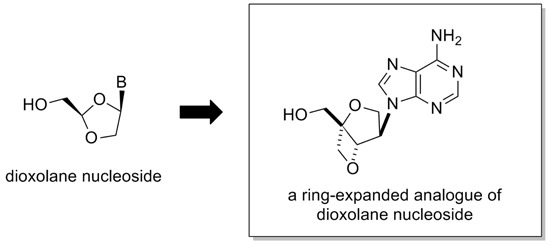
Construction of an Isonucleoside on a 2,6-Dioxobicyclo[3.2.0]-heptane Skeleton
Abstract
:1. Introduction
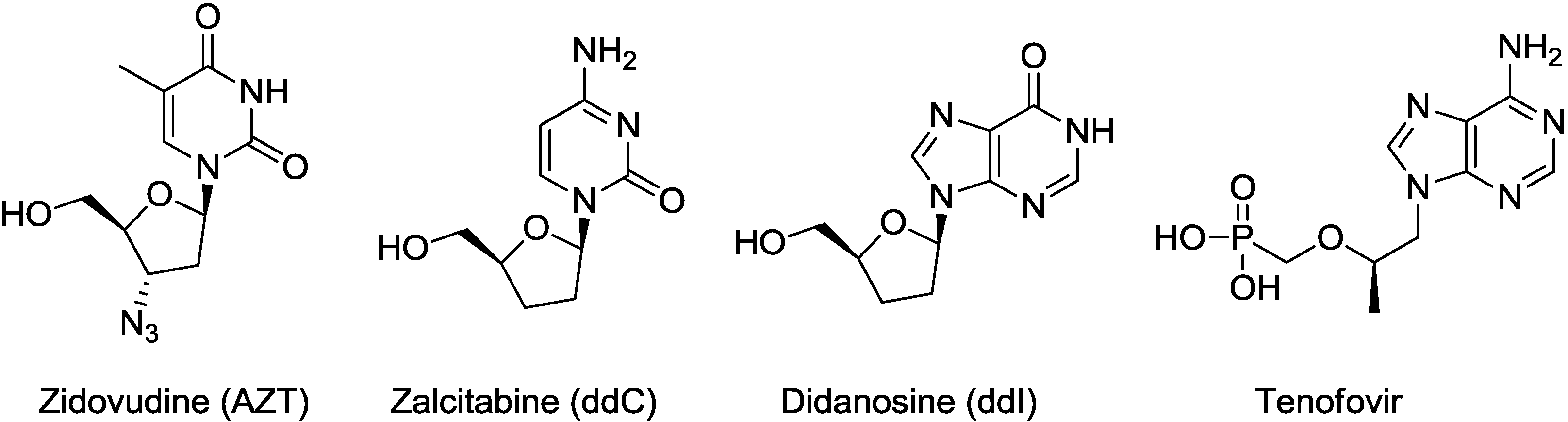
