Optimal Timing of External Ventricular Drainage after Severe Traumatic Brain Injury: A Systematic Review
Abstract
:1. Introduction
2. Methods
2.1. Protocol and Registration
2.2. Ethics Approval and Consent
2.3. Search Strategy
2.4. Inclusion Criteria/Exclusion Criteria
2.5. Study Selection
2.6. Data Extraction and Risk of Bias Assessment
2.7. Outcomes
2.8. Statistical Analyses
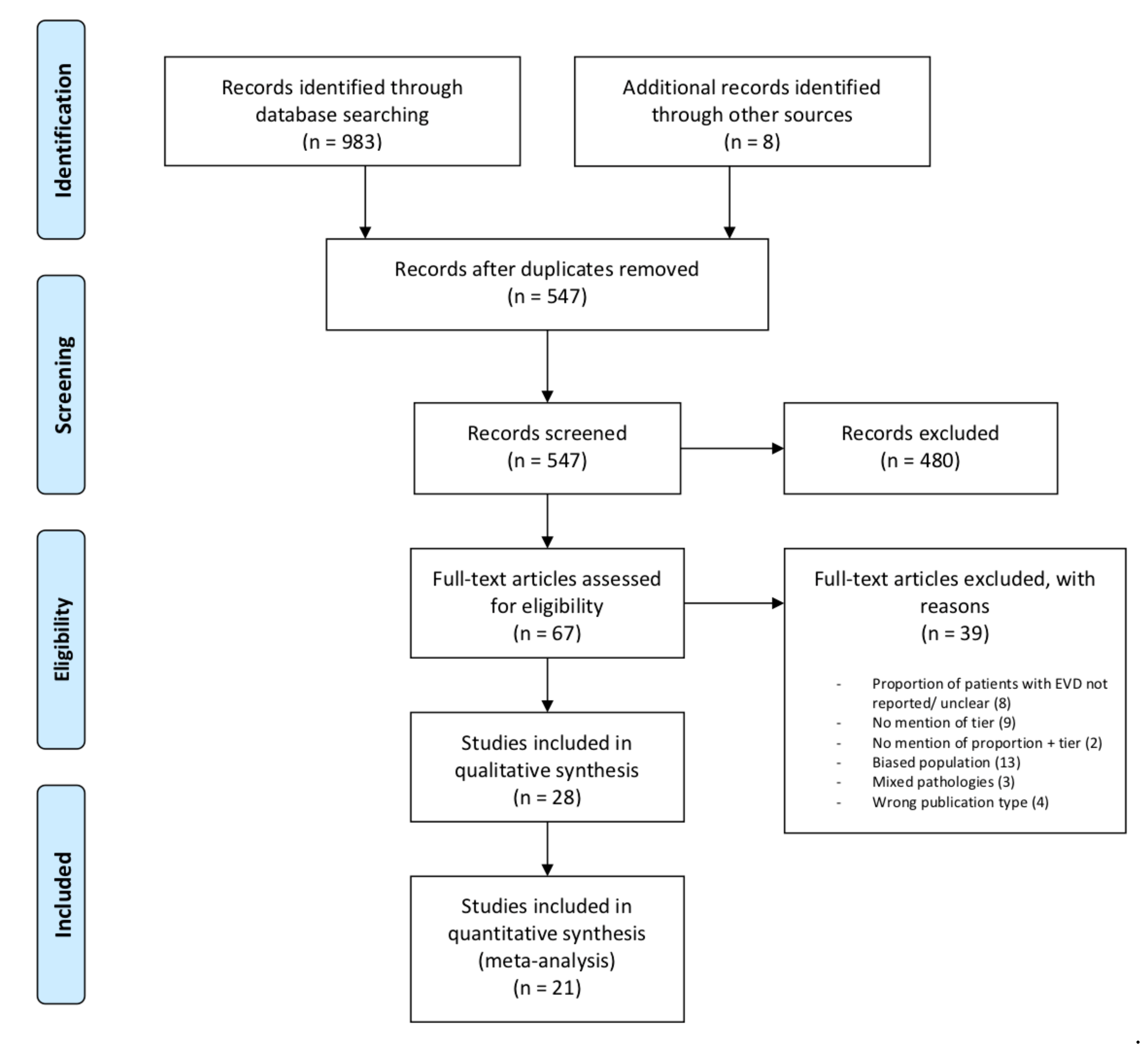
3. Results
3.1. Study Characteristics
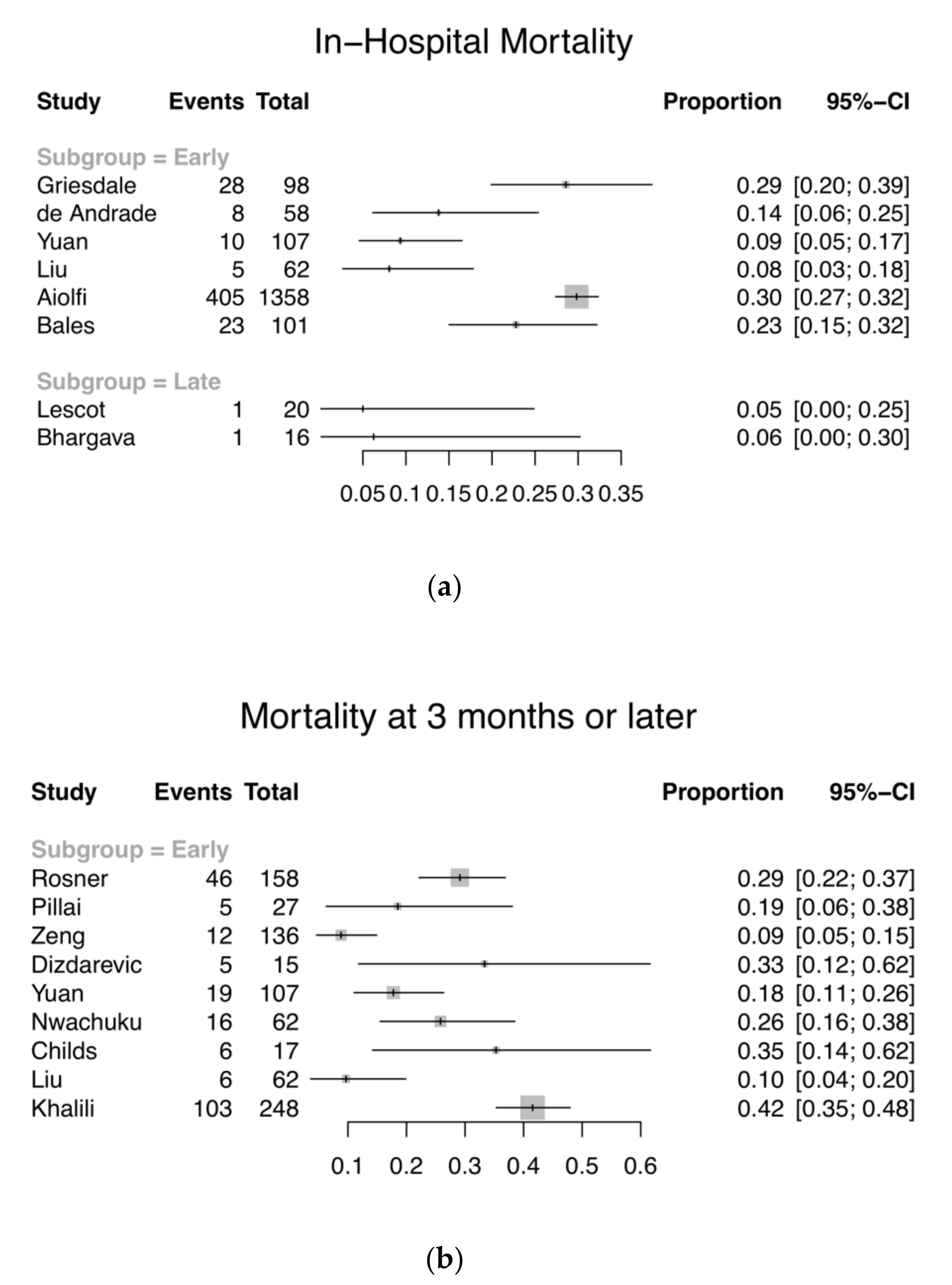
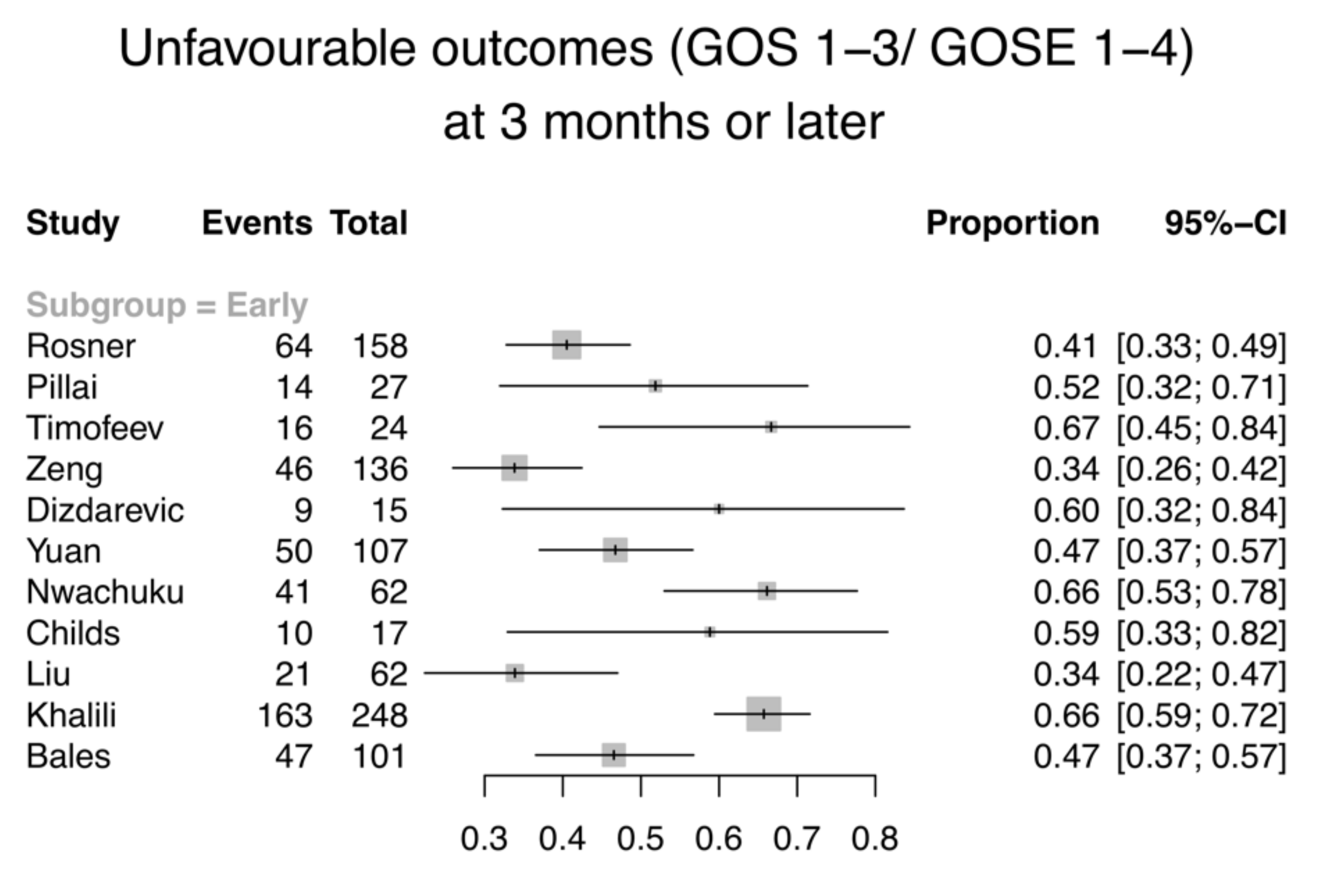
3.2. Primary Outcome—Mortality and GOS/GOS-E
3.3. Secondary Outcomes
3.3.1. ICP Control
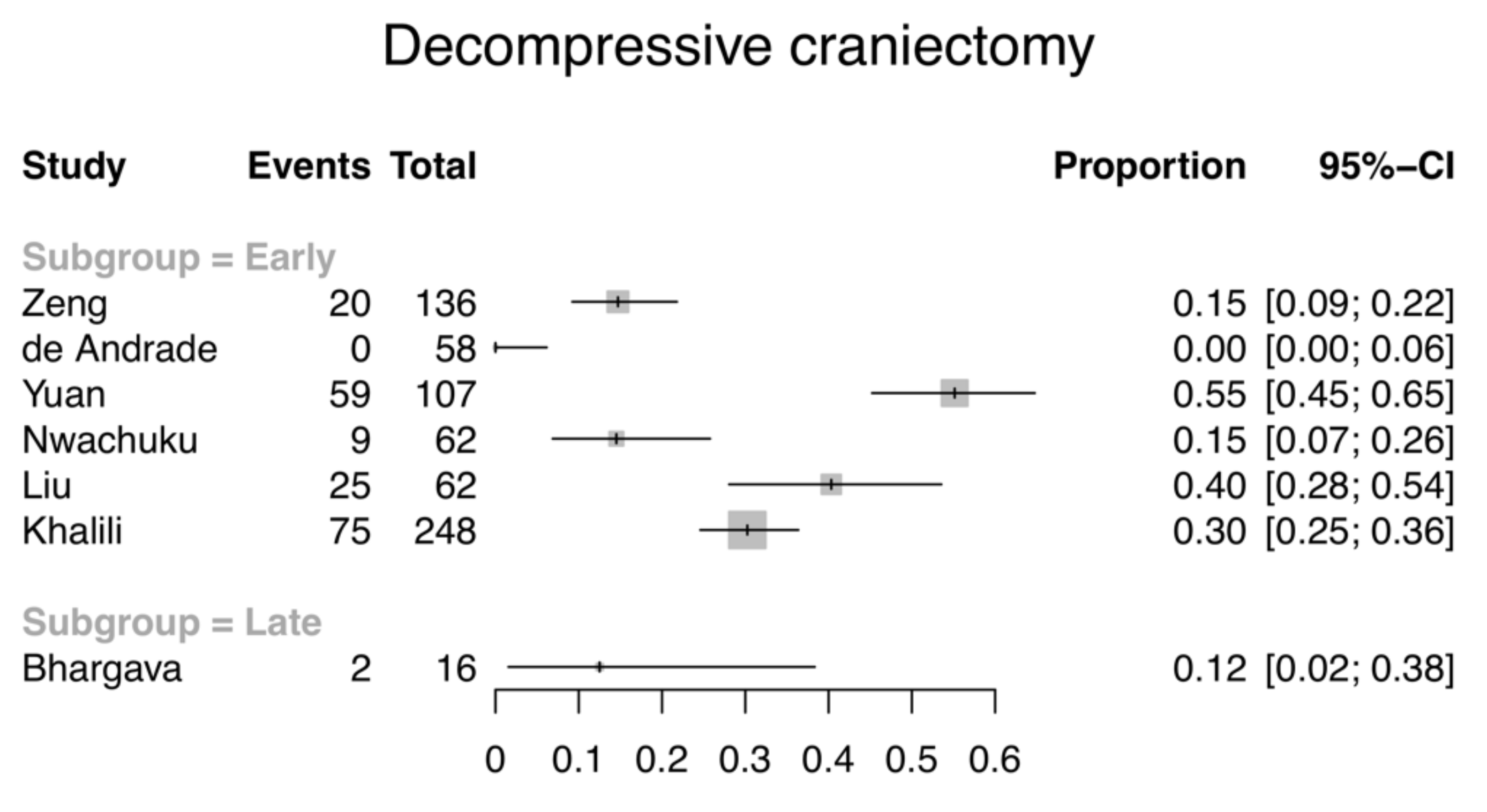
3.3.2. ICP-Lowering Interventions
3.3.3. Length of Stay
3.3.4. Device-Related Complications
3.4. Stratified Analysis of Drainage Strategy
3.5. Risk of Bias Assessment
4. Discussion
4.1. Variation in Reporting of Study Variables
4.1.1. Drainage Strategy
4.1.2. Intracranial Pressure Outcome Reporting
4.2. Comparison with Existing Literature
4.3. Study Limitations
4.4. Deviations from the Protocol
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hutchinson, P.J.; Kolias, A.G.; Czosnyka, M.; Kirkpatrick, P.J.; Pickard, J.D.; Menon, D.K. Intracranial pressure monitoring in severe traumatic brain injury. BMJ Br. Med. J. 2013, 346, f1000. [Google Scholar] [CrossRef]
- Timofeev, I.; Dahyot-Fizelier, C.; Keong, N.; Nortje, J.; Al-Rawi, P.G.; Czosnyka, M.; Menon, D.K.; Kirkpatrick, P.J.; Gupta, A.K.; Hutchinson, P.J. Ventriculostomy for control of raised ICP in acute traumatic brain injury. Acta Neurochir. Suppl. 2008, 102, 99–104. [Google Scholar]
- Lescot, T.; Boroli, F.; Reina, V.; Chauvet, D.; Boch, A.L.; Puybasset, L. Effect of continuous cerebrospinal fluid drainage on therapeutic intensity in severe traumatic brain injury. Neurochirurgie 2012, 58, 235–240. [Google Scholar] [CrossRef]
- Woernle, C.M.; Burkhardt, J.-K.; Bellut, D.; Krayenbuehl, N.; Bertalanffy, H. Do Iatrogenic Factors Bias the Placement of External Ventricular Catheters?—A Single Institute Experience and Review of the Literature. Neurol. Med. Chir. 2011, 51, 180–186. [Google Scholar] [CrossRef] [Green Version]
- Ghajar, J. Intracranial pressure monitoring techniques. New Horiz. 1995, 3, 395–399. [Google Scholar]
- Dey, M.; Stadnik, A.; Riad, F.; Zhang, L.; McBee, N.; Kase, C.; Carhuapoma, J.R.; Ram, M.; Lane, K.; Ostapkovich, N.; et al. Bleeding and Infection With External Ventricular Drainage: A Systematic Review in Comparison to Adjudicated Adverse Events in the Ongoing CLEAR III Trial. Neurosurgery 2015, 76, 291–301. [Google Scholar] [CrossRef] [Green Version]
