Clinical Evidence on the Potential Beneficial Effects of Diet and Dietary Supplements against COVID-19 Infection Risk and Symptoms’ Severity
Abstract
:1. Introduction
1.1. Dietary Supplements
1.2. SARS-CoV-2
2. Methods
3. Results
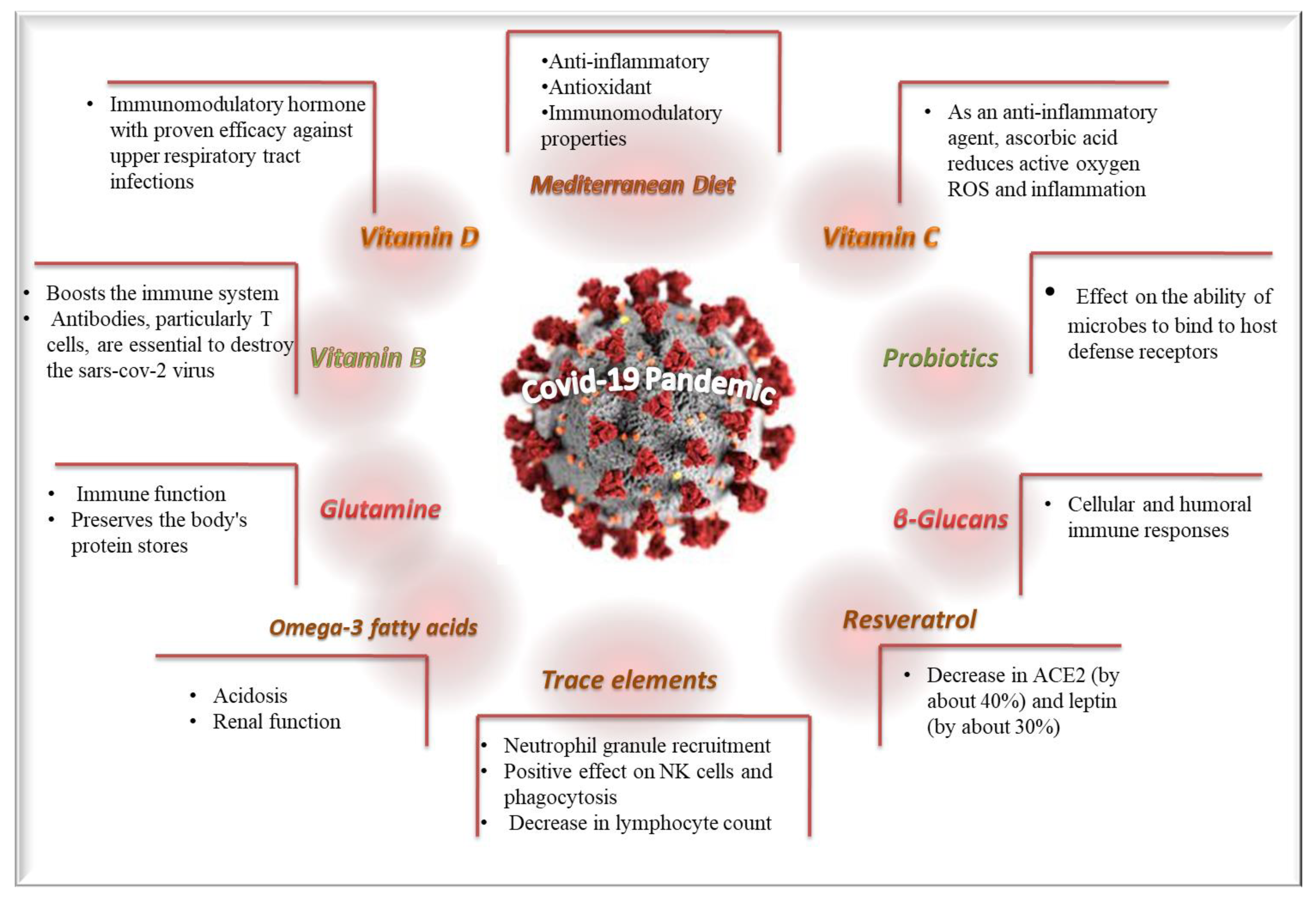
3.1. SARS-CoV-2 and Dietary Supplements
3.1.1. Trace Elements
| Dietary Supplements/Patterns | Study Design | Main Findings | Ref. |
|---|---|---|---|
| Zn Control group: 84.86 ± 19.65 μg/dL, Patients group: 70.32 ± 15.94 μg/dL | Prospective cohort study 88 COVID-19-positive pediatric patients and 88 healthy children. | Zn mean concentrations of COVID-19 positive children were significantly decreased compared to the healthy children. | Doğan et al., 2022 [24] |
| Vitamin D Control group: 18.14 ± 8.81 ng/mL, Patients group: 11.73 ± 4.7 ng/mL | Prospective cohort study 88 COVID-19 positive pediatric patients and 88 healthy children. | The mean serum vitamin D levels of COVID-19 positive patients were statistically significantly lower than the control group. Severe serum vitamin D deficiency in COVID-19-positive patients was statistically significantly higher than in the control group. | Doğan et al., 2022 [24] |
| Zn Control group: 86.66 ± 11.76 μg/dL, Patients group: 67.61 ± 15.10 μg/dL | Case-control study 93 hospitalized patients with COVID-19 and 186 healthy subjects with no symptoms of COVID-19. | Serum Zn concentrations were reduced in individuals with COVID-19 compared to healthy ones. | Elham et al., 2021 [25] |
| Ca (Control group: 9.50 ± 0.52 mg/dL, Patients group: 9.46 ± 0.58 mg/dL) | Case-control study 93 hospitalized patients with COVID-19 and 186; healthy subjects with no symptoms of COVID-19. | The serum calcium level significantly differed between patients and healthy groups among men and women. | Elham et al., 2021 [25] |
| Vitamin D Control group 22.83 ± 12.97 ng/mL, Patients groups: 27.50 ± 15.35 ng/mL) | Case-control study 93 hospitalized patients with COVID-19 and 186 healthy subjects with no symptoms of COVID-19. | Serum vitamin D levels in COVID-19 patients are lower than in the control group. | Elham et al., 2021 [25] |
| Zn 50 mg for 10 days | Randomized clinical open-label trial Standard care patients: n = 50; Interventional group: n = 58. | Zn supplementation was not significantly related with reduced COVID-19 symptomatology intensity, as well as duration compared with standard care. | Thomas et al., 2021 [26] |
| Vitamin C Control patients: standard care Interventional group: 8000 mg for 10 days | Randomized clinical open-label trial Standard care patients: n = 50; Interventional group: n = 48. | Vitamin C supplementation was not significantly related with reduced COVID-19 symptomatology intensity, as well as duration compared with standard care. | Thomas et al., 2021 [26] |
| Se | Population-based, retrospective study | COVID-19 cure rates were associated with Se levels. | Zhang et al., 2020 [29] |
| Mg-Ca ratio cut-off: 0.20 | Cross-sectional retrospective study Recovery patients: n = 510; Death patients: n = 554. | A Mg-to-Ca ratio ≤ 0.20 was highly associated with death rates in individuals presenting severe COVID-19. | Guerrero-Romero et al., 2022 [31] |
| Vitamin A Control group: hydroxychloroquine Experimental group: hydroxychloroquine + 25,000 IU/d oral vitamin A for 10 days | Triple-blind controlled randomized clinical trial Control group: n = 91; Experimental group: n = 91. | There were significant differences in reduction in fever, body pain, exhaustion, and white blood cell count for the intervention group compared to control group. | Rohani et al., 2022 [32] |
| Fe Outpatients group = 8.6 (5.0–14.9) μmol/L Inpatients group = 2.6 (1.8–3.9) μmol/L Outpatients admitted to hospital because of health deterioration = 3.2 (2.4–4.6) μmol/L | Small, retrospective cohort study 204 outpatients; 81 inpatients; 23 outpatients later admitted to hospital because of health deterioration. | An association between low serum Fe levels with COVID-19 related mortality and disease severity was noted. Serum iron and ferritin were significantly associated with hospitalization, whereby doubling of serum iron was associated with a 6.7-fold lower odds of hospitalization. | Hippchen et al., 2020 [36] |
| Folic acid (Vitamin B9), 5 mg or greater | Randomized, double-blind, placebo-controlled trial | No impact on disease progression was observed. | Wiltshire et al., 2020 [37] |
| Vitamin C | Retrospective cohort study Participants: n = 372 720 UK, n = 45 757 Sweden n = 27 373. | Vitamin C supplementation did not exert any significant effect against SARS-CoV-2 infection. There was only a positive association in male participants aged >60 years, receiving vitamin C supplements for testing positive for SARS-CoV-2. | Louca et al., 2021 [38] |
