Hepatoprotective Effects of Royal Jelly Against Vincristine-Induced Hepatotoxicity in Rats: A Biochemical and Molecular Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals
2.3. Experimental Design
2.4. Sample Collection
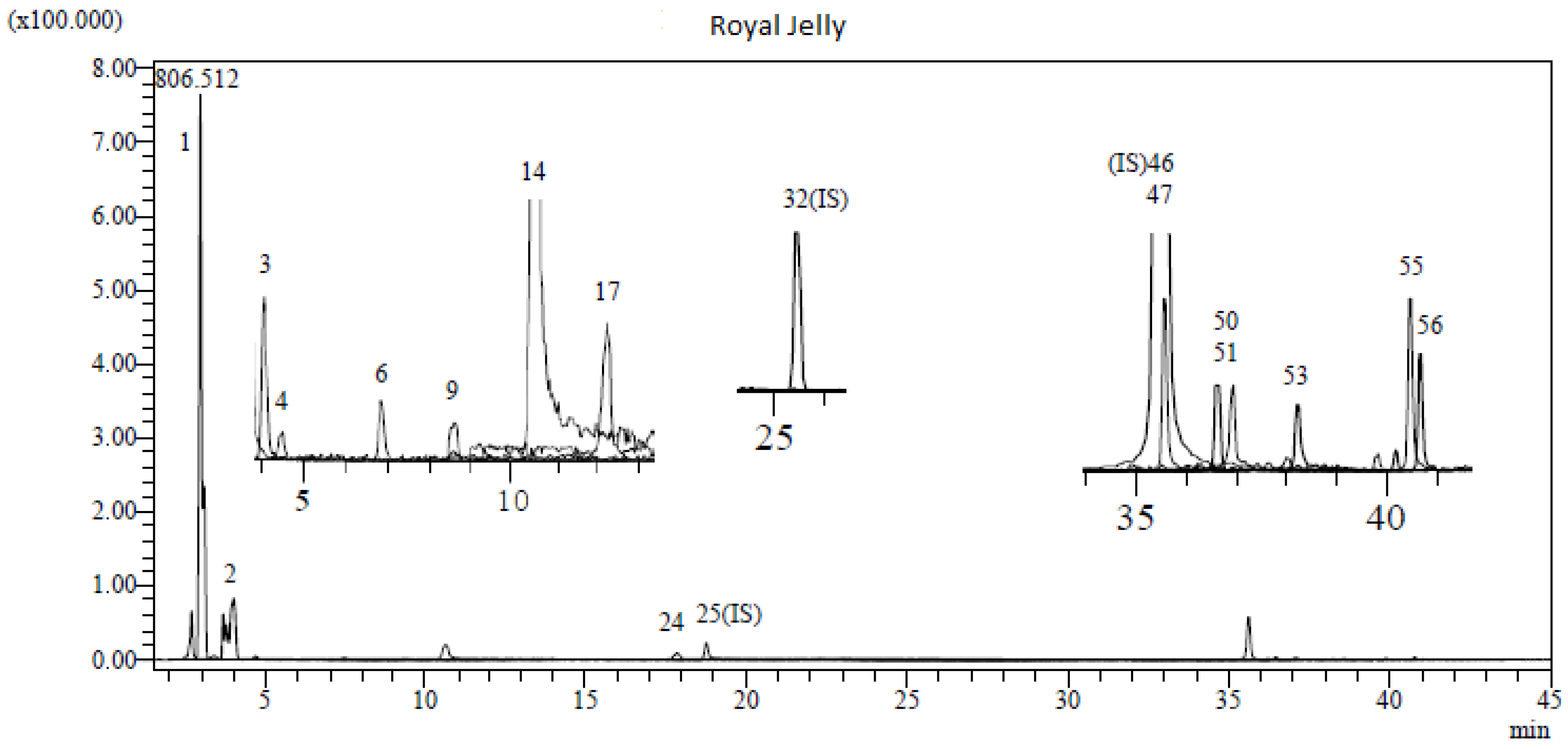
2.5. Determination of Chemical Content of RJ by LC-MS/MS
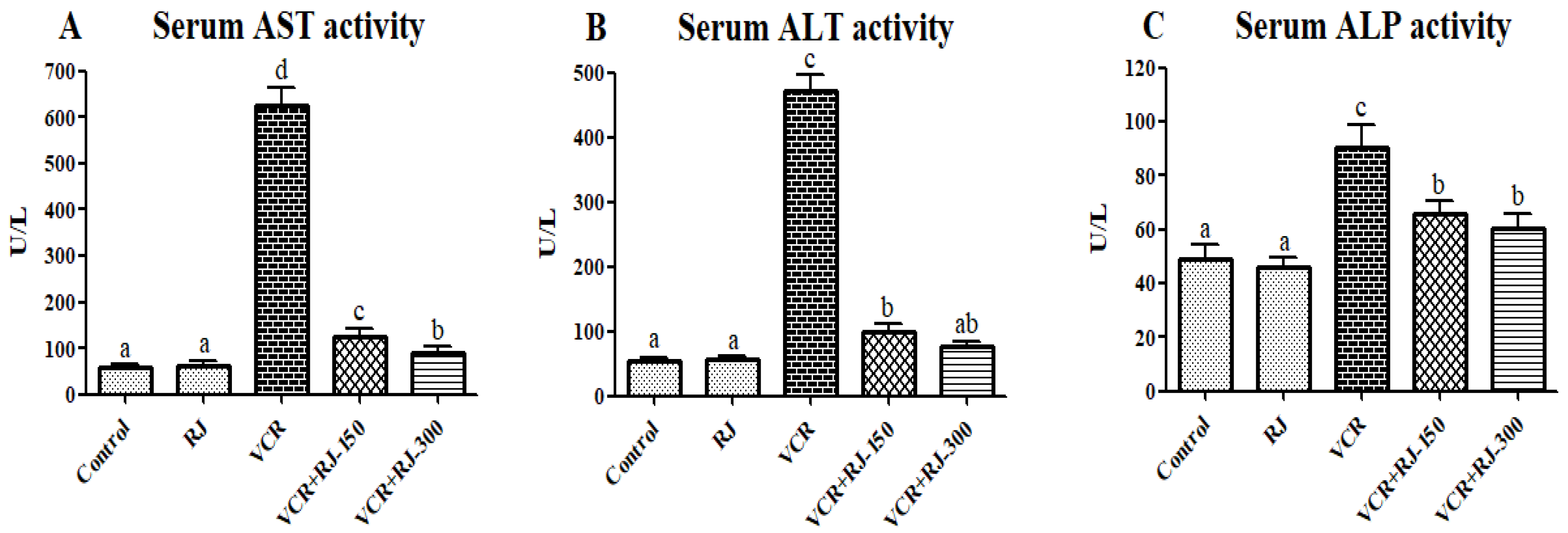
2.6. Liver Function Tests
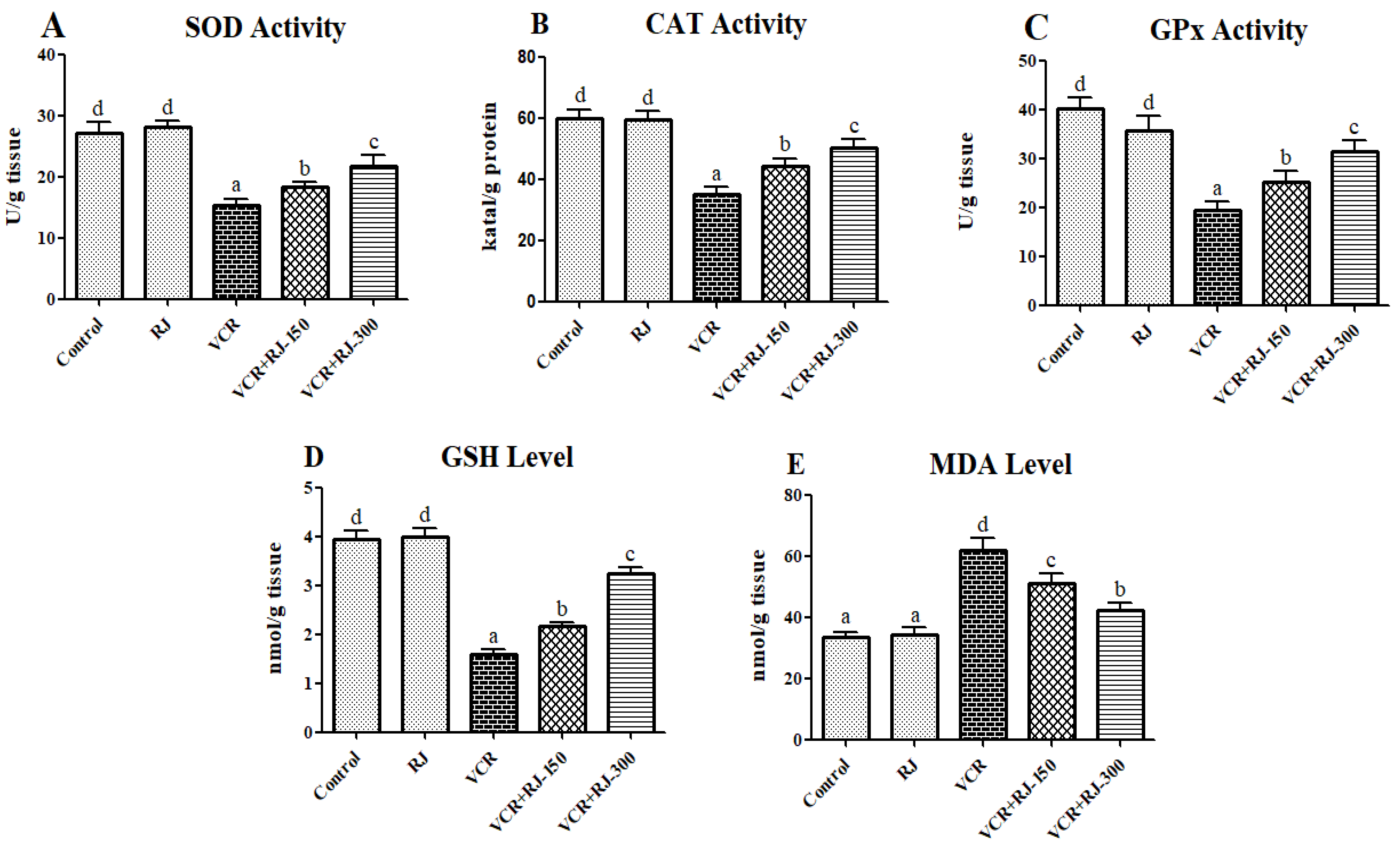
2.7. Oxidative Stress Markers in the Liver Tissue
2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. Total RNA Isolation and cDNA Synthesis from Liver Tissue
2.10. Determination of Relative mRNA Transcript Levels (RT-PCR)
2.11. Western Blot Analysis for Apoptosis
2.12. Statistical Analysis
3. Results
3.1. LC-MS/MS Analysis Results of RJ
3.2. Effects of RJ on VCR-Induced Liver Function Parameters
3.3. Effects of RJ on VCR-Induced Oxidative Stress and Lipid Peroxidation in Liver Tissue
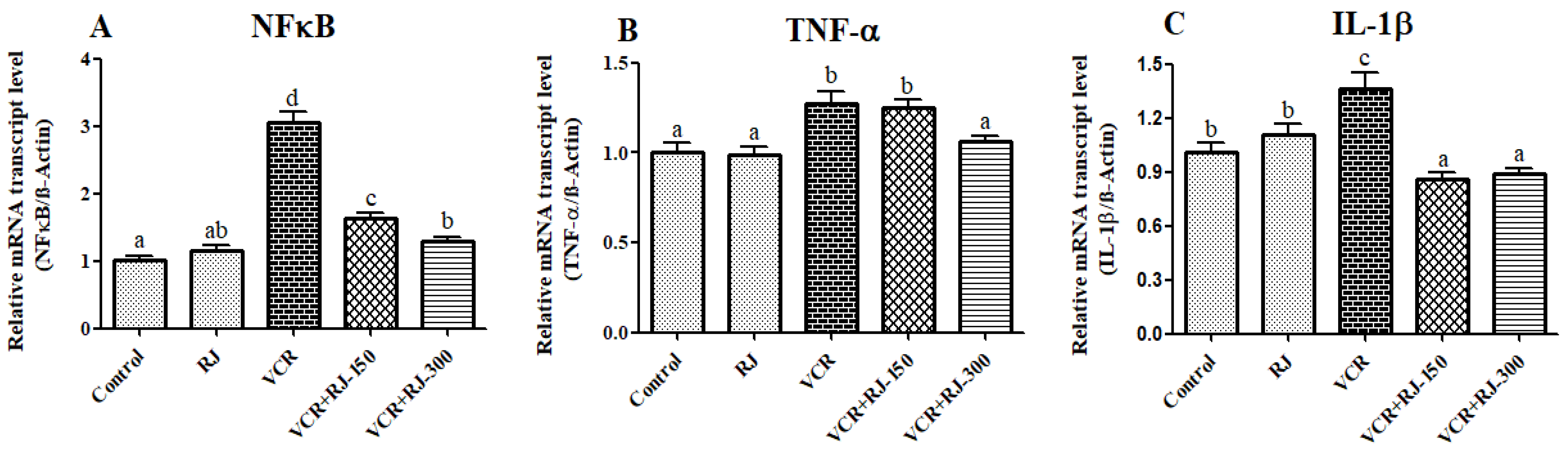
3.4. Effects of RJ and VCR on Relative mRNA Transcript Levels of NF-κB, TNF-α, and IL-1β Genes in Liver Tissue
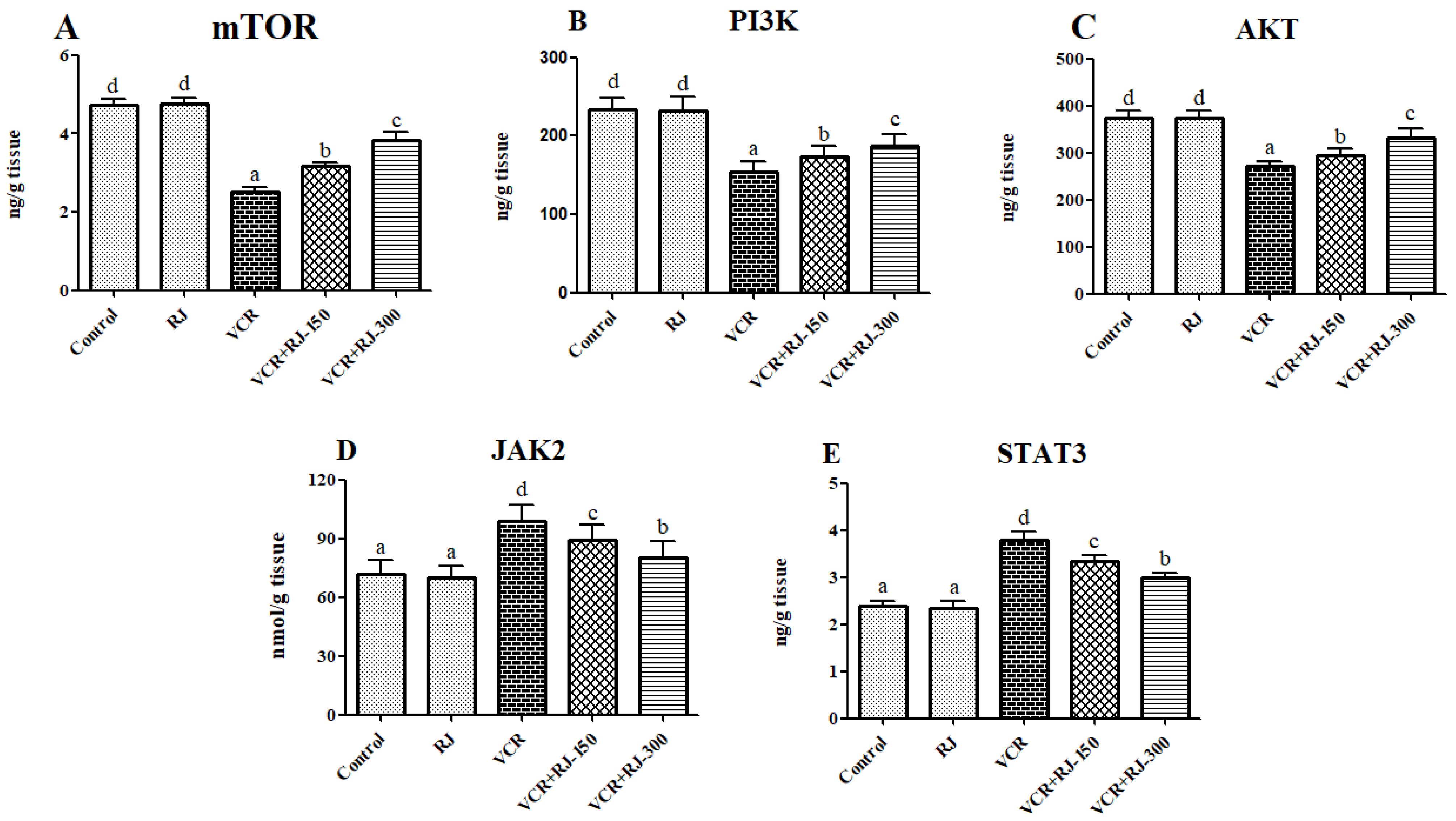
3.5. Effects of RJ on VCR-Induced PI3K/Akt/mTOR Pathway in Liver Tissue
3.6. Effects of RJ on VCR-Induced JAK2 and STAT3 Levels in Liver Tissue
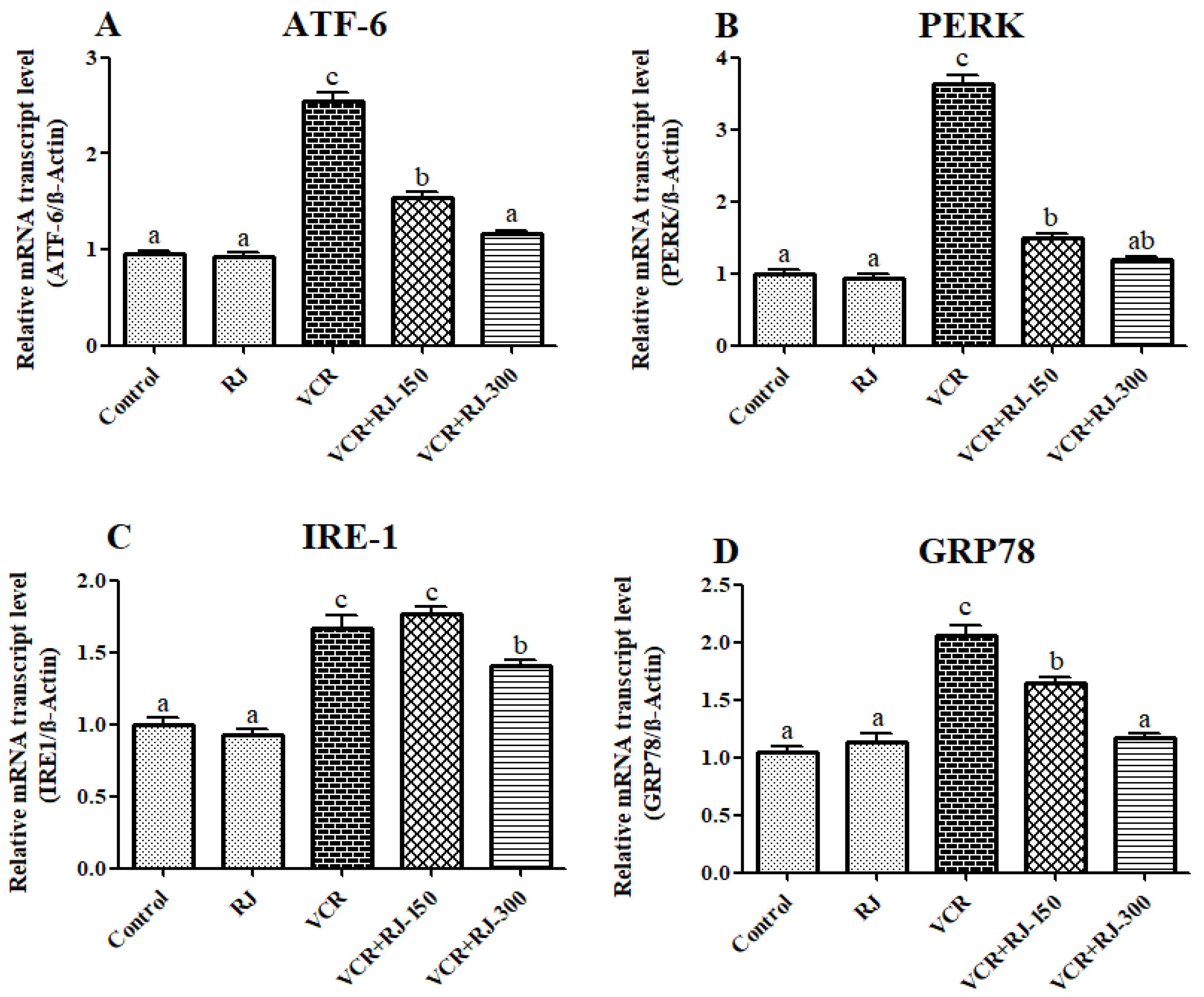
3.7. Effects of RJ and VCR on Relative mRNA Transcript Levels of ATF-6, PERK, IRE1, and GRP-78 Genes in Liver Tissue
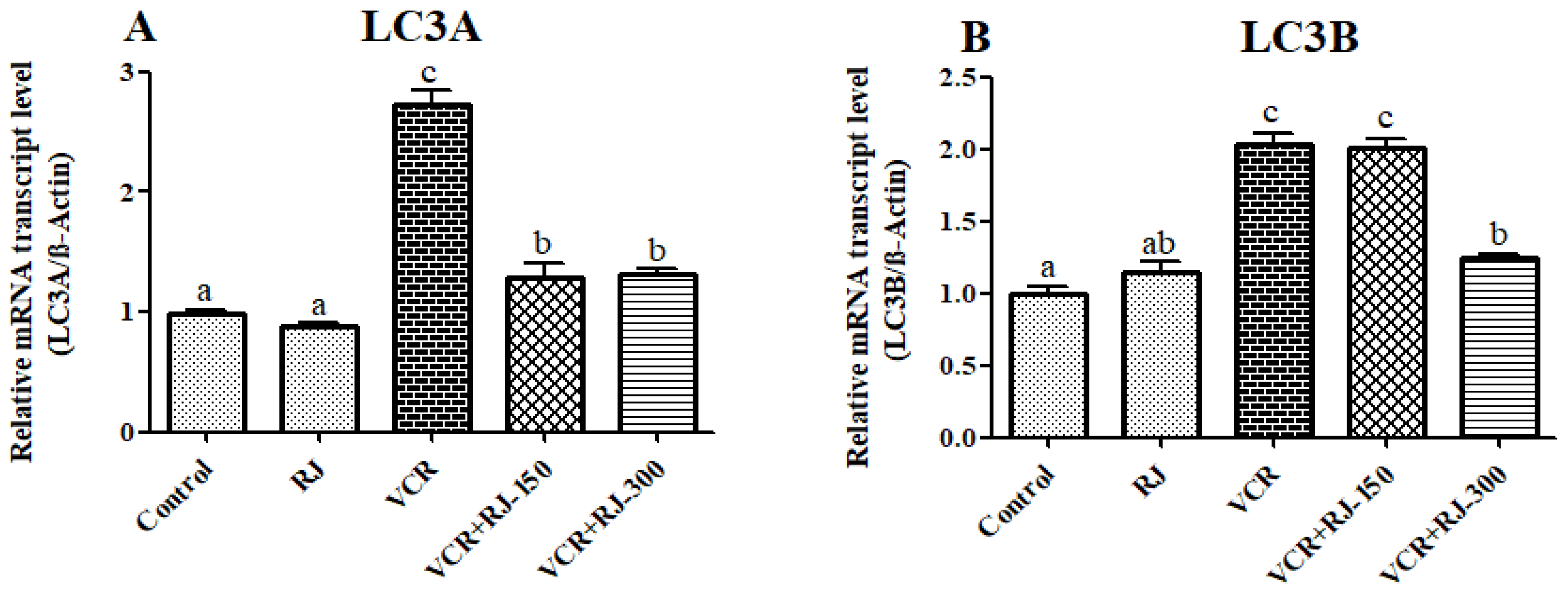
3.8. Effects of RJ and VCR on Relative mRNA Transcript Levels of LC3A and LC3B Genes in Liver Tissue
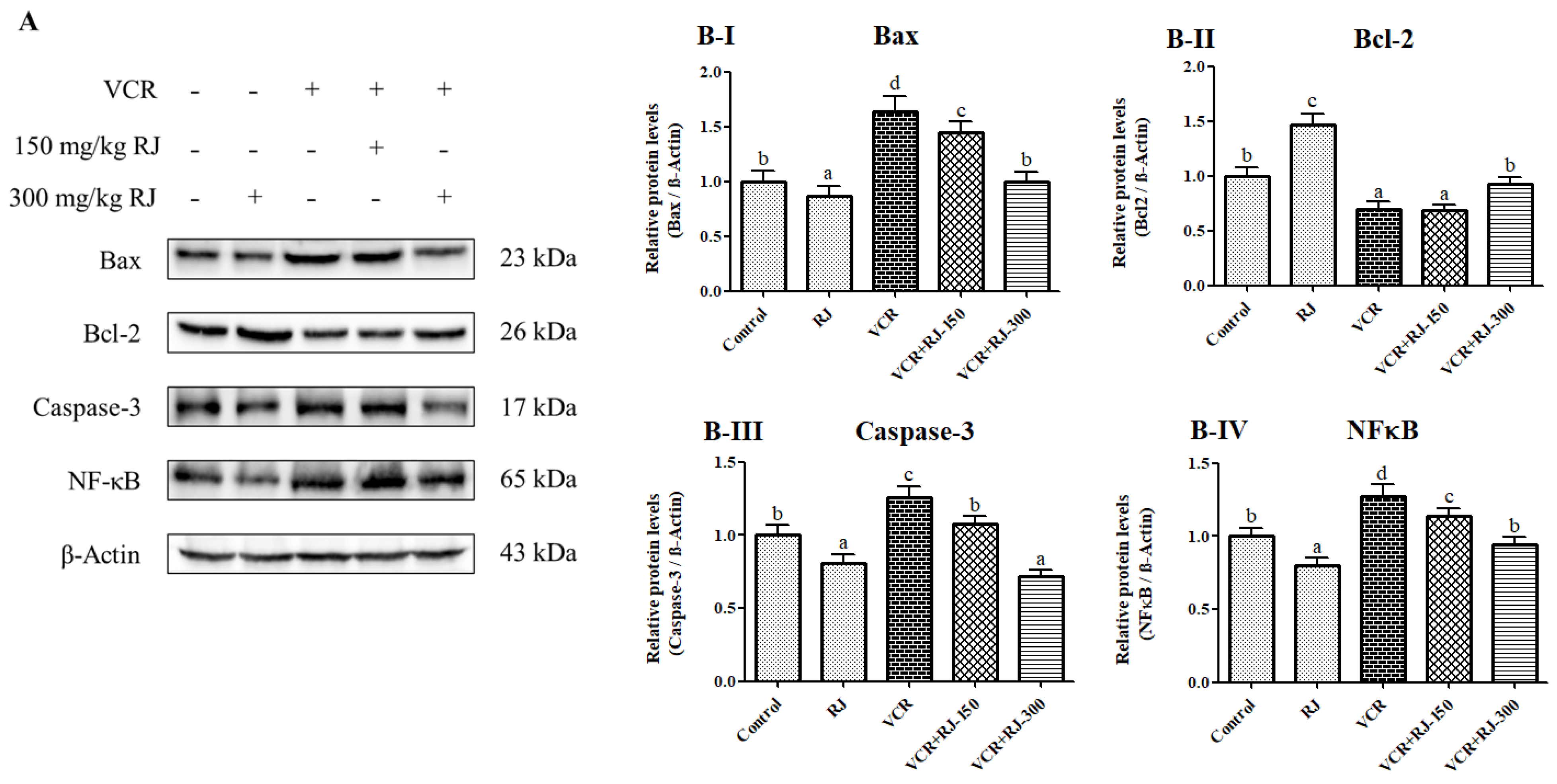
3.9. Effects of RJ and VCR on Apoptosis in Liver Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, Z.; Li, M.; Dey, R.; Chen, Y. Nanomaterials for cancer therapy: Current progress and perspectives. J. Hematol. Oncol. 2021, 14, 85. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Khan, A.R.; Yang, X.; Dong, B.; Ji, J.; Zhai, G. The reversal of chemotherapy-induced multidrug resistance by nanomedicine for cancer therapy. J. Control. Release 2021, 335, 1–20. [Google Scholar] [CrossRef]
- Dodurga, Y.; Seçme, M.; Elmas, L.; Demirkıran, N.; Sağ, S.; Adamcı, U.; Akdağ, Z. Cisplatin impact on Kasumi-1 leukemia cell line: Gene expression and DNA damage. Pamukkale Med. J. 2025, 18, 63–71. [Google Scholar]
- Behranvand, N.; Nasri, F.; Zolfaghari Emameh, R.; Khani, P.; Hosseini, A.; Garssen, J.; Falak, R. Chemotherapy: A double-edged sword in cancer treatment. Cancer Immunol. Immunother. 2022, 71, 507–526. [Google Scholar] [CrossRef]
- Dodurga, Y.; Elmas, L.; Seçme, M.; Demirkıran, N.; Avcı, Ç.B.; Bağcı, G.; Sağ, S.; Tufan, L.Ş. Investigation of the apoptotic and cell cycle effects of sorafenib and doxorubicin on URG4/URGCP in leukemia cells. Pamukkale Med. J. 2024, 17, 498–508. [Google Scholar] [CrossRef]
- Herradón, E.; González, C.; González, A.; Uranga, J.A.; López-Miranda, V. Cardiovascular toxicity induced by chronic vincristine treatment. Front. Pharmacol. 2021, 12, 692970. [Google Scholar] [CrossRef]
