Gluten and Autism Spectrum Disorder
Abstract
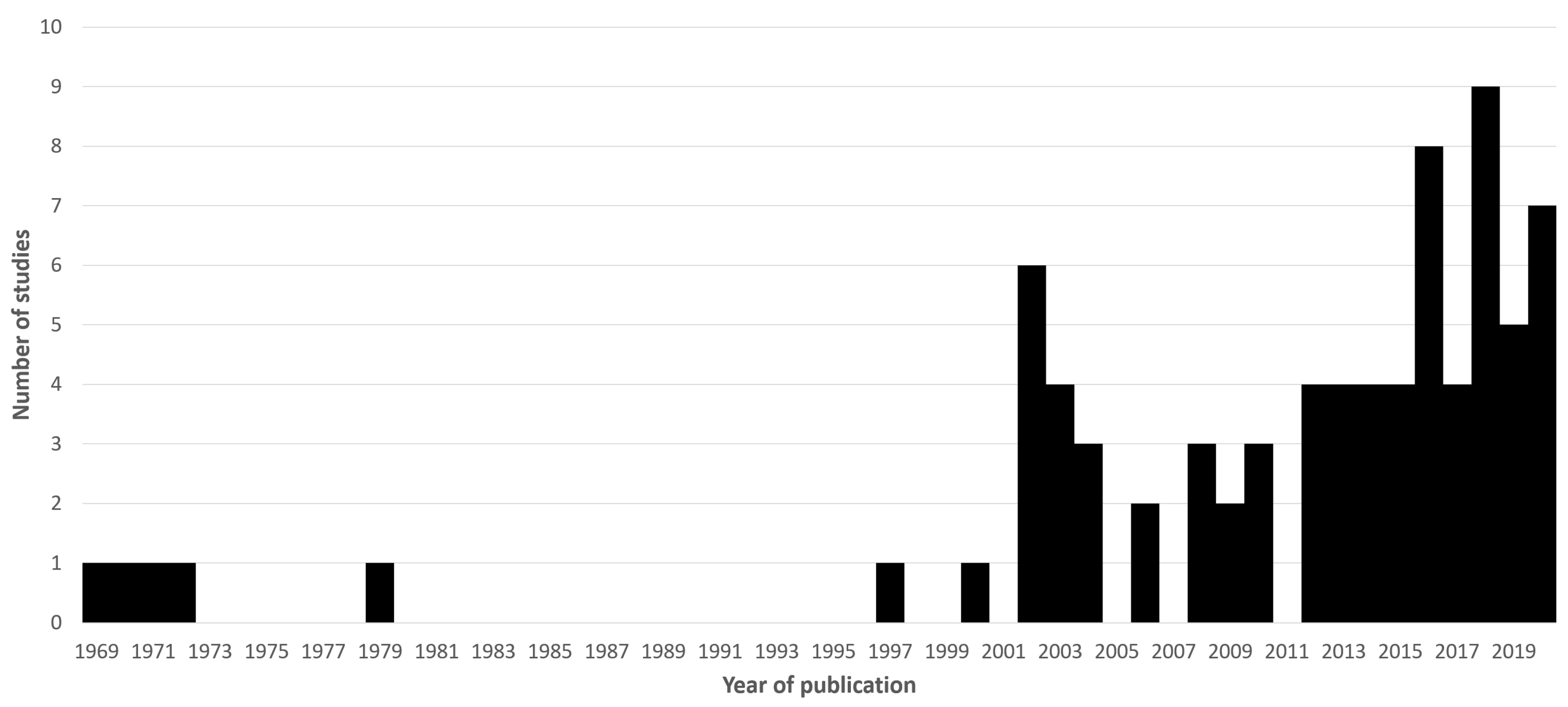
1. Motivation and Literature Search Methods
- Autism coeliac
- Autism celiac
- Autism gluten
- Autism wheat
- Autistic coeliac
- Autistic celiac
- Autistic gluten
- Autistic wheat
2. Historical Context
3. Gastrointestinal Symptoms in ASD
4. The Co-Morbidity between ASD and CD
5. Hypothetical Mechanisms of Action
6. Trials of the GFD in ASD
7. Adoption of the GFD and GCFD in ASD
8. Nutritional Considerations
9. Synthesis of Literature and Future Directions for Research
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar]
- Maenner, M.J.; Shaw, K.A.; Baio, J.; Washington, A.; Patric, M.; DiRienzo, M.; Christensen, D.L.; Wiggins, L.D.; Pettygrove, S.; Andrews, J.G.; et al. Prevalence of Autism Spectrum Disorder among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2016. MMWR Surveill. Summ. 2020, 69, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sapone, A.; Bai, J.C.; Ciacci, C.; Dolinsek, J.; Green, P.H.; Hadjivassiliou, M.; Kaukinen, K.; Rostami, K.; Sanders, D.S.; Schumann, M.; et al. Spectrum of gluten-related disorders: Consensus on new nomenclature and classification. BMC Med. 2012, 10, 13. [Google Scholar] [CrossRef]
- Jones, A.L. The Gluten-Free Diet: Fad or Necessity? Diabetes Spectr. 2017, 30, 118–123. [Google Scholar] [CrossRef]
- Goodwin, M.S.; Goodwin, T.C. In a dark mirror. Ment. Hyg. 1969, 53, 550–563. [Google Scholar] [PubMed]
- Gujral, N.; Freeman, H.J.; Thomson, A.B. Celiac disease: Prevalence, diagnosis, pathogenesis and treatment. World J. Gastroenterol. 2012, 18, 6036–6059. [Google Scholar] [CrossRef]
- Dohan, F.C. Coeliac disease and schizophrenia. Lancet 1970, 1, 897–898. [Google Scholar] [CrossRef][Green Version]
- Fitzgerald, M. Overlap between autism and schizophrenia: History and current status. Adv. Ment. Health Intell. Disabil. 2013, 8, 15–23. [Google Scholar] [CrossRef]
- Goodwin, M.S.; Cowen, M.A.; Goodwin, T.C. Malabsorption and cerebral dysfunction: A multivariate and comparative study of autistic children. J. Autism Child. Schizophr. 1971, 1, 48–62. [Google Scholar] [CrossRef]
- Walker-Smith, J.; Andrews, J. Alpha-1-antitrypsin, autism, and coeliac disease. Lancet 1972, 2, 883–884. [Google Scholar] [CrossRef]
- Coleman, M.; Landgrebe, M.A.; Landgrebe, A.R. Celiac autism: Calcium studies and their relationship to celiac disease in autistic patients. In The Autistic Syndrome, 1st ed.; Coleman, M., Ed.; Elsevier/North Holland Publishing Co.: New York, NY, USA, 1976; pp. 197–205. [Google Scholar]
- McCarthy, D.M.; Coleman, M. Response of intestinal mucosa to gluten challenge in autistic subjects. Lancet 1979, 2, 877–878. [Google Scholar] [CrossRef]
- Chaidez, V.; Hansen, R.L.; Hertz-Picciotto, I. Gastrointestinal problems in children with autism, developmental delays or typical development. J. Autism Dev. Disord. 2014, 44, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- McElhanon, B.O.; McCracken, C.; Karpen, S.; Sharp, W.G. Gastrointestinal symptoms in autism spectrum disorder: A meta-analysis. Pediatrics 2014, 133, 872–883. [Google Scholar] [CrossRef]
- Afzal, N.; Murch, S.; Thirrupathy, K.; Berger, L.; Fagbemi, A.; Heuschkel, R. Constipation with acquired megarectum in children with autism. Pediatrics 2003, 112, 939–942. [Google Scholar] [CrossRef]
- Valicenti-McDermott, M.D.; McVicar, K.; Cohen, H.J.; Wershil, B.K.; Shinnar, S. Gastrointestinal symptoms in children with an autism spectrum disorder and language regression. Pediatr. Neurol. 2008, 39, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Babinska, K.; Celusakova, H.; Belica, I.; Szapuova, Z.; Waczulikova, I.; Nemcsicsova, D.; Tomova, A.; Ostatnikova, D. Gastrointestinal Symptoms and Feeding Problems and Their Associations with Dietary Interventions, Food Supplement Use, and Behavioural Characteristics in a Sample of Children and Adolescents with Autism Spectrum Disorders. Int. J. Environ. Res. Public Health 2020, 17, 6372. [Google Scholar] [CrossRef]
- Adams, J.B.; Johansen, L.J.; Powell, L.D.; Quig, D.; Rubin, R.A. Gastrointestinal flora and gastrointestinal status in children with autism--comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 2011, 11, 22. [Google Scholar] [CrossRef]
