Hereditary Renal Cancer Syndromes
Abstract
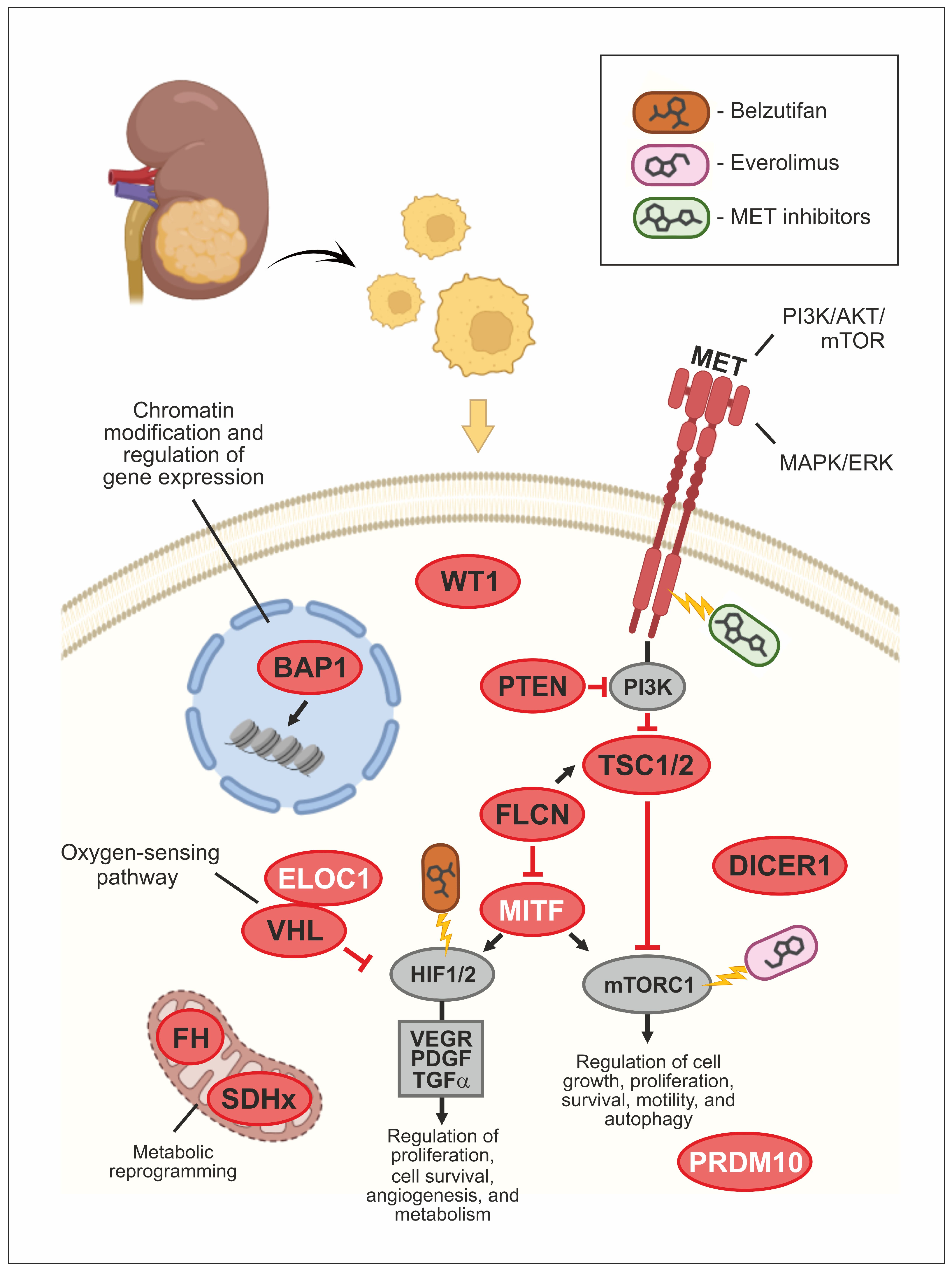
1. Introduction
2. Hereditary Conditions Associated with Increased Risk of Renal Malignancies
2.1. Von Hippel–Lindau Syndrome
2.2. Fumarate Hydratase Tumor Predisposition Syndrome (FHTPS)
2.3. Kidney Tumors Associated with Hereditary Paraganglioma and Pheochromocytoma (HPP) Syndrome
2.4. Hereditary Papillary Renal Cell Carcinoma (HPRCC)
2.5. Kidney Tumors Associated with Birt–Hogg–Dubé (BHD) Syndrome
2.6. Tuberous Sclerosis
2.7. Other Hereditary Cancer Syndromes Associated with Increased Risk of Kidney Tumors
3. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mighton, C.; Lerner-Ellis, J.P. Principles of molecular testing for hereditary cancer. Genes Chromosomes Cancer 2022, 61, 356–381. [Google Scholar] [CrossRef] [PubMed]
- Imyanitov, E.N.; Kuligina, E.S.; Sokolenko, A.P.; Suspitsin, E.N.; Yanus, G.A.; Iyevleva, A.G.; Ivantsov, A.O.; Aleksakhina, S.N. Hereditary cancer syndromes. World J. Clin. Oncol. 2023, 14, 40–68. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Knudson, A.G., Jr. Mutation and cancer: Statistical study of retinoblastoma. Proc. Natl. Acad. Sci. USA 1971, 68, 820–823. [Google Scholar] [CrossRef] [PubMed]
- Binderup, M.L.; Galanakis, M.; Budtz-Jørgensen, E.; Kosteljanetz, M.; Luise Bisgaard, M. Prevalence, birth incidence, and penetrance of von Hippel-Lindau disease (vHL) in Denmark. Eur. J. Hum. Genet. 2017, 25, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Binderup, M.; Smerdel, M.; Borgwadt, L.; Beck Nielsen, S.S.; Madsen, M.G.; Møller, H.U.; Kiilgaard, J.F.; Friis-Hansen, L.; Harbud, V.; Cortnum, S.; et al. von Hippel-Lindau disease: Updated guideline for diagnosis and surveillance. Eur. J. Med. Genet. 2022, 65, 104538. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.; Lanillos, J.; Roldan-Romero, J.M.; Caleiras, E.; Montero-Conde, C.; Cascón, A.; Climent, M.A.; Anguera, G.; Hernando, S.; Laínez, N.; et al. Prevalence of pathogenic germline variants in patients with metastatic renal cell carcinoma. Genet. Med. 2021, 23, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Abou Alaiwi, S.; Nassar, A.H.; Adib, E.; Groha, S.M.; Akl, E.W.; McGregor, B.A.; Esplin, E.D.; Yang, S.; Hatchell, K.; Fusaro, V.; et al. Trans-ethnic variation in germline variants of patients with renal cell carcinoma. Cell Rep. 2021, 34, 108926. [Google Scholar] [CrossRef]
- Carlo, M.I.; Mukherjee, S.; Mandelker, D.; Vijai, J.; Kemel, Y.; Zhang, L.; Knezevic, A.; Patil, S.; Ceyhan-Birsoy, O.; Huang, K.C.; et al. Prevalence of Germline Mutations in Cancer Susceptibility Genes in Patients with Advanced Renal Cell Carcinoma. JAMA Oncol. 2018, 4, 1228–1235. [Google Scholar] [CrossRef]
- Wu, J.; Wang, H.; Ricketts, C.J.; Yang, Y.; Merino, M.J.; Zhang, H.; Shi, G.; Gan, H.; Linehan, W.M.; Zhu, Y.; et al. Germline mutations of renal cancer predisposition genes and clinical relevance in Chinese patients with sporadic, early-onset disease. Cancer 2019, 125, 1060–1069. [Google Scholar] [CrossRef]
- Yngvadottir, B.; Andreou, A.; Bassaganyas, L.; Larionov, A.; Cornish, A.J.; Chubb, D.; Saunders, C.N.; Smith, P.S.; Zhang, H.; Cole, Y.; et al. Frequency of pathogenic germline variants in cancer susceptibility genes in 1336 renal cell carcinoma cases. Hum. Mol. Genet. 2022, 31, 3001–3011. [Google Scholar] [CrossRef]
- Truong, H.; Sheikh, R.; Kotecha, R.; Kemel, Y.; Reisz, P.A.; Lenis, A.T.; Mehta, N.N.; Khurram, A.; Joseph, V.; Mandelker, D.; et al. Germline Variants Identified in Patients with Early-onset Renal Cell Carcinoma Referred for Germline Genetic Testing. Eur. Urol. Oncol. 2021, 4, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Yang, T.; Wen, X.; Mu, Z.; Zhao, C.; Han, S.; Tian, J.; Zhang, X.; Zhou, T.; Zhang, Y.; et al. Germline Mutation Landscape and Associated Clinical Characteristics in Chinese Patients with Renal Cell Carcinoma. Front. Oncol. 2021, 11, 737547. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Cao, S.; Ouyang, Q.; Li, H.; Li, X.; Chen, K.; Zhang, X.; Huang, Y.; Zhang, X.; Ma, X. Prevalence of germline mutations in cancer susceptibility genes in Chinese patients with renal cell carcinoma. Transl. Androl. Urol. 2023, 12, 308–319. [Google Scholar] [CrossRef]
- Takami, H.; Graffeo, C.S.; Perry, A.; Brown, D.A.; Meyer, F.B.; Burns, T.C.; Parney, I.F. Presentation, imaging, patterns of care, growth, and outcome in sporadic and von Hippel-Lindau-associated central nervous system hemangioblastomas. J. Neurooncol. 2022, 159, 221–231. [Google Scholar] [CrossRef] [PubMed]
- De Mestier, L.; Hentic, O.; Cros, J.; Walter, T.; Roquin, G.; Brixi, H.; Lombard-Bohas, C.; Hammel, P.; Diebold, M.D.; Couvelard, A.; et al. Metachronous hormonal syndromes in patients with pancreatic neuroendocrine tumors: A case-series study. Ann. Intern. Med. 2015, 162, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Buffet, A.; Venisse, A.; Nau, V.; Roncellin, I.; Boccio, V.; Le Pottier, N.; Boussion, M.; Travers, C.; Simian, C.; Burnichon, N.; et al. A decade (2001–2010) of genetic testing for pheochromocytoma and paraganglioma. Horm. Metab. Res. 2012, 44, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Yonamine, M.; Wasano, K.; Aita, Y.; Sugasawa, T.; Takahashi, K.; Kawakami, Y.; Shimano, H.; Nishiyama, H.; Hara, H.; Naruse, M.; et al. Prevalence of Germline Variants in a Large Cohort of Japanese Patients with Pheochromocytoma and/or Paraganglioma. Cancers 2021, 13, 4014. [Google Scholar] [CrossRef] [PubMed]
- Bender, B.U.; Eng, C.; Olschewski, M.; Berger, D.P.; Laubenberger, J.; Altehöfer, C.; Kirste, G.; Orszagh, M.; van Velthoven, V.; Miosczka, H.; et al. VHL c.505 T>C mutation confers a high age related penetrance but no increased overall mortality. J. Med. Genet. 2001, 38, 508–514. [Google Scholar] [CrossRef]
- Gossage, L.; Eisen, T.; Maher, E.R. VHL, the story of a tumour suppressor gene. Nat. Rev. Cancer 2015, 15, 55–64. [Google Scholar] [CrossRef]
- Sebai, M.; Tulasne, D.; Caputo, S.M.; Verkarre, V.; Fernandes, M.; Guérin, C.; Reinhart, F.; Adams, S.; Maugard, C.; Caron, O.; et al. Novel germline MET pathogenic variants in French patients with papillary renal cell carcinomas type I. Hum. Mutat. 2022, 43, 316–327. [Google Scholar] [CrossRef]
- Smit, D.L.; Mensenkamp, A.R.; Badeloe, S.; Breuning, M.H.; Simon, M.E.; van Spaendonck, K.Y.; Aalfs, C.M.; Post, J.G.; Shanley, S.; Krapels, I.P.; et al. Hereditary leiomyomatosis and renal cell cancer in families referred for fumarate hydratase germline mutation analysis. Clin. Genet. 2011, 79, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Gardie, B.; Remenieras, A.; Kattygnarath, D.; Bombled, J.; Lefèvre, S.; Perrier-Trudova, V.; Rustin, P.; Barrois, M.; Slama, A.; Avril, M.F.; et al. Novel FH mutations in families with hereditary leiomyomatosis and renal cell cancer (HLRCC) and patients with isolated type 2 papillary renal cell carcinoma. J. Med. Genet. 2011, 48, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Rabban, J.T.; Chan, E.; Mak, J.; Zaloudek, C.; Garg, K. Prospective Detection of Germline Mutation of Fumarate Hydratase in Women with Uterine Smooth Muscle Tumors Using Pathology-based Screening to Trigger Genetic Counseling for Hereditary Leiomyomatosis Renal Cell Carcinoma Syndrome: A 5-Year Single Institutional Experience. Am. J. Surg. Pathol. 2019, 43, 639–655. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Dillon, J.; Beavis, A.L.; Liu, Y.; Lombardo, K.; Fader, A.N.; Hung, C.F.; Wu, T.C.; Vang, R.; Garcia, J.E.; et al. Prevalence of somatic and germline mutations of Fumarate hydratase in uterine leiomyomas from young patients. Histopathology 2020, 76, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.; Ferlicot, S.; Guillaud-Bataille, M.; Le Teuff, G.; Genestie, C.; Deveaux, S.; Slama, A.; Poulalhon, N.; Escudier, B.; Albiges, L.; et al. Reassessing the clinical spectrum associated with hereditary leiomyomatosis and renal cell carcinoma syndrome in French FH mutation carriers. Clin. Genet. 2017, 92, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Ebbehoj, A.; Stochholm, K.; Jacobsen, S.F.; Trolle, C.; Jepsen, P.; Robaczyk, M.G.; Rasmussen, Å.K.; Feldt-Rasmussen, U.; Thomsen, R.W.; Søndergaard, E.; et al. Incidence and Clinical Presentation of Pheochromocytoma and Sympathetic Paraganglioma: A Population-based Study. J. Clin. Endocrinol. Metab. 2021, 106, e2251–e2261. [Google Scholar] [CrossRef] [PubMed]
