Developments in the Application of Nanomaterials for Water Treatment and Their Impact on the Environment
Abstract
1. Introduction
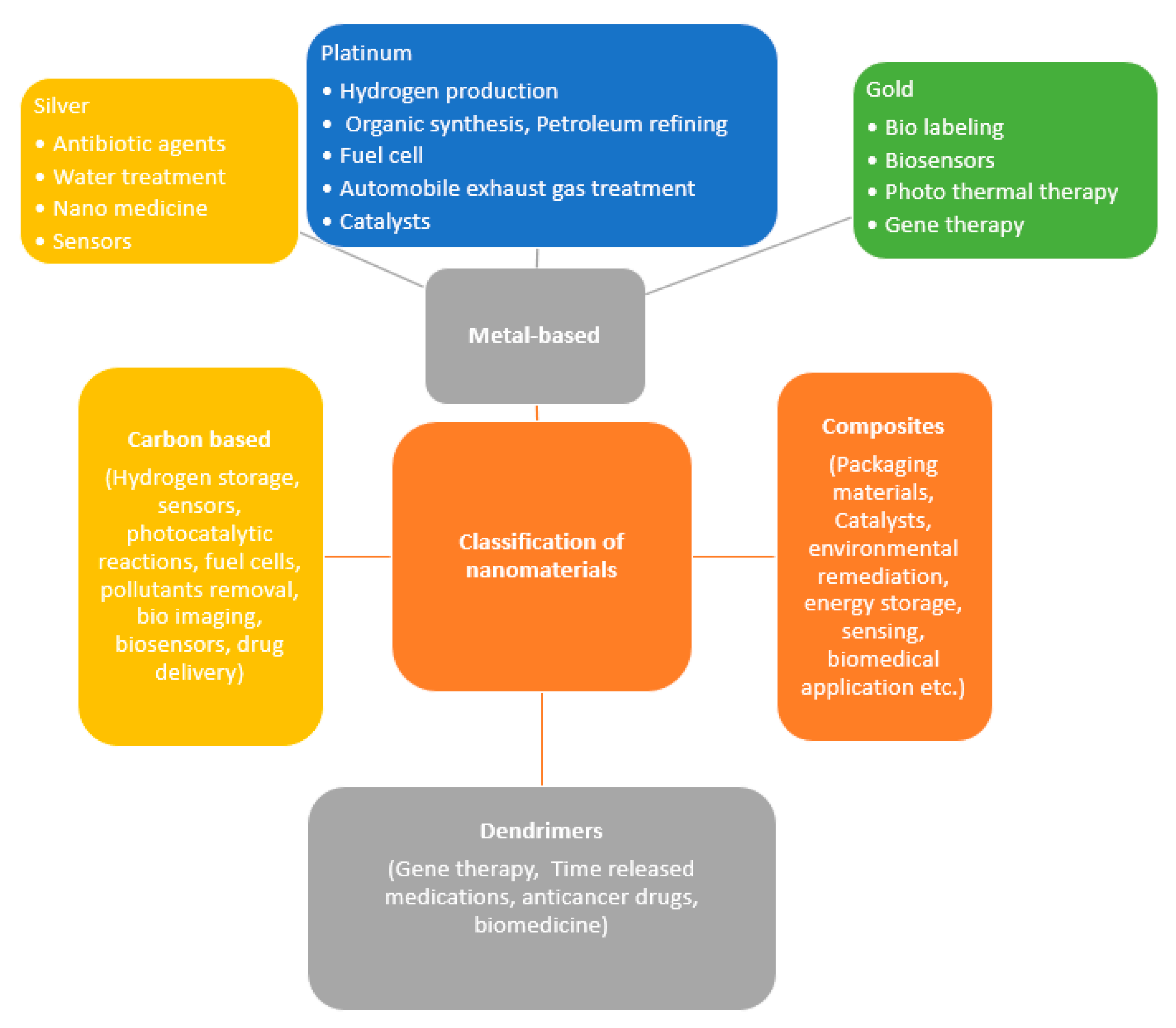
2. Different Types and Properties of Nanomaterials
3. Nanomaterials and the Aqueous Environment
4. Application of Nanomaterials in the Aqueous Environment
4.1. Adsorbents
4.1.1. Adsorption of Dyes
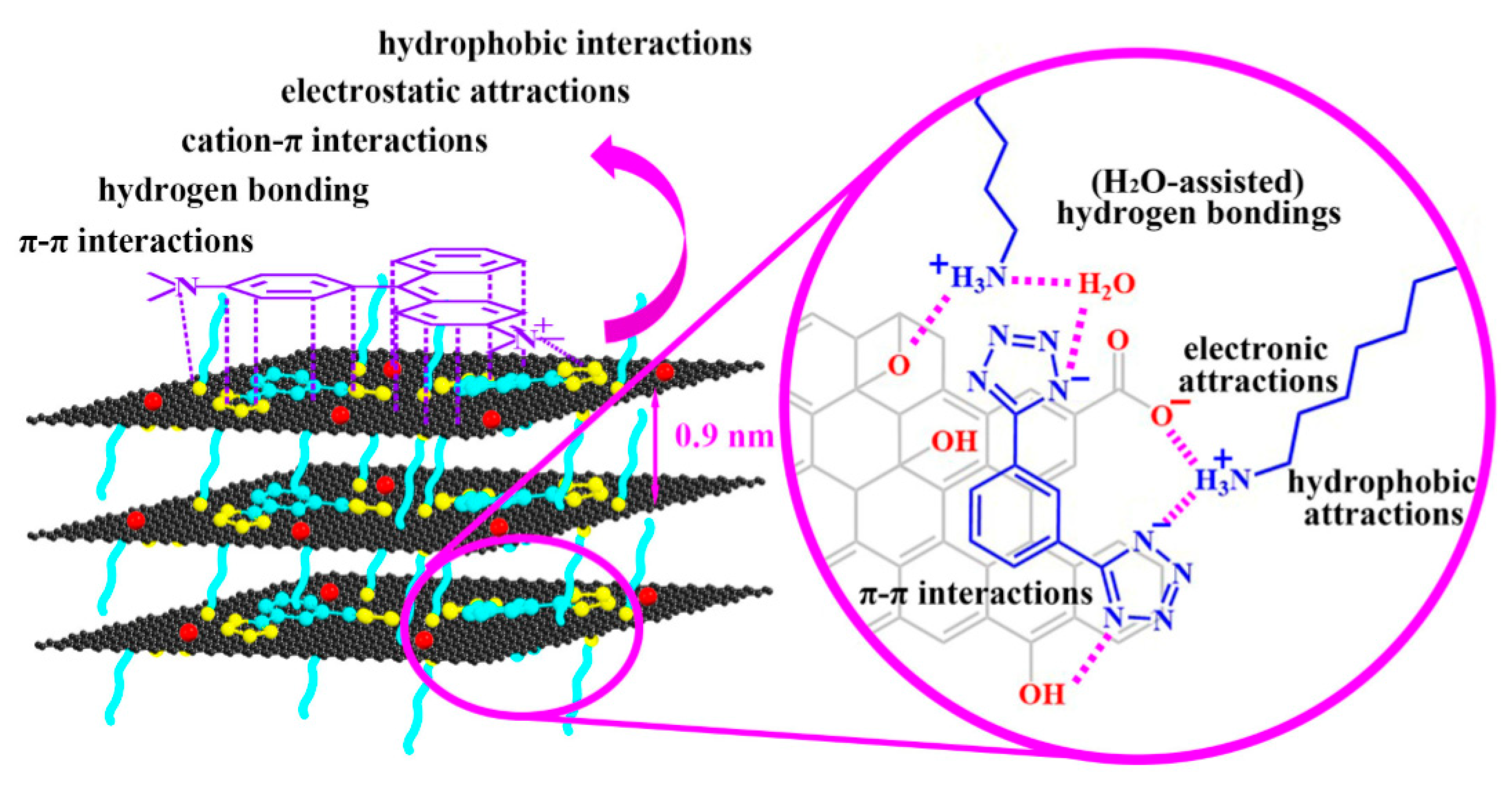
Carbon-Based Nanomaterials for the Adsorption of Dyes
Metal and Metal-Oxide-Based Nanomaterials for the Adsorption of Dyes
4.1.2. Adsorption of Heavy Metal Ions
Carbon-Based Nanomaterials for the Adsorption of Heavy Metals
Metal-Based Nanomaterials for Adsorption of Heavy Metals
4.1.3. Adsorption of the Surfactant
Carbon-Based Nanomaterials for the Adsorption of Surfactants
Other Nanomaterials for the Adsorption of Surfactants
4.1.4. Adsorption of Pharmaceuticals
Carbon-Based Nanomaterials for the Adsorption of Pharmaceuticals
Metal and Metal-Oxide-Based Nanomaterials for the Adsorption of Pharmaceuticals
4.1.5. Adsorption of Phenol and Other Toxic Contaminants
Carbon-Based Nanomaterials for the Adsorption of Phenol and Other Contaminants
Metal and Metal-Oxide-Based Nanomaterials for the Adsorption of Other Contaminants
4.2. Membranes and Filter Materials
4.2.1. Carbon-Based Nanomaterials for Membrane Applications
4.2.2. Metal and Metal-Oxide-Based Nanomaterials for Membrane Applications
4.3. Photocatalytic Degradation Application
4.3.1. Carbon-Based Nanomaterials for Photocatalytic Applications
4.3.2. Metal and Metal-Oxide-Based Nanomaterials for Photocatalytic Applications
4.4. Microbial Decontamination Applications
4.4.1. Carbon-Based Nanomaterials for Water Disinfection
4.4.2. Metals and Metal-Oxide-Based Nanomaterials for Water Disinfection
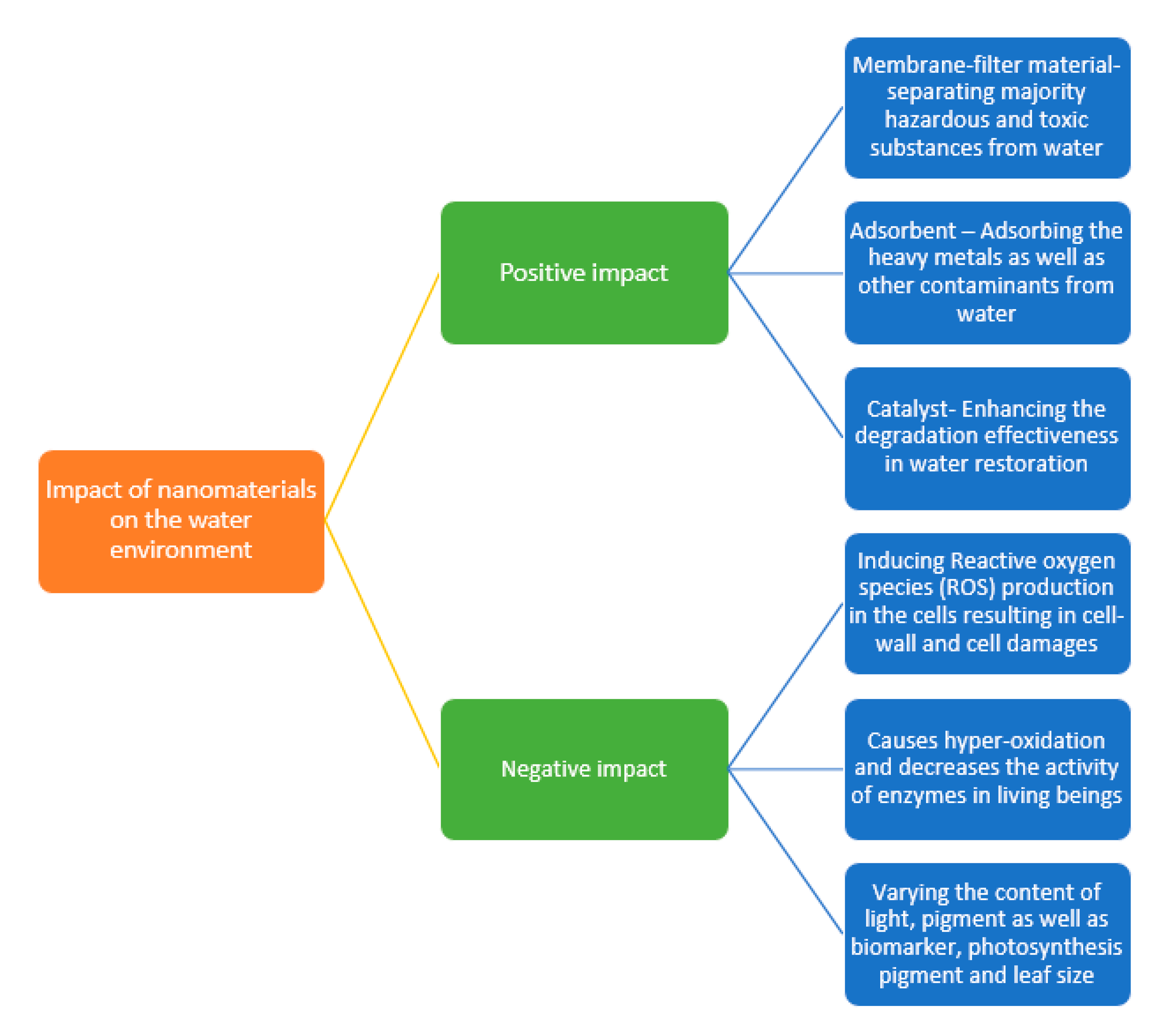
5. Toxicity of Nanomaterials in the Aqueous Environment
5.1. Cellular Damage/Membrane Damage
5.2. Other Negative Impacts
6. Regulations
7. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Capek, I. Nanocomposite Structures and Dispersions: Science and Nanotechnology—Fundamental Principles and Colloidal Particles Studies in Interface Science. In Nanotechnology and Nanomaterials; Mobius, D., Miller, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 23, Chapter 1. [Google Scholar] [CrossRef]
- Kabir, E.; Kumar, V.; Kim, K.H.; Yip, A.C.; Sohn, J.R. Environmental impacts of nanomaterials. J. Environ. Manag. 2018, 225, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, S.; Dave, P.N. Nanomaterials: Environmental, Human Health Risk. In Handbook of Nanomaterials for Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1055–1062. [Google Scholar] [CrossRef]
- Iavicoli, I.; Leso, V.; Ricciardi, W.; Hodson, L.L.; Hoover, M.D. Opportunities and challenges of nanotechnology in the green economy. Environ. Health 2014, 13, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Baruah, S.; Khan, M.N.; Dutta, J. Perspectives and applications of nanotechnology in water treatment. Environ. Chem. Lett. 2016, 14, 1–14. [Google Scholar] [CrossRef]
- Wu, Y.; Pang, H.; Liu, Y.; Wang, X.; Yu, S.; Fu, D.; Wang, X. Environmental remediation of heavy metal ions by novel-nanomaterials: A review. Environ. Pollut. 2019, 246, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Daer, S.; Kharraz, J.; Giwa, A.; Hasan, S.W. Recent applications of nanomaterials in water desalination: A critical review and future opportunities. Desalination 2015, 367, 37–48. [Google Scholar] [CrossRef]
- Tang, W.W.; Zeng, G.M.; Gong, J.L.; Liang, J.; Xu, P.; Zhang, C.; Huang, B.B. Impact of humic/fulvic acid on the removal of heavy metals from aqueous solutions using nanomaterials: A review. Sci. Total Environ. 2014, 468, 1014–1027. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ibrahim MN, M. A Review Article of Nanoparticles; Synthetic Approaches and Wastewater Treatment Methods. Int. J. Eng. Res. 2019, 6, 1–7. [Google Scholar]
- Umar, K. Water Contamination by Organic-Pollutants: TiO2 Photocatalysis. In Modern Age Environmental Problems and their Remediation; Springer: Cham, Switzerland, 2018; pp. 95–109. [Google Scholar] [CrossRef]
- Fang, X.; Li, J.; Li, X.; Pan, S.; Zhang, X.; Sun, X.; Wang, L. Internal pore decoration with polydopamine nanoparticle on polymeric ultrafiltration membrane for enhanced heavy metal removal. Chem. Eng. J. 2017, 314, 38–49. [Google Scholar] [CrossRef]
- Sekoai, P.T.; Ouma CN, M.; Du Preez, S.P.; Modisha, P.; Engelbrecht, N.; Bessarabov, D.G.; Ghimire, A. Application of nanoparticles in biofuels: An overview. Fuel 2019, 237, 380–397. [Google Scholar] [CrossRef]
- Adeleye, A.S.; Conway, J.R.; Garner, K.; Huang, Y.; Su, Y.; Keller, A.A. Engineered nanomaterials for water treatment and remediation: Costs, benefits, and applicability. Chem. Eng. J. 2016, 286, 640–662. [Google Scholar] [CrossRef]
- Lu, H.; Wang, J.; Stoller, M.; Wang, T.; Bao, Y.; Hao, H. An overview of nanomaterials for water and wastewater treatment. Adv. Mater. Sci. Eng. 2016, 2016. [Google Scholar] [CrossRef]
- Taghipour, S.; Hosseini, S.M.; Ataie-Ashtiani, B. Engineering nanomaterials for water and wastewater treatment: Review of classifications, properties and applications. New J. Chem. 2019, 43, 7902–7927. [Google Scholar] [CrossRef]
- Santhosh, C.; Velmurugan, V.; Jacob, G.; Jeong, S.K.; Grace, A.N.; Bhatnagar, A. Role of nanomaterials in water treatment applications: A review. Chem. Eng. J. 2016, 306, 1116–1137. [Google Scholar] [CrossRef]
- Ghasemzadeh, G.; Momenpour, M.; Omidi, F.; Hosseini, M.R.; Ahani, M.; Barzegari, A. Applications of nanomaterials in water treatment and environmental remediation. Front. Environ. Sci. Eng. 2014, 8, 471–482. [Google Scholar] [CrossRef]
- Saleh, T.A.; Gupta, V.K. Synthesis, Classification, and Properties of Nanomaterials. In Nanomaterial and Polymer Membranes: Synthesis, Characterization, and Applications; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 978-0-12-804703-3. [Google Scholar]
- Ding, Y.; Kuhlbusch, T.A.; Van Tongeren, M.; Jiménez, A.S.; Tuinman, I.; Chen, R.; Kaminski, H. Airborne engineered nanomaterials in the workplace—A review of release and worker exposure during nanomaterial production and handling processes. J. Hazard. Mater. 2017, 322, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, J.N.; Tiwari, R.N.; Kim, K.S. Zero-dimensional, one-dimensional, two-dimensional and three-dimensional nanostructured materials for advanced electrochemical energy devices. Prog. Mater. Sci. 2012, 57, 724–803. [Google Scholar] [CrossRef]
- European Commission, 2016. Recommendation on the Definition of a Nanomaterial. Available online: http://data.europa.eu/eli/reco/2011/696/oj (accessed on 29 August 2020).
- EPA 100/B-07/001, February 2007. Available online: https://archive.epa.gov/osa/pdfs/web/pdf/epa-nanotechnology-whitepaper-0207.pdf (accessed on 14 July 2020).
- Nasir, S.; Hussein, M.Z.; Zainal, Z.; Yusof, N.A. Carbon-based nanomaterials/allotropes: A glimpse of their synthesis, properties and some applications. Materials 2018, 11, 295. [Google Scholar] [CrossRef]
- Duc Vu Quyen, N.; Quang Khieu, D.; Tuyen, T.N.; Xuan Tin, D.; Thi Hoang Diem, B. Carbon nanotubes: Synthesis via chemical vapour deposition without hydrogen, surface modification, and application. J. Chem. 2019, 2019. [Google Scholar] [CrossRef]
- Kim, H.I.; Wang, M.; Lee, S.K.; Kang, J.; Nam, J.D.; Ci, L.; Suhr, J. Tensile properties of millimeter-long multi-walled carbon nanotubes. Sci. Rep. 2017, 7, 1–7. [Google Scholar] [CrossRef]