- Gorrindo, P.; Williams, K.C.; Lee, E.B.; Walker, L.S.; McGrew, S.G.; Levitt, P. Gastrointestinal dysfunction in autism: Parental report, clinical evaluation, and associated factors. Autism Res. Off. J. Int. Soc. Autism Res. 2012, 5, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Babinska, K.; Pivovarciova, A.; Filcikova, D.; Tomova, A.; Ostatnikova, D. Association of conduct problems and gastrointestinal symptoms in individuals with autism spectrum disorders. Act. Nerv. Super. Rediviva 2016, 58, 69–72. [Google Scholar]
- Pavone, L.; Fiumara, A.; Bottaro, G.; Mazzone, D.; Coleman, M. Autism and celiac disease: Failure to validate the hypothesis that a link might exist. Biol. Psychiatry 1997, 42, 72–75. [Google Scholar] [CrossRef]
- Atladóttir, H.O.; Pedersen, M.G.; Thorsen, P.; Mortensen, P.B.; Deleuran, B.; Eaton, W.W.; Parner, E.T. Association of family history of autoimmune diseases and autism spectrum disorders. Pediatrics 2009, 124, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Butwicka, A.; Lichtenstein, P.; Frisén, L.; Almqvist, C.; Larsson, H.; Ludvigsson, J.F. Celiac Disease Is Associated with Childhood Psychiatric Disorders: A Population-Based Study. J. Pediatr. 2017, 184, 87–93.e1. [Google Scholar] [CrossRef]
- Lebwohl, B.; Haggård, L.; Emilsson, L.; Söderling, J.; Roelstraete, B.; Butwicka, A.; Green, P.H.; Ludvigsson, J.F. Psychiatric disorders in patients with a diagnosis of celiac disease during childhood from 1973 to 2016. Clin. Gastroenterol. Hepatol. 2019. In Press. [Google Scholar] [CrossRef]
- Ludvigsson, J.F.; Reichenberg, A.; Hultman, C.M.; Murray, J.A. A nationwide study of the association between celiac disease and the risk of autistic spectrum disorders. JAMA Psychiatry 2013, 70, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
- Calderoni, S.; Santocchi, E.; Del Bianco, T.; Brunori, E.; Caponi, L.; Paolicchi, A.; Fulceri, F.; Prosperi, M.; Narzisi, A.; Cosenza, A.; et al. Serological screening for Celiac Disease in 382 pre-schoolers with Autism Spectrum Disorder. Ital. J. Pediatr. 2016, 42, 98. [Google Scholar] [CrossRef] [PubMed]
- Barcia, G.; Posar, A.; Santucci, M.; Parmeggiani, A. Autism and coeliac disease. J. Autism Dev. Disord. 2008, 38, 407–408. [Google Scholar] [CrossRef]
- Mazzone, L.; Reale, L.; Spina, M.; Guarnera, M.; Lionetti, E.; Martorana, S.; Mazzone, D. Compliant gluten-free children with celiac disease: An evaluation of psychological distress. BMC Pediatr. 2011, 11, 46. [Google Scholar] [CrossRef]
- Alabaf, S.; Gillberg, C.; Lundström, S.; Lichtenstein, P.; Kerekes, N.; Råstam, M.; Anckarsäter, H. Physical health in children with neurodevelopmental disorders. J. Autism Dev. Disord. 2019, 49, 83–95. [Google Scholar] [CrossRef]
- Juneja, M.; Venkatakrishnan, A.; Kapoor, S.; Jain, R. Autism Spectrum Disorders and Celiac Disease: Is there an Association? Indian Pediatr. 2018, 55, 912–914. [Google Scholar]
- Batista, I.C.; Gandolfi, L.; Nobrega, Y.K.; Almeida, R.C.; Almeida, L.M.; Campos, D., Jr.; Pratesi, R. Autism spectrum disorder and celiac disease: No evidence for a link. Arq. Neuro-Psiquiatr. 2012, 70, 28–33. [Google Scholar] [CrossRef]
- Zelnik, N.; Pacht, A.; Obeid, R.; Lerner, A. Range of neurological disorders in patients with celiac disease. Pediatrics 2004, 113, 1672–1676. [Google Scholar] [CrossRef]
- Black, C.; Kaye, J.A.; Jick, H. Relation of childhood gastrointestinal disorders to autism: Nested case-control study using data from the UK General Practice Research Database. BMJ (Clin. Res. Ed.) 2002, 325, 419–421. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Clappison, E.; Hadjivassiliou, M.; Zis, P. Psychiatric Manifestations of Coeliac Disease, a Systematic Review and Meta-Analysis. Nutrients 2020, 12, 142. [Google Scholar] [CrossRef] [PubMed]
- Money, J.; Bobrow, N.A.; Clarke, F.C. Autism and autoimmune disease: A family study. J. Autism Child. Schizophr. 1971, 1, 146–160. [Google Scholar] [CrossRef]
- Jyonouchi, H.; Sun, S.; Le, H. Proinflammatory and regulatory cytokine production associated with innate and adaptive immune responses in children with autism spectrum disorders and developmental regression. J. Neuroimmunol. 2001, 120, 170–179. [Google Scholar] [CrossRef]
- Vojdani, A.; Pangborn, J.B.; Vojdani, E.; Cooper, E.L. Infections, toxic chemicals and dietary peptides binding to lymphocyte receptors and tissue enzymes are major instigators of autoimmunity in autism. Int. J. Immunopathol. Pharmacol. 2003, 16, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Vojdani, A.; Bazargan, M.; Vojdani, E.; Samadi, J.; Nourian, A.A.; Eghbalieh, N.; Cooper, E.L. Heat shock protein and gliadin peptide promote development of peptidase antibodies in children with autism and patients with autoimmune disease. Clin. Diagn. Lab. Immunol. 2004, 11, 515–524. [Google Scholar] [CrossRef]