- Rijken, J.A.; Niemeijer, N.D.; Jonker, M.A.; Eijkelenkamp, K.; Jansen, J.C.; van Berkel, A.; Timmers, H.J.L.M.; Kunst, H.P.M.; Bisschop, P.H.L.T.; Kerstens, M.N.; et al. The penetrance of paraganglioma and pheochromocytoma in SDHB germline mutation carriers. Clin. Genet. 2018, 93, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Andrews, K.A.; Ascher, D.B.; Pires, D.E.V.; Barnes, D.R.; Vialard, L.; Casey, R.T.; Bradshaw, N.; Adlard, J.; Aylwin, S.; Brennan, P.; et al. Tumour risks and genotype-phenotype correlations associated with germline variants in succinate dehydrogenase subunit genes SDHB, SDHC and SDHD. J. Med. Genet. 2018, 55, 384–394. [Google Scholar] [CrossRef]
- Maniam, P.; Zhou, K.; Lonergan, M.; Berg, J.N.; Goudie, D.R.; Newey, P.J. Pathogenicity and Penetrance of Germline SDHA Variants in Pheochromocytoma and Paraganglioma (PPGL). J. Endocr. Soc. 2018, 2, 806–816. [Google Scholar] [CrossRef]
- Muller, M.E.; Daccord, C.; Taffé, P.; Lazor, R. Prevalence of Birt-Hogg-Dubé Syndrome Determined Through Epidemiological Data on Spontaneous Pneumothorax and Bayes Theorem. Front. Med. 2021, 8, 631168. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, Y.J.; Kim, M.J.; Kim, H.C. Epidemiology and clinical features of Birt-Hogg-Dubé syndrome: A nationwide population-based study in South Korea. PLoS ONE 2022, 17, e0269358. [Google Scholar] [CrossRef]
- Lagerstedt-Robinson, K.; Baranowska Körberg, I.; Tsiaprazis, S.; Björck, E.; Tham, E.; Poluha, A.; Hellström Pigg, M.; Paulsson-Karlsson, Y.; Nordenskjöld, M.; Johansson-Soller, M.; et al. A retrospective two centre study of Birt-Hogg-Dubé syndrome reveals a pathogenic founder mutation in FLCN in the Swedish population. PLoS ONE 2022, 17, e0264056. [Google Scholar] [CrossRef]
- Savatt, J.M.; Shimelis, H.; Moreno-De-Luca, A.; Strande, N.T.; Oetjens, M.T.; Ledbetter, D.H.; Martin, C.L.; Myers, S.M.; Finucane, B.M. Frequency of truncating FLCN variants and Birt-Hogg-Dubé-associated phenotypes in a health care system population. Genet. Med. 2022, 24, 1857–1866. [Google Scholar] [CrossRef] [PubMed]
- Toro, J.R.; Pautler, S.E.; Stewart, L.; Glenn, G.M.; Weinreich, M.; Toure, O.; Wei, M.H.; Schmidt, L.S.; Davis, L.; Zbar, B.; et al. Lung cysts, spontaneous pneumothorax, and genetic associations in 89 families with Birt-Hogg-Dubé syndrome. Am. J. Respir. Crit. Care Med. 2007, 175, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
- Bruinsma, F.J.; Dowty, J.G.; Win, A.K.; Goddard, L.C.; Agrawal, P.; Attina, D.; Bissada, N.; De Luise, M.; Eisen, D.B.; Furuya, M.; et al. Update of penetrance estimates in Birt-Hogg-Dubé syndrome. J. Med. Genet. 2023, 60, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Menko, F.H.; Johannesma, P.C.; van Moorselaar, R.J.; Reinhard, R.; van Waesberghe, J.H.; Thunnissen, E.; Houweling, A.C.; Leter, E.M.; Waisfisz, Q.; van Doorn, M.B.; et al. A de novo FLCN mutation in a patient with spontaneous pneumothorax and renal cancer; a clinical and molecular evaluation. Fam. Cancer 2013, 12, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Furuya, M.; Yao, M.; Tanaka, R.; Nagashima, Y.; Kuroda, N.; Hasumi, H.; Baba, M.; Matsushima, J.; Nomura, F.; Nakatani, Y. Genetic, epidemiologic and clinicopathologic studies of Japanese Asian patients with Birt-Hogg-Dubé syndrome. Clin. Genet. 2016, 90, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Strzelczyk, A.; Rosenow, F.; Zöllner, J.P.; Simon, A.; Wyatt, G.; Holland, R.; Schubert-Bast, S. Epidemiology, healthcare resource use, and mortality in patients with tuberous sclerosis complex: A population-based study on German health insurance data. Seizure 2021, 91, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Curatolo, P.; Moavero, R.; Roberto, D.; Graziola, F. Genotype/Phenotype Correlations in Tuberous Sclerosis Complex. Semin. Pediatr. Neurol. 2015, 22, 259–273. [Google Scholar] [CrossRef]
- Zöllner, J.P.; Franz, D.N.; Hertzberg, C.; Nabbout, R.; Rosenow, F.; Sauter, M.; Schubert-Bast, S.; Wiemer-Kruel, A.; Strzelczyk, A. A systematic review on the burden of illness in individuals with tuberous sclerosis complex (TSC). Orphanet J. Rare Dis. 2020, 15, 23. [Google Scholar] [CrossRef] [PubMed]
- Au, K.S.; Williams, A.T.; Roach, E.S.; Batchelor, L.; Sparagana, S.P.; Delgado, M.R.; Wheless, J.W.; Baumgartner, J.E.; Roa, B.B.; Wilson, C.M.; et al. Genotype/phenotype correlation in 325 individuals referred for a diagnosis of tuberous sclerosis complex in the United States. Genet. Med. 2007, 9, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Yehia, L.; Keel, E.; Eng, C. The Clinical Spectrum of PTEN Mutations. Annu. Rev. Med. 2020, 71, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.H.; Mester, J.; Peterson, C.; Yang, Y.; Chen, J.L.; Rybicki, L.A.; Milas, K.; Pederson, H.; Remzi, B.; Orloff, M.S.; et al. A clinical scoring system for selection of patients for PTEN mutation testing is proposed on the basis of a prospective study of 3042 probands. Am. J. Hum. Genet. 2011, 88, 42–56. [Google Scholar] [CrossRef] [PubMed]
- Tung, N.; Lin, N.U.; Kidd, J.; Allen, B.A.; Singh, N.; Wenstrup, R.J.; Hartman, A.R.; Winer, E.P.; Garber, J.E. Frequency of Germline Mutations in 25 Cancer Susceptibility Genes in a Sequential Series of Patients with Breast Cancer. J. Clin. Oncol. 2016, 34, 1460–1468. [Google Scholar] [CrossRef]
- Nagy, R.; Ringel, M.D. Genetic predisposition for nonmedullary thyroid cancer. Horm. Cancer 2015, 6, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Black, D.; Bogomolniy, F.; Robson, M.E.; Offit, K.; Barakat, R.R.; Boyd, J. Evaluation of germline PTEN mutations in endometrial cancer patients. Gynecol. Oncol. 2005, 96, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Mester, J.L.; Zhou, M.; Prescott, N.; Eng, C. Papillary renal cell carcinoma is associated with PTEN hamartoma tumor syndrome. Urology 2012, 79, 1187.e1–1187.e7. [Google Scholar] [CrossRef]
- Walpole, S.; Pritchard, A.L.; Cebulla, C.M.; Pilarski, R.; Stautberg, M.; Davidorf, F.H.; de la Fouchardière, A.; Cabaret, O.; Golmard, L.; Stoppa-Lyonnet, D.; et al. Comprehensive Study of the Clinical Phenotype of Germline BAP1 Variant-Carrying Families Worldwide. J. Natl. Cancer Inst. 2018, 110, 1328–1341. [Google Scholar] [CrossRef]
- Rai, K.; Pilarski, R.; Boru, G.; Rehman, M.; Saqr, A.H.; Massengill, J.B.; Singh, A.; Marino, M.J.; Davidorf, F.H.; Cebulla, C.M.; et al. Germline BAP1 alterations in familial uveal melanoma. Genes Chromosomes Cancer 2017, 56, 168–174. [Google Scholar] [CrossRef]
- Pagliuca, F.; Zito Marino, F.; Morgillo, F.; Della Corte, C.; Santini, M.; Vicidomini, G.; Guggino, G.; De Dominicis, G.; Campione, S.; Accardo, M.; et al. Inherited predisposition to malignant mesothelioma: Germline BAP1 mutations and beyond. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 4236–4246. [Google Scholar] [CrossRef] [PubMed]
- Masoomian, B.; Shields, J.A.; Shields, C.L. Overview of BAP1 cancer predisposition syndrome and the relationship to uveal melanoma. J. Curr. Ophthalmol. 2018, 30, 102–109. [Google Scholar] [CrossRef]
- Chau, C.; van Doorn, R.; van Poppelen, N.M.; van der Stoep, N.; Mensenkamp, A.R.; Sijmons, R.H.; van Paassen, B.W.; van den Ouweland, A.M.W.; Naus, N.C.; van der Hout, A.H.; et al. Families with BAP1-Tumor Predisposition Syndrome in The Netherlands: Path to Identification and a Proposal for Genetic Screening Guidelines. Cancers 2019, 11, 1114. [Google Scholar] [CrossRef] [PubMed]
