A Countermeasure Strategy against Peramine Developed by Chilesia rudis in the Endophyte–Ryegrass–Herbivore Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Larval Rearing
2.3. Evaluation, Isolation and Characterization of Endophytic Fungus in Lolium perenne
2.4. No-Choice Assay
2.5. Pupal Development Time and Adult Performance Evaluation
2.6. Carcasses, Feces and Guts Peramine Extraction
2.7. Plant Material Extraction
2.8. HPLC-DAD Analysis
2.9. Survival Rate Test
2.10. Statistical Analysis
3. Results
3.1. Evaluation, Isolation and Characterization of Endophytic Fungus in Lolium perenne
3.2. No-Choice Assay
3.3. Pupal Development Time and Adult Performance Evaluation and HPLC-DAD Analysis
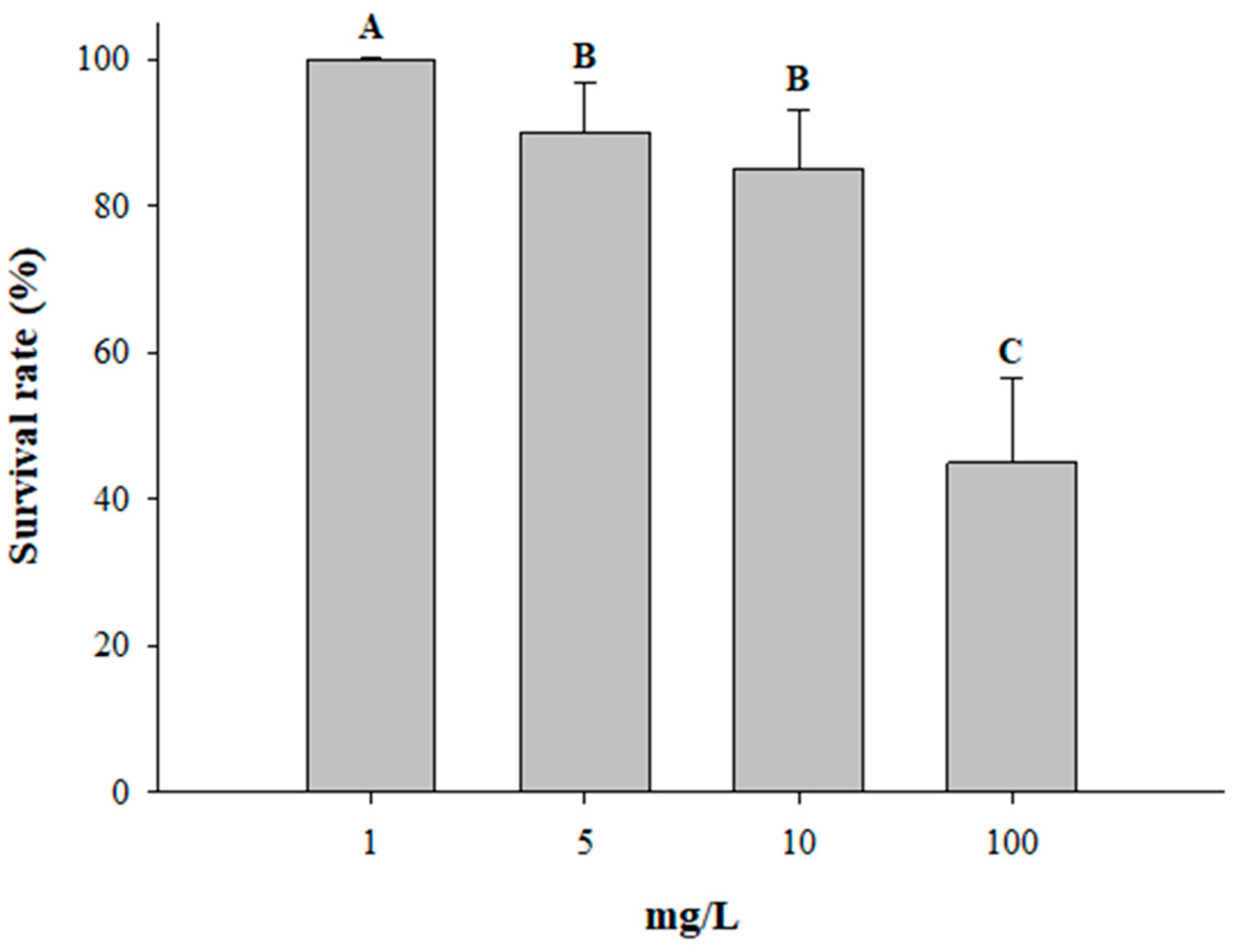
3.4. Survival Rate Test
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Khayri, J.M.; Rashmi, R.; Toppo, V.; Chole, P.B.; Banadka, A.; Sudheer, W.N.; Nagella, P.; Shehata, W.F.; Al-Mssallem, M.Q.; Alessa, F.M.; et al. Plant Secondary Metabolites: The Weapons for Biotic Stress Management. Metabolites 2023, 13, 716. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yan, W.; Liu, W.; Chen, H.; Yuan, Q.; Wang, Z.; Liu, H. Endophytic Beauveria bassiana of Tomato Resisted the Damage from Whitefly Bemisia tabaci by Mediating the Accumulation of Plant-Specialized Metabolites. J. Agric. Food Chem. 2023, 71, 13244–13254. [Google Scholar] [CrossRef] [PubMed]
- Hoogshagen, M.; Hastings, A.P.; Chavez, J.; Duckett, M.; Pettit, R.; Pahnke, A.A.; Agrawal, A.A.; de Roode, J.C. Mixtures of Milkweed Cardenolides Protect Monarch Butterflies against Parasites. J. Chem. Ecol. 2023, 50, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.; Zhou, S.; Shah, A.; Arafat, Y.; Arif Hussain Rizvi, S.; Shao, H. Plant Allelopathy in Response to Biotic and Abiotic Factors. Agronomy 2023, 13, 2358. [Google Scholar] [CrossRef]
- Erb, M.; Robert, C.A.M. Sequestration of plant secondary metabolites by insect herbivores: Molecular mechanisms and ecological consequences. Curr. Opin. Insect Sci. 2016, 14, 8–11. [Google Scholar] [CrossRef]
- Petschenka, G.; Agrawal, A. How herbivores coopt plant defenses: Natural selection, specialization, and sequestration. Curr. Opin. Insect Sci. 2016, 14, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Chacón-Fuentes, M.; Martínez-Cisterna, D.; Vera, W.; Ortega-Klose, F.; Reyes, C.; Matamala, I.; Bardehle, L. Feeding Performance of Argentine Stem Weevil Is Reduced by Peramine from Perennial Ryegrass Infected with Endophyte Fungus. Insects 2024, 15, 410. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Gao, C.; Wang, J.; Xu, W.; Wang, M.; Li, M.; Ma, B.; Tian, P. Effects of Drought Stress on Peramine and Lolitrem B in Epichloë-Endophyte-Infected Perennial Ryegrass. Life 2022, 12, 1207. [Google Scholar] [CrossRef] [PubMed]
