Rocaglamide Suppresses Allergic Reactions by Regulating IL-4 Receptor Signaling
Abstract
:1. Introduction
2. Results
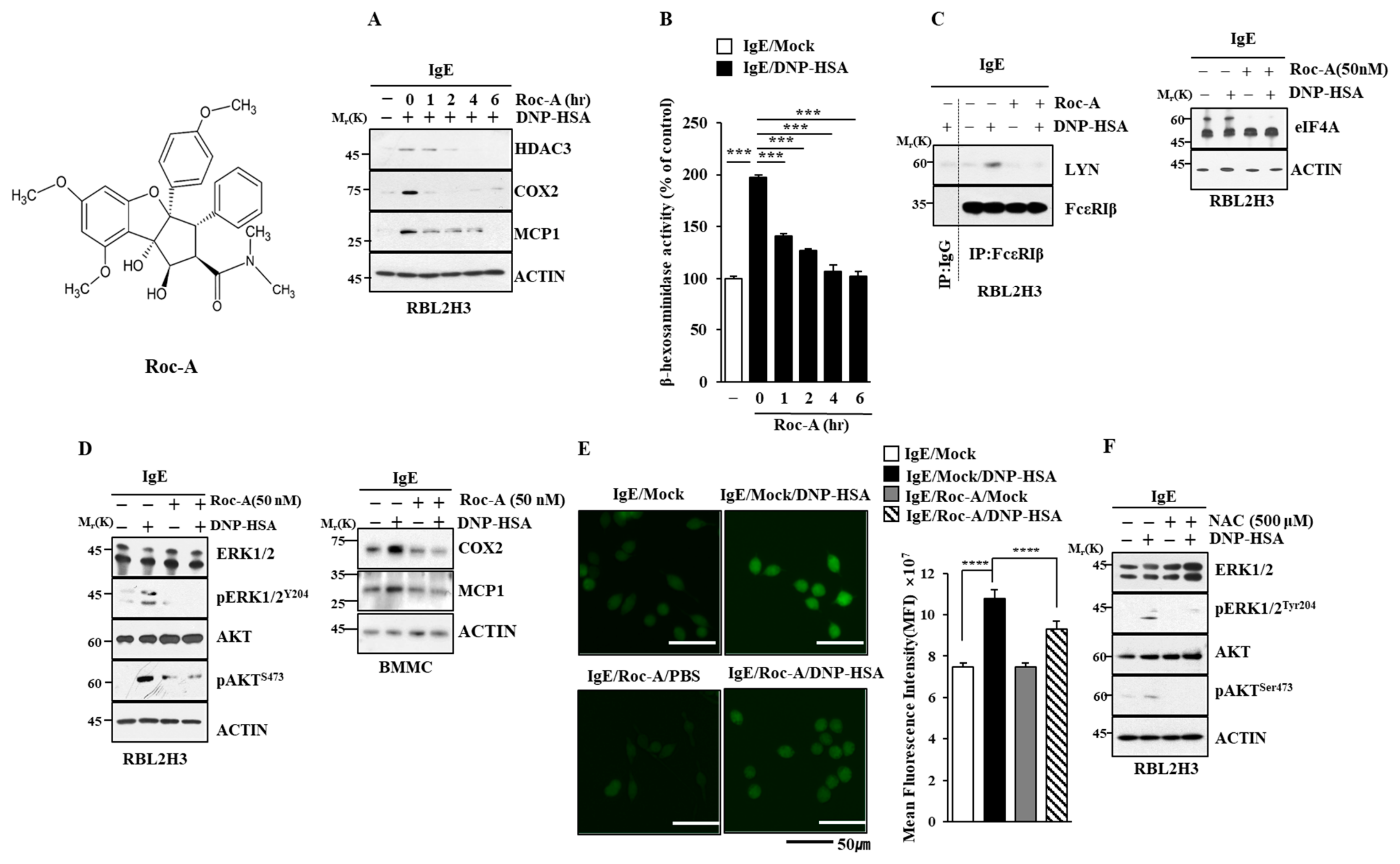
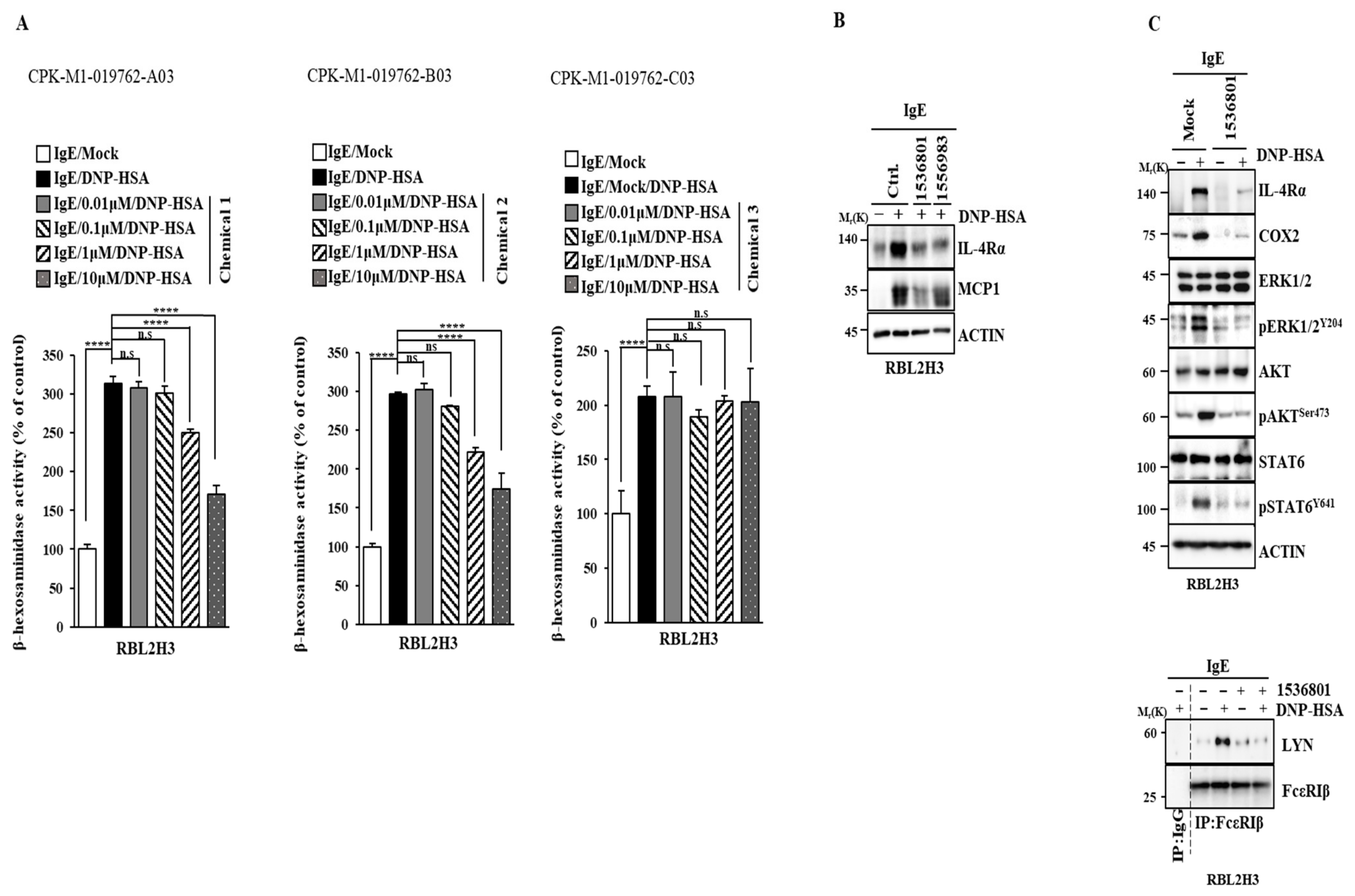
2.1. Roc-A Inhibits Allergic Reactions In Vitro
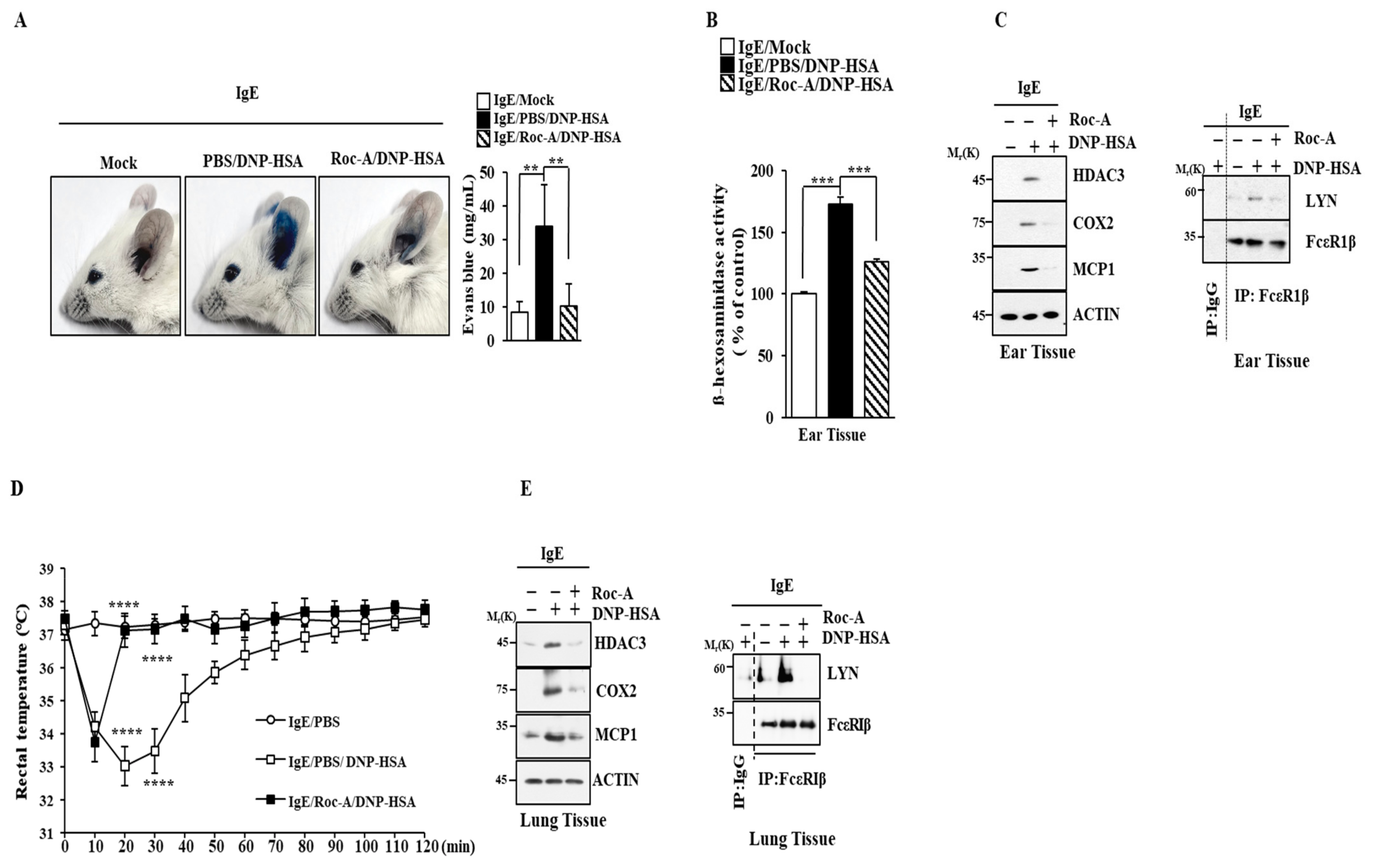
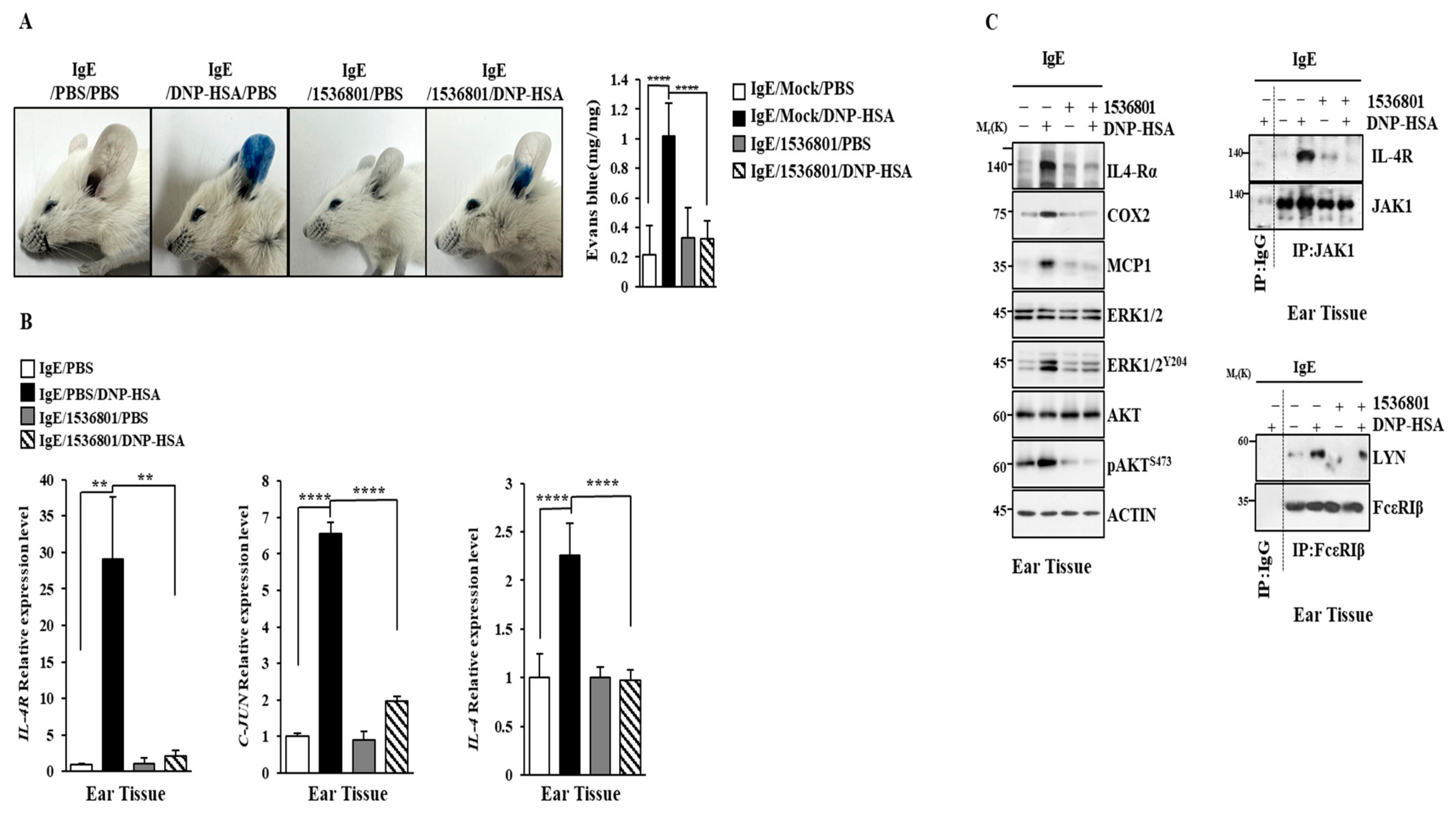
2.2. Roc-A Inhibits Anaphylaxis
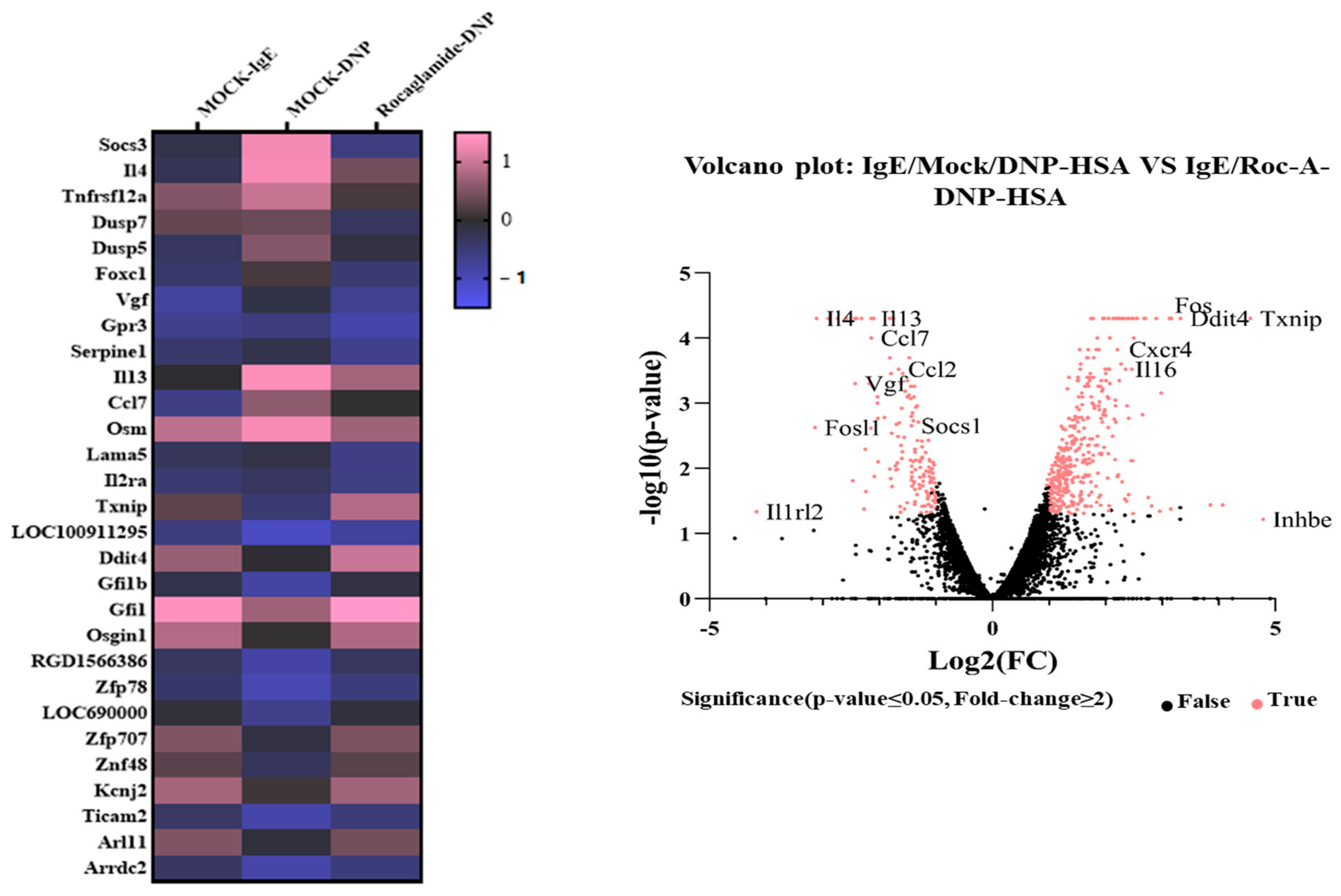
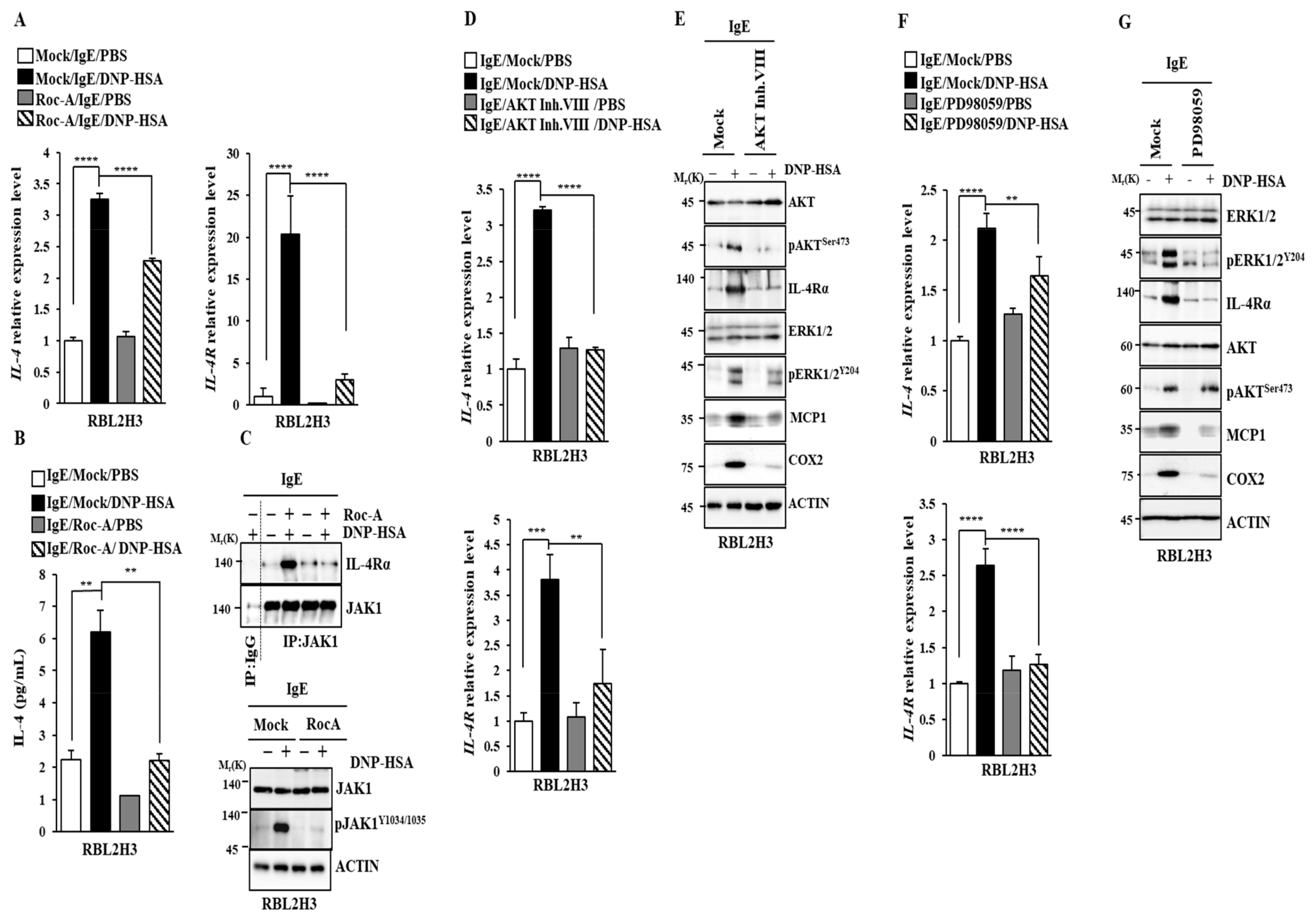
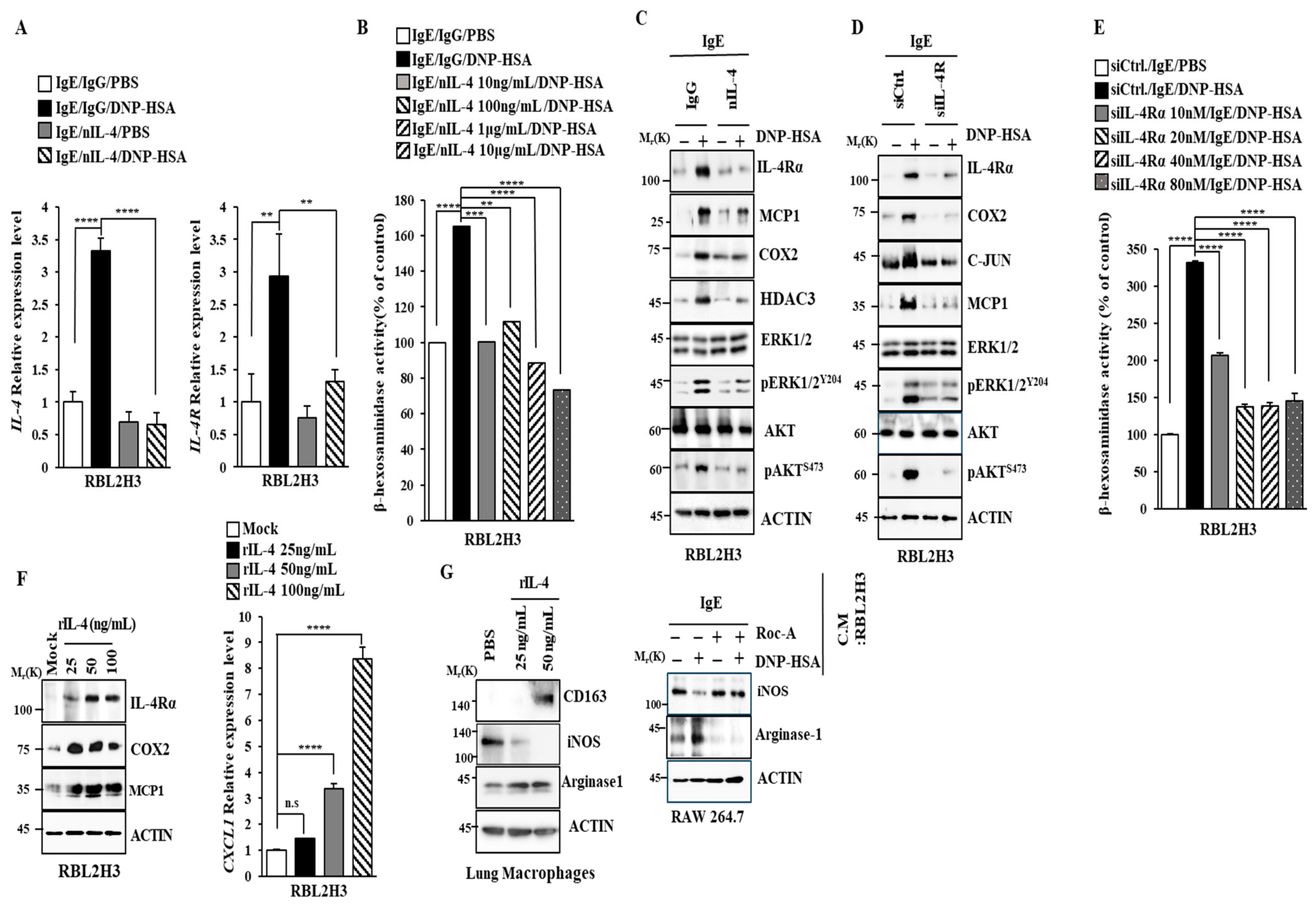
2.3. Roc-A Prevents Antigen from Increasing the Expression of IL-4 and IL-4R
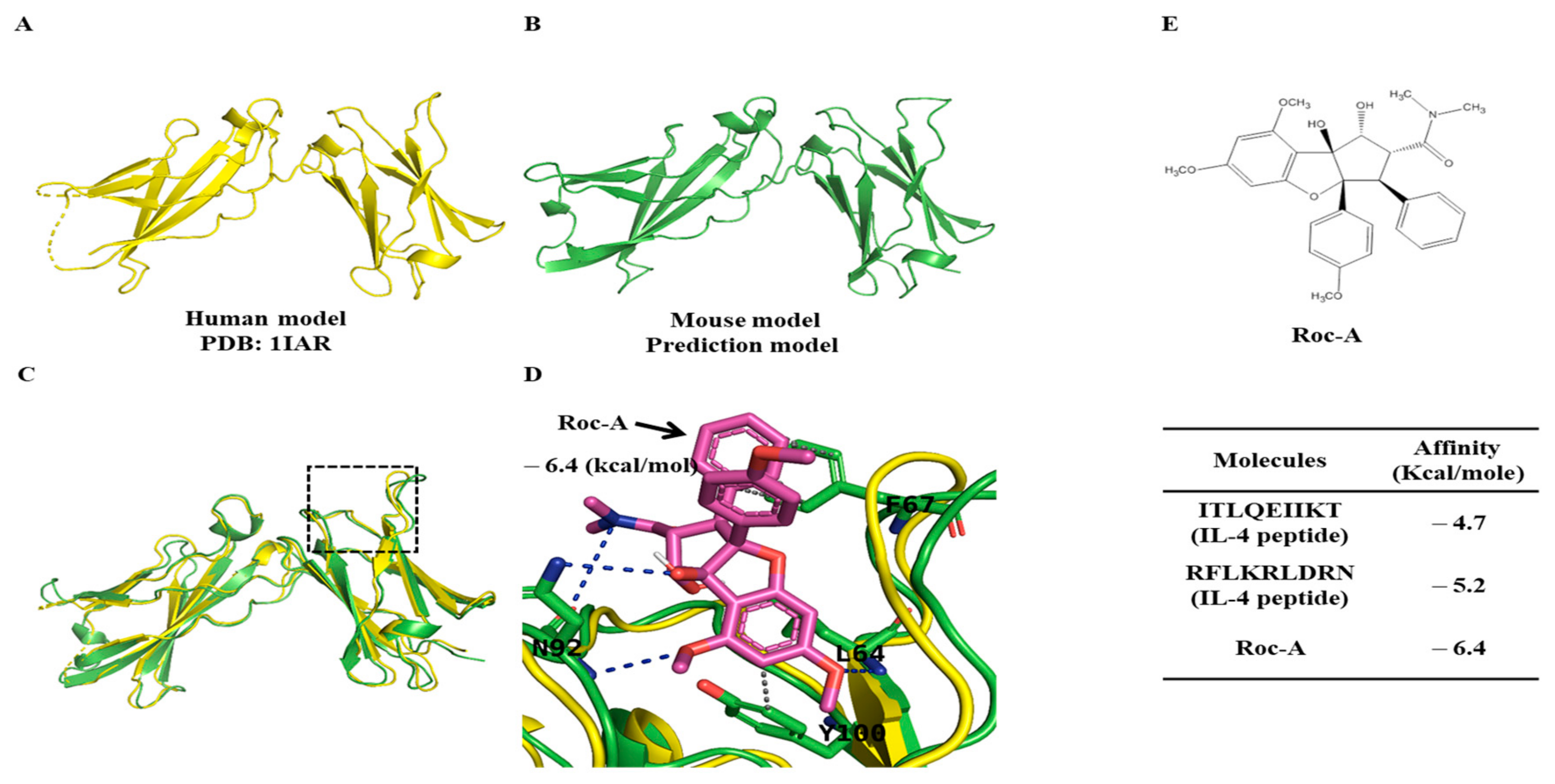
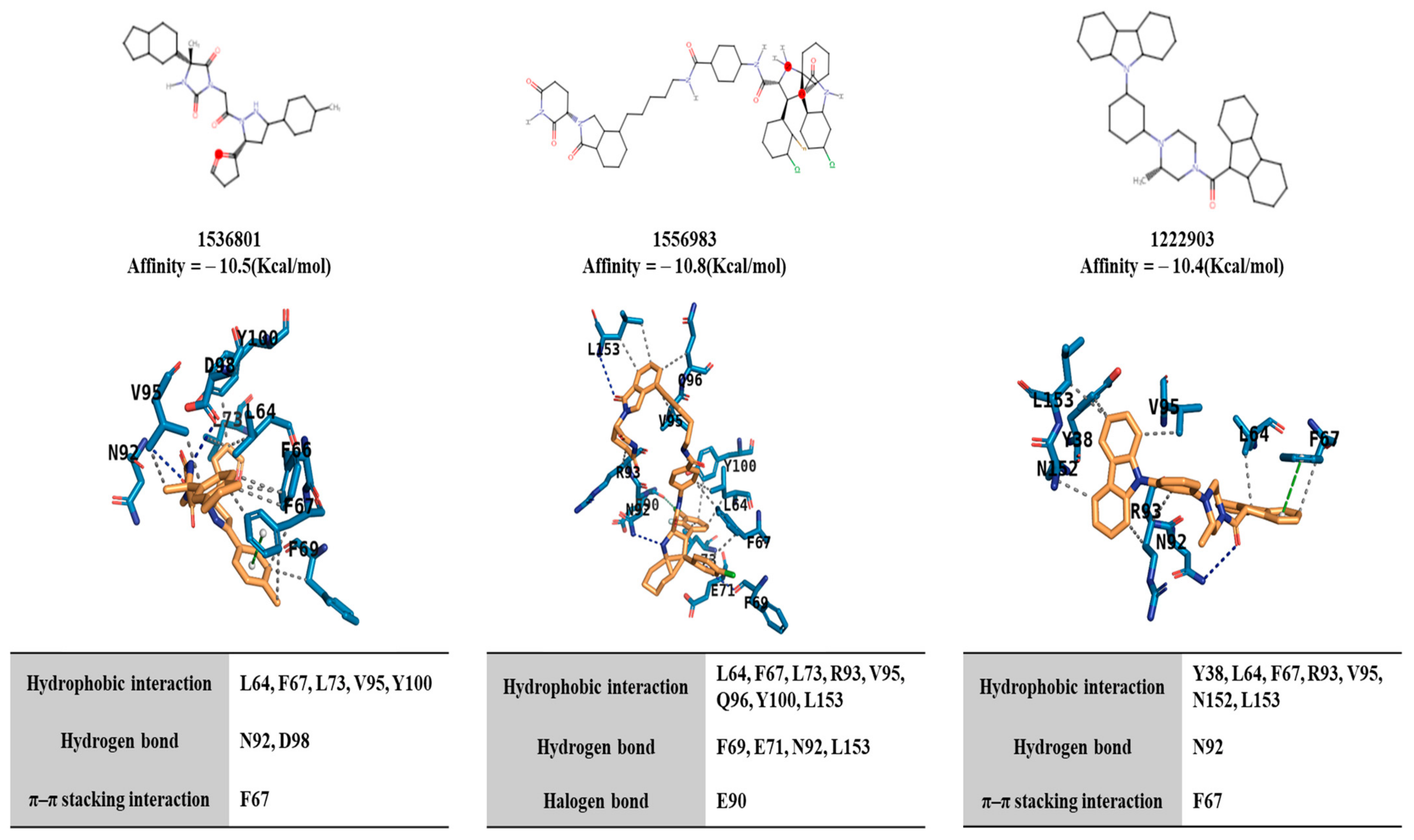
2.4. Potential Binding of Rocaglamide to IL-4R by Using In Silico Molecular Docking Analysis
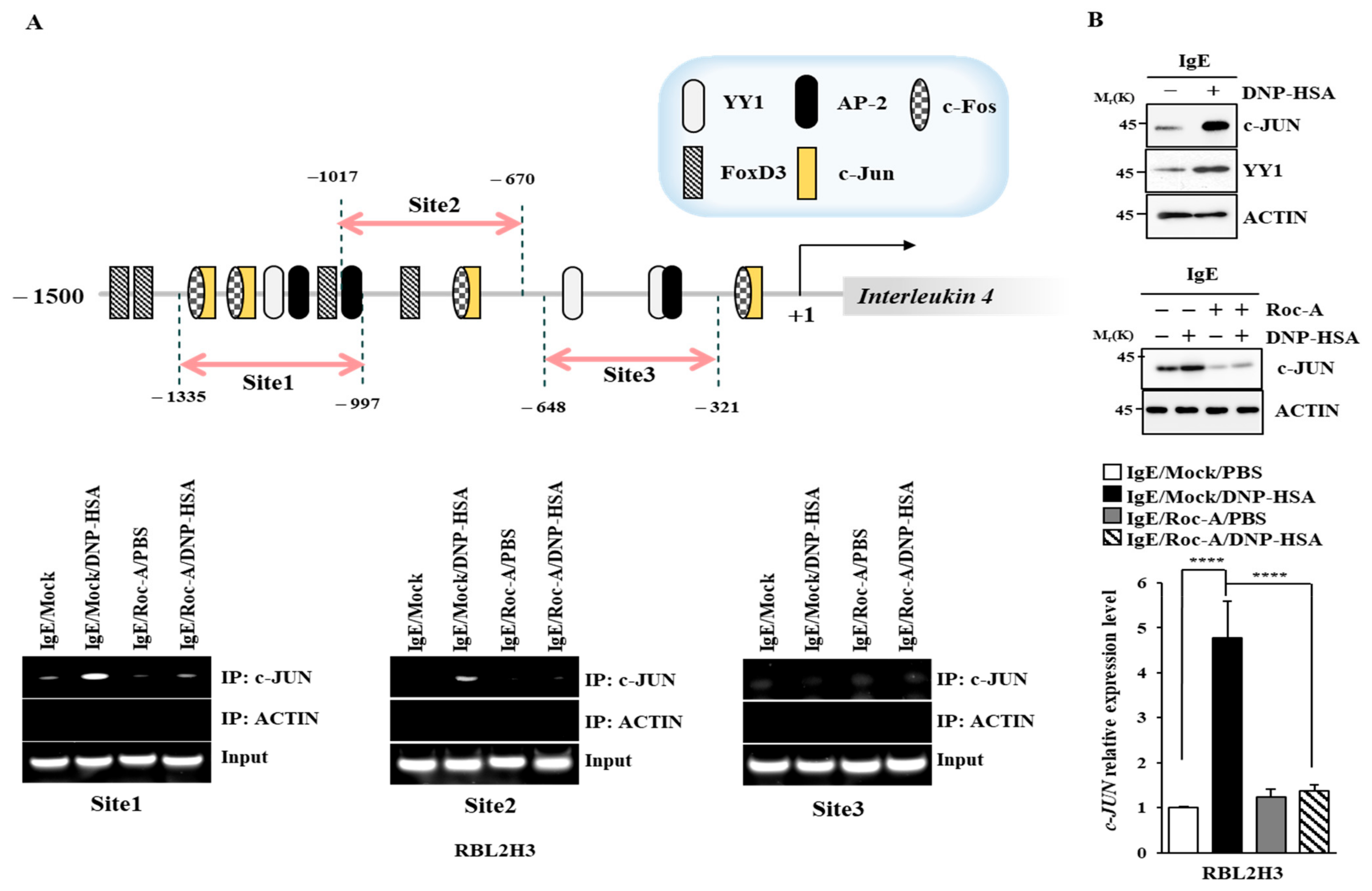
2.5. C-Jun Directly Regulates the Expression of IL-4 and IL-4R
2.6. IL-4 and IL-4R Mediate Allergic Reactions In Vitro
2.7. IL-4 and IL-4R Mediate Anaphylaxis
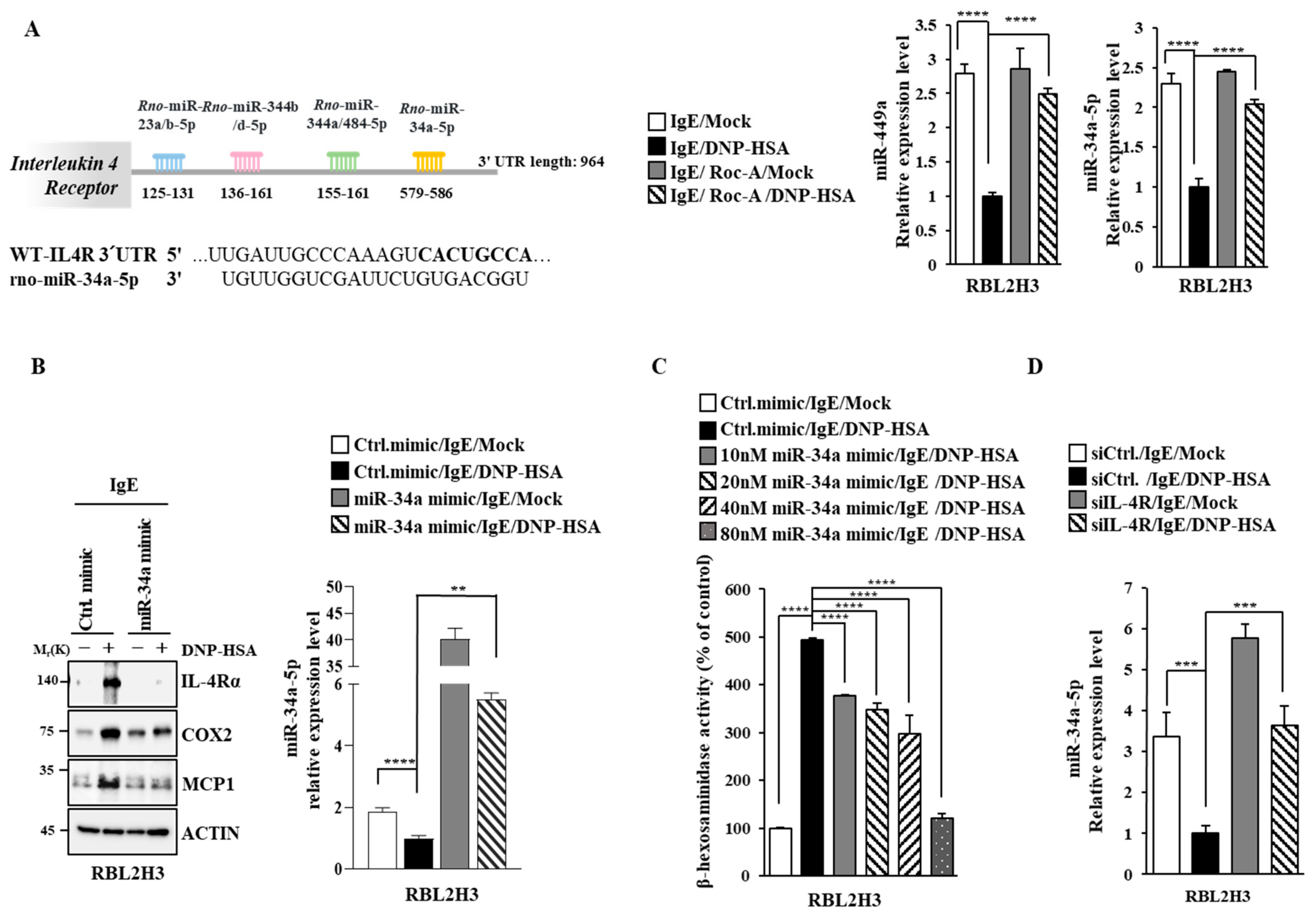
2.8. miR-34a Inhibits Allergic Reactions In Vitro by Decreasing IL-4R Expression
2.9. IL-4R Docking Chemical Inhibits Allergic Reactions In Vitro
2.10. IL-4R Docking Chemical Inhibits Anaphylaxis
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Animals
4.4. Reactive Oxygen Species Measurement
4.5. RNA Sequencing and Analysis
4.6. Transfections
4.7. β-Hexosaminidase Activity Assays
4.8. Quantitative Real-Time PCR
4.9. Chromatin Immunoprecipitation Assays
4.10. ELISA
4.11. Immunoblot and Immunoprecipitation
4.12. Passive Cutaneous Anaphylaxis
4.13. Passive Systemic Anaphylaxis
4.14. In Silico Molecular Docking Simulation
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, F.; Fang, J.; Yu, Y.; Hao, S.; Zou, Q.; Zeng, Q.; Yang, X. Reanalysis of ribosome profiling datasets reveals a function of rocaglamide A in perturbing the dynamics of translation elongation via eIF4A. Nat. Commun. 2023, 14, 553. [Google Scholar] [CrossRef] [PubMed]
- Howard, C.M.; Bearss, N.; Subramaniyan, B.; Tilley, A.; Sridharan, S.; Villa, N.; Fraser, C.S.; Raman, D. The CXCR4-LASP1-eIF4F Axis Promotes Translation of Oncogenic Proteins in Triple-Negative Breast Cancer Cells. Front. Oncol. 2019, 9, 284. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, S.; Robeson, M.; Bastihalli-Tukaramrao, D.; Howard, C.M.; Subramaniyan, B.; Tilley, A.M.C.; Tiwari, A.K.; Raman, D. Targeting of the Eukaryotic Translation Initiation Factor 4A Against Breast Cancer Stemness. Front. Oncol. 2019, 9, 1311. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, Y.; Chen, W.; Bai, S.; Peng, W.; Zheng, M.; Yang, Y.; Cheng, B.; Luan, Z. Targeted intervention of eIF4A1 inhibits EMT and metastasis of pancreatic cancer cells via c-MYC/miR-9 signaling. Cancer Cell Int. 2021, 21, 670. [Google Scholar] [CrossRef]
- Yurugi, H.; Marini, F.; Weber, C.; David, K.; Zhao, Q.; Binder, H.; Désaubry, L.; Rajalingam, K. Targeting prohibitins with chemical ligands inhibits KRAS-mediated lung tumours. Oncogene 2017, 36, 4778–4789. [Google Scholar] [CrossRef]