- Nordstrom-O’Brien, M.; van der Luijt, R.B.; van Rooijen, E.; van den Ouweland, A.M.; Majoor-Krakauer, D.F.; Lolkema, M.P.; van Brussel, A.; Voest, E.E.; Giles, R.H. Genetic analysis of von Hippel-Lindau disease. Hum. Mutat. 2010, 31, 521–537. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Kanazashi, Y.; Kawada, C.; Sekine, Y.; Maejima, K.; Ashida, S.; Karashima, T.; Kojima, S.; Parrish, N.F.; Kosugi, S.; et al. Variant spectrum of von Hippel-Lindau disease and its genomic heterogeneity in Japan. Hum. Mol. Genet. 2023, 32, 2046–2054. [Google Scholar] [CrossRef] [PubMed]
- Chuang, G.S.; Martinez-Mir, A.; Geyer, A.; Engler, D.E.; Glaser, B.; Cserhalmi-Friedman, P.B.; Gordon, D.; Horev, L.; Lukash, B.; Herman, E.; et al. Germline fumarate hydratase mutations and evidence for a founder mutation underlying multiple cutaneous and uterine leiomyomata. J. Am. Acad. Dermatol. 2005, 52, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, L.S.; Linehan, W.M. Hereditary leiomyomatosis and renal cell carcinoma. Int. J. Nephrol. Renovasc. Dis. 2014, 7, 253–260. [Google Scholar] [CrossRef]
- Donato, S.; Simões, H.; Pinto, A.T.; M Cavaco, B.; Leite, V. SDHx-related pheochromocytoma/paraganglioma—Genetic, clinical, and treatment outcomes in a series of 30 patients from a single center. Endocrine 2019, 65, 408–415. [Google Scholar] [CrossRef]
- Martins, R.G.; Cunha, N.; Simões, H.; Matos, M.J.; Silva, J.; Torres, I.; Rodrigues, F.; Leite, V.; Teixeira, M.R.; Bugalho, M.J. Surveillance of succinate dehydrogenase gene mutation carriers: Insights from a nationwide cohort. Clin. Endocrinol. 2020, 92, 545–553. [Google Scholar] [CrossRef]
- Cascón, A.; Landa, I.; López-Jiménez, E.; Díez-Hernández, A.; Buchta, M.; Montero-Conde, C.; Leskelä, S.; Leandro-García, L.J.; Letón, R.; Rodríguez-Antona, C.; et al. Molecular characterisation of a common SDHB deletion in paraganglioma patients. J. Med. Genet. 2008, 45, 233–238. [Google Scholar] [CrossRef]
- Fagundes, G.F.C.; Freitas-Castro, F.; Santana, L.S.; Afonso, A.C.F.; Petenuci, J.; Funari, M.F.A.; Guimaraes, A.G.; Ledesma, F.L.; Pereira, M.A.A.; Victor, C.R.; et al. Evidence for a Founder Effect of SDHB Exon 1 Deletion in Brazilian Patients with Paraganglioma. J. Clin. Endocrinol. Metab. 2023, 108, 2105–2114. [Google Scholar] [CrossRef]
- Manotas, M.C.; Rivera, A.L.; Gómez, A.M.; Abisambra, P.; Guevara, G.; Medina, V.; Tapiero, S.; Huertas, A.; Riaño-Moreno, J.; Mejía, J.C.; et al. SDHB exon 1 deletion: A recurrent germline mutation in Colombian patients with pheochromocytomas and paragangliomas. Front. Genet. 2023, 13, 999329. [Google Scholar] [CrossRef] [PubMed]
- Bourdeau, I.; Grunenwald, S.; Burnichon, N.; Khalifa, E.; Dumas, N.; Binet, M.C.; Nolet, S.; Gimenez-Roqueplo, A.P. A SDHC Founder Mutation Causes Paragangliomas (PGLs) in the French Canadians: New Insights on the SDHC-Related PGL. J. Clin. Endocrinol. Metab. 2016, 101, 4710–4718. [Google Scholar] [CrossRef] [PubMed]
- Peczkowska, M.; Erlic, Z.; Hoffmann, M.M.; Furmanek, M.; Cwikla, J.; Kubaszek, A.; Prejbisz, A.; Szutkowski, Z.; Kawecki, A.; Chojnowski, K.; et al. Impact of screening kindreds for SDHD p.Cys11X as a common mutation associated with paraganglioma syndrome type. J. Clin. Endocrinol. Metab. 2008, 93, 4818–4825. [Google Scholar] [CrossRef] [PubMed]
- Namba, Y.; Ebana, H.; Okamoto, S.; Kobayashi, E.; Kurihara, M.; Sekimoto, Y.; Tsuboshima, K.; Okura, M.K.; Mitsuishi, Y.; Takahashi, K.; et al. Clinical and genetic features of 334 Asian patients with Birt-Hogg-Dubé syndrome (BHDS) who presented with pulmonary cysts with or without a history of pneumothorax, with special reference to BHDS-associated pneumothorax. PLoS ONE 2023, 18, e0289175. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.H.; Rehal, P.K.; Nahorski, M.S.; Macdonald, F.; Claessens, T.; Van Geel, M.; Gijezen, L.; Gille, J.J.; Giraud, S.; Richard, S.; et al. A new locus-specific database (LSDB) for mutations in the folliculin (FLCN) gene. Hum. Mutat. 2010, 31, E1043–E1051. [Google Scholar] [CrossRef] [PubMed]
- Rossing, M.; Albrechtsen, A.; Skytte, A.B.; Jensen, U.B.; Ousager, L.B.; Gerdes, A.M.; Nielsen, F.C.; Hansen, T.V. Genetic screening of the FLCN gene identify six novel variants and a Danish founder mutation. J. Hum. Genet. 2017, 62, 151–157. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, M.; Jiang, X.; Lv, G.; Hu, D.; Zhang, G.; Liu, J.; Wei, W.; Xiao, J.; Shen, B.; et al. Exons 1-3 deletion in FLCN is associated with increased risk of pneumothorax in Chinese patients with Birt-Hogg-Dubé syndrome. Orphanet J. Rare Dis. 2023, 18, 115. [Google Scholar] [CrossRef]
- Repo, P.; Staskiewicz, A.; Sutinen, E.; Rönty, M.; Kivelä, T.T.; Myllärniemi, M.; Turunen, J.A. BAP1 germline variants in Finnish patients with malignant mesothelioma. Lung Cancer 2022, 165, 102–107. [Google Scholar] [CrossRef]
- Carbone, M.; Flores, E.G.; Emi, M.; Johnson, T.A.; Tsunoda, T.; Behner, D.; Hoffman, H.; Hesdorffer, M.; Nasu, M.; Napolitano, A.; et al. Combined Genetic and Genealogic Studies Uncover a Large BAP1 Cancer Syndrome Kindred Tracing Back Nine Generations to a Common Ancestor from the 1700s. PLoS Genet. 2015, 11, e1005633. [Google Scholar] [CrossRef]
- Carlo, M.I.; Hakimi, A.A.; Stewart, G.D.; Bratslavsky, G.; Brugarolas, J.; Chen, Y.B.; Linehan, W.M.; Maher, E.R.; Merino, M.J.; Offit, K.; et al. Familial Kidney Cancer: Implications of New Syndromes and Molecular Insights. Eur. Urol. 2019, 76, 754–764. [Google Scholar] [CrossRef]
- Carlo, M.I. Hereditary Renal Cell Carcinoma Syndromes. Hematol. Oncol. Clin. N. Am. 2023, 37, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Kaelin, W.G., Jr. Von Hippel-Lindau disease: Insights into oxygen sensing, protein degradation, and cancer. J. Clin. Investig. 2022, 132, e162480. [Google Scholar] [CrossRef] [PubMed]
- Rednam, S.P.; Erez, A.; Druker, H.; Janeway, K.A.; Kamihara, J.; Kohlmann, W.K.; Nathanson, K.L.; States, L.J.; Tomlinson, G.E.; Villani, A.; et al. Von Hippel-Lindau and Hereditary Pheochromocytoma/Paraganglioma Syndromes: Clinical Features, Genetics, and Surveillance Recommendations in Childhood. Clin. Cancer Res. 2017, 23, e68–e75. [Google Scholar] [CrossRef] [PubMed]
- Hudler, P.; Urbancic, M. The Role of VHL in the Development of von Hippel-Lindau Disease and Erythrocytosis. Genes 2022, 13, 362. [Google Scholar] [CrossRef] [PubMed]
- Latif, F.; Tory, K.; Gnarra, J.; Yao, M.; Duh, F.M.; Orcutt, M.L.; Stackhouse, T.; Kuzmin, I.; Modi, W.; Geil, L.; et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science 1993, 260, 1317–1320. [Google Scholar] [CrossRef] [PubMed]
- Vocke, C.D.; Ricketts, C.J.; Schmidt, L.S.; Ball, M.W.; Middelton, L.A.; Zbar, B.; Linehan, W.M. Comprehensive characterization of Alu-mediated breakpoints in germline VHL gene deletions and rearrangements in patients from 71 VHL families. Hum. Mutat. 2021, 42, 520–529. [Google Scholar] [CrossRef]
- Wang, N.; Lysenkov, V.; Orte, K.; Kairisto, V.; Aakko, J.; Khan, S.; Elo, L.L. Tool evaluation for the detection of variably sized indels from next generation whole genome and targeted sequencing data. PLoS Comput. Biol. 2022, 18, e1009269. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, L.; Parada-Fennen, L.; Solano, A.R. In Silico Copy Number Variation (CNVs) Bioinformatics Estimation: Dream or Nightmare? EJIFCC 2023, 34, 72–75. [Google Scholar]
- Brauch, H.; Kishida, T.; Glavac, D.; Chen, F.; Pausch, F.; Höfler, H.; Latif, F.; Lerman, M.I.; Zbar, B.; Neumann, H.P. Von Hippel-Lindau (VHL) disease with pheochromocytoma in the Black Forest region of Germany: Evidence for a founder effect. Hum. Genet. 1995, 95, 551–556. [Google Scholar] [CrossRef]
