Bidirectional Interactions between the Menstrual Cycle, Exercise Training, and Macronutrient Intake in Women: A Review
Abstract
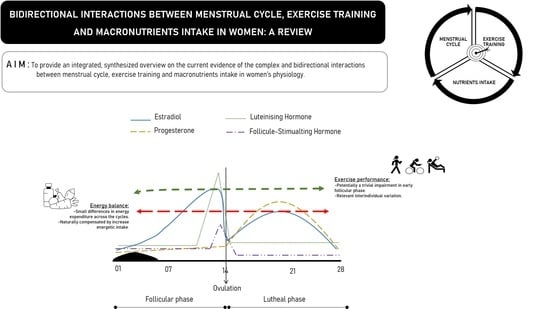
1. Introduction
2. State of Art
2.1. Bidirectional Relationships between Exercise Training and Sex Steroid Hormones
2.1.1. Estrogens
2.1.2. Progestogens
2.1.3. Estrogens, Progesterone and Exercise Training
2.2. Bidirectional Relationships between Exercise Training and the Menstrual Cycle
2.3. Bidirectional Relationships between Macronutrient Intake and Sex Steroid Hormones
2.3.1. Energy
2.3.2. Carbohydrates
2.3.3. Lipids
2.3.4. Protein
3. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, Y.J.; Tamadon, A.; Park, H.T.; Kim, H.; Ku, S.-Y. The role of sex steroid hormones in the pathophysiology and treatment of sarcopenia. Osteoporos. Sarcopenia 2016, 2, 140–155. [Google Scholar] [CrossRef]
- Acconcia, F.; Marino, M. Steroid Hormones: Synthesis, Secretion, and Transport. In Principles of Endocrinology and Hormone Action; Belfiore, A., LeRoith, D., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–31. [Google Scholar]
- Fuentes, N.; Silveyra, P. Chapter Three—Estrogen receptor signaling mechanisms. In Advances in Protein Chemistry and Structural Biology; Donev, R., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 116, pp. 135–170. [Google Scholar]
- Heldring, N.; Pike, A.; Andersson, S.; Matthews, J.; Cheng, G.; Hartman, J.; Gustafsson, J.-Å. Estrogen Receptors: How Do They Signal and What Are Their Targets. Physiol. Rev. 2007, 87, 905–931. [Google Scholar] [CrossRef]
- Kendall, B.; Eston, R. Exercise-Induced Muscle Damage and the Potential Protective Role of Estrogen. Sports Med. 2002, 32, 103–123. [Google Scholar] [CrossRef]
- Aizawa, K.; Iemitsu, M.; Otsuki, T.; Maeda, S.; Miyauchi, T.; Mesaki, N. Sex differences in steroidogenesis in skeletal muscle following a single bout of exercise in rats. J. Appl. Physiol. 2008, 104, 67–74. [Google Scholar] [CrossRef]
- Sato, K.; Iemitsu, M. Exercise and sex steroid hormones in skeletal muscle. J. Steroid Biochem. Mol. Biol. 2015, 145, 200–205. [Google Scholar] [CrossRef]
- Ahrens, K.A.; Vladutiu, C.J.; Mumford, S.L.; Schliep, K.C.; Perkins, N.J.; Wactawski-Wende, J.; Schisterman, E.F. The effect of physical activity across the menstrual cycle on reproductive function. Ann. Epidemiol. 2014, 24, 127–134. [Google Scholar] [CrossRef][Green Version]
- Janse De Jonge, X.A.K. Effects of the menstrual cycle on exercise performance. Sports Med. 2003, 33, 833–851. [Google Scholar] [CrossRef]
- Daniel, M.; Craig, S.; Simon, B.C.; Kirsty, J.E.-S. Period Prevalence and Perceived Side Effects of Hormonal Contraceptive Use and the Menstrual Cycle in Elite Athletes. Int. J. Sports Physiol. Perform. 2018, 13, 926–932. [Google Scholar] [CrossRef]
- Afonso, J.; Clemente, F.M.; Ribeiro, J.; Ferreira, M.; Fernandes, R.J. Towards a de facto Nonlinear Periodization: Extending Nonlinearity from Programming to Periodizing. Sports (Basel) 2020, 8, 110. [Google Scholar] [CrossRef]
- Devries, M.C.; Hamadeh, M.J.; Phillips, S.M.; Tarnopolsky, M.A. Menstrual cycle phase and sex influence muscle glycogen utilization and glucose turnover during moderate-intensity endurance exercise. Am. J. Physiol. -Regul. Integr. Comp. Physiol. 2006, 291, R1120–R1128. [Google Scholar] [CrossRef]
- Kammoun, I.; Ben Saâda, W.; Sifaou, A.; Haouat, E.; Kandara, H.; Ben Salem, L.; Ben Slama, C. Change in women’s eating habits during the menstrual cycle. Ann. D’endocrinologie 2017, 78, 33–37. [Google Scholar] [CrossRef]
- McLay, R.T.; Thomson, C.D.; Williams, S.M.; Rehrer, N.J. Carbohydrate Loading and Female Endurance Athletes: Effect of Menstrual-Cycle Phase. Int. J. Sport Nutr. Exerc. Metab. 2007, 17, 189–205. [Google Scholar] [CrossRef]
- Hatta, H.; Atomi, Y.; Shinohara, S.; Yamamoto, Y.; Yamada, S. The Effects of Ovarian Hormones on Glucose and Fatty Acid Oxidation during Exercise in Female Ovariectomized Rats. Horm. Metab. Res. 1988, 20, 609–611. [Google Scholar] [CrossRef]
- Oosthuyse, T.; Bosch, A.N. The effect of the menstrual cycle on exercise metabolism: Implications for exercise performance in eumenorrhoeic women. Sports Med. 2010, 40, 207–227. [Google Scholar] [CrossRef]
- Sims, S.T.; Heather, A.K. Myths and Methodologies: Reducing scientific design ambiguity in studies comparing sexes and/or menstrual cycle phases. Exp. Physiol. 2018, 103, 1309–1317. [Google Scholar] [CrossRef]
