1. Introduction
Rotator cuff (RC) tendinopathy is a prevailing orthopedic ailment, frequently causing discomfort [
1,
2,
3]. The incidence of RC tendinopathy rises with age, with more than half of the population experiencing a lesion by the time they are 60 years old [
4]. Older age, male sex, smoking, diabetes, hypertension, and a higher critical shoulder angle were found to be risk factors for RC tears [
5].
In comparison to complete tears, the frequency of partial RC tears is reported to be higher, and the majority of complete tears are attributed to partial tears [
6]. The prevalence of partial RC tears is substantial. In the general population, partial RC tear ranges from 15% to 32%, and in the dominant shoulder of professional overhead athletes, it can increase to 40% [
7]. Partial RC tears are potentially more painful than full-thickness tears, possibly due to the nonphysiological tension created within the remaining intact RC fibers [
8]. Conservative modalities predominate in the realm of RC tendinopathy management, notably in the absence of full tears. Injecting platelet-rich plasma (PRP) and using extracorporeal shockwave therapy (ESWT) have gained increasing popularity as treatment options [
7]. ESWT, with its diverse mechanisms, partially unfolds its therapeutic potential through a phenomenon referred to as “regenerative rehabilitation”. This involves physically stimulating damaged tissue, thereby amplifying regenerative processes and augmenting the efficacy of therapeutic procedures [
9]. ESWT delivers rapidly rising positive pressure impulses ranging from 5 to 120 MPa in approximately 5 ns, followed by a decrease to negative pressure values of −20 MPa at the treatment site [
10]. ESWT can induce hypervascularity in the ischemic rotator cuff tendon and may temporarily increase cell membrane permeability, enhancing the entry of treatment molecules into the cells [
11]. These key advantages distinguish ESWT from other potential stimulation methods.
Our prior work revealed that ESWT intervention can yield notable enhancements in visual analogue scale (VAS) assessments, muscle power, Constant–Murley scores (CMS), and range of motion (ROM) within six months post treatment among individuals recovering from RC partial tear [
12]. Platelet-rich plasma (PRP) injections exhibited superior early outcomes when juxtaposed with corticosteroid injections in patients presenting with RC partial tear [
13].
In the current study, our objectives are focused on delineating the additional therapeutic benefit derived from the combination of ESWT with PRP injection therapy in contrast to the isolated PRP injection therapy. It is our supposition that the synergistic embrace of ESWT alongside injection treatment could potentially yield augmented benefits beyond those engendered by PRP injection therapy.
3. Results
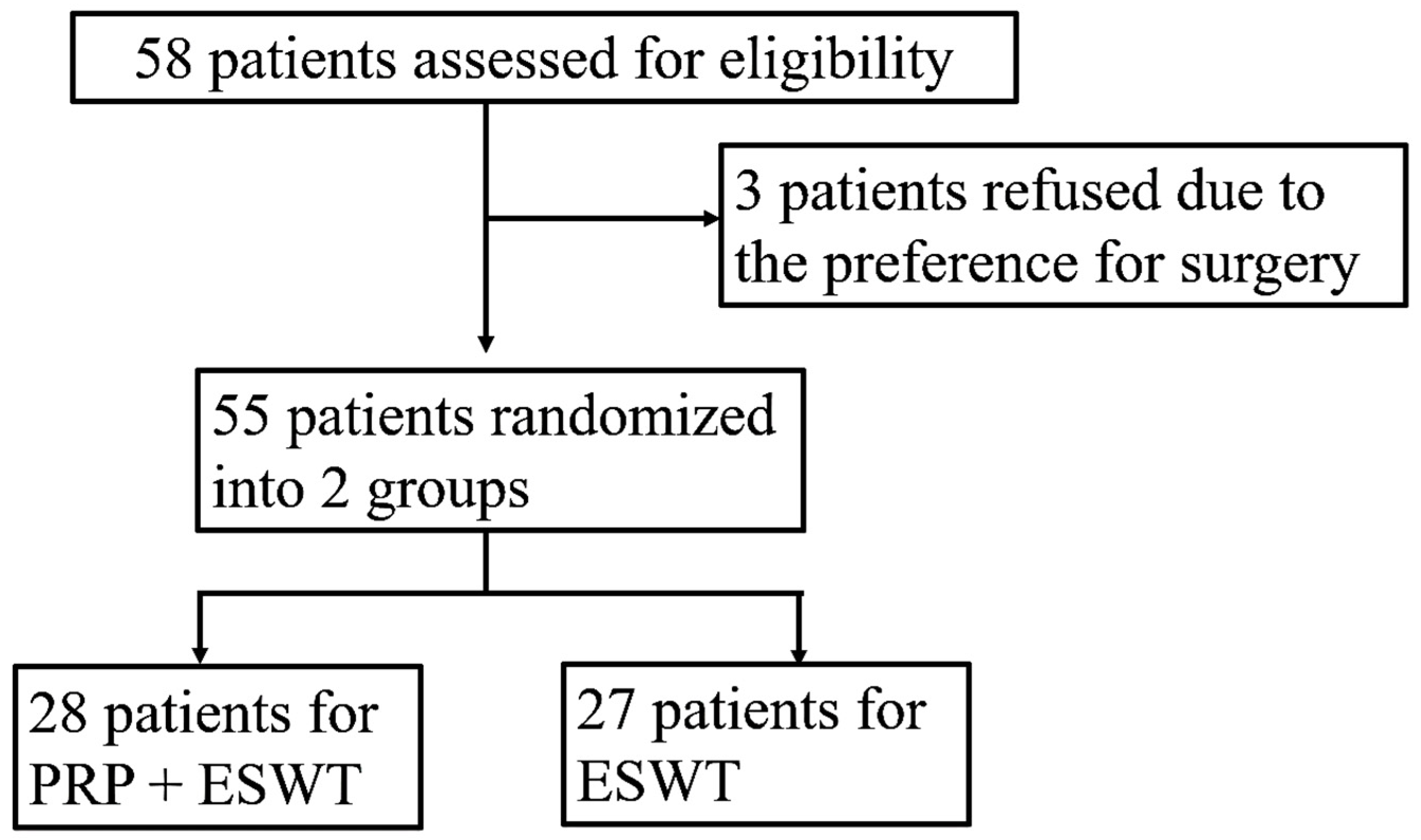
There were 28 and 27 patients in the PRP + ESWT and PRP groups, respectively. The randomization process is illustrated in
Figure 1. The demographic profiles are shown in
Table 2.