- Cybulski, C.; Matyjasik, J.; Soroka, M.; Szymaś, J.; Górski, B.; Debniak, T.; Jakubowska, A.; Bernaczyk, A.; Zimnoch, L.; Bierzyńska-Macyszyn, G.; et al. Mutations in the von hippel-lindau tumour suppressor gene in central nervous system hemangioblastomas. Hered. Cancer Clin. Pract. 2004, 2, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Larcher, A.; Belladelli, F.; Fallara, G.; Rowe, I.; Capitanio, U.; Marandino, L.; Raggi, D.; Capitanio, J.F.; Bailo, M.; Lattanzio, R.; et al. Multidisciplinary management of patients diagnosed with von Hippel-Lindau disease: A practical review of the literature for clinicians. Asian J. Urol. 2022, 9, 430–442. [Google Scholar] [CrossRef] [PubMed]
- Daniels, A.; Tirosh, A.; Huntoon, K.; Mehta, G.; Spiess, P.; Friedman, D.; Waguespack, S.; Kilkelly, J.; Rednam, S.; Pruthi, S.; et al. Guidelines for surveillance of patients with von Hippel-Lindau disease: Consensus statement of the International VHL Surveillance Guidelines Consortium and VHL Alliance. Cancer 2023, 129, 2927–2940. [Google Scholar] [CrossRef] [PubMed]
- Menko, F.; Maher, E.; Schmidt, L.; Middelton, L.; Aittomäki, K.; Tomlinson, I.; Richard, S.; Linehan, W. Hereditary leiomyomatosis and renal cell cancer (HLRCC): Renal cancer risk, surveillance and treatment. Fam. Cancer 2014, 13, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Amar, L.; Pacak, K.; Steichen, O.; Akker, S.; Aylwin, S.; Baudin, E.; Buffet, A.; Burnichon, N.; Clifton-Bligh, R.; Dahia, P.; et al. International consensus on initial screening and follow-up of asymptomatic SDHx mutation carriers. Nat. Rev. Endocrinol. 2021, 17, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Freifeld, Y.; Ananthakrishnan, L.; Margulis, V. Imaging for Screening and Surveillance of Patients with Hereditary Forms of Renal Cell Carcinoma. Curr. Urol. Rep. 2018, 19, 82. [Google Scholar] [CrossRef] [PubMed]
- Sattler, E.; Steinlein, O. Birt-Hogg-Dubé Syndrome; Adam, M., Feldman, J., Mirzaa, G., Pagon, R., Wallace, S., Bean, L., Gripp, K., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 2006; pp. 1993–2024. [Google Scholar]
- Northrup, H.; Aronow, M.; Bebin, E.; Bissler, J.; Darling, T.; de Vries, P.; Frost, M.; Fuchs, Z.; Gosnell, E.; Gupta, N.; et al. Updated International Tuberous Sclerosis Complex Diagnostic Criteria and Surveillance and Management Recommendations. Pediatr. Neurol. 2021, 123, 50–66. [Google Scholar] [CrossRef]
- Lalloo, F.; Kulkarni, A.; Chau, C.; Nielsen, M.; Sheaff, M.; Steele, J.; van Doorn, R.; Wadt, K.; Hamill, M.; Torr, B.; et al. Clinical practice guidelines for the diagnosis and surveillance of BAP1 tumour predisposition syndrome. Eur. J. Hum. Genet. 2023, 31, 1261–1269. [Google Scholar] [CrossRef]
- Tischkowitz, M.; Colas, C.; Pouwels, S.; Hoogerbrugge, N.; PHTS Guideline Development Group; European Reference Network GENTURIS. Cancer Surveillance Guideline for individuals with PTEN hamartoma tumour syndrome. Eur. J. Hum. Genet. 2020, 28, 1387–1393. [Google Scholar] [CrossRef]
- Kirste, S.; Rühle, A.; Zschiedrich, S.; Schultze-Seemann, W.; Jilg, C.A.; Neumann-Haefelin, E.; Lo, S.S.; Grosu, A.L.; Kim, E. Stereotactic Body Radiotherapy for Renal Cell Carcinoma in Patients with Von Hippel-Lindau Disease-Results of a Prospective Trial. Cancers 2022, 14, 5069. [Google Scholar] [CrossRef]
- Ooi, A. Advances in hereditary leiomyomatosis and renal cell carcinoma (HLRCC) research. Semin. Cancer Biol. 2020, 61, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Cinque, A.; Minnei, R.; Floris, M.; Trevisani, F. The Clinical and Molecular Features in the VHL Renal Cancers, Close or Distant Relatives with Sporadic Clear Cell Renal Cell Carcinoma? Cancers 2022, 14, 5352. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Bauer, T.M.; Papadopoulos, K.P.; Plimack, E.R.; Merchan, J.R.; McDermott, D.F.; Michaelson, M.D.; Appleman, L.J.; Thamake, S.; Perini, R.F.; et al. Inhibition of hypoxia-inducible factor-2α in renal cell carcinoma with belzutifan: A phase 1 trial and biomarker analysis. Nat. Med. 2021, 27, 802–805. [Google Scholar] [CrossRef]
- Hasanov, E.; Jonasch, E. MK-6482 as a potential treatment for von Hippel-Lindau disease-associated clear cell renal cell carcinoma. Expert. Opin. Investig. Drugs 2021, 30, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Jonasch, E.; Donskov, F.; Iliopoulos, O.; Rathmell, W.K.; Narayan, V.K.; Maughan, B.L.; Oudard, S.; Else, T.; Maranchie, J.K.; Welsh, S.J.; et al. Belzutifan for Renal Cell Carcinoma in von Hippel-Lindau Disease. N. Engl. J. Med. 2021, 385, 2036–2046. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, J.P.; Nikolovski, I.; Dinatale, R.; Zucker, M.; Knezevic, A.; Patil, S.; Ged, Y.; Kotecha, R.R.; Shapnik, N.; Murray, S.; et al. Comprehensive Molecular Characterization and Response to Therapy in Fumarate Hydratase-Deficient Renal Cell Carcinoma. Clin. Cancer Res. 2021, 27, 2910–2919. [Google Scholar] [CrossRef] [PubMed]
- Carril-Ajuria, L.; Colomba, E.; Cerbone, L.; Romero-Ferreiro, C.; Crouzet, L.; Laguerre, B.; Thibault, C.; Vicier, C.; de Velasco, G.; Fléchon, A.; et al. Response to systemic therapy in fumarate hydratase-deficient renal cell carcinoma. Eur. J. Cancer 2021, 151, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Keam, B.; Kim, M.; Yoon, S.; Kim, D.; Choi, J.G.; Seo, J.Y.; Park, I.; Lee, J.L. Bevacizumab Plus Erlotinib Combination Therapy for Advanced Hereditary Leiomyomatosis and Renal Cell Carcinoma-Associated Renal Cell Carcinoma: A Multicenter Retrospective Analysis in Korean Patients. Cancer Res. Treat. 2019, 4, 1549–1556. [Google Scholar] [CrossRef]
- Sun, G.; Zhang, X.; Liang, J.; Pan, X.; Zhu, S.; Liu, Z.; Armstrong, C.M.; Chen, J.; Lin, W.; Liao, B.; et al. Integrated Molecular Characterization of Fumarate Hydratase-deficient Renal Cell Carcinoma. Clin. Cancer Res. 2021, 27, 1734–1743. [Google Scholar] [CrossRef]
- Zecchini, V.; Paupe, V.; Herranz-Montoya, I.; Janssen, J.; Wortel, I.M.N.; Morris, J.L.; Ferguson, A.; Chowdury, S.R.; Segarra-Mondejar, M.; Costa, A.S.H.; et al. Fumarate induces vesicular release of mtDNA to drive innate immunity. Nature 2023, 615, 499–506. [Google Scholar] [CrossRef]
- Iribe, Y.; Furuya, M.; Shibata, Y.; Yasui, M.; Funahashi, M.; Ota, J.; Iwashita, H.; Nagashima, Y.; Hasumi, H.; Hayashi, N.; et al. Complete response of hereditary leiomyomatosis and renal cell cancer (HLRCC)-associated renal cell carcinoma to nivolumab and ipilimumab combination immunotherapy by: A case report. Fam. Cancer 2021, 20, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Sekito, T.; Takamoto, A.; Kobayashi, Y.; Mitsui, M.; Watari, S.; Kubota, R.; Sadahira, T.; Iwata, T.; Nishimura, S.; Edamura, K.; et al. A Case of Metastatic Fumarate Hydratase-Deficient-like Renal Cell Carcinoma Successfully Managed by Ipilimumab plus Nivolumab. Acta Med. Okayama 2021, 75, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Huang, Y.; Huang, X.; Lv, Z.; Tian, S.; Ma, X.; Zhang, X. Complete Response of Hereditary Leiomyomatosis and Renal Cell Cancer (HLRCC)-Associated Renal Cell Carcinoma to Pembrolizumab Immunotherapy: A Case Report. Front. Oncol. 2021, 11, 735077. [Google Scholar] [CrossRef] [PubMed]
- Howells, E.; Wigston, L.; Mackie, G.; Tran, B.; Nott, L. Advanced fumarate hydratase-deficient renal cell carcinoma responding to combination immune checkpoint inhibitors. Can. J. Urol. 2023, 30, 11558–11561. [Google Scholar] [PubMed]
- Ball, M.W.; An, J.Y.; Gomella, P.T.; Gautam, R.; Ricketts, C.J.; Vocke, C.D.; Schmidt, L.S.; Merino, M.J.; Srinivasan, R.; Malayeri, A.A.; et al. Growth Rates of Genetically Defined Renal Tumors: Implications for Active Surveillance and Intervention. J. Clin. Oncol. 2020, 38, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.N.; Hirshfield, K.M.; Zhong, H.; Singer, E.A.; Ali, S.M.; Ganesan, S. Response to crizotinib in a patient with MET-mutant papillary renal cell cancer after progression on tivantinib. Eur. Urol. 2015, 67, 353–354. [Google Scholar] [CrossRef] [PubMed]
- Schöffski, P.; Wozniak, A.; Escudier, B.; Rutkowski, P.; Anthoney, A.; Bauer, S.; Sufliarsky, J.; van Herpen, C.; Lindner, L.H.; Grünwald, V.; et al. Crizotinib achieves long-lasting disease control in advanced papillary renal-cell carcinoma type 1 patients with MET mutations or amplification. EORTC 90101 CREATE trial. Eur. J. Cancer 2017, 87, 147–163. [Google Scholar] [CrossRef]