- Goodman-Gruen, D.; Barrett-Connor, E. Sex differences in the association of endogenous sex hormone levels and glucose tolerance status in older men and women. Diabetes Care 2000, 23, 912. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.S.; Pal, L.; Seli, E. Speroff’s Clinical Gynecologic Endocrinology and Infertility, 9th ed.; Williams & Wilkins (LWW): Philadelphia, PA, USA, 2019. [Google Scholar]
- Horstman, A.M.; Dillon, E.L.; Urban, R.J.; Sheffield-Moore, M. The Role of Androgens and Estrogens on Healthy Aging and Longevity. J. Gerontol. Ser. A 2012, 67, 1140–1152. [Google Scholar] [CrossRef]
- Knowlton, A.A.; Lee, A.R. Estrogen and the cardiovascular system. Pharmacol. Ther. 2012, 135, 54–70. [Google Scholar] [CrossRef]
- Rosano, G.M.; Fini, M. Comparative cardiovascular effects of different progestins in menopause. Int. J. Fertil. Womens Med. 2001, 46, 248–256. [Google Scholar]
- Behan, M.; Wenninger, J.M. Sex steroidal hormones and respiratory control. Respir. Physiol. Neurobiol. 2008, 164, 213–221. [Google Scholar] [CrossRef]
- Kalkhoff, R.K. Metabolic effects of progesterone. Am. J. Obstet. Gynecol. 1982, 142, 735–738. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F.; Clegg, D.J.; Hevener, A.L. The role of estrogens in control of energy balance and glucose homeostasis. Endocr. Rev. 2013, 34, 309–338. [Google Scholar] [CrossRef]
- Westerlind, K.C.; Byrnes, W.C.; Freedson, P.S.; Katch, F.I. Exercise and Serum Androgens in Women. Physician Sportsmed. 1987, 15, 87–94. [Google Scholar] [CrossRef]
- Cui, J.; Shen, Y.; Li, R. Estrogen synthesis and signaling pathways during aging: From periphery to brain. Trends Mol. Med. 2013, 19, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Campos, C.; Casali, K.R.; Baraldi, D.; Conzatti, A.; Araújo, A.S.d.R.; Khaper, N.; Belló-Klein, A. Efficacy of a Low Dose of Estrogen on Antioxidant Defenses and Heart Rate Variability. Oxidative Med. Cell. Longev. 2014, 2014, 218749. [Google Scholar] [CrossRef]
- Strehlow, K.; Rotter, S.; Wassmann, S.; Adam, O.; Grohé, C.; Laufs, K.; Nickenig, G. Modulation of antioxidant enzyme expression and function by estrogen. Circ. Res. 2003, 93, 170–177. [Google Scholar] [CrossRef]
- Liedtke, S.; Schmidt, M.E.; Vrieling, A.; Lukanova, A.; Becker, S.; Kaaks, R.; Steindorf, K. Postmenopausal Sex Hormones in Relation to Body Fat Distribution. Obesity 2012, 20, 1088–1095. [Google Scholar] [CrossRef]
- Rocha-Rodrigues, S. Physical exercise and sex steroid hormones in breast cancer. Hum. Mov. 2020. [Google Scholar] [CrossRef]
- Tworoger, S.S.; Missmer, S.A.; Eliassen, A.H.; Barbieri, R.L.; Dowsett, M.; Hankinson, S.E. Physical activity and inactivity in relation to sex hormone, prolactin, and insulin-like growth factor concentrations in premenopausal women. Cancer Causes Control 2007, 18, 743–752. [Google Scholar] [CrossRef]
- Verkasalo, P.K.; Thomas, H.V.; Appleby, P.N.; Davey, G.K.; Key, T.J. Circulating levels of sex hormones and their relation to risk factors for breast cancer: A cross-sectional study in 1092 pre- and postmenopausal women (United Kingdom). Cancer Causes Control 2001, 12, 47–59. [Google Scholar] [CrossRef]
- Williams, N.I.; Reed, J.L.; Leidy, H.J.; Legro, R.S.; De Souza, M.J. Estrogen and progesterone exposure is reduced in response to energy deficiency in women aged 25–40 years. Hum. Reprod. 2010, 25, 2328–2339. [Google Scholar] [CrossRef][Green Version]
- Smith, A.J.; Phipps, W.R.; Arikawa, A.Y.; O’Dougherty, M.; Kaufman, B.; Thomas, W.; Kurzer, M.S. Effects of aerobic exercise on premenopausal sex hormone levels: Results of the WISER study, a randomized clinical trial in healthy, sedentary, eumenorrheic women. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1098–1106. [Google Scholar] [CrossRef]
- Ennour-Idrissi, K.; Maunsell, E.; Diorio, C. Effect of physical activity on sex hormones in women: A systematic review and meta-analysis of randomized controlled trials. Breast Cancer Res. 2015, 17, 139. [Google Scholar] [CrossRef]
- Kyröläinen, H.; Hackney, A.C.; Salminen, R.; Repola, J.; Häkkinen, K.; Haimi, J. Effects of Combined Strength and Endurance Training on Physical Performance and Biomarkers of Healthy Young Women. J. Strength Cond. Res. 2018, 32, 1554–1561. [Google Scholar] [CrossRef]