- Shymanovich, T.; Musso, A.M.; Cech, N.B.; Faeth, S.H. Epichloë endophytes of Poa alsodes employ alternative mechanisms for host defense: Insecticidal versus deterrence. Arthropod-Plant Interact. 2019, 13, 79–90. [Google Scholar] [CrossRef]
- Nelli, M.R. Total Synthesis of Peramine, a Defensive Alkaloid Produced by Endophytic Fungi of Cool Season Grasses Possessing Anti-Insect Properties. J. Nat. Prod. 2016, 71, 1370–1377. [Google Scholar]
- Nelli, M.R.; Scheerer, J.R. Synthesis of peramine, an anti-insect defensive alkaloid produced by endophytic fungi of cool season grasses. J. Nat. Prod. 2016, 79, 1189–1192. [Google Scholar] [CrossRef] [PubMed]
- Rowan, D.D.; Dymock, J.J.; Brimble, M.A. Effect of fungal metabolite peramine and analogs on feeding development of Argentine stem weevil (Listronotus bonariensis). J. Chem. Ecol. 1990, 16, 1683–1695. [Google Scholar] [CrossRef] [PubMed]
- Young, C.; Hume, D.; McCulley, R. Fungal endophytes of tall fescue and perennial ryegrass: Pasture friend or foe? J. Anim. Science. 2013, 91, 2379–2394. [Google Scholar] [CrossRef] [PubMed]
- Keogh, R.G.; Tapper, B.A.; Fletcher, R.H. Distributions of the fungal endophyte Acremonium lolii, and of the alkaloids Lolitrem B and Peramine, within perennial ryegrass. N. Z. J. Agricutural Res. 1996, 39, 121–127. [Google Scholar] [CrossRef]
- Siegel, M.R.; Bush, L.P. Defensive chemicals in grass-fungal endophyte associations. Recent Adv. Phytochem. 1996, 30, 81–118. [Google Scholar]
- Fuchs, B.; Krischke, M.; Mueller, M.J.; Krauss, J.J. Peramine and Lolitrem B from Endophyte-Grass Associations Cascade Up the Food Chain. Chem. Ecol. 2013, 39, 1385–1389. [Google Scholar] [CrossRef] [PubMed]
- Parra, L.; Mutis, A.; Chacón, M.; Lizama, M.; Rojas, C.; Catrileo, A.; Rubilar, O.; Tortella, G.; Birkett, M.; Quiroz, A. Horn fly larval survival in cattle dung is reduced by endophyte infection of tall fescue pasture. Pest Manag. Sci. 2016, 72, 1328–1334. [Google Scholar] [CrossRef] [PubMed]
- Faeth, S.H.; Saari, S. Fungal grass endophytes and arthropod communities: Lessons from plant defence theory and multitrophic interactions. Fungal Ecol. 2012, 5, 364–371. [Google Scholar] [CrossRef]
- Zust, T.; Agrawal, A.A. Population growth and sequestration of plant toxins along a gradient of specialization in four aphid species on the common milkweed, Asclepias syriaca. Funct. Ecol. 2016, 30, 547–556. [Google Scholar] [CrossRef]
