Topical Propranolol Improves Epistaxis Control in Hereditary Hemorrhagic Telangiectasia (HHT): A Randomized Double-Blind Placebo-Controlled Trial
Abstract
:1. Introduction
2. Experimental Section
Statistical Analysis
3. Results
3.1. Study Participants
3.2. Primary Outcome
3.3. Secondary Outcomes
3.4. Nasal Endoscopy
3.5. Side Effects
3.6. Results of the Open-Label Phase of the Study
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McDonald, J.; Bayrak-Toydemir, P.; Pyeritz, R.E. Hereditary hemorrhagic telangiectasia: An overview of diagnosis, management, and pathogenesis. Genet. Med. Off. J. Am. Coll. Med. Genet. 2011, 13, 607–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merlo, C.A.; Yin, L.X.; Hoag, J.B.; Mitchell, S.E.; Reh, D.D. The effects of epistaxis on health-related quality of life in patients with hereditary hemorrhagic telangiectasia. Int. Forum Allergy Rhinol. 2014, 4, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Ingrand, I.; Ingrand, P.; Gilbert-Dussardier, B.; Defossez, G.; Jouhet, V.; Migeot, V.; Dufour, X.; Klossek, J.M. Altered quality of life in Rendu-Osler-Weber disease related to recurrent epistaxis. Rhinology 2011, 49, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Loaëc, M.; Morinière, S.; Hitier, M.; Ferrant, O.; Plauchu, H.; Babin, E. Psychosocial quality of life in hereditary haemorrhagic telangiectasia patients. Rhinology 2011, 49, 164–167. [Google Scholar] [CrossRef]
- Sautter, N.B.; Smith, T.L. Treatment of Hereditary Hemorrhagic Telangiectasia-Related Epistaxis. Otolaryngol. Clin. N. Am. 2016, 49, 639–654. [Google Scholar] [CrossRef]
- Ardelean, D.S.; Letarte, M. Anti-angiogenic therapeutic strategies in hereditary hemorrhagic telangiectasia. Front. Genet. 2015, 6, 35. [Google Scholar] [CrossRef] [Green Version]
- Stokes, P.; Rimmer, J. Intranasal bevacizumab in the treatment of HHT -related epistaxis: A systematic review. Rhinology 2018, 56, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Halderman, A.A.; Ryan, M.W.; Marple, B.F.; Sindwani, R.; Reh, D.D.; Poetker, D.M. Bevacizumab for Epistaxis in Hereditary Hemorrhagic Telangiectasia: An Evidence-based Review. Am. J. Rhinol. Allergy 2018, 32, 258–268. [Google Scholar] [CrossRef]
- Rosenberg, T.; Fialla, A.D.; Kjeldsen, J.; Kjeldsen, A.D. Does severe bleeding in HHT patients respond to intravenous bevacizumab? Review of the literature and case series. Rhinology 2019, 57, 242–251. [Google Scholar] [CrossRef] [Green Version]
- Léauté-Labrèze, C.; Dumas de la Roque, E.; Hubiche, T.; Boralevi, F.; Thambo, J.-B.; Taïeb, A. Propranolol for severe hemangiomas of infancy. N. Engl. J. Med. 2008, 358, 2649–2651. [Google Scholar] [CrossRef]
- Storch, C.H.; Hoeger, P.H. Propranolol for infantile haemangiomas: Insights into the molecular mechanisms of action. Br. J. Dermatol. 2010, 163, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Mei-Zahav, M.; Blau, H.; Bruckheimer, E.; Zur, E.; Goldschmidt, N. Topical propranolol improves epistaxis in patients with hereditary hemorrhagic telangiectasia—A preliminary report. J. Otolaryngol. Head Neck Surg. J. Oto-Rhino-Laryngol. Chir. Cervico-Faciale 2017, 46, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shovlin, C.L.; Guttmacher, A.E.; Buscarini, E.; Faughnan, M.E.; Hyland, R.H.; Westermann, C.J.; Kjeldsen, A.D.; Plauchu, H. Diagnostic criteria for hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber syndrome). Am. J. Med. Genet. 2000, 91, 66–67. [Google Scholar] [CrossRef]
- Hoag, J.B.; Terry, P.; Mitchell, S.; Reh, D.; Merlo, C.A. An epistaxis severity score for hereditary hemorrhagic telangiectasia. Laryngoscope 2010, 120, 838–843. [Google Scholar] [CrossRef]
- Common Terminology Criteria for Adverse Events (CTCAE)|Protocol Development|CTEP. Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm (accessed on 28 August 2020).