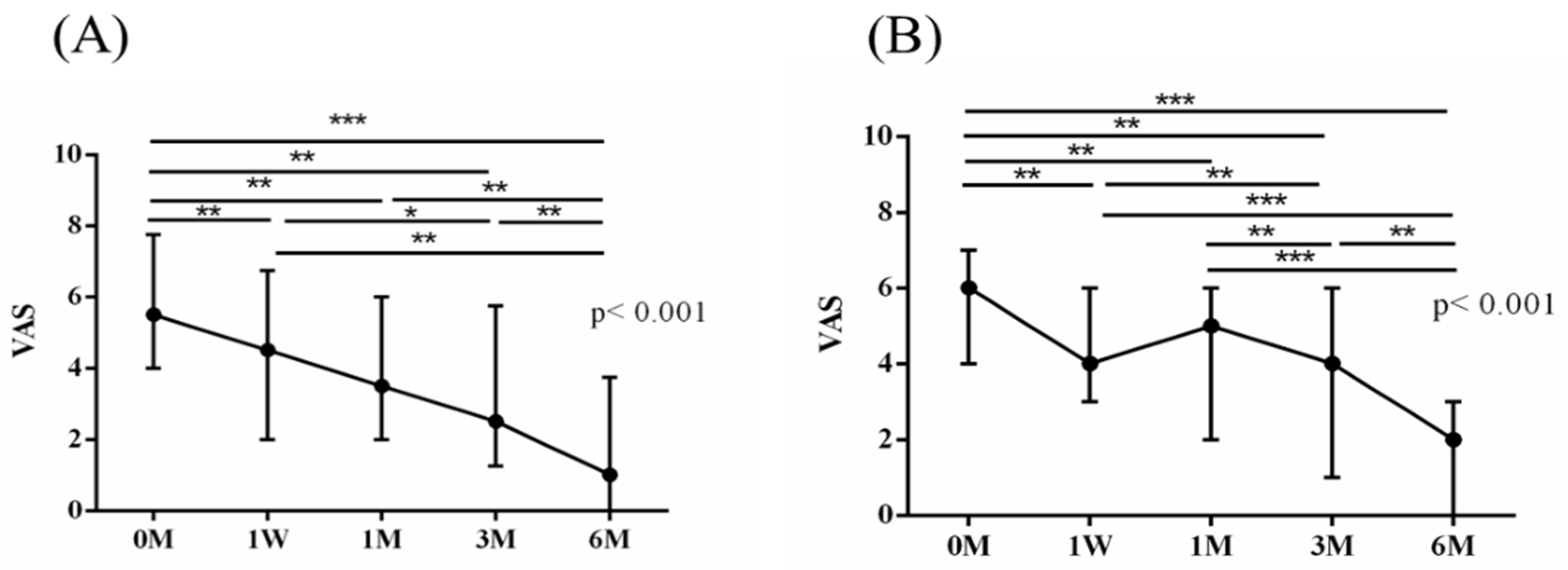
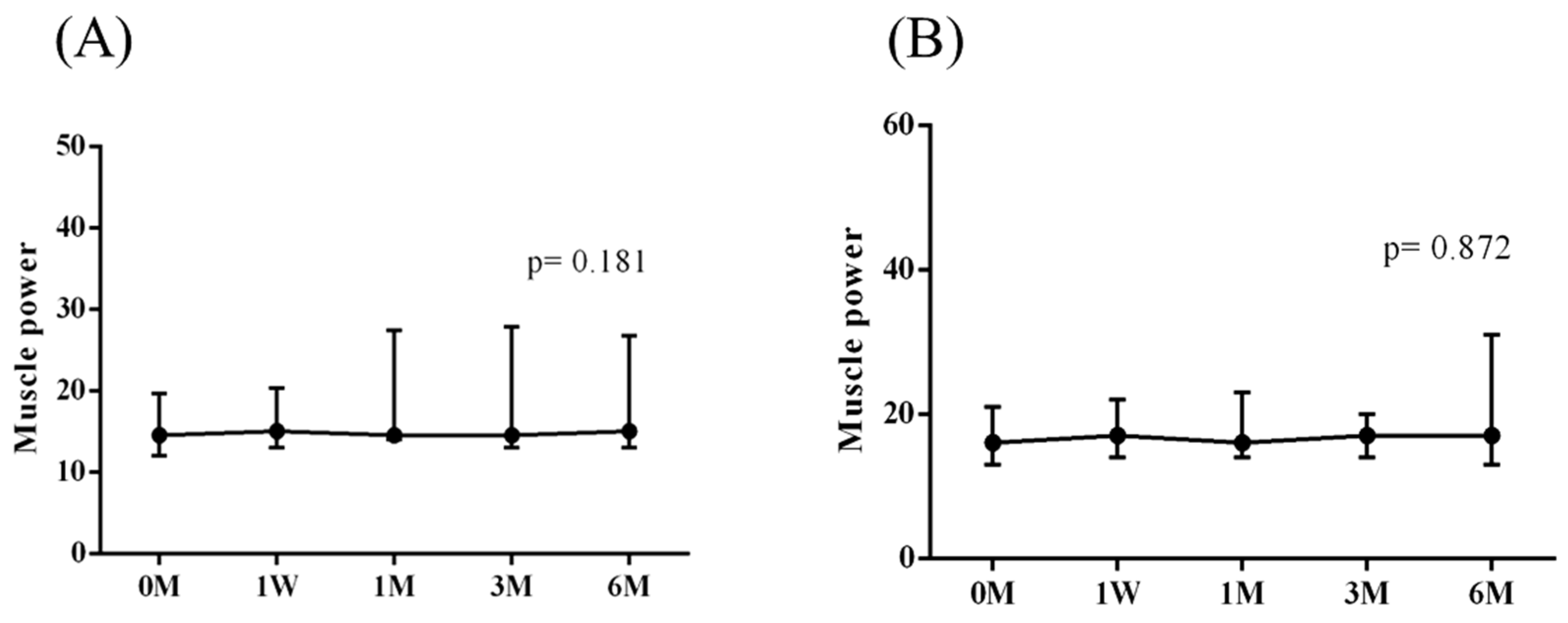
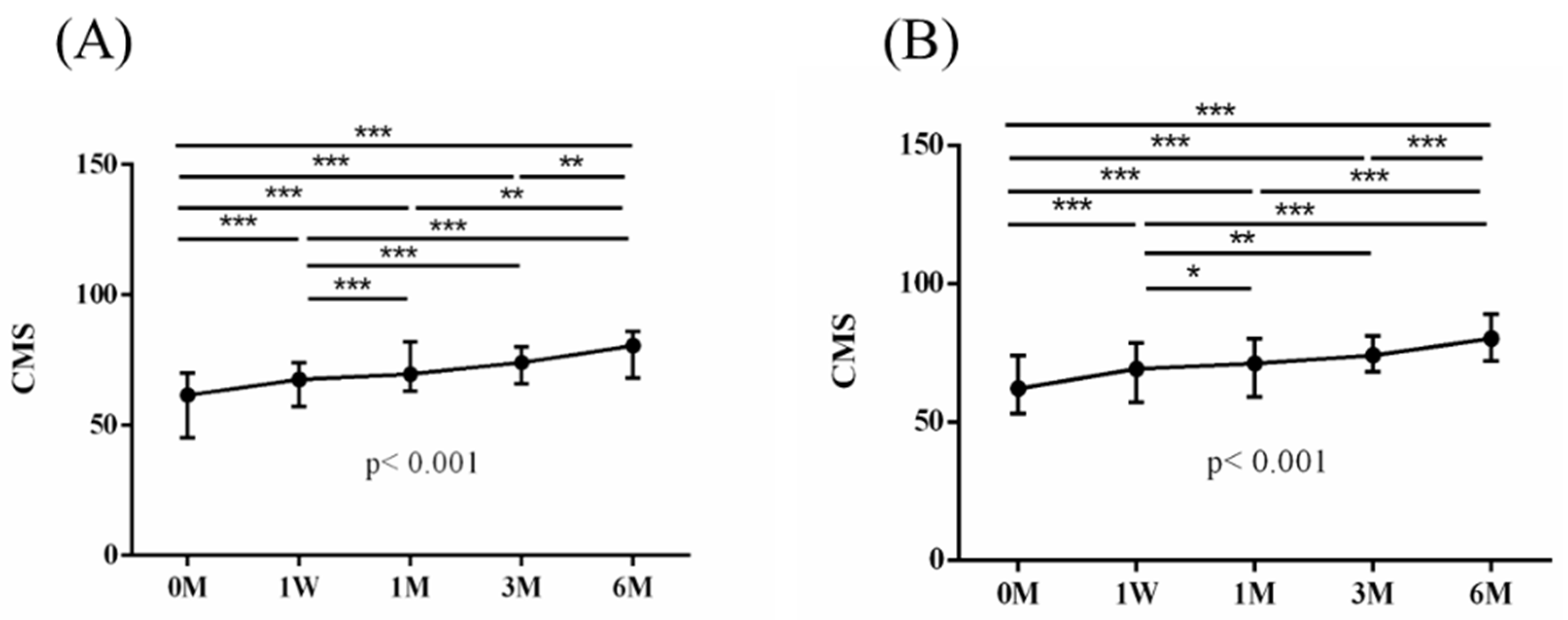
The intra-group alterations in visual analogue scale (VAS), muscle power of abduction, and the Constant–Murley score (CMS) over the course of this study are depicted in
Figure 2,
Figure 3 and
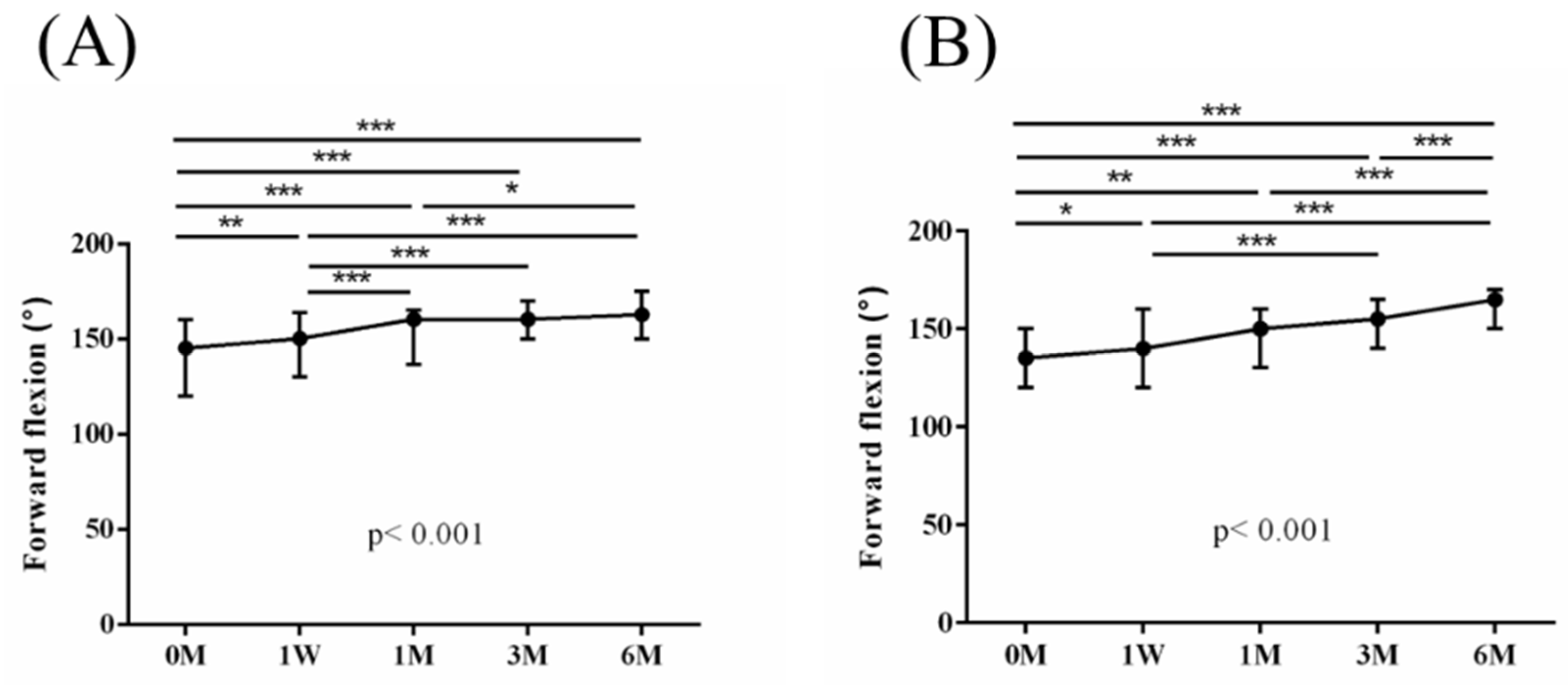
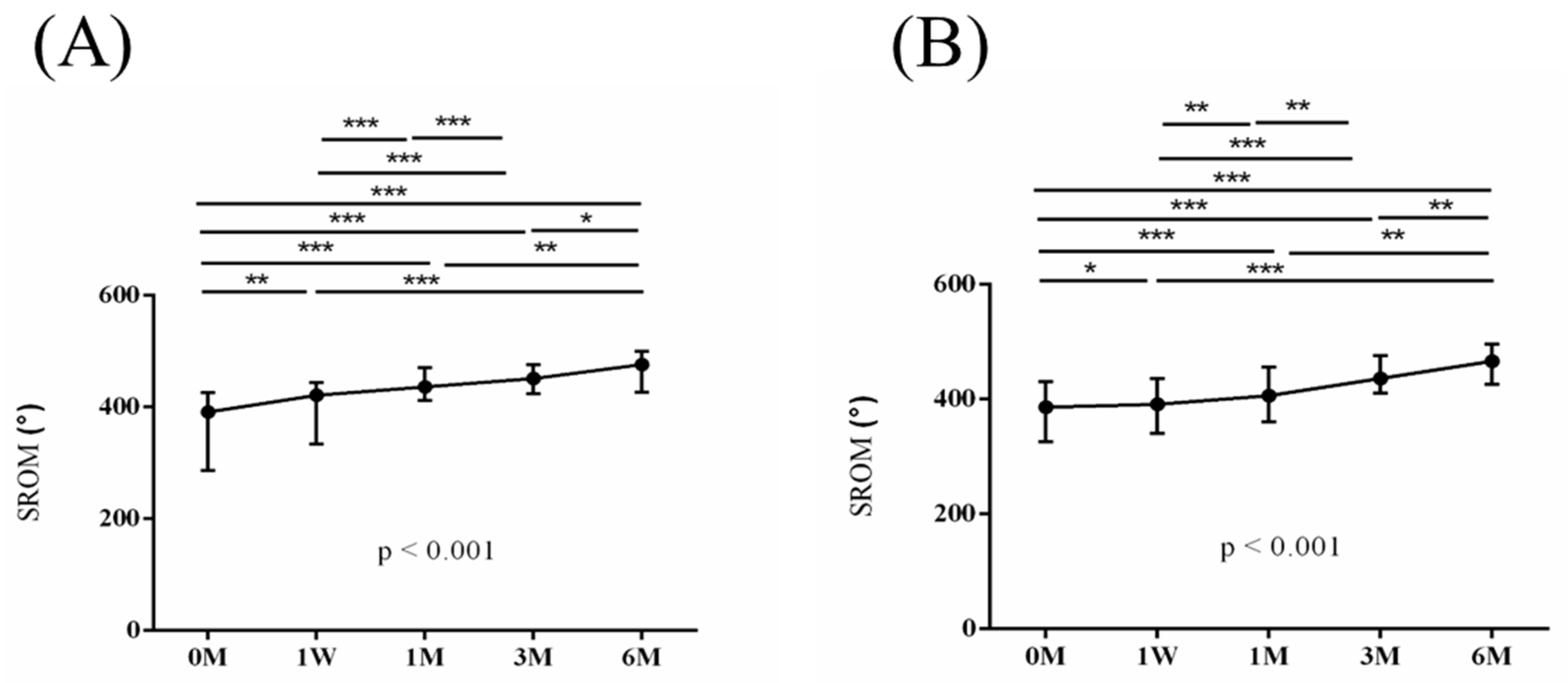
Figure 4. Similarly, the intra-group variations in the degrees (°) of forward flexion, abduction, internal rotation, external rotation, and the sum of range of motion (SROM) throughout the study duration are illustrated in