- Sutton-Tyrrell, K.; Wildman Rachel, P.; Matthews Karen, A.; Chae, C.; Lasley Bill, L.; Brockwell, S.; Torréns Javier, I. Sex Hormone–Binding Globulin and the Free Androgen Index Are Related to Cardiovascular Risk Factors in Multiethnic Premenopausal and Perimenopausal Women Enrolled in the Study of Women Across the Nation (SWAN). Circulation 2005, 111, 1242–1249. [Google Scholar] [CrossRef]
- Xu, W.-H.; Feng, L.; Liu, Y.; Cai, D.-Q.; Wen, N.; Zheng, W.-J. Estrogen enhances the bone regeneration potential of periodontal ligament stem cells derived from osteoporotic rats and seeded on nano-hydroxyapatite/collagen/poly(L-lactide). Int. J. Mol. Med. 2016, 37, 1475–1486. [Google Scholar] [CrossRef]
- Enns, D.L.; Tiidus, P.M. The Influence of Estrogen on Skeletal Muscle. Sports Med. 2010, 40, 41–58. [Google Scholar] [CrossRef]
- Hansen, M. Female hormones: Do they influence muscle and tendon protein metabolism? Proc. Nutr. Soc. 2018, 77, 32–41. [Google Scholar] [CrossRef]
- Collins, B.C.; Mader, T.L.; Cabelka, C.A.; Iñigo, M.R.; Spangenburg, E.E.; Lowe, D.A. Deletion of estrogen receptor α in skeletal muscle results in impaired contractility in female mice. J. Appl. Physiol. 2018, 124, 980–992. [Google Scholar] [CrossRef]
- Kitajima, Y.; Ono, Y. Estrogens maintain skeletal muscle and satellite cell functions. J. Endocrinol. 2016, 229, 267. [Google Scholar] [CrossRef]
- Liu, S.H.; Al-Shaikh, R.A.; Panossian, V.; Finerman, G.A.M.; Lane, J.M. Estrogen Affects the Cellular Metabolism of the Anterior Cruciate Ligament: A Potential Explanation for Female Athletic Injury. Am. J. Sports Med. 1997, 25, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Taraborrelli, S. Physiology, production and action of progesterone. Acta Obstet. Et Gynecol. Scand. 2015, 94, 8–16. [Google Scholar] [CrossRef]
- Chabbert-Buffeta, N.; Skinner, D.C.; Caraty, A.; Bouchard, P. Neuroendocrine effects of progesterone. Steroids 2000, 65, 613–620. [Google Scholar] [CrossRef]
- Chidi-Ogbolu, N.; Baar, K. Effect of Estrogen on Musculoskeletal Performance and Injury Risk. Front. Physiol. 2019, 9, 1834. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.; Mattern, C.; Ghoumari, A.; Oudinet, J.P.; Liere, P.; Labombarda, F.; Guennoun, R. Revisiting the roles of progesterone and allopregnanolone in the nervous system: Resurgence of the progesterone receptors. Prog. Neurobiol. 2014, 113, 6–39. [Google Scholar] [CrossRef] [PubMed]
- Mas, A.; Elam, L.; Diamond, M.P.; Simon, C.; Al-Hendy, A. Synergistic effect of estrogen and progesterone on myometrial stem cell expansion in vivo. Fertil. Steril. 2015, 104, e72. [Google Scholar] [CrossRef]
- Candolfi, M.; Jaita, G.; Zaldivar, V.; Zárate, S.; Ferrari, L.; Pisera, D.; Seilicovich, A. Progesterone Antagonizes the Permissive Action of Estradiol on Tumor Necrosis Factor-α-Induced Apoptosis of Anterior Pituitary Cells. Endocrinology 2005, 146, 736–743. [Google Scholar] [CrossRef]
- Janse De Jonge, X.A.K.; Boot, C.R.L.; Thom, J.M.; Ruell, P.A.; Thompson, M.W. The influence of menstrual cycle phase on skeletal muscle contractile characteristics in humans. J. Physiol. 2001, 530, 161–166. [Google Scholar] [CrossRef]
- Uotinen, N.; Puustinen, R.; Pasanen, S.; Manninen, T.; Kivineva, M.; Syvälä, H.; Ylikomi, T. Distribution of progesterone receptor in female mouse tissues. Gen. Comp. Endocrinol. 1999, 115, 429–441. [Google Scholar] [CrossRef]
- Greeves, J.P.; Cable, N.T.; Reilly, T. The Relationship between Maximal Muscle Strength and Reproductive Hormones during the Menstrual Cycle. In Proceedings of the 4th Annual Congress of the European College of Sport Science, Rome, Italy, 14–17 July 1999. [Google Scholar]
- Phillips, S.K.; Sanderson, A.G.; Birch, K.; Bruce, S.A.; Woledge, R.C. Changes in maximal voluntary force of human adductor pollicis muscle during the menstrual cycle. J. Physiol. 1996, 496 (Pt 2), 551–557. [Google Scholar] [CrossRef]
- Sarwar, R.; Niclos, B.B.; Rutherford, O.M. Changes in muscle strength, relaxation rate and fatiguability during the human menstrual cycle. J. Physiol. 1996, 493, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.E.; Febbraio, M.A. Effect of ovarian hormones on mitochondrial enzyme activity in the fat oxidation pathway of skeletal muscle. Am. J. Physiol. -Endocrinol. Metab. 2001, 281, E803–E808. [Google Scholar] [CrossRef]