- Kirmani, A.R.; Sarmast, A.H.; Bhat, A.R. Role of external ventricular drainage in the management of intraventricular hemorrhage; its complications and management. Surg. Neurol. Int. 2015, 6, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, C.; Tummala, R.P. Risk factors for hemorrhage associated with external ventricular drain placement and removal. J. Neurosurg. 2017, 126, 289–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, J.K.; Cha, S.H.; Choi, B.K.; Lee, J.I.; Yun, E.Y.; Choi, C.H. Hemorrhage rates associated with two methods of ventriculostomy: External ventricular drainage vs. ventriculoperitoneal shunt procedure. Neurol. Med. Chir. 2014, 54, 545–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramanan, M.; Lipman, J.; Shorr, A.; Shankar, A. A meta-analysis of ventriculostomy-associated cerebrospinal fluid infections. BMC Infect. Dis. 2015, 15, 3. [Google Scholar] [CrossRef] [Green Version]
- Carney, N.; Totten, A.M.; O’Reilly, C.; Ullman, J.S.; Hawryluk, G.W.J.; Bell, M.J.; Bratton, S.L.; Chesnut, R.; Harris, O.A.; Kissoon, N.; et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery 2017, 80, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Geeraerts, T.; Velly, L.; Abdennour, L.; Asehnoune, K.; Audibert, G.; Bouzat, P.; Bruder, N.; Carrillon, R.; Cottenceau, V.; Cotton, F.; et al. Management of severe traumatic brain injury (first 24hours). Anaesth. Crit. Care Pain Med. 2018, 37, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Zuercher, P.; Groen, J.L.; Aries, M.J.H.; Steyerberg, E.W.; Maas, A.I.R.; Ercole, A.; Menon, D.K. Reliability and Validity of the Therapy Intensity Level Scale: Analysis of Clinimetric Properties of a Novel Approach to Assess Management of Intracranial Pressure in Traumatic Brain Injury. J. Neurotrauma 2016, 33, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, P. Intracranial Pressure Monitoring and Management; Chapter 15; CRC Press: Boca Raton, FL, USA; Taylor and Francis Group: Abingdon, UK, 2016. [Google Scholar]
- Birrer, K.; Hunter, J.; Wisniewski, P.; Semon, G.; Liu-DeRyke, X.; Cress, M.; Farkas, J.; Hirschl, R.; Virgilio Matheus, M. Severe Traumatic Brain Injury Management; The Eastern Association for the Surgery of Trauma—Surgical Critical Care: Chicago, IL, USA, 2017. [Google Scholar]
- Society, N.C. Emergency Neurological Life Support Traumatic Brain Injury Protocol; Neurocritical Care Society: Chicago, IL, USA, 2016. [Google Scholar]
- Cooper, D.J.; Rosenfeld, J.V.; Murray, L.; Arabi, Y.M.; Davies, A.R.; D’Urso, P.; Kossmann, T.; Ponsford, J.; Seppelt, I.; Reilly, P.; et al. Decompressive Craniectomy in Diffuse Traumatic Brain Injury. N. Engl. J. Med. 2011, 364, 1493–1502. [Google Scholar] [CrossRef] [Green Version]
- Cnossen, M.C.; Huijben, J.A.; Jagt, M.V.d.; Volovici, V.; Essen, T.v.; Polinder, S.; Nelson, D.; Ercole, A.; Stocchetti, N.; Citerio, G.; et al. Variation in monitoring and treatment policies for intracranial hypertension in traumatic brain injury: A survey in 66 neurotrauma centers participating in the CENTER-TBI study. Crit. Care 2017, 21, 233. [Google Scholar] [CrossRef]