- Vojdani, A.; O’Bryan, T.; Green, J.A.; Mccandless, J.; Woeller, K.N.; Vojdani, E.; Nourian, A.A.; Cooper, E.L. Immune response to dietary proteins, gliadin and cerebellar peptides in children with autism. Nutr. Neurosci. 2004, 7, 151–161. [Google Scholar] [CrossRef]
- Pruimboom, L.; de Punder, K. The opioid effects of gluten exorphins: Asymptomatic celiac disease. J. Health Popul. Nutr. 2015, 33, 24. [Google Scholar] [CrossRef]
- Trivedi, M.S.; Shah, J.S.; Al-Mughairy, S.; Hodgson, N.W.; Simms, B.; Trooskens, G.A.; Van Criekinge, W.; Deth, R.C. Food-derived opioid peptides inhibit cysteine uptake with redox and epigenetic consequences. J. Nutr. Biochem. 2014, 25, 1011–1018. [Google Scholar] [CrossRef]
- Di Liberto, D.; D’Anneo, A.; Carlisi, D.; Emanuele, S.; De Blasio, A.; Calvaruso, G.; Giuliano, M.; Lauricella, M. Brain Opioid Activity and Oxidative Injury: Different Molecular Scenarios Connecting Celiac Disease and Autistic Spectrum Disorder. Brain Sci. 2020, 10, 437. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Johnson, S.K.; Busetti, F.; Solah, V.A. Formation and Degradation of Beta-casomorphins in Dairy Processing. Crit. Rev. Food Sci. Nutr. 2015, 55, 1955–1967. [Google Scholar] [CrossRef] [PubMed]
- Dohan, F.C. Genetic hypothesis of idiopathic schizophrenia: Its exorphin connection. Schizophr. Bull. 1988, 14, 489–494. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Panksepp, J. A neurochemical theory of autism. Trends Neurosci. 1979, 2, 174–177. [Google Scholar] [CrossRef]
- Pellissier, L.P.; Gandía, J.; Laboute, T.; Becker, J.; Le Merrer, J. μ opioid receptor, social behaviour and autism spectrum disorder: Reward matters. Br. J. Pharmacol. 2018, 175, 2750–2769. [Google Scholar] [CrossRef]
- Reichelt, K.L.; Knivsberg, A.M. The possibility and probability of a gut-to-brain connection in autism. Ann. Clin. Psychiatry 2009, 21, 205–211. [Google Scholar] [PubMed]
- Gu, F.; Chauhan, V.; Chauhan, A. Impaired synthesis and antioxidant defense of glutathione in the cerebellum of autistic subjects: Alterations in the activities and protein expression of glutathione-related enzymes. Free Radic. Biol. Med. 2013, 65, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Wrońska-Nofer, T.; Nofer, J.R.; Jajte, J.; Dziubałtowska, E.; Szymczak, W.; Krajewski, W.; Wąsowicz, W.; Rydzyński, K. Oxidative DNA damage and oxidative stress in subjects occupationally exposed to nitrous oxide (N2O). Mutat. Res. 2012, 731, 58–63. [Google Scholar] [CrossRef]
- Frye, R.E.; Slattery, J. The potential role of nitrous oxide in the etiology of autism spectrum disorder. Transl. Psychiatry 2016, 6, e812. [Google Scholar] [CrossRef] [PubMed]
- Monguzzi, E.; Marabini, L.; Elli, L.; Vaira, V.; Ferrero, S.; Ferretti, F.; Branchi, F.; Gaudioso, G.; Scricciolo, A.; Lombardo, V.; et al. Gliadin effect on the oxidative balance and DNA damage: An in-vitro, ex-vivo study. Dig. Liver Dis. 2019, 51, 47–54. [Google Scholar] [CrossRef]
- Stojiljković, V.; Todorović, A.; Radlović, N.; Pejić, S.; Mladenović, M.; Kasapović, J.; Pajović, S.B. Antioxidant enzymes, glutathione and lipid peroxidation in peripheral blood of children affected by coeliac disease. Ann. Clin. Biochem. 2007, 44, 537–543. [Google Scholar] [CrossRef]
- Bennabi, M.; Gaman, A.; Delorme, R.; Boukouaci, W.; Manier, C.; Scheid, I.; Si Mohammed, N.; Bengoufa, D.; Charron, D.; Krishnamoorthy, R.; et al. HLA-class II haplotypes and Autism Spectrum Disorders. Sci. Rep. 2018, 8, 7639. [Google Scholar] [CrossRef]
- Rahmoune, H.; Boutrid, N. Autism & Gluten: The Proof by Regression! Pediatr. Neurol. Briefs 2018, 32, 9. [Google Scholar]
- Autism Spectrum Disorders Working Group of The Psychiatric Genomics Consortium. Meta-analysis of GWAS of over 16,000 individuals with autism spectrum disorder highlights a novel locus at 10q24.32 and a significant overlap with schizophrenia. Mol. Autism 2017, 8, 21. [CrossRef]
- Cervio, E.; Volta, U.; Verri, M.; Boschi, F.; Pastoris, O.; Granito, A.; Barbara, G.; Parisi, C.; Felicani, C.; Tonini, M.; et al. Sera of patients with celiac disease and neurologic disorders evoke a mitochondrial-dependent apoptosis in vitro. Gastroenterology 2007, 133, 195–206. [Google Scholar] [CrossRef]
