should be the priority target for treatment. BMC Nutr. 2016, 26, 3. [Google Scholar] [CrossRef]
- Myatt, M.; Khara, T.; Collins, S. A review of methods to detect cases of severely malnourished children in the community for their admission into community-based therapeutic care programs. Food Nutr. Bull. 2006, 27, S7–S23. [Google Scholar] [CrossRef]
- Briend, A.; Maire, B.; Fontaine, O.; Garenne, M. Mid-upper arm circumference and weight-for-height to identify high-risk malnourished under-five children. Matern. Child. Nutr. 2012, 8, 130–133. [Google Scholar] [CrossRef]
- Bliss, J.; Lelijveld, N.; Briend, A.; Kerac, M.; Manary, M.; McGrath, M.; Weise Prinzo, Z.; Shepherd, S.; Marie Zagre, N.; Woodhead, S.; et al. Use of Mid-Upper Arm Circumference by Novel Community Platforms to Detect, Diagnose, and Treat Severe Acute Malnutrition in Children: A Systematic Review. Glob. Health Sci. Pract. 2018, 6, 552–564. [Google Scholar] [CrossRef]
- WHO. Community-Based Management of Severe Acute Malnutrition. A Joint Statement by the World Health Organization, World Food Programme, United Nations Standing Committee on Nutrition, United Nations Children’s Fund; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- WHO. Guideline: Updates on the Management of Severe Acute Malnutrition in Infants and Children; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Bailey, J.; Lelijveld, N.; Marron, B.; Onyoo, P.; Ho, L.S.; Manary, M.; Briend, A.; Opondo, C.; Kerac, M. Combined Protocol for Acute Malnutrition Study (ComPAS) in rural South Sudan and urban Kenya: Study protocol for a randomized controlled trial. Trials 2018, 19, 251. [Google Scholar] [CrossRef]
- Kangas, S.T.; Salpeteur, C.; Nikiema, V.; Talley, L.; Ritz, C.; Friis, H.; Briend, A.; Kaestel, P. Impact of reduced dose of ready-to-use therapeutic foods in children with uncomplicated severe acute malnutrition: A randomised non-inferiority trial in Burkina Faso. PLoS Med. 2019, 16, e1002887. [Google Scholar] [CrossRef]
- Phelan, K. OptiMA Study in Burkina Faso: Emerging Findings and Additional Insights. Field Exchange. 2019. Available online: https://www.ennonline.net/fex/60/optimastudyburkinafaso (accessed on 22 March 2021).
- Daures, M.; Phelan, K.; Issoufou, M.; Kouanda, S.; Sawadogo, O.; Issaley, K.; Cazes, C.; Séri, B.; Ouaro, B.; Akpakpo, B.; et al. New approach to simplifying and optimising acute malnutrition treatment in children aged 6–59 months: The OptiMA single-arm proof-of-concept trial in Burkina Faso. Br. J. Nutr. 2020, 123, 756–767. [Google Scholar] [CrossRef] [PubMed]
- Defining and Treating “High-Risk” Moderate Acute Malnutrition Using Expanded Admission Criteria (Hi-MAM Study): A Cluster-Randomised Controlled Trial Protocol. Field Exchange. 2019. Available online: https://www.ennonline.net/fex/60/himamstudy (accessed on 22 March 2021).
- Optimizing Acute Malnutrition Management in Children Aged 6 to 59 Months in Democratic Republic of Congo (OptiMA-DRC). Available online: https://clinicaltrials.gov/ct2/show/NCT03751475 (accessed on 22 March 2021).
- WHO. Joint UN Communiqué: Simplified Approaches for the Treatment of Child Wasting. 26 March 2019. Available online: https://www.who.int/news-room/events/detail/2019/03/26/default-calendar/simplified-approaches-for-the-treatment-of-child-wasting (accessed on 22 March 2021).