- Hutchinson, P.J.; Kolias, A.G.; Timofeev, I.S.; Corteen, E.A.; Czosnyka, M.; Timothy, J.; Anderson, I.; Bulters, D.O.; Belli, A.; Eynon, C.A.; et al. Trial of Decompressive Craniectomy for Traumatic Intracranial Hypertension. New Engl. J. Med. 2016, 375, 1119–1130. [Google Scholar] [CrossRef] [Green Version]
- Dinsmore, J. Traumatic brain injury: An evidence-based review of management. Contin. Educ. Anaesth. Crit. Care Pain 2013, 13, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Patel, H.C.; Menon, D.K.; Tebbs, S.; Hawker, R.; Hutchinson, P.J.; Kirkpatrick, P.J. Specialist neurocritical care and outcome from head injury. Intensive Care Med. 2002, 28, 547–553. [Google Scholar] [CrossRef]
- Bhargava, D.; Alalade, A.; Ellamushi, H.; Yeh, J.; Hunter, R. Mitigating effects of external ventricular drain usage in the management of severe head injury. Acta Neurochir. 2013, 155, 2129–2132. [Google Scholar] [CrossRef]
- Maas, A. Mitigating effects of external ventricular drain usage in the management of severe head injury. Acta Neurochir. 2013, 155, 1343–1344. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Group, P.-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- Chau, Y.C.C.; Mediratta, S.; Gregson, B.; Tülü, S.; Kolias, A.; Hutchinson, P. Optimal Timing of External Ventricular Drainage in Traumatic Brain Injury: A Systematic Review and Meta-Analysis; PROSPERO: International Prospective Register of Systematic Reviews; Centre for Reviews and Dissemination, University of York: York, UK, 2019. [Google Scholar]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.P.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing [Computer Software Manual]; R Core Team: Vienna, Austria, 2016. [Google Scholar]
- Balduzzi, S.; Rucker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Evid. Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Rosner, M.J.; Rosner, S.D.; Johnson, A.H. Cerebral perfusion pressure: Management protocol and clinical results. J. Neurosurg. 1995, 83, 949–962. [Google Scholar] [CrossRef]
- Kerr, M.E.; Weber, B.B.; Sereika, S.M.; Wilberger, J.; Marion, D.W. Dose response to cerebrospinal fluid drainage on cerebral perfusion in traumatic brain-injured adults. Neurosurg. Focus 2001, 11, E1. [Google Scholar] [CrossRef]
- Pillai, S.; Praharaj, S.S.; Rao, G.S.U.; Kolluri, V.R.S. Cerebral perfusion pressure management of severe diffuse head injury: Effect on brain compliance and intracranial pressure. Neurol. India 2004, 52, 67–71. [Google Scholar]
- Kinoshita, K.; Sakurai, A.; Utagawa, A.; Ebihara, T.; Furukawa, M.; Moriya, T.; Okuno, K.; Yoshitake, A.; Noda, E.; Tanjoh, K. Importance of cerebral perfusion pressure management using cerebrospinal drainage in severe traumatic brain injury. Acta Neurochir. Suppl. 2006, 96, 37–39. [Google Scholar]