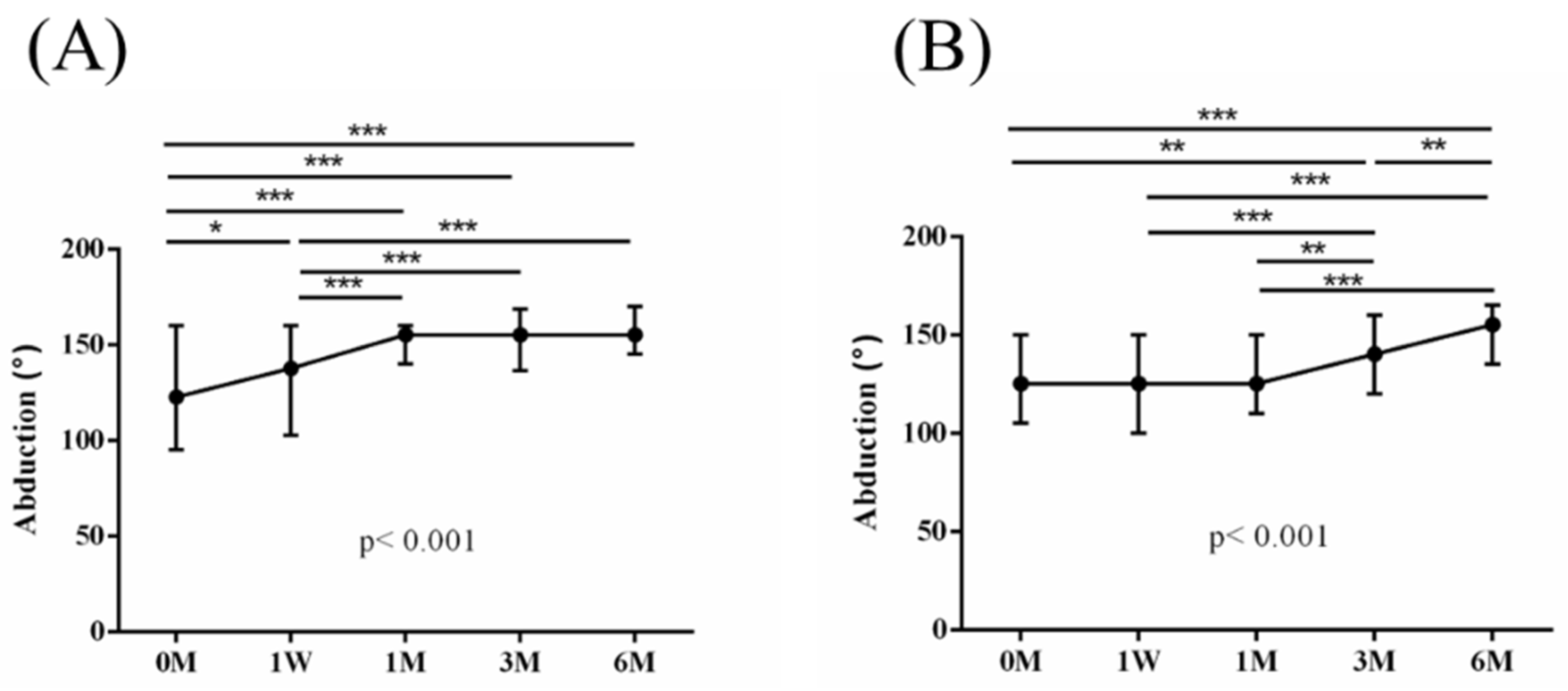
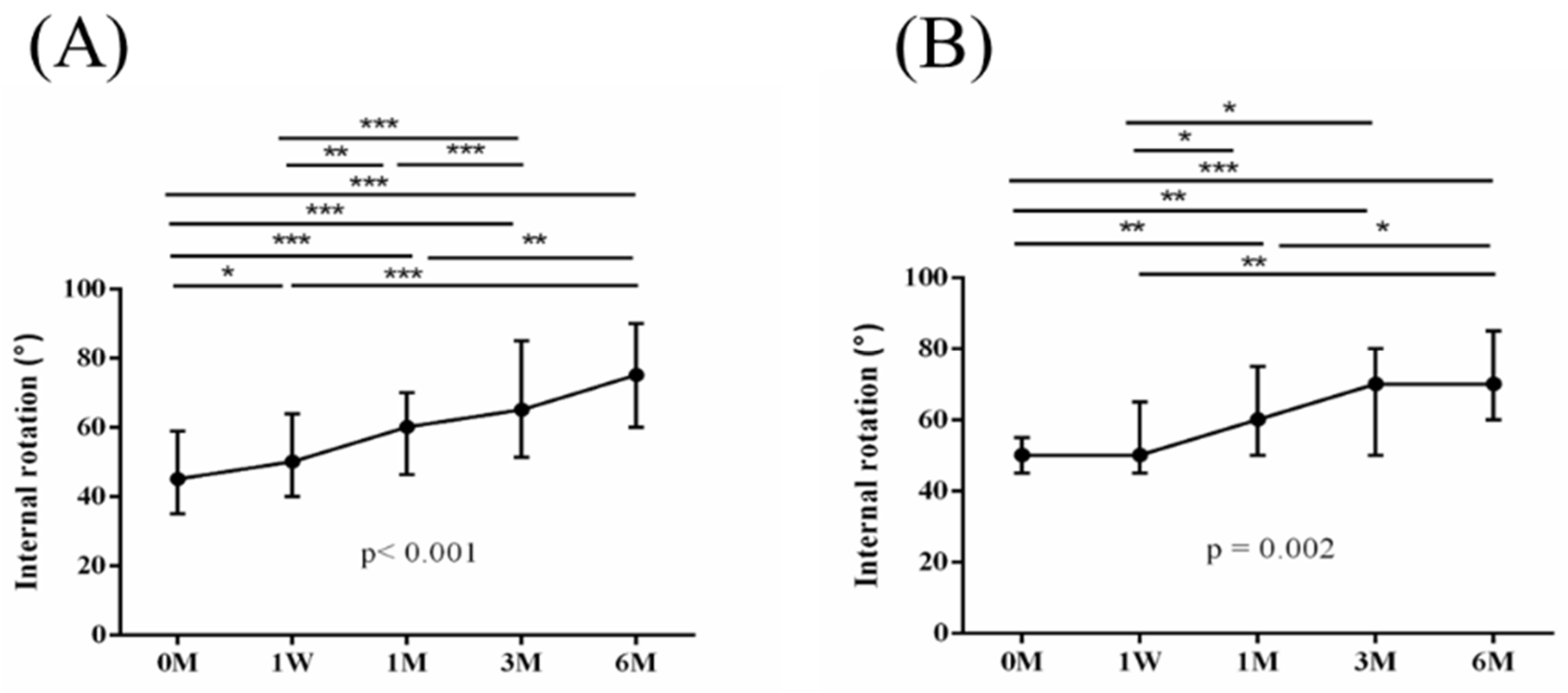
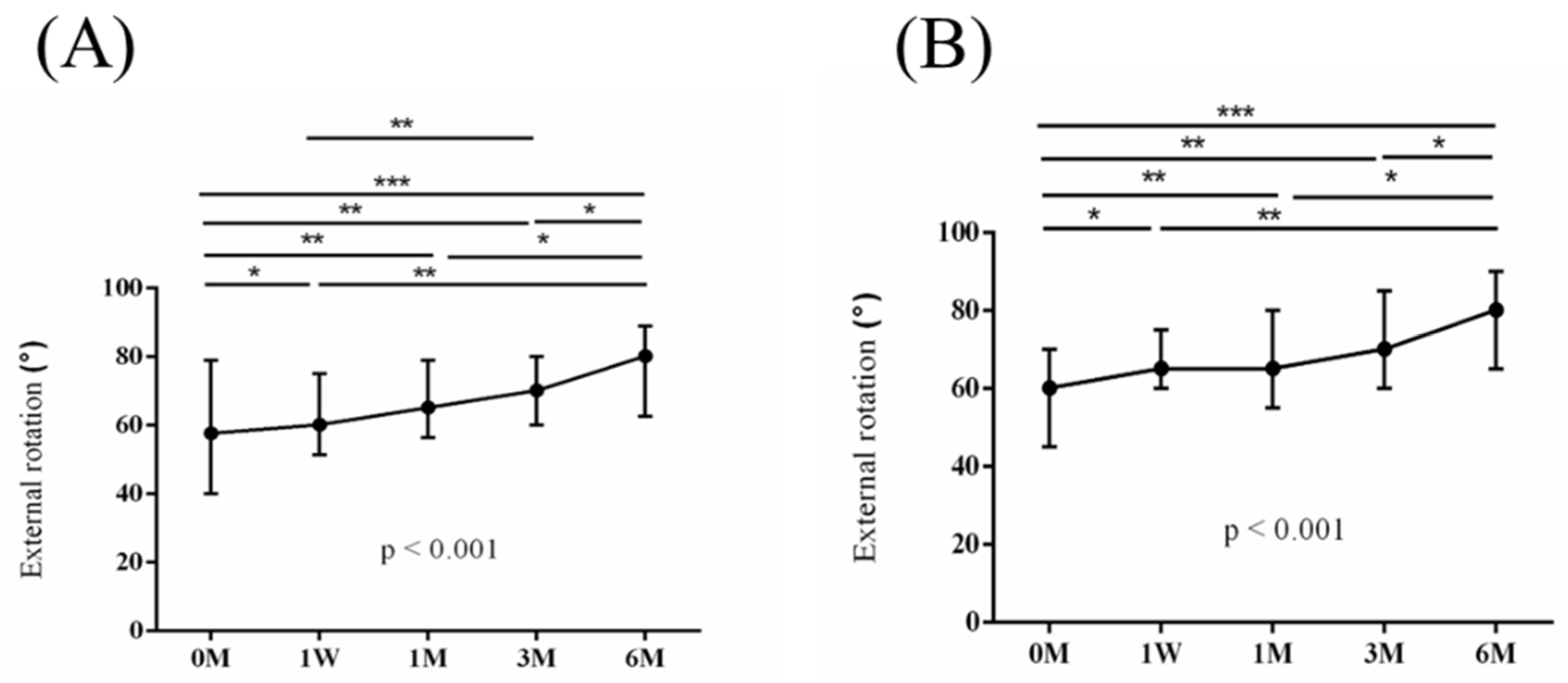
Figure 5,
Figure 6,
Figure 7,
Figure 8 and
Figure 9.
The baseline measurements of VAS, muscle power of abduction, CMS, and the degrees of forward flexion, abduction, internal rotation, external rotation, and the SROM were similar among the two groups. No additional benefits of ESWT were observed in terms of visual analogue scale (VAS), muscle power of abduction, and Constant–Murley score (CMS) throughout the study period from baseline to six months after intervention (
Table 3 and
