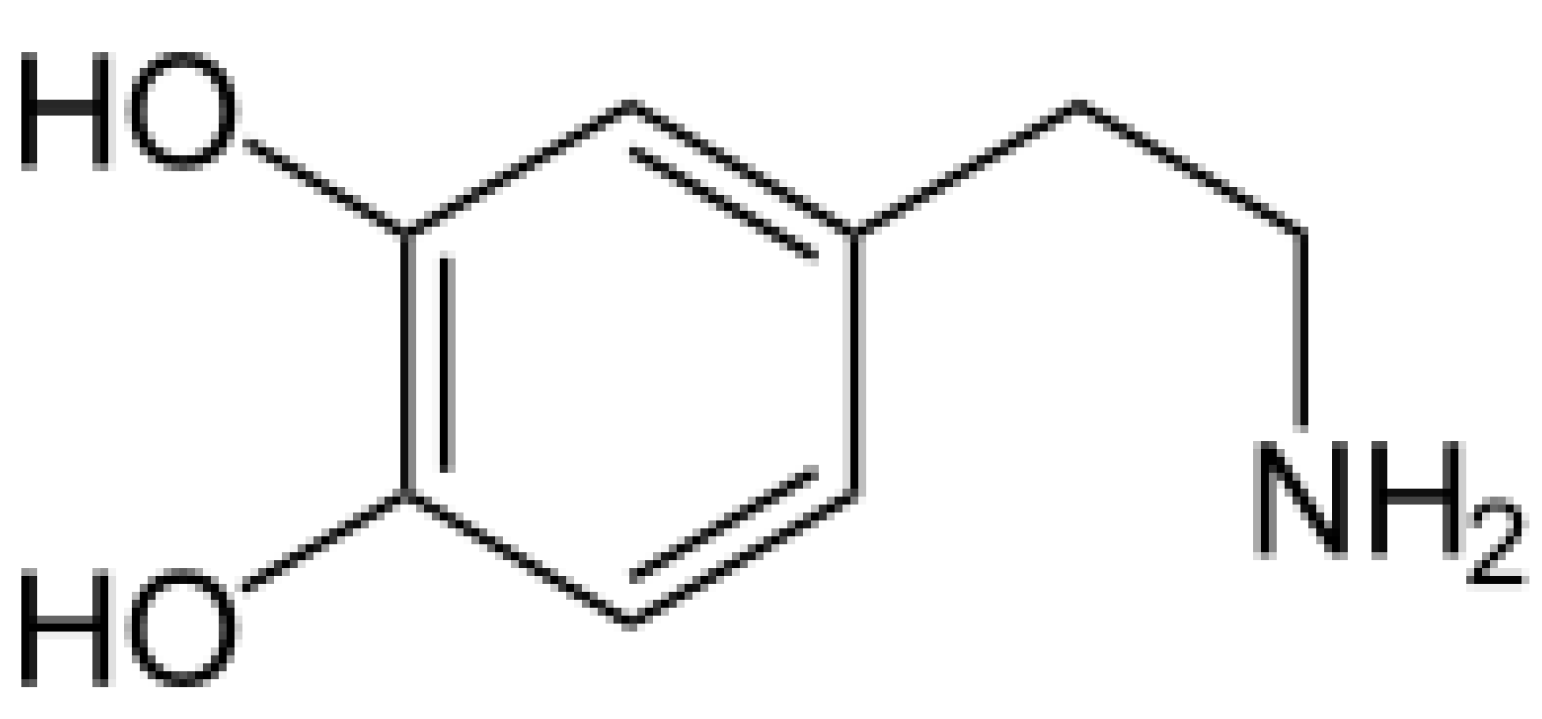
Scheme 1.
2-(3,4-Dihydroxyphenyl)ethylamine (dopamine).
Scheme 1.
2-(3,4-Dihydroxyphenyl)ethylamine (dopamine).
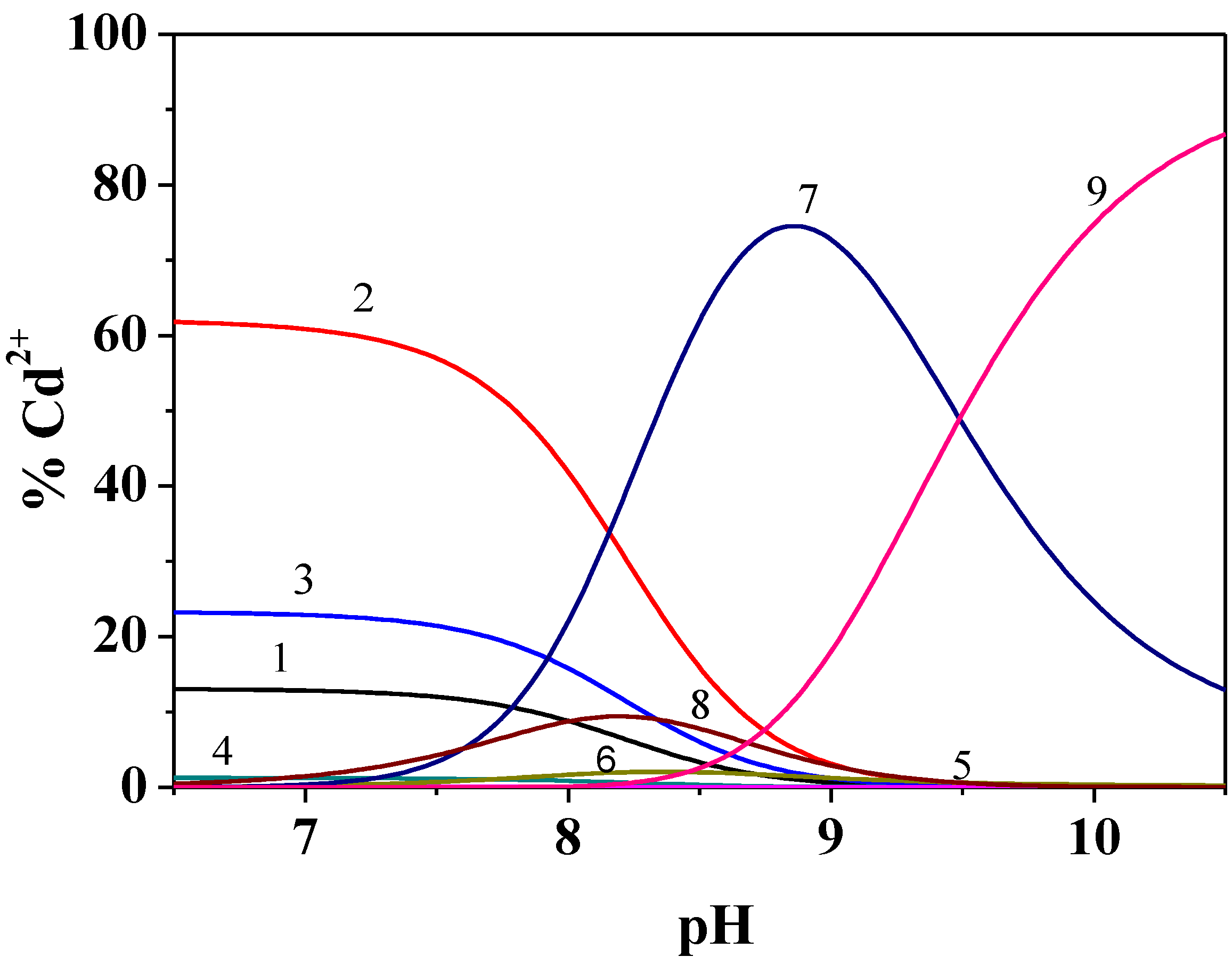
Figure 1.
Distribution diagram of the species of Cd2+/Dop− system at I = 0.15 mol dm−3 and T = 298.15 K (the formation percentages of the CdCli species are also reported). Species 1 free Cd2+; 2 CdCl; 3 CdCl2; 4 CdCl3; 5 CdCl4; 6 CdOHCl; 7 CdDop; 8 CdDopH; 9 CdDop2. Experimental conditions: cCd2+ = 1.5 mmol dm−3; cDOP− = 3.0 mmol dm−3. Charges omitted for simplicity.
Figure 1.
Distribution diagram of the species of Cd2+/Dop− system at I = 0.15 mol dm−3 and T = 298.15 K (the formation percentages of the CdCli species are also reported). Species 1 free Cd2+; 2 CdCl; 3 CdCl2; 4 CdCl3; 5 CdCl4; 6 CdOHCl; 7 CdDop; 8 CdDopH; 9 CdDop2. Experimental conditions: cCd2+ = 1.5 mmol dm−3; cDOP− = 3.0 mmol dm−3. Charges omitted for simplicity.
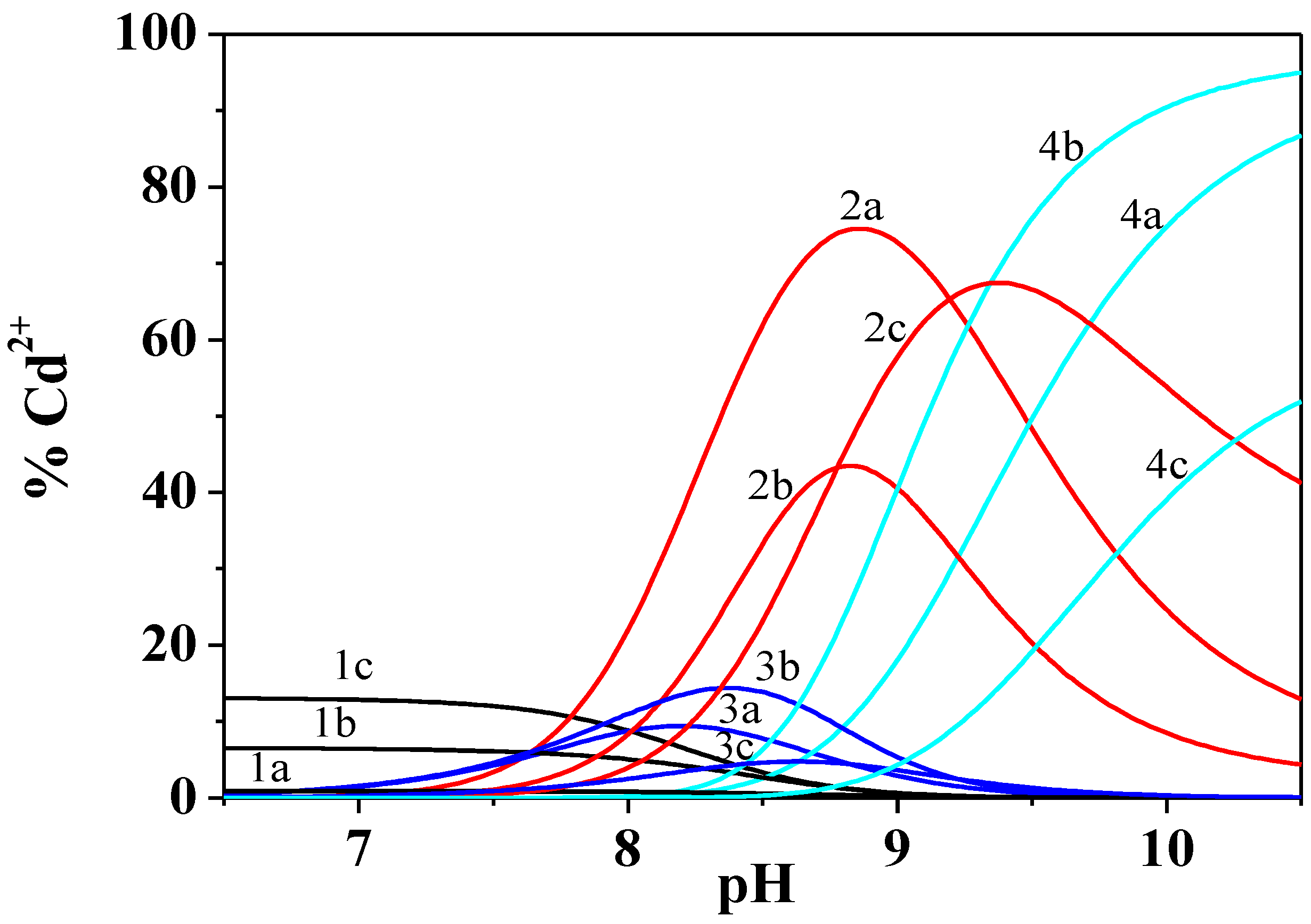
Figure 2.
