Pharmacological Insights into Halophyte Bioactive Extract Action on Anti-Inflammatory, Pain Relief and Antibiotics-Type Mechanisms
Abstract
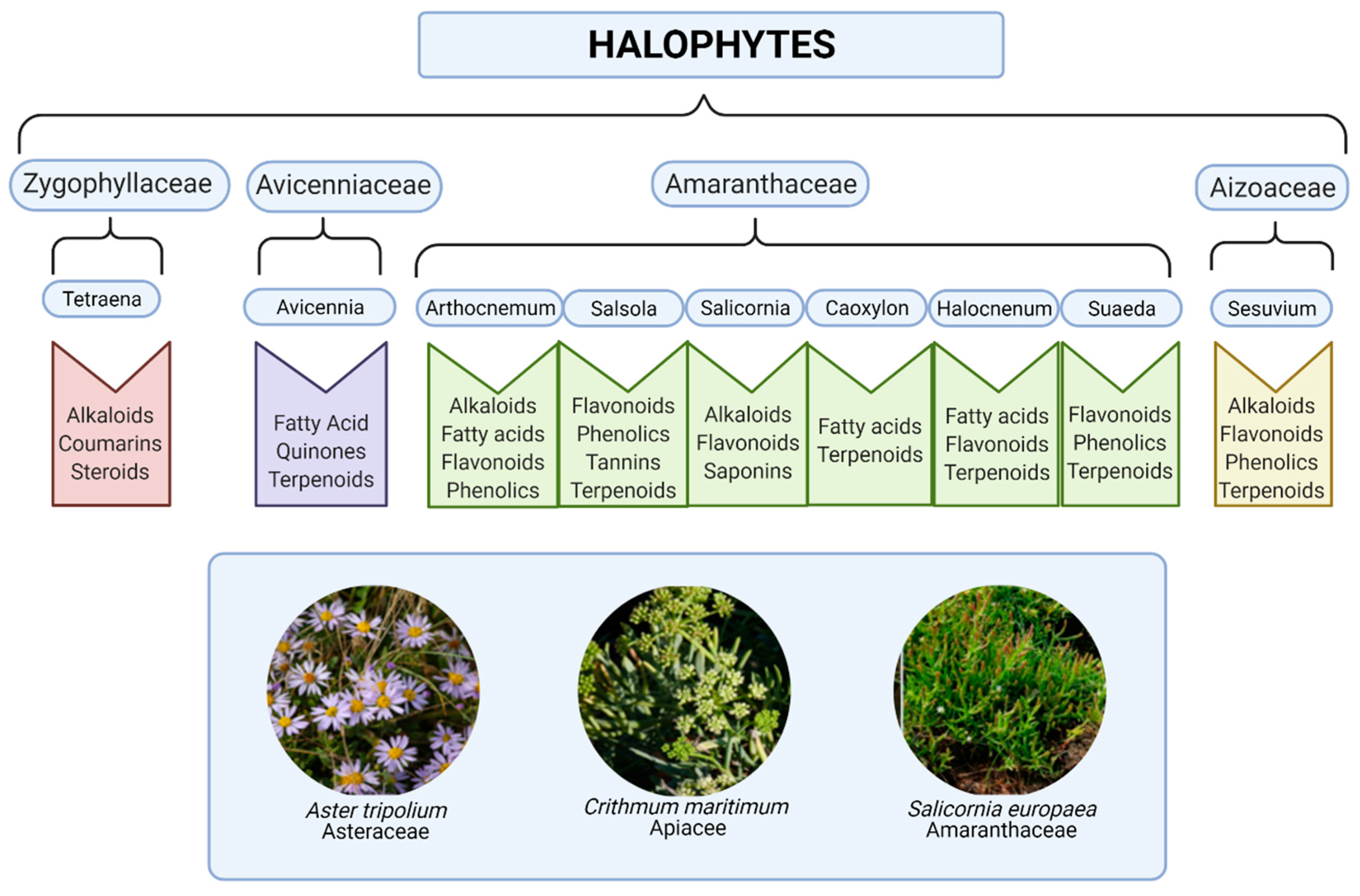
:1. Halophyte Species for Current and Future Biomedical Applications
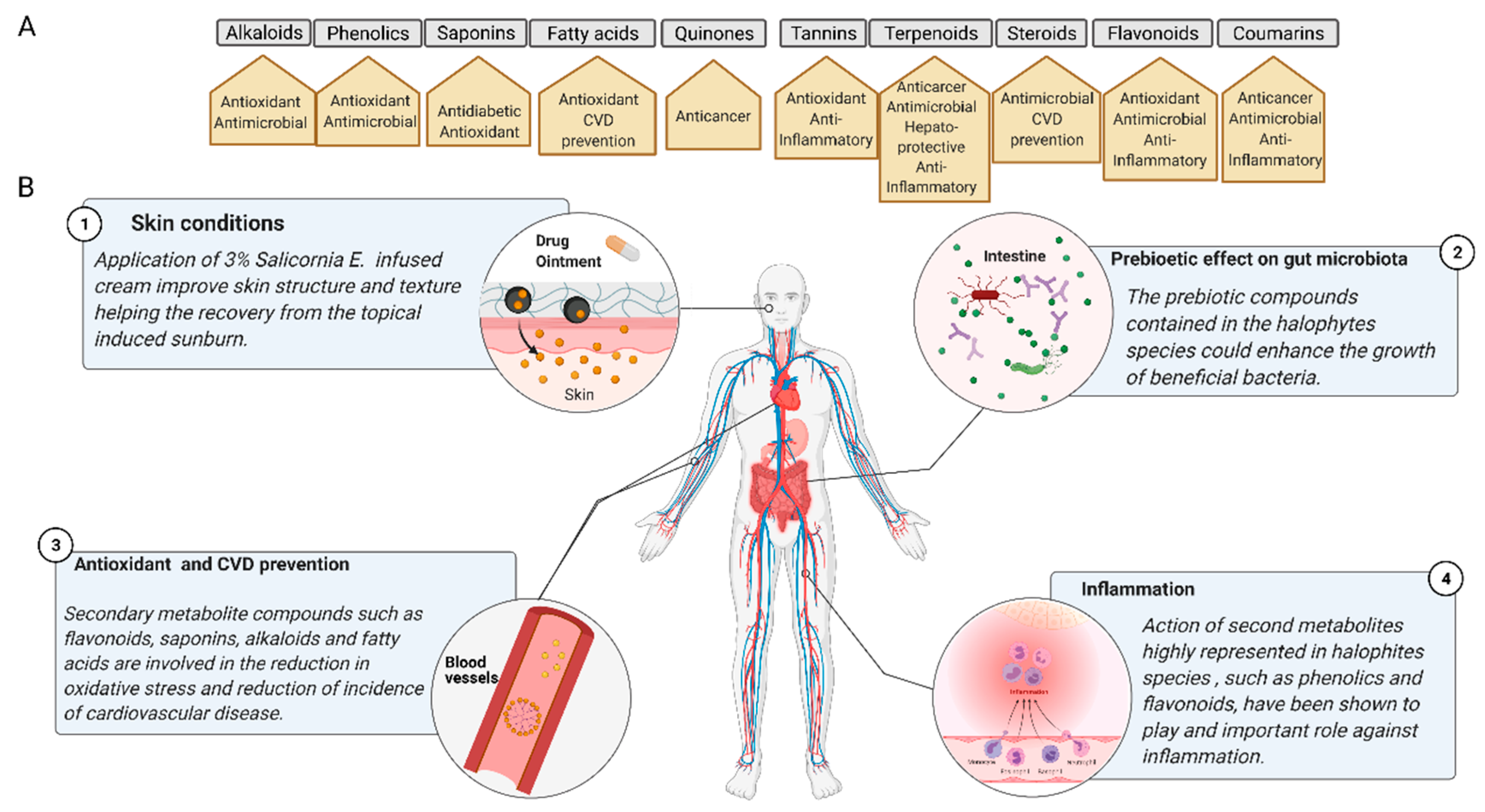
2. Bioactive Components Including Primary Metabolites, Phenolics and Their Antioxidant Properties
3. Nutraceutical and Pharmacological Mode-of-Action of Key Secondary Metabolites in Halophytes
4. Anti-Inflammatory and Antimicrobial Activities of the Three Halophytes
4.1. Characteristics and Medicinal Properties of Aster Tripolium
4.1.1. Anti-Inflammatory Compounds of Aster Tripolium
4.1.2. Antimicrobial Compounds of Aster Tripolium
4.2. Characteristics and Medicinal Properties of Crithmum maritimum
4.2.1. Anti-Inflammatory Compounds of Crithmum maritimum
4.2.2. Antimicrobial Compounds of Crithmum maritimum
4.3. Characteristics and Medicinal Properties of Salicornia Species
4.3.1. Anti-Inflammatory Compounds of Salicornia europaea and other Salicornia species
4.3.2. Antimicrobial Compounds of S. europaea
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes*. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Cybulska, I.; Brudecki, G.; Alassali, A.; Thomsen, M.; Brown, J. Phytochemical composition of some common coastal halophytes of the United Arab Emirates. Emirates J. Food Agric. 2014, 26, 1046–1056. [Google Scholar] [CrossRef] [Green Version]