- Chase, R.P.; Kerac, M.; Grant, A.; Manary, M.; Briend, A.; Opondo, C.; Bailey, J. Acute malnutrition recovery energy requirements based on mid-upper arm circumference: Secondary analysis of feeding program data from 5 countries, Combined Protocol for Acute Malnutrition Study (ComPAS) Stage 1. PLoS ONE 2020, 15, e0230452. [Google Scholar] [CrossRef]
- Bailey, J.; Opondo, C.; Lelijveld, N.; Marron, B.; Onyo, P.; Musyoki, E.N.; Adongo, S.W.; Manary, M.; Briend, A.; Kerac, M. A simplified, combined protocol versus standard treatment for acute malnutrition in children 6–59 months (ComPAS trial): A cluster-randomized controlled non-inferiority trial in Kenya and South Sudan. PLoS Med. 2020, 17, e1003192. [Google Scholar] [CrossRef] [PubMed]
- Maust, A.; Koroma, A.S.; Abla, C.; Molokwu, N.; Ryan, K.N.; Singh, L.; Manary, M.J. Severe and Moderate Acute Malnutrition Can Be Successfully Managed with an Integrated Protocol in Sierra Leone. J. Nutr. 2015, 145, 2604–2609. [Google Scholar] [CrossRef]
- Lelijveld, N.; Bailey, J.; Mayberry, A.; Trenouth, L.; N’Diaye, D.S.; Haghparast-Bidgoli, H.; Puett, C. The “ComPAS Trial” combined treatment model for acute malnutrition: Study protocol for the economic evaluation. Trials 2018, 19, 252. [Google Scholar] [CrossRef]
- Goossens, S.; Bekele, Y.; Yun, O.; Harczi, G.; Ouannes, M.; Shepherd, S. Mid-upper arm circumference based nutrition programming: Evidence for a new approach in regions with high burden of acute malnutrition. PLoS ONE 2012, 7, e49320. [Google Scholar] [CrossRef]
- Binns, P.; Dale, N.; Hoq, M.; Banda, C.; Myatt, M. Relationship between mid upper arm circumference and weight changes in children aged 6–59 months. Arch. Public Health 2015, 73, 54. [Google Scholar] [CrossRef]
- Bailey, J.; Opondo, C.; Lelijveld, N.; Marron, B.; Onyoo, P.; Musyoki, E.; Adongo, S.; Briend, A.; Manary, M.; Kerac, M. Combined Protocol for Acute Malnutrition Study (ComPAS) Dataset; London School of Hygiene and Tropical Medicine: London, UK, 2019. [Google Scholar] [CrossRef]
- BodyStat. Isle of Man: Douglas. Available online: https://www.bodystat.com/ (accessed on 22 March 2021).
- Wells, J.C.; Hawton, K.; Darch, T.; Lunn, P.G. Body composition by 2H dilution in Gambian infants: Comparison with UK infants and evaluation of simple prediction methods. Br. J. Nutr. 2009, 102, 1776–1782. [Google Scholar] [CrossRef] [PubMed]
- LeRoy, J. zscore06: Stata Command for the Calculation of Anthropometric z-Scores Using the 2006 WHO Child Growth Standards. 2011. Available online: https://econpapers.repec.org/software/bocbocode/s457279.htm (accessed on 22 March 2021).
- WHO. WHO Child Growth Standards: LFH, LFA, WFA, WFL, WFH, and BMI/A: Methods and Development; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr. Suppl. 2006, 450, 76–85. [Google Scholar]
- CMAM Report: A Comprehensive Monitoring and Reporting Package for Community-Based Management of Acute Malnutrition. Standardised Indicators and Categories for Better CMAM Reporting. April 2015. Available online: https://www.cmamreport.com/sites/all/themes/stc/cmam-assets/STANDARDISED%20CATEGORIES%20AND%20INDICATORS%20FOR%20BETTER%20CMAM%20REPORTING%20%20FINAL%20Apr%202015.pdf (accessed on 22 March 2021).