- Griesdale, D.E.G.; McEwen, J.; Kurth, T.; Chittock, D.R. External Ventricular Drains and Mortality in Patients with Severe Traumatic Brain Injury. Can. J. Neurol. Sci. 2010, 37, 43–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, T.; Gao, L. Management of patients with severe traumatic brain injury guided by intraventricular intracranial pressure monitoring: A report of 136 cases. Chin. J. Traumatol. 2010, 13, 146–151. [Google Scholar] [PubMed]
- Dizdarevic, K.; Hamdan, A.; Omerhodzic, I.; Kominlija-Smajic, E. Modified Lund concept versus cerebral perfusion pressure-targeted therapy: A randomised controlled study in patients with secondary brain ischaemia. Clin. Neurol. Neurosurg. 2012, 114, 142–148. [Google Scholar] [CrossRef] [PubMed]
- De Andrade, A.F.; Paiva, W.S.; de Amorim, R.L.O.; Figueiredo, E.G.; de Almeida, A.N.; Brock, R.S.; Bor-Seng-Shu, E.; Teixeira, M.J. Continuous ventricular cerebrospinal fluid drainage with intracranial pressure monitoring for management of posttraumatic diffuse brain swelling. Arq. Neuro Psiquiatr. 2011, 69, 79–84. [Google Scholar] [CrossRef] [Green Version]
- Kasotakis, G.; Michailidou, M.; Bramos, A.; Chang, Y.; Velmahos, G.; Alam, H.; King, D.; de Moya, M.A. Intraparenchymal vs extracranial ventricular drain intracranial pressure monitors in traumatic brain injury: Less is more? J. Am. Coll. Surg. 2012, 214, 950–957. [Google Scholar] [CrossRef]
- Yuan, Q.; Liu, H.; Wu, X.; Sun, Y.; Zhou, L.; Hu, J. Predictive value of initial intracranial pressure for refractory intracranial hypertension in persons with traumatic brain injury: A prospective observational study. Brain Inj. 2013, 27, 664–670. [Google Scholar] [CrossRef]
- Nwachuku, E.L.; Puccio, A.M.; Fetzick, A.; Scruggs, B.; Chang, Y.F.; Shutter, L.A.; Okonkwo, D.O. Intermittent versus continuous cerebrospinal fluid drainage management in adult severe traumatic brain injury: Assessment of intracranial pressure burden. Neurocrit. Care 2014, 20, 49–53. [Google Scholar] [CrossRef]
- Childs, C.; Shen, L. Regional pressure and temperature variations across the injured human brain: Comparisons between paired intraparenchymal and ventricular measurements. Crit. Care 2015, 19, 267. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Wang, W.; Cheng, F.; Yuan, Q.; Yang, J.; Hu, J.; Ren, G. External Ventricular Drains versus Intraparenchymal Intracranial Pressure Monitors in Traumatic Brain Injury: A Prospective Observational Study. World Neurosurg. 2015, 83, 794–800. [Google Scholar] [CrossRef]
- Khalili, H.; Sadraei, N.; Niakan, A.; Ghaffarpasand, F.; Sadraei, A. Role of Intracranial Pressure Monitoring in Management of Patients with Severe Traumatic Brain Injury: Results of a Large Level I Trauma Center in Southern Iran. World Neurosurg. 2016, 94, 120–125. [Google Scholar] [CrossRef]
- Akbik, O.S.; Krasberg, M.; Nemoto, E.M.; Yonas, H. Effect of Cerebrospinal Fluid Drainage on Brain Tissue Oxygenation in Traumatic Brain Injury. J. Neurotrauma 2017, 34, 3153–3157. [Google Scholar] [CrossRef] [PubMed]