- Siqueira, J.R., Jr.; Oliveira, O.N., Jr. Carbon-Based Nanomaterials. In Nanostructures; William Andrew Publishing, Elservier: London, UK, 2017; pp. 233–249. [Google Scholar] [CrossRef]
- Kulkarni, S.K. Types of Nanomaterials and Their Properties. In Nanotechnology: Principles and Practices; Cham, Ed.; Springer: Cham, Switzerland, 2015; pp. 199–239. [Google Scholar] [CrossRef]
- Palmerston Mendes, L.; Pan, J.; Torchilin, V.P. Dendrimers as nanocarriers for nucleic acid and drug delivery in cancer therapy. Molecules 2017, 22, 1401. [Google Scholar] [CrossRef]
- Kesharwani, P.; Amin MC, I.M.; Giri, N.; Jain, A.; Gajbhiye, V. Dendrimers in Targeting and Delivery of Drugs. In Nanotechnology-Based Approaches for Targeting and Delivery of Drugs and Genes; Academic Press, Elsevier: London, UK, 2017; pp. 363–388. [Google Scholar] [CrossRef]
- Luo, Y.H.; Chang, L.W.; Lin, P. Metal-based nanoparticles and the immune system: Activation, inflammation, and potential applications. BioMed Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Libralato, G.; Galdiero, E.; Falanga, A.; Carotenuto, R.; De Alteriis, E.; Guida, M. Toxicity effects of functionalized quantum dots, gold and polystyrene nanoparticles on target aquatic biological models: A review. Molecules 2017, 22, 1439. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zhang, C.; Zeng, G.; Chen, G.; Wan, J.; Guo, Z.; Liu, J. Metal-based quantum dots: Synthesis, surface modification, transport and fate in aquatic environments and toxicity to microorganisms. RSC Adv. 2016, 6, 78595–78610. [Google Scholar] [CrossRef]
- Ye, S.; Zeng, G.; Wu, H.; Zhang, C.; Liang, J.; Dai, J.; Cheng, M. Co-occurrence and interactions of pollutants, and their impacts on soil remediation—A review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1528–1553. [Google Scholar] [CrossRef]
- Ye, S.; Zeng, G.; Wu, H.; Liang, J.; Zhang, C.; Dai, J.; Yu, J. The effects of activated biochar addition on remediation efficiency of co-composting with contaminated wetland soil. Resour. Conserv. Recycl. 2019, 140, 278–285. [Google Scholar] [CrossRef]
- Ye, S.; Zeng, G.; Wu, H.; Zhang, C.; Dai, J.; Liang, J.; Zhang, C. Biological technologies for the remediation of co-contaminated soil. Crit. Rev. Biotechnol. 2017, 37, 1062–1076. [Google Scholar] [CrossRef]
- Dutta, S.; Huang, S.Y.; Chen, C.; Chen, J.E.; Alothman, Z.A.; Yamauchi, Y.; Wu, K.C.W. Cellulose framework directed construction of hierarchically porous carbons offering high-performance capacitive deionization of brackish water. ACS Sustain. Chem. Eng. 2016, 4, 1885–1893. [Google Scholar] [CrossRef]
- Wang, M.; Xu, X.; Tang, J.; Hou, S.; Hossain MS, A.; Pan, L.; Yamauchi, Y. High performance capacitive deionization electrodes based on ultrathin nitrogen-doped carbon/graphene nano-sandwiches. Chem. Commun. 2017, 53, 10784–10787. [Google Scholar] [CrossRef]
- Xu, X.; Allah, A.E.; Wang, C.; Tan, H.; Farghali, A.A.; Khedr, M.H.; Malgras, V.; Yang, T.; Yamauchi, Y. Capacitive deionization using nitrogen-doped mesostructured carbons for highly efficient brackish water desalination. Chem. Eng. J. 2019, 362, 887–896. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, X.; Kim, J.; Malgras, V.; Mo, R.; Li, C.; Bando, Y. Nanoarchitectured metal–organic framework/polypyrrole hybrids for brackish water desalination using capacitive deionization. Mater. Horiz. 2019, 6, 1433–1437. [Google Scholar] [CrossRef]
- Abdel-Fatah, M.A. Nanofiltration systems and applications in wastewater treatment. Ain Shams Eng. J. 2018, 9, 3077–3092. [Google Scholar] [CrossRef]
- Zekić, E.; Vuković, Ž.; Halkijević, I. Application of nanotechnology in wastewater treatment. Građevinar 2018, 70, 315–323. [Google Scholar] [CrossRef]
- Charitidis, C.A.; Georgiou, P.; Koklioti, M.A.; Trompeta, A.F.; Markakis, V. Manufacturing nanomaterials: From research to industry. Manuf. Rev. 2014, 1, 11. [Google Scholar] [CrossRef]
- Zhang, Z.M.; Duan, Y.S.; Xu, Q.; Zhang, B. A review on nanofiber fabrication with the effect of high-speed centrifugal force field. J. Eng. Fibers Fabr. 2019, 14. [Google Scholar] [CrossRef]
- Lim, J.Y.; Mubarak, N.M.; Abdullah, E.C.; Nizamuddin, S.; Khalid, M. Recent trends in the synthesis of graphene and graphene oxide based nanomaterials for removal of heavy metals—A review. J. Ind. Eng. Chem. 2018, 66, 29–44. [Google Scholar] [CrossRef]
- Singh, R.; Dutta, S. A review on H2 production through photocatalytic reactions using TiO2/TiO2-assisted catalysts. Fuel 2018, 220, 607–620. [Google Scholar] [CrossRef]
- Huang, X.Y.; Ling, L.; Zhang, W.X. Nanoencapsulation of hexavalent chromium with nanoscale zero-valent iron: High resolution chemical mapping of the passivation layer. J. Environ. Sci. 2018, 67, 4–13. [Google Scholar] [CrossRef]
- Lim, C.T. Nanofiber technology: Current status and emerging developments. Prog. Polym. Sci. 2017, 70, 1–17. [Google Scholar] [CrossRef]
- Rtimi, S.; Dionysiou, D.D.; Pillai, S.C.; Kiwi, J. Advances in catalytic/photocatalytic bacterial inactivation by nano Ag and Cu coated surfaces and medical devices. Appl. Catal. B Environ. 2019, 240, 291–318. [Google Scholar] [CrossRef]
- Rashed, M.N. Adsorption technique for the removal of organic pollutants from water and wastewater. Org. Pollut. Monit. Risk Treat. 2013, 167–194. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Taher, M.M.; Bajpai, A.K.; Heibati, B.; Tyagi, I.; Asif, M.; Gupta, V.K. Removal of noxious Cr (VI) ions using single-walled carbon nanotubes and multi-walled carbon nanotubes. Chem. Eng. J. 2015, 279, 344–352. [Google Scholar] [CrossRef]
- Li, Y.H.; Zhao, Y.M.; Hu, W.B.; Ahmad, I.; Zhu, Y.Q.; Peng, X.J.; Luan, Z.K. Carbon Nanotubes-the Promising Adsorbent in Wastewater Treatment. In Journal of Physics: Conference Series; IOP Publishing: Philadelphia, PA, USA, 2007; Volume 61, pp. 698–702. [Google Scholar] [CrossRef]
- Ncibi, M.C.; Sillanpää, M. Optimized removal of antibiotic drugs from aqueous solutions using single, double and multi-walled carbon nanotubes. J. Hazard. Mater. 2015, 298, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ng, C.W.; Wang, W.; Li, Q.; Hao, Z. Synergistic and competitive adsorption of organic dyes on multiwalled carbon nanotubes. Chem. Eng. J. 2012, 197, 34–40. [Google Scholar] [CrossRef]
- Liu, T.; Li, Y.; Du, Q.; Sun, J.; Jiao, Y.; Yang, G.; Wang, Z.; Xia, Y.; Zhang, W.; Wang, K. Adsorption of methylene blue from aqueous solution by graphene. Colloids Surf. B Biointerfaces 2012, 90, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Purwajanti, S.; Huang, X.; Liu, Y.; Yang, Y.; Noonan, O.; Song, H.; Yu, C. Mg(OH)2–MgO@ reduced graphene oxide nanocomposites: The roles of composition and nanostructure in arsenite sorption. J. Mater. Chem. A 2017, 5, 24484–24492. [Google Scholar] [CrossRef]
- Chandra, V.; Park, J.; Chun, Y.; Lee, J.W.; Hwang, I.C.; Kim, K.S. Water-dispersible magnetite-reduced graphene oxide composites for arsenic removal. ACS Nano 2010, 4, 3979–3986. [Google Scholar] [CrossRef]
- Malwal, D.; Gopinath, P. Efficient adsorption and antibacterial properties of electrospun CuO-ZnO composite nanofibers for water remediation. J. Hazard. Mater. 2017, 321, 611–621. [Google Scholar] [CrossRef]
- Belessi, V.; Romanos, G.; Boukos, N.; Lambropoulou, D.; Trapalis, C. Removal of Reactive Red 195 from aqueous solutions by adsorption on the surface of TiO2 nanoparticles. J. Hazard. Mater. 2009, 170, 836–844. [Google Scholar] [CrossRef]