- Essaidi, I.; Brahmi, Z.; Snoussi, A.; Ben Haj Koubaier, H.; Casabianca, H.; Abe, N.; El Omri, A.; Chaabouni, M.M.; Bouzouita, N. Phytochemical investigation of Tunisian Salicornia herbacea L., antioxidant, antimicrobial and cytochrome P450 (CYPs) inhibitory activities of its methanol extract. Food Control 2013, 32, 125–133. [Google Scholar] [CrossRef]
- Margină, D.; Ungurianu, A.; Purdel, C.; Tsoukalas, D.; Sarandi, E.; Thanasoula, M.; Tekos, F.; Mesnage, R.; Kouretas, D.; Tsatsakis, A. Chronic inflammation in the context of everyday life: Dietary changes as mitigating factors. Int. J. Environ. Res. Public Health 2020, 17, 4135. [Google Scholar] [CrossRef] [PubMed]
- Lautié, E.; Russo, O.; Ducrot, P.; Boutin, J.A. Unraveling plant natural chemical diversity for drug discovery purposes. Front. Pharmacol. 2020, 11, 397. [Google Scholar] [CrossRef]
- İbrahimova, U.; Kumari, P.; Yadav, S.; Rastogi, A.; Antala, M.; Suleymanova, Z.; Zivcak, M.; Tahjib-Ul-Arif, M.; Hussain, S.; Abdelhamid, M.; et al. Progress in understanding salt stress response in plants using biotechnological tools. J. Biotechnol. 2021, 329, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.H. Evidence for health benefits of plant phenols: Local or systemic effects? J. Sci. Food Agric. 2001, 81, 842–852. [Google Scholar] [CrossRef]
- Giada, M.D.L.R. Oxidative Stress and Chronic Degenerative Diseases—A Role for Antioxidants; Chapter: Food Phenolic Compounds: Main Classes, Sources and Their Antioxidant Power; Morales-Gonzalez, J.A., Ed.; IntechOpen Limited: London, UK, 2013; ISBN 953-51-1123-X. [Google Scholar]
- Hilbers, D.; van Kruijsbergen, W.; Boll, H. Photograph on Creative Commons Licence; Saxifraga. Available online: http://www.saxifraga.nl/ (accessed on 10 March 2021).