| Vitamin C Enteral vitamin C in a dose of 1000 mg daily with a median duration of administration of 11 days | Two-center, non-interventional, retrospective cohort study Control group: n = 581 patients; Interventional group: n = 158 patients. | Vitamin C as an adjunctive therapeutic agent against COVID-19 was not related with mortality benefits; however, it was associated with a reduced prevalence of thrombosis. | Al Sulaiman et al., 2021 [39] |
| Vitamin D Control group: 100,000 IU. Intervention group: 400,000 IU Duration: 28 days | Open-label, multicenter, randomized controlled superiority clinical trial Control group: 130 patients; Intervention group: 130 patients. | Enhanced vitamin D supplement seems to be an effective, well-tolerated, and easily and immediately accessible treatment for COVID-19. | Annweiler et al., 2020 [40] |
| Vitamin D Standard treatment: 1000 UI oral vitamin D3 Case treatment: 5000 IU oral vitamin D3 Duration: 2 weeks | Multicenter randomized clinical trial Standard control: n = 33 hospitalized patients; Case control, n = 36 hospitalized patients. | A 5000 IU daily oral vitamin D3 supplementation for 2 weeks reduces the time to recovery for cough and gustatory sensory loss among patients with sub-optimal vitamin D status and mild to moderate COVID-19 symptoms. | Sabico et al., 2021 [41] |
| Vitamin D Daily oral 1000IU dose of vitamin D3, 150 mg of Mg, and 500 μg vitamin B12 for ≤14 days | Retrospective observational cohort study Control group: n = 17 patients; Interventional group: n = 26 patients. | A vitamin D/Mg/vitamin B12 combination was associated with a considerable decrease in the percentage of patients with clinical weakening, requiring oxygen support, intensive care support, or both of them. | Tan et al., 2020 [42] |
| Vitamin D Group A: 25(OH)D levels ˂ 20 ng/mL, n = 309 Group B: 25(OH)D levels ± 20 ng/mL, n = 155 | Multicenter observational study Included patients: n = 464. | Serum 25(OH)D levels < 12 ng/mL were strongly associated with COVID-19 severity and mortality among a sample of affected people. | Al Safar et al., [43] |
| Vitamin D Higher-dose: 3200 IU/day Lower-dose: 800 IU/day Follow-up: 2 weeks | Randomized controlled trial Lower intake group: n = 1550; Higher intake group: n = 1550. | Vitamin D did not affect the defensive efficacy or immunogenicity of SARS-CoV-2 vaccination when administered to adults presenting lower levels of vitamin D at disease onset. | Jollife et al., 2022 [44] |
| Vitamin D Group 1: 52,000 IU monthly Group 2: dietary-hygienic measures Follow-up: 3- to 6-months | Randomized controlled clinical trial Hospital workers with 25(OH)D3 levels between 20 and 100 ng/mL and no previous SARS-CoV-2 infection: n = 198. | Vitamin D supplement in participants presenting 25(OH)D3 concentrations at a range of 20–100 ng/mL exhibited a decreased occurrence of SARS-CoV-2 infection compared to the utilization of nutritional-hygienic measures at a 6-month follow-up. | Romero-Ibarguengoitia et al., 2023 [45] |
| Omega-3 fatty acids Control group: Hydroxychloroquine Intervention group: Hydroxychloroquine plus 2 grams of Docosahexaenoic acid [DHA] + Eicosapen-taenoic acid [EPA]) Duration: 2 weeks | Single-blind randomized controlled study Control group: n = 15; Intervention group: n = 15. | Omega-3-supplemented COVID-19 patients showed improved clinical complaints except for bodily pain and tiredness, for appetite, and olfactory. Both CRP and ESR were also reduced by omega-3 supplement than the control group following therapy. | Sedighiyan et al., 2021 [46] |
| Omega-3 fatty acids Intervention: fortified formula with n3-PUFA for 2 weeks | Double-blind, randomized clinical trial Control group: n = 86; Intervention group: n = 42. | The intervention group had a significantly enhanced one-month survival rate, arterial pH, HCO3, and Be levels, as well as arterial BUN, Cr, and K levels, compared with the control group. | Doaei et al., 2021 [47] |
| Glutamine Case group consumed 10 g of glutamine supplement three times per day for a duration of 5 days | Case-control clinical study Control group: n = 230; Case group: n = 232. | Glutamine supplementation significantly increased patients’ appetite relative to the control group, and considerably decreased serum concentrations of TNF-a, high-sensitivity CRP, and IL-1. | Mohajeri et al., 2021 [48] |
| Resveratrol 30 days of 150 mg/day trans-resveratrol | Placebo-controlled cross-over clinical study Control group: n = 10; Intervention group: n = 11. | Resveratrol treatment reduced ACE2 in AT, which may inhibit the spread of SARS-CoV-2 in COVID-19. | De Ligt et al., 2022 [49] |
| Beta-glycan 516.67 mg of β-1,3/1,6-glucan for 30- 35 days | Single-center, randomized, double-blind, placebo-controlled study Control group: n = 34; Intervention group: n = 33. | A supplement including beta-glycan showed that COVID-19 patients group experienced higher increases in IgG and IgM than the placebo group. The beta-glycan supplement increased the ability to stimulate trained immunity. | Rodriguez et al., 2021 [50] |
