Alginate-Based 3D A549 Cell Culture Model to Study Paracoccidioides Infection
Abstract
1. Lay Summary
2. Introduction
3. Material and Methods
3.1. Two-Dimensional Cell Culture
3.2. Three-Dimensional Alginate Scaffold Cell Culture
3.3. Characterization of 3D Alginate Scaffolds
Microscopy
3.4. Viability and Density of A549 Cells inside 3D Alginate Scaffolds
3.4.1. Viability Trypan Blue Assay
3.4.2. Viability Resazurin Assay
3.5. Pb Culture
3.6. Infection Assay of Pb
Two-Dimensional Monolayers of Lung Cells
3.7. Three-Dimensional Alginate Scaffolds
3.8. Confocal Microscopy
3.9. Isolation of Pb from 3D Alginate Scaffold
3.10. Statistical Analysis
4. Results
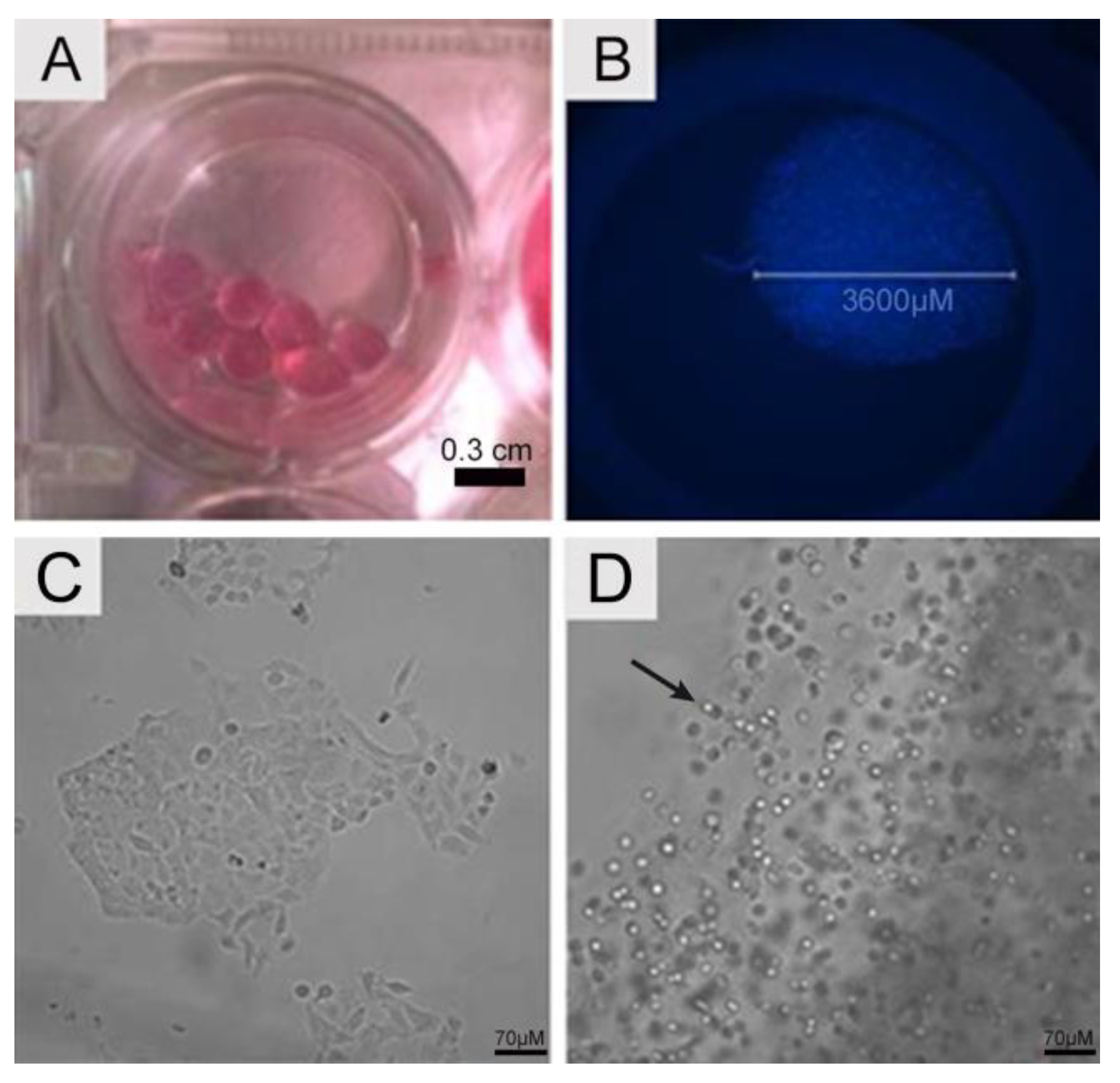
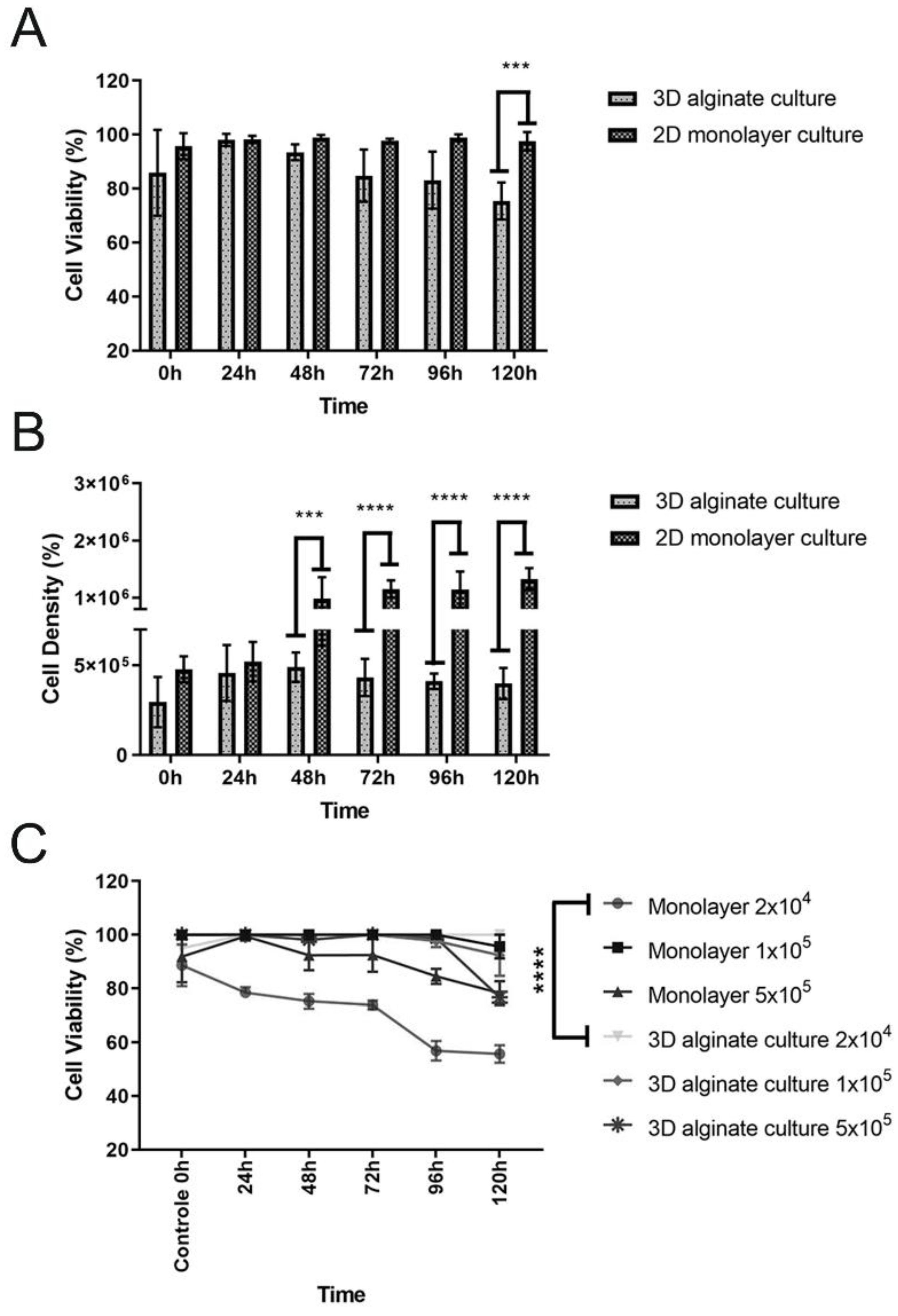
4.1. Cell Seeding, Viability, Total Cell Counts, and Cell Metabolic Activity
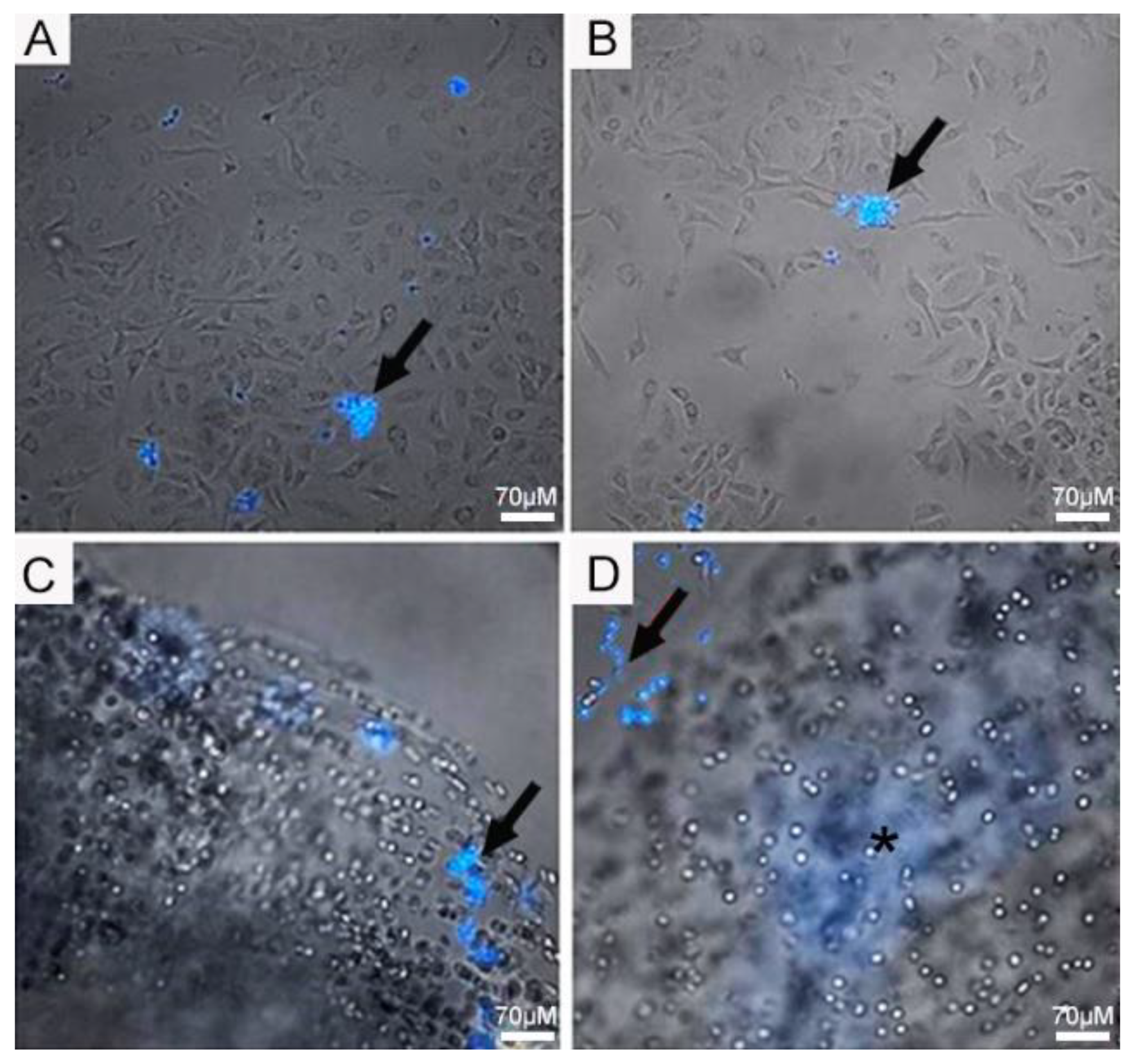
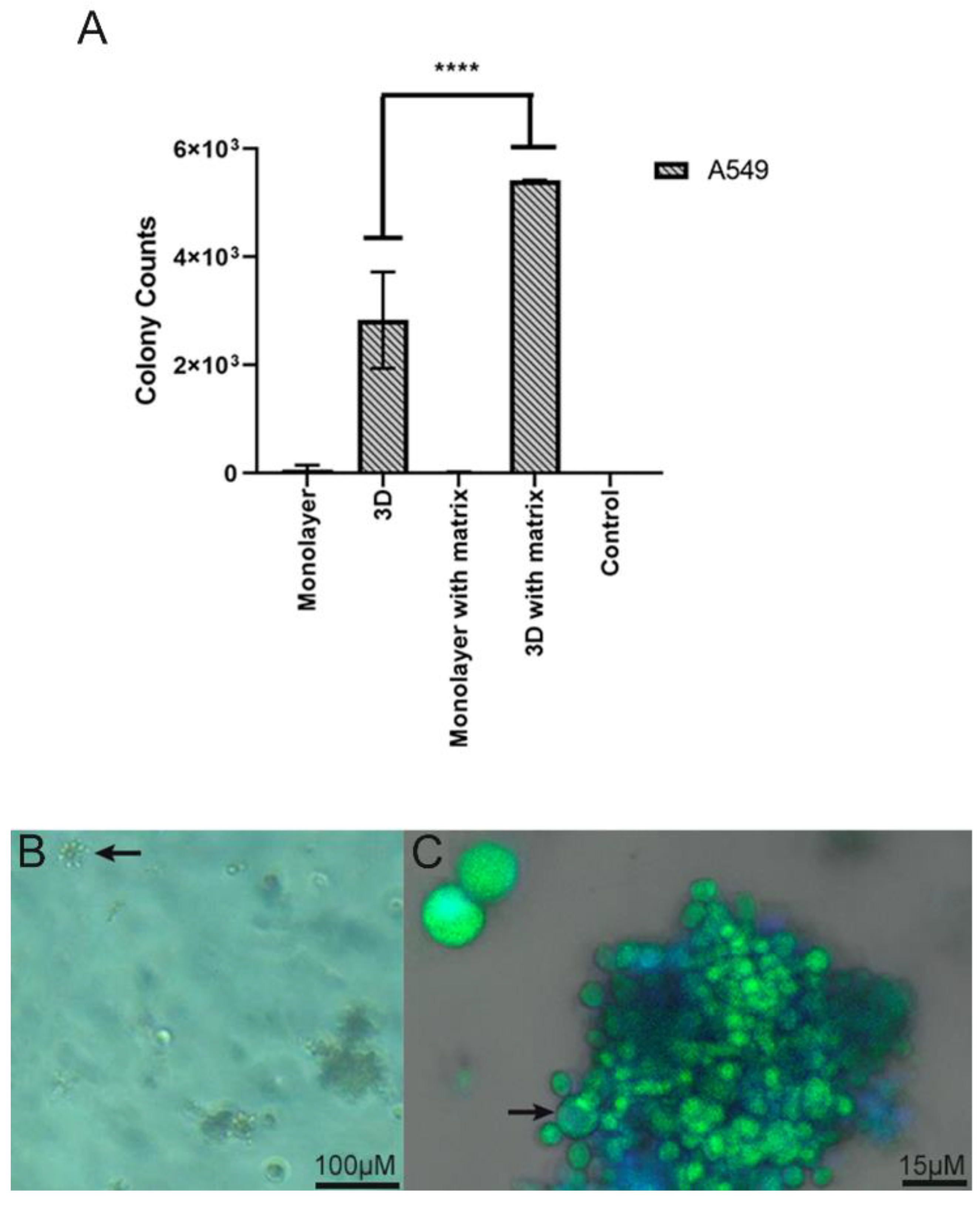
4.2. Infection Assay of Pb in 2D Monolayer and 3D-Cell-Seeded Alginate Scaffolds
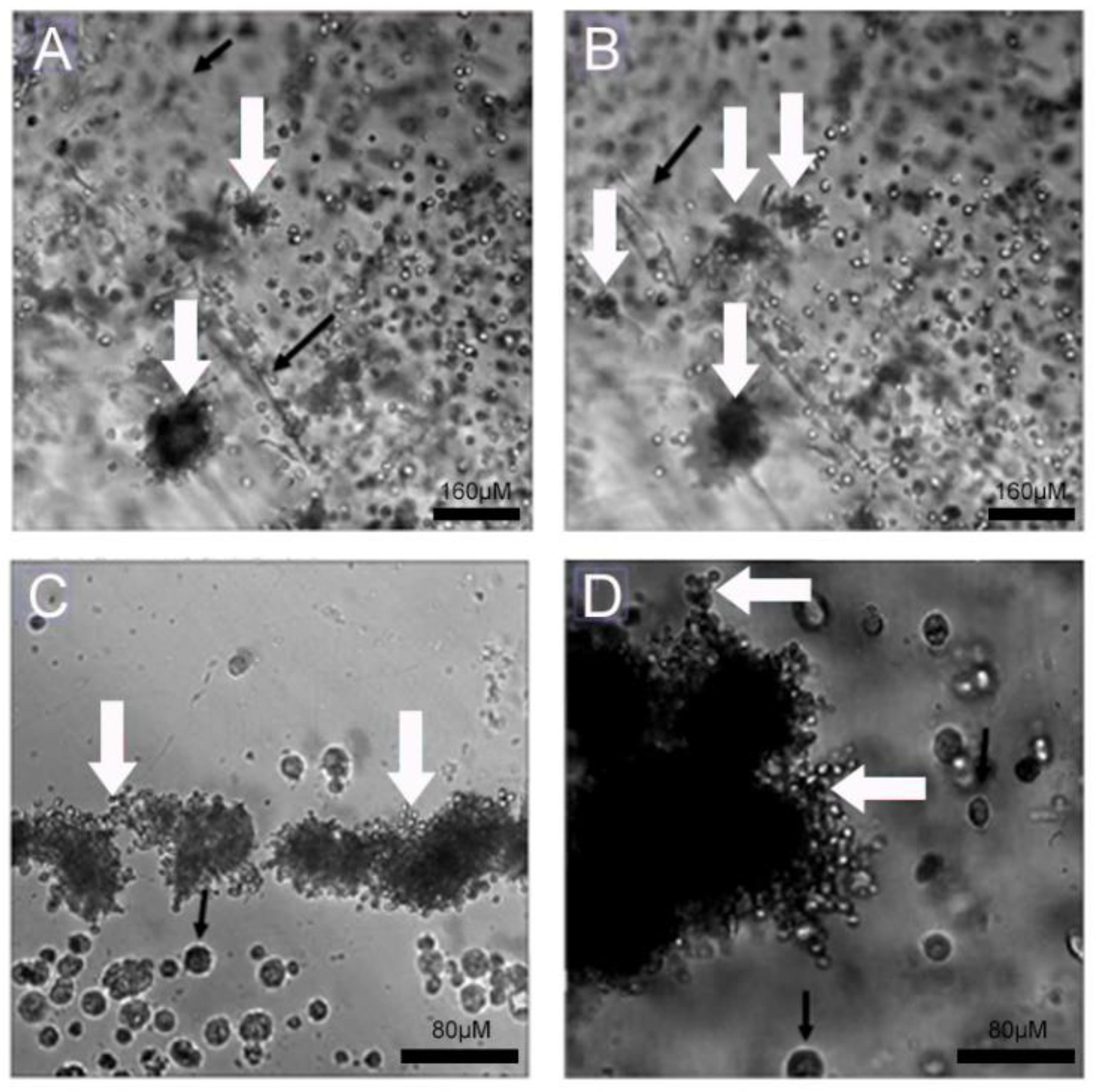
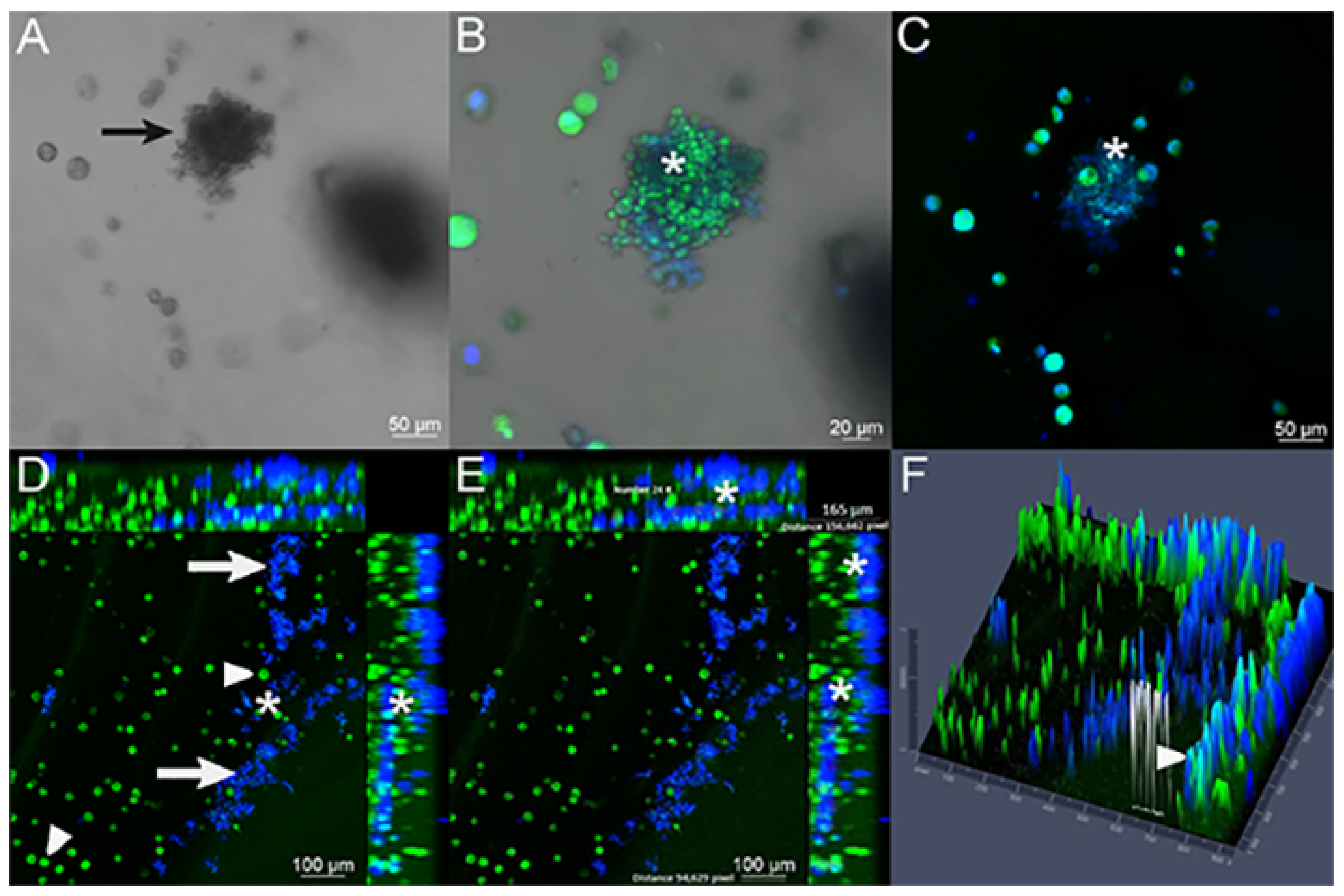
4.3. Interaction of Pb with Cell-Seeded 3D Alginate Scaffolds
4.4. Isolation of Pb from 3D Alginate Scaffold
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barrila, J.; Radtke, A.L.; Crabbé, A.; Sarker, S.F.; Herbst-Kralovetz, M.M.; Ott, C.M.; Nickerson, C.A. Organotypic 3D cell culture models: Using the rotating wall vessel to study host–pathogen interactions. Nat. Rev. Microbiol. 2010, 8, 791–801. [Google Scholar] [CrossRef]
- Barrila, J.; Crabbé, A.; Yang, J.; Franco, K.; Nydam, S.D.; Forsyth, R.J.; Davis, R.R.; Gangaraju, S.; Ott, C.M.; Coyne, C.B.; et al. Modeling Host-Pathogen Interactions in the Context of the Microenvironment: Three-Dimensional Cell Culture Comes of Age. Infect. Immun. 2018, 86, e00282-18. [Google Scholar] [CrossRef] [PubMed]
- Braz, J.D.; Sardi, J.d.C.O.; Pitangui, N.d.S.; Voltan, A.R.; Almeida, A.M.F.; Mendes-Giannini, M.J.S. Gene expression of Paracoccidioides virulence factors after interaction with macrophages and fibroblasts. Memórias Do Inst. Oswaldo Cruz 2021, 116, e200592. [Google Scholar] [CrossRef] [PubMed]
- Merad, Y.; Derrar, H.; Belmokhtar, Z.; Belkacemi, M. Aspergillus Genus and Its Various Human Superficial and Cutaneous Features. Pathogens 2021, 10, 643. [Google Scholar] [CrossRef]
- de Oliveira, H.C.; Assato, P.A.; Marcos, C.M.; Scorzoni, L.; de Paula E Silva, A.C.A.; Da Silva, J.D.F.; Singulani, J.d.L.; Alarcon, K.M.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S. Paracoccidioides-host Interaction: An Overview on Recent Advances in the Paracoccidioidomycosis. Front. Microbiol. 2015, 6, 1319. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Guvendiren, M. Recent Advances in Bioink Design for 3D Bioprinting of Tissues and Organs. Front. Bioeng. Biotechnol. 2017, 5, 5–23. [Google Scholar] [CrossRef]