- Bodas, R.; Prieto, N.; García-González, R.; Andrés, S.; Giráldez, F.J. Manipulation of rumen fermentation and methane production with plant secondary metabolites. Anim. Feed Sci. Technol. 2012, 176, 78–93. [Google Scholar] [CrossRef]
- Meot-Duros, L.; Le Floch, G.; Magné, C. Radical scavenging, antioxidant and antimicrobial activities of halophytic species. J. Ethnopharmacol. 2008, 116, 258–262. [Google Scholar] [CrossRef] [Green Version]
- Meot-Duros, L.; Cérantola, S.; Talarmin, H.; Le Meur, C.; Le Floch, G.; Magné, C. New antibacterial and cytotoxic activities of falcarindiol isolated in Crithmum maritimum L. leaf extract. Food Chem. Toxicol. 2010, 48, 553–557. [Google Scholar] [CrossRef]
- Alassali, A.; Cybulska, I.; Brudecki, G.P.; Farzanah, R.; Thomsen, M.H. Methods for upstream extraction and chemical characterization of secondary metabolites from algae biomass. Adv. Tech. Biol. Med. 2015, 4, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Meot-Duros, L.; Magné, C. Antioxidant activity and phenol content of Crithmum maritimum L. leaves. Plant Physiol. Biochem. 2009, 47, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, E.-Y.; Hillman, P.F.; Ko, J.; Yang, I.; Nam, S.-J. Chemical structure and biological activities of secondary metabolites from Salicornia europaea L. Molecules 2021, 26, 2252. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M.; Barnes, J.; Gibbons, S.; Williamson, E.M.; Kinghorn, A.D. Fundamentals of Pharmacognosy And Phytotherapy; Elsevier Ltd: London, UK, 2012; ISBN 9780702033889. [Google Scholar]
- de Whalley, C.V.; Rankin, S.M.; Hoult, J.R.; Jessup, W.; Leake, D.S. Flavonoids inhibit the oxidative modification of low density lipoproteins by macrophages. Biochem. Pharmacol. 1990, 39, 1743–1750. [Google Scholar] [CrossRef]
- Elegir, G.; Kindl, A.; Sadocco, P.; Orlandi, M. Development of antimicrobial cellulose packaging through laccase-mediated grafting of phenolic compounds. Enzyme Microb. Technol. 2008, 43, 84–92. [Google Scholar] [CrossRef]
- Kȩpa, M.; Miklasińska-Majdanik, M.; Wojtyczka, R.D.; Idzik, D.; Korzeniowski, K.; Smoleń-Dzirba, J.; Wasik, T.J. Antimicrobial potential of caffeic acid against staphylococcus aureus clinical strains. Biomed Res. Int. 2018. [Google Scholar] [CrossRef] [Green Version]
- Hertog, M.G.L.; Hollman, P.C.H.; Katan, M.B.; Daan, D.; Kromhout, K. Intake of potentially anticarcinogenic flavonoids and their determinants in adults in The Netherlands. Nutr. Cancer 1993, 20, 21–29. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Satti, N.K.; Sharma, P.; Sharma, V.K.; Suri, K.A.; Bani, S. Differential effects of chlorogenic acid on various immunological parameters relevant to rheumatoid arthritis. Phyther. Res. 2012, 26, 1156–1165. [Google Scholar] [CrossRef]
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical scheme for LC-MSn identification of chlorogenic acids. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef]
- Jallali, I.; Zaouali, Y.; Missaoui, I.; Smeoui, A.; Abdelly, C.; Ksouri, R. Variability of antioxidant and antibacterial effects of essential oils and acetonic extracts of two edible halophytes: Crithmum maritimum L. and Inula crithmoïdes L. Food Chem. 2014, 145, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Ruberto, G.; Baratta, M.T.; Deans, S.G.; Dorman, H.J.D. Antioxidant and antimicrobial activity of Foeniculum vulgare and Crithmum maritimum essential oils. Planta Med. 2000, 66, 687–693. [Google Scholar] [CrossRef]
- Kim, J.; Karthivashan, G.; Kweon, M.H.; Kim, D.H.; Choi, D.K. The ameliorative effects of the ethyl acetate extract of Salicornia europaea L. and its bioactive candidate, Irilin B, on LPS-induced microglial inflammation and MPTP-intoxicated PD-like mouse model. Oxid. Med. Cell. Longev. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.C.; Zhang, H.B.; Gu, C.D.; Guo, S.D.; Li, G.; Lian, R.; Yao, Y.; Zhang, G.Q. Protective effect of acacetin on sepsis-induced acute lung injury via its anti-inflammatory and antioxidative activity. Arch. Pharm. Res. 2018, 41, 1199–1210. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.H.; Kim, M.E.; Cho, J.H.; Lee, Y.; Lee, J.; Park, Y.D.; Lee, J.S. Hesperetin inhibits neuroinflammation on microglia by suppressing inflammatory cytokines and MAPK pathways. Arch. Pharm. Res. 2019, 42, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Aumeeruddy-Elalfi, Z.; Mollica, A.; Yilmaz, M.A.; Mahomoodally, M.F. In vitro and in silico perspectives on biological and phytochemical profile of three halophyte species—A source of innovative phytopharmaceuticals from nature. Phytomedicine 2018, 38, 35–44. [Google Scholar] [CrossRef]