- Wilmore, S.; Rogers-Broadway, K.R.; Taylor, J.; Lemm, E.; Fell, R.; Stevenson, F.K.; Forconi, F.; Steele, A.J.; Coldwell, M.; Packham, G.; et al. Targeted inhibition of eIF4A suppresses B-cell receptor-induced translation and expression of MYC and MCL1 in chronic lymphocytic leukemia cells. Cell. Mol. Life Sci. 2021, 78, 6337–6349. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Lavrik, I.N.; Mahlknecht, U.; Giaisi, M.; Proksch, P.; Krammer, P.H.; Li-Weber, M. The traditional Chinese herbal compound rocaglamide preferentially induces apoptosis in leukemia cells by modulation of mitogen-activated protein kinase activities. Int. J. Cancer 2007, 121, 1839–1846. [Google Scholar] [CrossRef]
- Nalli, A.D.; Brown, L.E.; Thomas, C.L.; Sayers, T.J.; Porco, J.A., Jr.; Henrich, C.J. Sensitization of renal carcinoma cells to TRAIL-induced apoptosis by rocaglamide and analogs. Sci. Rep. 2018, 8, 17519. [Google Scholar] [CrossRef]
- Kim, M.; Park, Y.; Kwon, Y.; Kim, Y.; Byun, J.; Jeong, M.S.; Kim, H.U.; Jung, H.S.; Mun, J.Y.; Jeoung, D. MiR-135-5p-p62 Axis Regulates Autophagic Flux, Tumorigenic Potential, and Cellular Interactions Mediated by Extracellular Vesicles During Allergic Inflammation. Front. Immunol. 2019, 10, 738. [Google Scholar] [CrossRef]
- Kwon, Y.; Kim, M.; Kim, Y.; Jeong, M.S.; Jung, H.S.; Jeoung, D. EGR3-HDAC6-IL-27 Axis Mediates Allergic Inflammation and Is Necessary for Tumorigenic Potential of Cancer Cells Enhanced by Allergic Inflammation-Promoted Cellular Interactions. Front. Immunol. 2021, 12, 680441. [Google Scholar] [CrossRef]
- Yu, X.; Li, L.; Cai, B.; Zhang, W.; Liu, Q.; Li, N.; Shi, X.; Yu, L.; Chen, R.; Qiu, C. Single-cell analysis reveals alterations in cellular composition and cell-cell communication associated with airway inflammation and remodeling in asthma. Respir. Res. 2024, 25, 76. [Google Scholar] [CrossRef] [PubMed]
- So, L.; Obata-Ninomiya, K.; Hu, A.; Muir, V.S.; Takamori, A.; Song, J.; Buckner, J.H.; Savan, R.; Ziegler, S.F. Regulatory T cells suppress CD4+ effector T cell activation by controlling protein synthesis. J. Exp. Med. 2023, 220, e20221676. [Google Scholar] [CrossRef] [PubMed]
- Galván Morales, M.A.; Montero-Vargas, J.M.; Vizuet-de-Rueda, J.C.; Teran, L.M. New Insights into the Role of PD-1 and Its Ligands in Allergic Disease. Int. J. Mol. Sci. 2021, 22, 11898. [Google Scholar] [CrossRef] [PubMed]
- Ogino, H.; Okuno, T.; Murano, K.; Ueno, H. Naturally Oxidized Olive Oil Promotes Active Cutaneous Anaphylaxis and Th2 Cytokine Production. Biol. Pharm. Bull. 2021, 44, 838–843. [Google Scholar] [CrossRef]
- Ashley, S.E.; Jones, A.C.; Anderson, D.; Holt, P.G.; Bosco, A.; Tang, M.L.K. Remission of peanut allergy is associated with rewiring of allergen-driven T helper 2-related gene networks. Allergy 2022, 77, 3015–3027. [Google Scholar] [CrossRef]