- Harchegani, A.B.; Khor, A.; Niha, M.M.; Kaboutaraki, H.B.; Shirvani, H.; Shahriary, A. The hepatoprotective and antioxidative effect of saffron stigma alcoholic extract against vincristine sulfate induced toxicity in rats. Interdiscip. Toxicol. 2019, 12, 186–191. [Google Scholar] [CrossRef]
- Shati, A.A.; Elsaid, F.G. Hepatotoxic effect of subacute vincristine administration activates necrosis and intrinsic apoptosis in rats: Protective roles of broccoli and Indian mustard. Arch. Physiol. Biochem. 2019, 125, 1–11. [Google Scholar] [CrossRef]
- Mollinedo, F.; Gajate, C. Microtubules, microtubule-interfering agents and apoptosis. Apoptosis 2003, 8, 413–450. [Google Scholar] [CrossRef]
- Vashistha, B.; Sharma, A.; Jain, V. Ameliorative potential of ferulic acid in vincristine-induced painful neuropathy in rats: An evidence of behavioral and biochemical examination. Nutr. Neurosci. 2017, 20, 60–70. [Google Scholar] [CrossRef]
- Çomaklı, S.; Özdemir, S.; Küçükler, S.; Kandemir, F.M. Beneficial effects of quercetin on vincristine-induced liver injury in rats: Modulating the levels of Nrf2/HO-1, NF-kB/STAT3, and SIRT1/PGC-1α. J. Biochem. Mol. Toxicol. 2023, 37, e23326. [Google Scholar] [CrossRef] [PubMed]
- Shati, A.A. Sub-Chronic Administration of Vincristine Sulfate Induces Renal Damage and Apoptosis in Rats via Induction of Oxidative Stress and Activation of Raf1-MEK1/2-Erk1/2 Signal Transduction. Int. J. Morphol. 2019, 37, 273–283. [Google Scholar] [CrossRef]
- Nevalainen, T.J. Cytotoxicity of vinblastine and vincristine to pancreatic acinar cells. Virchows Arch. B 1975, 18, 119–127. [Google Scholar] [CrossRef]
- İzol, E.; Çağlayan, C. Protective Effects of Royal Jelly on Heavy Metal Toxicity. In The Significance of Bee Products in Health; İzol, E., Haspolat, Y.K., Gülçin, İ., Eds.; Orient Publications: New Delhi, UK, 2023; pp. 1–8. [Google Scholar]
- İzol, E.; Yapıcı, İ.; Gülçin, İ. 10-Hydroxy-2-Decenoic Acid (10-HDA) and Bioactive Components in Royal Jelly. In Biological Activities of Honeybee Products; İzol, E., Haspolat, Y.K., Gülçin, İ., Eds.; Orient Publications: New Delhi, UK, 2023; pp. 1–9. [Google Scholar]
- Karadeniz, A.; Simsek, N.; Karakus, E.; Yildirim, S.; Kara, A.; Can, I.; Kisa, F.; Emre, H.; Turkeli, M. Royal jelly modulates oxidative stress and apoptosis in liver and kidneys of rats treated with cisplatin. Oxidative Med. Cell. Longev. 2011, 2011, 981793. [Google Scholar] [CrossRef]
- Guendouz, M.; Haddi, A.; Grar, H.; Kheroua, O.; Saidi, D.; Kaddouri, H. Preventive effects of royal jelly against anaphylactic response in a murine model of cow’s milk allergy. Pharm. Biol. 2017, 55, 2145–2152. [Google Scholar] [CrossRef] [PubMed]