- Salehi, R.; Arami, M.; Mahmoodi, N.M.; Bahrami, H.; Khorramfar, S. Novel biocompatible composite (chitosan–zinc oxide nanoparticle): Preparation, characterization and dye adsorption properties. Colloids Surf. B Biointerfaces 2010, 80, 86–93. [Google Scholar] [CrossRef]
- Husein, D.Z.; Hassanien, R.; Al-Hakkani, M.F. Green-synthesized copper nano-adsorbent for the removal of pharmaceutical pollutants from real wastewater samples. Heliyon 2019, 5, e02339. [Google Scholar] [CrossRef]
- Madrakian, T.; Afkhami, A.; Ahmadi, M.; Bagheri, H. Removal of some cationic dyes from aqueous solutions using magnetic-modified multi-walled carbon nanotubes. J. Hazard. Mater. 2011, 196, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Albukhari, S.M.; Ismail, M.; Akhtar, K.; Danish, E.Y. Catalytic reduction of nitrophenols and dyes using silver nanoparticles@ cellulose polymer paper for the resolution of waste water treatment challenges. Colloids Surf. A Physicochem. Eng. Asp. 2019, 577, 548–561. [Google Scholar] [CrossRef]
- Fugetsu, B.; Satoh, S.; Shiba, T.; Mizutani, T.; Lin, Y.B.; Terui, N.; Shindoh, M. Caged multiwalled carbon nanotubes as the adsorbents for affinity-based elimination of ionic dyes. Environ. Sci. Technol. 2004, 38, 6890–6896. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Yan, L.; Liu, C.; Su, C.; Zhou, Q.; Zhang, X.; Ye, Z. Non-covalent functionalized graphene oxide (GO) adsorbent with an organic gelator for co-adsorption of dye, endocrine-disruptor, pharmaceutical and metal ion. Chem. Eng. J. 2018, 349, 791–799. [Google Scholar] [CrossRef]
- Hayeeye, F.; Sattar, M.; Chinpa, W.; Sirichote, O. Kinetics and thermodynamics of Rhodamine B adsorption by gelatin/activated carbon composite beads. Colloids Surf. A Physicochem. Eng. Asp. 2017, 513, 259–266. [Google Scholar] [CrossRef]
- Li, J.F.; Rupa, E.J.; Hurh, J.; Huo, Y.; Chen, L.; Han, Y.; Yang, D.C. Cordyceps militaris fungus mediated Zinc Oxide nanoparticles for the photocatalytic degradation of Methylene blue dye. Optik 2019, 183, 691–697. [Google Scholar] [CrossRef]
- Jain, A.; Vaya, D. Photocatalytic activity of TiO2 nanomaterial. J. Chil. Chem. Soc. 2017, 62, 3683–3690. [Google Scholar] [CrossRef]
- Shim, H.E.; Yang, J.E.; Jeong, S.W.; Lee, C.H.; Song, L.; Mushtaq, S.; Jeon, J. Silver nanomaterial-immobilized desalination systems for efficient removal of radioactive iodine species in water. Nanomaterials 2018, 8, 660. [Google Scholar] [CrossRef]
- Van, H.T.; Nguyen TM, P.; Thao, V.T.; Vu, X.H.; Nguyen, T.V.; Nguyen, L.H. Applying activated carbon derived from coconut shell loaded by silver nanoparticles to remove methylene blue in aqueous solution. Water Air Soil Pollut. 2018, 229, 393. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef]
- Yap, P.L.; Kabiri, S.; Tran, D.N.; Losic, D. Multifunctional binding chemistry on modified graphene composite for selective and highly efficient adsorption of mercury. ACS Appl. Mater. Interfaces 2018, 11, 6350–6362. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ma, R.; Wang, X.; Ma, Y.; Yang, Y.; Zhuang, L.; Wang, X. Graphene oxide-based materials for efficient removal of heavy metal ions from aqueous solution: A review. Environ. Pollut. 2019, 252, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Wang, X.; Liu, Y.; Chen, Z.; Wu, Y.; Liu, Y.; Song, G.; Chen, J.; Wang, X. Efficient removal of uranium (VI) by layered double hydroxides supported nanoscale zero-valent iron: A combined experimental and spectroscopic studies. Chem. Eng. J. 2019, 365, 51–59. [Google Scholar] [CrossRef]
- Lim, J.Y.; Mubarak, N.M.; Khalid, M.; Abdullah, E.C.; Arshid, N. Novel fabrication of functionalized graphene oxide via magnetite and 1-butyl-3-methylimidazolium tetrafluoroborate. Nano-Struct. Nano-Objects 2018, 16, 403–411. [Google Scholar] [CrossRef]
- Das, R.; Hamid, S.B.A.; Ali, M.; Annuar, M.S.M.; Samsudin, E.M.B.; Bagheri, S. Covalent functionalization schemes for tailoring solubility of multi-walled carbon nanotubes in water and acetone solvents. Sci. Adv. Mater. 2015, 7, 2726–2737. [Google Scholar] [CrossRef]
- Mubarak, N.M.; Sahu, J.N.; Abdullah, E.C.; Jayakumar, N.S.; Ganesan, P. Microwave-assisted synthesis of multi-walled carbon nanotubes for enhanced removal of Zn (II) from wastewater. Res. Chem. Intermed. 2016, 42, 3257–3281. [Google Scholar] [CrossRef]
- Xu, J.; Cao, Z.; Zhang, Y.; Yuan, Z.; Lou, Z.; Xu, X.; Wang, X. A review of functionalized carbon nanotubes and graphene for heavy metal adsorption from water: Preparation, application, and mechanism. Chemosphere 2018, 195, 351–364. [Google Scholar] [CrossRef]
- Lee, K.M.; Wong CP, P.; Tan, T.L.; Lai, C.W. Functionalized carbon nanotubes for adsorptive removal of water pollutants. Mater. Sci. Eng. B 2018, 236, 61–69. [Google Scholar] [CrossRef]
- Alsohaimi, I.H.; Khan, M.A.; Alothman, Z.A.; Khan, M.R.; Kumar, M. Synthesis, characterization, and application of Fe-CNTs nanocomposite for BrO3− remediation from water samples. J. Ind. Eng. Chem. 2015, 26, 218–225. [Google Scholar] [CrossRef]
- Brasili, E.; Bavasso, I.; Petruccelli, V.; Vilardi, G.; Valletta, A.; Dal Bosco, C.; Di Palma, L. Remediation of hexavalent chromium contaminated water through zero-valent iron nanoparticles and effects on tomato plant growth performance. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Badruddoza, A.Z.M.; Shawon, Z.B.Z.; Rahman, M.T.; Hao, K.W.; Hidajat, K.; Uddin, M.S. Ionically modified magnetic nanomaterials for arsenic and chromium removal from water. Chem. Eng. J. 2013, 225, 607–615. [Google Scholar] [CrossRef]
- Bagbi, Y.; Sarswat, A.; Tiwari, S.; Mohan, D.; Pandey, A.; Solanki, P.R. Synthesis of l-cysteine stabilized zero-valent iron (nZVI) nanoparticles for lead remediation from water. Environ. Nanotechnol. Monit. Manag. 2017, 7, 34–45. [Google Scholar] [CrossRef]
- Ren, J.; Yao, M.; Woo, Y.C.; Tijing, L.D.; Kim, J.H.; Shon, H.K. Recyclable nanoscale zerovalent iron (nZVI)-immobilized electrospun nanofiber composites with improved mechanical strength for groundwater remediation. Compos. Part B Eng. 2019, 171, 339–346. [Google Scholar] [CrossRef]
- Ivanković, T.; Hrenović, J. Surfactants in the environment. Arch. Ind. Hyg. Toxicol. 2010, 61, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Prediger, P.; Cheminski, T.; de Figueiredo Neves, T.; Nunes, W.B.; Sabino, L.; Picone, C.S.F.; Correia, C.R.D. Graphene oxide nanomaterials for the removal of non-ionic surfactant from water. J. Environ. Chem. Eng. 2018, 6, 1536–1545. [Google Scholar] [CrossRef]
- Gao, Q.; Wu, F.; Hu, J.; Chen, W.; Zhang, X.; Guo, X.; Wang, X. Chemical composition-dependent removal of cationic surfactants by carbon nanotubes. Sci. Total Environ. 2020, 716, 137017. [Google Scholar] [CrossRef]
- Cheminski, T.; de Figueiredo Neves, T.; Silva, P.M.; Guimarães, C.H.; Prediger, P. Insertion of phenyl ethyleneglycol units on graphene oxide as stabilizers and its application for surfactant removal. J. Environ. Chem. Eng. 2019, 7, 102976. [Google Scholar] [CrossRef]
- Dasgupta, N.; Ranjan, S.; Lichtfouse, E. Environmental Nanotechnology: Volume 2; Dasgupta, N., Ranjan, S., Lichtfouse, E., Eds.; Springer, International Publishing: New York, NY, USA, 2018. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Ali, M.M.K.; Saleh, M.M. Adsorption and removal of cationic and anionic surfactants using zero-valent iron nanoparticles. J. Mol. Liq. 2018, 268, 497–505. [Google Scholar] [CrossRef]
- Sui, Q.; Cao, X.; Lu, S.; Zhao, W.; Qiu, Z.; Yu, G. Occurrence, sources and fate of pharmaceuticals and personal care products in the groundwater: A review. Emerg. Contam. 2015, 1, 14–24. [Google Scholar] [CrossRef]
