Daily Eating Frequency in US Adults: Associations with Low-Calorie Sweeteners, Body Mass Index, and Nutrient Intake (NHANES 2007–2016)
Abstract
:1. Introduction
2. Subjects and Methods
2.1. Definitions
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BMI | body mass index |
| CI | confidence interval |
| CSFII | Continuing Survey of Food Intakes of Individuals |
| DG | Dietary Guidelines for Americans |
| DGAC | Dietary Guidelines Advisory Committee |
| FBA | food and beverage additions |
| FDA | United States Food and Drug Administration |
| INTERMAP | International Study on Macro/Micronutrients and Blood Pressure |
| LCS | low-calorie sweetener |
| NCHS | National Center for Health Statistics |
| NHANES | National Health and Nutrition Examination Survey |
| NHW | non-Hispanic white |
| NHB | non-Hispanic black |
| NS | nutritive sweeteners |
| US | United States |
| USDA | United States Department of Agriculture |
| y | year |
References
- Dietary Guidelines for Americans. Topics and Questions to be Examined by the Committee. Available online: https://www.dietaryguidelines.gov/work-under-way/review-science/topics-and-questions-under-review (accessed on 29 April 2020).
- Centers for Disease Control and Prevention (CDC); National Center for Health Statistics (NCHS). ANALYTIC AND REPORTING GUIDELINES: The National Health and Nutrition Examination Survey (NHANES). Available online: http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/nhanes_analytic_guidelines_dec_2005.pdf (accessed on 16 May 2018).
- Kant, A.K.; Ballard-Barbash, R.; Schatzkin, A. Evening eating and its relation to self-reported body weight and nutrient intake in women, CSFII 1985–1986. J. Am. Coll. Nutr. 1995, 14, 358–363. [Google Scholar] [CrossRef]
- Kant, A.K.; Schatzkin, A.; Graubard, B.I.; Ballard-Barbash, R. Frequency of eating occasions and weight change in the NHANES I Epidemiologic Follow-up Study. Int. J. Obes. Relat. Metab. Disord. 1995, 19, 468–474. [Google Scholar]
- Marriott, B.P.; Hunt, K.J.; Malek, A.M.; St Peter, J.V.; Greenberg, D. Low-Calorie Sweeteners: Exploring Underutilized Database Resources to Understand Dietary Patterns and Obesity. Obesity (Silver Spring) 2018, 26 (Suppl. 3), S5–S8. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Hollis, J.H. Associations between eating frequency and energy intake, energy density, diet quality and body weight status in adults from the USA. Br. J. Nutr. 2016, 115, 2138–2144. [Google Scholar] [CrossRef] [Green Version]
- Murakami, K.; Livingstone, M.B. Eating Frequency Is Positively Associated with Overweight and Central Obesity in U.S. Adults. J. Nutr. 2015, 145, 2715–2724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kant, A.K.; Graubard, B.I. Association of self-reported sleep duration with eating behaviors of American adults: NHANES 2005-2010. Am. J. Clin. Nutr. 2014, 100, 938–947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aljuraiban, G.S.; Chan, Q.; Oude Griep, L.M.; Brown, I.J.; Daviglus, M.L.; Stamler, J.; Van Horn, L.; Elliott, P.; Frost, G.S.; Group, I.R. The impact of eating frequency and time of intake on nutrient quality and Body Mass Index: The INTERMAP Study, a Population-Based Study. J. Acad. Nutr. Diet. 2015, 115, 528–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franko, D.L.; Striegel-Moore, R.H.; Thompson, D.; Affenito, S.G.; Schreiber, G.B.; Daniels, S.R.; Crawford, P.B. The relationship between meal frequency and body mass index in black and white adolescent girls: More is less. Int. J. Obes. (Lond.) 2008, 32, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Howarth, N.C.; Huang, T.T.; Roberts, S.B.; Lin, B.H.; McCrory, M.A. Eating patterns and dietary composition in relation to BMI in younger and older adults. Int. J. Obes. (Lond.) 2007, 31, 675–684. [Google Scholar] [CrossRef] [Green Version]
- Hartline-Grafton, H.L.; Rose, D.; Johnson, C.C.; Rice, J.C.; Webber, L.S. The influence of weekday eating patterns on energy intake and BMI among female elementary school personnel. Obesity (Silver Spring) 2010, 18, 736–742. [Google Scholar] [CrossRef]
- Leech, R.M.; Worsley, A.; Timperio, A.; McNaughton, S.A. Characterizing eating patterns: A comparison of eating occasion definitions. Am. J. Clin. Nutr. 2015, 102, 1229–1237. [Google Scholar] [CrossRef]