- Kim, S.D.; Kang, S.A.; Mun, S.J.; Yu, H.S.; Roh, H.J.; Cho, K.S. SCGB1C1 Plays a Critical Role in Suppression of Allergic Airway Inflammation through the Induction of Regulatory T Cell Expansion. Int. J. Mol. Sci. 2024, 25, 6282. [Google Scholar] [CrossRef]
- Chen, C.J.; Wang, H.C.; Hou, Y.C.; Wu, Y.Y.; Shieh, C.C.; Shan, Y.S. Blocking M2-like macrophage polarization using decoy oligodeoxynucleotide-based gene therapy prevents immune evasion for pancreatic cancer treatment. Mol. Cancer Ther. 2024, 23, 1431–1445. [Google Scholar] [CrossRef]
- Oh, S.G.; Choi, J.Y.; Lee, J.E.; Jeon, S.; Lee, B.R.; Son, K.H.; Lee, S.B.; An, B.S.; Hwang, D.Y.; Kim, S.J.; et al. Visualizing mast cell migration to tumor sites using sodium iodide symporter of nuclear medicine reporter gene. Neoplasia 2023, 43, 100925. [Google Scholar] [CrossRef]
- Chlastáková, A.; Kaščáková, B.; Kotál, J.; Langhansová, H.; Kotsyfakis, M.; Kutá Smatanová, I.; Tirloni, L.; Chmelař, J. Iripin-1, a new anti-inflammatory tick serpin, inhibits leukocyte recruitment in vivo while altering the levels of chemokines and adhesion molecules. Front. Immunol. 2023, 14, 1116324. [Google Scholar] [CrossRef]
- Weng, C.M.; Lee, M.J.; Chao, W.; Lin, Y.R.; Chou, C.J.; Chen, M.C.; Chou, C.L.; Tsai, I.L.; Lin, C.H.; Fan Chung, K.; et al. Airway epithelium IgE-FcεRI cross-link induces epithelial barrier disruption in severe T2-high asthma. Mucosal Immunol. 2023, 16, 685–698. [Google Scholar] [CrossRef]
- Tu, W.; Xiao, X.; Lu, J.; Liu, X.; Wang, E.; Yuan, R.; Wan, R.; Shen, Y.; Xu, D.; Yang, P.; et al. Vanadium exposure exacerbates allergic airway inflammation and remodeling through triggering reactive oxidative stress. Front. Immunol. 2023, 13, 1099509. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.; Dijoux, E.; Cheminant, M.A.; Intes, L.; Bouchaud, G. GliSODin® prevents airway inflammation by inhibiting T-cell differentiation and activation in a mouse model of asthma. Front. Allergy 2023, 4, 1199355. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, L.; Kalm, F.; Björkander, S.; Melén, E.; Lundahl, J.; Nopp, A. Sequential engagement of adhesion molecules and cytokine receptors impacts both piecemeal and anaphylactic degranulation of human basophils. Immunology 2024, 171, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Fan, Y.J.; Nguyen, T.V.; Yu, Z.N.; Song, C.H.; Lee, S.Y.; Shin, H.S.; Chai, O.H. Fallopia japonica Root Extract Ameliorates Ovalbumin-Induced Airway Inflammation in a CARAS Mouse Model by Modulating the IL-33/TSLP/NF-κB Signaling Pathway. Int. J. Mol. Sci. 2023, 24, 12514. [Google Scholar] [CrossRef]
- Elesela, S.; Arzola-Martínez, L.; Rasky, A.; Ptaschinski, C.; Hogan, S.P.; Lukacs, N.W. Mucosal IgA immune complex induces immunomodulatory responses in allergic airway and intestinal TH2 disease. J. Allergy Clin. Immunol. 2023, 152, 1607–1618. [Google Scholar] [CrossRef]