- Zheng, S.; Shi, J.; Zhang, J.; Yang, Y.; Hu, J.; Shao, B. Identification of the disinfection byproducts of bisphenol S and the disrupting effect on peroxisome proliferator-activated receptor gamma (PPARγ) induced by chlorination. Water Res. 2018, 132, 167–176. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Shafieizadeh, M.; Taher, M.A.; Opoku, F.; Kiarii, E.M.; Govender, P.P.; Orooji, Y. The role of magnetite/graphene oxide nano-composite as a high-efficiency adsorbent for removal of phenazopyridine residues from water samples, an experimental/theoretical investigation. J. Mol. Liq. 2020, 298, 112040. [Google Scholar] [CrossRef]
- Zhang, B.; Ji, J.; Liu, X.; Li, C.; Yuan, M.; Yu, J.; Ma, Y. Rapid adsorption and enhanced removal of emodin and physcion by nano zirconium carbide. Sci. Total Environ. 2019, 647, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Awfa, D.; Ateia, M.; Fujii, M.; Johnson, M.S.; Yoshimura, C. Photodegradation of pharmaceuticals and personal care products in water treatment using carbonaceous-TiO2 composites: A critical review of recent literature. Water Res. 2018, 142, 26–45. [Google Scholar] [CrossRef] [PubMed]
- Rahmatinia, Z.; Rahmatinia, M. Removal of the metronidazole from aqueous solution by heterogeneous electro-Fenton process using nano-Fe3O4. Data Brief 2018, 19, 2139–2145. [Google Scholar] [CrossRef] [PubMed]
- Moradi, F.; Ganji, M.D.; Sarrafi, Y. Remediation of phenol-contaminated water by pristine and functionalized SWCNTs: Ab initio van der Waals DFT investigation. Diam. Relat. Mater. 2018, 82, 7–18. [Google Scholar] [CrossRef]
- Ersan, G.; Apul, O.G.; Perreault, F.; Karanfil, T. Adsorption of organic contaminants by graphene nanosheets: A review. Water Res. 2017, 126, 385–398. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, Y.; Liu, S.; Zeng, G.; Hu, X.; Hu, X.; Wu, Z. Adsorption of estrogen contaminants by graphene nanomaterials under natural organic matter preloading: Comparison to carbon nanotube, biochar, and activated carbon. Environ. Sci. Technol. 2017, 51, 6352–6359. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, S.; Zhu, Y.; Sun, Y.; Zeng, G.; Yang, C.; Zhang, W. Toxicity of carbon nanomaterials to plants, animals and microbes: Recent progress from 2015-present. Chemosphere 2018, 206, 255–264. [Google Scholar] [CrossRef]
- Kang, S.; Zhu, Y.; Chen, M.; Zeng, G.; Li, Z.; Zhang, C.; Xu, P. Can microbes feed on environmental carbon nanomaterials? Nano Today 2019, 25, 10–12. [Google Scholar] [CrossRef]
- Das, R.; Leo, B.F.; Murphy, F. The toxic truth about carbon nanotubes in water purification: A perspective view. Nanoscale Res. Lett. 2018, 13, 183. [Google Scholar] [CrossRef]
- Gora, S.L.; Andrews, S.A. Adsorption of natural organic matter and disinfection byproduct precursors from surface water onto TiO2 nanoparticles: pH effects, isotherm modelling and implications for using TiO2 for drinking water treatment. Chemosphere 2017, 174, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Jiao, T.; Yin, J.; Zhang, L.; Zhang, L.; Zhou, J.; Peng, Q. Facile preparation of carbon nanotube-Cu2O nanocomposites as new catalyst materials for reduction of p-nitrophenol. Nanoscale Res. Lett. 2019, 14, 78. [Google Scholar] [CrossRef]
- Maysoon, M.; Ahmed, K.; Amera, H.; Duread, E. Treatment of Contaminated Water with Industrial Dyes by Using Nano Zero Valent Iron (NZVI) and Supported on Pillared Clay. Adv. Anal. Chem. 2019, 9, 1–7. [Google Scholar] [CrossRef]
- Alvarez, P.J.; Chan, C.K.; Elimelech, M.; Halas, N.J.; Villagrán, D. Emerging opportunities for nanotechnology to enhance water security. Nat. Nanotechnol. 2018, 13, 634. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.K.; Kim, H.B.; Dutta, S.D.; Ganguly, K.; Lim, K.T. Carbon Nanotubes-Based Nanomaterials and Their Agricultural and Biotechnological Applications. Materials 2020, 13, 1679. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; He, F.; Guo, J.; Peng, S.; Cheng, X.Q.; Zhang, Y.; Shao, L. The stability of a graphene oxide (GO) nanofiltration (NF) membrane in an aqueous environment: Progress and challenges. Mater. Adv. 2020, 1, 554–568. [Google Scholar] [CrossRef]
- Liang, B.; Zhang, P.; Wang, J.; Qu, J.; Wang, L.; Wang, X.; Pan, K. Membranes with selective laminar nanochannels of modified reduced graphene oxide for water purification. Carbon 2016, 103, 94–100. [Google Scholar] [CrossRef]
- Zheng, J.; Li, M.; Yu, K.; Hu, J.; Zhang, X.; Wang, L. Sulfonated multiwall carbon nanotubes assisted thin-film nanocomposite membrane with enhanced water flux and anti-fouling property. J. Membr. Sci. 2017, 524, 344–353. [Google Scholar] [CrossRef]
- Zdarta, J.; Antecka, K.; Frankowski, R.; Zgoła-Grześkowiak, A.; Ehrlich, H.; Jesionowski, T. The effect of operational parameters on the biodegradation of bisphenols by Trametes versicolor laccase immobilized on Hippospongia communis spongin scaffolds. Sci. Total Environ. 2018, 615, 784–795. [Google Scholar] [CrossRef]
- Qu, Y.; Zhang, X.; Shen, W.; Ma, Q.; You, S.; Pei, X.; Zhou, J. Illumina MiSeq sequencing reveals long-term impacts of single-walled carbon nanotubes on microbial communities of wastewater treatment systems. Bioresour. Technol. 2016, 211, 209–215. [Google Scholar] [CrossRef]
- Pendergast, M.M.; Hoek, E.M. A review of water treatment membrane nanotechnologies. Energy Environ. Sci. 2011, 4, 1946–1971. [Google Scholar] [CrossRef]
- He, J.; Lin, X.M.; Chan, H.; Vukovic, L.; Král, P.; Jaeger, H.M. Diffusion and filtration properties of self-assembled gold nanocrystal membranes. Nano Lett. 2011, 11, 2430–2435. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Bhattacharya, A. Drinking water contamination and treatment techniques. Appl. Water Sci. 2017, 7, 1043–1067. [Google Scholar] [CrossRef]
- Yi, H.; Huang, D.; Qin, L.; Zeng, G.; Lai, C.; Cheng, M.; Guo, X. Selective prepared carbon nanomaterials for advanced photocatalytic application in environmental pollutant treatment and hydrogen production. Appl. Catal. B Environ. 2018, 239, 408–424. [Google Scholar] [CrossRef]
- Lee, J.; Mackeyev, Y.; Cho, M.; Wilson, L.J.; Kim, J.H.; Alvarez, P.J. C60 aminofullerene immobilized on silica as a visible-light-activated photocatalyst. Environ. Sci. Technol. 2010, 44, 9488–9495. [Google Scholar] [CrossRef]
- Fu, D.; Han, G.; Chang, Y.; Dong, J. The synthesis and properties of ZnO–graphene nano hybrid for photodegradation of organic pollutant in water. Mater. Chem. Phys. 2012, 132, 673–681. [Google Scholar] [CrossRef]
- Liu, L.; Liu, J.; Sun, D.D. Graphene oxide enwrapped Ag3PO4 composite: Towards a highly efficient and stable visible-light-induced photocatalyst for water purification. Catal. Sci. Technol. 2012, 2, 2525–2532. [Google Scholar] [CrossRef]
- Ameen, S.; Seo, H.K.; Akhtar, M.S.; Shin, H.S. Novel graphene/polyaniline nanocomposites and its photocatalytic activity toward the degradation of rose Bengal dye. Chem. Eng. J. 2012, 210, 220–228. [Google Scholar] [CrossRef]
- Laishram, D.; Shejale, K.P.; Gupta, R.; Sharma, R.K. Heterostructured HfO2/TiO2 spherical nanoparticles for visible photocatalytic water remediation. Mater. Lett. 2018, 231, 225–228. [Google Scholar] [CrossRef]
- Alkaykh, S.; Mbarek, A.; Ali-Shattle, E.E. Photocatalytic degradation of methylene blue dye in aqueous solution by MnTiO3 nanoparticles under sunlight irradiation. Heliyon 2020, 6, e03663. [Google Scholar] [CrossRef]