- Nishida, R. Sequestration of defensive substances from plants by lepidoptera. Annu. Rev. Entomol. 2002, 47, 57–92. [Google Scholar] [CrossRef]
- Opitz, S.E.W.; Müller, C. Plant chemistry and insect sequestration. Chemoecology 2009, 1, 71. [Google Scholar] [CrossRef]
- Weintraub, J.D. Host plant association patterns and phylogeny in the tribe Troidini (Lepidoptera: Papilionidae). In Swallowtail Butterflies: Their Ecology and Evolutionary Biology; Scriber, J.M., Tsubaki, Y., Lederhouse, R.C., Eds.; Scientific Publishers: Gainesville, FL, USA, 1995; pp. 307–316. [Google Scholar]
- Pinto, C.F.; Troncoso, A.J.; Urzua, A.; Niemeyer, H.M. Use of volatiles of Aristolochia chilensis (aristolochiaceae) in host searching by fourth-instar larvae and adults of battus Polydamas archidamas (Lepidoptera: Papilionidae: Troidini). Eur. J. Entomol. 2009, 106, 63–68. [Google Scholar] [CrossRef]
- Pinto, C.F.; Urzúa, A.; Niemeyer, H.M. Sequestration of aristolochic acids from meridic diets by larvae of Battus Polydamas archidamas (Papilionidae: Troidini). Eur. J. Entomol. 2011, 108, 41–45. [Google Scholar] [CrossRef]
- Ángulo, A.; Ruiz, V. Maenas rudis (Butler): Cuncuna colorada de prados y jardines; biología y estados inmaduros (Lepidoptera: Arctiidae). Boletín Soc. Biológica Concepción 1975, 139–147. [Google Scholar]
- Chacón-Fuentes, M.; Parra, L.; Rodriguez-Saona, C.; Seguel, I.; Ceballos, R.; Quiroz, A. Domestication in murtilla (Ugni molinae) reduced defensive flavonol levels but increased resistance against a native herbivorous insect. Environ. Entomol. 2015, 44, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Chacón-Fuentes, M.; Parra, L.; Lizama, M.; Seguel, I.; Urzúa, A.; Quiroz, A. Plant flavonoid content modified by domestication. Environ. Entomol. 2017, 46, 1080–1089. [Google Scholar] [CrossRef]
- Chacon-Fuentes, M.; Bardehle, L.; Seguel, I.; Rubilar, F.; Martínez-Cisterna, D.; Quiróz, A. Domestication of Plants of Ugni molinae Turcz (Myrtaceae) Interferes in the Biology of Chilesia rudis (Lepidoptera: Erebidae) Larvae. Molecules 2021, 26, 2063. [Google Scholar] [CrossRef] [PubMed]
- Nickisch-Rosenegk, E.; Wink, M. Sequestration of pyrrolizidine alkaloids in several arctiid moths (Lepidoptera: Arctiidae). J. Chem. Ecol. 1993, 19, 1189–1903. [Google Scholar] [CrossRef] [PubMed]
- Dickel, F.; Freitak, D.; Mappes, J. Long-term prophylactic antibiotic treatment: Effects on survival, immunocompetence and reproduction success of Parasemia plantaginis (Lepidoptera: Erebidae). J. Insect Sci. 2016, 16, 46. [Google Scholar] [CrossRef]