| Probiotics Bifidobacterium strains (25 billion CFUs per capsule), galactooligosaccharides, xylooligosaccharide, and resistant dextrin Treatment for 4 weeks Follow-up for 9 months | Longitudinal fecal metagenomic profiling study Control group: n = 10; Intervention group, n = 22. | Probiotics dramatically lowered the ARGs reservoir in the intestinal microbiome of individuals with COVID-19 infection. | Su et al., 2022 [51] |
| Probiotics 4 capsules ImmunoSEB (500 mg/capsule) + ProbioSEB CSC3 (5 billion CFUs/capsule) for 14 days | Randomized, multicentric, double blind, placebo-controlled clinical trial Control group: n = 100; Intervention group: n = 100. | Probiotics’ supplement treatment resulted in the resolution of fatigue in a higher proportion of patients of the interventional group compared to the control group. Patients in the interventional group had a significantly elevated decrease in total, physical, and mental fatigue scoring at all time points compared to the non-interventional group. | Rathi et al., 2021 [52] |
| Mediterranean diet adherence Medi-lite adherence score | Observational retrospective study No COVID-19 infected: n = 752; COVID-19 infected: n = 148. | Patients presenting SARS-CoV-2 infection had a considerably reduced MD compliance (e.g., decreased intake of fruits, vegetables, cereals, and olive oil). Patients presenting SARS-CoV-2 infection with no symptoms documented a decreased consumption of saturated fats compared to those with symptoms. Patients needing hospitalization stated more unhealthy nutritional behaviors compared to both asymptomatic and symptomatic individuals. | Ponzo et al., 2013 [53] |
| Mediterranean diet adherence Mediterranean diet score (MDS) | Prospective and multipurpose cohort study n = 9413 participants; n = 369 participants infected by COVID-19. | Individuals with intermediate MD adherence had significantly decreased probability of developing COVID-19. | Perez-Araluce et al., 2022 [54] |
| Mediterranean diet adherence MedDietScore | Cross-sectional clinical study n = 3721 participants. | Higher MD compliance was independently related with a decreased probability of abdominal obesity, enhanced physical activity, higher incidence of good sleep quality, improved quality of life, and reduced probability of anxiety and depression throughout the COVID-19 pandemic period. | Pavlidou et al., 2021 [55] |
3.1.2. Vitamin A
3.1.3. Vitamins B Complex
3.1.4. Vitamin C
3.1.5. Vitamin D
3.1.6. Omega-3 Fatty Acids
3.1.7. Glutamine
3.1.8. Resveratrol
3.1.9. Beta-Glucans
3.1.10. Probiotics
3.2. SARS-CoV-2 and Dietary Patterns
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Almubarak, S.H.; Alsaif, A.K.; Almulla, S.J.; Alfayez, A.S.; Alnujaidi, H.Y.; Alsalman, D.M. Teleworking during COVID-19: Experiences from Saudi Arabia. Ind. Health 2023, 61, 291–303. [Google Scholar] [CrossRef]
- Pérez-Cano, H.J.; Moreno-Murguía, M.B.; Morales-López, O.; Crow-Buchanan, O.; English, J.A.; Lozano-Alcázar, J.; Somilleda-Ventura, S.A. Anxiety, depression, and stress in response to the coronavirus disease-19 pandemic. Cir. Cir. 2020, 88, 562–568. [Google Scholar] [CrossRef]
- Mrityunjaya, M.; Pavithra, V.; Neelam, R.; Janhavi, P.; Halami, P.M.; Ravindra, P.V. Immune-Boosting, Antioxidant and Anti-Inflammatory Food Supplements Targeting Pathogenesis of COVID-19. Front. Immunol. 2020, 11, 570122. [Google Scholar] [CrossRef]
- Lordan, R.; Rando, H.M.; Greene, C.S. Dietary Supplements and Nutraceuticals under Investigation for COVID-19 Prevention and Treatment. mSystems 2021, 6, e00122-21. [Google Scholar] [CrossRef]
- Mortaz, E.; Bezemer, G.; Alipoor, S.D.; Varahram, M.; Mumby, S.; Folkerts, G.; Garssen, J.; Adcock, I.M. Nutritional Impact and Its Potential Consequences on COVID-19 Severity. Front. Nutr. 2021, 8, 698617. [Google Scholar] [CrossRef] [PubMed]
- Paces, J.; Strizova, Z.; Smrz, D.; Cerny, J. COVID-19 and the Immune System. Physiol. Res. 2020, 69, 379–388. [Google Scholar] [CrossRef]
- Tyrovolas, S.; Giné-Vázquez, I.; Fernández, D.; Morena, M.; Koyanagi, A.; Janko, M.; Haro, J.M.; Lin, Y.; Lee, P.; Pan, W.; et al. Estimating the COVID-19 Spread through Real-Time Population Mobility Patterns: Surveillance in Low- and Middle-Income Countries. J. Med. Internet Res. 2021, 23, e22999. [Google Scholar] [CrossRef]
- Mey, J.T.; Kirwan, J.P.; Axelrod, C.L. The Role of Nutrition in Mitigating the Effects of COVID-19 from Infection through PASC. Nutrients 2023, 15, 866. [Google Scholar] [CrossRef]