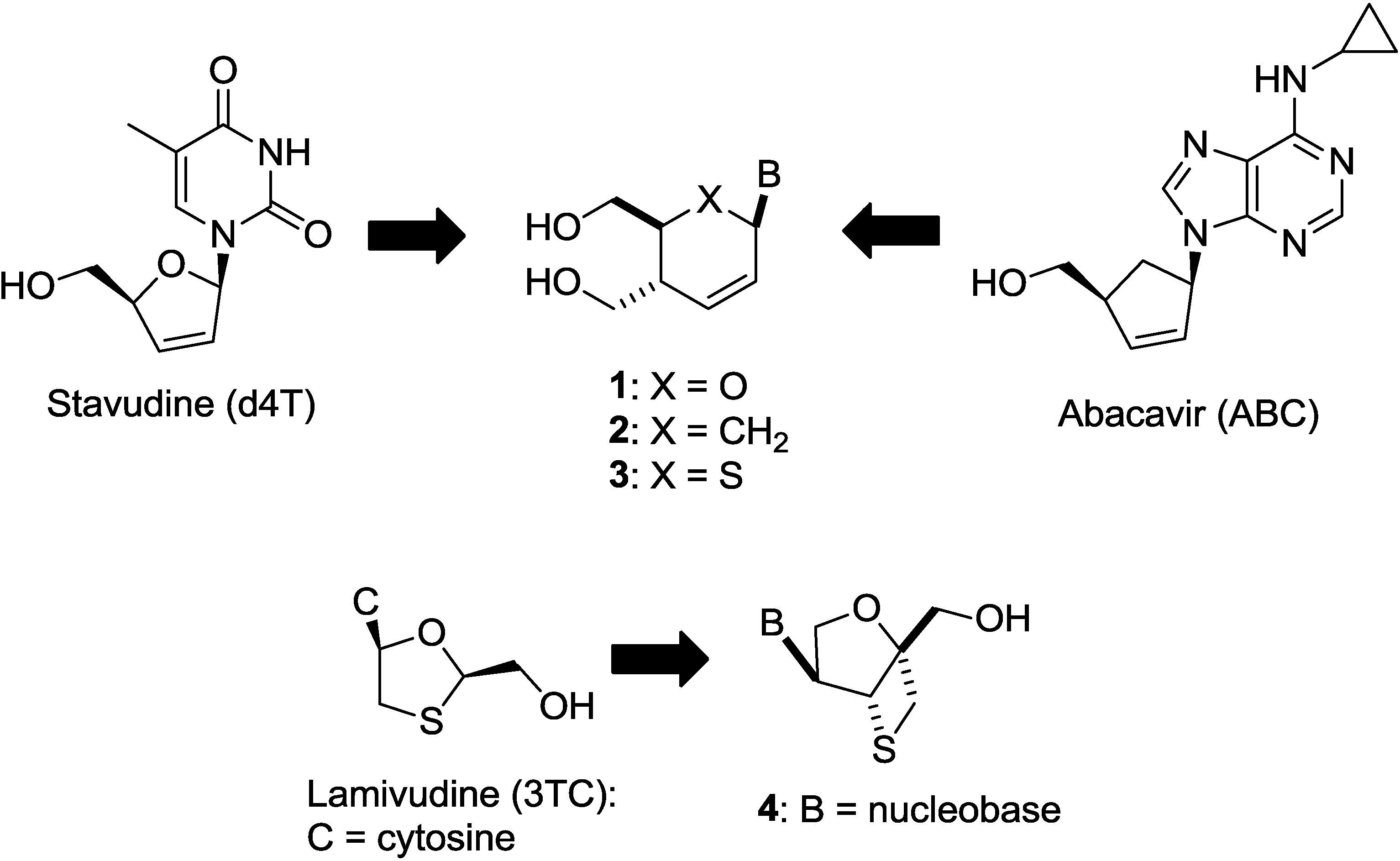
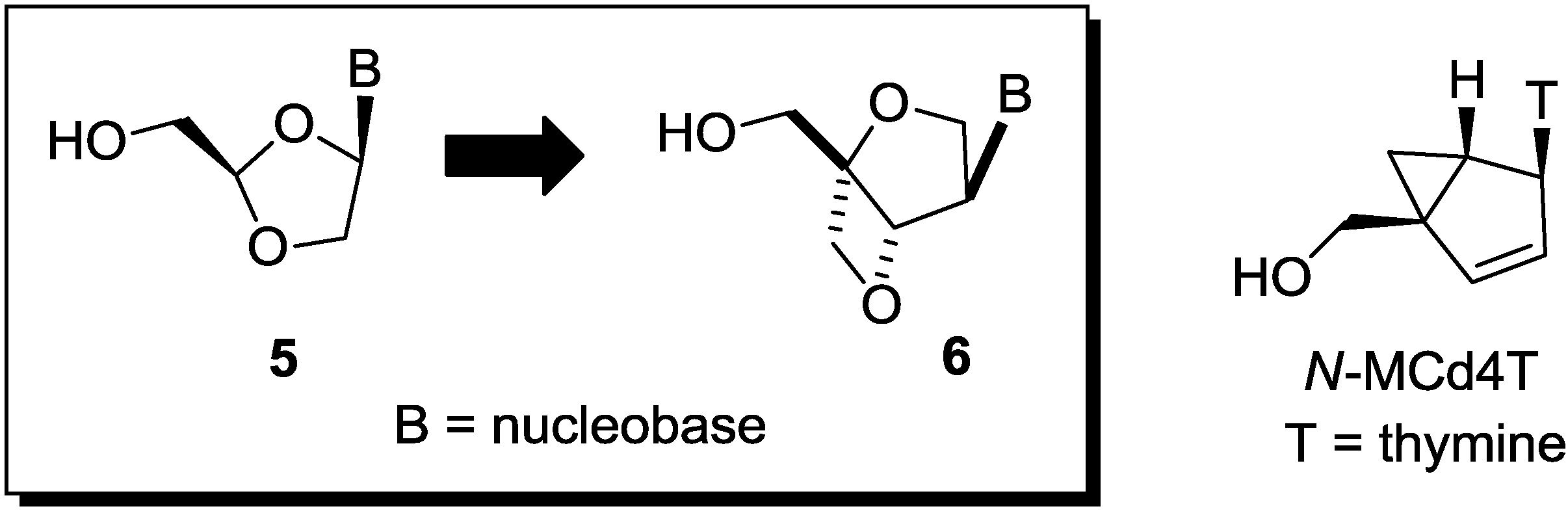
2. Results and Discussion
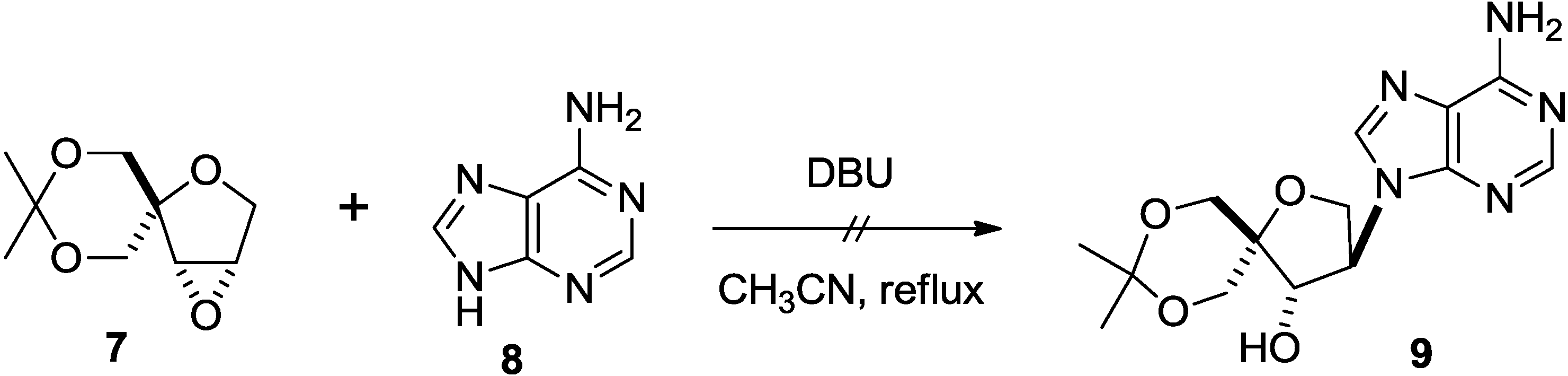
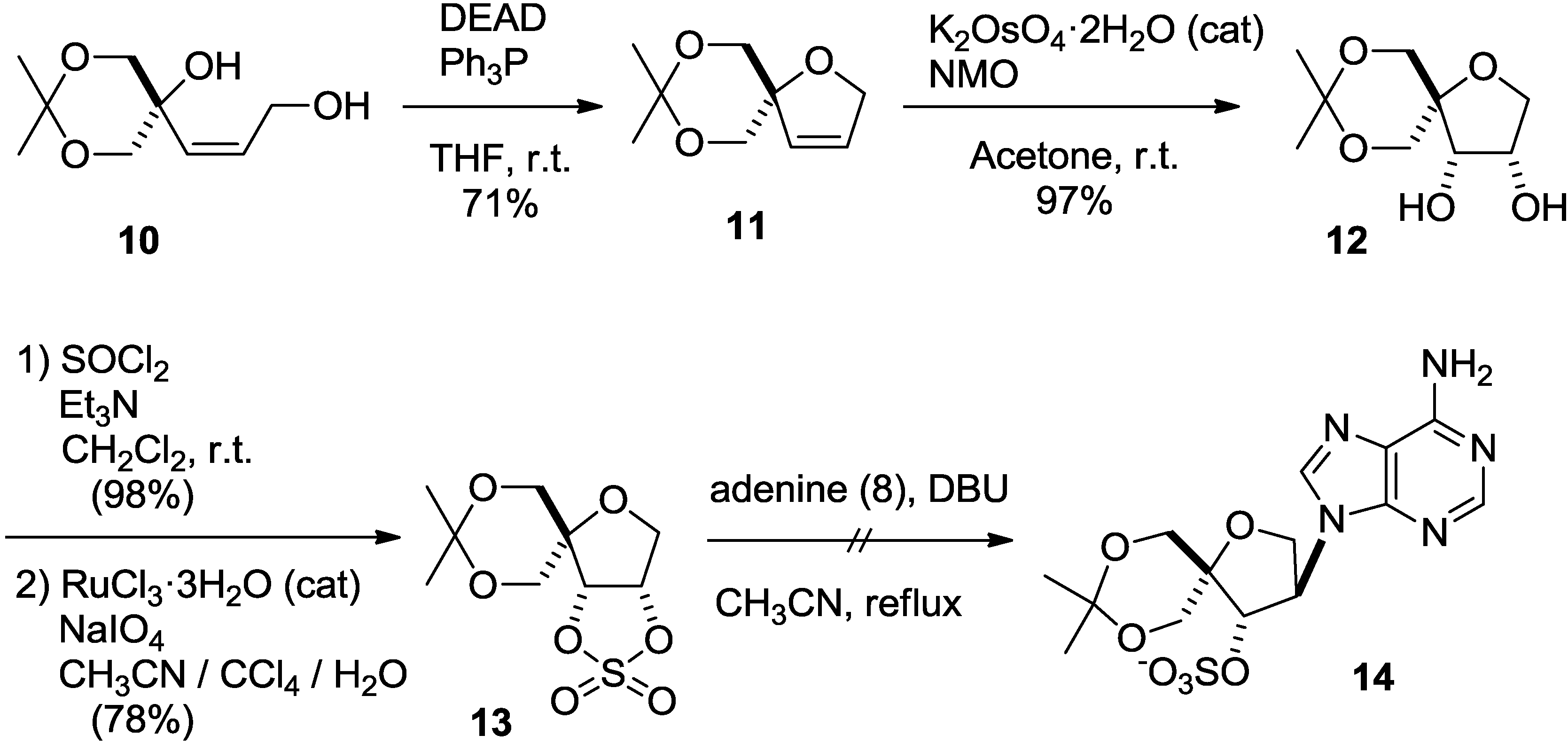