- Uchida, A.M.; Ro, G.; Qiang, L.; Peterson, K.A.; Round, J.; Dougan, M.; Dougan, S.K. Human differentiated eosinophils release IL-13 in response to IL-33 stimulation. Front. Immunol. 2022, 13, 946643. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, Y.; Dang, B.; Wang, Y.; Zheng, G.; Zhang, T.; An, H. Myricetin alleviated immunologic contact urticaria and mast cell degranulation via the PI3K/Akt/NF-κB pathway. Phytother. Res. 2023, 37, 2024–2035. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, W.; Wu, X. Isorhynchophylline exerts anti-asthma effects in mice by inhibiting the proliferation of airway smooth muscle cells: The involvement of miR-200a-mediated FOXC1/NF-κB pathway. Biochem. Biophys. Res. Commun. 2020, 521, 1055–1060. [Google Scholar] [CrossRef]
- Delgado-Dolset, M.I.; Obeso, D.; Rodríguez-Coira, J.; Tarin, C.; Tan, G.; Cumplido, J.A.; Cabrera, A.; Angulo, S.; Barbas, C.; Sokolowska, M.; et al. Understanding uncontrolled severe allergic asthma by integration of omic and clinical data. Allergy 2022, 77, 1772–1785. [Google Scholar] [CrossRef]
- Bitton, A.; Avlas, S.; Reichman, H.; Itan, M.; Karo-Atar, D.; Azouz, N.P.; Rozenberg, P.; Diesendruck, Y.; Nahary, L.; Rothenberg, M.E.; et al. A key role for IL-13 signaling via the type 2 IL-4 receptor in experimental atopic dermatitis. Sci. Immunol. 2020, 5, eaaw2938. [Google Scholar] [CrossRef]
- Ramsey, N.; Kazmi, W.; Phelan, M.; Lozano-Ojalvo, D.; Berin, M.C. JAK1 inhibition with abrocitinib decreases allergen-specific basophil and T-cell activation in pediatric peanut allergy. J Allergy Clin. Immunol. Glob. 2023, 2, 100103. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.X.; Tang, X.; Zhu, J.Y.; Zhang, W.; Tang, W.Q.; Yan, J.; Xu, X.; Liang, H.P. Cytochrome P450 1A1 enhances Arginase-1 expression, which reduces LPS-induced mouse peritonitis by targeting JAK1/STAT6. Cell. Immunol. 2020, 349, 104047. [Google Scholar] [CrossRef]
- Kim, M.; Jo, H.; Kwon, Y.; Jeong, M.S.; Jung, H.S.; Kim, Y.; Jeoung, D. MiR-154-5p-MCP1 Axis Regulates Allergic Inflammation by Mediating Cellular Interactions. Front. Immunol. 2021, 12, 663726. [Google Scholar] [CrossRef] [PubMed]
- Crozier, R.W.E.; Fajardo, V.A.; MacNeil, A.J. Targeting glycogen synthase kinase 3 with CHIR99021 negatively regulates allergen-induced mast cell activation. Eur. J. Immunol. 2023, 53, e2250104. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.S.; Jang, J.H.; Lee, Y.; Park, H.S. Long-term efficacy of anti-IL-4 receptor antibody in a patient with aspirin-exacerbated respiratory disease and IgG4-related disease. Allergy Asthma Clin. Immunol. 2023, 19, 67. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Castillo, J.M.; McGurk, A.; Strakosha, M.; Vega-Mendoza, D.; Smith, S.E.M.; Stafstrom, K.; Elkins, M.; Chou, J.; Wang, Y.H.; Geha, R.S. IL-4 receptor alpha blockade dampens allergic inflammation and upregulates IL-17A expression to promote Saureus clearance in antigen sensitized mouse skin. J. Allergy Clin. Immunol. 2023, 152, 907–915. [Google Scholar] [CrossRef]