- Yin, L.X.; Reh, D.D.; Hoag, J.B.; Mitchell, S.E.; Mathai, S.C.; Robinson, G.M.; Merlo, C.A. The minimal important difference of the epistaxis severity score in hereditary hemorrhagic telangiectasia. Laryngoscope 2015, 126, 1029–1032. [Google Scholar] [CrossRef]
- Snellings, D.A.; Gallione, C.J.; Clark, D.S.; Vozoris, N.T.; Faughnan, M.E.; Marchuk, D.A. Somatic Mutations in Vascular Malformations of Hereditary Hemorrhagic Telangiectasia Result in Bi-allelic Loss of ENG or ACVRL1. Am. J. Hum. Genet. 2019, 105, 894–906. [Google Scholar] [CrossRef]
- Sadick, H.; Naim, R.; Gössler, U.; Hörmann, K.; Riedel, F. Angiogenesis in hereditary hemorrhagic telangiectasia: VEGF165 plasma concentration in correlation to the VEGF expression and microvessel density. Int. J. Mol. Med. 2005, 15, 15–19. [Google Scholar] [CrossRef]
- Xu, G.; Lv, R.; Zhao, Z.; Huo, R. Topical propranolol for treatment of superficial infantile hemangiomas. J. Am. Acad. Dermatol. 2012, 67, 1210–1213. [Google Scholar] [CrossRef]
- Przewratil, P.; Sitkiewicz, A.; Andrzejewska, E. Local serum levels of vascular endothelial growth factor in infantile hemangioma: Intriguing mechanism of endothelial growth. Cytokine 2010, 49, 141–147. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, X.; Wang, W.; Zhuang, X.; Dong, J.; Qi, Z.; Hu, Q. Circulating level of vascular endothelial growth factor in differentiating hemangioma from vascular malformation patients. Plast. Reconstr. Surg. 2005, 116, 200–204. [Google Scholar] [CrossRef]
- Albiñana, V.; Recio-Poveda, L.; Zarrabeitia, R.; Bernabéu, C.; Botella, L.M. Propranolol as antiangiogenic candidate for the therapy of hereditary haemorrhagic telangiectasia. Thromb. Haemost. 