- Cleveland, W.S. Robust locally weighted regression and smoothing scatterplots. J. Am. Statist. Assoc. 1979, 74, 829–836. [Google Scholar] [CrossRef]
- Briend, A.; Khara, T.; Dolan, C. Wasting and stunting--similarities and differences: Policy and programmatic implications. Food Nutr. Bull. 2015, 36, S15–S23. [Google Scholar] [CrossRef]
- Grellety, E.; Golden, M.H. Severely malnourished children with a low weight-for-height have a higher mortality than those with a low mid-upper-arm-circumference: I. Empirical data demonstrates Simpson’s paradox. Nutr. J. 2018, 17, 79. [Google Scholar] [CrossRef]
- Pelletier, D.L. The relationship between child anthropometry and mortality in developing countries: Implications for policy, programs and future research. J. Nutr. 1994, 124, 2047S–2081S. [Google Scholar]
- Mwangome, M.; Ngari, M.; Fegan, G.; Mturi, N.; Shebe, M.; Bauni, E.; Berkley, J.A. Diagnostic criteria for severe acute malnutrition among infants aged under 6 mo. Am. J. Clin. Nutr. 2017, 105, 1415–1423. [Google Scholar] [CrossRef]
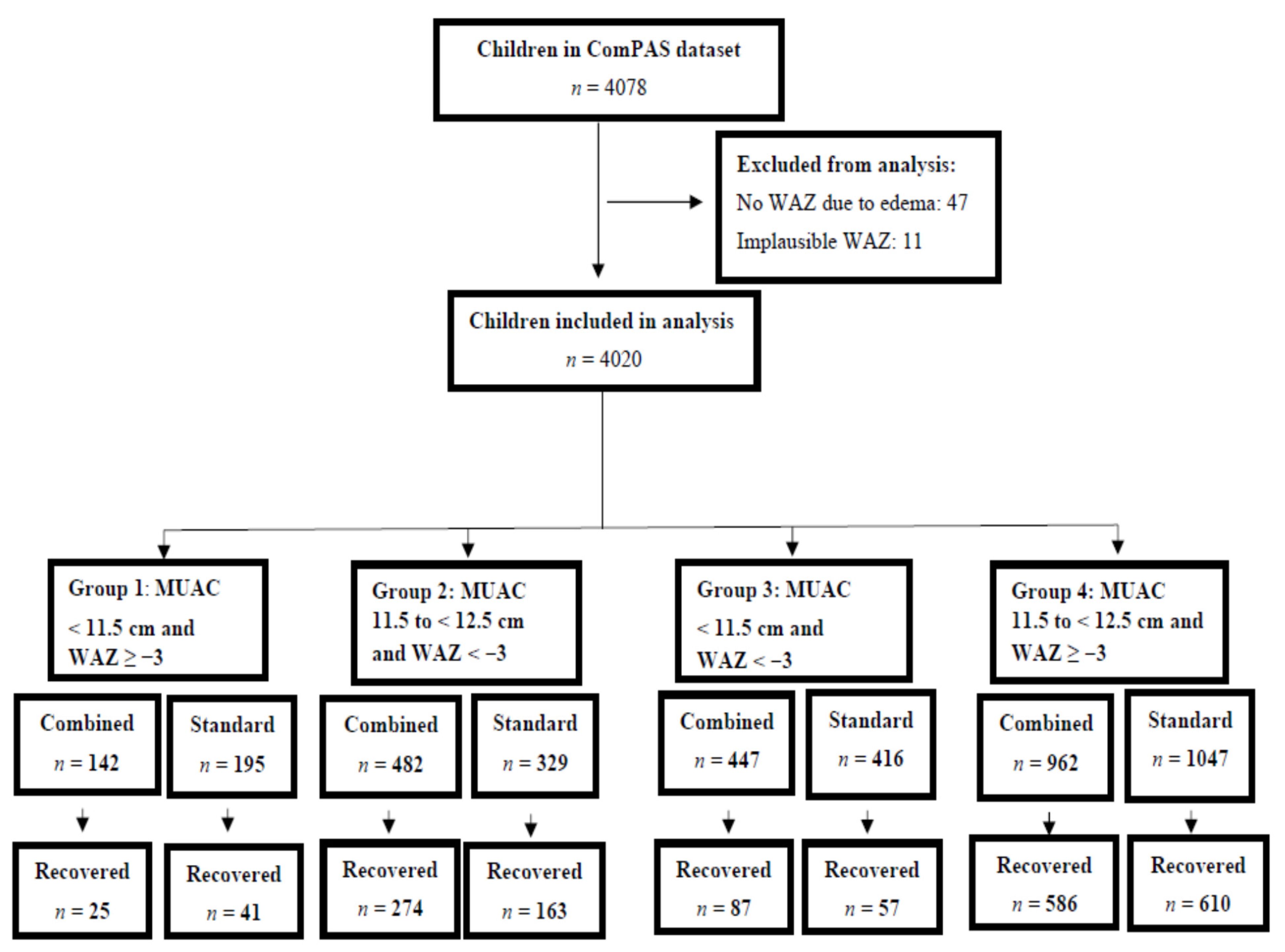
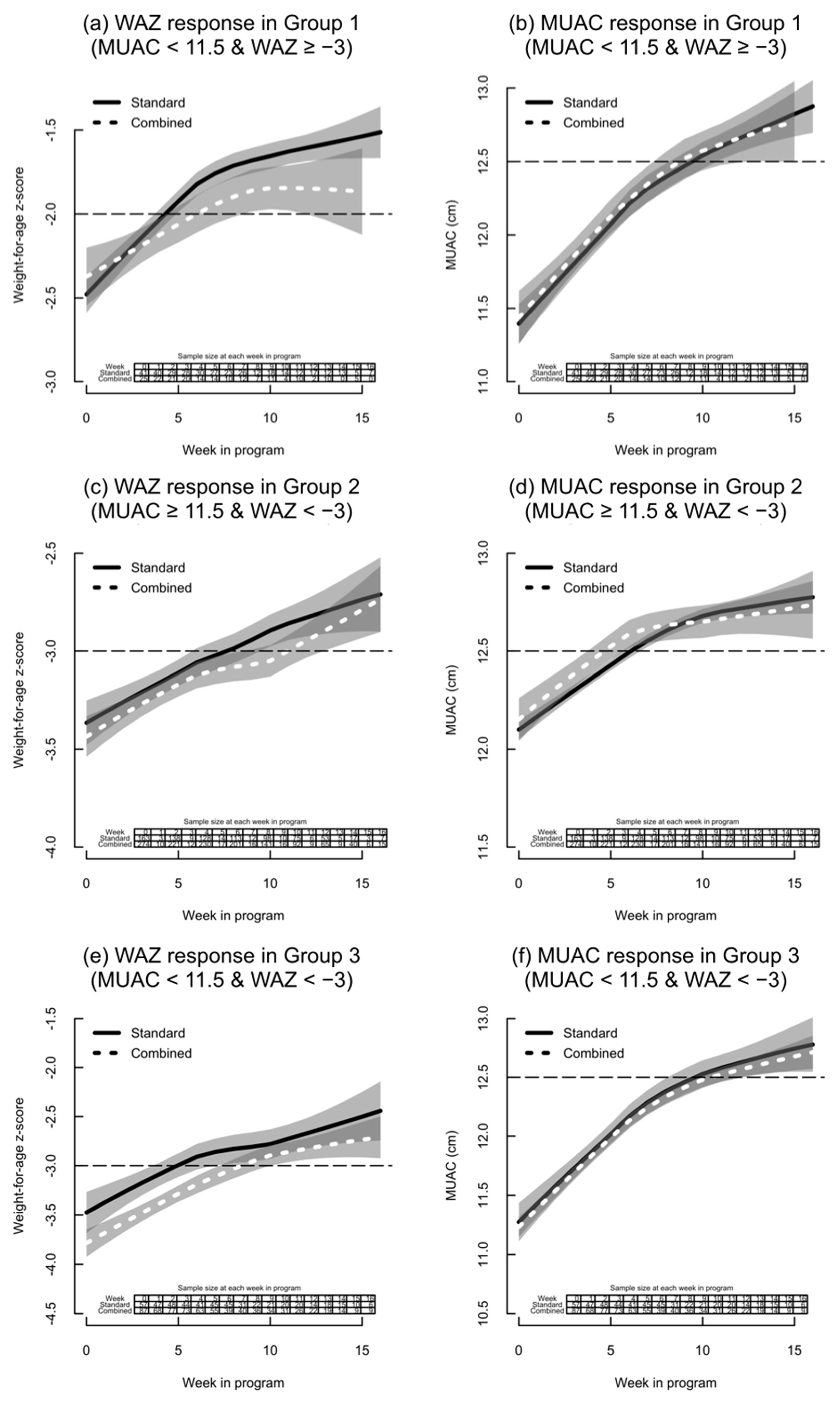
| Group | Combined Protocol | Standard Protocol |
|---|---|---|