- Kenagy, R.; Weinstein, I.R.A.; Heimberg, M. The Effects of 17β-Estradiol and Progesterone on the Metabolism of Free Fatty Acid by Perfused Livers from Normal Female and Ovariectomized Rats*. Endocrinology 1981, 108, 1613–1621. [Google Scholar] [CrossRef] [PubMed]
- Hagan, C.R.; Lange, C.A. Molecular determinants of context-dependent progesterone receptor action in breast cancer. BMC Med. 2014, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Tseng, L.; Gusberg, S.B.; Gurpide, E. Estradiol receptor and 17β-Dehydrogenase in normal and abnormal human endometrium. Ann. N. Y. Acad. Sci. 1977, 286, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Mesaki, N.; Sasaki, J.; Shoji, M.; Iwasaki, H.; Asano, K.; Eda, M. Hormonal changes during incremental exercise in athletic women. Nihon Sanka Fujinka Gakkai Zasshi 1986, 38, 45–52. [Google Scholar] [PubMed]
- Bonen, A.; Belcastro, A.N.; Ling, W.Y.; Simpson, A.A. Profiles of selected hormones during menstrual cycles of teenage athletes. J. Appl. Physiol. 1981, 50, 545–551. [Google Scholar] [CrossRef]
- Montagnani, C.F.; Arena, B.; Maffulli, N. Estradiol and progesterone during exercise in healthy untrained women. Med. Sci. Sports Exerc. 1992, 24, 764–768. [Google Scholar] [CrossRef]
- Sato, K.; Iemitsu, M.; Katayama, K.; Ishida, K.; Kanao, Y.; Saito, M. Responses of sex steroid hormones to different intensities of exercise in endurance athletes. Exp. Physiol. 2016, 101, 168–175. [Google Scholar] [CrossRef]
- Ziomkiewicz, A.; Ellison, P.T.; Lipson, S.F.; Thune, I.; Jasienska, G. Body fat, energy balance and estradiol levels: A study based on hormonal profiles from complete menstrual cycles. Hum. Reprod. 2008, 23, 2555–2563. [Google Scholar] [CrossRef]
- González-Alonso, J.; Teller, C.; Andersen, S.L.; Jensen, F.B.; Hyldig, T.; Nielsen, B. Influence of body temperature on the development of fatigue during prolonged exercise in the heat. J. Appl. Physiol. 1999, 86, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Janse De Jonge, X.A.K.; Thompson, M.W.; Chuter, V.H.; Silk, L.N.; Thom, J.M. Exercise performance over the menstrual cycle in temperate and hot, humid conditions. Med. Sci. Sports Exerc. 2012, 44, 2190–2198. [Google Scholar] [CrossRef] [PubMed]
- D’Eon, T.M.; Sharoff, C.; Chipkin, S.R.; Grow, D.; Ruby, B.C.; Braun, B. Regulation of exercise carbohydrate metabolism by estrogen and progesterone in women. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E1046–E1055. [Google Scholar] [CrossRef] [PubMed]
- Lamont, L.S.; Lemon, P.W.; Bruot, B.C. Menstrual cycle and exercise effects on protein catabolism. Med. Sci. Sports Exerc. 1987, 19, 106–110. [Google Scholar] [PubMed]
- Braun, B.; Horton, T. Endocrine regulation of exercise substrate utilization in women compared to men. Exerc. Sport. Sci. Rev. 2001, 29, 149–154. [Google Scholar] [CrossRef]
- Draper, C.F.; Duisters, K.; Weger, B.; Chakrabarti, A.; Harms, A.C.; Brennan, L.; van der Greef, J. Menstrual cycle rhythmicity: Metabolic patterns in healthy women. Sci. Rep. 2018, 8, 14568. [Google Scholar] [CrossRef]
- Kenney, W.L.; Wilmore, J.H.; Costill, D.L. Physiology of Sport and Exercise, 5th ed.; Human Kinetics: Champaign, IL, USA, 2012. [Google Scholar]
- Ekenros, L.; Papoutsi, Z.; Fridén, C.; Dahlman Wright, K.; Lindén Hirschberg, A. Expression of sex steroid hormone receptors in human skeletal muscle during the menstrual cycle. Acta Physiol. (Oxf.) 2017, 219, 486–493. [Google Scholar] [CrossRef]
- Teixeira, A.L.d.S.; Fernandes Júnior, W.; Marques, F.A.D.; Lacio, M.L.d.; Dias, M.R.C. Influência das diferentes fases do ciclo menstrual na flexibilidade de mulheres jovens. Rev. Bras. De Med. Do Esporte 2012, 18, 361–364. [Google Scholar]
- Matsuda, T.; Furuhata, T.; Ogata, H.; Kamemoto, K.; Yamada, M.; Sakamaki-Sunaga, M. Effects of the Menstrual Cycle on Serum Carnitine and Endurance Performance of Women. Int. J. Sports Med. 2020, 41, 443–449. [Google Scholar] [CrossRef]
- Sim, M.; Dawson, B.; Landers, G.; Trinder, D.; Peeling, P. Iron Regulation in Athletes: Exploring the Menstrual Cycle and Effects of Different Exercise Modalities on Hepcidin Production. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 177. [Google Scholar] [CrossRef]