Distribution diagram of the species of Cd2+/Dop− system at different ionic strengths and at T = 298.15 K. Species 1 free Cd2+; 2 CdDop; 3 CdDopH; 4 CdDop2. a I = 0.15 mol dm−3; b I = 0.50 mol dm−3; c I = 1.00 mol dm−3. Experimental conditions: cCd2+ = 1.5 mmol dm−3; cDOP− = 3.0 mmol dm−3. Charges omitted for simplicity.
Figure 2.
Distribution diagram of the species of Cd2+/Dop− system at different ionic strengths and at T = 298.15 K. Species 1 free Cd2+; 2 CdDop; 3 CdDopH; 4 CdDop2. a I = 0.15 mol dm−3; b I = 0.50 mol dm−3; c I = 1.00 mol dm−3. Experimental conditions: cCd2+ = 1.5 mmol dm−3; cDOP− = 3.0 mmol dm−3. Charges omitted for simplicity.
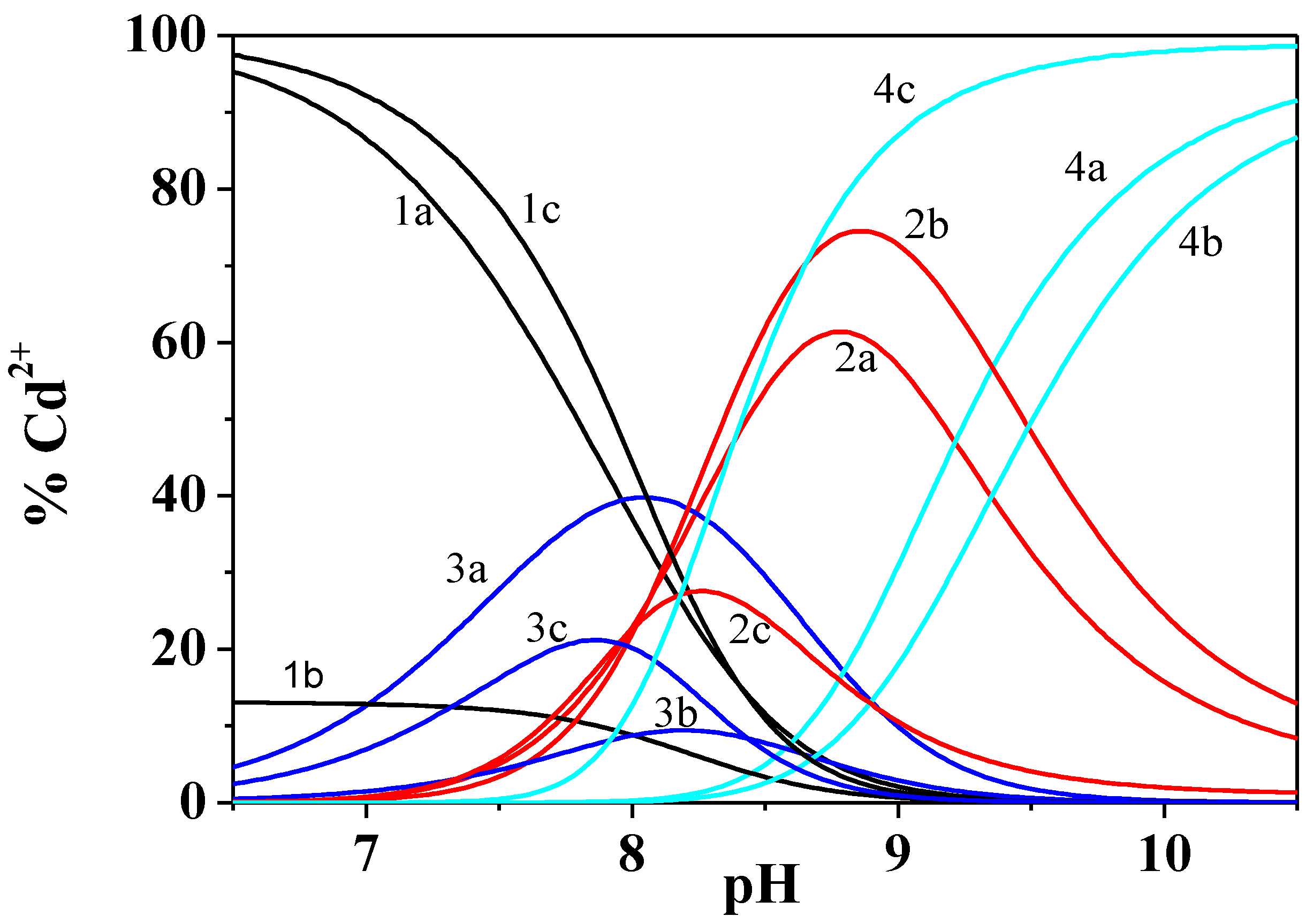
Figure 3.
Distribution diagram of the species Cd2+/Dop− system at different temperatures and at ionic strength I = 0.15 mol dm−3. Species 1 free Cd2+; 2 CdDop; 3 CdDopH; 4 CdDop2. a T = 288.15 K; b T = 298.15 K; c T = 310.15 K. Experimental conditions: cCd2+ = 1.5 mmol dm−3; cDOP− = 3.0 mmol dm−3. Charges omitted for simplicity.
Figure 3.
Distribution diagram of the species Cd2+/Dop− system at different temperatures and at ionic strength I = 0.15 mol dm−3. Species 1 free Cd2+; 2 CdDop; 3 CdDopH; 4 CdDop2. a T = 288.15 K; b T = 298.15 K; c T = 310.15 K. Experimental conditions: cCd2+ = 1.5 mmol dm−3; cDOP− = 3.0 mmol dm−3. Charges omitted for simplicity.
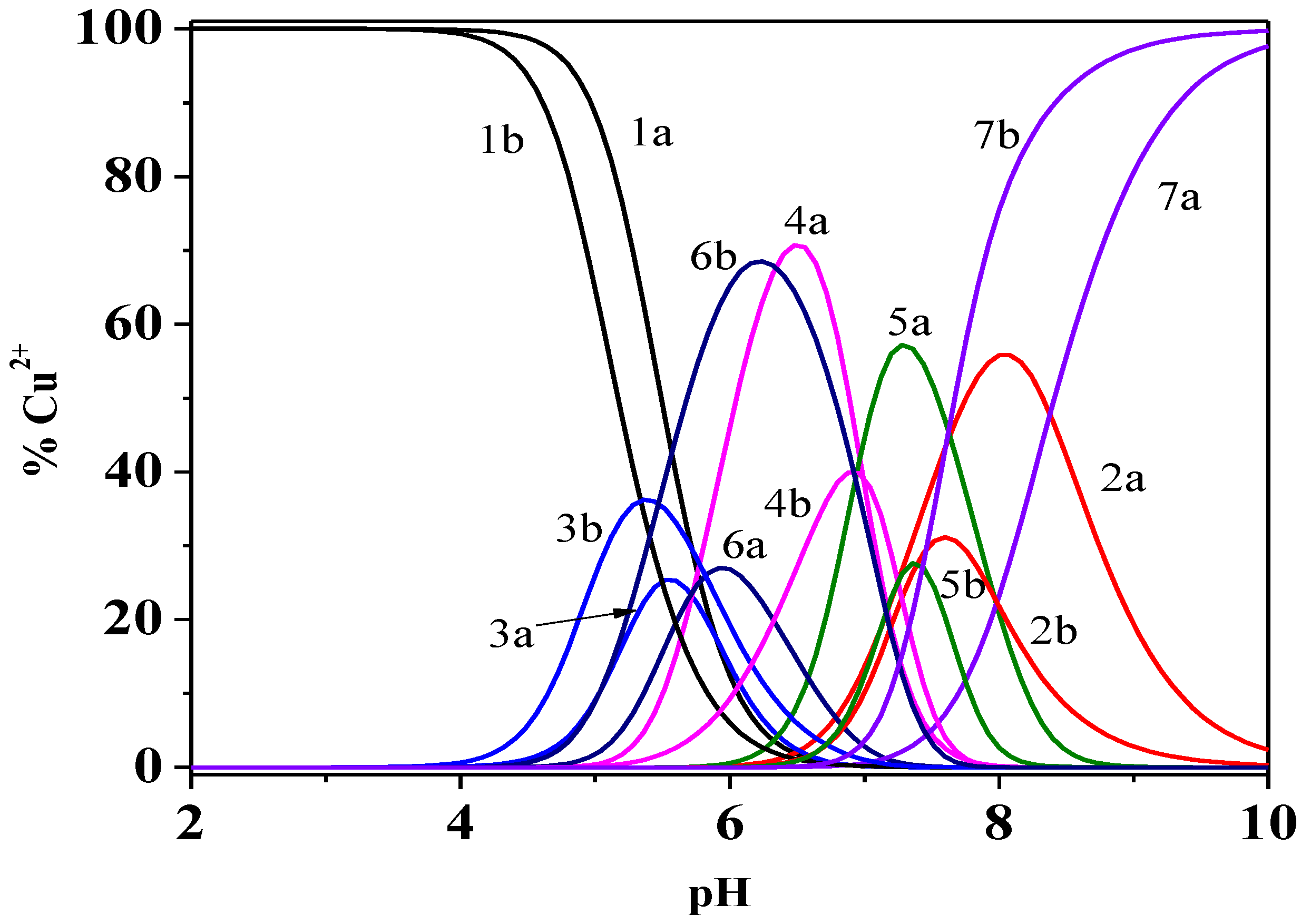
Figure 4.