- Ladds, E.; Rushforth, A.; Wieringa, S.; Taylor, S.; Rayner, C.; Husain, L.; Greenhalgh, T. Persistent symptoms after COVID-19: Qualitative study of 114 “long Covid” patients and draft quality principles for services. BMC Health Serv. Res. 2020, 20, 1144. [Google Scholar] [CrossRef] [PubMed]
- Carfì, A.; Bernabei, R.; Landi, F.; Gemelli against COVID-19 Post-Acute Care Study Group. Persistent Symptoms in Patients after Acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Kompaniyets, L.; Goodman, A.B.; Belay, B.; Freedman, D.S.; Sucosky, M.S.; Lange, S.J.; Gundlapalli, A.V.; Boehmer, T.K.; Blanck, H.M. Body Mass Index and Risk for COVID-19-Related Hospitalization, Intensive Care Unit Admission, Invasive Mechanical Ventilation, and Death—United States, March-December 2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 355–361. [Google Scholar] [CrossRef]
- Maughan, R.J.; King, D.S.; Lea, T. Dietary supplements. J. Sports Sci. 2004, 22, 95–113. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y. Potential Interventions for Novel Coronavirus in China: A Systematic Review. J. Med. Virol. 2020, 92, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Vanherwegen, A.-S.; Gysemans, C.; Mathieu, C. Regulation of Immune Function by Vitamin D and Its Use in Diseases of Immunity. Endocrinol. Metab. Clin. N. Am. 2017, 46, 1061–1094. [Google Scholar] [CrossRef] [PubMed]
- Maares, M.; Haase, H. Zinc and Immunity: An Essential Interrelation. Arch. Biochem. Biophys. 2016, 611, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Kanauchi, O.; Andoh, A.; AbuBakar, S.; Yamamoto, N. Probiotics and Paraprobiotics in Viral Infection: Clinical Application and Effects on the Innate and Acquired Immune Systems. Curr. Pharm. Des. 2018, 24, 710–717. [Google Scholar] [CrossRef]
- Arora, I.; White, S.; Mathews, R. Global Dietary and Herbal Supplement Use during COVID-19-A Scoping Review. Nutrients 2023, 15, 771. [Google Scholar] [CrossRef]
- Feng, Z.; Yang, J.; Xu, M.; Lin, R.; Yang, H.; Lai, L.; Wang, Y.; Wahner-Roedler, D.L.; Zhou, X.; Shin, K.M.; et al. Dietary supplements and herbal medicine for COVID-19: A systematic review of randomized control trials. Clin. Nutr. ESPEN 2021, 44, 50–60. [Google Scholar] [CrossRef]
- Yadav, P.D.; Sarkale, P.; Razdan, A.; Gupta, N.; Nyayanit, D.A.; Sahay, R.R.; Potdar, V.; Patil, D.Y.; Baradkar, S.; Kumar, A.; et al. Isolation and Characterization of SARS-CoV-2 Beta Variant from UAE Travelers. J. Infect. Public Health 2022, 15, 182–186. [Google Scholar] [CrossRef]
- Raghavan, K.; Dedeepiya, V.D.; Suryaprakash, V.; Rao, K.-S.; Ikewaki, N.; Sonoda, T.; Levy, G.G.; Iwasaki, M.; Senthilkumar, R.; Preethy, S.; et al. Beneficial effects of novel aureobasidium pullulans strains produced beta-1,3-1,6 glucans on interleukin-6 and D-dimer levels in COVID-19 patients; results of a randomized multiple-arm pilot clinical study. Biomed. Pharmacother. 2022, 145, 112243. [Google Scholar] [CrossRef]
- Razzaque, M.S. COVID-19 Pandemic: Can Zinc Supplementation Provide an Additional Shield against the Infection? Comput. Struct. Biotechnol. J. 2021, 19, 1371–1378. [Google Scholar] [CrossRef]
- Science, M.; Johnstone, J.; Roth, D.E.; Guyatt, G.; Loeb, M. Zinc for the Treatment of the Common Cold: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. CMAJ 2012, 184, E551–E561. [Google Scholar] [CrossRef]
- Balboni, E.; Zagnoli, F.; Filippini, T.; Fairweather-Tait, S.J.; Vinceti, M. Zinc and Selenium Supplementation in COVID-19 Prevention and Treatment: A Systematic Review of the Experimental Studies. J. Trace Elem. Med. Biol. 2022, 71, 126956. [Google Scholar] [CrossRef] [PubMed]
- Doğan, A.; Dumanoğlu Doğan, İ.; Uyanık, M.; Köle, M.T.; Pişmişoğlu, K. The Clinical Significance of Vitamin D and Zinc Levels with Respect to Immune Response in COVID-19 Positive Children. J. Trop. Pediatr. 2022, 68, fmac072. [Google Scholar] [CrossRef] [PubMed]
- Elham, A.S.; Azam, K.; Azam, J.; Mostafa, L.; Nasrin, B.; Marzieh, N. Serum vitamin D, calcium, and zinc levels in patients with COVID-19. Clin. Nutr. ESPEN 2021, 43, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Patel, D.; Bittel, B.; Wolski, K.; Wang, Q.; Kumar, A.; Il’Giovine, Z.J.; Mehra, R.; McWilliams, C.; Nissen, S.E.; et al. Effect of High-Dose Zinc and Ascorbic Acid Supplementation vs. Usual Care on Symptom Length and Reduction among Ambulatory Patients with SARS-CoV-2 Infection: The COVID A to Z Randomized Clinical Trial. JAMA Netw. Open 2021, 4, e210369. [Google Scholar] [CrossRef] [PubMed]
- SeyedAlinaghi, S.; Shahidi, R.; Mojdeganlou, H.; Akhtaran, F.K.; Maroufi, S.F.; Maroufi, S.P.; Mirzapour, P.; Karimi, A.; Khodaei, S.; Pour, M.M.; et al. The effect of macronutrient and micronutrient supplements on COVID-19: An umbrella review. J. Health Popul. Nutr. 2024, 43, 16. [Google Scholar] [CrossRef] [PubMed]