- Herzberg, S.D.; Motu’apuaka, M.L.; Lambert, W.; Fu, R.; Brady, J.; Guise, J.-M. The Effect of Menstrual Cycle and Contraceptives on ACL Injuries and Laxity: A Systematic Review and Meta-analysis. Orthop. J. Sports Med. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Balachandar, V.; Marciniak, J.-L.; Wall, O.; Balachandar, C. Effects of the menstrual cycle on lower-limb biomechanics, neuromuscular control, and anterior cruciate ligament injury risk: A systematic review. Musclesligaments Tendons J. 2017, 7, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Fridén, C.; Saartok, T.; Bäckström, C.; Leanderson, J.; Renström, P. The influence of premenstrual symptoms on postural balance and kinesthesia during the menstrual cycle. Gynecol. Endocrinol. 2003, 17, 433–440. [Google Scholar] [CrossRef]
- Lee, B.J.; Cho, K.H.; Lee, W.H. The effects of the menstrual cycle on the static balance in healthy young women. J. Phys. Ther. Sci. 2017, 29, 1964–1966. [Google Scholar] [CrossRef] [PubMed]
- Emami, F.; Kordi Yoosefinejad, A.; Motealleh, A. Comparison of static and dynamic balance during early follicular and ovulation phases in healthy women, using simple, clinical tests: A cross sectional study. Gynecol. Endocrinol. 2019, 35, 257–260. [Google Scholar] [CrossRef]
- Melegario, S.M.; Simão, R.; Vale, R.G.S.; Batista, L.A.; Novaes, J.S. A influência do ciclo menstrual na flexibilidade em praticantes de ginástica de academia. Rev. Bras. De Med. Do Esporte 2006, 12, 125–128. [Google Scholar] [CrossRef][Green Version]
- Findlay, R.J.; Macrae, E.H.R.; Whyte, I.Y.; Easton, C.; Forrest, L.J. How the menstrual cycle and menstruation affect sporting performance: Experiences and perceptions of elite female rugby players. Br. J. Sports Med. 2020, 54, 1108. [Google Scholar] [CrossRef]
- Hooper, A.E.C.; Bryan, A.D.; Eaton, M. Menstrual cycle effects on perceived exertion and pain during exercise among sedentary women. J. Women Health (2002) 2011, 20, 439–446. [Google Scholar] [CrossRef]
- Crewther, B.T.; Cook, C.J. A longitudinal analysis of salivary testosterone concentrations and competitiveness in elite and non-elite women athletes. Physiol. Behav. 2018, 188, 157–161. [Google Scholar] [CrossRef]
- Spieth, P.M.; Kubasch, A.S.; Penzlin, A.I.; Illigens, B.M.-W.; Barlinn, K.; Siepmann, T. Randomized controlled trials - a matter of design. Neuropsychiatr. Dis. Treat. 2016, 12, 1341–1349. [Google Scholar] [CrossRef]
- Carneiro, L.; Afonso, J.; Ramirez-Campillo, R.; Murawska-Cialowciz, E.; Marques, A.; Clemente, F.M. The Effects of Exclusively Resistance Training-Based Supervised Programs in People with Depression: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2020, 17, 6715. [Google Scholar] [CrossRef] [PubMed]
- Pereira, H.M.; Larson, R.D.; Bemben, D.A. Menstrual Cycle Effects on Exercise-Induced Fatigability. Front. Physiol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Blagrove, R.C.; Bruinvels, G.; Pedlar, C.R. Variations in strength-related measures during the menstrual cycle in eumenorrheic women: A systematic review and meta-analysis. J. Sci. Med. Sport 2020, 23, 1220–1227. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Hayashida, H.; Shimura, M.; Sugama, K.; Kanda, K.; Suzuki, K. Exercise-Induced Inflammation during Different Phases of the Menstrual Cycle. J. Physiother. Phys. Rehabil. 2016, 1. [Google Scholar] [CrossRef]
- Suzuki, K.; Hayashida, H. Effect of Exercise Intensity on Cell-Mediated Immunity. Sports (Basel) 2021, 9, 8. [Google Scholar] [CrossRef]
- Bernstein, L.; Ross, R.K.; Lobo, R.A.; Hanisch, R.; Krailo, M.D.; Henderson, B.E. The effects of moderate physical activity on menstrual cycle patterns in adolescence: Implications for breast cancer prevention. Br. J. Cancer 1987, 55, 681–685. [Google Scholar] [CrossRef]
- Constantini, N.W.; Warren, M.P. Menstrual dysfunction in swimmers: A distinct entity. J. Clin. Endocrinol. Metab. 1995, 80, 2740–2744. [Google Scholar] [CrossRef]
- McNulty, K.L.; Elliott-Sale, K.J.; Dolan, E.; Swinton, P.A.; Ansdell, P.; Goodall, S.; Hicks, K.M. The Effects of Menstrual Cycle Phase on Exercise Performance in Eumenorrheic Women: A Systematic Review and Meta-Analysis. Sports Med. 2020, 50, 1813–1827. [Google Scholar] [CrossRef]
- Minkin, M.J. Menopause: Hormones, Lifestyle, and Optimizing Aging. Obstet. Gynecol. Clin. North Am. 2019, 46, 501–514. [Google Scholar] [CrossRef]
- Bertone-Johnson, E.R.; Tworoger, S.S.; Hankinson, S.E. Recreational Physical Activity and Steroid Hormone Levels in Postmenopausal Women. Am. J. Epidemiol. 2009, 170, 1095–1104. [Google Scholar] [CrossRef]