- Dombrowski, J.; Baldwin, J.; Azevedo, M.; Banowetz, G. A sensitive PCR-based assay to detect Neotyphodium fungi in seed and plant tissue of tall, fescue and ryegrass species. Crop Sci. 2006, 46, 1064–1070. [Google Scholar] [CrossRef]
- Chacon-Fuentes, M.; Martínez-Cisterna, D.; Reyes, C.; Vera, W.; Fincheira, P.; Lizama, M.; Quiroz, A.; Bardehle, L. Infection of perennial ryegrass (Lolium perenne) by an endophyte fungus (Neotyphodium lolii) decreases the abundance and diversity of predators and parasitoids. Rev. Bras. De Entomol. 2023, 67, e20230012. [Google Scholar] [CrossRef]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S. Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef] [PubMed]
- Kholghahmadi, M.; Karimi-Malati, A.; Jalali Sendi, J. Ecophysiological responses of individually and group reared Cydalima perspectalis (Lepidoptera: Crambidae) to alkaloid-containing host plants. Environ. Entomol. 2023, 52, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Ait Lhaj, R.; Saffaj, T.; Belmir, H.; Ihssane, B. The uncertainty profile used for full validation of the HPLC Method to determine 22 azo amines in fabrics. J. AOAC Int. 2023, 106, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Chacón-Fuentes, M.; Bardehle, L.; Lizama, M.; Seguel, I.; Quiroz, A. Restoration of flavonols and isoflavonoids in Ugni molinae subjected to a reciprocal transplant experiment in a domestication framework. Chem. Ecol. 2019, 35, 115–127. [Google Scholar] [CrossRef]
- Guerre, P. Ergot alkaloid produced by endophytic fungi of the genus Epichloe. Toxins 2015, 7, 773–790. [Google Scholar] [CrossRef] [PubMed]
- Schardl, C.L.; Young, C.A.; Hesse, U.; Amyotte, S.G.; Andreeva, K.; Calie, P.J.; Fleetwood, D.J.; Haws, D.C.; Moore, N.; Oeser, B.; et al. Plant-symbiotic fungi as chemical engineers: Multi-genome analysis of the clavicipitaceae reveals dynamics of alkaloid loci. PLoS Genet 2013, 9, e1003323. [Google Scholar] [CrossRef] [PubMed]
- Shymanovich, T.; Faeth, S.H. Environmental factors affect the distribution of two Epichloë fungal endophyte species inhabiting a common host grove bluegrass (Poa alsodes). Ecol. Evol. 2019, 9, 6624–6642. [Google Scholar] [CrossRef]
- Newton, E.; Bullock, J.M.; Hodgson, D. Bottom-up effects of glucosinolate variation on aphid colony dynamics in wild cabbage populations. Ecol. Entomol. 2009, 34, 614–623. [Google Scholar] [CrossRef]