- Golin, A.; Tinkov, A.A.; Aschner, M.; Farina, M.; da Rocha, J.B. Relationship between Selenium Status, Selenoproteins and COVID-19 and Other Inflammatory Diseases: A Critical Review. J. Trace Elem. Med. Biol. 2023, 75, 127099. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Taylor, E.W.; Bennett, K.; Saad, R.; Rayman, M.P. Association between regional selenium status and reported outcome of COVID-19 cases in China. Am. J. Clin. Nutr. 2020, 111, 1297–1299. [Google Scholar] [CrossRef] [PubMed]
- Bermano, G.; Méplan, C.; Mercer, D.K.; Hesketh, J.E. Selenium and viral infection: Are there lessons for COVID-19? Br. J. Nutr. 2021, 125, 618–627. [Google Scholar] [CrossRef]
- Guerrero-Romero, F.; Mercado, M.; Rodriguez-Moran, M.; Ramírez-Renteria, C.; Martínez-Aguilar, G.; Marrero-Rodríguez, D.; Ferreira-Hermosillo, A.; Simental-Mendía, L.E.; Remba-Shapiro, I.; Gamboa-Gómez, C.I.; et al. Magnesium-to-Calcium Ratio and Mortality from COVID-19. Nutrients 2022, 14, 1686. [Google Scholar] [CrossRef]
- Rohani, M.; Mozaffar, H.; Mesri, M.; Shokri, M.; Delaney, D.; Karimy, M. Evaluation and Comparison of Vitamin a Supplementation with Standard Therapies in the Treatment of Patients with COVID-19. East. Mediterr. Health J. 2022, 28, 673–681. [Google Scholar] [CrossRef]
- Kumar, V.; Choudhry, V.P. Iron deficiency and infection. Indian J. Pediatr. 2010, 77, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Litton, E.; Lim, J. Iron Metabolism: An Emerging Therapeutic Target in Critical Illness. Crit. Care 2019, 23, 81. [Google Scholar] [CrossRef] [PubMed]
- Sonnweber, T.; Boehm, A.; Sahanic, S.; Pizzini, A.; Aichner, M.; Sonnweber, B.; Kurz, K.; Koppelstätter, S.; Haschka, D.; Petzer, V.; et al. Persisting alterations of iron homeostasis in COVID-19 are associated with non-resolving lung pathologies and poor patients’ performance: A prospective observational cohort study. Respir. Res. 2020, 21, 276. [Google Scholar] [CrossRef] [PubMed]
- Hippchen, T.; Altamura, S.; Muckenthaler, M.U.; Merle, U. Hypoferremia is Associated with Increased Hospitalization and Oxygen Demand in COVID-19 Patients. Hemasphere 2020, 10, e492. [Google Scholar] [CrossRef] [PubMed]
- Wiltshire, E.; Peña, A.S.; MacKenzie, K.; Shaw, G.; Couper, J. High dose folic acid is a potential treatment for pulmonary hypertension, including when associated with COVID-19 pneumonia. Med. Hypotheses 2020, 143, 110142. [Google Scholar] [CrossRef] [PubMed]
- Louca, P.; Murray, B.; Klaser, K.; Graham, M.S.; Mazidi, M.; Leeming, E.R.; Thompson, E.; Bowyer, R.; Drew, D.A.; Nguyen, L.H.; et al. Modest effects of dietary supplements during the COVID-19 pandemic: Insights from 445 850 users of the COVID-19 Symptom Study app. BMJ Nutr. Prev. Health 2021, 4, 149–157. [Google Scholar] [CrossRef]
- Al Sulaiman, K.; Aljuhani, O.; Saleh, K.B.; Badreldin, H.A.; Al Harthi, A.; Alenazi, M.; Alharbi, A.; Algarni, R.; Al Harbi, S.; Alhammad, A.M.; et al. Ascorbic acid as an adjunctive therapy in critically ill patients with COVID-19: A propensity score matched study. Sci. Rep. 2021, 11, 17648. [Google Scholar] [CrossRef] [PubMed]
- Annweiler, C.; Beaudenon, M.; Gautier, J.; Simon, R.; Dubée, V.; Gonsard, J.; Parot-Schinkel, E. COVIT-TRIAL study group. COVID-19 and high-dose VITamin D supplementation TRIAL in high-risk older patients (COVIT-TRIAL): Study protocol for a randomized controlled trial. Trials 2020, 21, 1031. [Google Scholar] [CrossRef]
- Sabico, S.; Enani, M.A.; Sheshah, E.; Aljohani, N.J.; Aldisi, D.A.; Alotaibi, N.H.; Alshingetti, N.; Alomar, S.Y.; Alnaami, A.M.; Amer, O.E.; et al. Effects of a 2-Week 5000 IU versus 1000 IU Vitamin D3 Supplementation on Recovery of Symptoms in Patients with Mild to Moderate COVID-19: A Randomized Clinical Trial. Nutrients 2021, 13, 2170. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.W.; Ho, L.P.; Kalimuddin, S.; Cherng, B.P.Z.; The, Y.E.; Thien, S.Y.; Wong, H.M.; Tern, P.J.W.; Chandran, M.; Chay, J.W.M.; et al. Cohort study to evaluate the effect of vitamin D, magnesium, and vitamin B12 in combination on progression to severe outcomes in older patients with coronavirus (COVID-19). Nutrition 2020, 79–80, 111017. [Google Scholar] [CrossRef] [PubMed]
- AlSafar, H.; Grant, W.B.; Hijazi, R.; Uddin, M.; Alkaabi, N.; Tay, G.; Mahboub, B.; Al Anouti, F. COVID-19 Disease Severity and Death in Relation to Vitamin D Status among SARS-CoV-2-Positive UAE Residents. Nutrients 2021, 13, 1714. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, D.A.; Vivaldi, G.; Chambers, E.S.; Cai, W.; Li, W.; Faustini, S.E.; Gibbons, J.M.; Pade, C.; Coussens, A.K.; Richter, A.G.; et al. Vitamin D Supplementation Does Not Influence SARS-CoV-2 Vaccine Efficacy or Immunogenicity: Sub-Studies Nested within the CORONAVIT Randomised Controlled Trial. Nutrients 2022, 14, 3821. [Google Scholar] [CrossRef]