- Kanbur, M.; Eraslan, G.; Beyaz, L.; Silici, S.; Liman, B.C.; Altınordulu, Ş.; Atasever, A. The effects of royal jelly on liver damage induced by paracetamol in mice. Exp. Toxicol. Pathol. 2009, 61, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Abdelhafiz, H.A.; El-kott, A.F.; Elesh, M.R. Hepatoprotective effect of royal jelly against cisplatin-induced biochemical, oxidative stress, anti-oxidants and histopathological abnormalities. Adv. Life Sci. Technol. 2014, 27, 28–38. [Google Scholar]
- Mostafa, R.E.; El-Marasy, S.A.; Jaleel, G.A.A.; Bakeer, R.M. Protective effect of royal jelly against diclofenac-induced hepato-renal damage and gastrointestinal ulcerations in rats. Heliyon 2020, 6, e03330. [Google Scholar] [CrossRef]
- Djemil, R.; Djemli, S.; Derouiche, F.; Maamar, H.; Ati, S.; Arrouf, D.; Houggas, A.; Khellili, K. Study of the preventive effect of royal jelly against the hepatotoxicity of two anti-tuberculosis drugs. Uttar Pradesh J. Zool. 2022, 43, 56–64. [Google Scholar] [CrossRef]
- Rzetecka, N.; Matuszewska, E.; Plewa, S.; Matysiak, J.; Klupczynska-Gabryszak, A. Bee products as valuable nutritional ingredients: Determination of broad free amino acid profiles in bee pollen, royal jelly, and propolis. J. Food Compos. Anal. 2024, 126, 105860. [Google Scholar] [CrossRef]
- Yardim, A.; Kandemir, F.M.; Ozdemir, S.; Kucukler, S.; Comakli, S.; Gur, C.; Celik, H. Quercetin provides protection against the peripheral nerve damage caused by vincristine in rats by suppressing caspase 3, NF-κB, ATF-6 pathways and activating Nrf2, Akt pathways. NeuroToxicology 2020, 81, 137–146. [Google Scholar] [CrossRef] [PubMed]
- İzol, E. Molecular docking-guided in-depth investigation of the biological activities and phytochemical and mineral profiles of endemic Phlomis capitata. J. Sci. Food Agric. 2025. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Placer, Z.A.; Cushman, L.L.; Johnson, B.C. Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. Anal. Biochem. 1966, 16, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Oberley, L.W.; Li, Y. A simple method for clinical assay of superoxide dismutase. Clin. Chem. 1988, 34, 497–500. [Google Scholar] [CrossRef]
- Aebi, H. [13] Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Lawrence, R.A.; Burk, R.F. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem. Biophys. Res. Commun. 1976, 71, 952–958. [Google Scholar] [CrossRef]
- Sedlak, J.; Lindsay, R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Darendelioglu, E.; Caglayan, C.; Küçükler, S.; Bayav, İ.; Kandemir, F.M.; Ayna, A.; Sağ, S. 18β-glycyrrhetinic acid Mitigates bisphenol A-induced liver and renal damage: Inhibition of TNF-α/NF-κB/p38-MAPK, JAK1/STAT1 pathways, oxidative stress and apoptosis. Food Chem. Toxicol. 2025, 196, 115218. [Google Scholar] [CrossRef]
- Akinrinde, A.S.; Ajao, J.J.; Oyagbemi, A.A.; Ola-Davies, O.E. Taurine and protocatechuic acid attenuate Vincristine sulphate-induced bone marrow, liver and intestinal injuries via anti-oxidative, anti-inflammatory and anti-apoptotic activities. Comp. Clin. Pathol. 2024, 33, 545–562. [Google Scholar] [CrossRef]