- Aiolfi, A.; Khor, D.; Cho, J.; Benjamin, E.; Inaba, K.; Demetriades, D. Intracranial pressure monitoring in severe blunt head trauma: Does the type of monitoring device matter? J. Neurosurg. 2018, 128, 828–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, S.P.; Bruyninckx, D.; Callebaut, I.; Depreitere, B. Comparison of Intracranial Pressure and Pressure Reactivity Index Obtained Through Pressure Measurements in the Ventricle and in the Parenchyma During and Outside Cerebrospinal Fluid Drainage Episodes in a Manipulation-Free Patient Setting. Acta Neurochir. Suppl. 2018, 126, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Bales, J.W.; Bonow, R.H.; Buckley, R.T.; Barber, J.; Temkin, N.; Chesnut, R.M. Primary External Ventricular Drainage Catheter Versus Intraparenchymal ICP Monitoring: Outcome Analysis. Neurocrit. Care 2019, 31, 11–21. [Google Scholar] [CrossRef] [PubMed]
- McMillan, T.M.; Weir, C.J.; Ireland, A.; Stewart, E. The Glasgow Outcome at Discharge Scale: An inpatient assessment of disability after brain injury. J. Neurotrauma 2013, 30, 970–974. [Google Scholar] [CrossRef] [Green Version]
- Hawryluk, G.W.J.; Aguilera, S.; Buki, A.; Bulger, E.; Citerio, G.; Cooper, D.J.; Arrastia, R.D.; Diringer, M.; Figaji, A.; Gao, G.; et al. A management algorithm for patients with intracranial pressure monitoring: The Seattle International Severe Traumatic Brain Injury Consensus Conference (SIBICC). Intensive Care Med. 2019, 45, 1783–1794. [Google Scholar] [CrossRef] [Green Version]
- Maas, A.I.; Harrison-Felix, C.L.; Menon, D.; Adelson, P.D.; Balkin, T.; Bullock, R.; Engel, D.C.; Gordon, W.; Langlois-Orman, J.; Lew, H.L.; et al. Standardizing data collection in traumatic brain injury. J. Neurotrauma 2011, 28, 177–187. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.S.; Amato, A.; James, M.L.; Britz, G.W.; Zomorodi, A.; Graffagnino, C.; Zomorodi, M.; Olson, D.M. Continuous and Intermittent CSF Diversion after Subarachnoid Hemorrhage: A Pilot Study. Neurocrit. Care 2011, 14, 68–72. [Google Scholar] [CrossRef]
- Volovici, V.; Huijben, J.A.; Ercole, A.; Stocchetti, N.; Dirven, C.M.F.; van der Jagt, M.; Steyerberg, E.W.; Lingsma, H.; Menon, D.; Maas, A.; et al. Ventricular drainage catheters versus intracranial parenchymal catheters for intracranial pressure monitoring-based management of traumatic brain injury: A systematic review and meta-analysis. J. Neurotrauma 2018, 36, 988–995. [Google Scholar] [CrossRef]
| Defining Domains | Timing of EVD | |
|---|---|---|
| Early | Late | |
| Time of EVD Insertion | EITHER
| Inserted at a later stage after IPM insertion |
| Tier/step of CSF drainage in a tiered/stepwise ICP management protocol | First tier/step | Second tier/step or later |