- Pal, S.K.; Tangen, C.; Thompson, I.M., Jr.; Balzer-Haas, N.; George, D.J.; Heng, D.Y.C.; Shuch, B.; Stein, M.; Tretiakova, M.; Humphrey, P.; et al. A comparison of sunitinib with cabozantinib, crizotinib, and savolitinib for treatment of advanced papillary renal cell carcinoma: A randomised, open-label, phase 2 trial. Lancet 2021, 397, 695–703. [Google Scholar] [CrossRef]
- Webster, B.R.; Gopal, N.; Ball, M.W. Tumorigenesis Mechanisms Found in Hereditary Renal Cell Carcinoma: A Review. Genes 2022, 13, 2122. [Google Scholar] [CrossRef]
- Nakamura, M.; Yao, M.; Sano, F.; Sakata, R.; Tatenuma, T.; Makiyama, K.; Nakaigawa, N.; Kubota, Y. A case of metastatic renal cell carcinoma associated with Birt-Hogg-Dubé syndrome treated with molecular-targeting agents. Hinyokika Kiyo 2013, 59, 503–506. [Google Scholar]
- Bissler, J.J.; McCormack, F.X.; Young, L.R.; Elwing, J.M.; Chuck, G.; Leonard, J.M.; Schmithorst, V.J.; Laor, T.; Brody, A.S.; Bean, J.; et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N. Engl. J. Med. 2008, 358, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Bissler, J.J.; Kingswood, J.C.; Radzikowska, E.; Zonnenberg, B.A.; Frost, M.; Belousova, E.; Sauter, M.; Nonomura, N.; Brakemeier, S.; de Vries, P.J.; et al. Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST-2): A multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2013, 381, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Dun, S.; Wang, Y.Y.; Wan, L.; Wang, Q.H.; Lu, Q.; Yang, X.Y.; Zhang, Q.; Chen, H.M.; Qiu, L.P.; Zou, L.P. Sirolimus can promote the disappearance of renal angiomyolipoma associated with tuberous sclerosis complex: A prospective cohort study. World J. Pediatr, 2023, in press. [CrossRef]
- Kapur, P.; Brugarolas, J.; Trpkov, K. Recent Advances in Renal Tumors with TSC/mTOR Pathway Abnormalities in Patients with Tuberous Sclerosis Complex and in the Sporadic Setting. Cancers 2023, 15, 4043. [Google Scholar] [CrossRef] [PubMed]
- Henske, E.P.; Cornejo, K.M.; Wu, C.L. Renal Cell Carcinoma in Tuberous Sclerosis Complex. Genes 2021, 12, 1585. [Google Scholar] [CrossRef] [PubMed]
- Komiya, T.; Blumenthal, G.M.; DeChowdhury, R.; Fioravanti, S.; Ballas, M.S.; Morris, J.; Hornyak, T.J.; Wank, S.; Hewitt, S.M.; Morrow, B.; et al. A Pilot Study of Sirolimus in Subjects with Cowden Syndrome or Other Syndromes Characterized by Germline Mutations in PTEN. Oncologist 2019, 24, 1510.e1265. [Google Scholar] [CrossRef] [PubMed]
- Poliakova, L.A. Familial erythrocytosis among the residents of the Chuvash ASSR. Probl. Gematol. Pereliv. Krovi. 1974, 19, 30–33. [Google Scholar] [PubMed]
- Sergeyeva, A.; Gordeuk, V.R.; Tokarev, Y.N.; Sokol, L.; Prchal, J.F.; Prchal, J.T. Congenital polycythemia in Chuvashia. Blood 1997, 89, 2148–2154. [Google Scholar] [CrossRef]
- Semenza, G.L. Heritable disorders of oxygen sensing. Am. J. Med. Genet. A 2021, 185, 3334–3339. [Google Scholar] [CrossRef]
- Ang, S.O.; Chen, H.; Gordeuk, V.R.; Sergueeva, A.I.; Polyakova, L.A.; Miasnikova, G.Y.; Kralovics, R.; Stockton, D.W.; Prchal, J.T. Endemic polycythemia in Russia: Mutation in the VHL gene. Blood Cells Mol. Dis. 2002, 28, 57–62. [Google Scholar] [CrossRef]
- Pastore, Y.; Jedlickova, K.; Guan, Y.; Liu, E.; Fahner, J.; Hasle, H.; Prchal, J.F.; Prchal, J.T. Mutations of von Hippel-Lindau tumor-suppressor gene and congenital polycythemia. Am. J. Hum. Genet. 2003, 73, 412–419. [Google Scholar] [CrossRef]
- Sarangi, S.; Lanikova, L.; Kapralova, K.; Acharya, S.; Swierczek, S.; Lipton, J.M.; Wolfe, L.; Prchal, J.T. The homozygous VHL (D126N) missense mutation is associated with dramatically elevated erythropoietin levels, consequent polycythemia, and early onset severe pulmonary hypertension. Pediatr. Blood Cancer 2014, 61, 2104–2106. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, S.; McLeod, M.; Torchia, D.; Romanelli, P. Multiple cutaneous and uterine leiomyomatosis syndrome: A review. J. Clin. Aesthet. Dermatol. 2013, 6, 16–21. [Google Scholar] [PubMed]
- Launonen, V.; Vierimaa, O.; Kiuru, M.; Isola, J.; Roth, S.; Pukkala, E.; Sistonen, P.; Herva, R.; Aaltonen, L.A. Inherited susceptibility to uterine leiomyomas and renal cell cancer. Proc. Natl. Acad. Sci. USA 2001, 98, 3387–3392. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, I.P.; Alam, N.A.; Rowan, A.J.; Barclay, E.; Jaeger, E.E.; Kelsell, D.; Leigh, I.; Gorman, P.; Lamlum, H.; Rahman, S.; et al. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat. Genet. 2002, 30, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, L.; Zhang, Y.; Zeng, C. Hereditary Leiomyomatosis and Renal Cell Cancer: Recent Insights into Mechanisms and Systemic Treatment. Front. Oncol. 2021, 11, 686556. [Google Scholar] [CrossRef] [PubMed]
- Lorenzato, A.; Olivero, M.; Perro, M.; Brière, J.J.; Rustin, P.; Di Renzo, M.F. A cancer-predisposing “hot spot” mutation of the fumarase gene creates a dominant negative protein. Int. J. Cancer 2008, 122, 947–951. [Google Scholar] [CrossRef]
- Zhang, L.; Walsh, M.; Jairam, S.; Mandelker, D.; Zhong, Y.; Kemel, Y.; Chen, Y.; Musheyev, D.; Zehir, A.; Jayakumaran, G.; et al. Fumarate hydratase FH c.1431_1433dupAAA (p.Lys477dup) variant is not associated with cancer including renal cell carcinoma. Hum. Mutat. 2020, 41, 103–109. [Google Scholar] [CrossRef]
- Lu, E.; Hatchell, K.E.; Nielsen, S.M.; Esplin, E.D.; Ouyang, K.; Nykamp, K.; Zavoshi, S.; Li, S.; Zhang, L.; Wilde, B.R.; et al. Fumarate hydratase variant prevalence and manifestations among individuals receiving germline testing. Cancer 2022, 128, 675–684. [Google Scholar] [CrossRef]
- Sulkowski, P.L.; Sundaram, R.K.; Oeck, S.; Corso, C.D.; Liu, Y.; Noorbakhsh, S.; Niger, M.; Boeke, M.; Ueno, D.; Kalathil, A.N.; et al. Krebs-cycle-deficient hereditary cancer syndromes are defined by defects in homologous-recombination DNA repair. Nat. Genet. 2018, 50, 1086–1092. [Google Scholar] [CrossRef]
- Kancherla, P.; Daneshvar, M.; Sager, R.A.; Mollapour, M.; Bratslavsky, G. Fumarate hydratase as a therapeutic target in renal cancer. Expert Opin. Ther. Targets 2020, 24, 923–936. [Google Scholar] [CrossRef]
- Ricketts, C.J.; Killian, J.K.; Vocke, C.D.; Wang, Y.; Merino, M.J.; Meltzer, P.S.; Linehan, W.M. Kidney tumors associated with germline mutations of FH and SDHB show a CpG island methylator phenotype (CIMP). PLoS ONE 2022, 17, e0278108. [Google Scholar] [CrossRef]
- Forde, C.; Lim, D.H.K.; Alwan, Y.; Burghel, G.; Butland, L.; Cleaver, R.; Dixit, A.; Evans, D.G.; Hanson, H.; Lalloo, F.; et al. Hereditary Leiomyomatosis and Renal Cell Cancer: Clinical, Molecular, and Screening Features in a Cohort of 185 Affected Individuals. Eur. Urol. Oncol. 2020, 3, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zhu, Z.R.; Sneh, T.; Zhang, W.T.; Wang, Z.Y.; Wu, G.Y.; He, W.; Qi, H.G.; Wang, H.; Wu, X.Y.; et al. Circulating succinate-modifying metabolites accurately classify and reflect the status of fumarate hydratase-deficient renal cell carcinoma. J. Clin. Investig. 2023, 133, e165028. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Kong, W.; Cao, M.; Wang, J.; Wang, Z.; Zheng, L.; Wu, X.; Cheng, R.; He, W.; Yang, B.; et al. Genomic Profiling and Response to Immune Checkpoint Inhibition plus Tyrosine Kinase Inhibition in FH-Deficient Renal Cell Carcinoma. Eur. Urol. 2023, 83, 163–172. [Google Scholar] [CrossRef]
- MacFarlane, J.; Seong, K.C.; Bisambar, C.; Madhu, B.; Allinson, K.; Marker, A.; Warren, A.; Park, S.M.; Giger, O.; Challis, B.G.; et al. A review of the tumour spectrum of germline succinate dehydrogenase gene mutations: Beyond phaeochromocytoma and paraganglioma. Clin. Endocrinol. 2020, 93, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Imyanitov, E.N.; Iyevleva, A.G.; Levchenko, E.V. Molecular testing and targeted therapy for non-small cell lung cancer: Current status and perspectives. Crit. Rev. Oncol. Hematol. 2021, 157, 103194. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, L.; Duh, F.M.; Chen, F.; Kishida, T.; Glenn, G.; Choyke, P.; Scherer, S.W.; Zhuang, Z.; Lubensky, I.; Dean, M.; et al. Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat. Genet. 1997, 16, 68–73. [Google Scholar] [CrossRef]