- Hadjivassiliou, M.; Aeschlimann, P.; Sanders, D.S.; Mäki, M.; Kaukinen, K.; Grünewald, R.A.; Bandmann, O.; Woodroofe, N.; Haddock, G.; Aeschlimann, D.P. Transglutaminase 6 antibodies in the diagnosis of gluten ataxia. Neurology 2013, 80, 1740–1745. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.T.; Tang, B.S.; Lan, W.; Song, N.N.; Huang, Y.; Zhang, L.; Guan, W.J.; Shi, Y.T.; Shen, L.; Jiang, H.; et al. Distribution of transglutaminase 6 in the central nervous system of adult mice. Anat. Rec. 2013, 296, 1576–1587. [Google Scholar] [CrossRef] [PubMed]
- Józefczuk, J.; Konopka, E.; Bierła, J.B.; Trojanowska, I.; Sowińska, A.; Czarnecki, R.; Sobol, L.; Józefczuk, P.; Surdy, W.; Cukrowska, B. The Occurrence of Antibodies Against Gluten in Children with Autism Spectrum Disorders Does Not Correlate with Serological Markers of Impaired Intestinal Permeability. J. Med. Food 2018, 21, 181–187. [Google Scholar] [CrossRef]
- Pratesi, R.; Gandolfi, L.; Friedman, H.; Farage, L.; de Castro, C.A.; Catassi, C. Serum IgA antibodies from patients with coeliac disease react strongly with human brain blood-vessel structures. Scand. J. Gastroenterol. 1998, 33, 817–821. [Google Scholar] [PubMed]
- Alaedini, A.; Okamoto, H.; Briani, C.; Wollenberg, K.; Shill, H.A.; Bushara, K.O.; Sander, H.W.; Green, P.H.; Hallett, M.; Latov, N. Immune cross-reactivity in celiac disease: Anti-gliadin antibodies bind to neuronal synapsin I. J. Immunol. 2007, 178, 6590–6595. [Google Scholar] [CrossRef] [PubMed]
- Ruuskanen, A.; Kaukinen, K.; Collin, P.; Huhtala, H.; Valve, R.; Mäki, M.; Luostarinen, L. Positive serum antigliadin antibodies without celiac disease in the elderly population: Does it matter? Scand. J. Gastroenterol. 2010, 45, 1197–1202. [Google Scholar] [CrossRef]
- Cade, R.; Privette, M.; Fregly, M.; Rowland, N.; Sun, Z.; Zele, V.; Wagemaker, H.; Edelstein, C. Autism and Schizophrenia: Intestinal Disorders. Nutr. Neurosci. 2000, 3, 57–72. [Google Scholar] [CrossRef]
- Kawashti, M.I.; Amin, O.R.; Rowehy, N.G. Possible immunological disorders in autism: Concomitant autoimmunity and immune tolerance. Egypt. J. Immunol. 2006, 13, 99–104. [Google Scholar]
- Lau, N.M.; Green, P.H.; Taylor, A.K.; Hellberg, D.; Ajamian, M.; Tan, C.Z.; Kosofsky, B.E.; Higgins, J.J.; Rajadhyaksha, A.M.; Alaedini, A. Markers of Celiac Disease and Gluten Sensitivity in Children with Autism. PLoS ONE 2013, 8, e66155. [Google Scholar] [CrossRef]
- De Magistris, L.; Picardi, A.; Siniscalco, D.; Riccio, M.P.; Sapone, A.; Cariello, R.; Abbadessa, S.; Medici, N.; Lammers, K.M.; Schiraldi, C.; et al. Antibodies against food antigens in patients with autistic spectrum disorders. BioMed Res. Int. 2013, 2013, 729349. [Google Scholar] [CrossRef]
- Abdel-Maksoud, M.; Aly El-Gabry, D.; Al Kayoumi, T.; Alketbi, J.; Mohamednour, D.; Elhassan Elamin, M.; Subhash Reddy, M.; Al Yafei, Z.A.; Stip, E.; Abdel Aziz, K.; et al. Measures of gluten-related reactivity in children with autism spectrum disorders in the absence of overt gastrointestinal symptoms: A pilot study from the United Arab Emirates. J. Int. Med. Res. 2020, 48, 0300060520952655. [Google Scholar] [CrossRef]
- Torrente, F.; Ashwood, P.; Day, R.; Machado, N.; Furlano, R.I.; Anthony, A.; Davies, S.E.; Wakefield, A.J.; Thomson, M.A.; Walker-Smith, J.A.; et al. Small intestinal enteropathy with epithelial IgG and complement deposition in children with regressive autism. Mol. Psychiatry 2002, 7, 375–382. [Google Scholar] [CrossRef]
- Jyonouchi, H.; Sun, S.; Itokazu, N. Innate immunity associated with inflammatory responses and cytokine production against common dietary proteins in patients with autism spectrum disorder. Neuropsychobiology 2002, 46, 76–84. [Google Scholar] [CrossRef]
- Ashwood, P.; Anthony, A.; Pellicer, A.A.; Torrente, F.; Walker-Smith, J.A.; Wakefield, A.J. Intestinal lymphocyte populations in children with regressive autism: Evidence for extensive mucosal immunopathology. J. Clin. Immunol. 2003, 23, 504–517. [Google Scholar] [CrossRef]
- Ashwood, P.; Anthony, A.; Torrente, F.; Wakefield, A.J. Spontaneous mucosal lymphocyte cytokine profiles in children with autism and gastrointestinal symptoms: Mucosal immune activation and reduced counter regulatory interleukin-10. J. Clin. Immunol. 2004, 24, 664–673. [Google Scholar] [CrossRef]
- Souza, N.C.; Mendonca, J.N.; Portari, G.V.; Jordao, A.A., Jr.; Marchini, J.S.; Chiarello, P.G. Intestinal permeability and nutritional status in developmental disorders. Altern. Ther. Health Med. 2012, 18, 19–24. [Google Scholar]