- Popkin, B.M.; Duffey, K.J. Does hunger and satiety drive eating anymore? Increasing eating occasions and decreasing time between eating occasions in the United States. Am. J. Clin. Nutr. 2010, 91, 1342–1347. [Google Scholar] [CrossRef] [Green Version]
- Mills, J.P.; Perry, C.D.; Reicks, M. Eating frequency is associated with energy intake but not obesity in midlife women. Obesity (Silver Spring) 2011, 19, 552–559. [Google Scholar] [CrossRef]
- Kant, A.K. Evidence for efficacy and effectiveness of changes in eating frequency for body weight management. Adv. Nutr. 2014, 5, 822–828. [Google Scholar] [CrossRef] [Green Version]
- Sylvetsky, A.C.; Jin, Y.; Clark, E.J.; Welsh, J.A.; Rother, K.I.; Talegawkar, S.A. Consumption of Low-Calorie Sweeteners among Children and Adults in the United States. J. Acad. Nutr. Diet. 2017, 117, 441–448. [Google Scholar] [CrossRef]
- Sylvetsky, A.C.; Rother, K.I. Nonnutritive Sweeteners in Weight Management and Chronic Disease: A Review. Obesity (Silver Spring) 2018, 26, 635–640. [Google Scholar] [CrossRef] [Green Version]
- Sun, L. A Foolish Take: American soda consumption plunges to a 31-year low. USA Today, 1 August 2017. [Google Scholar]
- Haley, S.; Suarez, N.R. Sugar and Sweeteners Outlook; SSS-M-283; U.S. Department of Agriculture, Economic Research Service: Springfield, VA, USA, 2012. [Google Scholar]
- McConnell, M.J.; Olson, D. Sugar and Sweeteners Outlook; SSS-M-368; U.S. Department of Agriculture, Economic Research Service: Springfield, VA, USA, 2019. [Google Scholar]
- Drewnowski, A.; Rehm, C.D. Consumption of low-calorie sweeteners among U.S. adults is associated with higher Healthy Eating Index (HEI 2005) scores and more physical activity. Nutrients 2014, 6, 4389–4403. [Google Scholar] [CrossRef]
- Fernstrom, J.D. Non-nutritive sweeteners and obesity. Annu. Rev. Food Sci. Technol. 2015, 6, 119–136. [Google Scholar] [CrossRef] [Green Version]
- Rogers, P.J.; Hogenkamp, P.S.; de Graaf, C.; Higgs, S.; Lluch, A.; Ness, A.R.; Penfold, C.; Perry, R.; Putz, P.; Yeomans, M.R.; et al. Does low-energy sweetener consumption affect energy intake and body weight? A systematic review, including meta-analyses, of the evidence from human and animal studies. Int. J. Obes. (Lond.) 2016, 40, 381–394. [Google Scholar] [CrossRef] [Green Version]
- Gardner, C.; Wylie-Rosett, J.; Gidding, S.S.; Steffen, L.M.; Johnson, R.K.; Reader, D.; Lichtenstein, A.H.; American Heart Association Nutrition Committee of the Council on Nutrition; Physical Activity and Metabolism; Council on Arteriosclerosis; et al. Nonnutritive sweeteners: Current use and health perspectives: A scientific statement from the American Heart Association and the American Diabetes Association. Circulation 2012, 126, 509–519. [Google Scholar] [CrossRef]
- Tate, D.F.; Turner-McGrievy, G.; Lyons, E.; Stevens, J.; Erickson, K.; Polzien, K.; Diamond, M.; Wang, X.; Popkin, B. Replacing caloric beverages with water or diet beverages for weight loss in adults: Main results of the Choose Healthy Options Consciously Everyday (CHOICE) randomized clinical trial. Am. J. Clin. Nutr. 2012, 95, 555–563. [Google Scholar] [CrossRef] [Green Version]
- Peters, J.C.; Wyatt, H.R.; Foster, G.D.; Pan, Z.; Wojtanowski, A.C.; Vander Veur, S.S.; Herring, S.J.; Brill, C.; Hill, J.O. The effects of water and non-nutritive sweetened beverages on weight loss during a 12-week weight loss treatment program. Obesity (Silver Spring) 2014, 22, 1415–1421. [Google Scholar] [CrossRef]
- Raben, A.; Vasilaras, T.H.; Moller, A.C.; Astrup, A. Sucrose compared with artificial sweeteners: Different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am. J. Clin. Nutr. 2002, 76, 721–729. [Google Scholar] [CrossRef]
- De Koning, L.; Malik, V.S.; Rimm, E.B.; Willett, W.C.; Hu, F.B. Sugar-sweetened and artificially sweetened beverage consumption and risk of type 2 diabetes in men. Am. J. Clin. Nutr. 2011, 93, 1321–1327. [Google Scholar] [CrossRef] [Green Version]
- Fowler, S.P.; Williams, K.; Resendez, R.G.; Hunt, K.J.; Hazuda, H.P.; Stern, M.P. Fueling the obesity epidemic? Artificially sweetened beverage use and long-term weight gain. Obesity (Silver Spring) 2008, 16, 1894–1900. [Google Scholar] [CrossRef]