Distribution diagram of the species of Cu2+/Dop− system at different ionic strengths and T = 298.15 K, in NaCl(aq). a I = 0.15 mol dm−3; b I = 1.00 mol dm−3. Species: 1 free Cu2+; 2. CuL2; 3. Cu2L; 4. Cu2L2; 5. Cu2L2(OH)2: 6. Cu2LOH; 7. CuL2OH. Experimental conditions: cCu2+ = 0.5 mmol dm−3 and cDop− = 1.5 mmol dm−3. Charges omitted for simplicity.
Figure 4.
Distribution diagram of the species of Cu2+/Dop− system at different ionic strengths and T = 298.15 K, in NaCl(aq). a I = 0.15 mol dm−3; b I = 1.00 mol dm−3. Species: 1 free Cu2+; 2. CuL2; 3. Cu2L; 4. Cu2L2; 5. Cu2L2(OH)2: 6. Cu2LOH; 7. CuL2OH. Experimental conditions: cCu2+ = 0.5 mmol dm−3 and cDop− = 1.5 mmol dm−3. Charges omitted for simplicity.
Figure 5.
Distribution diagram of the Cu2+/Dop− system at I = 0.5 mol dm−3 in NaCl(aq) and different temperatures. a. T = 288.15 K; b. T = 318.15 K. Species: 1 free Cu2+; 2. CuL2; 3. Cu2L; 4. Cu2L2; 5. Cu2L2(OH)2: 6. Cu2LOH; 7. CuL2OH. Experimental conditions: cCu2+ = 0.5 mmol dm−3 and cDop− = 1.5 mol dm−3. Charges omitted for simplicity.
Figure 5.
Distribution diagram of the Cu2+/Dop− system at I = 0.5 mol dm−3 in NaCl(aq) and different temperatures. a. T = 288.15 K; b. T = 318.15 K. Species: 1 free Cu2+; 2. CuL2; 3. Cu2L; 4. Cu2L2; 5. Cu2L2(OH)2: 6. Cu2LOH; 7. CuL2OH. Experimental conditions: cCu2+ = 0.5 mmol dm−3 and cDop− = 1.5 mol dm−3. Charges omitted for simplicity.
Figure 6.
Distribution diagram of the UO2-Ac/Dop− system at T = 298.15 K and different ionic strengths. a. I = 0.15 mol dm−3; b. I = 0.50 mol dm−3; c. I = 1.00 mol dm−3). Experimental conditions: cUO22+ = 1.0 mmol dm−3; cDop− = 1.5 mmol dm−3. Species: 1. MLOH; 2. MLAc; 3. ML2. Charges omitted for simplicity.
Figure 6.
Distribution diagram of the UO2-Ac/Dop− system at T = 298.15 K and different ionic strengths. a. I = 0.15 mol dm−3; b. I = 0.50 mol dm−3; c. I = 1.00 mol dm−3). Experimental conditions: cUO22+ = 1.0 mmol dm−3; cDop− = 1.5 mmol dm−3. Species: 1. MLOH; 2. MLAc; 3. ML2. Charges omitted for simplicity.
Figure 7.
Distribution diagram of the UO2-Ac/Dop− system at I = 0.15 mol dm−3 and different temperatures. a. T = 288.15 K; b. T = 298.15 K; c. T = 310.15 K. Experimental conditions: cUO22+ = 1.0 mmol dm−3; cDOP− = 1.5 mmol dm−3. Species: 1. MLOH; 2. MLAc; 3. ML2. Charges omitted for simplicity.
Figure 7.
Distribution diagram of the UO2-Ac/Dop− system at I = 0.15 mol dm−3 and different temperatures. a. T = 288.15 K; b. T = 298.15 K; c. T = 310.15 K. Experimental conditions: cUO22+ = 1.0 mmol dm−3; cDOP− = 1.5 mmol dm−3. Species: 1. MLOH; 2. MLAc; 3. ML2. Charges omitted for simplicity.
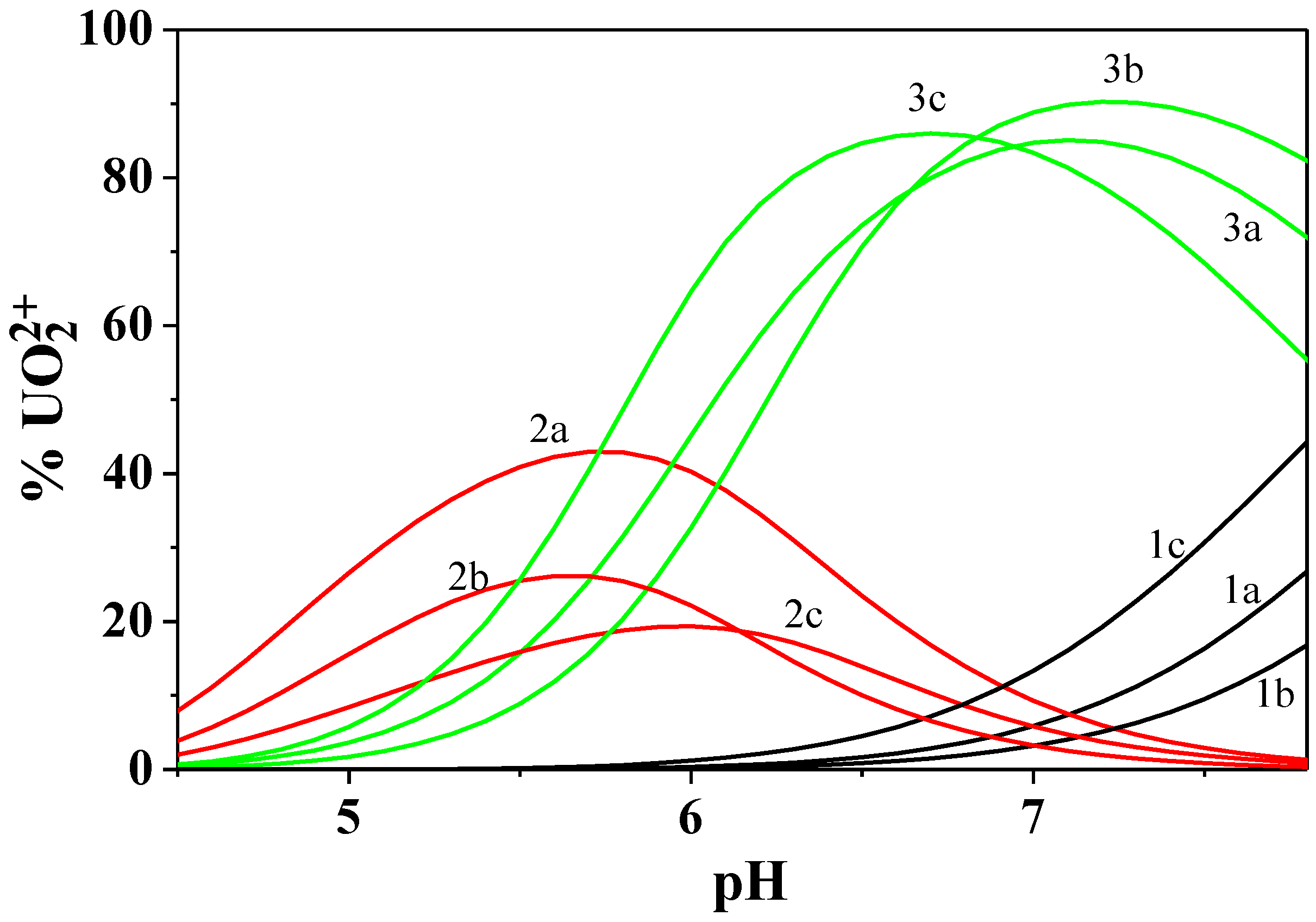
Figure 8.
Distribution diagram of the UO22+/Cd2+/Dop− system at I = 0.15 mol dm−3 and T = 298.15 K. Experimental conditions: cUO22+ = 14.0 mmol dm−3; cCd2+ = 8.0 mmol dm−3; cDop− = 16.0 mmol dm−3. Charges omitted for simplicity.
Figure 8.
Distribution diagram of the UO22+/Cd2+/Dop− system at I = 0.15 mol dm−3 and T = 298.15 K. Experimental conditions: cUO22+ = 14.0 mmol dm−3; cCd2+ = 8.0 mmol dm−3; cDop− = 16.0 mmol dm−3. Charges omitted for simplicity.