2012, 108, 41–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Contis, A.; Gensous, N.; Viallard, J.F.; Goizet, C.; Léauté-Labrèze, C.; Duffau, P. Efficacy and safety of propranolol for epistaxis in hereditary haemorrhagic telangiectasia: Retrospective, then prospective study, in a total of 21 patients. Clin. Otolaryngol. Off. J. ENT-UK Off. J. Neth. Soc. Oto-Rhino-Laryngol. Cervico-Facial Surg. 2017, 42, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Casado, S.; Martín de Rosales Cabrera, A.M.; Usarralde Pérez, A.; Martínez Simón, J.J.; Zhan Zhou, E.; Marcos Salazar, M.S.; Pérez Encinas, M.; Botella Cubells, L. Sclerotherapy and Topical Nasal Propranolol: An Effective and Safe Therapy for HHT-Epistaxis. Laryngoscope 2019, 129, 2216–2223. [Google Scholar] [CrossRef] [PubMed]
- Epperla, N.; Brilliant, M.H.; Vidaillet, H. Topical timolol for treatment of epistaxis in hereditary haemorrhagic telangiectasia associated with bradycardia: A look at CYP2D6 metabolising variants. BMJ Case Rep. 2014, 2014. [Google Scholar] [CrossRef] [Green Version]
- Olitsky, S.E. Topical timolol for the treatment of epistaxis in hereditary hemorrhagic telangiectasia. Am. J. Otolaryngol. 2012, 33, 375–376. [Google Scholar] [CrossRef]
- Garg, N.; Khunger, M.; Gupta, A.; Kumar, N. Optimal management of hereditary hemorrhagic telangiectasia. J. Blood Med. 2014, 5, 191–206. [Google Scholar] [CrossRef] [Green Version]
- Dupuis-Girod, S.; Ginon, I.; Saurin, J.-C.; Marion, D.; Guillot, E.; Decullier, E.; Roux, A.; Carette, M.-F.; Gilbert-Dussardier, B.; Hatron, P.-Y.; et al. Bevacizumab in patients with hereditary hemorrhagic telangiectasia and severe hepatic vascular malformations and high cardiac output. JAMA 2012, 307, 948–955. [Google Scholar] [CrossRef] [Green Version]
- Riss, D.; Burian, M.; Wolf, A.; Kranebitter, V.; Kaider, A.; Arnoldner, C. Intranasal submucosal bevacizumab for epistaxis in hereditary hemorrhagic telangiectasia: A double-blind, randomized, placebo-controlled trial. Head Neck 2015, 37, 783–787. [Google Scholar] [CrossRef]
- Iyer, V.N.; Apala, D.R.; Pannu, B.S.; Kotecha, A.; Brinjikji, W.; Leise, M.D.; Kamath, P.S.; Misra, S.; Begna, K.H.; Cartin-Ceba, R.; et al. Intravenous Bevacizumab for Refractory Hereditary Hemorrhagic Telangiectasia-Related Epistaxis and Gastrointestinal Bleeding. Mayo Clin. Proc. 2018, 93, 155–166. [Google Scholar] [CrossRef]