- Azad, M.B.; Abou-Setta, A.M.; Chauhan, B.F.; Rabbani, R.; Lys, J.; Copstein, L.; Mann, A.; Jeyaraman, M.M.; Reid, A.E.; Fiander, M.; et al. Nonnutritive sweeteners and cardiometabolic health: A systematic review and meta-analysis of randomized controlled trials and prospective cohort studies. CMAJ 2017, 189, E929–E939. [Google Scholar] [CrossRef] [Green Version]
- Miller, P.E.; Perez, V. Low-calorie sweeteners and body weight and composition: A meta-analysis of randomized controlled trials and prospective cohort studies. Am. J. Clin. Nutr. 2014, 100, 765–777. [Google Scholar] [CrossRef] [Green Version]
- Malik, V.S.; Pan, A.; Willett, W.C.; Hu, F.B. Sugar-sweetened beverages and weight gain in children and adults: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2013, 98, 1084–1102. [Google Scholar] [CrossRef] [Green Version]
- Davidson, T.L.; Swithers, S.E. A Pavlovian approach to the problem of obesity. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 933–935. [Google Scholar] [CrossRef] [Green Version]
- Swithers, S.E.; Baker, C.R.; Davidson, T.L. General and persistent effects of high-intensity sweeteners on body weight gain and caloric compensation in rats. Behav. Neurosci. 2009, 123, 772–780. [Google Scholar] [CrossRef] [Green Version]
- Swithers, S.E.; Martin, A.A.; Davidson, T.L. High-intensity sweeteners and energy balance. Physiol. Behav. 2010, 100, 55–62. [Google Scholar] [CrossRef] [Green Version]
- Swithers, S.E.; Sample, C.H.; Davidson, T.L. Adverse effects of high-intensity sweeteners on energy intake and weight control in male and obesity-prone female rats. Behav. Neurosci. 2013, 127, 262–274. [Google Scholar] [CrossRef] [Green Version]
- Schoenfeld, B.J.; Aragon, A.A.; Krieger, J.W. Effects of meal frequency on weight loss and body composition: A meta-analysis. Nutr. Rev. 2015, 73, 69–82. [Google Scholar] [CrossRef] [Green Version]
- U.S. Office of Management and Budget. Standards for the Classification of Federal Data on Race and Ethnicity; Office of the President: Washington, DC, USA, 1995. [Google Scholar]
- DellaValle, D.M.; Malek, A.M.; Hunt, K.J.; St Peter, J.V.; Greenberg, D.; Marriott, B.P. Low Calorie Sweeteners in Foods, Beverages, and Food and Beverage Additions: NHANES 2007–2012. Curr. Dev. Nutr. 2018, 2, nzy024. [Google Scholar] [CrossRef]
- Malek, A.M.; Hunt, K.J.; DellaValle, D.M.; Greenberg, D.; Peter, J.V.S.; Marriott, B.P. Reported Consumption of Low-Calorie Sweetener in Foods, Beverages, and Food and Beverage Additions by US Adults: NHANES 2007–2012. Curr. Dev. Nutr. 2018, 2, nzy054. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Industry Resources on the Changes to the Nutrition Facts Label. Added Sugars. Available online: https://www.fda.gov/food/food-labeling-nutrition/industry-resources-changes-nutrition-facts-label#AddedSugars (accessed on 30 April 2020).
- Kant, A.K. Eating patterns of US adults: Meals, snacks, and time of eating. Physiol. Behav. 2018, 193, 270–278. [Google Scholar] [CrossRef]
- Ahluwalia, N.; Dwyer, J.; Terry, A.; Moshfegh, A.; Johnson, C. Update on NHANES Dietary Data: Focus on Collection, Release, Analytical Considerations, and Uses to Inform Public Policy. Adv. Nutr. 2016, 7, 121–134. [Google Scholar] [CrossRef] [Green Version]
- Dodd, K.W.; Guenther, P.M.; Freedman, L.S.; Subar, A.F.; Kipnis, V.; Midthune, D.; Tooze, J.A.; Krebs-Smith, S.M. Statistical methods for estimating usual intake of nutrients and foods: A review of the theory. J. Am. Diet. Assoc. 2006, 106, 1640–1650. [Google Scholar] [CrossRef]
- Tooze, J.A.; Midthune, D.; Dodd, K.W.; Freedman, L.S.; Krebs-Smith, S.M.; Subar, A.F.; Guenther, P.M.; Carroll, R.J.; Kipnis, V. A new statistical method for estimating the usual intake of episodically consumed foods with application to their distribution. J. Am. Diet. Assoc. 2006, 106, 1575–1587. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention; National Center for Health Statistics. Available online: http://www.cdc.gov/nchs/nhanes/survey_methods.htm (accessed on 20 November 2018).
- U.S. Department of Agriculture; Agricultural Research Service; Beltsville Human Nutrition Research Center; Food Surveys Research Group (Beltsville; MD) and U.S. Department of Health and Human Services; Centers for Disease Control and Prevention; National Center for Health Statistics. What We Eat in America. Available online: https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/wweianhanes-overview/ (accessed on 1 May 2020).