- Fiorentino, M.; Sapone, A.; Senger, S.; Camhi, S.S.; Kadzielski, S.M.; Buie, T.M.; Kelly, D.L.; Cascella, N.; Fasano, A. Blood-brain barrier and intestinal epithelial barrier alterations in autism spectrum disorders. Mol. Autism 2016, 7, 49. [Google Scholar] [CrossRef]
- Dalton, N.; Chandler, S.; Turner, C.; Charman, T.; Pickles, A.; Loucas, T.; Simonoff, E.; Sullivan, P.; Baird, G. Gut permeability in autism spectrum disorders. Autism Res. 2014, 7, 305–313. [Google Scholar] [CrossRef]
- Wasilewska, J.; Klukowski, M. Gastrointestinal symptoms and autism spectrum disorder: Links and risks—A possible new overlap syndrome. Pediatr. Health Med. Ther. 2015, 6, 153–166. [Google Scholar] [CrossRef]
- Knivsberg, A.M.; Wiig, K.; Lind, G.; Nødland, M. Dietary intervention in autistic syndromes. Brain Dysfunct. 1990, 3, 315–327. [Google Scholar]
- Knivsberg, A.M.; Reichelt, K.L.; Nodland, M.; Hoien, T. Autistic syndromes and diet: A follow-up study. Scand. J. Educ. Res. 1995, 39, 223–236. [Google Scholar] [CrossRef]
- Whiteley, P.; Rodgers, J.; Savery, D.; Shattock, P. A gluten-free diet as an intervention for autism and associated spectrum disorders: Preliminary findings. Autism 1999, 3, 45–65. [Google Scholar] [CrossRef]
- Knivsberg, A.M.; Reichelt, K.L.; Høien, T.; Nødland, M. A randomised, controlled study of dietary intervention in autistic syndromes. Nutr. Neurosci. 2002, 5, 251–261. [Google Scholar] [CrossRef] [PubMed]
- González-Domenech, P.J.; Díaz Atienza, F.; García Pablos, C.; Fernández Soto, M.L.; Martínez-Ortega, J.M.; Gutiérrez-Rojas, L. Influence of a Combined Gluten-Free and Casein-Free Diet on Behavior Disorders in Children and Adolescents Diagnosed with Autism Spectrum Disorder: A 12-Month Follow-Up Clinical Trial. J. Autism Dev. Disord. 2020, 50, 935–948. [Google Scholar] [CrossRef]
- Piwowarczyk, A.; Horvath, A.; Pisula, E.; Kawa, R.; Szajewska, H. Gluten-Free Diet in Children with Autism Spectrum Disorders: A Randomized, Controlled, Single-Blinded Trial. J. Autism Dev. Disord. 2020, 50, 482–490. [Google Scholar] [CrossRef]
- Grimaldi, R.; Gibson, G.R.; Vulevic, J.; Giallourou, N.; Castro-Mejía, J.L.; Hansen, L.H.; Leigh Gibson, E.; Nielsen, D.S.; Costabile, A. A prebiotic intervention study in children with autism spectrum disorders (ASDs). Microbiome 2018, 6, 133. [Google Scholar] [CrossRef]
- Adams, J.B.; Audhya, T.; Geis, E.; Gehn, E.; Fimbres, V.; Pollard, E.L.; Mitchell, J.; Ingram, J.; Hellmers, R.; Laake, D.; et al. Comprehensive Nutritional and Dietary Intervention for Autism Spectrum Disorder-A Randomized, Controlled 12-Month Trial. Nutrients 2018, 10, 369. [Google Scholar] [CrossRef]
- El-Rashidy, O.; El-Baz, F.; El-Gendy, Y.; Khalaf, R.; Reda, D.; Saad, K. Ketogenic diet versus gluten free casein free diet in autistic children: A case-control study. Metab. Brain Dis. 2017, 32, 1935–1941. [Google Scholar] [CrossRef] [PubMed]
- Ghalichi, F.; Ghaemmaghami, J.; Malek, A.; Ostadrahimi, A. Effect of gluten free diet on gastrointestinal and behavioral indices for children with autism spectrum disorders: A randomized clinical trial. World J. Pediatr. 2016, 12, 436–442. [Google Scholar] [CrossRef]
- Hyman, S.L.; Stewart, P.A.; Foley, J.; Cain, U.; Peck, R.; Morris, D.D.; Wang, H.; Smith, T. The Gluten-Free/Casein-Free Diet: A Double-Blind Challenge Trial in Children with Autism. J. Autism Dev. Disord. 2016, 46, 205–220. [Google Scholar] [CrossRef]
- Pusponegoro, H.D.; Ismael, S.; Firmansyah, A.; Sastroasmoro, S.; Vandenplas, Y. Gluten and casein supplementation does not increase symptoms in children with autism spectrum disorder. Acta Paediatr. 2015, 104, e500–e505. [Google Scholar] [CrossRef]
- Navarro, F.; Pearson, D.A.; Fatheree, N.; Mansour, R.; Hashmi, S.S.; Rhoads, J.M. Are ‘leaky gut’ and behavior associated with gluten and dairy containing diet in children with autism spectrum disorders? Nutr. Neurosci. 2015, 18, 177–185. [Google Scholar] [CrossRef]
- Johnson, C.R.; Handen, B.L.; Zimmer, M.; Sacco, K.; Kylan, T. Effects of gluten free / casein free diet in young children with autism: A pilot study. J. Dev. Phys. Disabil. 2011, 23, 213–225. [Google Scholar] [CrossRef]
- Whiteley, P.; Haracopos, D.; Knivsberg, A.M.; Reichelt, K.L.; Parlar, S.; Jacobsen, J.; Seim, A.; Pedersen, L.; Schondel, M.; Shattock, P. The ScanBrit randomised, controlled, single-blind study of a gluten- and casein-free dietary intervention for children with autism spectrum disorders. Nutr. Neurosci. 2010, 13, 87–100. [Google Scholar] [CrossRef]