- Wang, T.; Wei, J.; Shi, H.; Zhou, M.; Zhang, Y.; Chen, Q.; Zhang, Z. Preparation of electrospun Ag/TiO2 nanotubes with enhanced photocatalytic activity based on water/oil phase separation. Phys. E Low-Dimens. Syst. Nano Struct. 2017, 86, 103–110. [Google Scholar] [CrossRef]
- Praveen, R.; Chandreshia, C.B.; Ramaraj, R. Silicate sol–gel matrix stabilized ZnO–Ag nanocomposites materials and their environmental remediation applications. J. Environ. Chem. Eng. 2018, 6, 3702–3708. [Google Scholar] [CrossRef]
- Singh, J.; Kumar, V.; Jolly, S.S.; Kim, K.H.; Rawat, M.; Kukkar, D.; Tsang, Y.F. Biogenic synthesis of silver nanoparticles and its photocatalytic applications for removal of organic pollutants in water. J. Ind. Eng. Chem. 2019, 80, 247–257. [Google Scholar] [CrossRef]
- Gallo, A.; Bianco, C.; Tosco, T.; Tiraferri, A.; Sethi, R. Synthesis of eco-compatible bimetallic silver/iron nanoparticles for water remediation and reactivity assessment on bromophenol blue. J. Clean. Prod. 2019, 211, 1367–1374. [Google Scholar] [CrossRef]
- Li, Q.; Mahendra, S.; Lyon, D.Y.; Brunet, L.; Liga, M.V.; Li, D.; Alvarez, P.J. Antimicrobial nanomaterials for water disinfection and microbial control: Potential applications and implications. Water Res. 2008, 42, 4591–4602. [Google Scholar] [CrossRef] [PubMed]
- Hassouna, M.E.M.; ElBably, M.A.; Mohammed, A.N.; Nasser, M.A.G. Assessment of carbon nanotubes and silver nanoparticles loaded clays as adsorbents for removal of bacterial contaminants from water sources. J. Water Health 2017, 15, 133–144. [Google Scholar] [CrossRef]
- Vance, C.C.; Vaddiraju, S.; Karthikeyan, R. Water disinfection using zinc phosphide nanowires under visible light conditions. J. Environ. Chem. Eng. 2018, 6, 568–573. [Google Scholar] [CrossRef]
- Andrade, P.F.; de Faria, A.F.; Oliveira, S.R.; Arruda MA, Z.; do Carmo Gonçalves, M. Improved antibacterial activity of nanofiltration polysulfone membranes modified with silver nanoparticles. Water Res. 2015, 81, 333–342. [Google Scholar] [CrossRef]
- Fan, M.; Gong, L.; Huang, Y.; Wang, D.; Gong, Z. Facile preparation of silver nanoparticle decorated chitosan cryogels for point-of-use water disinfection. Sci. Total Environ. 2018, 613, 1317–1323. [Google Scholar] [CrossRef]
- Zhang, B.; Zou, S.; Cai, R.; Li, M.; He, Z. Highly-efficient photocatalytic disinfection of Escherichia coli under visible light using carbon supported Vanadium Tetrasulfide nanocomposites. Appl. Catal. B Environ. 2018, 224, 383–393. [Google Scholar] [CrossRef]
- Sharma, B.; Boruah, P.K.; Yadav, A.; Das, M.R. TiO2–Fe2O3 nanocomposite heterojunction for superior charge separation and the photocatalytic inactivation of pathogenic bacteria in water under direct sunlight irradiation. J. Environ. Chem. Eng. 2018, 6, 134–145. [Google Scholar] [CrossRef]
- Maddigpu, P.R.; Sawant, B.; Wanjari, S.; Goel, M.D.; Vione, D.; Dhodapkar, R.S.; Rayalu, S. Carbon nanoparticles for solar disinfection of water. J. Hazard. Mater. 2018, 343, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Thines, R.K.; Mubarak, N.M.; Nizamuddin, S.; Sahu, J.N.; Abdullah, E.C.; Ganesan, P. Application potential of carbon nanomaterials in water and wastewater treatment: A review. J. Taiwan Inst. Chem. Eng. 2017, 72, 116–133. [Google Scholar] [CrossRef]
- Smith, S.C.; Rodrigues, D.F. Carbon-based nanomaterials for removal of chemical and biological contaminants from water: A review of mechanisms and applications. Carbon 2015, 91, 122–143. [Google Scholar] [CrossRef]
- Gunawan, P.; Guan, C.; Song, X.; Zhang, Q.; Leong, S.S.J.; Tang, C.; Xu, R. Hollow fiber membrane decorated with Ag/MWNTs: Toward effective water disinfection and biofouling control. ACS Nano 2011, 5, 10033–10040. [Google Scholar] [CrossRef]
- Ahmed, F.; Santos, C.M.; Vergara, R.A.M.V.; Tria, M.C.R.; Advincula, R.; Rodrigues, D.F. Antimicrobial applications of electroactive PVK-SWNT nanocomposites. Environ. Sci. Technol. 2012, 46, 1804–1810. [Google Scholar] [CrossRef]
- Hu, W.; Peng, C.; Luo, W.; Lv, M.; Li, X.; Li, D.; Fan, C. Graphene-based antibacterial paper. ACS Nano 2010, 4, 4317–4323. [Google Scholar] [CrossRef]
- Bao, Q.; Zhang, D.; Qi, P. Synthesis and characterization of silver nanoparticle and graphene oxide nanosheet composites as a bactericidal agent for water disinfection. J. Colloid Interface Sci. 2011, 360, 463–470. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Chen, Y. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: Membrane and oxidative stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef]
- Chen, J.; Peng, H.; Wang, X.; Shao, F.; Yuan, Z.; Han, H. Graphene oxide exhibits broad-spectrum antimicrobial activity against bacterial phytopathogens and fungal conidia by intertwining and membrane perturbation. Nanoscale 2014, 6, 1879–1889. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, M.; Gu, C.; Zhang, C. Water disinfection processes change the cytotoxicity of C60 fullerene: Reactions at the nano-bio interface. Water Res. 2019, 163, 114867. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, H.; Lee, J.Y.; Park, K.H.; Kim, W.; Lee, J.H.; Lee, J.H. Photosensitized Production of Singlet Oxygen via C60 Fullerene Covalently Attached to Functionalized Silica-coated Stainless-Steel Mesh: Remote Bacterial and Viral Inactivation. Appl. Catal. B Environ. 2020, 270, 118862. [Google Scholar] [CrossRef]
- Dutta, V.; Singh, P.; Shandilya, P.; Sharma, S.; Raizada, P.; Saini, A.K.; Rahmani-Sani, A. Review on advances in photocatalytic water disinfection utilizing graphene and graphene derivatives-based nanocomposites. J. Environ. Chem. Eng. 2019, 7, 103132. [Google Scholar] [CrossRef]
- Cruz-Ortiz, B.R.; Hamilton, J.W.; Pablos, C.; Díaz-Jiménez, L.; Cortés-Hernández, D.A.; Sharma, P.K.; Byrne, J.A. Mechanism of photocatalytic disinfection using titania-graphene composites under UV and visible irradiation. Chem. Eng. J. 2017, 316, 179–186. [Google Scholar] [CrossRef]
- Kumar, P.; Huo, P.; Zhang, R.; Liu, B. Antibacterial properties of graphene-based nanomaterials. Nanomaterials 2019, 9, 737. [Google Scholar] [CrossRef]
- Deng, C.H.; Gong, J.L.; Zhang, P.; Zeng, G.M.; Song, B.; Liu, H.Y. Preparation of melamine sponge decorated with silver nanoparticles-modified graphene for water disinfection. J. Colloid Interface Sci. 2017, 488, 26–38. [Google Scholar] [CrossRef]
- Dimapilis, E.A.S.; Hsu, C.S.; Mendoza, R.M.O.; Lu, M.C. Zinc oxide nanoparticles for water disinfection. Sustain. Environ. Res. 2018, 28, 47–56. [Google Scholar] [CrossRef]
- Li, J.; Zhu, Q.; Su, Y.; Wang, D.; Xing, Z.; Fang, L. High-efficiency bacteriostatic material modified by nano zinc oxide and polyelectrolyte diallyl dimethylammonium chloride based on red mud. Colloids Surf. B Biointerfaces 2019, 177, 260–266. [Google Scholar] [CrossRef]
- Jiang, D.; Zeng, G.; Huang, D.; Chen, M.; Zhang, C.; Huang, C.; Wan, J. Remediation of contaminated soils by enhanced nanoscale zero valent iron. Environ. Res. 2018, 163, 217–227. [Google Scholar] [CrossRef]
- Schirinzi, G.F.; Pérez-Pomeda, I.; Sanchís, J.; Rossini, C.; Farré, M.; Barceló, D. Cytotoxic effects of commonly used nanomaterials and microplastics on cerebral and epithelial human cells. Environ. Res. 2017, 159, 579–587. [Google Scholar] [CrossRef]