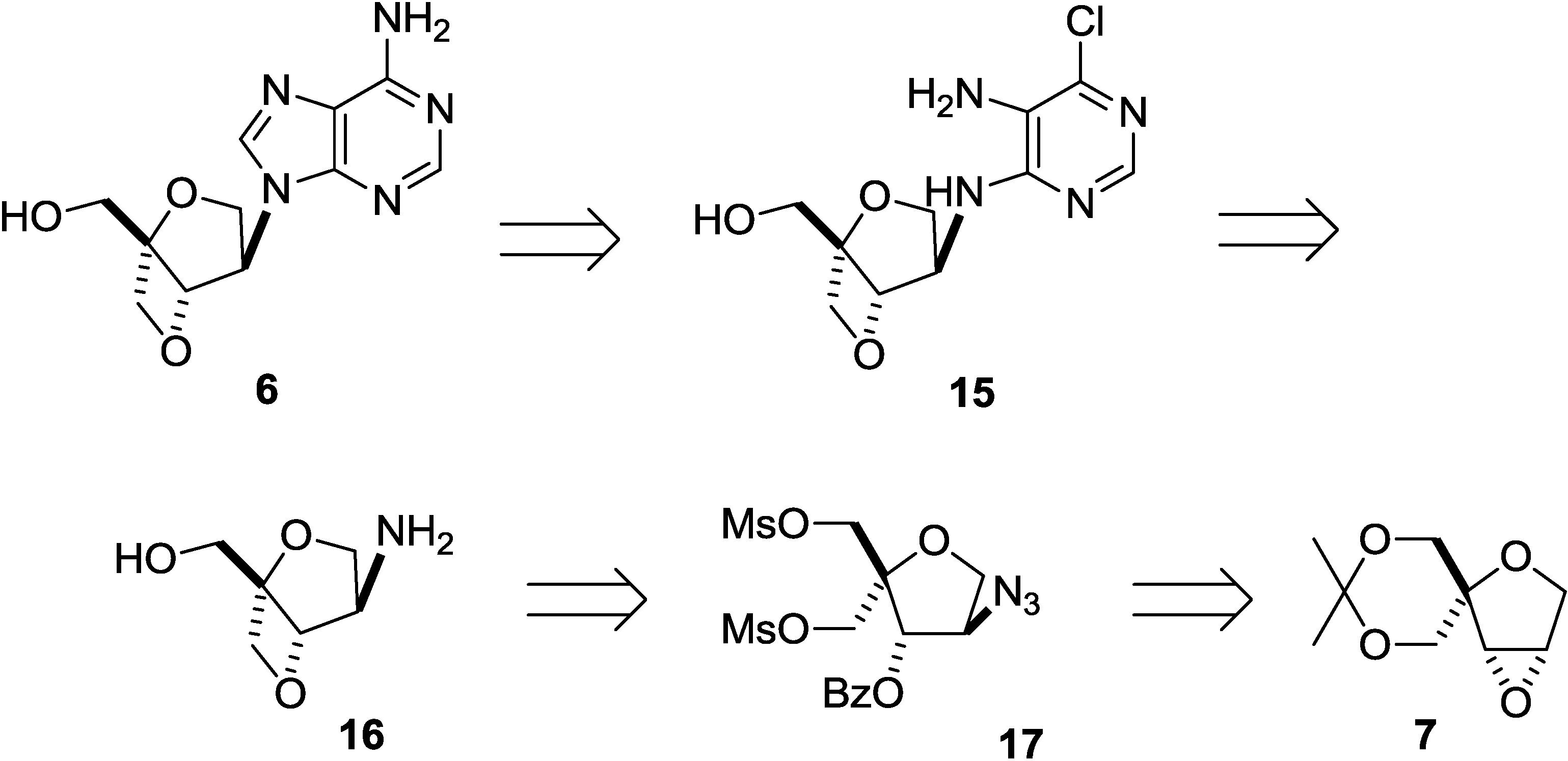
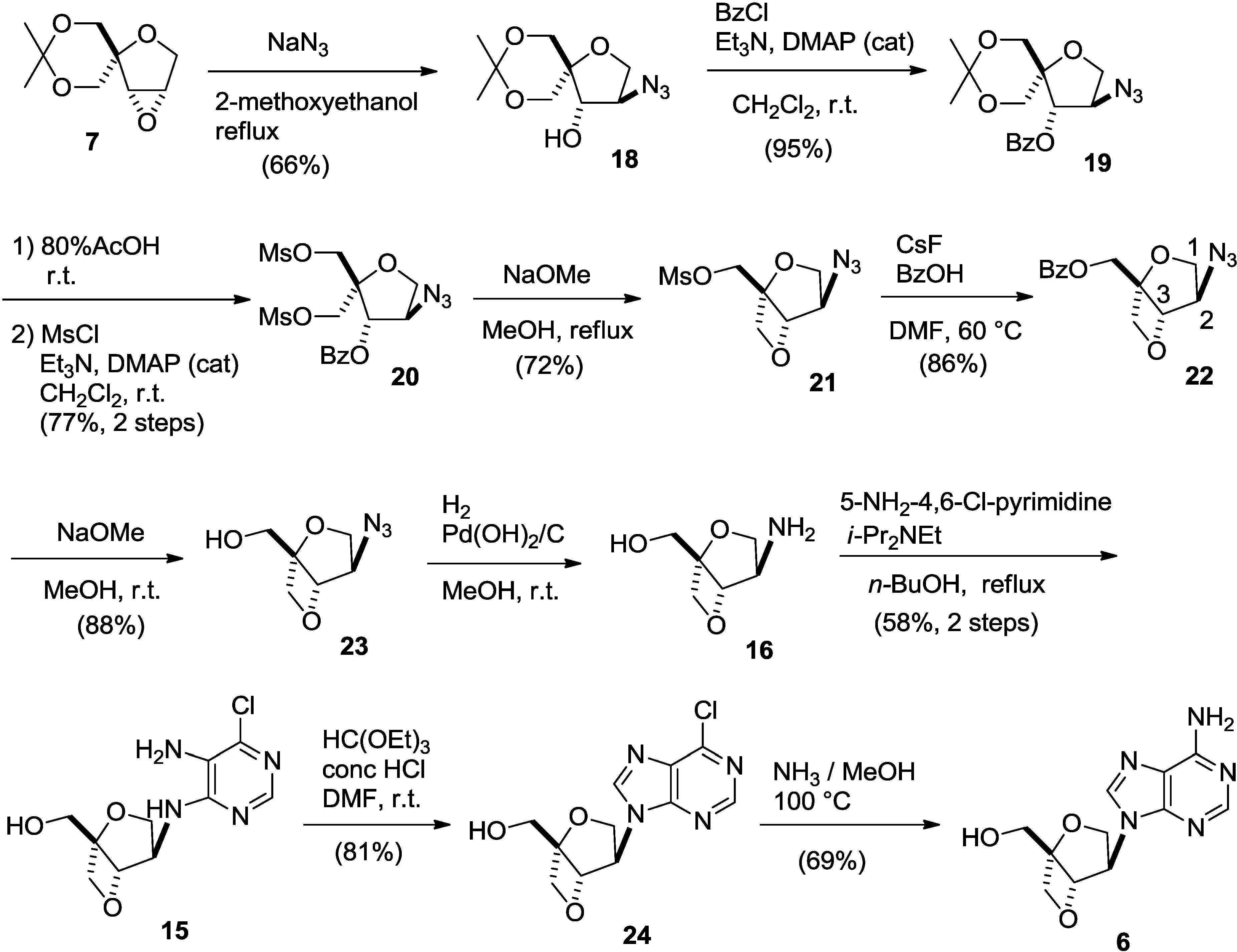
3. Experimental Section
General Information
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cihlar, T.; Ray, A.S. Nucleoside and nucleotide HIV reverse transcriptase inhibitors: 25 years after zidovudine. Antivir. Res. 2010, 85, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Mehellou, Y.; de Clercq, E. Twenty-six years of anti-HIV drug discovery: Where do we stand and where do we go? J. Med. Chem. 2010, 53, 521–538. [Google Scholar] [CrossRef] [PubMed]
- WHO. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection. Available online: http://www.who.int/hiv/pub/guidelines/arv2013/download/en/ (accessed on 30 June 2013).