- Verjans, E.-T.; Doijen, J.; Luyten, W.; Landuyt, B.; Schoofs, L. Three-dimensional cell culture models for anticancer drug screening: Worth the effort? J. Cell Physiol. 2018, 233, 2993–3003. [Google Scholar] [CrossRef]
- Organisation for Economic Co-Operation and Development. Guidance Document on Using Cytotoxicity Tests to Estimate Starting Doses for Acute Oral Systemic Toxicity Tests. Ser. Test. Assess. 2010, 8, 129. [Google Scholar]
- Mizuno, C.S.; Ampomaah, W.; Mendonça, F.R.; Andrade, G.C.; Silva, A.M.N.d.; Goulart, M.O.; Santos, R.A.d. Cytotoxicity and genotoxicity of stilbene derivatives in CHO-K1 and HepG2 cell lines. Genet. Mol. Biol. 2017, 40, 656–664. [Google Scholar] [CrossRef]
- Daud, M.F.B.; Pawar, K.C.; Claeyssens, F.; Ryan, A.J.; Haycock, J.W. An aligned 3D neuronal-glial co-culture model for peripheral nerve studies. Biomaterials 2012, 33, 5901–5913. [Google Scholar] [CrossRef]
- Skrobanska, R.; Evangelatov, A.; Stefanova, N.; Topouzova-Hristova, T.; Momchilova, A.; Pankov, R. Cell proliferation in in vivo -like three-dimensional cell culture is regulated by sequestration of ERK1/2 to lipid rafts. Cell Prolif. 2014, 47, 336–346. [Google Scholar] [CrossRef]
- Yi, S.; Ding, F.; Gong, L.; Gu, X. Extracellular Matrix Scaffolds for Tissue Engineering and Regenerative Medicine. Curr. Stem Cell Res. Ther. 2017, 12, 233–246. [Google Scholar] [CrossRef]
- Pasqua, M.; Pereira, U.; Messina, A.; de Lartigue, C.; Vigneron, P.; Dubart-Kupperschmitt, A.; Legallais, C. HepaRG Self-Assembled Spheroids in Alginate Beads Meet the Clinical Needs for Bioartificial Liver. Tissue Eng. Part A 2020, 26, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Saleh, F.; Harb, A.; Soudani, N.; Zaraket, H. A three-dimensional A549 cell culture model to study respiratory syncytial virus infections. J. Infect. Public Health 2020, 13, 1142–1147. [Google Scholar] [CrossRef]
- Carterson, A.J.; Höner zu Bentrup, K.; Ott, C.M.; Clarke, M.S.; Pierson, D.L.; Vanderburg, C.R.; Buchanan, K.L.; Nickerson, C.A.; Schurr, M.J. A549 Lung Epithelial Cells Grown as Three-Dimensional Aggregates: Alternative Tissue Culture Model for Pseudomonas aeruginosa Pathogenesis. Infect. Immun. 