Table 4). The supplementary advantages of ESWT in conjunction with PRP injection became evident in forward flexion (
p = 0.033) and abduction (
p = 0.015) after one month of intervention, and in SROM (
p < 0.001) after six months of intervention (
Table 5). The outcomes of inter-group comparisons are outlined in
Table 3,
Table 4 and
Table 5.
The iTRAQ gel-free proteomic technology was employed to detect differential plasma protein levels across distinct serum samples. Samples were collected, including one from PRP and another from PRP + ESWT, 1 month after intervention. By applying identification parameters of a false determinate rate <0.01 for protein and peptide identification and requiring protein identification with at least one unique peptide, 688 proteins were assessed and quantified. Abundance quantification of these proteins was further analyzed by using Partek [
25]. With parameters set at
p-value < 0.05 and variation >1.25, 15 proteins exhibited differential abundances between the two groups. Notably, several of these proteins were linked to inflammation. These findings suggest that PRP + ESWT may yield superior treatment efficacy compared to PRP alone, possibly by counteracting the inflammation-promoting effect of standalone PRP treatment.
Among the proteins displayed in
Table 6, we are especially interested in S100A9. S100A8 and S100A9 are well-known inflammatory plasma proteins involved in the inflammatory disorders, including osteoarthritis [
29,
30]. The serum samples were procured from the participants within the two cohorts one month post intervention. Notably, the serum concentrations of S100A8 and S100A9 were markedly diminished among the patients in the PRP+ ESWT group (
Table 7).
4. Discussion
The potential therapeutic benefits of ESWT for patients with incomplete RC tears have been previously investigated. In our prior prospective research, we observed that ESWT, when compared to a sham treatment, exhibited significant improvements in VAS, muscle power, CMS, and ROM in both the 6-month and 12-month post-intervention assessments. This underscores the therapeutic efficacy of ESWT in addressing incomplete RC tears associated with shoulder stiffness [
12]. In Chou et al.’s retrospective study, ESWT yielded a satisfaction rate surpassing 50% among patients with incomplete RC tears. Additionally, 53.8% of athletes returned to their prior activity levels, a result comparable to surgery [
31].