- Meadows, D.C.; Gervey-Hague, J. Current developments in HIV chemotherapy. ChemMedChem 2006, 1, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Imamichi, T. Action of anti-HIV drugs and resistance: Reverse transcriptase inhibitors and protease inhibitors. Curr. Pharm. Des. 2004, 10, 4039–4053. [Google Scholar] [CrossRef] [PubMed]
- Mitsuya, H.; Broder, S. Inhibition of the in vitro infectivity and cytopathic effect of human T-lymphotrophic virus type III/lymphadenopathy-associated virus (HTLV-III/LAV) by 2',3'-dideoxynucleosides. Proc. Natl. Acad. Sci. USA 1986, 83, 1911–1915. [Google Scholar] [CrossRef] [PubMed]
- Mitsuya, H.; Weinhold, K.J.; Furman, P.A.; St Clair, M.H.; Lehrman, S.N.; Gallo, R.C.; Bolognesi, D.; Barry, D.W.; Broder, S. 3'-Azido-3'-deoxythymidine (BW A509U): An antiviral agent that inhibits the infectivity and cytopathic effect of human T-lymphotropic virus type III/lymphadenopathy-associated virus in vitro. Proc. Natl. Acad. Sci. USA 1985, 82, 7096–7100. [Google Scholar] [CrossRef] [PubMed]
- Schinazi, R.F.; Chu, C.K.; Peck, A.; McMillan, A.; Mathis, R.; Cannon, D.; Jeong, L.S.; Beach, J.W.; Choi, W.B.; Yeola, S.; et al. Activities of the four optical isomers of 2',3'-dideoxy-3'-thiacytidine (BCH-189) against human immunodeficiency virus type 1 in human lymphocytes. Antimicrob. Agents Chemother. 1992, 36, 672–676. [Google Scholar] [CrossRef]
- Balzarini, J.; Holy, A.; Jindrich, J.; Naesens, L.; Snoeck, R.; Schols, D.; de Clercq, E. Differential antiherpesvirus and antiretrovirus effects of the (S) and (R) enantiomers of acyclic nucleoside phosphonates: Potent and selective in vitro and in vivo antiretrovirus activities of (R)-9-(2-phosphonomethoxypropyl)-2,6-diaminopurine. Antimicrob. Agents Chemother. 1993, 37, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, Y.; Yamazaki, Y.; Kawahata, M.; Yamaguchi, K.; Takahata, H. Design and synthesis of a novel ring-expanded 4'-Thio-apio-nucleoside derivatives. Tetrahedron Lett. 2007, 48, 4519–4522. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Asami, K.; Matsui, H.; Tanaka, H.; Takahata, H. New synthesis of (±)-isonucleosides. Org. Lett. 2006, 8, 6015–6018. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, Y.; Yamazaki, Y.; Saito, Y.; Takahata, H. Synthesis of 1-(5,6-dihydro-2H-thiopyran-2-yl)uracil by a Pummerer-type thioglycosylation reaction: The regioselectivity of allylic substitution. Tetrahedron 2009, 65, 9091–9102. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Ohta, M.; Imahori, T.; Imamichi, T.; Takahata, H. A new entry to carbocyclic nucleosides: Oxidative coupling reaction of cycloalkenylsilanes with a nucleobase mediated by hypervalent iodine reagent. Org. Lett. 2008, 10, 3449–3452. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, Y.; Asami, K.; Imamichi, T.; Okuda, T.; Shiraki, K.; Takahata, H. Design and synthesis of isonucleosides constructed on a 2-oxa-6-thiabicyclo[3.2.0]heptane scaffold. J. Org. Chem. 2010, 75, 4161–4171. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, Y.; Yamazaki, Y.; Saito, Y.; Natori, Y.; Imamichi, T.; Takahata, H. Synthesis of 5-thiodidehydropyranylcytosine derivatives as potential anti-HIV agents. Bioorg. Med. Chem. Lett. 2011, 21, 3313–3316. [Google Scholar] [CrossRef] [PubMed]
- Kiran, Y.B.; Wakamatsu, H.; Natori, Y.; Takahata, H.; Yoshimura, Y. Design and synthesis of a nucleoside and a phosphonate analogue constructed on a branched-threo-tetrofuranose skeleton. Tetrahedron Lett. 2013, 54, 3949–3952. [Google Scholar] [CrossRef]
