Differential Role of CD318 in Tumor Immunity Affecting Prognosis in Colorectal Cancer Compared to Other Adenocarcinomas
Abstract
1. Introduction
2. Materials and Methods
2.1. STRING
2.2. GEPIA2
2.3. Human Protein Atlas (HPA)
2.4. TIMER 2.0
2.5. TISIDB
2.6. ClusPro
2.7. Data Availability
2.8. Statistical Significance
3. Results
3.1. Involvement of CD318 in Biological Pathways
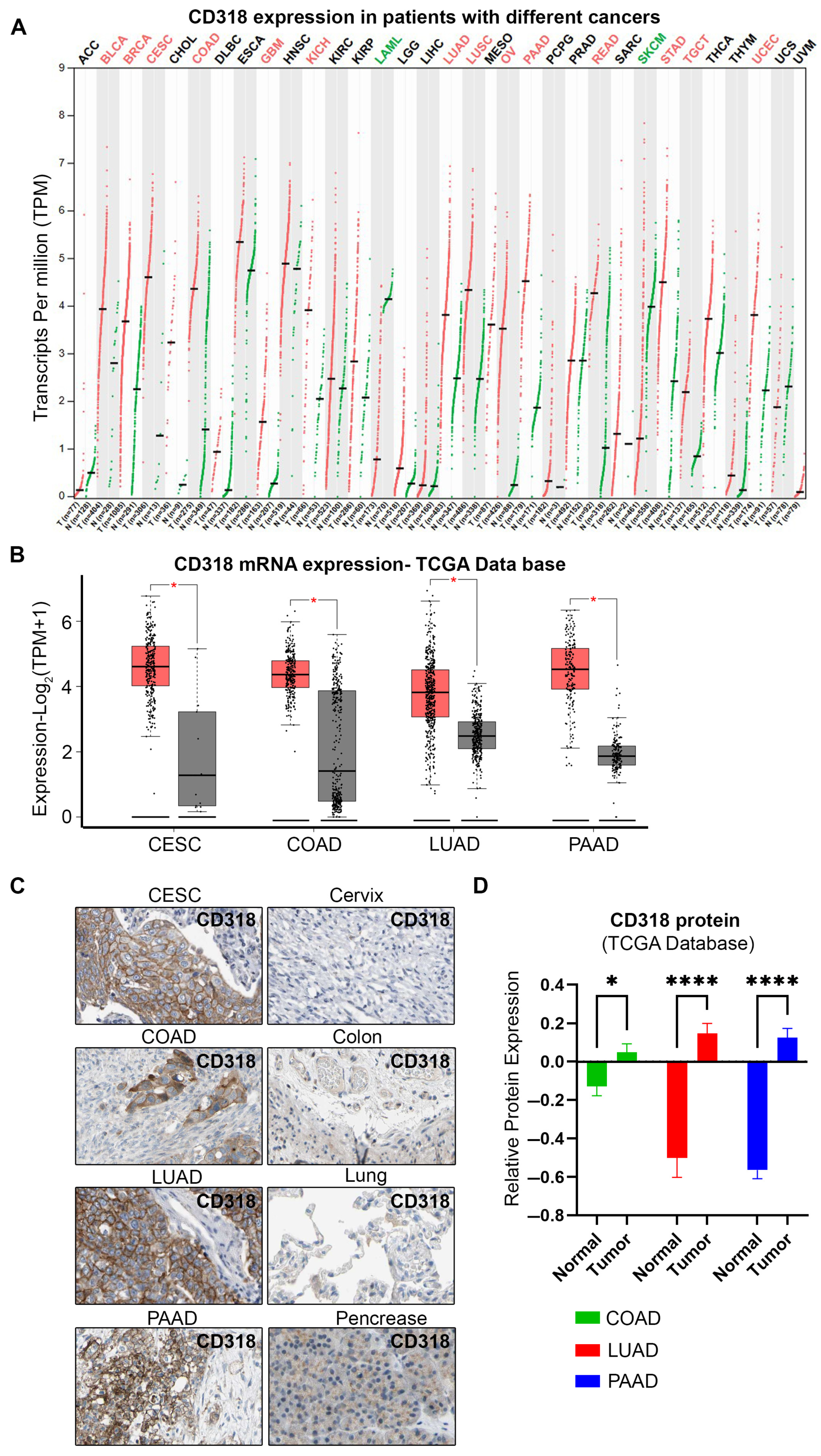
3.2. High Expression of CD318 in Multiple Cancers
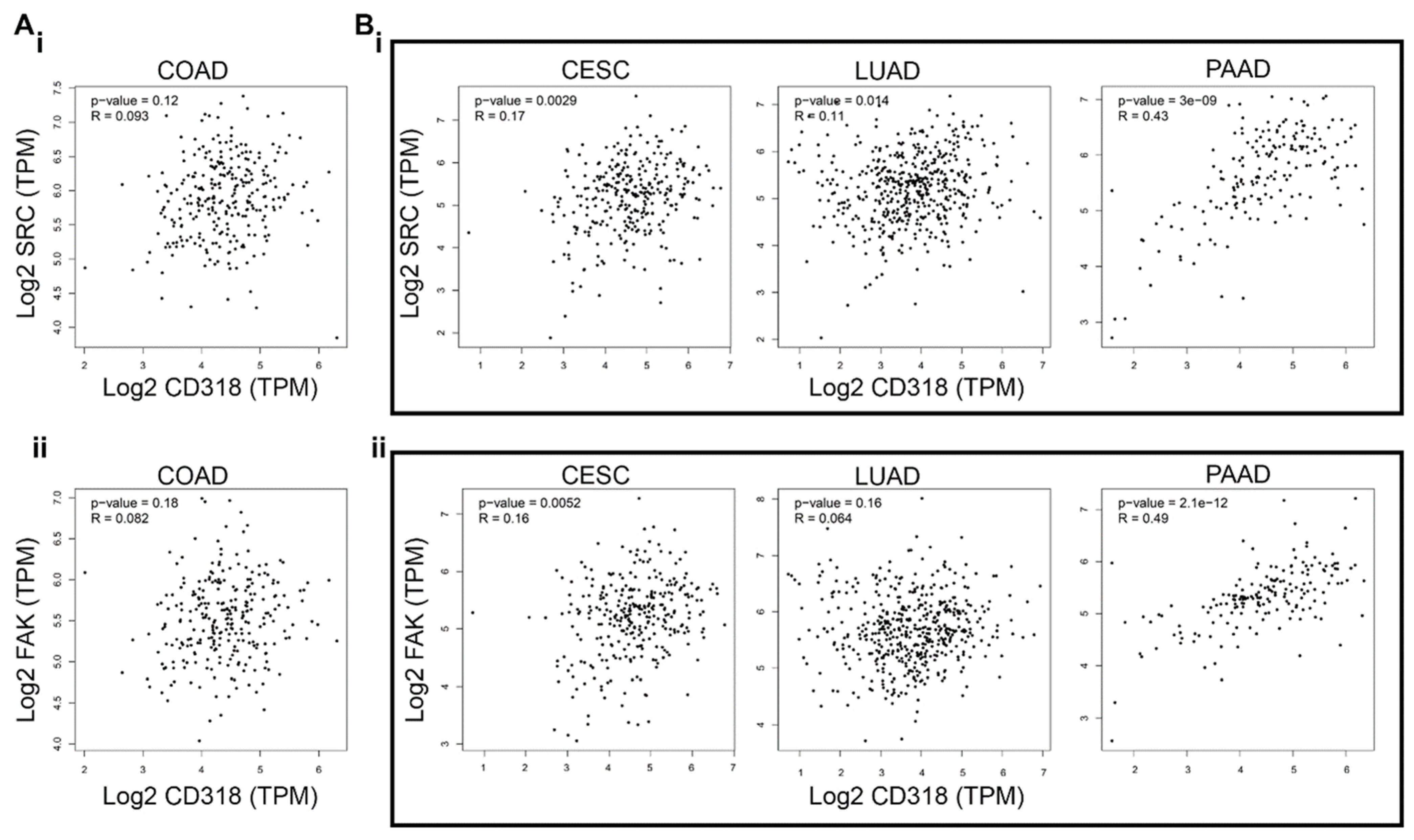