PRP is a concentrated solution of platelets that is rich in growth factors, showing the ability to facilitate angiogenesis, neuroprotection, neural regeneration, regulation of inflammation with broad therapeutic applications [
17,
32,
33,
34,
35,
36,
37,
38,
39,
40,
41,
42]. Comparative studies have shown the clinical advantages of PRP injection for patients with incomplete RC tears. Ahmed Shams et al. conducted a study comparing PRP and corticosteroid injections in 40 patients. After 12 weeks, they found a statistically significant advantage in the PRP group over the corticosteroid group in terms of VAS, American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form (ASES), CMS, and Simple Shoulder Test (SST) [
13]. Thanathep Tanpowpong et al. showed that PRP effectively reduces the size of supraspinatus tendon tears, surpassing corticosteroid [
43]. Lutz von Wehren observed significant improvements in shoulder scores for both PRP and cortisone groups. Assessments at 12 weeks, including VAS, ASES, SST, and CMS, favored the PRP group [
44]. Damjanov et al. showed that PRP significantly improved shoulder pain, surpassing glucocorticoid effects, with sustained benefits at 4 and 24 weeks. PRP patients also exhibited significantly greater CMS improvements at 24 weeks, and none reported adverse events, unlike the betamethasone group (
n = 8) [
45]. Aylin Sari et al. divided 129 patients into PRP, corticosteroid, prolotherapy, and lidocaine groups. In the PRP group at week 24, VAS and western Ontario rotator cuff (WORC) scores were notably lower than the corticosteroid group [
46]. Cai et al. found significant differences in CMS, VAS, and ASES scores at 12 months in the SH (sodium hyaluronate) + PRP group. MRI results indicated a notable reduction in tear size, especially in the SH + PRP group. The study concludes that PRP injection effectively heals partial RC tears, with SH + PRP providing superior clinical outcomes compared to SH or PRP alone [
47]. Collectively, these studies provide evidence that PRP surpasses other injection therapies as the preferred modality for treating incomplete RC tears.
The preceding studies underscore the therapeutic merits of ESWT and PRP as standalone treatments for incomplete RC tears. Our research extends this literature by revealing the added benefits of combining ESWT with PRP for patients with incomplete RC tears. Notably, 1 month post intervention, there were significant improvements in forward flexion (p = 0.033) and abduction (p = 0.015). Additionally, sustained enhancement in SROM (p < 0.001) was observed after 6 months of intervention, emphasizing the synergistic advantages of ESWT with PRP for shoulder ROM.
Besides improved shoulder ROM, we aim to elucidate the mechanistic underpinnings of this additional benefit. In our study, the distinct serum protein expression pattern among patients undergoing ESWT + PRP or PRP alone was detected through the iTRAQ assay one month post intervention. Reduced serum levels of S100A8 and S100A9 were confirmed through ELISA in patients undergoing ESWT + PRP. S100A8 and S100A9 are noteworthy alarmins—endogenous immune-activating proteins released into the extracellular milieu following tissue damage to initiate and augment inflammatory responses [
48,
49,
50,
51].
Both S100A8 and S100A9 exhibit chemotactic properties towards monocytes and are linked to myeloid cell maturation. They have the potential to induce both pro- and anti-inflammatory effects by modulating the cytokine profile through interaction with pattern recognition receptors (PRRs). This chemotaxis and cytokine modulation are particularly prominent in the early stages of tendinopathy, establishing a mechanistic link between S100A8 and S100A9 proteins and RC tendinopathy development. Exposing primary human tenocytes to exogenous S100A8/A9 resulted in a significant increase in the release of IL-6, IL-8, CCL2, CCL20, and CXCL10 proteins. This implies that S100A8/S100A9 modulates the inflammatory profile through a positive feedback mechanism, involving increased recruitment of leukocytes and the release of pro-inflammatory cytokines from tenocytes, sustaining the inflammatory response in the early stages of tendinopathy [
52]. Our research provides novel evidence indicating an association between supplementary ESWT and an anti-inflammatory effect, as evidenced by reduced systemic levels of S100A8/A9.
Our study has certain limitations. Firstly, not all of the markers of differential expression as determined by iTRAQ were quantified by ELISA. The decision to focus our investigation on proteins S100A8 and S100A9 was rooted in our review of the existing literature. Secondly, the present study did not establish a direct correlation between the serum levels of S100A8/S100A9 and the functional assessments. This is an avenue that warrants exploration in subsequent research. Thirdly, our study did not provide conclusive evidence of differential image improvement, which highlights the need for further inquiry in this domain. Fourthly, ultrasonic guidance was not utilized during the PRP injection, as unguided injections into the subacromial bursa are considered less optimal compared to ultrasound-guided procedures [
53]. Fifthly, the specific mode of ESWT application was referenced in our previous publication, and whether it is possible to use stimuli of lower or higher intensity warrants further investigation. Finally, shoulder abduction and forward flexion may occur due to deltoid contraction. We cannot rule out the possibility that the noted enhancements in shoulder abduction and forward flexion might be attributed to the application of ESWT on the potential deltoid contracture site rather than the site of the RC partial tear. Despite these limitations, our study represents a pioneering effort in demonstrating the advantages of combining ESWT and PRP treatments over PRP alone. We have shed light on the distinct serum levels of protein S100A8 and protein S100A9 between the two groups. These findings offer clinicians valuable insight into the potential benefits of employing a combined approach of ESWT and PRP injection for the treatment of partial RC tears.