| Group 1: MUAC < 11.5 cm and WAZ ≥ −3.0 | 4184 kJ (1000 kcal)/day RUTF | 836.8 kJ (200 kcal)/kg/day RUTF |
| Group 2: MUAC 11.5 to <12.5 and WAZ < −3.0 | 2092 kJ (500 kcal)/day RUTF | 2092 kJ (500 kcal)/day RUSF |
| Group 3: MUAC < 11.5 cm and WAZ < −3.0 | 4184 kJ (1000 kcal)/day RUTF | 836.8 kJ (200 kcal)/kg/day RUTF |
| Group 4: MUAC 11.5 to <12.5 and WAZ ≥ −3.0 | 2092 kJ (500 kcal)/day RUTF | 2092 kJ (500 kcal)/day RUSF |
| Admission Groups | Total by Category | By Intervention Arm | By Country | ||
|---|---|---|---|---|---|
| Combined Protocol | Standard Protocol | Kenya | South Sudan | ||
| Group 1 MUAC < 11.5 cm and WAZ ≥ −3.0 | 337 (8.4%) | 142 (7%) | 195 (9.8%) | 126 (6.4%) | 211 (10.3%) |
| Group 2 MUAC 11.5 to <12.5 cm and WAZ < −3.0 | 811 (20.2%) | 482 (23.7%) | 329 (16.6%) | 294 (15%) | 517 (25.1%) |
| Group 3 MUAC < 11.5 cm and WAZ < −3.0 | 863 (21.5%) | 447 (22%) | 416 (20.9%) | 215 (11%) | 648 (31.5%) |
| Group 4 MUAC 11.5 to <12.5 cm and WAZ ≥ −3.0 | 2009 (50%) | 962 (47.3%) | 1047 (52.7%) | 1327 (67.6%) | 682 (33.1%) |
| Total | 4020 | 2033 | 1987 | 1962 | 2058 |
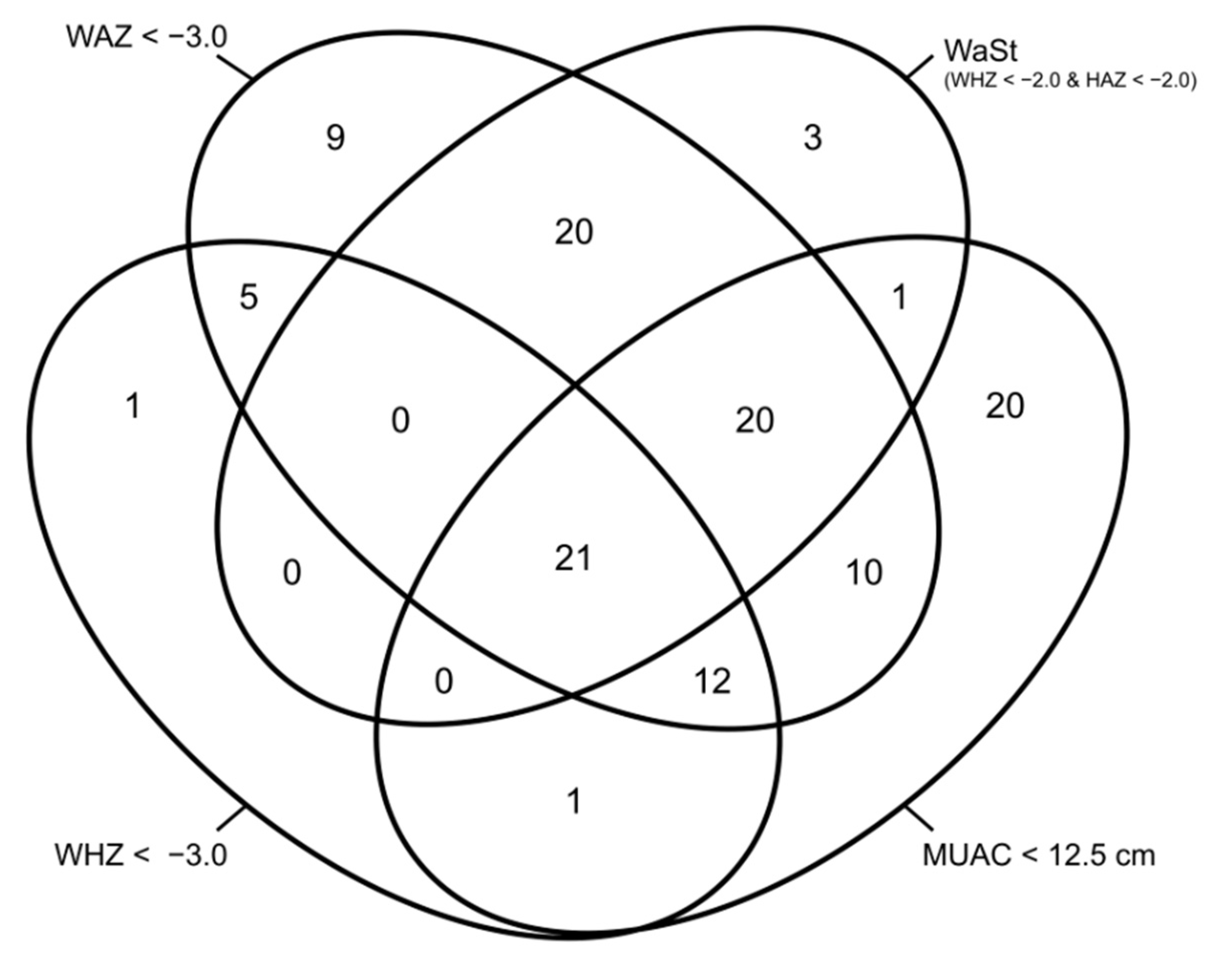
| Country | Criterion | WAZ < −3.0 | WAZ ≥ −3.0 | Total |
|---|---|---|---|---|
| Kenya | WHZ < −2 and HAZ < −2 | 322 (80.9%) | 76 (19.1%) | 398 |