- Romero-Ibarguengoitia, M.E.; Gutiérrez-González, D.; Cantú-López, C.; Sanz-Sánchez, M.Á.; González-Cantú, A. Effect of Vitamin D3 Supplementation vs. Dietary-Hygienic Measures on SARS-CoV-2 Infection Rates in Hospital Workers with 25-Hydroxyvitamin D3 [25(OH)D3] Levels ≥20 ng/mL. Microorganisms 2023, 11, 282. [Google Scholar] [CrossRef]
- Sedighiyan, M.; Abdollahi, H.; Karimi, E.; Badeli, M.; Erfanian, R.; Raeesi, S.; Hashemi, R.; Vahabi, Z.; Asanjarani, B.; Mansouri, F.; et al. Omega-3 polyunsaturated fatty acid supplementation improve clinical symptoms in patients with 64 COVID-19: A randomized clinical trial. Int. J. Clin. Pract. 2021, 75, e14854. [Google Scholar] [CrossRef]
- Doaei, S.; Gholami, S.; Rastgoo, S.; Gholamalizadeh, M.; Bourbour, F.; Bagheri, S.E.; Samipoor, F.; Akbari, M.E.; Shadnoush, M.; Ghorat, F.; et al. The effect of omega-3 fatty acid supplementation on clinical and biochemical parameters of critically ill patients with COVID-19: A randomized clinical trial. J. Transl. Med. 2021, 19, 128. [Google Scholar] [CrossRef]
- Mohajeri, M.; Horriatkhah, E.; Mohajery, R. The effect of glutamine supplementation on serum levels of some inflammatory factors, oxidative stress, and appetite in COVID-19 patients: A case–control study. Inflammopharmacology 2021, 29, 1769–1776. [Google Scholar] [CrossRef] [PubMed]
- de Ligt, M.; Hesselink, M.K.C.; Jorgensen, J.; Hoebers, V.; Blaak, E.E.; Goossens, G.H. Resveratrol supplementation reduces ACE2 expression in human adipose tissue. Adipocyte 2021, 10, 408–411. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.A.M.; Bifano, M.; Roca Goma, E.; Plasencia, C.M.; Torralba, A.O.; Font, M.S.; Millán, P.R. Effect and Tolerability of a Nutritional Supplement Based on a Synergistic Combination of β-Glucans and Selenium-and Zinc-Enriched Saccharomyces cerevisiae (ABB C1®) in Volunteers Receiving the Influenza or the COVID-19 Vaccine: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2021, 13, 4347. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Liu, Q.; Zhang, L.; Xu, Z.; Liu, C.; Lu, W.; Ching, J.Y.L.; Li, A.; Mak, J.W.Y.; Lui, G.C.Y.; et al. Antibiotics and probiotics impact gut antimicrobial resistance gene reservoir in COVID-19 patients. Gut Microbes 2022, 14, 2128603. [Google Scholar] [CrossRef]
- Rathi, A.; Jadhav, S.B.; Shah, N. A Randomized Controlled Trial of the Efficacy of Systemic Enzymes and Probiotics in the Resolution of Post-COVID Fatigue. Medicines 2021, 8, 47. [Google Scholar] [CrossRef]
- Ponzo, V.; Pellegrini, M.; D’eusebio, C.; Bioletto, F.; Goitre, I.; Buscemi, S.; Bo, S. Mediterranean diet and SARS-CoV-2 infection: Is there any association? A proof-of-concept study. Nutrients 2021, 13, 1721. [Google Scholar] [CrossRef]
- Perez-Araluce, R.; Martinez-Gonzalez, M.A.; Fernández-Lázaro, C.I.; Bes-Rastrollo, M.; Gea, A.; Carlos, S. Mediterranean diet and the risk of COVID-19 in the ‘Seguimiento Universidad de Navarra’ cohort. Clin. Nutr. 2022, 41, 3061–3068. [Google Scholar] [CrossRef]
- Pavlidou, E.; Papadopoulou, S.K.; Mentzelou, M.; Dakanalis, A.; Vorvolakos, T.; Antasouras, G.; Spanoudaki, M.; Pandi, A.L.; Serdari, A.; Chrysafi, M.; et al. Association of Mediterranean Diet Adherence with Sociodemographic, Anthropometric, and Lifestyle Factors during the COVID-19 Pandemic: A Cross-Sectional Study in Greece. Nutrients 2023, 15, 4123. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, K.; Hosomi, K.; Sawane, K.; Kunisawa, J. Metabolism of Dietary and Microbial Vitamin B Family in the Regulation of Host Immunity. Front. Nutr. 2019, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Paul, S.; Roy, S.; Sutradhar, H.; Bin Emran, T.; Nainu, F.; Khandaker, M.U.; Almalki, M.; Wilairatana, P.; Mubarak, M.S. Exploring the Immune-Boosting Functions of Vitamins and Minerals as Nutritional Food Bioactive Compounds: A Comprehensive Review. Molecules 2022, 27, 555. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, H.; Feehan, J.; Mikkelsen, K.; Al Dhaheri, A.S.; Ali, H.I.; Platat, C.; Ismail, L.C.; Stojanovska, L.; Apostolopoulos, V. Be Well: A Potential Role for Vitamin B in COVID-19. Maturitas 2021, 144, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Ragan, I.; Hartson, L.; Pidcoke, H.; Bowen, R.; Goodrich, R. Pathogen Reduction of SARS-CoV-2 Virus in Plasma and Whole Blood Using Riboflavin and UV Light. PLoS ONE 2020, 15, e0233947. [Google Scholar] [CrossRef]
- Beigmohammadi, M.T.; Bitarafan, S.; Hoseindokht, A.; Abdollahi, A.; Amoozadeh, L.; Soltani, D. The effect of supplementation with vitamins A, B, C, D, and E on disease severity and inflammatory responses in patients with COVID-19: A randomized clinical trial. Trials 2021, 22, 802. [Google Scholar] [CrossRef] [PubMed]
- Ibrahimagić, O.Ć.; Kunić, S. Comment on an article: “High dose folic acid is a potential treatment for pulmonary hypertension, including when associated with COVID-19 pneumonia”. Med. Hypotheses 2020, 145, 110338. [Google Scholar] [CrossRef]