| Study | Study Type | Country | sTBI Patients | Mean Age | Gender (M:F) | Timing by Tier | Drainage Strategy | ||
|---|---|---|---|---|---|---|---|---|---|
| Total (N) | EVD (n) | CSF (n) | |||||||
| Rosner, 1995 [32] | PS | USA | 158 | 158 (100%) | NR | 27.9 | 117:41 | Early | I |
| Kerr, 2001 [33] | RCT | USA | 58 | 58 (100%) | 58 | 31.6 | 45:13 | Early | I |
| Pillai, 2004 [34] | PS | India | 27 | 27 (100%) | 25 | 31 | NR | Early | I |
| Kinoshita, 2006 [35] | NRS | Japan | 26 | 12 (46.2%) | 12 | 55.3 | NR | Early | I |
| Timofeev, 2008 [2] | PS | UK | 24 | 24 (100%) | 24 | 41 | 18:6 | Early | C |
| Griesdale, 2010 [36] | RS | Canada | 171 | 98 (57.3%) | NR | 35 | 77:21 | Early | I |
| Zeng, 2010 [37] | RS | China | 136 | 136 (100%) | 136 | 44.8 | 91:45 | Early | I |
| Dizdarevic, 2012 [38] | RCT | BIH | 15 | 15 (100%) | 15 | 43 | 12:3 | Early | I |
| de Andrade, 2011 [39] | PS | Brazil | 58 | 58 (100%) | 58 | 29 | 48:10 | Early | I |
| Kasotakis, 2012 [40] | RS | USA | 378 | 119 (31.5%) | NR | 48.7 | NS | Early | I |
| Yuan, 2013 [41] | PS | China | 107 | 107 (100%) | NR | 49.1 | 79:28 | Early | I |
| Nwachuku, 2014 [42] | RS | USA | 62 | 62 (100%) | 62 | 34.7 | 42:20 | Early | C (n = 31); I (n = 31) |
| Childs, 2015 [43] | PS | UK | 17 | 17 (100%) | 17 | Median: 47 | 12:5 | Early | NR |
| Liu, 2015 [44] | PS | China | 62 | 62 (100%) | NR | 41.7 | 50:12 | Early | I |
| Khalili, 2016 [45] | PS | Iran | 248 | 248 (100%) | NR | 34.6 | 216:32 | Early | I |
| Akbik, 2017 [46] | RS | USA | 40 | 40 (100%) | 40 | 39 | 30:10 | Early | I |
| Aiolfi, 2018 [47] | RS | USA | 2562 | 1358 (53%) | NR | Median: 52 | 1013:345 | Early | NR |
| Klein, 2018 [48] | PS | Belgium | 10 | 10 (100%) | 10 | 51.9 | 8:2 | Early | I |
| Bales, 2019 [49] | PS | USA | 224 | 101 (45%) | 86 | 33.6 | 74:27 | Early | NR |
| Lescot, 2012 [3] | RS | France | 20 | 20 (100%) | 20 | 46.8 | 14:6 | Late | C |
| Bhargava, 2013 [22] | RS | UK | 139 | 16 (100%) | 16 | 24 | 13:3 | Late | NR |
| Study | Timing by Tier | Guidelines | ICP Monitoring | CSF Drainage Step/Tier | CSF Drainage Details |
|---|---|---|---|---|---|
| Rosner, 1995 [32] | Early | NR | EVD ± subdural | First step | Whenever CPP <70 mm Hg; Drain as needed: “pop-off” at 15 mmHg |
| Kerr, 2001 [33] | Early | BTF (1996) | EVD | First step (ICP >20 mm Hg) | CSF drained in random order: 1 mL (16 drops), 2 mL (32 drops), 3 mL (48 drops) |
| Pillai, 2004 [34] | Early | NR | EVD | First step (in the three-step therapeutic ladder) | NR |
| Kinoshita, 2006 [35] | Early | BTF (1996) | EVD | First step (of CPP management therapy) | NR |
| Timofeev, 2008 [2] | Early | Institutional | IPM | First tier (when ICP failed to maintain <20 mmHg and CPP >60–70 mmHg despite initial measures) | Continuous free drainage of CSF was allowed, limited only by the height of the collecting reservoir (≈15 mmHg above the external projection of foramen of Monro) |