2005, 73, 1129–1140. [Google Scholar] [CrossRef]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-Dimensional Cell Culture Systems and Their Applications in Drug Discovery and Cell-Based Biosensors. ASSAY Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Farach-Carson, M.C.; Jia, X. Three-dimensional in vitro tumor models for cancer research and drug evaluation. Biotechnol. Adv. 2014, 32, 1256–1268. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Gastélum, G.R.; Aguilar-Medina, E.M.; Soto-Sainz, E.; Ramos-Payán, R.; Silva-Benítez, E.L. Antimicrobial Properties of Extracellular Matrix Scaffolds for Tissue Engineering. BioMed Res. Int. 2019, 2019, 1–7. [Google Scholar] [CrossRef]
- Moroz, A.; Delella, F.K.; Lacorte, L.M.; Deffune, E.; Felisbino, S.L. Fibronectin induces MMP2 expression in human prostate cancer cells. Biochem. Biophys. Res. Commun. 2013, 430, 1319–1321. [Google Scholar] [CrossRef]
- Moroz, A.; Bittencourt, R.A.C.; Almeida, R.P.; Felisbino, S.L.; Deffune, E. Platelet lysate 3D scaffold supports mesenchymal stem cell chondrogenesis: An improved approach in cartilage tissue engineering. Platelets 2013, 24, 219–225. [Google Scholar] [CrossRef]
- Moroz, A.; Bittencourt, R.A.C.; Felisbino, S.L.; Pereira, H.d.R.; Rossi-Ferreira, R.; Deffune, E. Gel de plaquetas: Arcabouço 3D para cultura celular. Acta Ortopédica Bras. 2009, 17, 43–45. [Google Scholar] [CrossRef]
- Ravi, M.; Paramesh, V.; Kaviya, S.R.; Anuradha, E.; Solomon, F.D.P. 3D Cell Culture Systems: Advantages and Applications. J. Cell Physiol. 2015, 230, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Bertanha, M.; Moroz, A.; Jaldin, R.G.; Silva, R.A.M.; Rinaldi, J.C.; Golim, M.A.; Felisbino, S.L.; Domingues, M.A.C.; Sobreira, M.L.; Reis, P.P.; et al. Morphofunctional characterization of decellularized vena cava as tissue engineering scaffolds. Exp. Cell Res. 2014, 326, 103–111. [Google Scholar] [CrossRef]
- Bertanha, M.; Sobreira, M.L.; Bovolato, A.L.d.C.; Rinaldi, J.d.C.; Reis, P.P.; Moroz, A.; Moraes, L.N.d.; Deffune, E. Ultrastructural analysis and residual DNA evaluation of rabbit vein scaffold. Acta Cir. Bras. 2017, 32, 706–711. [Google Scholar] [CrossRef]
- Baker, B.M.; Chen, C.S. Deconstructing the third dimension—How 3D culture microenvironments alter cellular cues. J. Cell Sci. 2012, 125, 3015–3024. [Google Scholar] [CrossRef]
- Andersen, T.; Auk-Emblem, P.; Dornish, M. 3D Cell Culture in Alginate Hydrogels. Microarrays 2015, 4, 133–161. [Google Scholar] [CrossRef]
- Uemura, T.; Dong, J.; Wang, Y.; Kojima, H.; Saito, T.; Iejima, D.; Kikuchi, M.; Tanaka, J.; Tateishi, T. Transplantation of cultured bone cells using combinations of scaffolds and culture techniques. Biomaterials 2003, 24, 2277–2286. [Google Scholar] [CrossRef] [PubMed]