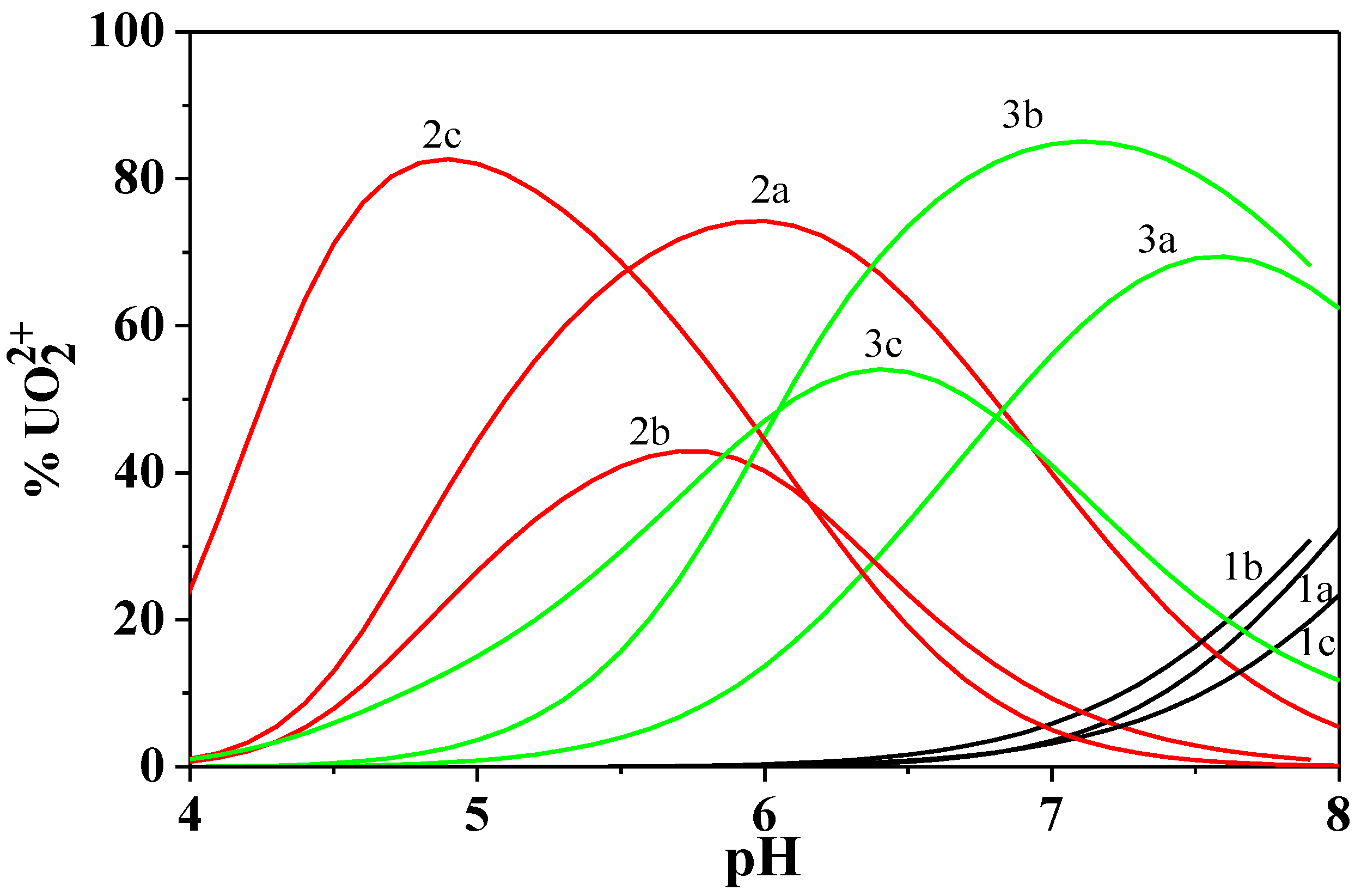
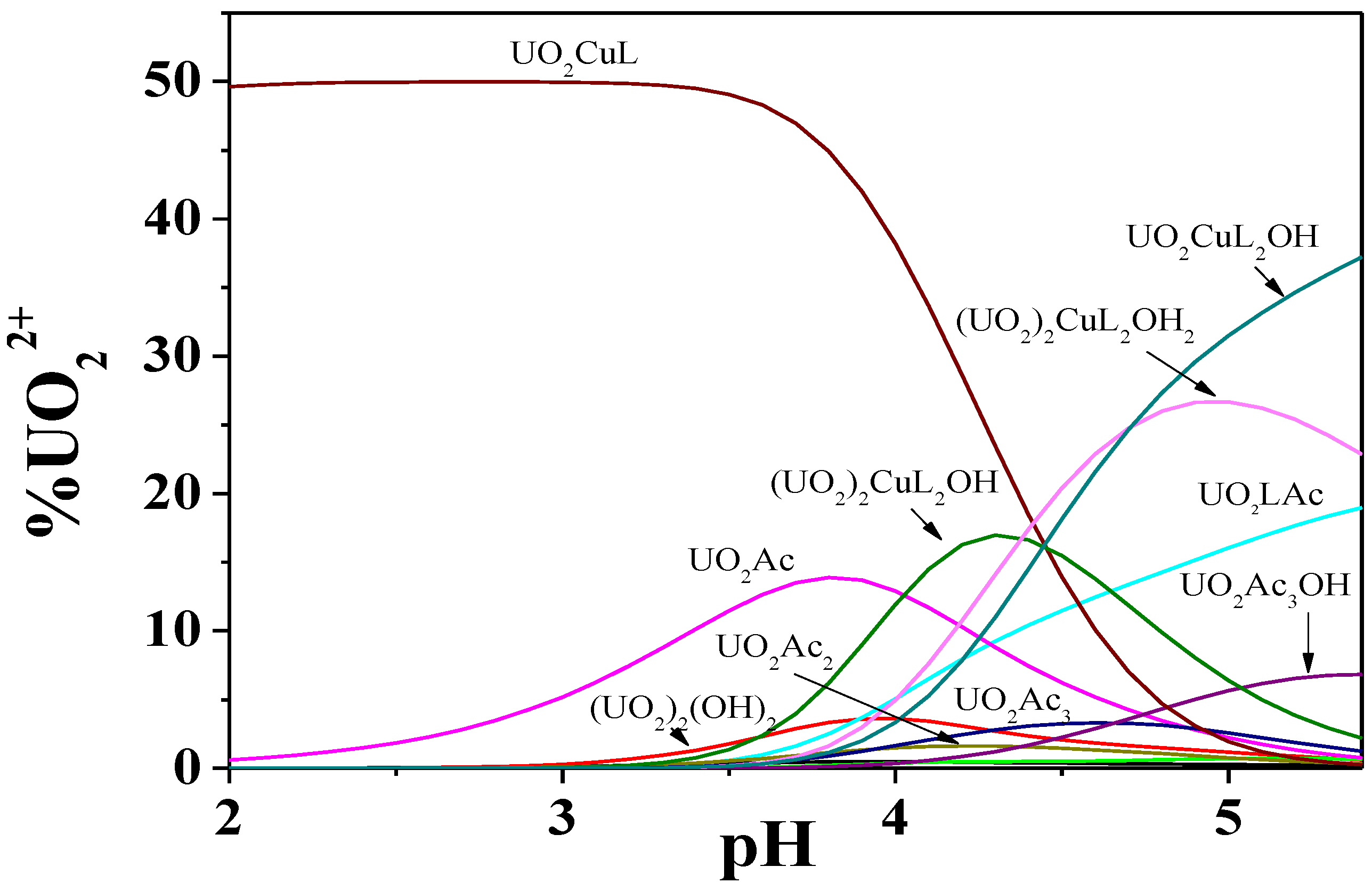
Figure 9.
Distribution diagram of the UO22+/Cu2+/Dop− system at I = 0.15 mol dm−3 and T = 298.15 K. Experimental conditions: cUO22+ = 8.0 mmol dm−3; cCd2+ = 4.0 mmol dm−3; cDop− = 10.0 mmol dm−3. Charges omitted for simplicity.
Figure 9.
Distribution diagram of the UO22+/Cu2+/Dop− system at I = 0.15 mol dm−3 and T = 298.15 K. Experimental conditions: cUO22+ = 8.0 mmol dm−3; cCd2+ = 4.0 mmol dm−3; cDop− = 10.0 mmol dm−3. Charges omitted for simplicity.
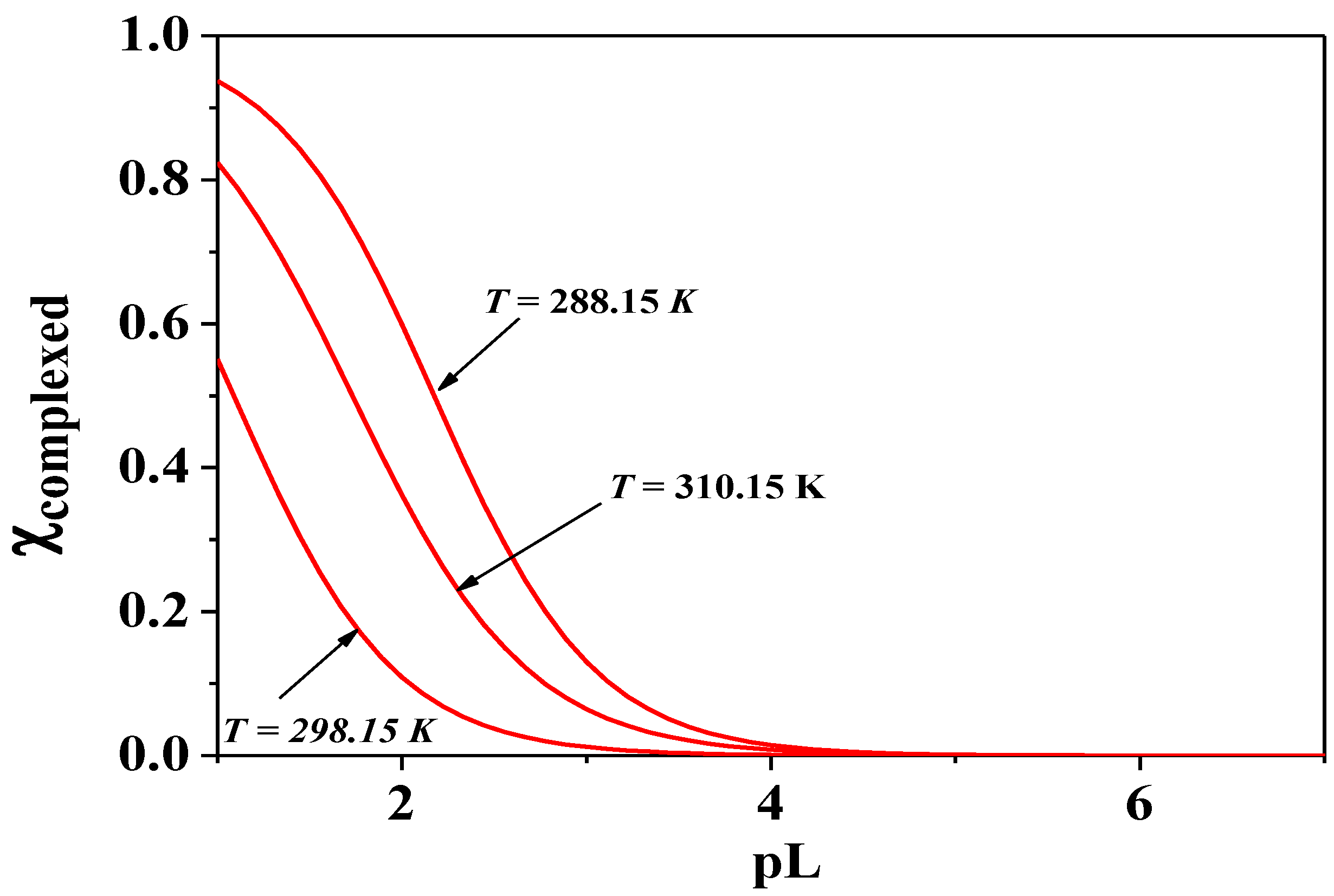
Figure 10.
Sequestering diagram of Cd2+ at I = 0.15 mol dm−3, pH = 7.4 and different temperatures. pL0.5: T = 288.15 K, 2.17; T = 298.15 K, 1.08; T = 310.15 K, 1.73.