| Griesdale, 2010 [36] | Early | Institutional | EVD | First step | If ICP >20 mmHg for >5 min without stimulation: EVD opened to 26 cm H2O; EVD closed every hour to check ICP |
| Zeng, 2010 [37] | Early | NR | EVD | First step | Monitoring with persistent intraventricular drainage; volume drained: 30–300 mL/d |
| Dizdarevic, 2012 [38] | Early | AANS (2004) | EVD | First step (when ICP >15–20 mm Hg) | NR |
| de Andrade, 2011 [39] | Early | BTF (1996) | EVD | First step | EVD kept open for 45 min with continuous drainage for 15 min if ICP overcame calibration value (10 mm Hg over foramen of Monro); EVD closed every hr to monitor ICP |
| Kasotakis, 2012 [40] | Early | NR | EVD | First step | NR |
| Yuan, 2013 [41] | Early | BTF (2007) | EVD | First tier | If ventricular pressure >20 mm Hg; Intermittent (5 min drainage) to remove the smallest volume of fluid necessary to control ICP in the shortest time |
| Nwachuku, 2014 [42] | Early | Institutional | Continuous group (n = 31): IPM Intermittent group (n = 31): EVD | First tier (when ICP > 20 mmHg for ≥5 min) | Intermittent: amount drained was variable based on individual needs to target ICP |
| Childs, 2015 [43] | Early | NR | IPM + EVD | First step | NR |
| Liu, 2015 [44] | Early | BTF (2007) | EVD | First tier | If ventricular pressure >20 mm Hg; intermittent (5 min drainage) to remove the smallest volume of fluid necessary to control ICP in the shortest time |
| Khalili, 2016 [45] | Early | Virginia stepwise ICP control | EVD | First tier | NR |
| Akbik, 2017 [46] | Early | NR | IPM + EVD | First tier | If ICP >20 mm Hg for >10 min, EVD opened to drain for 10 min and re-clamped; If ICP remains >20 mm Hg, EVD kept open at 20 cm H2O with ICP (IPM) recorded continuously and ICP (EVD) checked hourly |
| Aiolfi, 2018 [47] | Early | NR | EVD | First step | NR |
| Klein, 2018 [48] | Early | Institutional | IPM + EVD | First step | 30 min of drainage (O1), 30 min EVD closed (C), and 30 min of drainage (O2) |
| Bales, 2019 [49] | Early | AANS | EVD | First step | NR |
| Lescot, 2012 [3] | Late | Institutional | IPM | Second-line (persistent ICP elevation > 20 mm Hg after exclusion of new surgical lesions by a repeat CT scan) | Continuous CSF drainage via EVD placed 10 cm above the external acoustic meatus. |
| Bhargava, 2013 [22] | Late | BTF (2007) | IPM | Last tier, comparing with DC/BC (definitive measures for ICP control) | NR |
| Author, Year | EVD | ICP Control Description | Results |
|---|---|---|---|
| A. Change in ICP before and after CSF drainage | |||
| Kerr, 2001 [33] | E; I | - Mean ICP value at baseline, 1 min, 5 min, 10 min following drainage - Decrease in ICP from baseline at various timepoints after drainage | - 1 mL CSF drained: −2.4 (1 min), −1 (10 min) mmHg * - 2 mL CSF drained: −3.4 (1 min), −1.7 (10 min) mmHg * - 3 mL CSF drained: −4.5 (1 min), −2.6 (10 min) mmHg * * values represented relative to baseline |