- Hollland, P.C.; Rescorla, R.A. The effect of two ways of devaluing the unconditioned stimulus after first- and second-order appetitive conditioning. J. Exp. Psychol. Anim. Behav. Process. 1975, 1, 355–363. [Google Scholar] [CrossRef]
- Swithers, S.E. Artificial sweeteners produce the counterintuitive effect of inducing metabolic derangements. Trends Endocr. Metab. 2013, 24, 431–441. [Google Scholar] [CrossRef] [Green Version]
- Dalenberg, J.R.; Patel, B.P.; Denis, R.; Veldhuizen, M.G.; Nakamura, Y.; Vinke, P.C.; Luquet, S.; Small, D.M. Short-Term Consumption of Sucralose with, but Not without, Carbohydrate Impairs Neural and Metabolic Sensitivity to Sugar in Humans. Cell Metab. 2020, 31, 493–502.e497. [Google Scholar] [CrossRef]
- Keim, N.L.; Van Loan, M.D.; Horn, W.F.; Barbieri, T.F.; Mayclin, P.L. Weight loss is greater with consumption of large morning meals and fat-free mass is preserved with large evening meals in women on a controlled weight reduction regimen. J. Nutr. 1997, 127, 75–82. [Google Scholar] [CrossRef]
- Zhao, I.; Bogossian, F.; Song, S.; Turner, C. The association between shift work and unhealthy weight: A cross-sectional analysis from the Nurses and Midwives’ e-cohort Study. J. Occup. Environ. Med. 2011, 53, 153–158. [Google Scholar] [CrossRef] [Green Version]
- Parkes, K.R. Shift work and age as interactive predictors of body mass index among offshore workers. Scand. J. Work Environ. Health 2002, 28, 64–71. [Google Scholar] [CrossRef]
- Di Lorenzo, L.; De Pergola, G.; Zocchetti, C.; L’Abbate, N.; Basso, A.; Pannacciulli, N.; Cignarelli, M.; Giorgino, R.; Soleo, L. Effect of shift work on body mass index: Results of a study performed in 319 glucose-tolerant men working in a Southern Italian industry. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 1353–1358. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Bertone, E.R.; Stanek, E.J., 3rd; Reed, G.W.; Hebert, J.R.; Cohen, N.L.; Merriam, P.A.; Ockene, I.S. Association between eating patterns and obesity in a free-living US adult population. Am. J. Epidemiol. 2003, 158, 85–92. [Google Scholar] [CrossRef]
- Ritchie, L.D. Less frequent eating predicts greater BMI and waist circumference in female adolescents. Am. J. Clin. Nutr. 2012, 95, 290–296. [Google Scholar] [CrossRef] [Green Version]
- Metzner, H.L.; Lamphiear, D.E.; Wheeler, N.C.; Larkin, F.A. The relationship between frequency of eating and adiposity in adult men and women in the Tecumseh Community Health Study. Am. J. Clin. Nutr. 1977, 30, 712–715. [Google Scholar] [CrossRef]
- McCrory, M.A.; Howarth, N.C.; Roberts, S.B.; Huang, T.T. Eating frequency and energy regulation in free-living adults consuming self-selected diets. J. Nutr. 2011, 141, 148–153. [Google Scholar] [CrossRef] [Green Version]
- Andersson, I.; Rossner, S. Meal patterns in obese and normal weight men: The ‘Gustaf’ study. Eur. J. Clin. Nutr. 1996, 50, 639–646. [Google Scholar]
- Summerbell, C.D.; Moody, R.C.; Shanks, J.; Stock, M.J.; Geissler, C. Relationship between feeding pattern and body mass index in 220 free-living people in four age groups. Eur. J. Clin. Nutr. 1996, 50, 513–519. [Google Scholar]
- Zick, C.D.; Stevens, R.B. Time Spent Eating and Its Implications for Americans’ Energy Balance. Soc. Indic. Res. 2011, 101, 267–273. [Google Scholar] [CrossRef]
- Moshfegh, A.J.; Rhodes, D.G.; Baer, D.J.; Murayi, T.; Clemens, J.C.; Rumpler, W.V.; Paul, D.R.; Sebastian, R.S.; Kuczynski, K.J.; Ingwersen, L.A.; et al. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am. J. Clin. Nutr. 2008, 88, 324–332. [Google Scholar] [CrossRef]
- Ahuja, J.K.A.; Montville, B.J.; Omolewa-Tomobi, G.; Heendeniya, K.Y.; Martin, C.L.; Steinfeldt, L.C.; Anand, J.; Adler, M.E.; LaComb, R.P.; Moshfegh, A.J. USDA Food and Nutrient Database for Dietary Studies, 5.0. Available online: https://www.ars.usda.gov/ARSUserFiles/80400530/pdf/fndds/fndds5_doc.pdf (accessed on 1 January 2020).