- Chan, M.-F.; Dowsett, M.; Folkerd, E.; Bingham, S.; Wareham, N.; Luben, R.; Khaw, K.-T. Usual Physical Activity and Endogenous Sex Hormones in Postmenopausal Women: The European Prospective Investigation into Cancer–Norfolk Population Study. Cancer Epidemiol. Biomark. Prev. 2007, 16, 900. [Google Scholar] [CrossRef]
- Kantyka, J.; Herman, D.; Roczniok, R.; Kuba, L. Effects of aqua aerobics on body composition, body mass, lipid profile, and blood count in middle-aged sedentary women. Hum. Mov. 2015, 16, 9–14. [Google Scholar] [CrossRef]
- McTiernan, A.; Wu, L.; Chen, C.; Chlebowski, R.; Mossavar-Rahmani, Y.; Modugno, F.; Women’s Health Initiative Investigators. Relation of BMI and Physical Activity to Sex Hormones in Postmenopausal Women. Obesity 2006, 14, 1662–1677. [Google Scholar] [CrossRef] [PubMed]
- De Roon, M.; May, A.M.; McTiernan, A.; Scholten, R.J.P.M.; Peeters, P.H.M.; Friedenreich, C.M.; Monninkhof, E.M. Effect of exercise and/or reduced calorie dietary interventions on breast cancer-related endogenous sex hormones in healthy postmenopausal women. Breast Cancer Res. 2018, 20, 81. [Google Scholar] [CrossRef] [PubMed]
- Van Gemert, W.A.M.; Schuit, A.J.; van der Palen, J.; May, A.M.; Iestra, J.A.; Wittink, H.; Monninkhof, E.M. Effect of weight loss, with or without exercise, on body composition and sex hormones in postmenopausal women: The SHAPE-2 trial. Breast Cancer Res. 2015, 17, 120. [Google Scholar] [CrossRef]
- Sipilä, S.; Poutamo, J. Muscle performance, sex hormones and training in peri-menopausal and post-menopausal women. Scand. J. Med. Sci. Sports 2003, 13, 19–25. [Google Scholar] [CrossRef]
- Bamman, M.M.; Hill, V.J.; Adams, G.R.; Haddad, F.; Wetzstein, C.J.; Gower, B.A.; Hunter, G.R. Gender Differences in Resistance-Training-Induced Myofiber Hypertrophy Among Older Adults. J. Gerontol. Ser. A 2003, 58, B108–B116. [Google Scholar] [CrossRef]
- Pöllänen, E.; Fey, V.; Törmäkangas, T.; Ronkainen, P.H.A.; Taaffe, D.R.; Takala, T.; Kovanen, V. Power training and postmenopausal hormone therapy affect transcriptional control of specific co-regulated gene clusters in skeletal muscle. AGE 2010, 32, 347–363. [Google Scholar] [CrossRef]
- Ronkainen, P.H.A.; Kovanen, V.; Alén, M.; Pöllänen, E.; Palonen, E.-M.; Ankarberg-Lindgren, C.; Sipilä, S. Postmenopausal hormone replacement therapy modifies skeletal muscle composition and function: A study with monozygotic twin pairs. J. Appl. Physiol. 2009, 107, 25–33. [Google Scholar] [CrossRef]
- Sipilä, S.; Taaffe, D.R.; Cheng, S.; Puolakka, J.; Toivanen, J.; Suominen, H. Effects of hormone replacement therapy and high-impact physical exercise on skeletal muscle in post-menopausal women: A randomized placebo-controlled study. Clin. Sci. (Lond.) 2001, 101, 147–157. [Google Scholar] [CrossRef]
- Javed, A.A.; Mayhew, A.J.; Shea, A.K.; Raina, P. Association between Hormone Therapy and Muscle Mass in Postmenopausal Women: A Systematic Review and Meta-analysis. JAMA Netw. Open 2019, 2, e1910154. [Google Scholar] [CrossRef] [PubMed]
- Tarnopolsky, M.A.; Zawada, C.; Richmond, L.B.; Carter, S.L.; Shearer, J.; Graham, T.; Phillips, S.M. Gender differences in carbohydrate loading are related to energy intake. J. Appl. Physiol. 2001, 91, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Melin, A.K.; Heikura, I.A.; Tenforde, A.; Mountjoy, M. Energy Availability in Athletics: Health, Performance, and Physique. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Benton, M.J.; Hutchins, A.M.; Dawes, J.J. Effect of menstrual cycle on resting metabolism: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0236025. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.; Deakin, V. Clinical Sports Nutrition, 5th ed.; Australia McGraw-Hill Education: New York, NY, USA, 2015. [Google Scholar]
- Barr, S.I.; Janelle, K.C.; Prior, J.C. Energy intakes are higher during the luteal phase of ovulatory menstrual cycles. Am. J. Clin. Nutr. 1995, 61, 39–43. [Google Scholar] [CrossRef]
- Hirschberg, A.L. Sex hormones, appetite and eating behaviour in women. Maturitas 2012, 71, 248–256. [Google Scholar] [CrossRef]
- Carter, S.L.; Rennie, C.; Tarnopolsky, M.A. Substrate utilization during endurance exercise in men and women after endurance training. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E898–E907. [Google Scholar] [CrossRef]