3.3. CD318 Is Associated with SRC and FAK Proteins in CESC, LUAD, and PAAD but Not in COAD
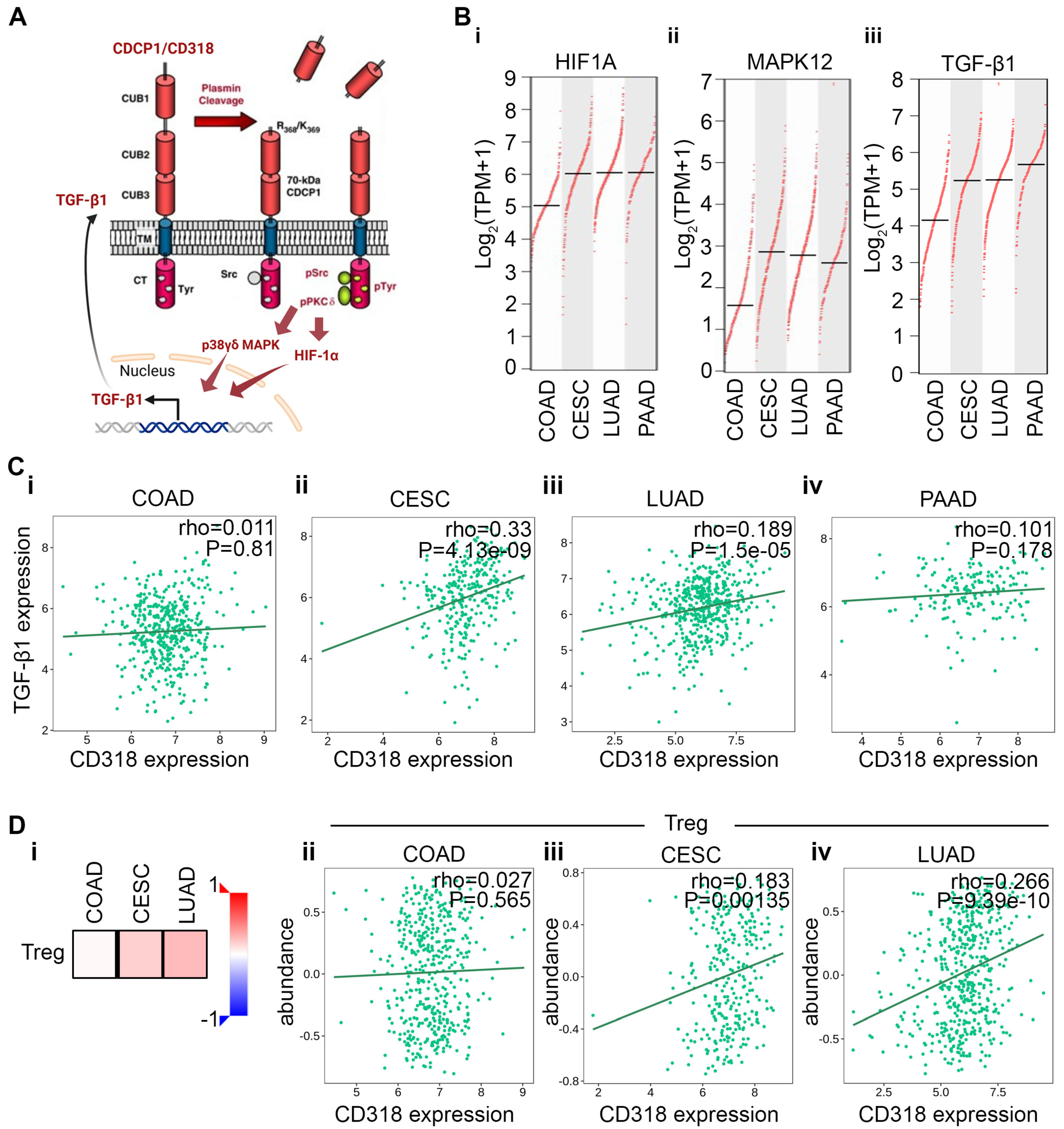
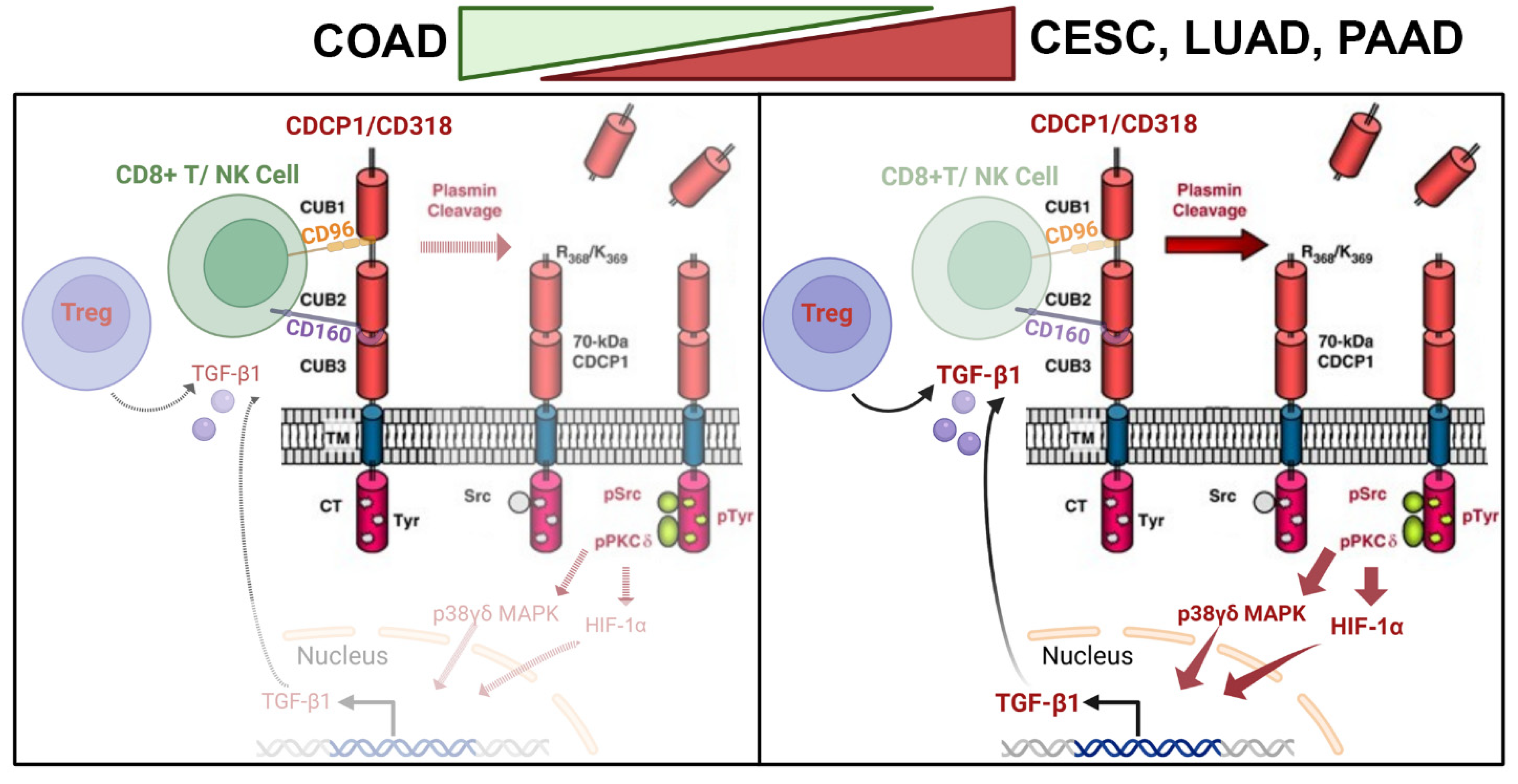