- Briefel, R.R.; Sempos, C.T.; McDowell, M.A.; Chien, S.; Alaimo, K. Dietary methods research in the third National Health and Nutrition Examination Survey: Underreporting of energy intake. Am. J. Clin. Nutr. 1997, 65, 1203S–1209S. [Google Scholar] [CrossRef] [Green Version]
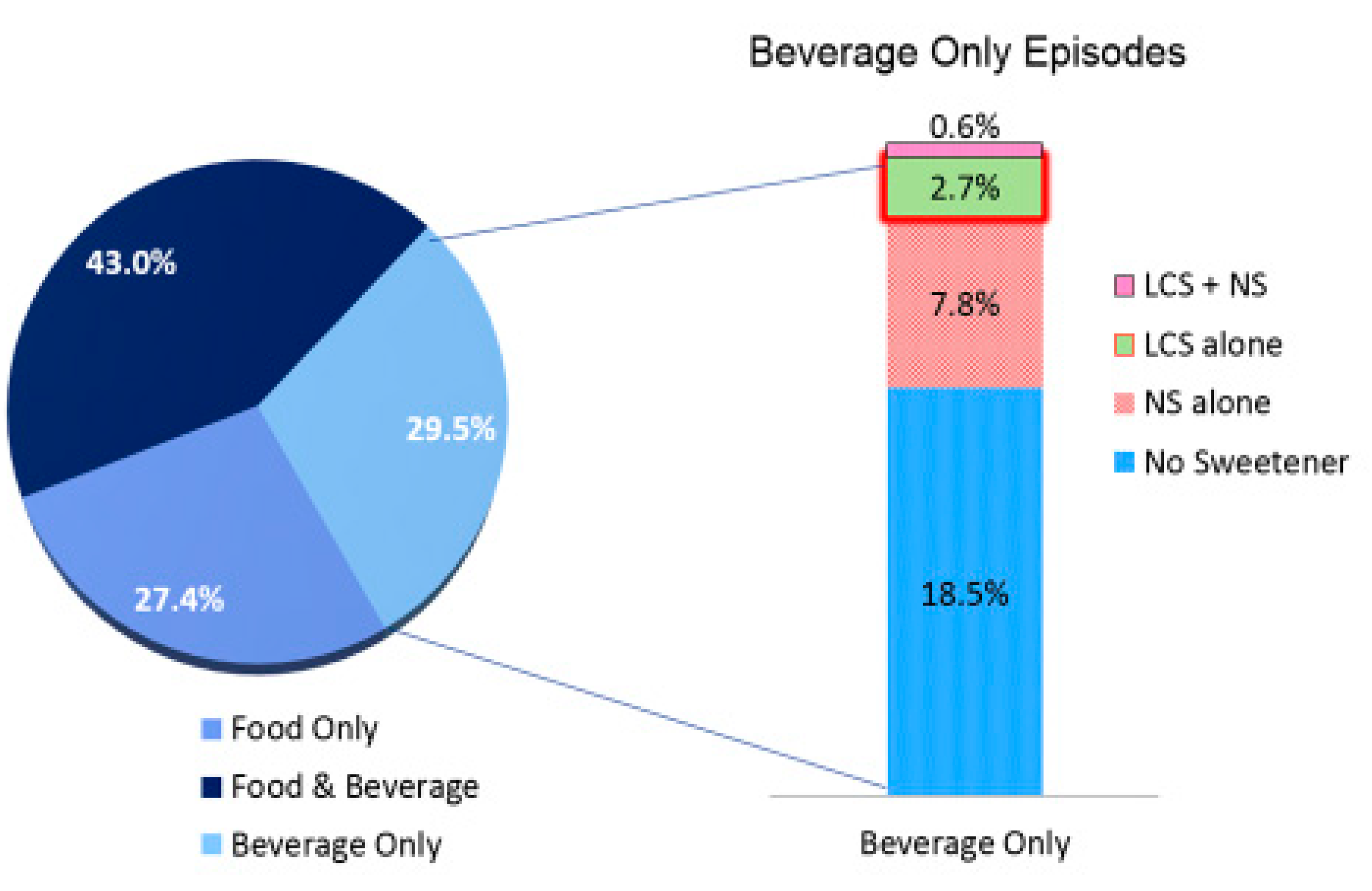

| Total Population | Total Population | Normal Weight (29.4%) | Overweight (33.6%) | Obese (37.1%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| No LCS (64.8%) | LCS (35.2%) | No LCS (21.2%) | LCS (8.2%) | No LCS (21.7%) | LCS (11.9%) | No LCS (21.9%) | LCS (15.1%) | ||
| Type of Episode 4 (%) | |||||||||
| Food Only | 27.44 ± 0.40 | 27.91 ± 0.42 | 26.65 ± 0.50 * | 29.06 ± 0.52 | 27.43 ± 0.92 * | 27.47 ± 0.46 | 26.09 ± 0.74 | 27.19 ± 0.60 | 26.66 ± 0.64 |
| Food and Beverage | 43.01 ± 0.38 | 42.48 ± 0.43 | 43.90 ± 0.46 | 41.23 ± 0.53 | 43.84 ± 0.75 | 43.14 ± 0.53 | 44.42 ± 0.63 | 43.09 ± 0.56 | 43.52 ± 0.67 |
| Beverage Only | 29.55 ± 0.30 | 29.61 ± 0.30 | 29.45 ± 0.37 | 29.71 ± 0.41 | 28.73 ± 0.74 | 29.40 ± 0.45 | 29.49 ± 0.58 | 29.72 ± 0.46 | 29.82 ± 0.44 |
| LCS and NS per Episode (%) | |||||||||
| No LCS or NS | 35.66 ± 0.32 | 38.65 ± 0.40 | 30.62 ± 0.33 ** | 38.84 ± 0.57 | 30.43 ± 0.74 ** | 38.42 ± 0.55 | 30.96 ± 0.52 ** | 38.69 ± 0.52 | 30.46 ± 0.42 ** |
| NS only | 53.07 ± 0.29 | 61.35 ± 0.40 | 39.14 ± 0.32 | 61.16 ± 0.57 | 42.37 ± 0.76 | 61.58 ± 0.55 | 39.46 ± 0.51 | 61.31 ± 0.52 | 37.07 ± 0.46 |
| LCS only | 4.85 ± 0.11 | -- | 13.02 ± 0.22 | -- | 11.05 ± 0.51 | -- | 12.91 ± 0.35 | -- | 14.21 ± 0.36 |
| LCS and NS | 6.42 ± 0.16 | -- | 17.22 ± 0.27 | -- | 16.15 ± 0.59 | -- | 16.67 ± 0.44 | -- | 18.26 ± 0.38 |
| Time of First Episode (mean ± min) | 7:30am ± 2.4 | 7:39am ± 2.6 | 7:13am ± 3.0 ** | 7:37am ± 3.6 | 7:22am ± 5.4 | 7:34am ± 3.5 | 7:04am ± 4.8 ** | 7:47am ± 3.4 | 7:16am ± 4.1 ** |
| Time of Last Episode (mean ± min) | 8:23pm ± 1.2 | 8:21pm ± 1.4 | 8:27pm ± 1.9 * | 8:25pm ± 2.6 | 8:29pm ± 4.0 | 8:20pm ± 2.2 | 8:24pm ± 2.9 | 8:19pm ± 1.9 | 8:28pm ± 3.0 * |
| Number of Episodes (n/d, mean) | 5.60 ± 0.03 | 5.42 ± 0.04 | 5.93 ± 0.03 ** | 5.56 ± 0.05 | 6.04 ± 0.08 ** | 5.44 ± 0.04 | 5.98 ± 0.06 ** | 5.26 ± 0.04 | 5.83 ± 0.04 ** |
| Eating Hours 5 (h/d, mean) | 12.60 ± 0.04 | 12.40 ± 0.04 | 12.97 ± 0.05 ** | 12.43 ± 0.06 | 12.86 ± 0.10 ** | 12.49 ± 0.05 | 13.09 ± 0.07 ** | 12.27 ± 0.06 | 12.93 ± 0.06 ** |
| Eating Hours 5 (%) | |||||||||