- Ogulur, I.; Pat, Y.; Ardicli, O.; Barletta, E.; Cevhertas, L.; Fernandez-Santamaria, R.; Huang, M.; Bel Imam, M.; Koch, J.; Ma, S.; et al. Advances and highlights in biomarkers of allergic diseases. Allergy 2021, 76, 3659–3686. [Google Scholar] [CrossRef]
- Noval Rivas, M.; Burton, O.T.; Oettgen, H.C.; Chatila, T. IL-4 production by group 2 innate lymphoid cells promotes food allergy by blocking regulatory T-cell function. J. Allergy Clin. Immunol. 2016, 138, 801–811. [Google Scholar] [CrossRef]
- Bruton, K.; Spill, P.; Vohra, S.; Baribeau, O.; Manzoor, S.; Gadkar, S.; Davidson, M.; Walker, T.D.; Koenig, J.F.E.; Ellenbogen, Y.; et al. Interrupting reactivation of immunologic memory diverts the allergic response and prevents anaphylaxis. J. Allergy Clin. Immunol. 2021, 147, 1381–1392. [Google Scholar] [CrossRef]
- Russkamp, D.; Aguilar-Pimentel, A.; Alessandrini, F.; Gailus-Durner, V.; Fuchs, H.; Ohnmacht, C.; Chaker, A.; de Angelis, M.H.; Ollert, M.; Schmidt-Weber, C.B.; et al. IL-4 receptor α blockade prevents sensitization and alters acute and long-lasting effects of allergen-specific immunotherapy of murine allergic asthma. Allergy 2019, 74, 1549–1560. [Google Scholar] [CrossRef]
- Kim, E.G.; Leem, J.S.; Baek, S.M.; Kim, H.R.; Kim, K.W.; Kim, M.N.; Sohn, M.H. Interleukin-18 Receptor α Modulates the T Cell Response in Food Allergy. Allergy Asthma Immunol. Res. 2022, 14, 424–438. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Lv, J.; Ge, D.; Bai, H.; Yang, Y.; Wu, J. Heme oxygenase-1 alleviates eosinophilic inflammation by inhibiting STAT3-SOCS3 signaling. Pediatr. Pulmonol. 2020, 55, 1440–1447. [Google Scholar] [CrossRef] [PubMed]
- Santana, F.P.R.; da Silva, R.C.; Ponci, V.; Pinheiro, A.J.M.C.R.; Olivo, C.R.; Caperuto, L.C.; Arantes-Costa, F.M.; Claudio, S.R.; Ribeiro, D.A.; Tibério, I.F.L.C.; et al. Dehydrodieugenol improved lung inflammation in an asthma model by inhibiting the STAT3/SOCS3 and MAPK pathways. Biochem. Pharmacol. 2020, 180, 114175. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Rasool, R.; Shafi, T.; Gull, A.; Jan, R.; Bhat, I.A.; Haq, M.G.; Shah, Z.A. Gene variants and mRNA expression analysis of SOCS3 and its association with serum IL-4 levels in atopic diseases. Immunobiology 2023, 228, 152387. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Oliveira, A.R.; Aquino-Junior, J.; Abbasi, A.; Santos-Dias, A.; Oliveira-Junior, M.C.; Alberca-Custodio, R.W.; Rigonato-Oliveira, N.C.; Salles-Dias, L.P.; Damaceno-Rodrigues, N.R.; Caldini, E.G.; et al. Effects of aerobic exercise on molecular aspects of asthma: Involvement of SOCS-JAK-STAT. Exerc. Immunol. Rev. 2019, 25, 50–62. [Google Scholar]
- Santos, S.S.; Miranda, V.C.; Trindade, L.M.; Cardoso, V.N.; Reis, D.C.; Cassali, G.D.; Nicoli, J.R.; Cara, D.C.; Martins, F.S. Bifidobacterium longum subsp. longum 51A Attenuates Signs of Inflammation in a Murine Model of Food Allergy. Probiotics Antimicrob. Proteins 2023, 15, 63–73. [Google Scholar] [CrossRef]