| South Sudan | WHZ < −2 and HAZ < −2 | 828 (93.3%) | 59 (6.7%) | 887 |
| Both | WHZ < −2 and HAZ < −2 | 1150 (89.5%) | 135 (10.5%) | 1285 |
| Characteristic | Group 1 MUAC < 11.5 cm and WAZ ≥ −3.0 (n = 337) | Group 2 MUAC 11.5 to <12.5 cm and WAZ < −3.0 (n = 811) | Group 3 MUAC < 11.5 cm and WAZ < −3.0 (n = 863) | |||
|---|---|---|---|---|---|---|
| Combined (m § = 12, n † = 142) | Standard (m = 12, n = 195) | Combined (m = 12, n = 482) | Standard (m = 12, n = 329) | Combined (m = 12, n = 447) | Standard (m = 12, n = 416) | |
| Sex and Age | ||||||
| Males, n (%) | 31 (21.8%) | 45 (23.1%) | 258 (53.5%) | 194 (59%) | 221 (49.4%) | 196 (47.1%) |
| Age at admission (months), median (IQR) | 10 (8, 14) | 10 (7, 14) | 18 (12, 30) | 21 (14, 30) | 14 (9, 24) | 15 (10, 24) |
| Age 6–24 months, n (%) | 119 (83.8%) | 168 (86.2%) | 274 (56.8%) | 173 (52.6%) | 306 (68.5%) | 287 (69.0%) |
| Anthropometry | ||||||
| Weight (kg), mean (SD) | 7.00 (1.54) | 6.97 (1.62) | 7.30 (1.42) | 7.52 (1.39) | 6.39 (1.34) | 6.50 (1.31) |
| Height (cm) *, mean (SD) | 72.6 (9.28) | 72.0 (9.29) | 74.0 (8.34) | 74.9 (8.06) | 70.8 (8.07) | 71.5 (8.09) |
| MUAC (cm), mean (SD) | 11.2 (0.23) | 11.2 (0.29) | 12.0 (0.27) | 12.0 (0.26) | 11.0 (0.44) | 11.0 (0.49) |