| Less than 11.0 h/24 | 20.48 ± 0.51 | 22.93 ± 0.60 | 15.96 ± 0.59 ** | 21.24 ± 0.89 | 18.28 ± 1.33 * | 21.86 ± 0.70 | 15.32 ± 0.90 ** | 25.63 ± 0.92 | 15.21 ± 0.77 ** |
| ≥11.0 to <13.0 h/24 | 28.46 ± 0.41 | 29.22 ± 0.44 | 27.06 ± 0.78 | 30.59 ± 0.88 | 26.56 ± 1.46 | 28.09 ± 0.82 | 24.91 ± 1.23 | 29.02 ± 0.71 | 29.02 ± 1.15 |
| ≥13.0 to <14.5 h/24 | 26.03 ± 0.39 | 25.17 ± 0.43 | 27.63 ± 0.62 | 25.63 ± 0.86 | 26.34 ± 1.37 | 26.01 ± 0.75 | 28.89 ± 1.24 | 23.88 ± 0.76 | 27.34 ± 0.95 |
| 14.5 h or greater/24 | 25.03 ± 0.54 | 22.68 ± 0.58 | 29.35 ± 0.83 | 22.53 ± 0.82 | 28.82 ± 1.62 | 24.03 ± 0.87 | 30.89 ± 1.29 | 21.48 ± 0.79 | 28.43 ± 1.35 |
| Evening/Morning Energy Ratio (median) | 1.69 (0.73, 4.00) | 1.69 (0.71, 4.30) | 1.67 (0.76, 3.59) ** | 1.84 (0.82, 4.46) | 1.79 (0.83, 3.91) ** | 1.66 (0.70, 4.00) | 1.64 (0.76, 3.20) ** | 1.60 (0.66, 4.48) | 1.66 (0.70, 3.87) ** |
| Evening/Morning Energy (%) | |||||||||
| Lower 25th (<0.73, morning) | 25.00 ± 0.64 | 25.51 ± 0.62 | 24.05 ± 0.74 ** | 22.81 ± 0.88 | 22.44 ± 1.47 | 26.00 ± 0.83 | 23.19 ± 1.30 ** | 27.64 ± 1.03 | 25.60 ± 0.91 |
| 25th to 50th (≥0.73 to <1.69) | 25.00 ± 0.53 | 24.35 ± 0.57 | 26.20 ± 0.77 | 24.25 ± 0.88 | 25.39 ± 1.85 | 24.74 ± 0.88 | 28.30 ± 1.40 | 24.05 ± 0.82 | 24.98 ± 0.98 |
| 50th to 75th (≥1.69 to <4.00) | 25.00 ± 0.45 | 23.87 ± 0.50 | 27.05 ± 0.68 | 25.63 ± 0.81 | 27.67 ± 1.38 | 24.17 ± 0.92 | 28.87 ± 1.37 | 21.87 ± 0.68 | 25.28 ± 1.06 |
| Upper 75th (≥4.00, evening) | 25.00 ± 0.39 | 26.27 ± 0.48 | 22.70 ± 0.71 | 27.31 ± 0.89 | 24.49 ± 1.35 | 25.08 ± 0.77 | 19.64 ± 1.16 | 26.44 ± 0.83 | 24.13 ± 1.07 |
| Total Population | Total Population | Normal Weight (29.4%) | Overweight (33.6%) | Obese (37.1%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| No LCS (64.8%) | LCS (35.2%) | No LCS (21.2%) | LCS (8.2%) | No LCS (21.7%) | LCS (11.9%) | No LCS (21.9%) | LCS (15.1%) | ||
| Nutrients per episode (mean ± SEM) | |||||||||
| Grams (g) | 630 ± 5 | 644 ± 5 | 605 ± 6 ** | 616 ± 8 | 568 ± 11 ** | 648 ± 8 | 603 ± 9 ** | 668 ± 7 | 628 ± 7 ** |
| Energy (kcal) | 383 ± 2 | 406 ± 3 | 344 ± 3 ** | 400 ± 5 | 327 ± 5 ** | 408 ± 4 | 347 ± 5 ** | 408 ± 4 | 352 ± 4 ** |
| Energy from Food (kcal) | 292 ± 2 | 303 ± 2 | 274 ± 2 ** | 298 ± 4 | 255 ± 4 ** | 304 ± 3 | 273 ± 4 ** | 306 ± 3 | 286 ± 4 ** |
| Energy from Beverage (kcal) | 66 ± 1 | 79 ± 1 | 46 ± 1 ** | 78 ± 2 | 46 ± 2 ** | 79 ± 2 | 49 ± 2 ** | 79 ± 1 | 43 ± 1 ** |
| Energy from FBA (kcal) | 24 ± 0.3 | 24 ± 0.4 | 25 ± 0.5 | 24 ± 1 | 27 ± 1 | 25 ± 1 | 25 ± 1 | 23 ± 1 | 23 ± 1 |