Figure 10.
Sequestering diagram of Cd2+ at I = 0.15 mol dm−3, pH = 7.4 and different temperatures. pL0.5: T = 288.15 K, 2.17; T = 298.15 K, 1.08; T = 310.15 K, 1.73.
Figure 11.
Sequestering diagrams of Cd2+, UO2Ac+, Cu2+ at T = 298.15 K, pH = 7.4 and different ionic strengths. (A) Cd2+; pL0.5: I = 0.15 mol dm−3, 1.08; I = 0.50 mol dm−3, 1.40; I = 1.0 mol dm−3, 1.65; (B) UO2Ac+; pL0.5: I = 0.15 mol dm−3, 7.58; I = 0.50 mol dm−3, 7.76; I = 0.75 mol dm−3, 8.01; I = 1.0 mol dm−3, 8.13; (C) Cu2+; pL0.5: I = 0.15 mol dm−3, 4.63; I = 0.50 mol dm−3, 4.64; I = 0.75 mol dm−3, 4.76; I = 1.0 mol dm−3, 4.82.
Figure 11.
Sequestering diagrams of Cd2+, UO2Ac+, Cu2+ at T = 298.15 K, pH = 7.4 and different ionic strengths. (A) Cd2+; pL0.5: I = 0.15 mol dm−3, 1.08; I = 0.50 mol dm−3, 1.40; I = 1.0 mol dm−3, 1.65; (B) UO2Ac+; pL0.5: I = 0.15 mol dm−3, 7.58; I = 0.50 mol dm−3, 7.76; I = 0.75 mol dm−3, 8.01; I = 1.0 mol dm−3, 8.13; (C) Cu2+; pL0.5: I = 0.15 mol dm−3, 4.63; I = 0.50 mol dm−3, 4.64; I = 0.75 mol dm−3, 4.76; I = 1.0 mol dm−3, 4.82.
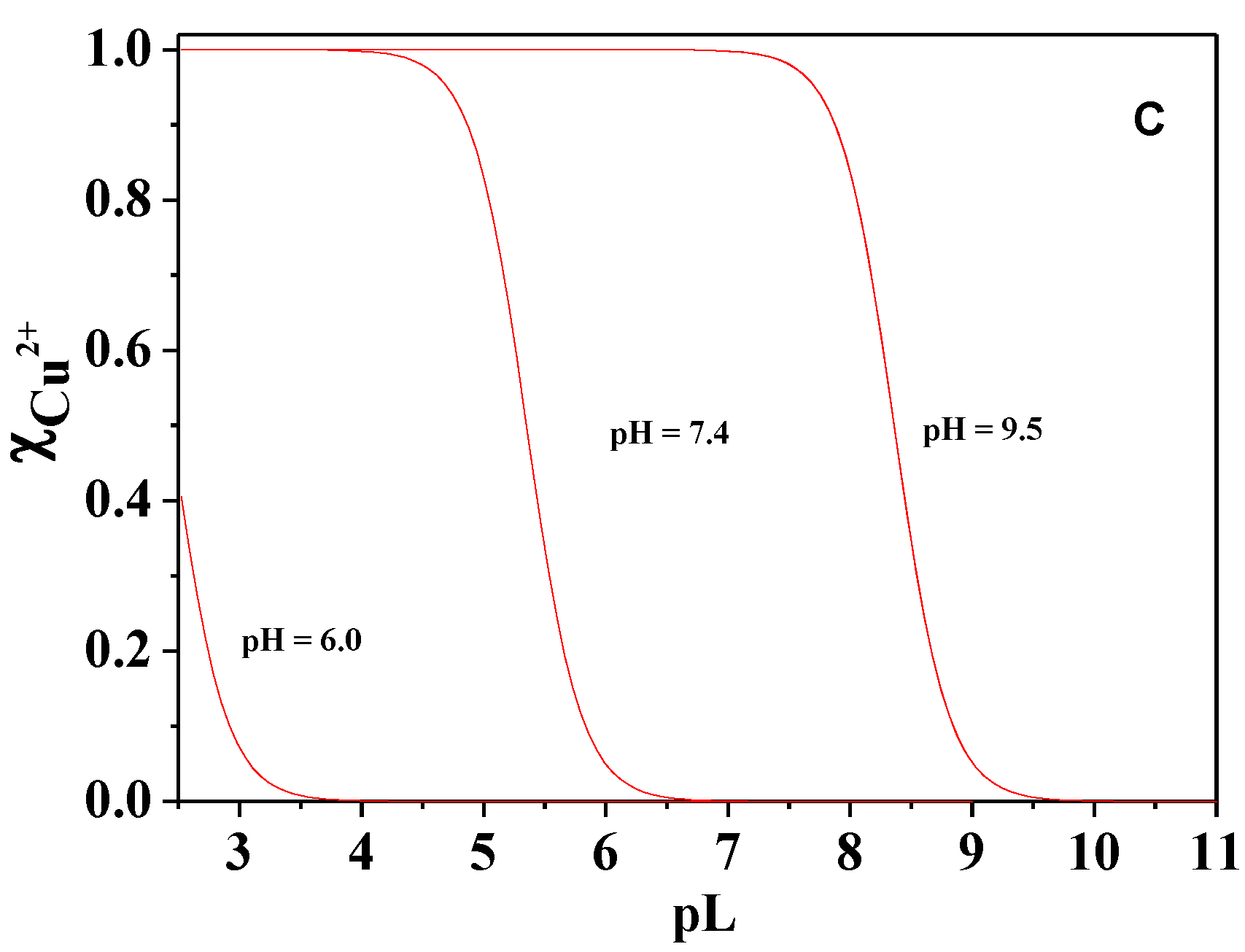
Figure 12.
Sequestering diagrams of Cd2+, UO2Ac+, Cu2+ at T = 310.15 K for UO22+, I = 0.15 mol dm−3 and different pHs (A) Cd2+; pL0.5: pH = 7.4, 1.08; pH = 8.0, 1.99; pH = 9.0, 3.56; (B) UO2Ac+; pL0.5: pH = 5.0, 3.91; pH = 6.0, 6.27; pH = 7.4, 8.87; (C) Cu2+; pL0.5: pH = 6, 2.4; pH = 7.4, 5.35; pH = 9.5, 8.36.
Figure 12.
Sequestering diagrams of Cd2+, UO2Ac+, Cu2+ at T = 310.15 K for UO22+, I = 0.15 mol dm−3 and different pHs (A) Cd2+; pL0.5: pH = 7.4, 1.08; pH = 8.0, 1.99; pH = 9.0, 3.56; (B) UO2Ac+; pL0.5: pH = 5.0, 3.91; pH = 6.0, 6.27; pH = 7.4, 8.87; (C) Cu2+; pL0.5: pH = 6, 2.4; pH = 7.4, 5.35; pH = 9.5, 8.36.
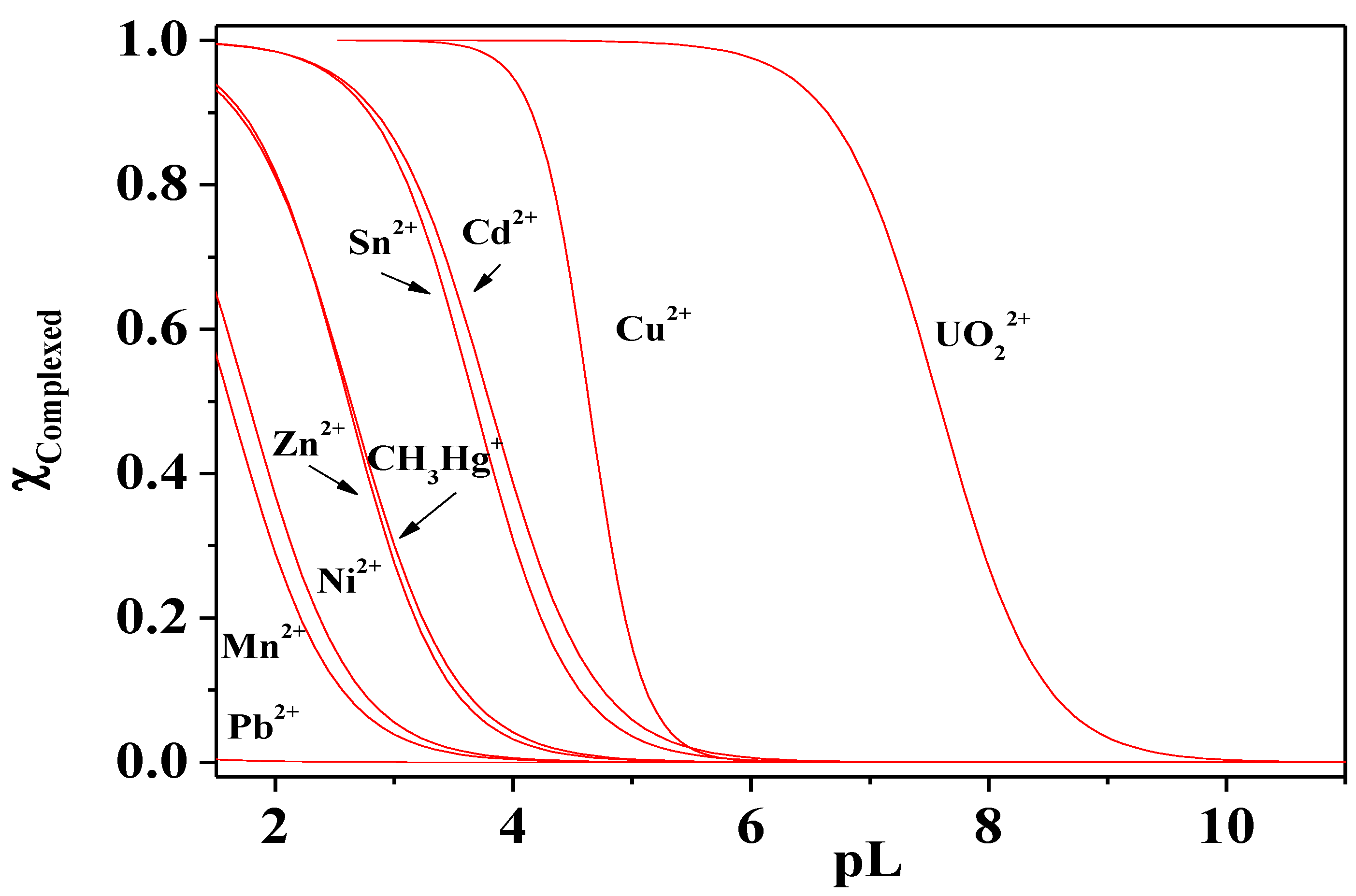
Figure 13.