- Adam, V.; Caballero-Guzman, A.; Nowack, B. Considering the forms of released engineered nanomaterials in probabilistic material flow analysis. Environ. Pollut. 2018, 243, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Qin, X.; Zeng, G. Biodegradation of carbon nanotubes, graphene, and their derivatives. Trends Biotechnol. 2017, 35, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Semerád, J.; Čvančarová, M.; Filip, J.; Kašlík, J.; Zlotá, J.; Soukupová, J.; Cajthaml, T. Novel assay for the toxicity evaluation of nanoscale zero-valent iron and derived nanomaterials based on lipid peroxidation in bacterial species. Chemosphere 2018, 213, 568–577. [Google Scholar] [CrossRef]
- Xie, Y.; Dong, H.; Zeng, G.; Tang, L.; Jiang, Z.; Zhang, C.; Zhang, Y. The interactions between nanoscale zero-valent iron and microbes in the subsurface environment: A review. J. Hazard. Mater. 2017, 321, 390–407. [Google Scholar] [CrossRef]
- De Marchi, L.; Pretti, C.; Gabriel, B.; Marques, P.A.; Freitas, R.; Neto, V. An overview of graphene materials: Properties, applications and toxicity on aquatic environments. Sci. Total Environ. 2018, 631, 1440–1456. [Google Scholar] [CrossRef]
- Pretti, C.; Oliva, M.; Di Pietro, R.; Monni, G.; Cevasco, G.; Chiellini, F.; Chiappe, C. Ecotoxicity of pristine graphene to marine organisms. Ecotoxicol. Environ. Saf. 2014, 101, 138–145. [Google Scholar] [CrossRef]
- Vale, G.; Mehennaoui, K.; Cambier, S.; Libralato, G.; Jomini, S.; Domingos, R.F. Manufactured nanoparticles in the aquatic environment-biochemical responses on freshwater organisms: A critical overview. Aquat. Toxicol. 2016, 170, 162–174. [Google Scholar] [CrossRef]
- Yamindago, A.; Lee, N.; Woo, S.; Choi, H.; Mun, J.Y.; Jang, S.W.; Yang, S.I.; Anton-Erxleben, F.; Bosch, T.C.G.; Yum, S. Acute toxic effects of zinc oxide nanoparticles on Hydra magnipapillata. Aquat. Toxicol. 2018, 205, 130–139. [Google Scholar] [CrossRef]
- Özgür, M.E.; Balcıoğlu, S.; Ulu, A.; Özcan, İ.; Okumuş, F.; Köytepe, S.; Ateş, B. The in vitro toxicity analysis of titanium dioxide (TiO2) nanoparticles on kinematics and biochemical quality of rainbow trout sperm cells. Environ. Toxicol. Pharmacol. 2018, 62, 11–19. [Google Scholar] [CrossRef]
- Bian, Z.; Zhu, J.; Li, H. Solvothermal alcoholysis synthesis of hierarchical TiO2 with enhanced activity in environmental and energy photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2016, 28, 72–86. [Google Scholar] [CrossRef]
- Liu, W.; Zeng, Z.; Chen, A.; Zeng, G.; Xiao, R.; Guo, Z.; Hu, L. Toxicity effects of silver nanoparticles on the freshwater bivalve Corbicula fluminea. J. Environ. Chem. Eng. 2018, 6, 4236–4244. [Google Scholar] [CrossRef]
- Tao, W.; Chen, G.; Zeng, G.; Yan, M.; Chen, A.; Guo, Z.; Wang, L. Influence of silver nanoparticles on heavy metals of pore water in contaminated river sediments. Chemosphere 2016, 162, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, Z.; Raza, M.A.; Manzoor, F.; Riaz, S.; Jabeen, G.; Fatima, S.; Naseem, S. A comparative assessment of nanotoxicity induced by metal (silver, nickel) and metal oxide (cobalt, chromium) nanoparticles in Labeo rohita. Nanomaterials 2019, 9, 309. [Google Scholar] [CrossRef]
- Syafiuddin, A.; Salmiati, S.; Hadibarata, T.; Salim, M.R.; Kueh AB, H.; Suhartono, S. Removal of silver nanoparticles from water environment: Experimental, mathematical formulation, and cost analysis. Water Air Soil Pollut. 2019, 230, 102. [Google Scholar] [CrossRef]
- Thanh, D.N.; Novák, P.; Vejpravova, J.; Vu, H.N.; Lederer, J.; Munshi, T. Removal of copper and nickel from water using nanocomposite of magnetic hydroxyapatite nanorods. J. Magn. Magn. Mater. 2018, 456, 451–460. [Google Scholar] [CrossRef]
- Khezami, L.; Taha, K.K.; Modwi, A. Efficient Removal of Cobalt from Aqueous Solution by Zinc Oxide Nanoparticles: Kinetic and Thermodynamic Studies. Z. Für Nat. A 2017, 72. [Google Scholar] [CrossRef]
- Le, A.T.; Pung, S.Y.; Sreekantan, S.; Matsuda, A. Mechanisms of removal of heavy metal ions by ZnO particles. Heliyon 2019, 5, e01440. [Google Scholar] [CrossRef]
- Burks, T.; Uheida, A.; Saleemi, M.; Eita, M.; Toprak, M.S.; Muhammed, M. Removal of chromium (VI) using surface modified superparamagnetic iron oxide nanoparticles. Sep. Sci. Technol. 2013, 48, 1243–1251. [Google Scholar] [CrossRef]
- Ale, A.; Rossi, A.S.; Bacchetta, C.; Gervasio, S.; de la Torre, F.R.; Cazenave, J. Integrative assessment of silver nanoparticles toxicity in Prochilodus lineatus fish. Ecol. Indic. 2018, 93, 1190–1198. [Google Scholar] [CrossRef]
- Song, B.; Xu, P.; Zeng, G.; Gong, J.; Wang, X.; Yan, J.; Ye, S. Modeling the transport of sodium dodecyl benzene sulfonate in riverine sediment in the presence of multi-walled carbon nanotubes. Water Res. 2018, 129, 20–28. [Google Scholar] [CrossRef]
- Zhu, B.; Zhu, S.; Li, J.; Hui, X.; Wang, G.X. The developmental toxicity, bioaccumulation and distribution of oxidized single walled carbon nanotubes in Artemia salina. Toxicol. Res. 2018, 7, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Parks, A.N.; Portis, L.M.; Schierz, P.A.; Washburn, K.M.; Perron, M.M.; Burgess, R.M.; Ferguson, P.L. Bioaccumulation and toxicity of single-walled carbon nanotubes to benthic organisms at the base of the marine food chain. Environ. Toxicol. Chem. 2013, 32, 1270–1277. [Google Scholar] [CrossRef]
- Da Rocha, A.M.; Kist, L.W.; Almeida, E.A.; Silva, D.G.H.; Bonan, C.D.; Altenhofen, S.; Kaufmann, C.G.; Bogo, M.R.; Barros, D.M.; Oliveira, S. Neurotoxicity in zebrafish exposed to carbon nanotubes: Effects on neurotransmitters levels and antioxidant system. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 218, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Qin, X.; Zeng, G. Single-walled carbon nanotube release affects the microbial enzyme-catalyzed oxidation processes of organic pollutants and lignin model compounds in nature. Chemosphere 2016, 163, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Tabei, Y.; Fukui, H.; Nishioka, A.; Hagiwara, Y.; Sato, K.; Yoneda, T.; Horie, M. Effect of iron overload from multi walled carbon nanotubes on neutrophil-like differentiated HL-60 cells. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Paz, P.; Negri, V.; Esteban-Arranz, A.; Martínez-Guitarte, J.L.; Ballesteros, P.; Morales, M. Effects at molecular level of multi-walled carbon nanotubes (MWCNT) in Chironomus riparius (DIPTERA) aquatic larvae. Aquat. Toxicol. 2019, 209, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Nogueira PF, M.; Nakabayashi, D.; Zucolotto, V. The effects of graphene oxide on green algae Raphidocelis subcapitata. Aquat. Toxicol. 2015, 166, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Ouyang, S.; Mu, L.; An, J.; Zhou, Q. Effects of graphene oxide and oxidized carbon nanotubes on the cellular division, microstructure, uptake, oxidative stress, and metabolic profiles. Environ. Sci. Technol. 2015, 49, 10825–10833. [Google Scholar] [CrossRef]
- Ouyang, S.; Hu, X.; Zhou, Q. Envelopment–internalization synergistic effects and metabolic mechanisms of graphene oxide on single-cell Chlorella vulgaris are dependent on the nanomaterial particle size. ACS Appl. Mater. Interfaces 2015, 7, 18104–18112. [Google Scholar] [CrossRef]
- Du, S.; Zhang, P.; Zhang, R.; Lu, Q.; Liu, L.; Bao, X.; Liu, H. Reduced graphene oxide induces cytotoxicity and inhibits photosynthetic performance of the green alga Scenedesmus obliquus. Chemosphere 2016, 164, 499–507. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, Q.; Zou, W.; Hu, X. Molecular mechanisms of developmental toxicity induced by graphene oxide at predicted environmental concentrations. Environ. Sci. Technol. 2017, 51, 7861–7871. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Selck, H.; Tangaa, S.R.; Pang, C.; Zhao, B. Bioaccumulation and effects of sediment-associated gold-and graphene oxide nanoparticles on Tubifex tubifex. J. Environ. Sci. 2017, 51, 138–145. [Google Scholar] [CrossRef] [PubMed]