- Narayanan, N.; Nair, D.T. Vitamin b12 May Inhibit Rna-Dependent-Rna Polymerase Activity of NSP12 from the SARS-CoV-2 Virus. IUBMB Life 2020, 72, 2112–2120. [Google Scholar] [CrossRef]
- Tengelmann, C.; Joos, S.; Kaußner, Y.; Malzahn, U.; Lunden, L.; Klug, A.; Häusler, K.G.; Escales, C.; Maetzler, W.; Hügen, K.; et al. Feasibility, safety and effectiveness of prednisolone and vitamin B1, B6, and B12 in patients with post-COVID-19-syndrome (PreVitaCOV)—Protocol of a randomised, double-blind, placebo-controlled multicentre trial in primary care (phase IIIb). BMC Infect. Dis. 2024, 24, 56. [Google Scholar] [CrossRef] [PubMed]
- Shahbaz, U.; Fatima, N.; Basharat, S.; Bibi, A.; Yu, X.; Hussain, M.I.; Nasrullah, M. Role of vitamin C in preventing of COVID-19 infection, progression and severity. AIMS Microbiol. 2022, 8, 108–124. [Google Scholar] [CrossRef]
- Tehrani, S.; Yadegarynia, D.; Abrishami, A.; Moradi, H.; Gharaei, B.; Rauofi, M.; Nejad, F.M.; Sali, S.; Khabiri, N.; Abolghasemi, S. An Investigation into the Effects of Intravenous Vitamin c on Pulmonary CT Findings and Clinical Outcomes of Patients with COVID 19 Pneumonia a Randomized Clinical Trial. Urol. J. 2021, 19, 460–465. [Google Scholar] [CrossRef]
- Tosato, M.; Calvani, R.; Picca, A.; Ciciarello, F.; Galluzzo, V.; Coelho-Júnior, H.J.; Di Giorgio, A.; Di Mario, C.; Gervasoni, J.; Gremese, E.; et al. Effects of L-Arginine plus Vitamin c Supplementation on Physical Performance, Endothelial Function, and Persistent Fatigue in Adults with Long COVID: A Single-Blind Randomized Controlled Trial. Nutrients 2022, 14, 4984. [Google Scholar] [CrossRef]
- Jamali Moghadam Siahkali, S.; Zarezade, B.; Koolaji, S.; SeyedAlinaghi, S.; Zendehdel, A.; Tabarestani, M.; Sekhavati Moghadam, E.; Abbasian, L.; Dehghan Manshadi, S.A.; Salehi, M.; et al. Safety and Effectiveness of High-Dose Vitamin c in Patients with COVID-19: A Randomized Open-Label Clinical Trial. Eur. J. Med. Res. 2021, 26, 20. [Google Scholar] [CrossRef]
- Fogleman, C.; Cohen, D.; Mercier, A.; Farrell, D.; Rutz, J.; Bresz, K.; Vernon, T. A Pilot of a Randomized Control Trial of Melatonin and Vitamin c for Mild-To-Moderate COVID-19. J. Am. Board Fam. Med. 2022, 35, 695–707. [Google Scholar] [CrossRef]
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef] [PubMed]
- Nicoll, R.; Henein, M.Y. COVID-19 Prevention: Vitamin D Is Still a Valid Remedy. J. Clin. Med. 2022, 11, 6818. [Google Scholar] [CrossRef]
- D’Ecclesiis, O.; Gavioli, C.; Martinoli, C.; Raimondi, S.; Chiocca, S.; Miccolo, C.; Bossi, P.; Cortinovis, D.; Chiaradonna, F.; Palorini, R.; et al. Vitamin D and SARS-CoV2 infection, severity and mortality: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0268396. [Google Scholar] [CrossRef]
- Oppedisano, F.; Macrì, R.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Bosco, F.; Nucera, S.; Caterina Zito, M.; Guarnieri, L.; et al. The Anti-Inflammatory and Antioxidant Properties of N-3 Pufas: Their Role in Cardiovascular Protection. Biomedicines 2020, 8, 306. [Google Scholar] [CrossRef]
- Morita, M.; Kuba, K.; Ichikawa, A.; Nakayama, M.; Katahira, J.; Iwamoto, R.; Watanebe, T.; Sakabe, S.; Daidoji, T.; Nakamura, S.; et al. The Lipid Mediator Protectin D1 Inhibits Influenza Virus Replication and Improves Severe Influenza. Cell 2013, 153, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Lakshimi, V.I.; Kavitha, M. New Insights into Prospective Health Potential of ω-3 PUFAs. Curr. Nutr. Rep. 2023, 12, 813–829. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, D.; Lavie, C.J.; Elagizi, A.; Milani, R.V. Update on Omega-3 Polyunsaturated Fatty Acids on Cardiovascular Health. Nutrients 2022, 14, 5146. [Google Scholar] [CrossRef] [PubMed]
- Zárate, R.; Jaber-Vazdekis, N.; Tejera, N.; Pérez, J.A.; Rodríguez, C. Significance of Long Chain Polyunsaturated Fatty Acids in Human Health. Clin. Transl. Med. 2017, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Nicodemo, M.; Spreghini, M.R.; Manco, M.; Wietrzykowska Sforza, R.; Morino, G. Childhood Obesity and COVID-19 Lockdown: Remarks on Eating Habits of Patients Enrolled in a Food-Education Program. Nutrients 2021, 13, 383. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Castrellón, P.; Gandara-Martí, T.; Abreu, A.T.Y.; Nieto-Rufino, C.D.; López-Orduña, E.; Jiménez-Escobar, I.; Jiménez-Gutiérrez, C.; López-Velazquez, G.; Espadaler-Mazo, J. Probiotic improves symptomatic and viral clearance in COVID-19 outpatients: A randomized, quadruple-blinded, placebo-controlled trial. Gut Microbes 2022, 14, 2018899. [Google Scholar] [CrossRef] [PubMed]
- Brahma, S.; Naik, A.; Lordan, R. Probiotics: A gut response to the COVID-19 pandemic but what does the evidence show? Clin. Nutr. ESPEN 2022, 51, 17–27. [Google Scholar] [CrossRef]