| Timofeev, 2008 [2] | E; C | Mean ICP before (≥24 h prior) and after (≥24 h) EVD | Pooled mean daily values of ICP remained <20 mmHg for at least 72 h after ventriculostomy and were significantly lower than before the procedure (p < 0.001). |
| Lescot, 2012 [3] | L; C | Mean ICP before (12 h, 24 h prior) and after (12 h, 24 h) EVD | Mean ICP before EVD: 18 ± 6 (24 h), 19 ± 7 (12 h) mmHg Mean ICP after EVD: 11 ± 5 (12 h), 12 ± 7 (24 h) mmHg Significant reduction in ICP (p < 0.05) |
| Akbik, 2017 [46] | E; I | Mean ICP change before and after EVD opening (4 min) | ICP decreased by 5.7 ± 0.6 mmHg |
| Klein, 2018 [48] | E; I | Mean ICP change before and after EVD (30 min) | Mean decrease after opening EVD: 2.12 ± 6.23 mmHg (p < 0.001) |
| B. ICP burden | |||
| Nwachuku, 2014 [42] | E; C + I | Area under the ICP curve (amount of time with ICP > 20 mmHg) | Patients with intermittent drainage had significantly higher ICP burden than continuous drainage (59.7 ± 72.9 vs. 17.2 ± 36.8; p = 0.0002). |
| C. ICP amplitude | |||
| Klein, 2018 [48] | E; I | Mean change in ICP amplitude (AMP) before and after CSF drainage | Significant reduction of amplitude of ICP signal |
| D. Number of patients with normal/raised ICP values after CSF drainage | |||
| Bhargava, 2013 [22] | L; NR | - Number of patients with sustained control of ICP (ICP values not specified) - Number of patients with further elevation of ICP (ICP values not specified) | - Sustained control of ICP in 14 patients (87.5%) - Further elevation of ICP in 2 patients (12.5%) |
| EVD-Related Complications | Number of Patients | |
|---|---|---|
| Early EVD | Late EVD | |
| Infection | 88 (12.8%) | 3 (8.3%) |
| Haemorrhage | 6 (1.5%) | NR |
| Device Failure | 27 (14.9%) | NR |
| Malposition | 12 (10.1%) | NR |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chau, C.Y.C.; Mediratta, S.; McKie, M.A.; Gregson, B.; Tulu, S.; Ercole, A.; Solla, D.J.F.; Paiva, W.S.; Hutchinson, P.J.; Kolias, A.G. Optimal Timing of External Ventricular Drainage after Severe Traumatic Brain Injury: A Systematic Review. J. Clin. Med. 2020, 9, 1996. https://doi.org/10.3390/jcm9061996
Chau CYC, Mediratta S, McKie MA, Gregson B, Tulu S, Ercole A, Solla DJF, Paiva WS, Hutchinson PJ, Kolias AG. Optimal Timing of External Ventricular Drainage after Severe Traumatic Brain Injury: A Systematic Review. Journal of Clinical Medicine. 2020; 9(6):1996. https://doi.org/10.3390/jcm9061996
Chicago/Turabian StyleChau, Charlene Y. C., Saniya Mediratta, Mikel A. McKie, Barbara Gregson, Selma Tulu, Ari Ercole, Davi J. F. Solla, Wellingson S. Paiva, Peter J. Hutchinson, and Angelos G. Kolias. 2020. "Optimal Timing of External Ventricular Drainage after Severe Traumatic Brain Injury: A Systematic Review" Journal of Clinical Medicine 9, no. 6: 1996. https://doi.org/10.3390/jcm9061996