- Tarnopolsky, L.J.; MacDougall, J.D.; Atkinson, S.A.; Tarnopolsky, M.A.; Sutton, J.R. Gender differences in substrate for endurance exercise. J. Appl. Physiol. 1990, 68, 302–308. [Google Scholar] [CrossRef]
- Esbjörnsson-Liljedahl, M.; Sundberg, C.J.; Norman, B.; Jansson, E. Metabolic response in type I and type II muscle fibers during a 30-s cycle sprint in men and women. J. Appl. Physiol. 1999, 87, 1326–1332. [Google Scholar] [CrossRef]
- Geer, E.B.; Shen, W. Gender differences in insulin resistance, body composition, and energy balance. Gend. Med. 2009, 6 (Suppl. 1), 60–75. [Google Scholar] [CrossRef]
- Wallis, G.A.; Yeo, S.E.; Blannin, A.K.; Jeukendrup, A.E. Dose-response effects of ingested carbohydrate on exercise metabolism in women. Med. Sci. Sports Exerc. 2007, 39, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Jeukendrup, A. A step towards personalized sports nutrition: Carbohydrate intake during exercise. Sports Med. (Auckl. N. Z.) 2014, 44 (Suppl. 1), S25–S33. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.L.; Heigenhauser, G.J.; Hultman, E.; Spriet, L.L. Dietary carbohydrate, muscle glycogen content, and endurance performance in well-trained women. J. Appl. Physiol. (1985) 2000, 88, 2151–2158. [Google Scholar] [CrossRef] [PubMed]
- Rehrer, N.J.; McLay-Cooke, R.T.; Sims, S.T. Nutritional strategies and sex hormone interactions in women. In Sex Hormones, Exercise and Women; Hackney, A.C., Ed.; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Hashimoto, H.; Ishijima, T.; Hayashida, H.; Suzuki, K.; Higuchi, M. Menstrual cycle phase and carbohydrate ingestion alter immune response following endurance exercise and high intensity time trial performance test under hot conditions. J. Int. Soc. Sports Nutr. 2014, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Chenevière, X.; Borrani, F.; Sangsue, D.; Gojanovic, B.; Malatesta, D. Gender differences in whole-body fat oxidation kinetics during exercise. Appl. Physiol. Nutr. Metab. 2011, 36, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Devries, M.C. Sex-based differences in endurance exercise muscle metabolism: Impact on exercise and nutritional strategies to optimize health and performance in women. Exp. Physiol. 2016, 101, 243–249. [Google Scholar] [CrossRef]
- Devries, M.C.; Lowther, S.A.; Glover, A.W.; Hamadeh, M.J.; Tarnopolsky, M.A. IMCL area density, but not IMCL utilization, is higher in women during moderate-intensity endurance exercise, compared with men. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R2336–R2342. [Google Scholar] [CrossRef] [PubMed]
- Hamadeh, M.J.; Devries, M.C.; Tarnopolsky, M.A. Estrogen supplementation reduces whole body leucine and carbohydrate oxidation and increases lipid oxidation in men during endurance exercise. J. Clin. Endocrinol. Metab. 2005, 90, 3592–3599. [Google Scholar] [CrossRef]
- Murphy, N.E.; Carrigan, C.T.; Margolis, L.M. High-Fat Ketogenic Diets and Physical Performance: A Systematic Review. Adv. Nutr. 2020. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies. Scientific Opinion on Dietary Reference Values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. Efsa J. 2010, 8, 1461. [Google Scholar] [CrossRef]
- Lariviere, F.; Moussalli, R.; Garrel, D.R. Increased leucine flux and leucine oxidation during the luteal phase of the menstrual cycle in women. Am. J. Physiol. 1994, 267 (Pt 1), E422–E428. [Google Scholar] [CrossRef] [PubMed]
- Sawai, A.; Tsuzuki, K.; Yamauchi, M.; Kimura, N.; Tsushima, T.; Sugiyama, K.; Tochikubo, O. The effects of estrogen and progesterone on plasma amino acids levels: Evidence from change plasma amino acids levels during the menstrual cycle in women. Biol. Rhythm Res. 2020, 51, 151–164. [Google Scholar] [CrossRef]
- Bailey, S.P.; Zacher, C.M.; Mittleman, K.D. Effect of menstrual cycle phase on carbohydrate supplementation during prolonged exercise to fatigue. J. Appl. Physiol. (1985) 2000, 88, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Kriengsinyos, W.; Wykes, L.J.; Goonewardene, L.A.; Ball, R.O.; Pencharz, P.B. Phase of menstrual cycle affects lysine requirement in healthy women. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E489–E496. [Google Scholar] [CrossRef]
- Obayashi, M.; Shimomura, Y.; Nakai, N.; Jeoung, N.H.; Nagasaki, M.; Murakami, T.; Harris, R.A. Estrogen controls branched-chain amino acid catabolism in female rats. J. Nutr. 2004, 134, 2628–2633. [Google Scholar] [CrossRef]