- Elder, J.H.; Shankar, M.; Shuster, J.; Theriaque, D.; Burns, S.; Sherrill, L. The gluten-free, casein-free diet in autism: Results of a preliminary double blind clinical trial. J. Autism Dev. Disord. 2006, 36, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Lionetti, E.; Catassi, C. New clues in celiac disease epidemiology, pathogenesis, clinical manifestations, and treatment. Int. Rev. Immunol. 2011, 30, 219–231. [Google Scholar] [CrossRef]
- Caio, G.; Volta, U.; Tovoli, F.; De Giorgio, R. Effect of gluten free diet on immune response to gliadin in patients with non-celiac gluten sensitivity. BMC Gastroenterol. 2014, 14, 26. [Google Scholar] [CrossRef]
- Bowers, L. An audit of referrals of children with autistic spectrum disorder to the dietetic service. J. Hum. Nutr. Diet. 2002, 15, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Harris, C.; Card, B. A pilot study to evaluate nutritional influences on gastrointestinal symptoms and behavior patterns in children with Autism Spectrum Disorder. Complement. Ther. Med. 2012, 20, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.A.; Hyman, S.L.; Schmidt, B.L.; Macklin, E.A.; Reynolds, A.; Johnson, C.R.; James, S.J.; Manning-Courtney, P. Dietary Supplementation in Children with Autism Spectrum Disorders: Common, Insufficient, and Excessive. J. Acad. Nutr. Diet. 2015, 115, 1237–1248. [Google Scholar] [CrossRef] [PubMed]
- Hopf, K.P.; Madren, E.; Santianni, K.A. Use and Perceived Effectiveness of Complementary and Alternative Medicine to Treat and Manage the Symptoms of Autism in Children: A Survey of Parents in a Community Population. J. Altern. Complement. Med. 2016, 22, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Trudeau, M.S.; Madden, R.F.; Parnell, J.A.; Gibbard, W.B.; Shearer, J. Dietary and Supplement-Based Complementary and Alternative Medicine Use in Pediatric Autism Spectrum Disorder. Nutrients 2019, 11, 1783. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, E.; Schieve, L.; Bradley, C.; DiGuiseppi, C.; Moody, E.; Thomas, K.; Daniels, J. The prevalence of gluten free diet use among preschool children with autism spectrum disorder. Autism Res. 2018, 11, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Blackett, J.W.; Shamsunder, M.; Reilly, N.R.; Green, P.; Lebwohl, B. Characteristics and comorbidities of inpatients without celiac disease on a gluten-free diet. Eur. J. Gastroenterol. Hepatol. 2018, 30, 477–483. [Google Scholar] [CrossRef]
- Pennesi, C.M.; Klein, L.C. Effectiveness of the gluten-free, casein-free diet for children diagnosed with autism spectrum disorder: Based on parental report. Nutr. Neurosci. 2012, 15, 85–91. [Google Scholar] [CrossRef]
- Ferguson, B.J.; Dovgan, K.; Severns, D.; Martin, S.; Marler, S.; Gross Margolis, K.; Bauman, M.L.; Veenstra-VanderWeele, J.; Sohl, K.; Beversdorf, D.Q. Lack of Associations Between Dietary Intake and Gastrointestinal Symptoms in Autism Spectrum Disorder. Front. Psychiatry 2019, 10, 528. [Google Scholar] [CrossRef]
- Marsden, R.; Francis, J.; Garner, I. Use of GFCF Diets in Children with ASD. An Investigation into Parents’ Beliefs Using the Theory of Planned Behaviour. J. Autism Dev. Disord. 2019, 49, 3716–3731. [Google Scholar] [CrossRef] [PubMed]
- Tarnowska, K.; Gruczyńska-Sękowska, E.; Kowalska, D.; Kozłowska, M.; Majewska, E.; Winkler, R. Difficulties and factors influencing purchase decision. The perspective of families with children with autism spectrum disorders on a gluten-free and casein-free diet. Preliminary study. Rocz. Panstw. Zakl. Hig. 2020, 71, 321–328. [Google Scholar] [PubMed]
- Winburn, E.; Charlton, J.; McConachie, H.; McColl, E.; Parr, J.; O’Hare, A.; Baird, G.; Gringras, P.; Wilson, D.C.; Adamson, A.; et al. Parents’ and child health professionals’ attitudes towards dietary interventions for children with autism spectrum disorders. J. Autism Dev. Disord. 2014, 44, 747–757. [Google Scholar] [CrossRef]
- Herndon, A.C.; DiGuiseppi, C.; Johnson, S.L.; Leiferman, J.; Reynolds, A. Does nutritional intake differ between children with autism spectrum disorders and children with typical development? J. Autism Dev. Disord. 2009, 39, 212–222. [Google Scholar] [CrossRef]
- Srinivasan, S.; O’Rourke, J.; Bersche Golas, S.; Neumeyer, A.; Misra, M. Calcium and Vitamin D Supplement Prescribing Practices among Providers Caring for Children with Autism Spectrum Disorders: Are We Addressing Bone Health? Autism Res. Treat. 2016, 2016, 6763205. [Google Scholar] [CrossRef]