- Ghanbari, E.; Nejati, V.; Khazaei, M. Improvement in serum biochemical alterations and oxidative stress of liver and pancreas following use of royal jelly in streptozotocin-induced diabetic rats. Cell J. (Yakhteh) 2016, 18, 362. [Google Scholar]
- Li, S.; Tan, H.-Y.; Wang, N.; Zhang, Z.-J.; Lao, L.; Wong, C.-W.; Feng, Y. The role of oxidative stress and antioxidants in liver diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef] [PubMed]
- İzol, E.; Turhan, M.; Yılmaz, M.A.; Çağlayan, C.; Gülçin, İ. Determination of Antioxidant, Antidiabetic, Anticholinergic, Antiglaucoma Properties and Comprehensive Phytochemical Content by LC-MS/MS of Bingöl Honeybee Pollen. Food Sci. Nutr. 2025, 13, e4531. [Google Scholar] [CrossRef]
- Bayav, I.; Darendelioğlu, E.; Caglayan, C. 18β-Glycyrrhetinic acid exerts cardioprotective effects against BPA-induced cardiotoxicity through antiapoptotic and antioxidant mechanisms. J. Biochem. Mol. Toxicol. 2024, 38, e23655. [Google Scholar] [CrossRef]
- Palma, F.R.; Gantner, B.N.; Sakiyama, M.J.; Kayzuka, C.; Shukla, S.; Lacchini, R.; Cunniff, B.; Bonini, M.G. ROS production by mitochondria: Function or dysfunction? Oncogene 2024, 43, 295–303. [Google Scholar] [CrossRef]
- Elshamy, A.M.; Salem, O.M.; Safa, M.A.; Barhoma, R.A.; Eltabaa, E.F.; Shalaby, A.M.; Alabiad, M.A.; Arakeeb, H.M.; Mohamed, H.A. Possible protective effects of CO Q10 against vincristine-induced peripheral neuropathy: Targeting oxidative stress, inflammation, and sarmoptosis. J. Biochem. Mol. Toxicol. 2022, 36, e22976. [Google Scholar] [CrossRef]
- Gu, H.; Song, I.-B.; Han, H.-J.; Lee, N.-Y.; Cha, J.-Y.; Son, Y.-K.; Kwon, J. Antioxidant activity of royal jelly hydrolysates obtained by enzymatic treatment. Korean J. Food Sci. Anim. Resour. 2018, 38, 135. [Google Scholar]
- Hamza, R.Z.; Al-Eisa, R.A.; El-Shenawy, N.S. Possible Ameliorative Effects of the Royal Jelly on Hepatotoxicity and Oxidative Stress Induced by Molybdenum Nanoparticles and/or Cadmium Chloride in Male Rats. Biology 2022, 11, 450. [Google Scholar] [CrossRef]
- Omar, E.M.; El-Sayed, N.S.; Elnozahy, F.Y.; Hassan, E.; Amr, A.; Augustyniak, M.; El-Samad, L.M.; El Wakil, A. Reversal Effects of Royal Jelly and Propolis Against Cadmium-Induced Hepatorenal Toxicity in Rats. Biol. Trace Elem. Res. 2024, 202, 1612–1627. [Google Scholar] [CrossRef]
- Kaur, G.; Shivanandappa, T.B.; Kumar, M.; Kushwah, A.S. Fumaric acid protect the cadmium-induced hepatotoxicity in rats: Owing to its antioxidant, anti-inflammatory action and aid in recast the liver function. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 1911–1920. [Google Scholar] [CrossRef]
- Shariati, S.; Mohtadi, S.; Khodayar, M.J.; Salehcheh, M.; Azadnasab, R.; Mansouri, E.; Moosavi, M. Quinic acid alleviates liver toxicity induced by acetaminophen in mice via anti-oxidative and anti-inflammatory effects. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025, 1–12. [Google Scholar] [CrossRef]