- Jaber, B.A.; Qureshi, R.; Abd-Alrazaq, A.; Rahman, M.A.; Househ, M.; Shah, Z.; Alam, T. Clinical Trials on Alternative Medicines for COVID-19. Stud. Health Technol. Inform. 2022, 295, 366–369. [Google Scholar] [CrossRef]
- Sharma, S.; Di Castelnuovo, A.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; Iacoviello, L.; Bonaccio, M. Diet Quality and Risk of SARS-CoV-2 Infection or COVID-19: A Systematic Review of Observational Studies. Adv. Nutr. 2023, 14, 1596–1616. [Google Scholar] [CrossRef] [PubMed]
- Ferro, Y.; Pujia, R.; Maurotti, S.; Boragina, G.; Mirarchi, A.; Gnagnarella, P.; Mazza, E. Mediterranean Diet a Potential Strategy against SARS-CoV-2 Infection: A Narrative Review. Medicina 2021, 57, 1389. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, R.F.W.; Silva, A.P.; Santana, A.I.C.; Silva, D.S.E.; Ramos, M.S.; Souza, M.C.; Suen, V.M.M.; Maduro, I.P.N.N.; Ribas Filho, D.; D’ Oliveira, A.J.; et al. Effect of immunonutrition on serum levels of C-reactive protein and lymphocytes in patients with COVID-19: A randomized, controlled, double-blind clinical trial. Nutr. Hosp. 2022, 39, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.L.; Murai, I.H.; Reis, B.Z.; Sales, L.P.; Santos, M.D.; Pinto, A.J.; Goessler, K.F.; Duran, C.S.C.; Silva, C.B.R.; Franco, A.S.; et al. Effect of a single high dose of vitamin D3 on cytokines, chemokines, and growth factor in patients with moderate to severe COVID-19. Am. J. Clin. Nutr. 2022, 115, 790–798. [Google Scholar] [CrossRef]
- Majidi, N.; Rabbani, F.; Gholami, S.; Gholamalizadeh, M.; BourBour, F.; Rastgoo, S.; Hajipour, A.; Shadnoosh, M.; Akbari, M.E.; Bahar, B.; et al. The Effect of Vitamin C on Pathological Parameters and Survival Duration of Critically Ill Coronavirus Disease 2019 Patients: A Randomized Clinical Trial. Front. Immunol. 2021, 12, 717816. [Google Scholar] [CrossRef] [PubMed]
- Karonova, T.L.; Chernikova, A.T.; Golovatyuk, K.A.; Bykova, E.S.; Grant, W.B.; Kalinina, O.V.; Grineva, E.N.; Shlyakhto, E.V. Vitamin D Intake May Reduce SARS-CoV-2 Infection Morbidity in Health Care Workers. Nutrients 2022, 14, 505. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, H.; Feehan, J.; Al Dhaheri, A.S.; Ali, H.I.; Platat, C.; Ismail, L.C.; Apostolopoulos, V.; Stojanovska, L. Immune-Boosting Role of Vitamins D, C, E, Zinc, Selenium and Omega-3 Fatty Acids: Could They Help against COVID-19? Maturitas 2021, 143, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ghelani, D.; Alesi, S.; Mousa, A. Vitamin D and COVID-19: An Overview of Recent Evidence. Int. J. Mol. Sci. 2021, 22, 10559. [Google Scholar] [CrossRef]
- Dubey, S.; Biswas, P.; Ghosh, R.; Chatterjee, S.; Dubey, M.J.; Chatterjee, S.; Lahiri, D.; Lavie, C.J. Psychosocial impact of COVID-19. Diabetol. Metab. Syndr. 2020, 14, 779–788. [Google Scholar] [CrossRef]
- Calder, P.C. Nutrition and immunity: Lessons for COVID-19. Nutr. Diabetes 2021, 11, 19. [Google Scholar] [CrossRef]
- Iddir, M.; Brito, A.; Dingeo, G.; Fernandez Del Campo, S.S.; Samouda, H.; La Frano, M.R.; Bohn, T. Strengthening the Immune System and Reducing Inflammation and Oxidative Stress through Diet and Nutrition: Considerations during the COVID-19 Crisis. Nutrients 2020, 12, 1562. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Food Supplements. Available online: https://www.efsa.europa.eu/en/topics/topic/food-supplements (accessed on 12 August 2022).
- Augustin, M.; Schommers, P.; Stecher, M.; Dewald, F.; Gieselmann, L.; Gruell, H.; Horn, C.; Vanshylla, K.; Cristanziano, V.D.; Osebold, L.; et al. Post-COVID syndrome in non-hospitalised patients with COVID-19: A longitudinal prospective cohort study. Lancet Reg. Health Eur. 2021, 6, 100122. [Google Scholar] [CrossRef] [PubMed]
- Holmes, C.J.; Racette, S.B. The Utility of Body Composition Assessment in Nutrition and Clinical Practice: An Overview of Current Methodology. Nutrients 2021, 13, 2493. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavlidou, E.; Poulios, E.; Papadopoulou, S.K.; Fasoulas, A.; Dakanalis, A.; Giaginis, C. Clinical Evidence on the Potential Beneficial Effects of Diet and Dietary Supplements against COVID-19 Infection Risk and Symptoms’ Severity. Med. Sci. 2024, 12, 11. https://doi.org/10.3390/medsci12010011
Pavlidou E, Poulios E, Papadopoulou SK, Fasoulas A, Dakanalis A, Giaginis C. Clinical Evidence on the Potential Beneficial Effects of Diet and Dietary Supplements against COVID-19 Infection Risk and Symptoms’ Severity. Medical Sciences. 2024; 12(1):11. https://doi.org/10.3390/medsci12010011
Chicago/Turabian StylePavlidou, Eleni, Efthymios Poulios, Sousana K. Papadopoulou, Aristeidis Fasoulas, Antonios Dakanalis, and Constantinos Giaginis. 2024. "Clinical Evidence on the Potential Beneficial Effects of Diet and Dietary Supplements against COVID-19 Infection Risk and Symptoms’ Severity" Medical Sciences 12, no. 1: 11. https://doi.org/10.3390/medsci12010011