- Yu, J.; Huang, L.; Cao, L. M2 macrophages regulate KDM6B/PFKFB2 metabolic reprogramming of cervical squamous cell carcinoma through CXCL1. Cell. Mol. Biol. 2024, 70, 78–84. [Google Scholar]
- Wang, N.; Wang, J.; Zhang, Y.; Zeng, Y.; Hu, S.; Bai, H.; Hou, Y.; Wang, C.; He, H.; He, L. Imperatorin ameliorates mast cell-mediated allergic airway inflammation by inhibiting MRGPRX2 and CamKII/ERK signaling pathway. Biochem. Pharmacol. 2021, 184, 114401. [Google Scholar] [CrossRef]
- Liang, X.; Li, X.; Sun, S.; Zhang, H.; Wang, B.; Xu, F.; Zhang, Y.; Liu, Z. Effects and potential mechanisms of Saposhnikovia divaricata (Turcz.) Schischk. On type I allergy and pseudoallergic reactions in vitro and in vivo. J. Ethnopharmacol. 2024, 318, 116942. [Google Scholar] [CrossRef]
- Wang, L.; Fang, Y.; Ma, Y.; Zhao, Z.; Ma, R.; Zhang, Y.; Qiao, Y.; Wang, X.; Zhang, Y. A novel natural Syk inhibitor suppresses IgE-mediated mast cell activation and passive cutaneous anaphylaxis. Bioorganic Chem. 2024, 146, 107320. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, J.; Zhang, L.; Zhang, Z.; He, L.; Mou, Y.; Deng, Y.; Cao, Y.; Yang, P.; Su, Y.; et al. Role of C/EBP homologous protein and endoplasmic reticulum stress in asthma exacerbation by regulating the IL-4/signal transducer and activator of transcription 6/transcription factor EC/IL-4 receptor α positive feedback loop in M2 macrophages. J. Allergy Clin. Immunol. 2017, 140, 1550–1561. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.N.; Lee, J.W.; Ryu, H.W.; Lee, J.K.; Oh, E.S.; Kim, D.Y.; Ro, H.; Yoon, D.; Park, J.Y.; Hong, S.T.; et al. Black Ginseng Extract Exerts Potentially Anti-Asthmatic Activity by Inhibiting the Protein Kinase Cθ-Mediated IL-4/STAT6 Signaling Pathway. Int. J. Mol. Sci. 2023, 24, 11970. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Long, Q.; Zhang, W.; Zeng, D.; Hu, B.; Liu, S.; Chen, L. miRNA-221-3p derived from M2-polarized tumor-associated macrophage exosomes aggravates the growth and metastasis of osteosarcoma through SOCS3/JAK2/STAT3 axis. Aging 2021, 13, 19760–19775. [Google Scholar] [CrossRef] [PubMed]
- Zak, M.; Hanan, E.J.; Lupardus, P.; Brown, D.G.; Robinson, C.; Siu, M.; Lyssikatos, J.P.; Romero, F.A.; Zhao, G.; Kellar, T.; et al. Discovery of a class of highly potent Janus Kinase 1/2 (JAK1/2) inhibitors demonstrating effective cell-based blockade of IL-13 signaling. Bioorganic Med. Chem. Lett. 2019, 29, 1522–1531. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Zhao, B.; Zhang, D.; Rui, X.; Hou, X.; Chen, X.; Zhang, B.; Yuan, Y.; Deng, H.; Ge, G. Viola yedoensis Makino formula alleviates DNCB-induced atopic dermatitis by activating JAK2/STAT3 signaling pathway and promoting M2 macrophages polarization. Phytomedicine 2022, 103, 154228. [Google Scholar] [CrossRef]