- Melvik, J.E.; Dornish, M. Alginate as a Carrier for Cell Immobilisation; Springer: Berlin/Heidelberg, Germany, 2004; pp. 33–51. [Google Scholar] [CrossRef]
- Takezawa, T.; Ozaki, K.; Nitani, A.; Takabayashi, C.; Shimo-Oka, T. Collagen Vitrigel: A Novel Scaffold that can Facilitate a Three-Dimensional Culture for Reconstructing Organoids. Cell Transplant. 2004, 13, 463–474. [Google Scholar] [CrossRef]
- Bittencourt, R.A.d.C.; Pereira, H.R.; Felisbino, S.L.; Ferreira, R.R.; Guilherme, G.R.B.; Moroz, A.; Deffune, E. Cultura de condrócitos em arcabouço tridimensional: Hidrogel de alginato. Acta Ortopédica Bras. 2009, 17, 242–246. [Google Scholar] [CrossRef]
- Patil, J.S.; Kamalapur, M.V.; Marapur, S.C.; Kadam, D.V. Ionotropic Gelation and Polyelectrolyte Complexation: THE Novel Techniques to Design Hydrogel Particulate Sustained, Modulated Drug Delivery System: A Review. Dig. J. Nanomater. Biostructures 2010, 5, 241–248. [Google Scholar]
- Caliari, S.R.; Burdick, J.A. A practical guide to hydrogels for cell culture. Nat. Methods 2016, 13, 405–414. [Google Scholar] [CrossRef]
- Ning, L.; Xu, Y.; Chen, X.; Schreyer, D.J. Influence of mechanical properties of alginate-based substrates on the performance of Schwann cells in culture. J. Biomater. Sci. 2016, 27, 898–915. [Google Scholar] [CrossRef]
- Mohanty, S.; Wu, Y.; Chakraborty, N.; Mohanty, P.; Ghosh, G. Impact of alginate concentration on the viability, cryostorage, and angiogenic activity of encapsulated fibroblasts. Mater. Sci. Eng. C 2016, 65, 269–277. [Google Scholar] [CrossRef]
- Chen, Y.-W.; Kuo, C.W.; Chueh, D.-Y.; Chen, P. Surface modified alginate microcapsules for 3D cell culture. Surf. Sci. 2016, 648, 47–52. [Google Scholar] [CrossRef]
- Ciraldo, F.E.; Boccardi, E.; Melli, V.; Westhauser, F.; Boccaccini, A.R. Tackling bioactive glass excessive in vitro bioreactivity: Preconditioning approaches for cell culture tests. Acta Biomater. 2018, 75, 3–10. [Google Scholar] [CrossRef]
- Dhamecha, D.; Movsas, R.; Sano, U.; Menon, J.U. Applications of alginate microspheres in therapeutics delivery and cell culture: Past, present and future. Int. J. Pharm. 2019, 569, 118627. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Staufer, U.; Accardo, A. Engineered 3D Polymer and Hydrogel Microenvironments for Cell Culture Applications. Bioengineering 2019, 6, 113. [Google Scholar] [CrossRef] [PubMed]
- Todd, R.D. Micro Encapsulation and Flavor Industry. Flavour Ind. 1970, 8, 768–771. [Google Scholar]