- Pang, C.; Shi, L.; Sheng, Y.; Zheng, Z.; Wei, H.; Wang, Z.; Ji, L. Caffeic acid attenuated acetaminophen-induced hepatotoxicity by inhibiting ERK1/2-mediated early growth response-1 transcriptional activation. Chem.-Biol. Interact. 2016, 260, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, Z.; Moaiedi, M.Z.; Gorji, A.V.; Mansouri, E. p-Coumaric acid alleviates adriamycin-induced hepatotoxicity in rats. Asian Pac. J. Trop. Biomed. 2021, 11, 115–121. [Google Scholar] [CrossRef]
- İzol, E.; Turhan, M.; Yapıcı, İ.; Necip, A.; Yılmaz, M.A.; Zengin, G. Research of Peganum harmala: Phytochemical Content, Mineral Profile, Antioxidant, 1 Antidiabetic, Anticholinergic Properties, and Molecular Docking. Chem. Biodivers. 2025, e202403178. [Google Scholar] [CrossRef]
- Li, G.-z.; Hu, Y.-h.; Li, D.-y.; Zhang, Y.; Guo, H.-l.; Li, Y.-m.; Chen, F.; Xu, J. Vincristine-induced peripheral neuropathy: A mini-review. NeuroToxicology 2020, 81, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Singh, A.; Singh, P.; Bhatti, R. Bergapten Ameliorates Vincristine-Induced Peripheral Neuropathy by Inhibition of Inflammatory Cytokines and NFκB Signaling. ACS Chem. Neurosci. 2019, 10, 3008–3017. [Google Scholar] [CrossRef]
- Gur, C.; Kandemir, F.M.; Caglayan, C.; Satıcı, E. Chemopreventive effects of hesperidin against paclitaxel-induced hepatotoxicity and nephrotoxicity via amendment of Nrf2/HO-1 and caspase-3/Bax/Bcl-2 signaling pathways. Chem.-Biol. Interact. 2022, 365, 110073. [Google Scholar] [CrossRef]
- Hartung, J.E.; Eskew, O.; Wong, T.; Tchivileva, I.E.; Oladosu, F.A.; O’Buckley, S.C.; Nackley, A.G. Nuclear factor-kappa B regulates pain and COMT expression in a rodent model of inflammation. Brain Behav. Immun. 2015, 50, 196–202. [Google Scholar] [CrossRef]
- Shih, R.-H.; Wang, C.-Y.; Yang, C.-M. NF-kappaB signaling pathways in neurological inflammation: A mini review. Front. Mol. Neurosci. 2015, 8, 77. [Google Scholar] [CrossRef]
- Semis, H.S.; Gur, C.; Ileriturk, M.; Kandemir, F.M.; Kaynar, O. Evaluation of therapeutic effects of quercetin against achilles tendinopathy in rats via oxidative stress, inflammation, apoptosis, autophagy, and metalloproteinases. Am. J. Sports Med. 2022, 50, 486–498. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-J.; Wang, L.; Song, X.-Y. Mitoquinone alleviates vincristine-induced neuropathic pain through inhibiting oxidative stress and apoptosis via the improvement of mitochondrial dysfunction. Biomed. Pharmacother. 2020, 125, 110003. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Banerjee, S.; Mondal, A.; Chakraborty, U.; Pumarol, J.; Croley, C.R.; Bishayee, A. Targeting the JAK/STAT signaling pathway using phytocompounds for cancer prevention and therapy. Cells 2020, 9, 1451. [Google Scholar] [CrossRef] [PubMed]
- Eriten, B.; Kucukler, S.; Gur, C.; Ayna, A.; Diril, H.; Caglayan, C. Protective Effects of Carvacrol on Mercuric Chloride-Induced Lung Toxicity Through Modulating Oxidative Stress, Apoptosis, Inflammation, and Autophagy. Environ. Toxicol. 2024, 39, 5227–5237. [Google Scholar] [CrossRef] [PubMed]