- Al-Samkari, H.; Kasthuri, R.S.; Parambil, J.G.; Albitar, H.A.; Almodallal, Y.A.; Vázquez, C.; Serra, M.M.; Dupuis-Girod, S.; Wilsen, C.B.; McWilliams, J.P.; et al. An international, multicenter study of intravenous bevacizumab for bleeding in hereditary hemorrhagic telangiectasia: The InHIBIT-Bleed study. Haematologica 2020. [Google Scholar] [CrossRef]
- Kabbinavar, F.; Hurwitz, H.I.; Fehrenbacher, L.; Meropol, N.J.; Novotny, W.F.; Lieberman, G.; Griffing, S.; Bergsland, E. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2003, 21, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Iriarte, A.; Figueras, A.; Cerdà, P.; Mora, J.M.; Jucglà, A.; Penín, R.; Viñals, F.; Riera-Mestre, A. PI3K (Phosphatidylinositol 3-Kinase) Activation and Endothelial Cell Proliferation in Patients with Hemorrhagic Hereditary Telangiectasia Type 1. Cells 2019, 8, 971. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, S.; Zhao, H.; Chandakkar, P.; Papoin, J.; Choi, H.; Nomura-Kitabayashi, A.; Patel, R.; Gillen, M.; Diao, L.; Chatterjee, P.K.; et al. Correcting Smad1/5/8, mTOR, and VEGFR2 treats pathology in hereditary hemorrhagic telangiectasia models. J. Clin. Investig. 2020, 130, 942–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lesca, G.; Olivieri, C.; Burnichon, N.; Pagella, F.; Carette, M.-F.; Gilbert-Dussardier, B.; Goizet, C.; Roume, J.; Rabilloud, M.; Saurin, J.-C.; et al. Genotype-phenotype correlations in hereditary hemorrhagic telangiectasia: Data from the French-Italian HHT network. Genet. Med. Off. J. Am. Coll. Med. Genet. 2007, 9, 14–22. [Google Scholar] [CrossRef] [Green Version]
- Mora-Luján, J.M.; Iriarte, A.; Alba, E.; Sánchez-Corral, M.A.; Cerdà, P.; Cruellas, F.; Ordi, Q.; Corbella, X.; Ribas, J.; Castellote, J.; et al. Gender differences in hereditary hemorrhagic telangiectasia severity. Orphanet J. Rare Dis. 2020, 15, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