- De Marchi, L.; Neto, V.; Pretti, C.; Figueira, E.; Brambilla, L.; Rodriguez-Douton, M.J.; Freitas, R. Physiological and biochemical impacts of graphene oxide in polychaetes: The case of Diopatra neapolitana. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 193, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.G.; Rong, Z.H.O.U.; Jiang, D.; Jing, E.S.; Qian, X.U.; Jing, S.I.; Zhang, H. Toxicity of graphene quantum dots in zebrafish embryo. Biomed. Environ. Sci. 2015, 28, 341–351. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, G.; Zeng, G.; Guo, Z.; He, K.; Hu, L.; Song, Z. Toxicity mechanisms and synergies of silver nanoparticles in 2, 4-dichlorophenol degradation by Phanerochaete chrysosporium. J. Hazard. Mater. 2017, 321, 37–46. [Google Scholar] [CrossRef]
- Navarro, E.; Wagner, B.; Odzak, N.; Sigg, L.; Behra, R. Effects of differently coated silver nanoparticles on the photosynthesis of Chlamydomonas reinhardtii. Environ. Sci. Technol. 2015, 49, 8041–8047. [Google Scholar] [CrossRef]
- Buffet, P.E.; Zalouk-Vergnoux, A.; Châtel, A.; Berthet, B.; Métais, I.; Perrein-Ettajani, H.; Guibbolini, M. A marine mesocosm study on the environmental fate of silver nanoparticles and toxicity effects on two endobenthic species: The ragworm Hediste diversicolor and the bivalve mollusc Scrobicularia plana. Sci. Total Environ. 2014, 470, 1151–1159. [Google Scholar] [CrossRef]
- Thakkar, M.; Mitra, S.; Wei, L. Effect on growth, photosynthesis, and oxidative stress of single walled carbon nanotubes exposure to marine alga Dunaliella tertiolecta. J. Nanomater. 2016, 2016. [Google Scholar] [CrossRef]
- Shi, D.; Mi, G.; Webster, T.J. The Synthesis, Application, and Related Neurotoxicity of Carbon Nanotubes. In Neurotoxicity of Nanomaterials and Nanomedicine; Academic Press, Elsevier: New York, NY, USA, 2017; pp. 259–284. [Google Scholar] [CrossRef]
- Jang, M.H.; Hwang, Y.S. Effects of functionalized multi-walled carbon nanotubes on toxicity and bioaccumulation of lead in Daphnia magna. PLoS ONE 2018, 13, e0194935. [Google Scholar] [CrossRef]
- Kim, K.T.; Edgington, A.J.; Klaine, S.J.; Cho, J.W.; Kim, S.D. Influence of multiwalled carbon nanotubes dispersed in natural organic matter on speciation and bioavailability of copper. Environ. Sci. Technol. 2009, 43, 8979–8984. [Google Scholar] [CrossRef]
- Chambers, B.A.; Afrooz, A.N.; Bae, S.; Aich, N.; Katz, L.; Saleh, N.B.; Kirisits, M.J. Effects of chloride and ionic strength on physical morphology, dissolution, and bacterial toxicity of silver nanoparticles. Environ. Sci. Technol. 2014, 48, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.Y.; Bornhöft, N.A.; Hungerbühler, K.; Nowack, B. Dynamic probabilistic modeling of environmental emissions of engineered nanomaterials. Environ. Sci. Technol. 2016, 50, 4701–4711. [Google Scholar] [CrossRef] [PubMed]
- Khosravi-Katuli, K.; Prato, E.; Lofrano, G.; Guida, M.; Vale, G.; Libralato, G. Effects of nanoparticles in species of aquaculture interest. Environ. Sci. Pollut. Res. 2017, 24, 17326–17346. [Google Scholar] [CrossRef] [PubMed]
- Kriebel, D.; Tickner, J.; Epstein, P.; Lemons, J.; Levins, R.; Loechler, E.L.; Stoto, M. The precautionary principle in environmental science. Environ. Health Perspect. 2001, 109, 871–876. [Google Scholar] [CrossRef]
- OECD Environment Directorate. Ecotoxicology and Environmental Fate of Manufactured Nano-materials: Test Guidelines. Expert Meeting Report. Series on the Safety of Manufactured Nanomaterials No. 40 (ENV/JM/MONO(2014)1). 2014. Available online: http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=ENV/JM/MONO(2014)1&doclanguage=en (accessed on 20 June 2020).


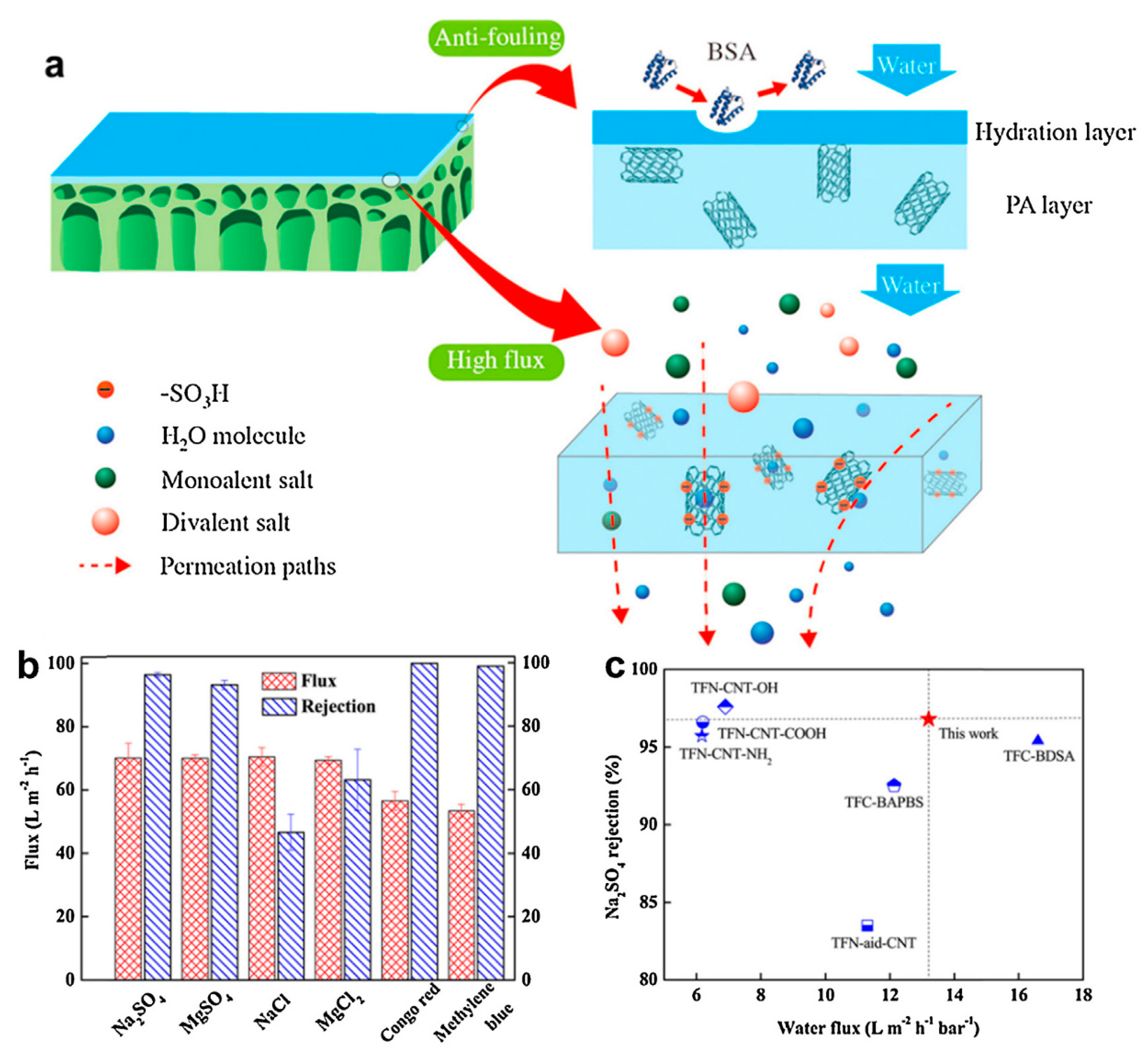
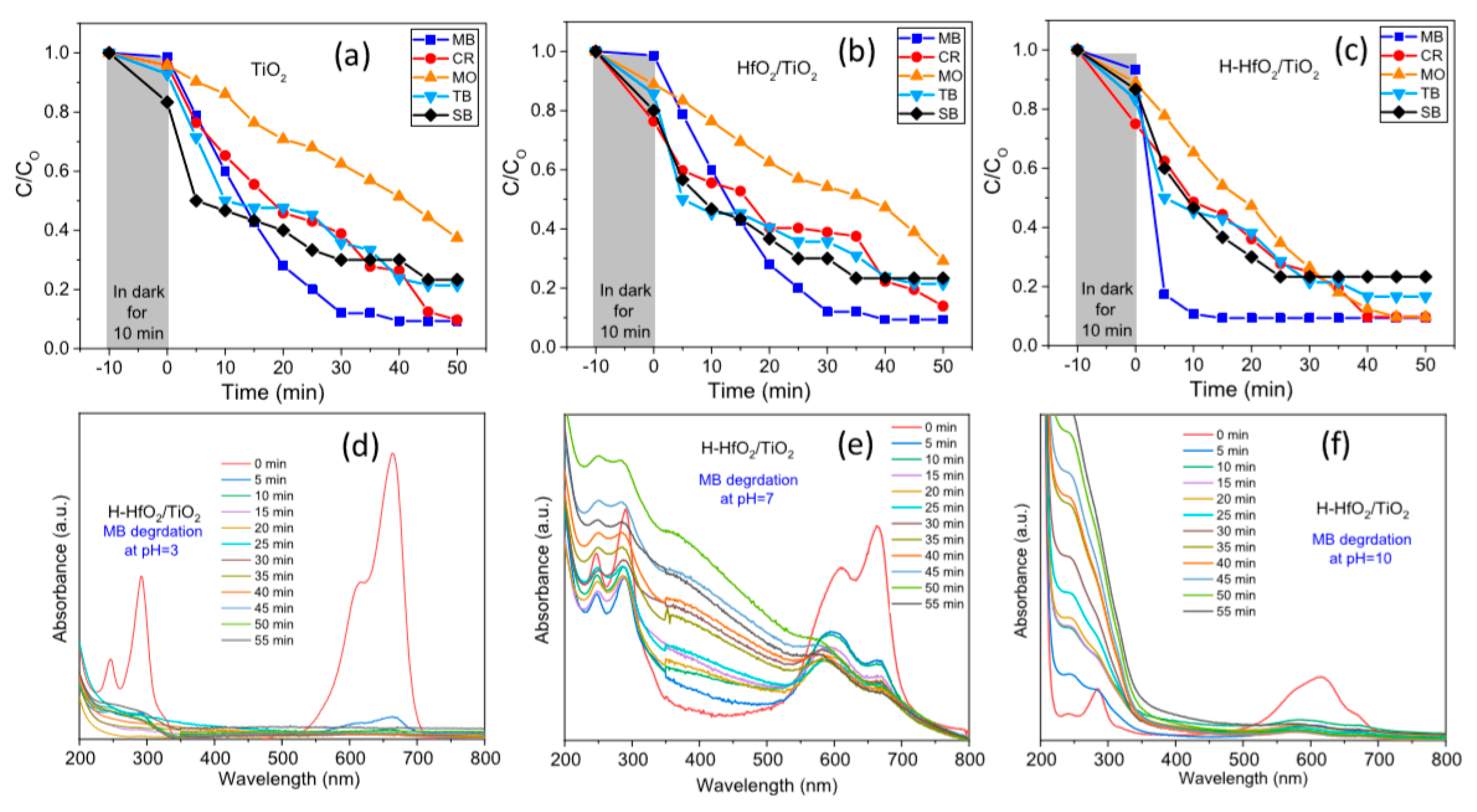
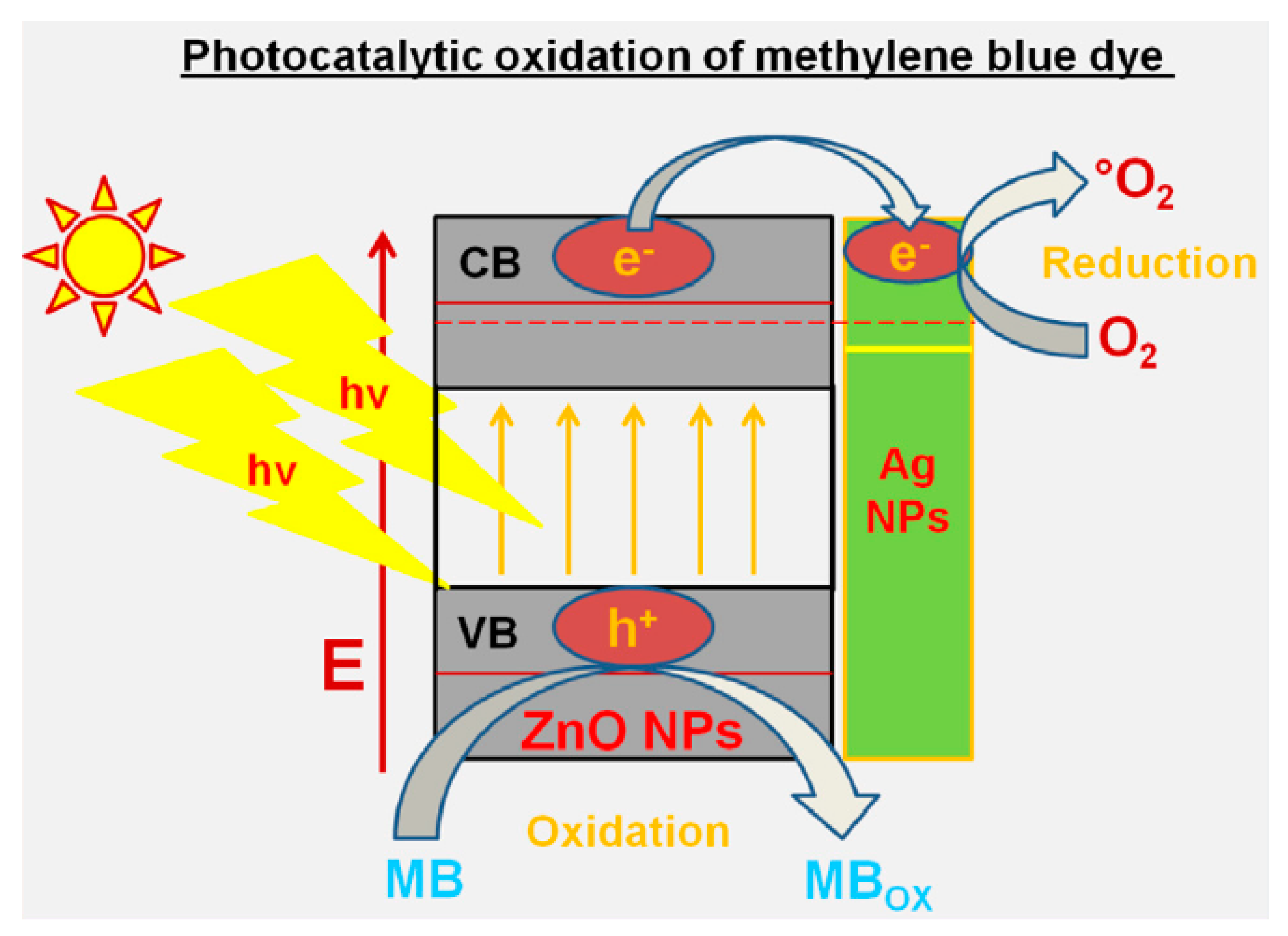
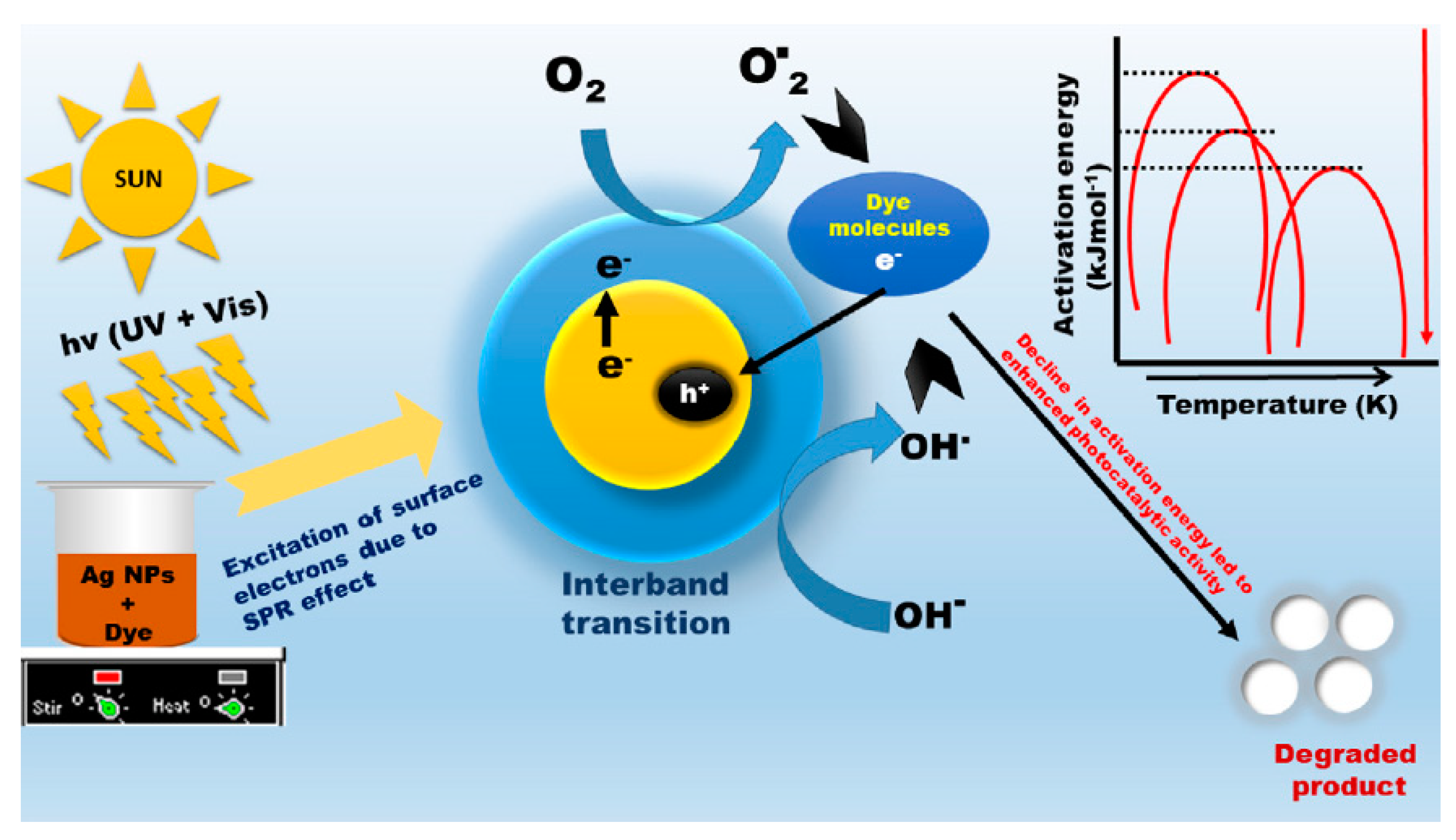
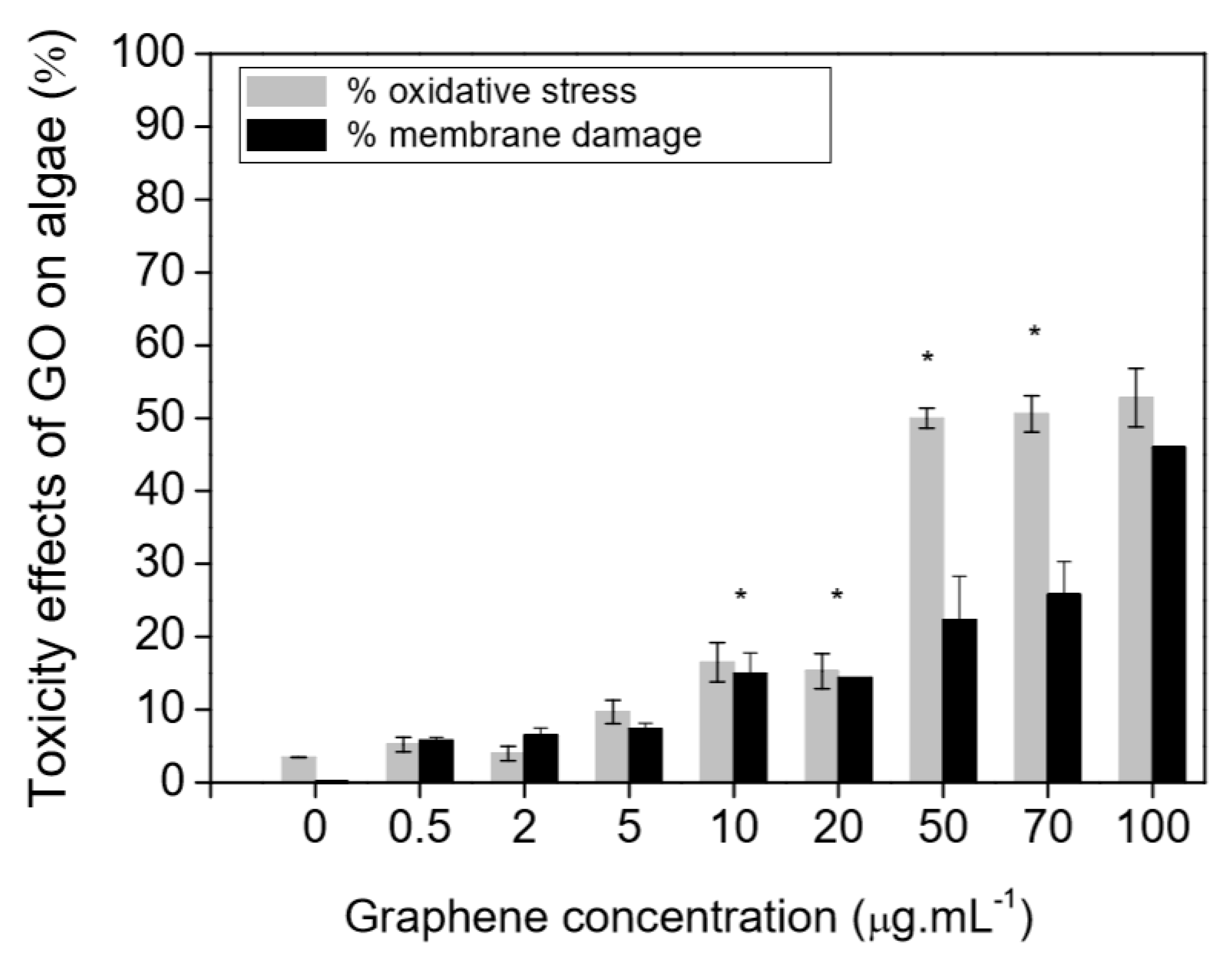
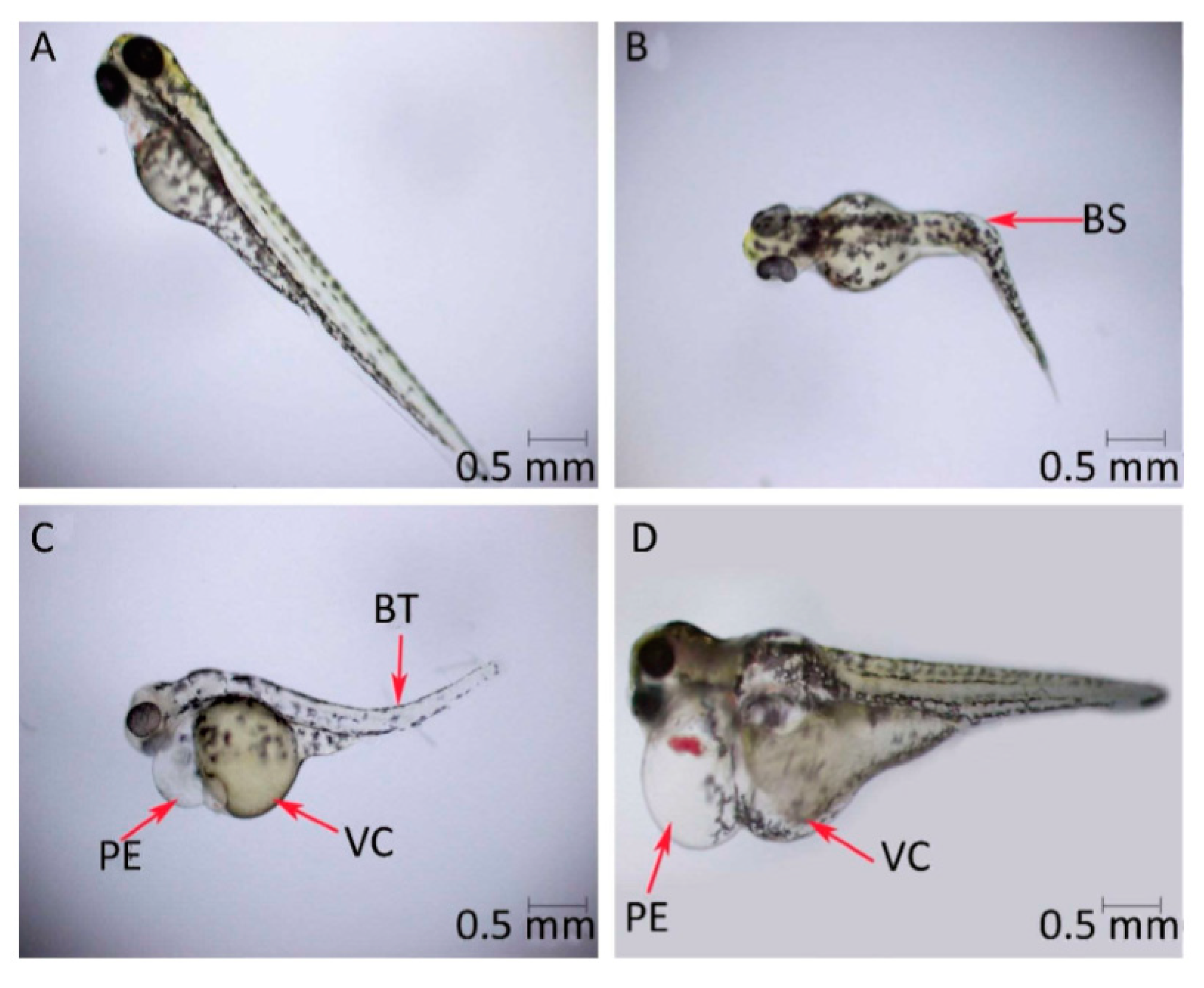
| Sl. No. | Nanomaterials | Common Manufacturing Techniques | Mechanism | Remediation | Reference |
|---|---|---|---|---|---|
| 1 | Graphene nanomaterials | Photocatalytic reduction, carbon nanotube (CNT) conversion, chemical oxidation–reduction, mechanical milling, pyrolysis, and plasma synthesis | The porous structures present in the nanomaterial adsorb the contaminants present in water | Dyes and heavy metals | [44] |
| 2 | Titanium dioxide (TiO2) nanoparticles | Liquid phase technique and gas-phase technique | The ultraviolet (UV) light in light could stimulate the TiO2 nanoparticles and develop the free-radicals having high catalytic activity, that could generate a stronger photo-oxidation as well as reduction capacity | Certain inorganic substances and different organic substances like formaldehyde | [45] |
| 3 | Zerovalent iron (nZVI) | Reduction | The thin iron oxide core could support the pollutant adsorption by means of the surface complexation and electrostatic interaction. | Azodyes, nitro-aromatic compounds, chlorinated aromatic compounds, heavy metals, and organo-chlorine. | [46] |
| 4 | Nanofiber material | Electrohydraulic dynamic (EHD) direct writing, centrifugal jet spinning (CJS), solution blowing, electrospinning (ES), molecular techniques, etc. | Particles having various diameters are filtered using membranes possessing distinct pore sizes. | Different contaminants present in eutrophicated landscape water | [47] |
| 5 | Silver nanoparticles | Radiation, electroplating, laser ablation method, photoreduction, and chemical vapor deposition. | By coupling with the bacterial metabolic enzymes, the bacteria will get dried up as well as later die. Superior germicidal performance can be observed in smaller size particles | Role of a water photocatalyst and antibacterial agent | [48] |
| Sl. No. | Nanomaterial | Contaminant | Max Adsorption Capacity (mg/g) | Model | Conditions | Important Findings | Reference |
|---|---|---|---|---|---|---|---|
| 1 | Single-walled CNT | Cr (VI) | 2.35 | Langmuir | Cr (VI) Cin = 0.2–1.0 mg/L CNT Cin = 25–200 mg/L | pH dependent (Optimum = 2.5) and time dependent | [50] |
| 2 | Multiwalled CNT | Cr (VI) | 1.26 | Langmuir | Cr (VI) Cin = 0.2–1.0 mg/L CNT Cin = 25–200 mg/L | pH dependent (Optimum = 2.5) and time dependent | [50] |
| 3 | Single-walled CNT | Ciprofloxacin (CIP) | 724 | Brouers-Sotolongo | CIP Cin = 50 mg/L CNT Cin = 100 mg/L | pH dependent (Optimum= 7) and time dependent | [51] |
| 4 | Multiwalled CNT | Ciprofloxacin (CIP) | 475 | Brouers-Sotolongo | CIP Cin = 50 mg/L CNT Cin = 100 mg/L | pH dependent (Optimum = 7) and time dependent | [51] |
| 5 | Single-walled CNT | Oxytetracycline (OXY) | 554 | Brouers-Sotolongo | OXY Cin = 50 mg/L CNT Cin = 100 mg/L | pH dependent (Optimum = 7) and time dependent | [52] |
| 6 | Multiwalled CNT | Oxytetracycline (OXY) | 391 | Brouers-Sotolongo | OXY Cin = 50 mg/L CNT Cin = 100 mg/L | pH dependent (Optimum = 7) and time dependent | [52] |
| 7 | Multiwalled CNT | Methylene blue | 59.7 | Langmuir | MB Cin = 5–15 mg/L Cin = 0.02 g | pH dependent (Optimum = 6) | [53] |
| 8 | Multiwalled CNT | Acid dye | 45.2 | Langmuir | Acid dye Cin = 10–50 mg/L graphene Cin = 0.02 g | pH dependent (Optimum = 6) | [53] |
| 9 | Graphene | Methylene blue (MB) | 204.08 | Langmuir | MB Cin = 20–120 mg/L graphene Cin = 0.02–0.17 g | Adsorption is pH insensitive | [54] |