| Energy density (kcal/g) | 1.14 ± 0.01 | 1.17 ± 0.01 | 1.09 ± 0.01 ** | 1.19 ± 0.01 | 1.13 ± 0.02 * | 1.15 ± 0.01 | 1.07 ± 0.02 ** | 1.16 ± 0.01 | 1.08 ± 0.02 ** |
| Protein (g) | 14.8 ± 0.10 | 15.3 ± 0.12 | 14.1 ± 0.13 ** | 15.0 ± 0.21 | 13.2 ± 0.3 ** | 15.6 ± 0.2 | 14.3 ± 0.2 ** | 15.2 ± 0.2 | 14.5 ± 0.2 ** |
| Carbohydrate (g) | 45.7 ± 0.27 | 49.3 ± 0.35 | 39.6 ± 0.31 ** | 48.9 ± 0.62 | 39.2 ± 0.7 ** | 49.2 ± 0.5 | 39.5 ± 0.6 ** | 49.8 ± 0.8 | 39.9 ± 0.5 ** |
| Total Sugars (g) | 20.3 ± 0.18 | 22.6 ± 0.24 | 16.3 ± 0.16 ** | 22.1 ± 0.38 | 16.6 ± 0.3 ** | 22.7 ± 0.5 | 16.2 ± 0.3 ** | 23.1 ± 0.3 | 16.1 ± 0.3 ** |
| Dietary Fiber (g) | 3.05 ± 0.03 | 3.09 ± 0.02 | 2.97 ± 0.04* | 3.15 ± 0.05 | 3.10 ± 0.08 | 3.12 ± 0.04 | 3.01 ± 0.06 | 3.01 ± 0.04 | 2.87 ± 0.04 * |
| Total Fat (g) | 14.7 ± 0.10 | 15.2 ± 0.12 | 13.7 ± 0.14 ** | 14.7 ± 0.21 | 12.5 ± 0.3 ** | 15.4 ± 0.2 | 13.7 ± 0.2 ** | 15.6 ± 0.2 | 14.5 ± 0.2 ** |
| Nutrients for all eating episodes (Person level 2; mean ± SEM) | |||||||||
| Energy (kcal) | 2143 ± 9 | 2197 ± 13 | 2044 ± 14 ** | 2225 ± 24 | 1977 ± 26 ** | 2220 ± 19 | 2075 ± 26 ** | 2147 ± 19 | 2055 ± 20 * |
| Protein (g) | 83.1 ± 0.5 | 82.7 ± 0.5 | 83.8 ± 0.7 | 83.2 ± 1.0 | 79.7 ± 1.0 * | 84.7 ± 1.1 | 85.4 ± 1.1 | 80.1 ± 0.7 | 84.7 ± 1.0 ** |
| Carbohydrate (g) | 256 ± 1 | 267 ± 2 | 235 ± 2 ** | 272 ± 3 | 237.1 ± 4 ** | 267 ± 2 | 236 ± 3 ** | 262 ± 3 | 232 ± 2 ** |
| Total Sugars (g) | 113 ± 1 | 123 ± 1 | 96.5 ± 0.9 ** | 123 ± 2 | 100.2 ± 2 ** | 123 ± 1. | 96.9 ± 1.7 ** | 122 ± 2 | 94 ± 1.3 ** |
| Dietary Fiber (g) | 17.1 ± 0.2 | 16.8 ± 0.2 | 17.6 ± 0.2 ** | 17.6 ± 0.3 | 18.7 ± 0.4 * | 17.0 ± 0.3 | 18.0 ± 0.4 * | 15.8 ± 0.2 | 16.8 ± 0.2 * |
| Total Fat (g) | 82.1 ± 0.5 | 82.5 ± 0.6 | 81.5 ± 0.7 | 81.9 ± 1.0 | 75.7 ± 1.4 ** | 83.6 ± 0.9 | 81.9 ± 1.3 | 81.8 ± 0.9 | 84.4 ± 1.2 |
| Mean BMI Difference per Specified Unit (95% CI) | |||
|---|---|---|---|
| Variable | Initial Models 1 | Secondary Models 2 | Final Models 3 |
| LCS (yes versus no) | 1.61 (1.32, 1.91) | ---- | ---- |
| Number of Episodes (n/day) | −0.16 (−0.23, −0.08) | −0.20 (−0.27, −0.12) | −0.22 (−0.29, −0.14) |
| Eating Hours 4 (h/day) | −0.02 (−0.07, 0.02) | −0.05 (−0.09, 0.004) | −0.05 (−0.10, −0.001) |
| Eating Hours 4 (categories) | |||
| Less than 11.0 h/24 | Referent | Referent | Referent |
| ≥11.0 to <13.0 h/24 | −0.21 (−0.55, 0.13) | −0.25 (−0.61, 0.10) | −0.27 (−0.62, 0.09) |
| ≥13.0 to <14.5 h/24 | −0.32 (−0.64, −0.001) | −0.42 (−0.74, −0.10) | −0.44 (−0.76, −0.12) |