- Evaristo, T.C.; CruzAlves, F.C.M.d.; Moroz, A.; Mion, W.; Acorci-Valério, M.J.; Felisbino, S.L.; Rossi-Ferreira, R.; Ruiz Júnior, R.L.; Deffune, E. Light-emitting diode effects on combined decellularization of tracheae. A Nov. Approach Obtain Biol. Scaffolds. Acta Cir. Bras. 2014, 29, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.M.F.; Soldi, V.; Borsali, R. Dynamic light scattering and viscosimetry of aqueous solutions of pectin, sodium alginate and their mixtures: Effects of added salt, concentration, counterions, temperature and chelating agent. J. Braz. Chem. Soc. 2009, 20, 1705–1714. [Google Scholar] [CrossRef]
- Sun, D.; Liu, Y.; Wu, H.; Ren, Y.; Ma, X.; Wu, H.; Sun, G. Effects of gelling bath on the physical properties of alginate gel beads and the biological characteristics of entrapped HepG2 cells. Biotechnol. Appl. Biochem. 2018, 65, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Dantas, K.C.; Mauad, T.; de André, C.D.S.; Bierrenbach, A.L.; Saldiva, P.H.N. A single-centre, retrospective study of the incidence of invasive fungal infections during 85 years of autopsy service in Brazil. Sci. Rep. 2021, 11, 3943. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R. Epidemiology of paracoccidioidomycosis. Rev. Do Inst. De Med. Trop. De São Paulo 2015, 57 (Suppl. 19), 11–20. [Google Scholar] [CrossRef] [PubMed]
- Marcos, C.M.; Fátima da Silva, J.; Oliveira, H.C.; Moraes da Silva, R.A.; Mendes-Giannini, M.J.S.; Fusco-Almeida, A.M. Surface-expressed enolase contributes to the adhesion of Paracoccidioides brasiliensis to host cells. FEMS Yeast Res. 2012, 12, 557–570. [Google Scholar] [CrossRef] [PubMed]
- Mendes-Giannini, M.J.S.; Soares, C.P.; Silva, J.L.M.; Andreotti, P.F. Interaction of pathogenic fungi with host cells: Molecular and cellular approaches. FEMS Immunol. Med. Microbiol. 2005, 45, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Monteiro da Silva, J.L.; Andreotti, P.F.; Benard, G.; Soares, C.P.; Miranda, E.T.; Mendes-Giannini, M.J.S. Epithelial cells treated with genistein inhibit adhesion and endocytosis of Paracoccidioides brasiliensis. Antonie Van Leeuwenhoek 2007, 92, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Peres da Silva, R.; Matsumoto, M.T.; Braz, J.D.; Voltan, A.R.; de Oliveira, H.C.; Soares, C.P.; Mendes Giannini, M.J.S. Differential gene expression analysis of Paracoccidioides brasiliensis during keratinocyte infection. J. Med. Microbiol. 2011, 60, 269–280. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, Y.; Wang, J.; Yu, W.; Wang, W.; Ma, X. Monitoring of Cell Viability and Proliferation in Hydrogel-Encapsulated System by Resazurin Assay. Appl. Biochem. Biotechnol. 2010, 162, 1996–2007. [Google Scholar] [CrossRef]