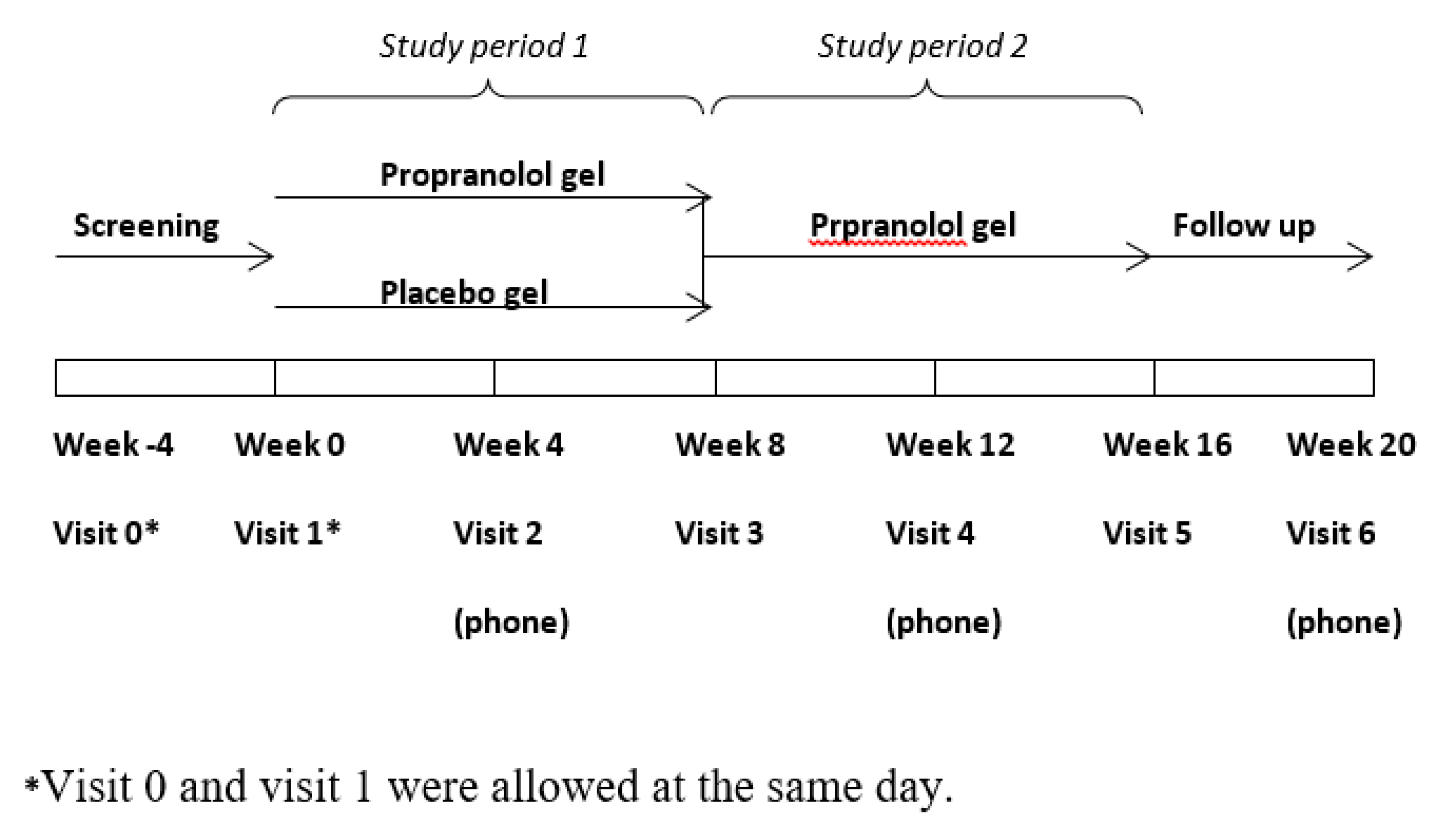
| Inclusion criteria: |
Exclusion criteria:
|
| Placebo (n = 10) | Propranolol (n = 10) | p | |
|---|---|---|---|
| Age—years (mean ± SD) | 51 ± 9 | 57 ± 11 | 0.262 |
| Gender (F,M) | 9:1 | 6:4 | 0.152 |
| Gene mutation (number of participants) | ACVRL1-8 Endoglin-1 ND-1 | ACVRL1-5 Endoglin-3 ND-2 | 0.223 |
| ESS | 5.68 ± 1.8 | 6.50 ± 1.84 | 0.323 |
| QOL | 34.75 ± 10.9 | 35.1 ± 6.7 | 0.932 |
| Hemoglobin (g/dL) | 10.7 ± 2.52 | 10.57 ± 2.6 | 0.918 |
| IV iron */IV PC * | 16/3 | 9/7 | 0.739/0.481 |
| Ferritin | 65.4 ± 86.19 | 89.65 ± 212.04 | 0.715 |
| Rhinology grading (number of patients) | 0.601 | ||
| Grade I | 4 | 3 | |
| Grade II | 5 | 5 | |
| Grade III | 1 | 2 | |
| Systolic BP mmHg (mean ± SD) | 110.2 ± 9.6 | 119.3 ± 12.3 | 0.09 |
| Diastolic BP mmHg (mean ± SD) | 63.3 ± 9.2 | 69.0 ± 9.2 | 0.20 |
| HR per minute (mean ± SD) | 76.0 ± 12.9 | 73.9 ± 111.7 | 0.71 |
| Outcome Measure | Placebo (n = 10) | Propranolol (n = 10) | ||||
|---|---|---|---|---|---|---|
| Baseline | End of DB Phase | p | Baseline | End of DB Phase | p | |
| Primary outcome | ||||||
| ESS | 5.68 ± 1.8 | 5.33 ± 2.1 | 0.133 | 6.50 ± 1.84 | 4.47 ± 1.75 | 0.004 |
| Secondary outcomes | ||||||
| Hb g/dL | 10.7 ± 2.5 | 10.7 ± 2.3 | 0.91 | 10.5 ± 2.6 | 11.4 ± 2.02 | <0.001 |
| Total PC units required * | 3 | 5 | 0.346 | 7 | 3 | <0.001 |
| Total IV iron ** | 16 | 14 | 0.233 | 9 | 4 | 0.15 |
| doses required * | ||||||
| QOL | 34.75 ± 10.9 | 40.6 ± 9.11 | 0.03 | 35.1 ± 6.7 | 41 ± 7.39 | 0.048 |
| Outcome Measure | Placebo | Propranolol | p |
|---|---|---|---|
| Primary outcome | |||
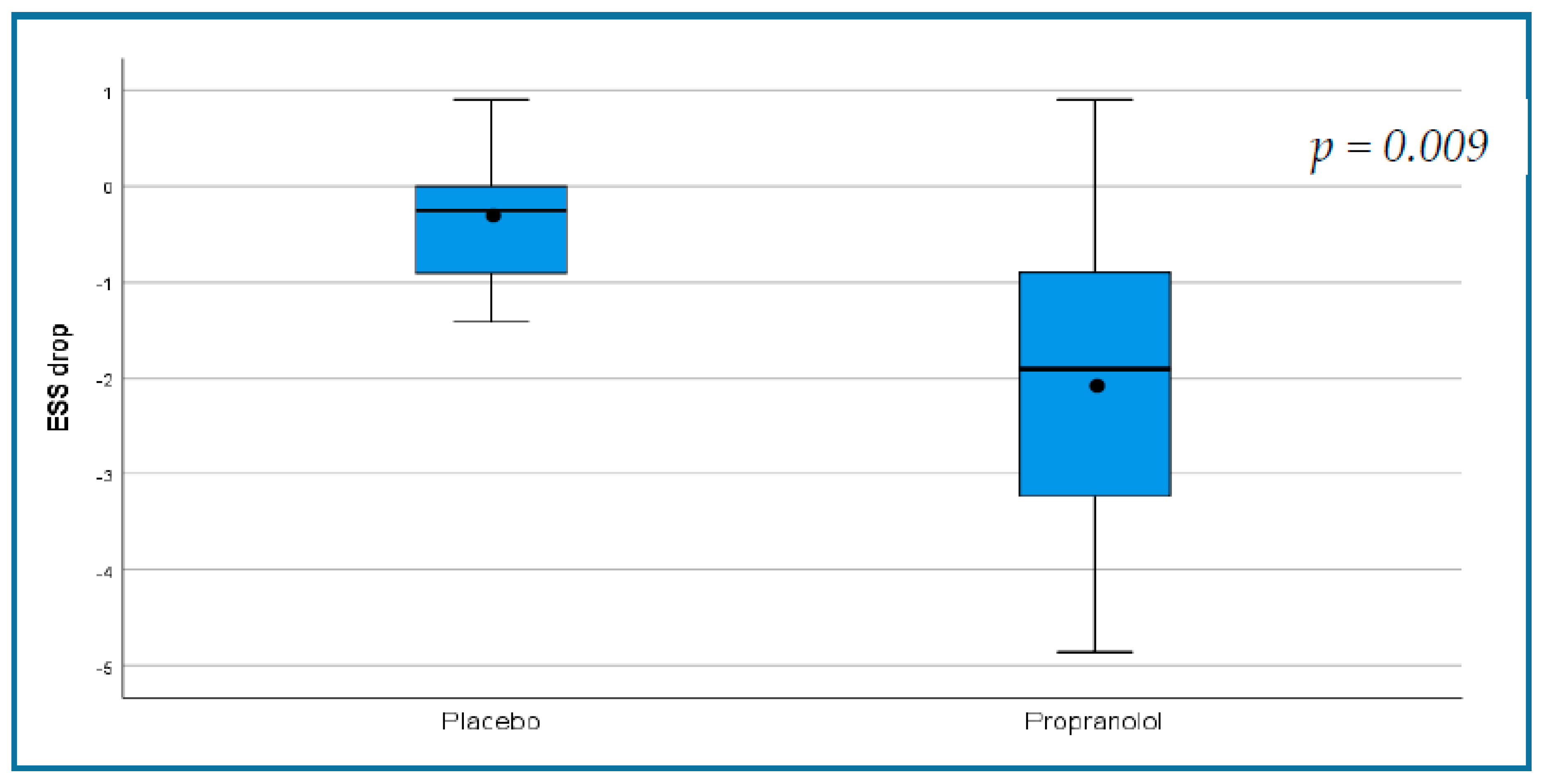
| Change in ESS | −0.35 ± 0.68 | −2.03 ± 1.7 | 0.009 |
| Secondary outcomes | |||
| Change in Hb level (g/dL) | 0.68 ± 0.01 | 1.96 ± 0.85 | 0.216 |
| Change in number of PC required | −0.20 ± 0.63 | −0.40 ± 0.69 | 0.029 |
| Change in number of IV iron doses required * | −0.20 ± 0.63 | −0.50 ± 0.97 | 0.304 |