- Lensing, M.; Jabbari, A. An overview of JAK/STAT pathways and JAK inhibition in alopecia areata. Front. Immunol. 2022, 13, 955035. [Google Scholar] [CrossRef]
- Scheer, M.; Polak, M.; Fritzsche, S.; Strauss, C.; Scheller, C.; Leisz, S. Nimodipine Used with Vincristine: Protects Schwann Cells and Neuronal Cells from Vincristine-Induced Cell Death but Increases Tumor Cell Susceptibility. Int. J. Mol. Sci. 2024, 25, 10389. [Google Scholar] [CrossRef]
- Chen, H.; Chen, Y.; Wang, X.; Yang, J.; Huang, C. Edaravone attenuates myocyte apoptosis through the JAK2/STAT3 pathway in acute myocardial infarction. Free Radic. Res. 2020, 54, 351–359. [Google Scholar] [CrossRef]
- Zai, W.; Chen, W.; Luan, J.; Fan, J.; Zhang, X.; Wu, Z.; Ding, T.; Ju, D.; Liu, H. Dihydroquercetin ameliorated acetaminophen-induced hepatic cytotoxicity via activating JAK2/STAT3 pathway and autophagy. Appl. Microbiol. Biotechnol. 2018, 102, 1443–1453. [Google Scholar] [CrossRef]
- Yu, H.C.; Qin, H.Y.; He, F.; Wang, L.; Fu, W.; Liu, D.; Guo, F.C.; Liang, L.; Dou, K.F.; Han, H. Canonical notch pathway protects hepatocytes from ischemia/reperfusion injury in mice by repressing reactive oxygen species production through JAK2/STAT3 signaling. Hepatology 2011, 54, 979–988. [Google Scholar] [CrossRef]
- Zhang, K.; Kaufman, R.J. From endoplasmic-reticulum stress to the inflammatory response. Nature 2008, 454, 455–462. [Google Scholar] [CrossRef]
- Varışlı, B.; Caglayan, C.; Kandemir, F.M.; Gür, C.; Ayna, A.; Genç, A.; Taysı, S. Chrysin mitigates diclofenac-induced hepatotoxicity by modulating oxidative stress, apoptosis, autophagy and endoplasmic reticulum stress in rats. Mol. Biol. Rep. 2023, 50, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Kandemir, F.M.; Ileriturk, M.; Gur, C. Rutin protects rat liver and kidney from sodium valproate-induce damage by attenuating oxidative stress, ER stress, inflammation, apoptosis and autophagy. Mol. Biol. Rep. 2022, 49, 6063–6074. [Google Scholar] [CrossRef]
- Bertolotti, A.; Zhang, Y.; Hendershot, L.M.; Harding, H.P.; Ron, D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat. Cell Biol. 2000, 2, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Varışlı, B.; Caglayan, C.; Kandemir, F.M.; Gür, C.; Bayav, İ.; Genç, A. The impact of Nrf2/HO-1, caspase-3/Bax/Bcl2 and ATF6/IRE1/PERK/GRP78 signaling pathways in the ameliorative effects of morin against methotrexate-induced testicular toxicity in rats. Mol. Biol. Rep. 2022, 49, 9641–9649. [Google Scholar] [CrossRef]
- Akaras, N.; Gür, C.; Caglayan, C.; Kandemir, F.M. Protective effects of naringin against oxaliplatin-induced testicular damage in rats: Involvement of oxidative stress, inflammation, endoplasmic reticulum stress, apoptosis, and histopathology. Iran. J. Basic Med. Sci. 2024, 27, 466. [Google Scholar]