- Netto, C.F.; Vegas, V.S.; Sciannaméa, I.M.; Guarnieri, D.B. The polysaccharidic antigen from Paracoccidioides brasiliensis. Study of the time of cultivation necessary for the preparation of the antigen. Rev. Inst. Med. Trop Sao Paulo 1969, 11, 177–181. [Google Scholar]
- Mellor, L.F.; Baker, T.L.; Brown, R.J.; Catlin, L.W.; Oxford, J.T. Optimal 3D Culture of Primary Articular Chondrocytes for Use in the Rotating Wall Vessel Bioreactor. Aviat. Space Environ. Med. 2014, 85, 798–804. [Google Scholar] [CrossRef]
- Shikanai-Yasuda, M.A.; Mendes, R.P.; Colombo, A.L.; Telles, F.d.Q.; Kono, A.; Paniago, A.M.M.; Nathan, A.; Valle, A.C.F.d.; Bagagli, E.; Benard, G.; et al. II Consenso Brasileiro em Paracoccidioidomicose—2017. Epidemiol. E Serviços De Saúde 2018, 27. [Google Scholar] [CrossRef]
- Mendes-Giannini, M.J.S.; Andreotti, P.F.; Vincenzi, L.R.; Monteiro da Silva, J.L.; Lenzi, H.L.; Benard, G.; Zancopé-Oliveira, R.; de Matos Guedes, H.L.; Soares, C.P. Binding of extracellular matrix proteins to Paracoccidioides brasiliensis. Microbes Infect. 2006, 8, 1550–1559. [Google Scholar] [CrossRef]
- Camacho, E.; Niño-Vega, G.A. Paracoccidioides Spp.: Virulence Factors and Immune-Evasion Strategies. Mediat. Inflamm. 2017, 2017, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Scorzoni, L.; de Paula e Silva, A.C.A.; de Oliveira, H.C.; Marcos, C.M.; Singulani, J.d.L.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S. Can passage in Galleria mellonella activate virulence factors of Paracoccidioides brasiliensis as in the murine model? Med. Mycol. 2018, 56, 374–377. [Google Scholar] [CrossRef] [PubMed]
- De Lacorte Singulani, J.; De Fátima Da Silva, J.; Gullo, F.P.; Costa, M.C.; Fusco-Almeida, A.M.; Enguita, F.J.; Mendes-Giannini, M.J.S. Preliminary evaluation of circulating microRNAs as potential biomarkers in paracoccidioidomycosis. Biomed. Rep. 2017, 6, 353–357. [Google Scholar] [CrossRef]
- Taborda, C.P.; Urán, M.E.; Nosanchuk, J.D.; Travassos, L.R. Paracoccidioidomycosis: Challenges in the development of a vaccine against an endemic mycosis in the americas. Rev. Do Inst. De Med. Trop. De São Paulo 2015, 57 (Suppl. 19), 21–24. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, T.; da Silva, J.d.F.; Vicentin, J.; de Oliveira, H.C.; Assato, P.A.; Marcos, C.M.; de Paula e Silva, A.C.A.; da Silva, R.A.M.; Regasini, L.O.; Silva, D.H.S.; et al. Anti-apoptotic effects of decyl gallate on the induction of apoptosis in A549 pneumocytes by Paracoccidioides brasiliensis gp43. Med. Mycol. 2017, 55, 890–894. [Google Scholar] [CrossRef] [PubMed]