| 10 | Reduced GO/Mg(OH)2MgO | Arsenite (As (III)) | 681 | Langmuir | As Cin = 1.5 mg/L adsorbent Cin = 400 mg/L | Optimum time—360 min | [55] |
| 11 | GO/Fe3O4 | Arsenic (V) | 5.27 (Langmuir), 1.99 (Freundlich) | Langmuir and Freundlich | Ar Cin = 5 mg/L adsorbent Cin = 200 mg/L | Temperature dependent | [56] |
| 12 | CuO-ZnO nanofibers | Congo red dye | 126.4 Langmuir | Langmuir, Freundlich and Temkin | CR Cin = 10–90 mg/L nanofibers Cin = 2 mg | - | [57] |
| 13 | TiO2 nanoparticles | Reactive Red 195 | 87 | Langmuir | RR Cin = 10–50 mg/L adsorbent Cin = 0.02 – 0.2 mg | Followed pseudo-second-order expression | [58] |
| 14 | ZnO | Direct blue 78 | 34.48 | Langmuir, and Temkin | DB Cin = 50 mg/L adsorbent Cin = 0.05 – 0.2 mg | Followed pseudo-second-order kinetics | [59] |
| 15 | ZnO | Acid black 26 | 52.63 | Langmuir, and Temkin | AB Cin = 50 mg/L adsorbent Cin = 0.05 – 0.2 mg | Followed pseudo-second-order kinetics | [59] |
| 16 | Copper | Ibuprofen | 33.9 | Langmuir | Ibu Cin = 10–40 mg/L | Adsorption spontaneous and endothermic | [60] |
| 17 | Copper | Naproxen | 33.9 | Langmuir | Nap Cin = 10–40 mg/L | Adsorption spontaneous and endothermic | [60] |
| 18 | Copper | Diclofenac | 36.0 | Langmuir | Diclo Cin = 10–40 mg/L | Adsorption spontaneous and endothermic | [60] |
| 19 | Magnetic-modified multiwalled CNTs | Janus green | 250 mg/g | Langmuir | JG Cin = 20 mg/L | Optimal pH—7 | [61] |
| 20 | Magnetic-modified multiwalled CNTs | methylene blue | 48.1 mg/g | Langmuir | MB Cin = 20 mg/L | Optimal pH—7 | [61] |
| Sl. No. | Nanomaterials | Microorganisms Removed | Efficiency of Removal | Reference |
|---|---|---|---|---|
| 1 | Silver nanomaterials loaded kaolin clay | Escherichia coli, Salmonella spp. | 80% for Escherichia coli; 9 % for Salmonella spp. (concentration: 0.1 ppm) | [127] |
| 2 | Zinc phosphide nanowires | Escherichia coli | Greater than 4 log reduction | [128] |
| 3 | Silver nanomaterial in polysulfone membranes | Escherichia coli | 90 percent efficiency (silver leaching 2 μg L–1) | [129] |
| 4 | Silver nanomaterial loaded chitosan cryogels | Bacillus subtilis and Escherichia coli | 3 log reduction (silver content –7.5 mg/g) | [130] |
| 5 | Carbon powder-Vanadium Tetrasulfide nanocomposite | Escherichia coli | 9.7 log reduction (at 0.1 g/L) | [131] |
| 6 | Titanium dioxide-Iron oxide nanocomposite | Escherichia coli | 99.28% removal efficiency (initial concentration of bacteria: 10 mg/mL) | [132] |
| 7 | Carbon nanoparticles | Escherichia coli bacteria | 6 log reduction (25 mg/50 mL concentration) | [133] |
| Sl. No. | Nanomaterials | Concentration/Toxicity | Remediation Measure | Reference |
|---|---|---|---|---|
| 1 | Nano-zerovalent iron | Six bacteria were examined for nZVI concentration, and the EC50 of the bacteria against pristine pyrophoric nano zerovalent iron was noted to be 0.30–1.10 g/L. Irrespective of the difference in EC50 values, the development of malondialdehyde displayed a similar tendency for all the tested bacteria. | The iron present in the ageing nano zerovalent iron underwent a reduction to the Fe2+ ion, which could decrease the pollutants. | [154,155] |
| 2 | Graphene oxide | With graphene oxide only, lethal effects on Artemia salina have been noted just at a high concentration of graphene oxide (500 ppm), however sublethal toxicity (hindrance of growth) has been noted while the graphene oxide loading was as little as 1 ppm, which might be brought about by the oxidative stress stimulated by graphene oxide. In the acute toxicity analysis on Artemia salina, the pure graphene showed no toxicity at 10 mg/L maximal concentration. Other nanomaterials in other crustacean species confirmed a similar toxicity tendency. | Adsorbing heavy metal ions as well as organic pollutants. | [156,157] |
| 3 | Zinc oxide nanoparticles | The median lethal concentrations of 20 nm zinc oxide nanoparticles towards Hydra magnipapillata have been 7.0, 8.70, and 55.30 μg/mL after exposing to 96, 72, and 48 h, respectively, when the median lethal concentrations of 100 nm zinc oxide nanoparticles have been 9.90, 14.90, and 262.0 μg, respectively. | Absorbing different elements like Cd, Ni, Cu, Pb, Hg, Mo, Al, and As, altering their speciation in the media and thus the bioavailability. | [158,159] |
| 4 | Titanium dioxide nanoparticles | When the titanium dioxide nanoparticle concentration attained 10 ppm, the speediness of sperm cells reduced, also superoxide dismutase activity as well as the total glutathione level got enhanced. | Resilient absorbability to As(V), As(III), and Cd. | [160,161] |
| 5 | Silver nanoparticles | When the silver nanoparticle concentration was 0.1 ppm, the biological behavior of Corbicula fluminea has been restrained. When the silver nanoparticle concentration attained 2 ppm, the physiological metabolism of Corbicula fluminea has been restrained again. | Silver nanoparticles have the ability for absorbing the heavy metal ions in water, like zinc and copper ions. | [162,163] |
| 6 | Silver nanoparticle | Fresh water fish Labeo rohita exposed to the nanoparticle (25 mg/L) for 21 days. Survival of fish was unaltered. Exhibited less toxicity. | Activated carbon can be used as an adsorbent to remove silver from water. | [164,165] |
| 7 | Nickel nanoparticle | Fresh water fish Labeo rohita exposed to the nanoparticle (25 mg/L) for 21 days. Survival of fish was unaltered. Remarkable reduction in growth and hemoglobin observed. Significant total protein rise noted. | Nanocomposite of magnetic hydroxyapatite can be used as an adsorbent for the removal of copper nickel (Ni(II)) from water. | [164,166] |
| 8 | Cobalt oxide Nanoparticle | Fresh water fish Labeo rohita exposed to the nanoparticle (25 mg/L) for 21 days. Survival of fish was unaltered. Significant total protein rise noted. | Zinc oxide nanopowder can be used for the cobalt oxide nanoparticle removal. | [164,167,168] |
| 9 | Chromium oxide nanoparticle | Fresh water fish Labeo rohita exposed to the nanoparticle (25 mg/L) for 21 days. Cr3O4 nanoparticles caused early fish mortalities. Remarkable reduction in growth and hemoglobin. Significant total protein rise noted. | Superparamagnetic iron oxide nanoparticles can be used for chromium oxide removal. | [164,169] |
| 10 | Silver nanoparticles | Prochilodus lineatus fish exposed to 2.5 and 25.0 µg L−1 nanoparticle for 5 and 15 days. ACAP reduced in liver, all antioxidant enzymes activities increased, muscle protein concentration reduced, and glycogen content enhanced in liver and muscle. | Activated carbon can be used as an adsorbent to remove silver from water. | [165,170] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleem, H.; Zaidi, S.J. Developments in the Application of Nanomaterials for Water Treatment and Their Impact on the Environment. Nanomaterials 2020, 10, 1764. https://doi.org/10.3390/nano10091764
Saleem H, Zaidi SJ. Developments in the Application of Nanomaterials for Water Treatment and Their Impact on the Environment. Nanomaterials. 2020; 10(9):1764. https://doi.org/10.3390/nano10091764
Chicago/Turabian StyleSaleem, Haleema, and Syed Javaid Zaidi. 2020. "Developments in the Application of Nanomaterials for Water Treatment and Their Impact on the Environment" Nanomaterials 10, no. 9: 1764. https://doi.org/10.3390/nano10091764
APA StyleSaleem, H., & Zaidi, S. J. (2020). Developments in the Application of Nanomaterials for Water Treatment and Their Impact on the Environment. Nanomaterials, 10(9), 1764. https://doi.org/10.3390/nano10091764