Sequestering diagrams of metals at T = 298.15 K, I = 0.15 mol dm−3 and pH = 7.4. pL0.5: Pb2+, 0.23 < Mn2+, 1.61 <; Ni2+, 1.77 < Zn2+, 2.61 < CH3Hg+, 2.63 < Sn2+, 3.67 < Cd2+ (pH = 9.5) 3.80 < Cu2+, 4.64 < UO22+, 7.58.
Figure 13.
Sequestering diagrams of metals at T = 298.15 K, I = 0.15 mol dm−3 and pH = 7.4. pL0.5: Pb2+, 0.23 < Mn2+, 1.61 <; Ni2+, 1.77 < Zn2+, 2.61 < CH3Hg+, 2.63 < Sn2+, 3.67 < Cd2+ (pH = 9.5) 3.80 < Cu2+, 4.64 < UO22+, 7.58.
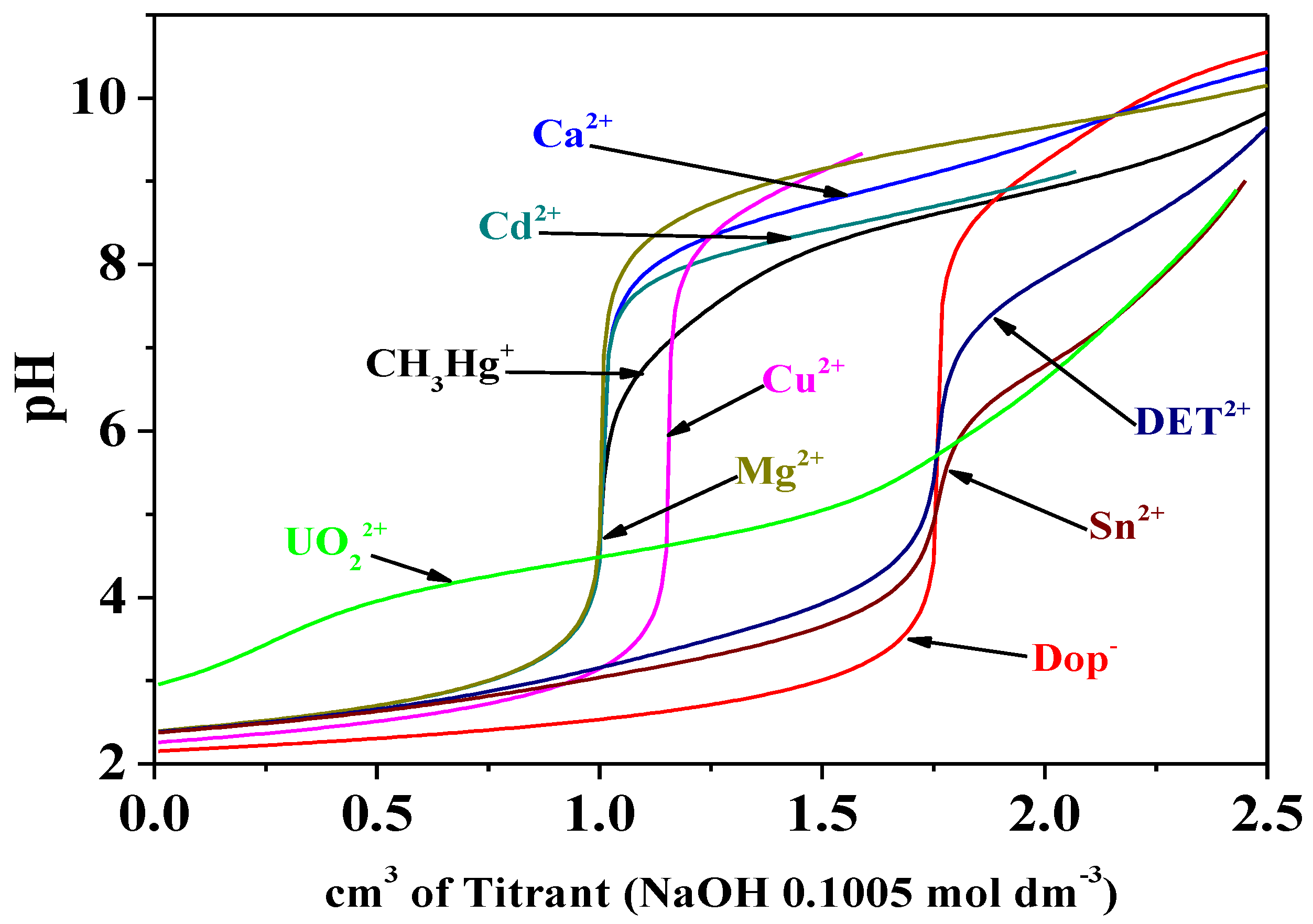
Figure 14.
Titration curves of dopamine and of the different metal/Dop− systems at T = 298.15 K and I = 0.15 mol dm−3. Experimental conditions: cDop− = 3 mmol dm−3; cMn+ = 1.5 mol dm−3. Titrant: NaOH 0.1005 mol dm−3.
Figure 14.
Titration curves of dopamine and of the different metal/Dop− systems at T = 298.15 K and I = 0.15 mol dm−3. Experimental conditions: cDop− = 3 mmol dm−3; cMn+ = 1.5 mol dm−3. Titrant: NaOH 0.1005 mol dm−3.
Table 1.
Experimental formation constants of the Cd2+/Dop− species in NaCl aqueous solutions in molar concentration scale.
Table 1.
Experimental formation constants of the Cd2+/Dop− species in NaCl aqueous solutions in molar concentration scale.
| I/mol dm−3 | logβMLH (a) | logβML (a) | logβML2 (a) |
|---|
| T = 288.15 K |
| 0.150 | 14.90 ± 0.09 (b) | 6.66 ± 0.03 | 11.62 ± 0.08 |
| T = 298.15 K |
| 0.148 | 14.08 ± 0.02 | 6.48 ± 0.03 | 10.88 ± 0.02 |
| 0.488 | 14.18 ± 0.01 | 6.06 ± 0.07 | 10.31 ± 0.02 |
| 0.730 | 14.71 ± 0.05 | 6.41 ± 0.02 | 10.35 ± 0.07 |
| 0.956 | 14.10 ± 0.05 | 6.30 ± 0.09 | 9.13 ± 0.06 |
| T = 310.15 K |
| 0.150 | 12.56 ± 0.10 | 4.61 ± 0.04 | 9.26 ± 0.02 |
Table 2.
Experimental formation constants of the Cu2+/Dop− species in NaCl aqueous solutions in molar concentration scale.
Table 2.
Experimental formation constants of the Cu2+/Dop− species in NaCl aqueous solutions in molar concentration scale.
| I/mol dm−3 | logβML2 (a) | logβM2L (a) | logβM2L2 (a) | logβM2L2(OH)2 (a) | logβM2L(OH) (a) | logβML2(OH) (a) |
|---|
| T = 288.15 K |
| 0.161 | 21.02 ± 0.08 (b) | 15.21 ± 0.06 | 27.34 ± 0.08 | 13.40 ± 0.08 | 8.87 ± 0.02 | 11.38 ± 0.06 |
| 0.492 | 20.50 ± 0.06 | 15.33 ± 0.04 | 26.86 ± 0.04 | 12.68 ± 0.06 | 9.02 ± 0.02 | 11.25 ± 0.04 |
| 0.743 | 20.21 ± 0.05 | 15.42 ± 0.03 | 26.60 ± 0.02 | 12.26 ± 0.04 | 9.21 ± 0.03 | 11.22 ± 0.03 |
| 0.995 | 19.95 ± 0.06 | 15.52 ± 0.04 | 26.38 ± 0.04 | 11.89 ± 0.04 | 9.42 ± 0.04 | 11.20 ± 0.03 |
| T = 298.15 K |
| 0.171 | 19.36 ± 0.06 | 14.57 ± 0.04 | 25.66 ± 0.06 | 11.72 ± 0.05 | 8.86 ± 0.02 | 11.01 ± 0.05 |
| 0.474 | 18.88 ± 0.04 | 14.68 ± 0.02 | 25.22 ± 0.03 | 11.04 ± 0.03 | 9.00 ± 0.02 | 10.88 ± 0.03 |
| 0.738 | 18.57 ± 0.04 | 14.78 ± 0.03 | 24.94 ± 0.03 | 10.60 ± 0.03 | 9.19 ± 0.02 | 10.85 ± 0.02 |
| 0.965 | 18.33 ± 0.05 | 14.87 ± 0.04 | 24.74 ± 0.05 | 10.26 ± 0.04 | 9.38 ± 0.04 | 10.84 ± 0.03 |
| T = 310.15 K |
| 0.145 | 17.58 ± 0.05 | 13.85 ± 0.02 | 23.87 ± 0.06 | 9.95 ± 0.03 | 8.85 ± 0.03 | 10.63 ± 0.04 |
| 0.482 | 17.02 ± 0.04 | 13.98 ± 0.02 | 23.35 ± 0.05 | 9.16 ± 0.02 | 8.99 ± 0.02 | 10.47 ± 0.03 |
| 0.728 | 16.73 ± 0.05 | 14.07 ± 0.04 | 23.09 ± 0.06 | 8.74 ± 0.04 | 9.16 ± 0.03 | 10.43 ± 0.03 |
| 0.982 | 16.46 ± 0.07 | 14.16 ± 0.06 | 22.85 ± 0.08 | 8.36 ± 0.07 | 9.37 ± 0.04 | 10.42 ± 0.04 |
| T = 318.15 K |
| 0.161 | 16.40 ± 0.06 | 13.41 ± 0.01 | 22.67 ± 0.06 | 8.73 ± 0.02 | 8.84 ± 0.04 | - |
| 0.505 | 15.83 ± 0.05 | 13.54 ± 0.03 | 22.15 ± 0.07 | 7.94 ± 0.04 | 8.98 ± 0.03 | 10.20 ± 0.04 |
| 0.732 | 15.56 ± 0.07 | 13.62 ± 0.05 | 21.91 ± 0.08 | 7.55 ± 0.06 | - | - |
| 0.994 | 15.28 ± 0.09 | 13.72 ± 0.08 | 21.66 ± 0.11 | 7.16 ± 0.09 | 9.36 ± 0.04 | 10.15 ± 0.06 |
Table 3.
Experimental formation constants of the UO22+/Dop− species in NaCl aqueous solutions in molar concentration scale.
Table 3.