- Bandaranayake, W.M. Bioactivities, bioactive compounds and chemical constituents of mangrove plants. Wetl. Ecol. Manag. 2002, 10, 421–452. [Google Scholar] [CrossRef]
- Im, S.A.; Kim, K.; Lee, C.K. Immunomodulatory activity of polysaccharides isolated from Salicornia herbacea. Int. Immunopharmacol. 2006, 6, 1451–1458. [Google Scholar] [CrossRef]
- Apea-Bah, F.B.; Minnaar, A.; Bester, M.J.; Duodu, K.G. Sorghum–cowpea composite porridge as a functional food, Part II: Antioxidant properties as affected by simulated in vitro gastrointestinal digestion. Food Chem. 2016, 197, 307–315. [Google Scholar] [CrossRef]
- Du, N.; Cao, S.; Yu, Y. Research on the adsorption property of supported ionic liquids for ferulic acid, caffeic acid and salicylic acid. J. Chromatogr. B. 2011, 879, 1697–1703. [Google Scholar] [CrossRef]
- Xiang, J.; Zhang, M.; Apea-Bah, F.B.; Beta, T. Hydroxycinnamic acid amide (HCAA) derivatives, flavonoid C-glycosides, phenolic acids and antioxidant properties of foxtail millet. Food Chem. 2019, 295, 214–223. [Google Scholar] [CrossRef]
- Tavares-Da-Silva, E.J.; Varela, C.L.; Pires, A.S.; Encarnação, J.C.; Abrantes, A.M.; Botelho, M.F.; Carvalho, R.A.; Proença, C.; Freitas, M.; Fernandes, E.; et al. Combined dual effect of modulation of human neutrophils’ oxidative burst and inhibition of colon cancer cells proliferation by hydroxycinnamic acid derivatives. Bioorg. Med. Chem. 2016, 24, 3556–3564. [Google Scholar] [CrossRef] [PubMed]
- Karthivashan, G.; Kweon, M.-H.; Park, S.-Y.; Kim, J.-S.; Kim, D.-H.; Ganesan, P.; Choi, D.-K. Cognitive-enhancing and ameliorative effects of acanthoside B in a scopolamine-induced amnesic mouse model through regulation of oxidative/inflammatory/cholinergic systems and activation of the TrkB/CREB/BDNF pathway. Food Chem. Toxicol. 2019, 129, 444–457. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, P.K.; Salem, A.Z.M.; Jena, R.; Kumar, S.; Singh, R.; Puniya, A.K. Rumen microbiology: An overview. In Rumen Microbiology: From Evolution to Revolution; Springer India: Punjab, India, 2015; pp. 1–379. ISBN 9788132224013. [Google Scholar]
- Manzano Durán, R.; Sánchez, J.E.F.; Velardo-Micharet, B.; Gómez, M.J.R. Multivariate optimization of ultrasound-assisted extraction for the determination of phenolic compounds in plums (Prunus salicina Lindl.) by high-performance liquid chromatography (HPLC). Instrum. Sci. Technol. 2020, 48, 113–127. [Google Scholar] [CrossRef]
- He, B.; Zhang, L.L.; Yue, X.Y.; Liang, J.; Jiang, J.; Gao, X.L.; Yue, P.X. Optimization of ultrasound-assisted extraction of phenolic compounds and anthocyanins from blueberry (Vaccinium ashei) wine pomace. Food Chem. 2016, 204, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Padalino, L.; Costa, C.; Del Nobile, M.A.; Conte, A. Extract of Salicornia europaea in fresh pasta to enhance phenolic compounds and antioxidant activity. Int. J. Food Sci. Technol. 2019, 54, 3051–3057. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Martí-Quijal, F.J.; Cilla, A.; Munekata, P.E.S.; Lorenzo, J.M.; Remize, F.; Barba, F.J. Influence of temperature, solvent and pH on the selective extraction of phenolic compounds from tiger nuts by-products: Triple-TOF-LC-MS-MS characterization. Molecules 2019, 24, 797. [Google Scholar] [CrossRef] [Green Version]
- Galanakis, C.; Goulas, V.; Tsakona, S.; Manganaris, G.; Gekas, V. A knowledge base for the recovery of natural phenols with different solvents. Int. J. food Prop. 2013, 16, 382–396. [Google Scholar] [CrossRef] [Green Version]
- Corbin, C.; Fidel, T.; Leclerc, E.A.; Barakzoy, E.; Sagot, N.; Falguiéres, A.; Renouard, S.; Blondeau, J.P.; Ferroud, C.; Doussot, J.; et al. Development and validation of an efficient ultrasound assisted extraction of phenolic compounds from flax (Linum usitatissimum L.) seeds. Ultrason. Sonochem. 2015, 26, 176–185. [Google Scholar] [CrossRef]