| 14.5 h or greater/24 | −0.32 (−0.69, 0.05) | −0.47 (−0.84, −0.11) | −0.50 (−0.88, −0.13) |
| Evening/Morning Energy | |||
| Lower 25th (<0.73, morning) | Referent | Referent | Referent |
| 25th to 50th (≥0.73 to <1.69) | −0.06 (−0.52, 0.08) | −0.24 (−0.54, 0.05) | −0.25 (−0.55, 0.05) |
| 50th to 75th (≥1.69 to <4.00) | −0.36 (−0.68, −0.05) | −0.41 (−0.73, −0.09) | −0.42 (−0.74, −0.10) |
| Upper 75th (≥4.00, evening) | −0.06 (−0.41, 0.29) | −0.06 (−0.41, 0.29) | −0.06 (−0.41, 0.28) |
| BMI—Difference per Specified Unit (Beta Values with 95% CI) | |||
|---|---|---|---|
| Stratified by LCS | |||
| Variable | LCS—No | LCS—Yes | p-Value Interaction |
| Number of Episodes (n/d) | −0.24 (−0.32, −0.15) | −0.18 (−0.31, −0.06) | 0.4407 |
| Eating Hours 2 (h/d) | −0.06 (−0.12, −0.01) | −0.02 (−0.12, 0.09) | 0.4231 |
| Eating Hours 2 (categories) | |||
| Less than 11.0 h/24 | Referent | Referent | |
| ≥11.0 to <13.0 h/24 | −0.60 (−0.99, −0.22) | 0.50 (−0.16, 1.16) | 0.0035 |
| ≥13.0 to <14.5 h/24 | −0.56 (−0.93, −0.19) | −0.07 (−0.70, 0.56) | 0.1823 |
| 14.5 h or greater/24 | −0.69 (−1.08, −0.30) | −0.04 (−0.76, 0.68) | 0.1027 |
| Evening/Morning Energy | |||
| Lower 25th (<0.73, morning) | Referent | Referent | |
| 25th to 50th (≥0.73 to <1.69) | −0.30 (−0.63, 0.04) | −0.16 (−0.73, 0.42) | 0.6697 |
| 50th to 75th (≥1.69 to <4.00) | −0.47 (−0.82, −0.12) | −0.33 (−0.91, 0.25) | 0.6658 |
| Upper 75th (≥4.00, evening) | −0.13 (−0.52, 0.26) | 0.06 (−0.52, 0.65) | 0.5656 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hunt, K.J.; St. Peter, J.V.; Malek, A.M.; Vrana-Diaz, C.; Marriott, B.P.; Greenberg, D. Daily Eating Frequency in US Adults: Associations with Low-Calorie Sweeteners, Body Mass Index, and Nutrient Intake (NHANES 2007–2016). Nutrients 2020, 12, 2566. https://doi.org/10.3390/nu12092566
Hunt KJ, St. Peter JV, Malek AM, Vrana-Diaz C, Marriott BP, Greenberg D. Daily Eating Frequency in US Adults: Associations with Low-Calorie Sweeteners, Body Mass Index, and Nutrient Intake (NHANES 2007–2016). Nutrients. 2020; 12(9):2566. https://doi.org/10.3390/nu12092566
Chicago/Turabian StyleHunt, Kelly J., John V. St. Peter, Angela M. Malek, Caroline Vrana-Diaz, Bernadette P. Marriott, and Danielle Greenberg. 2020. "Daily Eating Frequency in US Adults: Associations with Low-Calorie Sweeteners, Body Mass Index, and Nutrient Intake (NHANES 2007–2016)" Nutrients 12, no. 9: 2566. https://doi.org/10.3390/nu12092566