Experimental formation constants of the UO22+/Dop− species in NaCl aqueous solutions in molar concentration scale.
| I/mol dm−3 | logβML2 (a) | logβMLAc (a) | logβMLOH (a) |
|---|
| T = 288.15 K |
| 0.161 | 21.50 ± 0.12 (b) | 16.11 ± 0.11 | 6.66 ± 0.07 |
| 0.526 | 21.87 ± 0.11 | 15.88 ± 0.10 | 6.71 ± 0.05 |
| 0.742 | 22.26 ± 0.12 | 15.83 ± 0.11 | 6.83 ± 0.06 |
| 0.994 | 22.67 ± 0.15 | 15.81 ± 0.12 | 6.96 ± 0.09 |
| T = 298.15 K |
| 0.165 | 21.69 ± 0.10 | 16.14 ± 0.09 | 7.09 ± 0.06 |
| 0.504 | 21.35 ± 0.07 | 15.66 ± 0.06 | 6.83 ± 0.04 |
| 0.74 | 21.23 ± 0.08 | 15.44 ± 0.06 | 6.73 ± 0.05 |
| 0.992 | 21.13 ± 0.11 | 15.24 ± 0.07 | 6.64 ± 0.08 |
| T = 310.15 K |
| 0.160 | 22.15 ± 0.10 | 16.58 ± 0.10 | 7.48 ± 0.06 |
| 0.505 | 21.88 ± 0.05 | 16.28 ± 0.06 | 7.29 ± 0.03 |
| 0.738 | 21.80 ± 0.06 | 16.17 ± 0.04 | 7.23 ± 0.04 |
| 0.990 | 21.75 ± 0.09 | 16.10 ± 0.04 | 7.20 ± 0.07 |
| T = 318.15 K |
| 0.159 | 22.12 ± 0.12 | 17.22 ± 0.12 | 7.49 ± 0.06 |
| 0.502 | 21.97 ± 0.07 | 16.81 ± 0.08 | 7.51 ± 0.04 |
| 0.739 | 21.98 ± 0.07 | 16.63 ± 0.06 | 7.60 ± 0.05 |
| 0.988 | 22.02 ± 0.09 | 16.48 ± 0.05 | 7.71 ± 0.07 |
Table 4.
Formation constants of ternary MM′L species for the UO22+/Cu2+/Dop− and UO22+/Cd2+/Dop− systems at I = 0.15 mol dm−3 and T = 298.15 K.
Table 4.
Formation constants of ternary MM′L species for the UO22+/Cu2+/Dop− and UO22+/Cd2+/Dop− systems at I = 0.15 mol dm−3 and T = 298.15 K.
| | logβ (a) |
|---|
| I/mol dm−3 | MM’L | M2M’L2OH | M2M’L2(OH)2 | MM’L2OH | MM’2L2(OH)2 |
| UO22+/Cu2+/Dop− |
| 0.150 | 22.51 ± 0.11 (b) | 34.72 ± 0.10 (b) | 30.34 ± 0.12 (b) | 31.56 ± 0.11 (b) | - |
| UO22+/Cd2+/Dop− |
| 0.150 | 26.58 ± 0.06 | 38.57 ± 0.13 | - | - | 36.35 ± 0.08 |
Table 5.
Stepwise formation constants of some mixed species at I = 0.15 mol dm−3 and T = 298.15 K.
Table 5.
Stepwise formation constants of some mixed species at I = 0.15 mol dm−3 and T = 298.15 K.
| Species | Proposed Formation Reaction (a) | logK |
|---|
| | UO22+/Cu2+/Dop− system |
| M2M’L2OH | logβMLOH + logβM’2L = logβM2M’L2OH | logKM2M’L2OH = 22.51 − 7.09 − 14.57 = 0.85 |
| M2M’L2(OH)2 | logβMLOH + logβM’2LOH = logβM2M’L2(OH)2 | logKM2M’L2(OH)2 = 30.34 − 7.09 − 8.86 = 14.39 |
| | UO22+/Cd2+/Dop− system |
| MM’2L2(OH)2 | logβM’OH + logβM’L + logβMLOH = logβMM’2L2(OH)2 | logKMM’2L2(OH)2 = 36.35 + 10.36 − 6.48 − 7.09 = 33.14 |
Table 6.
Formation constants at infinite dilution, parameter for the dependence on ionic the strength and standard formation enthaly change values at T = 298.15 K of the Cd2+/Dop− species, in molar concentration scale.
Table 6.
Formation constants at infinite dilution, parameter for the dependence on ionic the strength and standard formation enthaly change values at T = 298.15 K of the Cd2+/Dop− species, in molar concentration scale.
| | logβTMLH (a) | logβTML (a) | logβTML2 (a) |
|---|
| I → 0 (b) | 14.33 ± 0.04 (c) | 6.87 ± 0.06 (c) | 11.93 ± 0.06 |
| C(d) | 0.19 ± 0.05 (c) | 0.21 ± 0.08 | −1.63 ± 0.08 |
| ΔH (e) | −183 ± 26 (c) | −162 ± 43 | −185 ± 29 |
| TΔS (e) | −103 ± 26 (c) | −125 ± 43 | −123 ± 29 |
Table 7.
Formation constants at infinite dilution, parameter for the dependence on ionic the strength and standard enthaly change values of formation at T = 298.15 K of the Cu2+/Dop− species, in molar concentration scale.
Table 7.
Formation constants at infinite dilution, parameter for the dependence on ionic the strength and standard enthaly change values of formation at T = 298.15 K of the Cu2+/Dop− species, in molar concentration scale.
| | logβTML2 (a) | logβTM2L (a) | logβTM2L2 (a) | logβTM2L2(OH)2 (a) | logβTM2L(OH) (a) | logβTML2(OH) (a) |
|---|
| I → 0 (b) | 20.27 ± 0.07 (c) | 14.51 ± 0.05 (c) | 26.55 ± 0.08 | 12.95 ± 0.07 | 9.21 ± 0.02 | 11.51 ± 0.06 |
| C(d) | −0.75 ± 011 (c) | 0.37 ± 0.09 | −0.62 ± 0.13 | −1.10 ± 0.11 | 1.01 ± 0.06 | 0.14 ± 0.08 |
| ΔH (e) | −265.5 ± 5.5 (c) | −105.1 ± 3.8 | −267.9 ± 5.1 | −266.7 ± 5.1 | 1.6 ± 2.5 | −56.6 ± 3.8 |
| TΔS (e) | 150 ± 6 | 22 ± 4 | 116 ± 5 | 193 ± 5 | −54 ± 3 | −9 ± 4 |
Table 8.
Formation constants at infinite dilution, parameter for the dependence on ionic the strength and standard enthalpy change values of formation at T = 298.15 K of the UO22+-Ac/Dop− species, in molar concentration scale.
Table 8.
Formation constants at infinite dilution, parameter for the dependence on ionic the strength and standard enthalpy change values of formation at T = 298.15 K of the UO22+-Ac/Dop− species, in molar concentration scale.
| | logβTML2 (a) | logβTMLAc (a) | logβTMLOH (a) |
|---|
| I → 0 (b) | 22.05 ± 0.12 (c) | 16.66 ± 0.10 | 7.01 ± 0.08 |
| C(d) | 0.16 ± 019 (c) | −0.51 ± 0.13 | −0.06 ± 0.14 |
| ΔH (e) | 15.0 ± 8.9 (c) | 54.8 ± 8.8 | 47.6 ± 4.1 |
| TΔS (e) | −141 ± 9 | −150 ± 9 | −88 ± 4 |
Table 9.
Formation constants at infinite dilutions (molal concentration scale) and at different temperatures for the Cd2+/Dop− species and SIT parameters (Equation (6)).
Table 9.
Formation constants at infinite dilutions (molal concentration scale) and at different temperatures for the Cd2+/Dop− species and SIT parameters (Equation (6)).
| | logβTMLH (a) | logβTML (a) | logβTML2 (a) |
|---|
| | | T = 288.15 K | |
| I → 0 (b) | 14.33 ± 0.04 (c) | 6.87 ± 0.05 | 11.93 ± 0.05 |
| Δε (d) | 0.95 ± 0.09 | 0.34 ± 0.11 | −0.68 ± 0.10 |
Table 10.
Formation constants at infinite dilution (molal concentration scale) at different temperatures for the Cu2+/Dop− species and SIT parameters (Equation (6)).
Table 10.
Formation constants at infinite dilution (molal concentration scale) at different temperatures for the Cu2+/Dop− species and SIT parameters (Equation (6)).
| | logβTML2 (a) | logβTM2L (a) | logβTM2L2 (a) | logβTM2L2(OH)2 (a) | logβTM2L(OH) (a) | logβTML2(OH) (a) |
|---|
| | T = 288.15 K |
| I → 0 (b) | 21.901 ± 0.001 (c) | 15.151 ± 0.001 | 28.198 ± 0.001 | 14.597 ± 0.001 | 9.222 ± 0.002 | 11.866 ± 0.002 |
| Δε (d) | −0.725 ± 0.001 | 0.346 ± 0.002 | −0.607 ± 0.001 | −1.060 ± 0.001 | 0.998 ± 0.003 | 0.149 ± 0.002 |
| | T = 298.15 K |
| I → 0 | 20.264 ± 0.001 | 14.510 ± 0.001 | 26.544 ± 0.001 | 12.945 ± 0.002 | 9.216 ± 0.002 | 11.506 ± 0.001 |
| Δε | −0.740 ± 0.002 | 0.344 ± 0.001 | −0.623 ± 0.002 | −1.080 ± 0.002 | 0.983 ± 0.002 | 0.137 ± 0.001 |
| | T = 310.15 K |
| I → 0 | 18.430 ± 0.004 | 13.794 ± 0.002 | 24.693 ± 0.004 | 11.095 ± 0.004 | 9.205 ± 0.001 | 11.100 ± 0.002 |
| Δε | −0.763 ± 0.006 | 0.341 ± 0.002 | −0.647 ± 0.005 | −1.108 ± 0.006 | 0.962 ± 0.002 | 0.120 ± 0.003 |
| | T = 318.15 K |
| I → 0 | 17.28 ± 0.01 | 13.35 ± 0.01 | 23.53 ± 0.01 | 9.93 ± 0.02 | 9.194 ± 0.009 | 10.83 ± 0.03 |
| Δε | −0.78 ± 0.02 | 0.34 ± 0.02 | −0.66 ± 0.02 | −1.13 ± 0.02 | 0.95 ± 0.02 | 0.12 ± 0.06 |
Table 11.
Formation constants at infinite dilution (molal concentration scale) at different temperatures for the UO22+-Ac/Dop− species and SIT parameters (Equation (6)).
Table 11.