- Kos, M.; Broekgaarden, C.; Kabouw, P.; Lenferink, K.O.; Poelman, E.H.; Vet, L.E.M.; Dicke, M.; Van Loon, J.J.A. Relative importance of plant mediated bottom-up and top-down forces on herbivore abundance on Brassica oleracea. Funct. Ecol. 2011, 25, 113–1124. [Google Scholar] [CrossRef]
- Cibils-Stewart, X.; Putra, R.; Islam, T.; Fanna, D.J.; Wuhrer, R.; Mace, W.J.; Johnson, S.N. Silicon and Epichloë-endophyte defences in a model temperate grass diminish feeding efficiency and immunity of an insect folivore. Funct. Ecol. 2023, 37, 3177–3192. [Google Scholar] [CrossRef]
- Lampert, E.C.; Bowers, M.D. Host plant influences on iridoid glycoside sequestration of generalist and specialist caterpillars. J. Chem. Ecol. 2010, 36, 1101–1104. [Google Scholar] [CrossRef]
- Lampert, E.C.; Dyer, L.A.; Bowers, M.D. Dietary specialization and the effects of plant species on potential multitrophic interactions of three species of nymphaline caterpillars. Entomol. Exp. Appl. 2014, 153, 207–216. [Google Scholar] [CrossRef]
- Abe, F.; Yamauchi, T.; Honda, K.; Omura, H.; Hayashi, N. Sequestration of phenanthroindolizidine alkaloids by an Asclepiadaceae-feeding danaid butterfly, Ideopsis similis. Phytochemistry 2001, 56, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Spiering, M.J.; Wilkinson, H.H.; Blankenship, J.D.; Schardl, C.L. Expressed sequence tags and genes associated with loline alkaloid expression by the fungal endophyte Neotyphodium uncinatum. Fungal Genet. Biol. 2002, 36, 242–254. [Google Scholar] [CrossRef]
- Leuchtmann, A.; Schmidt, D.; Bush, L.P. Different levels of protective alkaloids in grasses with stroma-forming and seed-transmitted Epichloë/Neotyphodium endophytes. J. Chem. Ecol. 2000, 26, 1025–1036. [Google Scholar] [CrossRef]

| Line Experimental | N° of Plants Tested | (E+) | (E−) | Endophyte (%) |
|---|---|---|---|---|
| L161 | 120 | 120 | 0 | 100 *a |
| L162 | 120 | 89 | 31 | 74 *d |
| L163 | 120 | 12 | 108 | 10 *e |
| L164 | 120 | 110 | 10 | 91.6 *b |
| L165 | 120 | 100 | 20 | 83.3 *c |
| L166 | 120 | 110 | 10 | 91.6 *b |
| L167 | 120 | 13 | 107 | 11 *e |
| JUMBO (E−) | 120 | 0 | 120 | 0 *f |
| ALTO AR1 (E+) | 120 | 120 | 0 | 100 *a |
| PCR (Tub2 Intron) | ||
|---|---|---|
| Experimental Lines or Commercial Cultivars | IS-RS-5′; IS-NS3′ | IS-tub2w-5′; IS-tub2w-3′ |
| L161 | + | + |
| L162 | + | + |
| L163 | + | + |
| L164 | + | + |
| L165 | + | + |
| L166 | + | + |
| L167 | + | + |
| JUMBO (E−) | − | − |
| ALTO AR1 (E+) | + | + |
| Experimental lines and Cultivars | Peramine in Leaves (µg/g) | Pupal Development Time (days) | Length (cm) | Weight (g) | Wing Length ♀ (cm) | Wing Length ♂ (cm) | Peramine in Carcasses (µg/g) | Peramine in Feces (µg/g) | Peramine in Guts (µg/g) |