| WAZ, mean (SD) | −2.4 (0.56) | −2.4 (0.52) | −3.6 (0.47) | −3.6 (0.52) | −4.1 (0.67) | −3.9 (0.66) |
| HAZ, mean (SD) | −0.91 (1.07) | −0.99 (1.16) | −3.05 (1.02) | −3.07 (1.03) | −3.13 (1.25) | −2.82 (1.17) |
| WHZ, mean (SD) | −2.61 (1.01) | −2.52 (0.73) | −2.72 (0.79) | −2.62 (0.81) | −3.23 (0.83) | −3.24 (0.86) |
| MUAC < 11.5 cm | MUAC 11.5 to <12.5 cm | |||||||
|---|---|---|---|---|---|---|---|---|
| Intention-to-Treat | Group 1 MUAC < 11.5 cm and WAZ ≥ −3.0 (n = 337) | Group 3 MUAC < 11.5 cm and WAZ < −3.0 (n = 863) | Group 2 MUAC 11.5 to <12.5 cm and WAZ < −3.0 (n = 811) | Group 4 MUAC 11.5 to <12.5 cm and WAZ ≥ −3.0 (n = 2009) | ||||
| n | % | n | % | n | % | n | % | |
| Recovered * | 66 | 19.6 | 144 | 16.7 | 437 | 53.9 | 1196 | 59.5 |
| Died | 3 | 0.9 | 16 | 1.9 | 7 | 0.9 | 18 | 0.9 |
| Defaulted | 131 | 38.9 | 340 | 39.4 | 176 | 21.7 | 480 | 23.9 |
| Non-recovered† | 102 | 30.3 | 225 | 26.1 | 69 | 8.5 | 176 | 8.8 |
| Transfer-inpatient | 9 | 2.7 | 29 | 3.4 | 10 | 1.2 | 8 | 0.4 |
| Transfer-new facility | 8 | 2.4 | 33 | 3.8 | 15 | 1.9 | 13 | 0.7 |
| Early discharge | 18 | 5.3 | 78 | 9.0 | 102 | 12.6 | 130 | 6.5 |
| Median | IQR | Median | IQR | Median | IQR | Median | IQR | |