- Camel, V. Microwave-assisted solvent extraction of environmental samples. TrAC-Trends Anal. Chem. 2000, 19, 229–248. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Pervin, M.; Unno, K.; Takagaki, A.; Isemura, M.; Nakamura, Y. Function of green tea catechins in the brain: Epigallocatechin gallate and its metabolites. Int. J. Mol. Sci. 2019, 20, 3630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, G.J.; Zhang, Z.; Wen, X.D.; Yu, C.; Calway, T.; Yuan, C.S.; Wang, C.Z. Epigallocatechin gallate (EGCG) is the most effective cancer chemopreventive polyphenol in green tea. Nutrients 2012, 4, 1679–1691. [Google Scholar] [CrossRef]
- Cano, A.; Arnao, M.; Williamson, G.; Garcia-Conesa, M.-T. Superoxide scavenging by polyphenols: Effect of conjugation and dimerization. Redox Rep. 2002, 7, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Lü, J.-M.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J. Cell. Mol. Med. 2010, 14, 840–860. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.; Akinloye, O. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Ungurianu, A.; Şeremet, O.; Gagniuc, E.; Olaru, O.T.; Guţu, C.; Grǎdinaru, D.; Ionescu-Tȋrgovişte, C.; Marginǎ, D.; Dǎnciulescu-Miulescu, R. Preclinical and clinical results regarding the effects of a plant-based antidiabetic formulation versus well established antidiabetic molecules. Pharmacol. Res. 2019, 150, 104522. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Rauf, A.; Abu-Izneid, T.; Nadeem, M.; Shariati, M.A.; Khan, I.A.; Imran, A.; Orhan, I.E.; Rizwan, M.; Atif, M.; et al. Luteolin, a flavonoid, as an anticancer agent: A review. Biomed. Pharmacother. 2019, 112, 108612. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [Green Version]
- Hollman, P.C.H.; Van Trijp, J.M.P.; Buysman, M.N.C.P.; Martijn, M.S.; Mengelers, M.J.B.; De Vries, J.H.M.; Katan, M.B. Relative bioavailability of the antioxidant flavonoid quercetin from various foods in man. FEBS Lett. 1997, 418, 152–156. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.A.; Kong, C.S.; Um, Y.R.; Lim, S.Y.; Yea, S.S.; Seo, Y. Evaluation of Salicornia herbacea as a potential antioxidant and anti-inflammatory agent. J. Med. Food 2009, 12, 661–668. [Google Scholar] [CrossRef]
- Pereira, A.G.; Fraga-Corral, M.; García-Oliveira, P.; Jimenez-Lopez, C.; Lourenço-Lopes, C.; Carpena, M.; Otero, P.; Gullón, P.; Prieto, M.A.; Simal-Gandara, J. Culinary and nutritional value of edible wild plants from northern Spain rich in phenolic compounds with potential health benefits. Food Funct. 2020, 11, 8493–8515. [Google Scholar] [CrossRef] [PubMed]
- Balkrishna, A.; Thakur, P.; Varshney, A. Phytochemical profile, pharmacological attributes and medicinal properties of Convolvulus prostratus–A cognitive enhancer herb for the management of neurodegenerative etiologies. Front. Pharmacol. 2020, 11, 171. [Google Scholar] [CrossRef] [Green Version]
- Chung, Y.C.; Chun, H.K.; Yang, J.Y.; Kim, J.Y.; Han, E.H.; Kho, Y.H.; Jeong, H.G. Tungtungmadic acid, a novel antioxidant, from Salicornia herbacea. Arch. Pharm. Res. 2005, 28, 1122–1126. [Google Scholar] [CrossRef]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, A.; Rojo, A.I.; Wells, G.; Hayes, J.D.; Cousin, S.P.; Rumsey, W.L.; Attucks, O.C.; Franklin, S.; Levonen, A.-L.; Kensler, T.W.; et al. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat. Rev. Drug Discov. 2019, 18, 295–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.S.; Seo, J.Y.; Oh, J.; Jang, Y.K.; Lee, C.H.; Kim, J.-S. Neuroprotective effect of halophyte Salicornia herbacea L. is mediated by activation of heme oxygenase-1 in mouse hippocampal HT22 cells. J. Med. Food 2017, 20, 140–151. [Google Scholar] [CrossRef]
- Taofiq, O.; González-Paramás, A.M.; Barreiro, M.F.; Ferreira, I.C.F.R.; McPhee, D.J. Hydroxycinnamic acids and their derivatives: Cosmeceutical significance, challenges and future perspectives, a review. Molecules 2017, 22, 281. [Google Scholar] [CrossRef]
- Doi, N.; Togari, H.; Minagi, K.; Nakaoji, K.; Hamada, K.; Tatsuka, M. Protective effects of Salicornia europaea on UVB-induced misoriented cell divisions in skin epithelium. Cosmetics 2020, 7, 44. [Google Scholar] [CrossRef]
- Dos Santos, M.D.; Almeida, M.C.; Lopes, N.P.; De Souza, G.E.P. Evaluation of the anti-inflammatory, analgesic and antipyretic activities of the natural polyphenol chlorogenic acid. Biol. Pharm. Bull. 2006, 29, 2236–2240. [Google Scholar] [CrossRef] [Green Version]