- Cornish, E. Gluten and casein free diets in autism: A study of the effects on food choice and nutrition. J. Hum. Nutr. Diet. 2002, 15, 261–269. [Google Scholar] [CrossRef]
- Marí-Bauset, S.; Llopis-González, A.; Zazpe, I.; Marí-Sanchis, A.; Suárez-Varela, M.M. Nutritional Impact of a Gluten-Free Casein-Free Diet in Children with Autism Spectrum Disorder. J. Autism Dev. Disord. 2016, 46, 673–684. [Google Scholar] [CrossRef]
- Arnold, G.L.; Hyman, S.L.; Mooney, R.A.; Kirby, R.S. Plasma amino acids profiles in children with autism: Potential risk of nutritional deficiencies. J. Autism Dev. Disord. 2003, 33, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Kałuzna-Czaplinska, J.; Michalska, M.; Rynkowski, J. Determination of tryptophan in urine of autistic and healthy children by gas chromatography/mass spectrometry. Med. Sci. Monit. 2010, 16, CR488–CR492. [Google Scholar]
| Citation | Cohort (Describes the ASD Group Unless Otherwise Specified) | Finding (% Where It Was Above an Abnormal Cutoff Given Where Possible) |
|---|---|---|
| Cade et al. (2000) [63] | 150 children and adolescents | IgG AGA raised (87% of group) |
| Vojdani et al. (2003; the two publications by Vojdani et al. in 2004 also report gliadin antibodies, but use the same dataset) [37,38,39] | 50 patients | IgG (44%)/IgA (46%)/IgM (36%) AGA raised compared to controls |
| Kawashti et al. (2006) [64] * | 30 children | AGA raised (50% of group) compared to controls |
| Batista et al. (2012) [31] | 147 patients | IgG/IgA AGA not different to controls |
| Lau et al. (2013) [65] | 37 children | IgG AGA raised (24.2% of group) compared to controls, and particularly in those with a GI medical history |
| de Magistris et al., 2013 [66] | 162 children | IgG AGA raised (25.3% of group) compared to controls, higher in both those on GFD and regular diets. |
| Józefczuk et al., 2018 [59] | 77 patients | IgG AGA raised (27.3% of group) |
| Abdel-Maksoud et al. (2020) [67] | 66 children | IgA AGA titre lowered compared to controls |
| Citation | N Randomized & Comment on Groupings | Participants Blinded? | Diet(s) Tested | Duration | Any Main Outcomes Significantly Affected by Intervention? |
|---|---|---|---|---|---|
| Gonzalez-Domenech et al., 2020 [80] | N = 37; crossover design. Mixed children and adolescents with ASD, without allergies to gluten or casein. Everyone on gluten & casein-containing diet at baseline. | No | GCFD vs. regular diet | 12 months (6 months per crossover block) | None; those tested included behavioural/cognitive measures (ERC = III, ATEC & ABC), and urinary beta-casomorphin as a marker of poor digestion of casein. |
| Piwowarczyk et al., 2020 [81] | N = 66; parallel group. Children with ASD, without celiac disease/wheat allergy. 8 week, GFD run-in period before start. | No | GFD vs. regular diet | 6 months | None; those tested included behavioural/cognitive measures (ADOS-2, SCQ, ASRS, VABS-2, LIPS), and Rome-III for GI symptoms. |
| Grimaldi et al., 2018 [82] | N = 30; parallel groups. Children with ASD who did not take dietary supplements. Baseline food diaries identified groups who already either followed GCFD or regular diets; randomization to receive the prebiotic mixture happened within these groups. | Yes | GCFD + “B-GOS” prebiotic mixture (vs. GCFD without B-GOS, vs. regular diet with/without B-GOS). | 6 weeks | Improvement in behavioural scores (ATEC & AQ) in children on GCFD + the prebiotic mixture (not observed in those on GCFD alone). No significant results reported for EQ-SQ or SCAS-P. Physiological changes (urine spectra, faecal samples, were observed in response to the prebiotic mixture, both across dietary groups and between them. |
| Adams et al., 2018 [83] | N = 67; parallel groups. Children and adults with ASD. 2 month run-in of no special diet or supplements. | No | Various interventions, added accumulatively (vs. no diet/modifications). At the end of the trial, interventions included GCFD (for 155 days) + supplementation of vitamins, minerals, essential fatty acids, carnitine, digestive enzymes & taking of Epsom salt baths. | 12 months | Improvement in behavioural/intellectual scores (RIAS non-verbal IQ, CARS, SAS Pro, VABS-II, PDDBI Composite, ATEC, ABC, SRS & SSP) Improvement in GI symptoms (measured by 6-GSI). Some changes to complete blood count and blood chemistry panel markers, fatty acid profile, vitamin levels, RBC elements, homocysteine, l-carnitine No changes in handgrip strength or C-reactive protein |