Formation constants at infinite dilution (molal concentration scale) at different temperatures for the UO22+-Ac/Dop− species and SIT parameters (Equation (6)).
| | logTβML2 (a) | logTβMLAc (a) | logTβMLOH (a) |
|---|
| | | T = 288.15 K | |
| I → 0 (b) | 21.48 ± 0.04 (c) | 16.36 ± 0.05 | 6.57 ± 0.05 |
| Δε (d) | 1.54 ± 0.06 | −0.19 ± 0.07 | 0.35 ± 0.06 |
| | | T = 298.15 K | |
| I → 0 | 21.99 ± 0.05 | 16.51 ± 0.05 | 7.15 ± 0.05 |
| Δε | −0.50 ± 0.07 | −0.90 ± 0.07 | −0.54 ± 0.07 |
| | | T = 310.15 K | |
| I → 0 | 22.42 ± 0.05 | 16.87 ± 0.05 | 7.51 ± 0.05 |
| Δε | −0.31 ± 0.07 | −0.41 ± 0.07 | −0.34 ± 0.07 |
| | | T = 318.15 K | |
| I → 0 | 22.33 ± 0.05 | 17.55 ± 0.05 | 7.43 ± 0.05 |
| Δε | 0.04 ± 0.07 | −0.71 ± 0.07 | 0.24 ± 0.06 |
Table 12.
Specific ion interaction parameters at T = 298.15 K for the Cd2+, Cu2+ and UO22+/Dop− species.
Table 12.
Specific ion interaction parameters at T = 298.15 K for the Cd2+, Cu2+ and UO22+/Dop− species.
| Species | | Cd2+ | Cu2+ | UO22+ |
|---|
| ε(M2+,CL−) | - | 0.09(NO3−) (a) | 0.08 (a) | 0.25 (b) |
| ε(Na+,L−) | −0.228 c) | - | - | - |
| ε(M2+,Ac−) | | - | - | 0.01 (b) |
| ε(Na+,Ac−) | 0.08 (b) | - | - | - |
| ε(H+,CL−) | 0.12 (d) | - | - | - |
| ε(ML+, Cl−) | - | −0.34 ± 0.10 | - | - |
| ε(MLH2+, Cl−) | - | −0.46 ± 0.20 | - | - |
| kMLAc | - | - | - | 1.00 ± 0.07 (e,f) |
| kMLOH | - | - | - | 0.42 ± 0.07 (e,f) |
| kML2 | - | 0.87 ± 0.25 | 0.364 ± 0.002 (e,g) | 0.29 ± 0.07 (e,f) |
| kMAcL | - | - | | 0.67 ± 0.07 (e,f) |
| ε(M2L3+, Cl−) | - | - | −0.412 ± 0.001 (f) | - |
| ε(M2L22+, Cl−) | - | - | 0.327 ± 0.002 (f) | - |
| kM2L2(OH)2 | - | - | 0.514 ± 0.002 (e,f) | - |
| ε(M2LOH2+, Cl−) | - | - | −1.186 ± 0.002( f) | - |
| ε(ML2OH−, Na+) | - | - | −0.648 ± 0.001 (f) | - |
Table 13.
Sequestering ability of dopamine towards the metals at I = 0.15 mol L−1, pH = 7.4 and different temperatures.
Table 13.
Sequestering ability of dopamine towards the metals at I = 0.15 mol L−1, pH = 7.4 and different temperatures.
| Metal | T/K | pL0.5 |
|---|
| Cd2+ | 288.15 | 2.17 |
| 298.15 | 1.08 |
| 310.15 | 1.73 |
| UO22+ | 288.15 | 7.00 |
| 298.15 | 7.58 |
| 310.15 | 8.87 |
| 318.15 | 9.02 |
| Cu2+ | 288.15 | 5.07 |
| 298.15 | 4.64 |
| 310.15 | 5.35 |
| 318.15 | 5.10 |
Table 14.
Sequestering ability of dopamine towards the metals at T = 298.15 K, pH = 7.4 and different ionic strengths.
Table 14.
Sequestering ability of dopamine towards the metals at T = 298.15 K, pH = 7.4 and different ionic strengths.
| Metal | I/mol dm−3 | pL0.5 |
|---|
| Cd2+ | 0.15 | 1.08 |
| 0.50 | 1.40 |
| 0.75 | 1.52 |
| 1.00 | 1.65 |
| UO22+ | 0.15 | 7.58 |
| 0.50 | 7.76 |
| 0.75 | 8.01 |
| 1.00 | 8.13 |
| Cu2+ | 0.15 | 4.63 |
| 0.50 | 4.64 |
| 0.75 | 4.76 |
| 1.00 | 4.82 |
Table 15.
Sequestering ability of dopamine towards the metals at T = 310.15 K, I = 0.15 mol dm−3 and different pHs.
Table 15.
Sequestering ability of dopamine towards the metals at T = 310.15 K, I = 0.15 mol dm−3 and different pHs.
| Metal | pH | pL0.5 |
|---|
| Cd2+ | 7.4 | 1.08 |
| 8.0 | 1.99 |
| 9.0 | 3.56 |
| UO22+ | 5.0 | 3.91 |
| 6.0 | 6.27 |
| 7.4 | 8.87 |
| Cu2+ | 6.0 | 2.40 |
| 7.4 | 5.35 |
| 9.5 | 8.36 |
Table 16.
Literature data for the complexation of dopamine towards metal ions.
Table 16.
Literature data for the complexation of dopamine towards metal ions.
| I/mol dm−3 | T/K | Metal | Medium | logβML | logβMLH | logβML2 | logβM(LH)2 | logβML2H | logβMLCl | logβMLOH | Ref. |
|---|
| 0.1 | 298.15 | Cu2+ | HClO4 | 20.56 | 26.91 | - | 48.26 | - | - | - | [60] |
| 0.2 | 298.15 | Y3+ | KCl | 7.95 | - | 14.84 | - | - | - | - | [56] |
| | | La3+ | | 6.35 | - | 11.7 | - | - | - | - | [61] |
| 0 | 298.15 | Mg2+ | NaCl | 3.56 | - | - | - | - | - | -5.36 | [12] |
| 0.2 | 288.15 | | | 4.57 | - | - | - | - | - | - | [62] |
| | 298.15 | | | 4.50 | - | - | - | - | - | - | |
| | 308.15 | | | 4.49 | - | - | - | - | - | - | |
| 0 | 298.15 | CH3Hg+ | NaCl | 10.62 | 19.64 | - | - | - | 11.41 | 2.00 | [12] |
| 0 | 298.15 | Ca2+ | NaCl | 5.30 | 13.80 | - | - | - | - | −6.18 | [12] |
| 0.2 | 288.15 | Zn2+ | NaCl | - | - | - | 52.94 | - | - | - | [63] |
| | 298.15 | | | - | - | - | 52.14 | - | - | - | |
| | 308.15 | | | - | - | - | 51.22 | - | - | - | |
| 0.15 | 310.15 | Cu2+ | NaClO4 | 15.44 | 22.07 | 23.25 | - | - | - | - | [64] |
| | | Ni2+ | | 9.66 | 18.12 | 26.07 | - | - | - | - | |
| | | Zn2+ | | 11.43 | 18.89 | - | - | - | - | - | |
| 0.2 | 288.15 | MoO42− | NaCl | - | - | - | 51.48 | - | - | - | [58] |
| | 298.15 | | | - | - | - | 51.08 | - | - | - | |
| | 308.15 | | | - | - | - | 50.66 | - | - | - | |
| 0.2 | 298.15 | Cu2+ | KCl | 16.60 | 24.22 | 24.78 | 45.83 | 35.66 | - | - | [31] |
| | | Ni2+ | | 9.42 | 19.38 | 14.81 | 35.66 | 25.61 | - | - | |
| | | Zn2+ | | - | 20.21 | 18.05 | 38.93 | 28.67 | - | - | |
| 0.37 | 293.15 | Ni2+ | NaNO3 | - | 18.373 | 13.86 | 34.05 | - | - | - | [65] |
| | | Cu2+ | | 16.01 | 23.29 | 23.47 | 44.26 | - | - | - | |
| | | Zn2+ | | - | 19.33 | - | - | - | - | - | |
| | | Cd2+ | | - | 17.991 | - | - | - | - | - | |
| | | Pb2+ | | - | 22.23 | - | - | - | - | - | |
| 0 | 298.15 | Sn2+ | NaCl | logβM2L2 | logβML2 | logβM2LOH | | | | logβMLOH | [12] |
| | | | | 36.40 | 25.56 | 16.11 | - | - | - | 10.43 | |
Table 17.
Chemicals used in this work, purchased from Sigma-Aldrich, Milan, Italy.
Table 17.
Chemicals used in this work, purchased from Sigma-Aldrich, Milan, Italy.
| Chemical | CAS Number | Purification Method | Purity (% wt.) | Purity Check |
|---|
| Hydrochloric acid | 7647-01-0 | none | ≥99% | Volumetric titrations with Na2CO3 |
| Potassium phthalate monobasic | 877-24-7 | none | ≥99.5% | - |
| Sodium carbonate | 497-19-8 | none | ≥99.5% | - |
| Sodium chloride | 7647-14-5 | none | ≥99% | - |
| Sodium hydroxide | 1310-73-2 | none | ≥99% | Volumetric titrations with potassium phthalate monobasic |
| CdCl2 | 10108-64-2 | none | 98% | Volumetric titrations with EDTA |
| CuCl2·2H2O | 10125-13-0 | none | ≥99% | Volumetric titrations with EDTA |
| UO2(Ac)2 | 541-09-3 | none | ≥99% | Volumetric titrations with EDTA |
| Dopamine HCl | 62-31-7 | none | ≥99.5% | Volumetric titrations |
Table 18.
Experimental conditions used for the M2+/Dop− systems in NaCl aqueous solutions.
Table 18.
Experimental conditions used for the M2+/Dop− systems in NaCl aqueous solutions.
| cM2+(mmol dm−3) | cDop−(mmol dm−3) | M:L |
|---|
| 1 | 1.5 | 1:1.5 |
| 1.5 | 3 | 1:2 |
| 1.5 | 5 | 1:3 |
| 2 | 5 | 1:2.5 |
Table 19.
Experimental conditions used for the ternary complexes M/M′/Dop− systems in NaCl aqueous solutions.
Table 19.
Experimental conditions used for the ternary complexes M/M′/Dop− systems in NaCl aqueous solutions.
| cM(mmol dm−3) (a) | cM’(mmol dm−3) (a) | CL(mmol dm−3) (a) | M:M′:L |
|---|
| 5 | 5 | 10 | 1:1:2 |
| 2 | 6 | 6 | 1:3:3 |
| 4 | 6 | 10 | 1:1.5:1.6 |
| 2 | 7 | 11 | 1:3.5:5.5 |
| 6 | 6 | 10 | 1:1:1.6 |
| 6 | 3 | 10 | 2:1:3 |