- Tovar, E.A.; Graveel, C.R. MET in human cancer: Germline and somatic mutations. Ann. Transl. Med. 2017, 5, 205. [Google Scholar] [CrossRef]
- Mikhaylenko, D.S.; Klimov, A.V.; Matveev, V.B.; Samoylova, S.I.; Strelnikov, V.V.; Zaletaev, D.V.; Lubchenko, L.N.; Alekseev, B.Y.; Nemtsova, M.V. Case of Hereditary Papillary Renal Cell Carcinoma Type I in a Patient with a Germline MET Mutation in Russia. Front. Oncol. 2020, 9, 1566. [Google Scholar] [CrossRef]
- Wolf, J.; Seto, T.; Han, J.Y.; Reguart, N.; Garon, E.B.; Groen, H.J.M.; Tan, D.S.W.; Hida, T.; de Jonge, M.; Orlov, S.V.; et al. Capmatinib in MET Exon 14-Mutated or MET-Amplified Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 944–957. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Vaishampayan, U.; Rosenberg, J.E.; Logan, T.F.; Harzstark, A.L.; Bukowski, R.M.; Rini, B.I.; Srinivas, S.; Stein, M.N.; Adams, L.M.; et al. Phase II and biomarker study of the dual MET/VEGFR2 inhibitor foretinib in patients with papillary renal cell carcinoma. J. Clin. Oncol. 2013, 31, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Hornstein, O.P.; Knickenberg, M. Perifollicular fibromatosis cutis with polyps of the colon--a cutaneo-intestinal syndrome sui generis. Arch. Dermatol. Res. 1975, 253, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Birt, A.R.; Hogg, G.R.; Dubé, W.J. Hereditary multiple fibrofolliculomas with trichodiscomas and acrochordons. Arch. Dermatol. 1977, 113, 1674–1677. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, L.S.; Linehan, W.M. Molecular genetics and clinical features of Birt-Hogg-Dubé syndrome. Nat. Rev. Urol. 2015, 12, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Furuya, M.; Hasumi, H.; Yao, M.; Nagashima, Y. Birt-Hogg-Dubé syndrome-associated renal cell carcinoma: Histopathological features and diagnostic conundrum. Cancer Sci. 2020, 111, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Liu, K.; Xu, K.F.; Liu, Y.; Tian, X. Clinical and Genetic Comparison of Birt-Hogg-Dubé Syndrome (Hornstein-Knickenberg Syndrome) in Chinese: A Systemic Review of Reported Cases. Int. J. Gen. Med. 2022, 15, 5111–5121. [Google Scholar] [CrossRef] [PubMed]
- Nickerson, M.L.; Warren, M.B.; Toro, J.R.; Matrosova, V.; Glenn, G.; Turner, M.L.; Duray, P.; Merino, M.; Choyke, P.; Pavlovich, C.P.; et al. Mutations in a novel gene lead to kidney tumors, lung wall defects, and benign tumors of the hair follicle in patients with the Birt-Hogg-Dubé syndrome. Cancer Cell 2002, 2, 157–164. [Google Scholar] [CrossRef]
- Trnka, P.; Kennedy, S.E. Renal tumors in tuberous sclerosis complex. Pediatr. Nephrol. 2021, 36, 1427–1438. [Google Scholar] [CrossRef]
- Marcinkowska, A.B.; Tarasewicz, A.; Jóźwiak, S.; Dębska-Ślizień, A.; Szurowska, E. Tuberous sclerosis complex-associated neuropsychiatric disorders. Psychiatr. Pol. 2022, 3, 1–20. [Google Scholar] [CrossRef]
- Suspitsin, E.N.; Yanus, G.A.; Dorofeeva, M.Y.; Ledashcheva, T.A.; Nikitina, N.V.; Buyanova, G.V.; Saifullina, E.V.; Sokolenko, A.P.; Imyanitov, E.N. Pattern of TSC1 and TSC2 germline mutations in Russian patients with tuberous sclerosis. J. Hum. Genet. 2018, 63, 597–604. [Google Scholar] [CrossRef]
- Klonowska, K.; Giannikou, K.; Grevelink, J.M.; Boeszoermenyi, B.; Thorner, A.R.; Herbert, Z.T.; Afrin, A.; Treichel, A.M.; Hamieh, L.; Kotulska, K.; et al. Comprehensive genetic and phenotype analysis of 95 individuals with mosaic tuberous sclerosis complex. Am. J. Hum. Genet. 2023, 110, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, P.; Roberts, P.; Dabora, S.; Franz, D.; Bissler, J.; Northrup, H.; Au, K.S.; Lazarus, R.; Domanska-Pakiela, D.; Kotulska, K.; et al. Identification of 54 large deletions/duplications in TSC1 and TSC2 using MLPA, and genotype-phenotype correlations. Hum. Genet. 2007, 121, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Brook-Carter, P.T.; Peral, B.; Ward, C.J.; Thompson, P.; Hughes, J.; Maheshwar, M.M.; Nellist, M.; Gamble, V.; Harris, P.C.; Sampson, J.R. Deletion of the TSC2 and PKD1 genes associated with severe infantile polycystic kidney disease—A contiguous gene syndrome. Nat. Genet. 1994, 8, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.H.; Mester, J.L.; Ngeow, J.; Rybicki, L.A.; Orloff, M.S.; Eng, C. Lifetime cancer risks in individuals with germline PTEN mutations. Clin. Cancer Res. 2012, 18, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Shuch, B.; Ricketts, C.J.; Vocke, C.D.; Komiya, T.; Middelton, L.A.; Kauffman, E.C.; Merino, M.J.; Metwalli, A.R.; Dennis, P.; Linehan, W.M. Germline PTEN mutation Cowden syndrome: An underappreciated form of hereditary kidney cancer. J. Urol. 2013, 190, 1990–1998. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Harbour, J.W.; Brugarolas, J.; Bononi, A.; Pagano, I.; Dey, A.; Krausz, T.; Pass, H.I.; Yang, H.; Gaudino, G. Biological Mechanisms and Clinical Significance of BAP1 Mutations in Human Cancer. Cancer Discov. 2020, 10, 1103–1120. [Google Scholar] [CrossRef] [PubMed]
- Näslund-Koch, C.; Nordestgaard, B.G.; Bojesen, S.E. Increased Risk for Other Cancers in Addition to Breast Cancer for CHEK2*1100delC Heterozygotes Estimated from the Copenhagen General Population Study. J. Clin. Oncol. 2016, 34, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Zlowocka-Perlowska, E.; Narod, S.A.; Cybulski, C. CHEK2 Alleles Predispose to Renal Cancer in Poland. JAMA Oncol. 2019, 5, 576. [Google Scholar] [CrossRef]
- Maciaszek, J.L.; Oak, N.; Nichols, K.E. Recent advances in Wilms’ tumor predisposition. Hum. Mol. Genet. 2020, 29, R138–R149. [Google Scholar] [CrossRef]
- Antonescu, C.R.; Reuter, V.E.; Keohan, M.L.; Hwang, S.; Chi, P. DICER1-Associated Anaplastic Sarcoma of the Kidney with Coexisting Activating PDGFRA D842V Mutations and Response to Targeted Kinase Inhibitors in One Patient. JCO Precis. Oncol. 2022, 6, e2100554. [Google Scholar] [CrossRef]
- Kroll-Wheeler, L.; Heider, A. Anaplastic Sarcoma of the Kidney with Heterologous Ganglioneuroblastic Differentiation: Another DICER1-Associated Tumor. Pediatr. Dev. Pathol. 2022, 25, 186–191. [Google Scholar] [CrossRef]
- Yamaoka, T.; Haga, Y.; Tochigi, N.; Yoshida, M.; Takahashi, H. Anaplastic sarcoma of the kidney with DICER1 mutation: A case report. Pediatr. Int. 2022, 64, e14851. [Google Scholar] [CrossRef]
- Bertolotto, C.; Lesueur, F.; Giuliano, S.; Strub, T.; de Lichy, M.; Bille, K.; Dessen, P.; d’Hayer, B.; Mohamdi, H.; Remenieras, A.; et al. A SUMOylation-defective MITF germline mutation predisposes to melanoma and renal carcinoma. Nature 2011, 480, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Guhan, S.M.; Artomov, M.; McCormick, S.; Njauw, C.; Stratigos, A.J.; Shannon, K.; Ellisen, L.W.; Tsao, H. Cancer risks associated with the germline MITF(E318K) variant. Sci. Rep. 2020, 10, 17051. [Google Scholar] [CrossRef] [PubMed]
- Lonati, C.; Simeone, C.; Suardi, N.; Spiess, P.E.; Necchi, A.; Moschini, M. Genitourinary manifestations of Lynch syndrome in the urological practice. Asian J. Urol. 2022, 9, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Nassour, A.J.; Jain, A.; Hui, N.; Siopis, G.; Symons, J.; Woo, H. Relative Risk of Bladder and Kidney Cancer in Lynch Syndrome: Systematic Review and Meta-Analysis. Cancers 2023, 15, 506. [Google Scholar] [CrossRef] [PubMed]
- Adolphe, F.; Ferlicot, S.; Verkarre, V.; Posseme, K.; Couvé, S.; Garnier, P.; Droin, N.; Deloger, M.; Job, B.; Giraud, S.; et al. Germline mutation in the NBR1 gene involved in autophagy detected in a family with renal tumors. Cancer Genet. 2021, 258–259, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Andreou, A.; Yngvadottir, B.; Bassaganyas, L.; Clark, G.; Martin, E.; Whitworth, J.; Cornish, A.J.; Genomics England Research Consortium; Houlston, R.S.; Rich, P.; et al. Elongin C (ELOC/TCEB1)-associated von Hippel-Lindau disease. Hum. Mol. Genet. 2022, 31, 2728–2737. [Google Scholar] [CrossRef]