| Length of stay (days)‡ | 94 | 73, 106 | 89.5 | 68, 101 | 64 | 43, 85 | 57 | 43, 76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bailey, J.; Lelijveld, N.; Khara, T.; Dolan, C.; Stobaugh, H.; Sadler, K.; Lino Lako, R.; Briend, A.; Opondo, C.; Kerac, M.; et al. Response to Malnutrition Treatment in Low Weight-for-Age Children: Secondary Analyses of Children 6–59 Months in the ComPAS Cluster Randomized Controlled Trial. Nutrients 2021, 13, 1054. https://doi.org/10.3390/nu13041054
Bailey J, Lelijveld N, Khara T, Dolan C, Stobaugh H, Sadler K, Lino Lako R, Briend A, Opondo C, Kerac M, et al. Response to Malnutrition Treatment in Low Weight-for-Age Children: Secondary Analyses of Children 6–59 Months in the ComPAS Cluster Randomized Controlled Trial. Nutrients. 2021; 13(4):1054. https://doi.org/10.3390/nu13041054
Chicago/Turabian StyleBailey, Jeanette, Natasha Lelijveld, Tanya Khara, Carmel Dolan, Heather Stobaugh, Kate Sadler, Richard Lino Lako, André Briend, Charles Opondo, Marko Kerac, and et al. 2021. "Response to Malnutrition Treatment in Low Weight-for-Age Children: Secondary Analyses of Children 6–59 Months in the ComPAS Cluster Randomized Controlled Trial" Nutrients 13, no. 4: 1054. https://doi.org/10.3390/nu13041054