- Bao, J.; Zhang, P.; Wu, B.; Wang, J.; Li, S.; Li, A.; Jie, Y. Gp130 Promotes Inflammation via the STAT3/JAK2 Pathway in Allergic Conjunctivitis. Investig. Ophthalmol. Vis. Sci. 2023, 64, 5. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, Y.; Zhang, Y.; Dang, B.; Hu, S.; Zhao, C.; Huang, Y.; Zheng, G.; Ma, T.; Zhang, T. Alpha-linolenic acid improves nasal mucosa epithelial barrier function in allergic rhinitis by arresting CD4+ T cell differentiation via IL-4Rα-JAK2-STAT3 pathway. Phytomedicine 2023, 116, 154825. [Google Scholar] [CrossRef]
- Bai, H.; Xue, Z.; Zhang, W.; Feng, C.; Zhou, Z.; Hu, S.; Zhang, Y.; Qin, Q.; Wu, Y.; Sun, X.; et al. α-Asarone alleviates allergic asthma by stabilizing mast cells through inhibition of ERK/JAK2-STAT3 pathway. Biofactors 2023, 49, 140–152. [Google Scholar] [CrossRef]
- Wang, X.; Xu, C.; Cai, Y.; Zou, X.; Chao, Y.; Yan, Z.; Zou, C.; Wu, X.; Tang, L. CircZNF652 promotes the goblet cell metaplasia by targeting the miR-452-5p/JAK2 signaling pathway in allergic airway epithelia. J. Allergy Clin. Immunol. 2022, 150, 192–203. [Google Scholar] [CrossRef]
- Nguyen, V.; Zhang, Q.; Pan, F.; Jin, Q.; Sun, M.; Tangthianchaichana, J.; Du, S.; Lu, Y. Zi-Su-Zi decoction improves airway hyperresponsiveness in cough-variant asthma rat model through PI3K/AKT1/mTOR, JAK2/STAT3 and HIF-1α/NF-κB signaling pathways. J. Ethnopharmacol. 2023, 314, 116637. [Google Scholar] [CrossRef]
- Yao, C.; Ni, Z.; Gong, C.; Zhu, X.; Wang, L.; Xu, Z.; Zhou, C.; Li, S.; Zhou, W.; Zou, C.; et al. Rocaglamide enhances NK cell-mediated killing of non-small cell lung cancer cells by inhibiting autophagy. Autophagy 2018, 14, 1831–1844. [Google Scholar] [CrossRef] [PubMed]
- Hussein, N.A.; Abdel Gawad, H.S.; Maklad, H.M.; El-Fakharany, E.M.; Aly, R.G.; Samy, D.M. Empagliflozin inhibits autophagy and mitigates airway inflammation and remodelling in mice with ovalbumin-induced allergic asthma. Eur. J. Pharmacol. 2023, 950, 175701. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, H.; Katsumoto, R.; Iida, S.; Miyake, T.; Higuchi, T.; Nagashima, T.; Nagayasu, K.; Nakagawa, T.; Kaneko, S. Sphingosine-1-phosphate induces Ca2+ signaling and CXCL1 release via TRPC6 channel in astrocytes. Glia 2017, 65, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Arenas, Y.M.; Balzano, T.; Ivaylova, G.; Llansola, M.; Felipo, V. The S1PR2-CCL2-BDNF-TrkB pathway mediates neuroinflammation and motor incoordination in hyperammonaemia. Neuropathol. Appl. Neurobiol. 2022, 48, e12799. [Google Scholar] [CrossRef]
- Xiang, P.; Chew, W.S.; Seow, W.L.; Lam, B.W.S.; Ong, W.Y.; Herr, D.R. The S1P2 receptor regulates blood-brain barrier integrity and leukocyte extravasation with implications for neurodegenerative disease. Neurochem. Int. 2021, 146, 105018. [Google Scholar] [CrossRef]
- Liu, H.; Li, L.; Chen, Z.; Song, Y.; Liu, W.; Gao, G.; Li, L.; Jiang, J.; Xu, C.; Yan, G.; et al. S1PR2 Inhibition Attenuates Allergic Asthma Possibly by Regulating Autophagy. Front. Pharmacol. 2021, 11, 598007. [Google Scholar] [CrossRef]