- Shan, S.; Shen, Z.; Song, F. Autophagy and acetaminophen-induced hepatotoxicity. Arch. Toxicol. 2018, 92, 2153–2161. [Google Scholar] [CrossRef]
- Muhammad, I.; Wang, X.; Li, S.; Li, R.; Zhang, X. Curcumin confers hepatoprotection against AFB1-induced toxicity via activating autophagy and ameliorating inflammation involving Nrf2/HO-1 signaling pathway. Mol. Biol. Rep. 2018, 45, 1775–1785. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, H.; Wang, K.; Yang, Z.; Liu, Z. Protective effect of quercetin on rat testes against cadmium toxicity by alleviating oxidative stress and autophagy. Environ. Sci. Pollut. Res. 2020, 27, 25278–25286. [Google Scholar] [CrossRef]
- Liao, D.; Shangguan, D.; Wu, Y.; Chen, Y.; Liu, N.; Tang, J.; Yao, D.; Shi, Y. Curcumin protects against doxorubicin induced oxidative stress by regulating the Keap1-Nrf2-ARE and autophagy signaling pathways. Psychopharmacology 2023, 240, 1179–1190. [Google Scholar] [CrossRef]
- Pi, H.; Xu, S.; Reiter, R.J.; Guo, P.; Zhang, L.; Li, Y.; Li, M.; Cao, Z.; Tian, L.; Xie, J. SIRT3-SOD2-mROS-dependent autophagy in cadmium-induced hepatotoxicity and salvage by melatonin. Autophagy 2015, 11, 1037–1051. [Google Scholar] [CrossRef]
- Gur, C.; Kandemir, O.; Kandemir, F.M. Investigation of the effects of hesperidin administration on abamectin-induced testicular toxicity in rats through oxidative stress, endoplasmic reticulum stress, inflammation, apoptosis, autophagy, and JAK2/STAT3 pathways. Environ. Toxicol. 2022, 37, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Temel, Y.; Kucukler, S.; Yıldırım, S.; Caglayan, C.; Kandemir, F.M. Protective effect of chrysin on cyclophosphamide-induced hepatotoxicity and nephrotoxicity via the inhibition of oxidative stress, inflammation, and apoptosis. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Kandemir, O.; Kucukler, S.; Comakli, S.; Gur, C.; İleriturk, M. Docetaxel-induced liver and kidney toxicity in rats can be alleviated by suppressing oxidative stress, endoplasmic reticulum stress, inflammation, apoptosis and autophagy signaling pathways after Silymarin treatment. Food Chem. Toxicol. 2025, 196, 115202. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of apoptosis in health and disease: The balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol. 2019, 20, 175–193. [Google Scholar] [CrossRef]

| Gene | Sequences (5′-3′) | Length (bp) | Accession No. |
|---|---|---|---|
| NF-κB | F: AGTCCCGCCCCTTCTAAAAC R: CAATGGCCTCTGTGTAGCCC | 106 | NM_001276711.1 |
| IL-1β | F: ATGGCAACTGTCCCTGAACT R: AGTGACACTGCCTTCCTGAA | 197 | NM_031512.2 |
| TNF-α | F: CTCGAGTGACAAGCCCGTAG R: ATCTGCTGGTACCACCAGTT | 139 | NM_012675.3 |
| ATF-6 | F: TCAACTCAGCACGTTCCTGA R: GACCAGTGACAGGCTTCTCT | 130 | NM_001107196.1 |
| PERK | F: GATGCCGAGAATCATGGGAA R: AGATTCGAGAAGGGACTCCA | 198 | NM_031599.2 |
| IRE1 | F: GCAGTTCCAGTACATTGCCATTG R: CAGGTCTCTGTGAACAATGTTGA | 163 | NM_001191926.1 |
| GRP78 | F: CATGCAGTTGTGACTGTACCAG R: CTCTTATCCAGGCCATATGCAA | 143 | NM_013083.2 |