- Moalem, G.; Tracey, D.J. Immune and inflammatory mechanisms in neuropathic pain. Brain Res. Rev. 2006, 51, 240–264. [Google Scholar] [CrossRef]
- Bagdas, D.; Cinkilic, N.; Ozboluk, H.Y.; Ozyigit, M.O.; Gurun, M.S. Antihyperalgesic activity of chlorogenic acid in experimental neuropathic pain. J. Nat. Med. 2013, 67, 698–704. [Google Scholar] [CrossRef]
- Bagdas, D.; Ozboluk, H.Y.; Cinkilic, N.; Gurun, M.S. Antinociceptive effect of chlorogenic acid in rats with painful diabetic neuropathy. J. Med. Food 2014, 17, 730–732. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.W.; Liu, T.T.; Qiu, C.Y.; Li, J.D.; Hu, W.P. Inhibition of acid-sensing ion channels by chlorogenic acid in rat dorsal root ganglion neurons. Neurosci. Lett. 2014, 567, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Hara, K.; Haranishi, Y.; Kataoka, K.; Takahashi, Y.; Terada, T.; Nakamura, M.; Sata, T. Chlorogenic acid administered intrathecally alleviates mechanical and cold hyperalgesia in a rat neuropathic pain model. Eur. J. Pharmacol. 2014, 723, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N. Chlorogenic acids and other cinnamates - Nature, occurrence and dietary burden. J. Sci. Food Agric. 1999, 79, 362–372. [Google Scholar] [CrossRef]
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 2011, 403, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Ramani, B.; Reeck, T.; Debez, A.; Stelzer, R.; Huchzermeyer, B.; Schmidt, A.; Papenbrock, J. Aster tripolium L. and Sesuvium portulacastrum L.: Two halophytes, two strategies to survive in saline habitats. Plant Physiol. Biochem. 2006, 44, 395–408. [Google Scholar] [CrossRef]
- Duarte, B.; Goessling, J.W.; Marques, J.C.; Caçador, I. Ecophysiological constraints of Aster tripolium under extreme thermal events impacts: Merging biophysical, biochemical and genetic insights. Plant Physiol. Biochem. 2015, 97, 217–228. [Google Scholar] [CrossRef]
- Geissler, N.; Hussin, S.; Koyro, H.W. Interactive effects of NaCl salinity and elevated atmospheric CO2 concentration on growth, photosynthesis, water relations and chemical composition of the potential cash crop halophyte Aster tripolium L. Environ. Exp. Bot. 2009, 65, 220–231. [Google Scholar] [CrossRef]
- Wubshet, S.G.; Schmidt, J.S.; Wiese, S.; Staerk, D. High-resolution screening combined with HPLC-HRMS-SPE-NMR for identification of potential health-promoting constituents in sea aster and searocket—New nordic food ingredients. J. Agric. Food Chem. 2013, 61, 8616–8623. [Google Scholar] [CrossRef]
- Ong, K.W.; Hsu, A.; Tan, B.K.H. Chlorogenic acid stimulates glucose transport in skeletal muscle via AMPK activation: A contributor to the beneficial effects of coffee on diabetes. PLoS ONE 2012, 7, e32718. [Google Scholar] [CrossRef] [Green Version]
- McCarty, M.F. A chlorogenic acid-induced increase in GLP-1 production may mediate the impact of heavy coffee consumption on diabetes risk. Med. Hypotheses 2005, 64, 848–853. [Google Scholar] [CrossRef]
- McCreight, L.J.; Bailey, C.J.; Pearson, E.R. Metformin and the gastrointestinal tract. Diabetologia 2016, 59, 426–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tousch, D.; Lajoix, A.D.; Hosy, E.; Azay-Milhau, J.; Ferrare, K.; Jahannault, C.; Cros, G.; Petit, P. Chicoric acid, a new compound able to enhance insulin release and glucose uptake. Biochem. Biophys. Res. Commun. 2008, 377, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Johnston, K.L.; Clifford, M.N.; Morgan, L.M. Coffee acutely modifies gastrointestinal hormone secretion and glucose tolerance in humans: Glycemic effects of chlorogenic acid and caffeine. Am. J. Clin. Nutr. 2003, 78, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Gaballah, H.H.; Zakaria, S.S.; Mwafy, S.E.; Tahoon, N.M.; Ebeid, A.M. Mechanistic insights into the effects of quercetin and/or GLP-1 analogue liraglutide on high-fat diet/streptozotocin-induced type 2 diabetes in rats. Biomed. Pharmacother. 2017, 92, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.V.; Mistry, B.M.; Shinde, S.K.; Syed, R.; Singh, V.; Shin, H.-S. Therapeutic potential of quercetin as a cardiovascular agent. Eur. J. Med. Chem. 2018, 155, 889–904. [Google Scholar] [CrossRef] [PubMed]