- Schmidt, L.S.; Vocke, C.D.; Ricketts, C.J.; Blake, Z.; Choo, K.K.; Nielsen, D.; Gautam, R.; Crooks, D.R.; Reynolds, K.L.; Krolus, J.L.; et al. PRDM10 RCC: A Birt-Hogg-Dubé-like Syndrome Associated with Lipoma and Highly Penetrant, Aggressive Renal Tumors Morphologically Resembling Type 2 Papillary Renal Cell Carcinoma. Urology 2023, 179, 58–70. [Google Scholar] [CrossRef]
- Van de Beek, I.; Glykofridis, I.E.; Oosterwijk, J.C.; van den Akker, P.C.; Diercks, G.F.H.; Bolling, M.C.; Waisfisz, Q.; Mensenkamp, A.R.; Balk, J.A.; Zwart, R.; et al. PRDM10 directs FLCN expression in a novel disorder overlapping with Birt-Hogg-Dubé syndrome and familial lipomatosis. Hum. Mol. Genet. 2023, 32, 1223–1235. [Google Scholar] [CrossRef]
- Gomella, P.T.; Linehan, W.M.; Ball, M.W. Precision Surgery and Kidney Cancer: Knowledge of Genetic Alterations Influences Surgical Management. Genes 2021, 12, 261. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.H.; Tu, H.P.; Hong, C.H. A population-based study to estimate survival and standardized mortality of tuberous sclerosis complex (TSC) in Taiwan. Orphanet J. Rare Dis. 2021, 16, 335. [Google Scholar] [CrossRef] [PubMed]
| Disease | Gene | Population Frequency of PVs * | Incidence of the Disease in Population | Contribution to Cancer Morbidity | PVs Penetrance | % De Novo | Comments | ||
|---|---|---|---|---|---|---|---|---|---|
| Patients Satisfying Clinical Criteria of the Syndrome | Kidney Tumors ** | Non-Kidney Tumors | |||||||
| Von Hippel–Lindau disease (VHL) | VHL | 16–32/141,456 (1.13–2.26 × 10−4/1:4424–1:8850) | Incidence in newborns: 1:27,000 [5] Prevalence: 1:46,900 (Denmark) [5]; 1:36,000–1:91,000 [6] | 90% [8] | 0.3–3.2%: 0.3% [7,8]; 0.4% [9]; 0.5% [10,11]; 1.7% [12]; 2.2% [13]; 3.2% [14] | Hemangioblastomas: 25–38% [15] Pancreatic neuroendocrine tumors: 10% [16] Pheochromocytomas/paragangliomas (PPGLs): 4–5% [17,18] | 87–95% [5,19] Hypomorphic missense mutations associated with isolated PPGLs may have reduced penetrance | 20% [3,5] | Genotype– phenotype correlations are observed. Type 1A disease (reduced risk of PPGL): truncating mutations, inactivating missense mutations; Type 1B disease (reduced risk of PPGL, RCC): large gene rearrangements (LGRs) involving neighboring BRK1 gene; type 2A disease (reduced risk of RCC), type 2B (high risk for all VHL-associated malignancies), type 2C disease (isolated PPGL): missense mutations. Some mutations are associated with autosomal-recessive congenital erythrocytosis [20] |
| Hereditary papillary renal cell carcinoma syndrome (HPRCC) | MET | 4/141,456 (2.83 × 10−5/1:35,335) | Unknown (this syndrome is considered to be very rare [7,14,21]) | N/A | 0.4% [9] Papillary type 1 renal tumors: 12.4% (sporadic: 5%; familial: 41%) [21] | None | ~100% [21] | Not reported | |
| Fumarate hydratase tumor predisposition syndrome (FHTPS) or hereditary leiomyomatosis and renal cell carcinoma (HLRCC) | FH | 29–35/141,456 (2.05–2.47 × 10−4/1:4049–1:4878) # | Unknown Leiomyomas are frequent finding in general population, and FH PVs have relatively low penetrance towards kidney cancer | 40% (multiple cutaneous leiomyomas and/or multiple uterine fibroids and/or papillary type 2 RCC) [22] 70% (familial HLRCC cases) [23] | 0.2–5.2%: 0.2% [11]; 0.6% [13]; 1% [7,10]; 1.6% [14]; 1.8% [11]; 2.8% [9]; 5.2% [12]; Papillary type 2 RCC: 17.4% [23] | Uterine leiomyomas: ~0.7% [24] Uterine leiomyomas in young patients (<30 years): 2% [25] | RCCs: ~19% [26] Cutaneous and uterine leiomyomas: almost complete penetrance Rarely, PPGLs are observed | Not reported | Individuals with biallelic germline FH inactivation demonstrate frequently fatal mitochondrial encephalopathy (constitutional fumarase deficiency). Cancer is not a feature of biallelic FH deficiency. Rare hypomorphic variants are described, which are associated with FH deficiency but not with HLRCC. FH-associated RCCs are very aggressive |
| Hereditary paraganglioma/ pheochromocytoma (HPP) syndrome | SDHB, SDHC, SDHD (paternally inherited), SDHA | SDHB: 51–53/141,456 (3.6–3.75 × 10−4/1:2668–1:2778) † SDHD: 16–51/141,456 (1.13–3.75 × 10−4/1:2668–1:8850) SDHC: 23–30/141,456 (1.62–2.12 × 10−4/1:4717–1:6173) SDHA: 136–146/141,456 (9.61 × 10−4–1.03 × 10−3/1:971–1:1041) | Unknown Prevalence of SDHx-associated PPGLs: 1:51,667–1:77,500 (Denmark) [27] | N/A | 0.7–0.9%: 0.8% [9]; 0.7% [11]; 0.8% [14]; 0.9% [8,12] | PPGLs: 20–43% [17,18,28] | PPGLs: SDHB: 21.8%; SDHD: 43.2%; SDHC: 25% [29] SDHA: clinical series: 10–30%; population-based estimates: 0.1–4.9% [30] SDHx-associated RCCs: SDHB: 4.2%; SDHD, SDHC, SDHA: the increased risk has not been proven [29] Rarely: thyroid carcinomas, GIST, pituitary adenomas, etc. [29] | Not reported | Individuals with biallelic germline SDHA, SDHB, SDHD inactivation frequently demonstrate fatal mitochondrial disorders. Cancer is not a feature of biallelic SDHx deficiency. Hypomorphic variants are described, which are associated with SDHx deficiency but not with familial PPGL Penetrance of SDHx (especially SDHA) mutations observed in relatives of PPGL patients is significantly higher than in accidentally identified individuals harboring the same PVs [30] |
| Birt–Hogg– Dubé syndrome (BHD) | FLCN | 25–38/141,456 (1.76–2.68 × 10−4/1:3731–1:5682) | Varying estimates (this disease seems to be underdiagnosed): 1:500,000 (worldwide) [31] 1:176,366 (South Korea) [32] 1:3265 (Sweden) [33] 1:3234 (Pennsylvania, USA) [34] | 67% [35]; 80–85% [35] | 0.3–1.6%: 0.3% [11,13]; 0.4% [13]; 0.5% [10]; 1.2% [8]; 1.6% [14] | RCCs: 19–21%; spontaneous pneumothorax/multiple bilateral pleural/subpleural cysts: 82–87%; skin lesions (fibrofolliculomas, acrochordons, angiofibromas): 78–87%; colonic polyps: 21–32% [36]. Population-based study [36]: cystic lung disease: 65.7%; pneumothorax: 17.1%; skin lesions: 8.6%; RCCs: 2.9% | A single report of de novo mutation [37] | In Asian patients, skin lesions are usually subtle and do not raise suspicion in patients or physicians. For example, in one Japanese study, skin lesions were noted in 49% of the patients, but only 1/76 (1.3%) subjects voluntarily consulted a dermatologist before the BHD diagnosis [38] | |
| Tuberous sclerosis (TS) | TSC1, TSC2 | TSC1: 5–10/141,456 (3.53 × 10−5–7.07 × 10−5/1:14,144–1:28,329) TSC2: 3–13/141,456 (2.12 × 10−5–9.19 × 10−5/1:10,881–1:47,170) | ~1:10,000 e.g., 1:12,658 (Germany) [39] Incidence in newborns: 1:5800–1:10,000 [40] | 75–90% [40] | 0.2–2.6%: 0.2% [8]; 0.4% [12]; 0.9% [13]; 2.6% [10] | Cortical tubers: 88–90% (associated with TSC2); SEGA (subependymal giant cell astrocytomas): 5–24.4%; cardiac rhabdomyomas: 34–58%; angiomyolipomas: 51.8%; RCCs: 1–2% (unusually early-onset); cystic kidney disease: 50%; lymphangioleiomyomatosis (female patients): 34–81%; angiofibromas: 57.3–74.5%; periungual fibromas: 15%; retinal hamartomas: 30–44% [41] | TSC1: 59%; TSC2: 85% (more severe phenotype) [42] | ||
| Cowden syndrome | PTEN | 24–27/141,456 (1.7–1.91 × 10−4/1:5236–1:5882) | 1:200,000 [43] | ~9.5% [44] | 0.3% [8] | Breast carcinomas:0.2% [45] Thyroid carcinomas: 0.8% [46] Endometrial carcinomas: <0.4% [47] | Breast carcinomas: 85%; thyroid carcinomas: 35%; kidney carcinomas: 34%; endometrial carcinomas: 28%; other cancers: 9% [43] | 10–48% [48] | |
| BAP1-associated tumor predisposition syndrome | BAP1 | 4–48/141,456 (2.82 × 10−5–3.39 × 10−4/1:2950–1:35,461) ‡ | Unknown (this syndrome is considered to be very rare [49]) | N/A | 0.3–1.6%: 0.3% [13]; 0.4% [8,12]; 1.2% [9]; 1.6% [10] | Uveal melanomas: 2–4% (familial uveal melanomas: 22%) [50] Malignant mesotheliomas: 1–5% [51] | Uveal melanomas: 8.5–31%; malignant mesotheliomas: 17–22%; melanocytic BAP1-mutated atypical intradermal tumors: 18%; cutaneous melanomas: 3–13%; RCC: 3–10% [52] | Up to 9.5% [53] | |