- Oskeritzian, C.A.; Hait, N.C.; Wedman, P.; Chumanevich, A.; Kolawole, E.M.; Price, M.M.; Falanga, Y.T.; Harikumar, K.B.; Ryan, J.J.; Milstien, S.; et al. The sphingosine-1-phosphate/sphingosine-1-phosphate receptor 2 axis regulates early airway T-cell infiltration in murine mast cell-dependent acute allergic responses. J. Allergy Clin. Immunol. 2015, 135, 1008–1018. [Google Scholar] [CrossRef]
- Chumanevich, A.; Wedman, P.; Oskeritzian, C.A. Sphingosine-1-Phosphate/Sphingosine-1-Phosphate Receptor 2 Axis Can Promote Mouse and Human Primary Mast Cell Angiogenic Potential through Upregulation of Vascular Endothelial Growth Factor-A and Matrix Metalloproteinase-2. Mediat. Inflamm. 2016, 2016, 1503206. [Google Scholar] [CrossRef]
- Okamoto, Y.; Kitakaze, K.; Takenouchi, Y.; Yamamoto, S.; Ishimaru, H.; Tsuboi, K. Sphingosine 1-phosphate receptor type 2 positively regulates interleukin (IL)-4/IL-13-induced STAT6 phosphorylation. Cell. Signal. 2021, 88, 110156. [Google Scholar] [CrossRef]
- Park, S.J.; Lee, K.P.; Kang, S.; Lee, J.; Sato, K.; Chung, H.Y.; Okajima, F.; Im, D.S. Sphingosine 1-phosphate induced anti-atherogenic and atheroprotective M2 macrophage polarization through IL-4. Cell. Signal. 2014, 26, 2249–2258. [Google Scholar] [CrossRef]
- Kwon, Y.; Choi, Y.; Kim, M.; Jo, H.; Jeong, M.S.; Jung, H.S.; Jeoung, D. HDAC6-MYCN-CXCL3 axis mediates allergic inflammation and is necessary for allergic inflammation-promoted cellular interactions. Mol. Immunol. 2024, 166, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript Assembly and Quantification by RNA-Seq Reveals Unannotated Transcripts and Isoform Switching During Cell Differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Hage, T.; Sebald, W.; Reinemer, P. Crystal structure of the interleukin-4/receptor alpha chain complex reveals a mosaic binding interface. Cell 1999, 97, 271–281. [Google Scholar] [CrossRef]
- Adasme, M.F.; Linnemann, K.L.; Bolz, S.N.; Kaiser, F.; Salentin, S.; Haupt, V.J.; Schroeder, M. PLIP 2021: Expanding the scope of the protein–ligand interaction profiler to DNA and RNA. Nucleic Acids Res. 2021, 49, W530–W534. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, H.; Kim, M.; Jeoung, J.; Kim, W.; Park, Y.H.; Jung, H.S.; Lee, W.; Jeoung, D. Rocaglamide Suppresses Allergic Reactions by Regulating IL-4 Receptor Signaling. Molecules 2025, 30, 840. https://doi.org/10.3390/molecules30040840
Jo H, Kim M, Jeoung J, Kim W, Park YH, Jung HS, Lee W, Jeoung D. Rocaglamide Suppresses Allergic Reactions by Regulating IL-4 Receptor Signaling. Molecules. 2025; 30(4):840. https://doi.org/10.3390/molecules30040840
Chicago/Turabian StyleJo, Hyein, Misun Kim, Jaewhoon Jeoung, Wonho Kim, Yoon Ho Park, Hyun Suk Jung, Wook Lee, and Dooil Jeoung. 2025. "Rocaglamide Suppresses Allergic Reactions by Regulating IL-4 Receptor Signaling" Molecules 30, no. 4: 840. https://doi.org/10.3390/molecules30040840
APA StyleJo, H., Kim, M., Jeoung, J., Kim, W., Park, Y. H., Jung, H. S., Lee, W., & Jeoung, D. (2025). Rocaglamide Suppresses Allergic Reactions by Regulating IL-4 Receptor Signaling. Molecules, 30(4), 840. https://doi.org/10.3390/molecules30040840