- Margina, D.; Ilie, M.; Gradinaru, D. Quercetin and epigallocatechin gallate induce in vitro a dose-dependent stiffening and hyperpolarizing effect on the cell membrane of human mononuclear blood cells. Int. J. Mol. Sci. 2012, 13, 4839–4859. [Google Scholar] [CrossRef] [Green Version]
- Blair, J.M.A.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J.V. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef]
- Nemeth, J.; Oesch, G.; Kuster, S.P. Bacteriostatic versus bactericidal antibiotics for patients with serious bacterial infections: Systematic review and meta-analysis. J. Antimicrob. Chemother. 2015, 70, 382–395. [Google Scholar] [CrossRef] [Green Version]
- Kohanski, M.A.; Dwyer, D.J.; Collins, J.J. How antibiotics kill bacteria: From targets to networks. Nat. Rev. Microbiol. 2010, 8, 423–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renna, M. Reviewing the prospects of sea fennel (Crithmum maritimum L.) as emerging vegetable crop. Plants 2018, 7, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flamini, G.; Mastrorilli, E.; Cioni, P.L.; Panizzi, L. Essential oil from crithmum maritimum grown in liguria (Italy): Seasonal variation and antimicrobial activity. J. Essent. Oil Res. 1999, 11, 788–792. [Google Scholar] [CrossRef]
- Zafeiropoulou, V.; Tomou, E.-M.; Douros, A.; Skaltsa, H. The effect of successive harvesting on the volatile constituents of two essential oils of cultivated populations of sea fennel (Crithmum maritimum L.) in Greece. J. Essent. Oil Bear. Plants 2021, 24, 1–11. [Google Scholar] [CrossRef]
- Watanabe, T.; Arai, Y.; Mitsui, Y.; Kusaura, T.; Okawa, W.; Kajihara, Y.; Saito, I. The blood pressure-lowering effect and safety of chlorogenic acid from green coffee bean extract in essential hypertension. Clin. Exp. Hypertens. 2006, 28, 439–449. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Chikama, A.; Mori, K.; Watanabe, T.; Shioya, Y.; Katsuragi, Y.; Tokimitsu, I. Hydroxyhydroquinone-free coffee: A double-blind, randomized controlled dose-response study of blood pressure. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 408–414. [Google Scholar] [CrossRef]
- Kozuma, K.; Tsuchiya, S.; Kohori, J.; Hase, T.; Tokimitsu, I. Antihypertensive effect of green coffee bean extract on mildly hypertensive subjects. Hypertens. Res. 2005, 28, 711–718. [Google Scholar] [CrossRef] [Green Version]
- Ochiai, R.; Jokura, H.; Suzuki, A.; Tokimitsu, I.; Ohishi, M.; Komai, N.; Rakugi, H.; Ogihara, T. Green coffee bean extract improves human vasoreactivity. Hypertens. Res. 2004, 27, 731–737. [Google Scholar] [CrossRef] [Green Version]
- Kanegae, M.P.P.; da Fonseca, L.M.; Brunetti, I.L.; de Oliveira Silva, S.; Ximenes, V.F. The reactivity of ortho-methoxy-substituted catechol radicals with sulfhydryl groups: Contribution for the comprehension of the mechanism of inhibition of NADPH oxidase by apocynin. Biochem. Pharmacol. 2007, 74, 457–464. [Google Scholar] [CrossRef]
- Pastori, C.; Wahlestedt, C. Involvement of long noncoding RNAs in diseases affecting the central nervous system. RNA Biol. 2012, 9, 860–870. [Google Scholar] [CrossRef] [Green Version]
- Bazool Farhood, H.; Balas, M.; Gradinaru, D.; Margină, D.; Dinischiotu, A. Hepatoprotective effects of chlorogenic acid under hyperglycemic conditions. Rom. Biotechnol. Lett. 2019, 24, 301–307. [Google Scholar] [CrossRef]
- Atia, A.; Barhoumi, Z.; Mokded, R.; Abdelly, C.; Smaoui, A. Environmental eco-physiology and economical potential of the halophyte Crithmum maritimum L. (Apiaceae). J. Med. Plants Res. 2011, 5, 3564–3571. [Google Scholar]
- Alves-Silva, J.M.; Guerra, I.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Figueirinha, A.; Salgueiro, L. Chemical composition of Crithmum maritimum L. essential oil and hydrodistillation residual water by GC-MS and HPLC-DAD-MS/MS, and their biological activities. Ind. Crops Prod. 2020, 149, 112329. [Google Scholar] [CrossRef]
- Cunsolo, F.; Ruberto, G.; Amico, V.; Plattelli, M. Bioactive metabolites from sicilian marine fennel, Crithmum maritimum. J. Nat. Prod. 1993, 56, 1598–1600. [Google Scholar] [CrossRef]
- Zidorn, C.; Jöhrer, K.; Ganzera, M.; Schubert, B.; Sigmund, E.M.; Mader, J.; Greil, R.; Ellmerer, E.P.; Stuppner, H. Polyacetylenes from the apiaceae vegetables carrot, celery, fennel, parsley, and parsnip and their cytotoxic activities. J. Agric. Food Chem. 2005, 53, 2518–2523. [Google Scholar] [CrossRef] [PubMed]