| Disease | Gene | Spectrum of PVs | Founder Mutations |
|---|---|---|---|
| Von Hippel–Lindau disease | VHL | Missense: 52%; frameshifts: 13%; nonsense: 11%; large gene rearrangements (LGRs): 11%; splice-site: 7%; in-frame deletions/insertions: 6% (945 VHL families) [54] Recent studies show an increased proportion of LGRs, e.g., 20% in a Japanese data set [55] | Regional founder variant, c.292T>C (p.Tyr98His or c.505T>C, according to old nomenclature), is associated with VHL type 2A disease (also known as “Black Forest mutation” (Germany, Bavaria)) [19] |
| Hereditary papillary renal cell carcinoma syndrome | MET | Activating missense mutations [21] | Not reported |
| Fumarate hydratase tumor predisposition syndrome | FH | Missense: ~50%, nonsense and frameshift mutations: 25–33%. LGRs are rarely described (~5%) [23,26] | Several minor founder variants exist, e.g., Iranian Jewish 905–1G>A mutation (4 families) [56]. There are known hot-spot mutations, e.g., those affecting codon 190 [57]. Recurrent mutations can be identified in the gnomAD database, e.g., c.698G>A (p.Arg233His) variant: 4/50,738 Northwestern Europeans |
| Hereditary paraganglioma/ pheochromocytoma (HPP) syndrome | SDHB, SDHC, SDHD, SDHA | SDHB, SDHC, SDHD: missense: 44%; nonsense: 15%; splice-site: 13%; frameshifts: 15%; in-frame deletions: 0.5%; large CNVs (frequently involve 1st exon/promoter of SDHB, SDHC, SDHD): 12% [29] | Multiple instances of founder mutations are known: Icelandic SDHA c.91C>T (p.Arg31Ter) mutation (7/9 (78%) SDHx-associated kidney cancer cases); this variant is also frequent in Sweden [13]; Dutch founder mutations in SDHB: exon 3 deletion (30% of pathogenic alleles), c.423+1G>A (20% of pathogenic alleles) [28]; Portuguese founder deletion in the 1st exon of SDHB: 26–37% of pathogenic SDHx alleles in Portuguese PPGL cases [58,59]; the same LGR occurs in Northern Spain [60] and is the most frequent SDHB mutation in Brazil (36% mutations) [61] and Colombia (90% mutations) [62]. SDHC p.Arg133Ter variant is a French Canadian founder mutation of French origin (69% SDHx mutations) [63]; SDHD p.Cys11Ter is a Polish founder variant [64] |
| Birt–Hogg– Dubé syndrome | FLCN | The majority of mutations are truncating, e.g., duplications (46.4%), deletions (29.0%), substitutions (7.1%), insertions (0.7%), deletions/insertions (0.3%), large genomic deletions (4.0%), and splice-site mutations (12.5%) (Japan) [65]. Similar results are reported in locus-specific FLCN mutation database: deletions (44.3%), substitutions (35.7%), duplications (14.3%), and deletions/insertions (5.7%) [66]. | In most populations, a recurrent hot-spot mutation is responsible for up to half of the cases [35]. Several founder variants were reported: Danish founder mutation c.1062+2T>G (11/31, 35% of all mutations) [67]; Chinese regional founder LGR (deletion of exons 1–3) [68]; Swedish founder mutation c.779+1G>T (57% pathogenic alleles) [33] |
| Tuberous sclerosis | TSC1, TSC2 | TSC1: the vast majority of mutations are truncating; TSC2: roughly 30% of variants are missense, and 6% are LGRs [40] | Not reported |
| Cowden syndrome | PTEN | Missense mutations: 29%; nonsense mutations: 32%; small deletions: 14%; small insertions: 8%; indels: 1%; large deletions 3%; splice-site mutations: 10%; promoter mutations 3% [44] | Not reported |
| BAP1-associated tumor predisposition syndrome | BAP1 | ~78% are truncating/null variants; 22% are missense mutations; LGRs are rare [49] | There are recurrent hot-spot variants, e.g., p.Arg60Ter. Finnish founder variant (p.G549Vfs*49) has been described [69]. An extremely large cancer family of Swiss origin, scattered across USA, carries p.Leu573fs*3 allele [70] |
| Disease | Kidney Tumors | Extrarenal Tumors |
|---|---|---|
| Von Hippel–Lindau disease [83] | Biannual MRI since 15 years | Since birth: physical examination; dilated eye examination (every 6–12 months); since 2 years: annual blood pressure and pulse assessment; since 5 years: annual measurement of plasma-free metanephrines; since 11 years: biannual brain and spine MRI and audiogram; 15–20 years: MRI of internal auditory canal (once if no findings detected) |
| Fumarate hydratase deficiency [84] | Annual MRI since 8–10 years | No specific surveillance |
| Hereditary pheochromocytoma/ paraganglioma syndrome [85] | MRI every 2–3 years since childhood | Annual blood pressure and pulse assessment; measurement of plasma-free metanephrines or urinary metanephrines (biannually in children, annually in adults); head and neck, thoracic, abdominal, and pelvic MRI (every 2–3 years) |
| Hereditary papillary renal cell carcinoma [86] | Annual MRI since 30 years | No specific surveillance |
| Birt–Hogg– Dubé syndrome [87] | Biannual MRI since 20 years | Dermatologic examination (every 6–12 months); annual evaluation of parotid glands; annual ultrasound of thyroid gland may be considered; lung CT for symptomatic patients (before scheduled general anesthesia or before long-distance flights); regular colonoscopies, especially in kindreds with family history of colorectal malignancies |
| Tuberous sclerosis [88] | MRI every 1–3 years since childhood; annual renal function assessment | Brain MRI every 1–3 years; in asymptomatic infants: electroencephalography (every 6 weeks before 1 year, then every 3 months until 2 years old); electrocardiography every 3–5 years; echocardiography every 1–3 years in asymptomatic patients until regression of cardiac rhabdomyomas; annual dermatologic and ophthalmic examination. Lung CT (every 5–7 years) and annual pulmonary function assessment in adult females |
| BAP1 tumor predisposition syndrome [89] | Annual MRI since 30 years | Annual dermatological examination; periodic ophthalmological examination |
| Cowden syndrome [90] | Biannual ultrasound since 40 years | Since 18 years: annual thyroid ultrasound; since 30 years: annual breast MRI; dermatologic examination (once, at time of diagnosis); Since 35–40 years: colonoscopy (once, at time of diagnosis); Since 40 years: annual mammography |
| Disease | Therapy | Surgical Approach |
|---|---|---|
| Von Hippel–Lindau disease | Antiangiogenic therapy and multikinase inhibitors; | “Watch-and-wait” approach (lesions < 3 cm in diameter) [82] |
| Anti-HIF2-alpha therapy (belzutifan) [93,94,95,96] | ||
| Fumarate hydratase deficiency | Bevacizumab + erlotinib; multikinase inhibitors; immune therapy [97,98,99,100,101,102,103,104,105] | Immediate surgical removal [106] |
| Hereditary pheochromocytoma/ paraganglioma syndrome | Immediate surgical removal [72] | |
| Hereditary papillary renal cell carcinoma | MET inhibitors (crizotinib, capmatinib) [107,108,109] | “Watch-and-wait” approach (lesions < 3 cm in diameter) [71,110] |
| Birt–Hogg– Dubé Syndrome | mTOR inhibitors (everolimus)? [111] | “Watch-and-wait” approach (lesions < 3 cm in diameter) [106] |
| Tuberous sclerosis | mTOR inhibitors (everolimus) [112,113,114] | “Watch-and-wait” approach for angiomyolipomas, insufficient evidence regarding RCC [115,116] |
| BAP1 tumor predisposition syndrome | Insufficient evidence, but immediate surgical removal is suggested [106] | |
| Cowden syndrome | mTOR inhibitors (everolimus)? [117] | Insufficient evidence |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yanus, G.A.; Kuligina, E.S.; Imyanitov, E.N. Hereditary Renal Cancer Syndromes. Med. Sci. 2024, 12, 12. https://doi.org/10.3390/medsci12010012
Yanus GA, Kuligina ES, Imyanitov EN. Hereditary Renal Cancer Syndromes. Medical Sciences. 2024; 12(1):12. https://doi.org/10.3390/medsci12010012
Chicago/Turabian StyleYanus, Grigory A., Ekaterina Sh. Kuligina, and Evgeny N. Imyanitov. 2024. "Hereditary Renal Cancer Syndromes" Medical Sciences 12, no. 1: 12. https://doi.org/10.3390/medsci12010012
APA StyleYanus, G. A., Kuligina, E. S., & Imyanitov, E. N. (2024). Hereditary Renal Cancer Syndromes. Medical Sciences, 12(1), 12. https://doi.org/10.3390/medsci12010012