| El-Rashidy et al., 2017 [84] | N = 45; 3 parallel groups (2 dietary interventions, and a controls). Children with ASD | No | GCFD vs. ketogenic vs. regular diet | 6 months | Improvement in behavioural/intellectual scores (CARS, ATEC); in both GCFD and ketogenic groups. Degree of change was not sig. different between these groups, but each appear markedly larger than change observed in the control group (this specific comparison does not appear to have been statistically evaluated) |
| Ghalichi et al., 2016 [85] | N = 80; parallel groups. Children and adolescents with ASD, not following any special diets. | No | GFD vs. regular diet | 6 weeks | Improvement in behavioural scores (GARS-2). Improvement in GI symptoms (ROME III) |
| Hyman et al., 2016 [86] | N = 14; crossover design. Children with ASD, without celiac disease or wheat/milk allergy. 6 week run-in period of GCFD. | Yes | GFD vs. CFD vs. GCFD vs. regular diet. | 12 weeks (Alternating diets delivered in “blocks” where every participant did each diet one week at a time. This was repeated 3 times, totalling 12 weeks) | None; those tested included behavioural scales (CARSA, RRLRS) and physiological scales (Bristol Stool Scale). |
| Pusponegoro et al., 2015 [87] | N = 74; parallel groups. Children with ASD, with high levels of urinary I-FABP excretion (indicating heightened intestinal permeability) | Yes | GCFD vs. regular diet | 1 week | No change in behavioural outcomes (AWPC) Worsening of gastrointestinal symptoms; significant in within-group analysis, but change in this measure was not different between groups. No change in urinary I-FABP |
| Navarro et al., 2015 [88] | N = 12; parallel groups. Children with ASD, without celiac disease or food allergies. 2 week GCFD run-in period. | Yes | GCFD vs. regular diet | 4 weeks | None; formal statistics generally avoided due to small sample size, but trends were generally absent in all outcomes which included behavioural/intellectual measures (CPRS-R, ABC) and physiological measures (lactulose/mannitol recovery ratio for intestinal permeability, or GI symptoms on a non-validated questionnaire) |
| Johnson et al., 2011 [89] | N = 22; parallel groups. Children with ASD. | No | GCFD vs regular diet | 3 months | None; those tested included behavioural scales (CBC, MSEL, blinded observations) and physiological measurements (likert scales RE constipation etc.). Isolated sub-scores of MSEL & CBC were sig., though authors note no consistent pattern and reject them |
| Whiteley et al., 2010 [90] | N = 73; parallel groups. Children with ASD | No | GCFD vs. regular diet | 24 months; interim analyses at 8 and 12 months would reassign regular diet participants to receive GCFD for the remainder, if sufficient improvement was observed in GCFD group. | Improvement in behavioural/intellectual outcomes (ADOS, GARS, VABS), no change in ADHD-IV at the 8 month analysis; the control group was added to the diet at 12 months making 24 month data un-comparable. |
| Elder et al., 2006 [91] | N = 13; crossover design. Children and adolescents with ASD, without celiac disease. | Yes | GCFD vs. regular diet | 12 weeks (6 weeks per crossover block) | None; those tested included behavioural/intellectual measures (CARS, ECO and observation of in-home behaviour). Authors do note parental reports indicating potential improvements in individual children when on the diet. Parents otherwise performed poorly at guessing which period of time they had been given the GCFD foodstuffs. |
| Knivsberg et al., 2002 [79] | N = 20; parallel groups Children with ASD in additional to abnormal urinary peptides. | No | GCFD vs regular diet | 12 months | Improvement in behavioural and intellectual outcomes (DIPAB, LIPS, ITPA, Reynells spraktest, MABC) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Croall, I.D.; Hoggard, N.; Hadjivassiliou, M. Gluten and Autism Spectrum Disorder. Nutrients 2021, 13, 572. https://doi.org/10.3390/nu13020572
Croall ID, Hoggard N, Hadjivassiliou M. Gluten and Autism Spectrum Disorder. Nutrients. 2021; 13(2):572. https://doi.org/10.3390/nu13020572
Chicago/Turabian StyleCroall, Iain D., Nigel Hoggard, and Marios Hadjivassiliou. 2021. "Gluten and Autism Spectrum Disorder" Nutrients 13, no. 2: 572. https://doi.org/10.3390/nu13020572
APA StyleCroall, I. D., Hoggard, N., & Hadjivassiliou, M. (2021). Gluten and Autism Spectrum Disorder. Nutrients, 13(2), 572. https://doi.org/10.3390/nu13020572