| LC3A | F: GACCATGTTAACATGAGCGA R: CCTGTTCATAGATGTCAGCG | 139 | NM_199500.2 |
| LC3B | F: GAGCTTCGAACAAAGAGTGG R: CGCTCATATTCACGTGATCA | 152 | NM_022867.2 |
| β-Actin | F: CAGCCTTCCTTCTTGGGTATG R: AGCTCAGTAACAGTCCGCCT | 360 | NM_031144.3 |
| No | Analytes | Royal Jelly (mg Analytes/g Sample) | No | Analytes | Royal Jelly (mg Analytes/g Sample) |
|---|---|---|---|---|---|
| 1 | Vanillin | ND | 28 | Ferulic acid | ND |
| 2 | Daidzin | ND | 29 | Salicylic acid | ND |
| 3 | Piceid | ND | 30 | Cyranoside | ND |
| 4 | Coumarin | ND | 31 | Miquelianin | ND |
| 5 | Hesperidin | ND | 32 | Isoquercitrin | ND |
| 6 | Quinic acid | 6.573 | 33 | Rutin | ND |
| 7 | Fumaric acid | 3.927 | 34 | Genistin | ND |
| 8 | Aconitic acid | 0.043 | 35 | O-Coumaric acid | ND |
| 9 | Gallic acid | 0.017 | 36 | Ellagic acid | ND |
| 10 | Protocatechuic acid | 0.043 | 37 | Rosmarinic acid | ND |
| 11 | Gentisic acid | ND | 38 | Fisetin | ND |
| 12 | Epigallocatechin | ND | 39 | Cosmosiin | ND |
| 13 | Protocatechuic aldehyde | ND | 40 | Quercitrin | ND |
| 14 | Catechin | ND | 41 | Astragalin | ND |
| 15 | Chlorogenic acid | 0.019 | 42 | Nicotiflorin | ND |
| 16 | Tannic acid | ND | 43 | Daidzein | ND |
| 17 | 4-OH Benzoic acid | 2.910 | 44 | Genistein | 0.004 |
| 18 | Epigallocatechin gallate | ND | 45 | Quercetin | 0.037 |
| 19 | Cynarin | ND | 46 | Luteolin | 0.005 |
| 20 | Vanilic acid | ND | 47 | Hesperetin | ND |
| 21 | Epicatechin | ND | 48 | Naringenin | ND |
| 22 | Caffeic acid | 0.023 | 49 | Kaempferol | ND |
| 23 | Syringic acid | ND | 50 | Apigenin | 0.003 |
| 24 | Syringic aldehyde | ND | 51 | Amentoflavone | ND |
| 25 | Epicatechin gallate | ND | 52 | Acacetin | 0.011 |
| 26 | p-Coumaric acid | 0.332 | 53 | Chrysin | 0.012 |
| 27 | Sinapic acid | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erzincan, R.; Caglayan, C.; Kandemir, F.M.; İzol, E.; Gür, C.; İleritürk, M. Hepatoprotective Effects of Royal Jelly Against Vincristine-Induced Hepatotoxicity in Rats: A Biochemical and Molecular Study. Life 2025, 15, 459. https://doi.org/10.3390/life15030459
Erzincan R, Caglayan C, Kandemir FM, İzol E, Gür C, İleritürk M. Hepatoprotective Effects of Royal Jelly Against Vincristine-Induced Hepatotoxicity in Rats: A Biochemical and Molecular Study. Life. 2025; 15(3):459. https://doi.org/10.3390/life15030459
Chicago/Turabian StyleErzincan, Rahime, Cuneyt Caglayan, Fatih Mehmet Kandemir, Ebubekir İzol, Cihan Gür, and Mustafa İleritürk. 2025. "Hepatoprotective Effects of Royal Jelly Against Vincristine-Induced Hepatotoxicity in Rats: A Biochemical and Molecular Study" Life 15, no. 3: 459. https://doi.org/10.3390/life15030459
APA StyleErzincan, R., Caglayan, C., Kandemir, F. M., İzol, E., Gür, C., & İleritürk, M. (2025). Hepatoprotective Effects of Royal Jelly Against Vincristine-Induced Hepatotoxicity in Rats: A Biochemical and Molecular Study. Life, 15(3), 459. https://doi.org/10.3390/life15030459