- Kan-no, H.; Saito, Y.; Omoto, S.; Minato, S.; Wakamatsu, H.; Natori, Y.; Imamichi, T.; Takahata, H.; Yoshimura, Y. Synthesis of a dihydropyranonucleoside using an oxidative glycosylation reaction mediated by hypervalent iodine. Synthesis 2014, 46, 879–886. [Google Scholar] [CrossRef]
- Chu, C.K.; Ahn, S.K.; Kim, H.O.; Beach, J.W.; Alves, A.J.; Jeong, L.S.; Islam, Q.; van Roey, P.; Schinazi, R.F. Asymmetric synthesis of enantiomerically pure (−)-(1'R,4'R)-dioxolane-thymine and its anti-HIV activity. Tetrahedron Lett. 1991, 32, 3791–3794. [Google Scholar] [CrossRef]
- Kim, H.O.; Schinazi, R.F.; Nampalli, S.; Shanmuganathan, K.; Cannon, D.L.; Alves, A.J.; Jeong, L.S.; Beach, J.W.; Chu, C.K. 1,3-dioxolanylpurine nucleosides (2R,4R) and (2R,4S) with selective anti-HIV-1 activity in human lymphocytes. J. Med. Chem. 1993, 36, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.O.; Schinazi, R.F.; Shanmuganathan, K.; Jeong, L.S.; Beach, J.W.; Nampalli, S.; Cannon, D.L.; Chu, C.K. l-beta-(2S,4S)- and l-alpha-(2S,4R)-dioxolanyl nucleosides as potential anti-HIV agents: Asymmetric synthesis and structure-activity relationships. J. Med. Chem. 1993, 36, 519–528. [Google Scholar] [CrossRef]
- Choi, Y.; George, C.; Comin, M.J.; Brachi, J.J.; Kim, H.S.; Jacobsen, K.A.; Balzarini, J.; Mitsuya, H.; Boyer, P.L.; Hughes, S.H.; et al. A conformationally locked analogue of the anti-HIV agent stavudine. An important correlation between pseudorotation and maximum amplitude. J. Med. Chem. 2003, 46, 3292–3299. [Google Scholar] [CrossRef] [PubMed]
- D’Alonzo, D.; van Aerschot, A.; Guaragna, A.; Palumbo, G.; Schepers, G.; Capone, S.; Rozenski, J.; Herdewijn, P. Synthesis and base pairing properties of 1',5'-anhydro-l-hexitol nucleic acids (l-HNA). Chem. Eur. J. 2009, 15, 10121–10131. [Google Scholar] [CrossRef] [PubMed]
- Bera, S.; Nair, V. A new general synthesis of isomeric nucleosides. Tetrahedron Lett. 2001, 42, 5813–5815. [Google Scholar] [CrossRef]
- Quadrelli, P.; Piccanello, A.; Mella, M.; Corsaro, A.; Pistarà, V. From cyclopentadiene to isoxazoline-carbocyclic nucleosides: A rapid access to biological molecules through aza-Dielse Alder reactions. Tetrahedron 2008, 64, 3541–3547. [Google Scholar] [CrossRef]
- Comin, M.J.; Vu, B.C.; Boyer, P.L.; Liao, C.; Hughes, S.H.; Marquez, V.E. d-(+)-iso-methanocarbathymidine: A high-affinity substrate for herpes simplex virus 1 thymidine kinase. ChemMedChem 2008, 3, 1129–1134. [Google Scholar] [CrossRef]
- Sample Availability: Sample of the final compound is available from the authors. About the other compounds, please contact the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshimura, Y.; Kobayashi, S.; Kaneko, H.; Suzuki, T.; Imamichi, T. Construction of an Isonucleoside on a 2,6-Dioxobicyclo[3.2.0]-heptane Skeleton. Molecules 2015, 20, 4623-4634. https://doi.org/10.3390/molecules20034623
Yoshimura Y, Kobayashi S, Kaneko H, Suzuki T, Imamichi T. Construction of an Isonucleoside on a 2,6-Dioxobicyclo[3.2.0]-heptane Skeleton. Molecules. 2015; 20(3):4623-4634. https://doi.org/10.3390/molecules20034623
Chicago/Turabian StyleYoshimura, Yuichi, Satoshi Kobayashi, Hitomi Kaneko, Takeshi Suzuki, and Tomozumi Imamichi. 2015. "Construction of an Isonucleoside on a 2,6-Dioxobicyclo[3.2.0]-heptane Skeleton" Molecules 20, no. 3: 4623-4634. https://doi.org/10.3390/molecules20034623
APA StyleYoshimura, Y., Kobayashi, S., Kaneko, H., Suzuki, T., & Imamichi, T. (2015). Construction of an Isonucleoside on a 2,6-Dioxobicyclo[3.2.0]-heptane Skeleton. Molecules, 20(3), 4623-4634. https://doi.org/10.3390/molecules20034623