|---|---|---|---|---|---|---|---|---|---|
| L161 | 179.3 ± 24.5 a | 28 ± 1.1 a | 1.7 ± 0.1 e | 0.5 ± 0.0 b | 0.17 ± 0.0 b | 0.63 ± 0.1 e | 12.1 ± 1.0 a | 31.7 ± 4.2 a | 0.0 ± 0.0 e |
| L162 | 99.8 ± 17.3 d | 25 ± 1.2 b | 1.8 ± 0.1 d | 0.6 ± 0.1 b | 0.14 ± 0.0 d | 0.99 ± 0.1 c | 8.2 ± 0.9 c | 13.7 ± 2.7 d | 0.8 ± 0.1 c |
| L163 | 15.2 ± 4.9 e | 24 ± 1.0 c | 2.9 ± 0.2 b | 0.7 ± 0.0 a | 0.13 ± 0.0 d | 1.32 ± 0.2 a | 1.6 ± 0.8 d | 1.1 ± 0.3 e | 0.0 ± 0.0 e |
| L164 | 160.8 ± 39.9 b | 26 ± 2.1 b | 1.9 ± 0.2 d | 0.5 ± 0.0 b | 0.15 ± 0.0 c | 0.70 ± 0.1 d | 11.3 ± 2.1 a | 27.9 ± 4.2 b | 0.2 ± 0.0 d |
| L165 | 127.0 ± 34.2 c | 25 ± 2.0 b | 2.4 ± 0.1 c | 0.6 ± 0.0 b | 0.16 ± 0.0 c | 1.19 ± 0.2 b | 9.5 ± 0.9 b | 19.7 ± 2.3 c | 1.5 ± 0.1 b |
| L166 | 146.4 ± 21.2 c | 26 ± 1.5 b | 2.0 ± 0.1 d | 0.5 ± 0.1 b | 0.12 ± 0.0 e | 0.80 ± 0.1 d | 10.1 ± 0.1 b | 24.0 ± 2.1 b | 1.7 ± 0.1 a |
| L167 | 17.1 ± 6.1 e | 24 ± 1.1 c | 3.1 ± 0.1 a | 0.8 ± 0.1 a | 0.15 ± 0.0 c | 1.20 ± 0.2 b | 2.2 ± 0.2 d | 1.1 ± 0.4 e | 0.0 ± 0.0 e |
| JUMBO (E−) | 0.0 ± 0.0 f | 23 ± 1.4 c | 3.2 ± 0.3 a | 0.7 ± 0.1 a | 0.20 ± 0.0 a | 1.25 ± 0.2 b | 0.0 ± 0.0 e | 0.0 ± 0.0 f | 0.0 ± 0.0 e |
| ALTO AR1 (E+) | 184.1 ± 29.8 a | 28 ± 1.2 a | 1.4 ± 0.1 f | 0.3 ± 0.0 c | 0.17 ± 0.0 b | 0.43 ± 0.1 f | 10.5 ± 1.1 a | 32.8 ± 4.4 a | 1.3 ± 0.1 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chacón-Fuentes, M.; Martínez-Cisterna, D.; Lizama, M.; Asencio-Cancino, V.; Matamala, I.; Bardehle, L. A Countermeasure Strategy against Peramine Developed by Chilesia rudis in the Endophyte–Ryegrass–Herbivore Model. J. Fungi 2024, 10, 512. https://doi.org/10.3390/jof10080512
Chacón-Fuentes M, Martínez-Cisterna D, Lizama M, Asencio-Cancino V, Matamala I, Bardehle L. A Countermeasure Strategy against Peramine Developed by Chilesia rudis in the Endophyte–Ryegrass–Herbivore Model. Journal of Fungi. 2024; 10(8):512. https://doi.org/10.3390/jof10080512
Chicago/Turabian StyleChacón-Fuentes, Manuel, Daniel Martínez-Cisterna, Marcelo Lizama, Valeria Asencio-Cancino, Ignacio Matamala, and Leonardo Bardehle. 2024. "A Countermeasure Strategy against Peramine Developed by Chilesia rudis in the Endophyte–Ryegrass–Herbivore Model" Journal of Fungi 10, no. 8: 512. https://doi.org/10.3390/jof10080512
APA StyleChacón-Fuentes, M., Martínez-Cisterna, D., Lizama, M., Asencio-Cancino, V., Matamala, I., & Bardehle, L. (2024). A Countermeasure Strategy against Peramine Developed by Chilesia rudis in the Endophyte–Ryegrass–Herbivore Model. Journal of Fungi, 10(8), 512. https://doi.org/10.3390/jof10080512