| QOL | 6.11 ± 5.85 | 6.06 ± 5.90 | 0.986 |
| Placebo (10) | Propranolol (10) | p | |
|---|---|---|---|
| Any burning sensation | 2 | 9 | 0.005 |
| Sustained burning sensation | 0 | 4 | 0.086 |
| Rhinorrhea | 0 | 3 | 0.06 |
| Nasal dryness | 1 | 0 | 1 |
| Otitis media | 0 | 1 | 1 |
| Outcome Measure (mean ± SD) n = 15 | Double Blind Period | Open-Label Period | p |
|---|---|---|---|
| Epistaxis frequency, bleeds/day | 1.71 ± 1.34 | 1.24 ± 1.24 | <0.001 |
| Epistaxis severity | 1.42 ± 0.64 | 1.03 ± 0.55 | <0.001 |
| Epistaxis duration, minutes/day | 10.68 ± 9.01 | 6.13 ± 4.67 | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mei-Zahav, M.; Gendler, Y.; Bruckheimer, E.; Prais, D.; Birk, E.; Watad, M.; Goldschmidt, N.; Soudry, E. Topical Propranolol Improves Epistaxis Control in Hereditary Hemorrhagic Telangiectasia (HHT): A Randomized Double-Blind Placebo-Controlled Trial. J. Clin. Med. 2020, 9, 3130. https://doi.org/10.3390/jcm9103130
Mei-Zahav M, Gendler Y, Bruckheimer E, Prais D, Birk E, Watad M, Goldschmidt N, Soudry E. Topical Propranolol Improves Epistaxis Control in Hereditary Hemorrhagic Telangiectasia (HHT): A Randomized Double-Blind Placebo-Controlled Trial. Journal of Clinical Medicine. 2020; 9(10):3130. https://doi.org/10.3390/jcm9103130
Chicago/Turabian StyleMei-Zahav, Meir, Yulia Gendler, Elchanan Bruckheimer, Dario Prais, Einat Birk, Muhamad Watad, Neta Goldschmidt, and Ethan Soudry. 2020. "Topical Propranolol Improves Epistaxis Control in Hereditary Hemorrhagic Telangiectasia (HHT): A Randomized Double-Blind Placebo-Controlled Trial" Journal of Clinical Medicine 9, no. 10: 3130. https://doi.org/10.3390/jcm9103130
APA StyleMei-Zahav, M., Gendler, Y., Bruckheimer, E., Prais, D., Birk, E., Watad, M., Goldschmidt, N., & Soudry, E. (2020). Topical Propranolol Improves Epistaxis Control in Hereditary Hemorrhagic Telangiectasia (HHT): A Randomized Double-Blind Placebo-Controlled Trial. Journal of Clinical Medicine, 9(10), 3130. https://doi.org/10.3390/jcm9103130