- Sardi, J.d.C.O.; Pitangui, N.d.S.; Voltan, A.R.; Braz, J.D.; Machado, M.P.; Fusco Almeida, A.M.; Mendes Giannini, M.J.S. In vitro Paracoccidioides brasiliensis biofilm and gene expression of adhesins and hydrolytic enzymes. Virulence 2015, 6, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Del Vecchio, A.; Silva, J.d.F.d.; Silva, J.L.M.d.; Andreotti, P.F.; Soares, C.P.; Benard, G.; Giannini, M.J.S.M. Induction of apoptosis in A549 pulmonary cells by two Paracoccidioides brasiliensis samples. Memórias Do Inst. Oswaldo Cruz 2009, 104, 749–754. [Google Scholar] [CrossRef]
- González, A.; Caro, E.; Muñoz, C.; Restrepo, A.; Hamilton, A.J.; Cano, L.E. Paracoccidioides brasiliensis conidia recognize fibronectin and fibrinogen which subsequently participate in adherence to human type II alveolar cells: Involvement of a specific adhesin. Microb. Pathog. 2008, 44, 389–401. [Google Scholar] [CrossRef]
- Marcos, C.M.; da Silva, J.d.F.; de Oliveira, H.C.; Assato, P.A.; Singulani, J.d.L.; Lopez, A.M.; Tamayo, D.P.; Hernandez-Ruiz, O.; McEwen, J.G.; Mendes-Giannini, M.J.S.; et al. Decreased expression of 14-3-3 in Paracoccidioides brasiliensis confirms its involvement in fungal pathogenesis. Virulence 2016, 7, 72–84. [Google Scholar] [CrossRef]
- Bonnier, F.; Keating, M.E.; Wróbel, T.P.; Majzner, K.; Baranska, M.; Garcia-Munoz, A.; Blanco, A.; Byrne, H.J. Cell viability assessment using the Alamar blue assay: A comparison of 2D and 3D cell culture models. Toxicol. Vitr. 2015, 29, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.; Stowers, R.; Lou, J.; Xia, Y.; Chaudhuri, O. Varying PEG density to control stress relaxation in alginate-PEG hydrogels for 3D cell culture studies. Biomaterials 2019, 200, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, X.; Dai, X.; Wang, X.; Li, X.; Diao, J.; Xu, T. Tumor-like lung cancer model based on 3D bioprinting. 3 Biotech 2018, 8, 501. [Google Scholar] [CrossRef]
- Smith, K.E.; Kelly, A.C.; Min, C.G.; Weber, C.S.; McCarthy, F.M.; Steyn, L.V.; Badarinarayana, V.; Stanton, J.B.; Kitzmann, J.P.; Strop, P.; et al. Acute Ischemia Induced by High-Density Culture Increases Cytokine Expression and Diminishes the Function and Viability of Highly Purified Human Islets of Langerhans. Transplantation 2017, 101, 2705–2712. [Google Scholar] [CrossRef] [PubMed]
- Sanketh, D.S.; Patil, S.; Rao, R.S. Estimating the frequency of Candida in oral squamous cell carcinoma using Calcofluor White fluorescent stain. J. Investig. Clin. Dent. 2016, 7, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Hernández, O.; Almeida, A.J.; Gonzalez, A.; Garcia, A.M.; Tamayo, D.; Cano, L.E.; Restrepo, A.; McEwen, J.G. A 32-Kilodalton Hydrolase Plays an Important Role in Paracoccidioides brasiliensis Adherence to Host Cells and Influences Pathogenicity. Infect. Immun. 2010, 78, 5280–5286. [Google Scholar] [CrossRef]