- Gorczyca, A.M.; Sjaarda, L.A.; Mitchell, E.M.; Perkins, N.J.; Schliep, K.C.; Wactawski-Wende, J.; Mumford, S.L. Changes in macronutrient, micronutrient, and food group intakes throughout the menstrual cycle in healthy, premenopausal women. Eur. J. Nutr. 2016, 55, 1181–1188. [Google Scholar] [CrossRef]
- Mercer, D.; Convit, L.; Condo, D.; Carr, A.J.; Hamilton, D.L.; Slater, G.; Snipe, R.M.J. Protein Requirements of Pre-Menopausal Female Athletes: Systematic Literature Review. Nutrients 2020, 12, 3527. [Google Scholar] [CrossRef]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. American College of Sports Medicine Joint Position Statement. Nutrition and Athletic Performance. Med. Sci. Sports Exerc. 2016, 48, 543–568. [Google Scholar] [CrossRef]
- West, D.W.D.; Burd, N.A.; Churchward-Venne, T.A.; Camera, D.M.; Mitchell, C.J.; Baker, S.K.; Phillips, S.M. Sex-based comparisons of myofibrillar protein synthesis after resistance exercise in the fed state. J. Appl. Physiol. 2012, 112, 1805–1813. [Google Scholar] [CrossRef]
- Morton, R.W.; McGlory, C.; Phillips, S.M. Nutritional interventions to augment resistance training-induced skeletal muscle hypertrophy. Front. Physiol. 2015, 6, 245. [Google Scholar] [CrossRef]
- Dalgaard, L.B.; Dalgas, U.; Andersen, J.L.; Rossen, N.B.; Møller, A.B.; Stødkilde-Jørgensen, H.; Hansen, M. Influence of Oral Contraceptive Use on Adaptations to Resistance Training. Front. Physiol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Burrows, M.; Peters, C.E. The influence of oral contraceptives on athletic performance in female athletes. Sports Med. 2007, 37, 557–574. [Google Scholar] [CrossRef] [PubMed]
- Elliott-Sale, K.J.; McNulty, K.L.; Ansdell, P.; Goodall, S.; Hicks, K.M.; Thomas, K.; Dolan, E. The Effects of Oral Contraceptives on Exercise Performance in Women: A Systematic Review and Meta-analysis. Sports Med. 2020, 50, 1785–1812. [Google Scholar] [CrossRef] [PubMed]
- Burkman, R.T. Noncontraceptive effects of hormonal contraceptives: Bone mass, sexually transmitted disease and pelvic inflammatory disease, cardiovascular disease, menstrual function, and future fertility. Am. J. Obstet. Gynecol. 1994, 170, 1569–1575. [Google Scholar] [CrossRef]
- Schaumberg, M.A.; Emmerton, L.M.; Jenkins, D.G.; Burton, N.W.; Janse De Jonge, X.A.K.; Skinner, T.L. Use of Oral Contraceptives to Manipulate Menstruation in Young, Physically Active Women. Int. J. Sports Physiol. Perform. 2018, 13, 82–87. [Google Scholar] [CrossRef]
- Hackney, A.C.; Constantini, N. Endocrinology of Physical Activity and Sport, 2nd ed.; Humana Press: Totowa, NJ, USA, 2013. [Google Scholar]
- Tucker, R.; Collins, M. What makes champions? A review of the relative contribution of genes and training to sporting success. Br. J. Sports Med. 2012, 46, 555. [Google Scholar] [CrossRef]
- Thompson, B.; Almarjawi, A.; Sculley, D.; Janse De Jonge, X.A.K. The Effect of the Menstrual Cycle and Oral Contraceptives on Acute Responses and Chronic Adaptations to Resistance Training: A Systematic Review of the Literature. Sports Med. 2020, 50, 171–185. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocha-Rodrigues, S.; Sousa, M.; Lourenço Reis, P.; Leão, C.; Cardoso-Marinho, B.; Massada, M.; Afonso, J. Bidirectional Interactions between the Menstrual Cycle, Exercise Training, and Macronutrient Intake in Women: A Review. Nutrients 2021, 13, 438. https://doi.org/10.3390/nu13020438
Rocha-Rodrigues S, Sousa M, Lourenço Reis P, Leão C, Cardoso-Marinho B, Massada M, Afonso J. Bidirectional Interactions between the Menstrual Cycle, Exercise Training, and Macronutrient Intake in Women: A Review. Nutrients. 2021; 13(2):438. https://doi.org/10.3390/nu13020438
Chicago/Turabian StyleRocha-Rodrigues, Sílvia, Mónica Sousa, Patrícia Lourenço Reis, César Leão, Beatriz Cardoso-Marinho, Marta Massada, and José Afonso. 2021. "Bidirectional Interactions between the Menstrual Cycle, Exercise Training, and Macronutrient Intake in Women: A Review" Nutrients 13, no. 2: 438. https://doi.org/10.3390/nu13020438
APA StyleRocha-Rodrigues, S., Sousa, M., Lourenço Reis, P., Leão, C., Cardoso-Marinho, B., Massada, M., & Afonso, J. (2021). Bidirectional Interactions between the Menstrual Cycle, Exercise Training, and Macronutrient Intake in Women: A Review. Nutrients, 13(2), 438. https://doi.org/10.3390/nu13020438