- Quan, C.C.; Lee, J.P.; Jin, W.Y.; Youn, U.J.; Kim, H.J.; Ik, S.L.; Zhang, X.F.; Song, K.S.; Seong, Y.H.; Bae, K.H. Cytotoxic constituents from Angelicae sinensis radix. Arch. Pharm. Res. 2007, 30, 565–569. [Google Scholar] [CrossRef]
- Lima, A.R.; Castañeda-Loaiza, V.; Salazar, M.; Nunes, C.; Quintas, C.; Gama, F.; Pestana, M.; Correia, P.J.; Santos, T.; Varela, J.; et al. Influence of cultivation salinity in the nutritional composition, antioxidant capacity and microbial quality of Salicornia ramosissima commercially produced in soilless systems. Food Chem. 2020, 333, 127525. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Cho, J.-Y.; Moon, J.-H.; Choi, G.-C.; Lee, K.-D.; Ham, K.-S.; Kim, S.-J. Change of phenylpropanoic acid and flavonol contents at different growth stage of glasswort (Salicornia herbacea L.). Food Sci. Biotechnol. 2014, 23, 685–691. [Google Scholar] [CrossRef]
- McNaught, A.D.; Wilkinson, A. Flavonoids (isoflavonoids and neoflavonoids). In IUPAC Compendium of Chemical Terminology; IUPAC: Research Triangle, NC, USA, 2008. [Google Scholar]
- Ferreira, D.; Isca, V.; Leal, P.; Seca, A.; Silva, H.; Pereira, M.; Silva, A.; Pinto, D. Salicornia ramosissima: Secondary metabolites and protective effect against acute testicular toxicity. Arab. J. Chem. 2018, 11, 70–80. [Google Scholar] [CrossRef] [Green Version]
- Isca, V.M.S.; Seca, A.M.L.; Pinto, D.C.G.A.; Silva, H.; Silva, A.M.S. Lipophilic profile of the edible halophyte Salicornia ramosissima. Food Chem. 2014, 165, 330. [Google Scholar] [CrossRef] [Green Version]
- Yousef, L.F.; Alkhoori, S.A.; Thomsen, M.H. Compositions from Halophyte Plants and Methods of Use Thereof. U.S. Patent WO 2018125694 A1, 5 July 2018. [Google Scholar]
- Ahn, H.J.; You, H.J.; Park, M.S.; Li, Z.; Choe, D.; Johnston, T.V.; Ku, S.; Ji, G.E. Microbial biocatalysis of quercetin-3-glucoside and isorhamnetin-3-glucoside in Salicornia herbacea and their contribution to improved anti-inflammatory activity. RSC Adv. 2020, 10, 5339–5350. [Google Scholar] [CrossRef] [Green Version]
- Pendás, A.M.; Santamaría, I.; Alvarez, M.V.; Pritchard, M.; López-Otín, C. Fine physical mapping of the human matrix metalloproteinase genes clustered on chromosome 11q22.3. Genomics 1996, 37, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Medeiros, R.; Furtado, A.A.; Zanatta, A.C.; Torres-Rêgo, M.; Guimarães Lourenço, E.M.; Ferreira Alves, J.S.; Galinari, É.; Alexandre de Oliveira Rocha, H.; Bernardo Guerra, G.C.; Vilegas, W.; et al. Mass spectrometry characterization of Commiphora leptophloeos leaf extract and preclinical evaluation of toxicity and anti-inflammatory potential effect. J. Ethnopharmacol. 2021, 264, 113229. [Google Scholar] [CrossRef] [PubMed]
| Anti-Inflammatory | Antibacterial | Antioxidative | References | |
|---|---|---|---|---|
| Aster tripolium |
|
|
| [11,12,14,16,17,18,19,20,21] |
| Crithmum maritimum |
|
|
| [11,12,14,21,22,23,24] |
| Salicornia europaea |
|
|
| [3,15,25,26,27,28] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giordano, R.; Saii, Z.; Fredsgaard, M.; Hulkko, L.S.S.; Poulsen, T.B.G.; Thomsen, M.E.; Henneberg, N.; Zucolotto, S.M.; Arendt-Nielsen, L.; Papenbrock, J.; et al. Pharmacological Insights into Halophyte Bioactive Extract Action on Anti-Inflammatory, Pain Relief and Antibiotics-Type Mechanisms. Molecules 2021, 26, 3140. https://doi.org/10.3390/molecules26113140
Giordano R, Saii Z, Fredsgaard M, Hulkko LSS, Poulsen TBG, Thomsen ME, Henneberg N, Zucolotto SM, Arendt-Nielsen L, Papenbrock J, et al. Pharmacological Insights into Halophyte Bioactive Extract Action on Anti-Inflammatory, Pain Relief and Antibiotics-Type Mechanisms. Molecules. 2021; 26(11):3140. https://doi.org/10.3390/molecules26113140
Chicago/Turabian StyleGiordano, Rocco, Zeinab Saii, Malthe Fredsgaard, Laura Sini Sofia Hulkko, Thomas Bouet Guldbæk Poulsen, Mikkel Eggert Thomsen, Nanna Henneberg, Silvana Maria Zucolotto, Lars Arendt-Nielsen, Jutta Papenbrock, and et al. 2021. "Pharmacological Insights into Halophyte Bioactive Extract Action on Anti-Inflammatory, Pain Relief and Antibiotics-Type Mechanisms" Molecules 26, no. 11: 3140. https://doi.org/10.3390/molecules26113140