- de Barros, B.C.S.C.; Almeida, B.R.; Suzuki, E. Paracoccidioides brasiliensis downmodulates α3 integrin levels in human lung epithelial cells in a TLR2-dependent manner. Sci. Rep. 2020, 10, 19483. [Google Scholar] [CrossRef]
- Almeida, B.R.; Barros, B.C.S.C.; Araújo, A.C.L.; Alcantara, C.; Suzuki, E. Paracoccidioides species present distinct fungal adherence to epithelial lung cells and promote different IL-8 secretion levels. Med. Microbiol. Immunol. 2020, 209, 59–67. [Google Scholar] [CrossRef]
- Neves, M.I.; Moroni, L.; Barrias, C.C. Modulating Alginate Hydrogels for Improved Biological Performance as Cellular 3D Microenvironments. Front. Bioeng. Biotechnol. 2020, 8. [Google Scholar] [CrossRef]
- Tezera, L.B.; Bielecka, M.K.; Chancellor, A.; Reichmann, M.T.; Shammari, B.A.; Brace, P.; Batty, A.; Tocheva, A.; Jogai, S.; Marshall, B.G.; et al. Dissection of the host-pathogen interaction in human tuberculosis using a bioengineered 3-dimensional model. eLife 2017, 6, e21283. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, A.; Beena, P.M.; Devnikar, A.V.; Mali, S. A systemic review on tuberculosis. Indian J. Tuberc. 2020, 67, 295–311. [Google Scholar] [CrossRef]
- Fogel, N. Tuberculosis: A disease without boundaries. Tuberculosis 2015, 95, 527–531. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Santos, K.S.; Oliveira, L.T.; de Lima Fontes, M.; Migliato, K.F.; Fusco-Almeida, A.M.; Mendes Giannini, M.J.S.; Moroz, A. Alginate-Based 3D A549 Cell Culture Model to Study Paracoccidioides Infection. J. Fungi 2023, 9, 634. https://doi.org/10.3390/jof9060634
dos Santos KS, Oliveira LT, de Lima Fontes M, Migliato KF, Fusco-Almeida AM, Mendes Giannini MJS, Moroz A. Alginate-Based 3D A549 Cell Culture Model to Study Paracoccidioides Infection. Journal of Fungi. 2023; 9(6):634. https://doi.org/10.3390/jof9060634
Chicago/Turabian Styledos Santos, Kelvin Sousa, Lariane Teodoro Oliveira, Marina de Lima Fontes, Ketylin Fernanda Migliato, Ana Marisa Fusco-Almeida, Maria José Soares Mendes Giannini, and Andrei Moroz. 2023. "Alginate-Based 3D A549 Cell Culture Model to Study Paracoccidioides Infection" Journal of Fungi 9, no. 6: 634. https://doi.org/10.3390/jof9060634
APA Styledos Santos, K. S., Oliveira, L. T., de Lima Fontes, M., Migliato, K. F., Fusco-Almeida, A. M., Mendes Giannini, M. J. S., & Moroz, A. (2023). Alginate-Based 3D A549 Cell Culture Model to Study Paracoccidioides Infection. Journal of Fungi, 9(6), 634. https://doi.org/10.3390/jof9060634