3.4. Differential Immune Suppression Through PKCδ/HIF-1α/TGFβ1 Axis in CESC, LUAD, and PAAD Compared to COAD
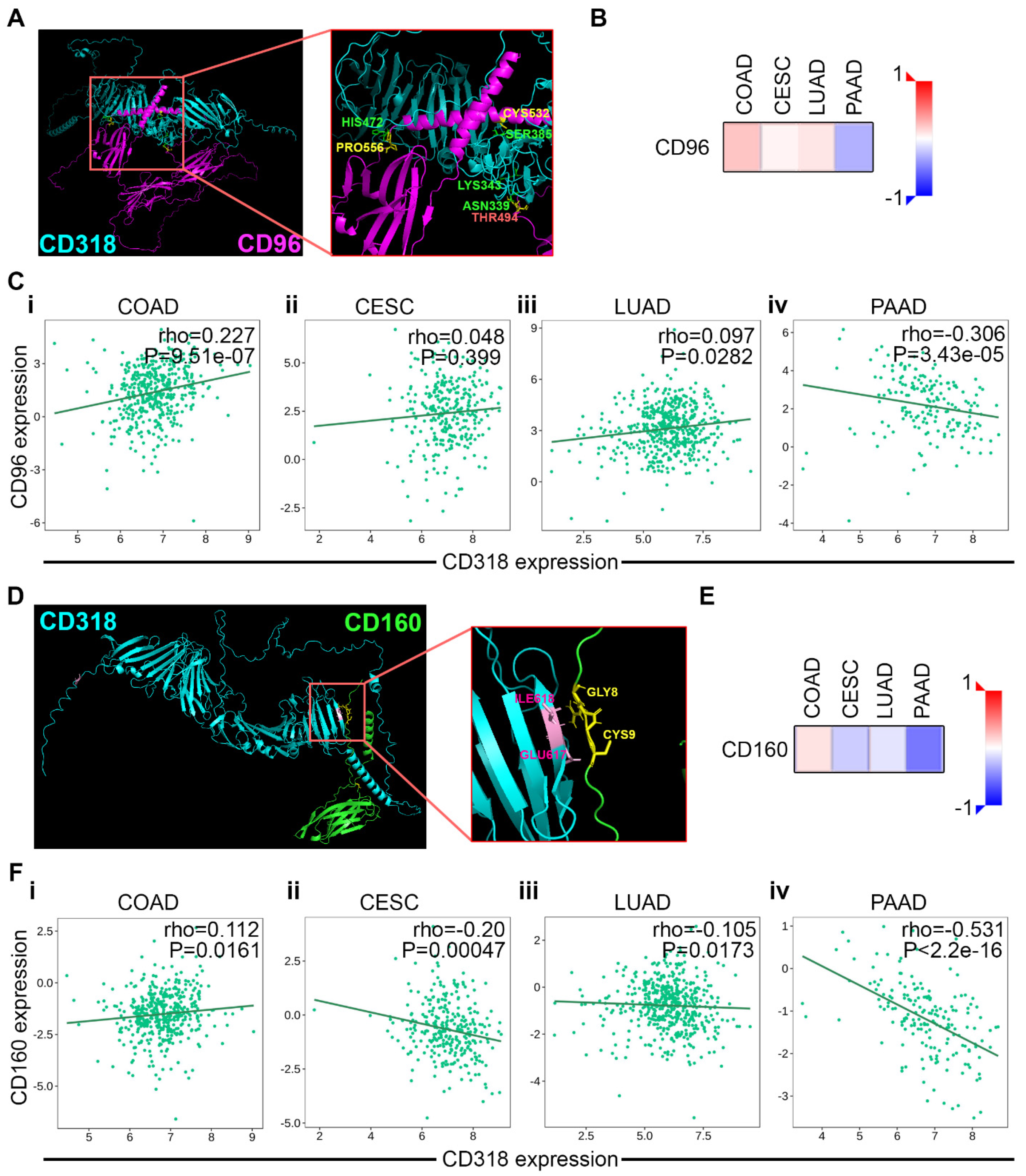
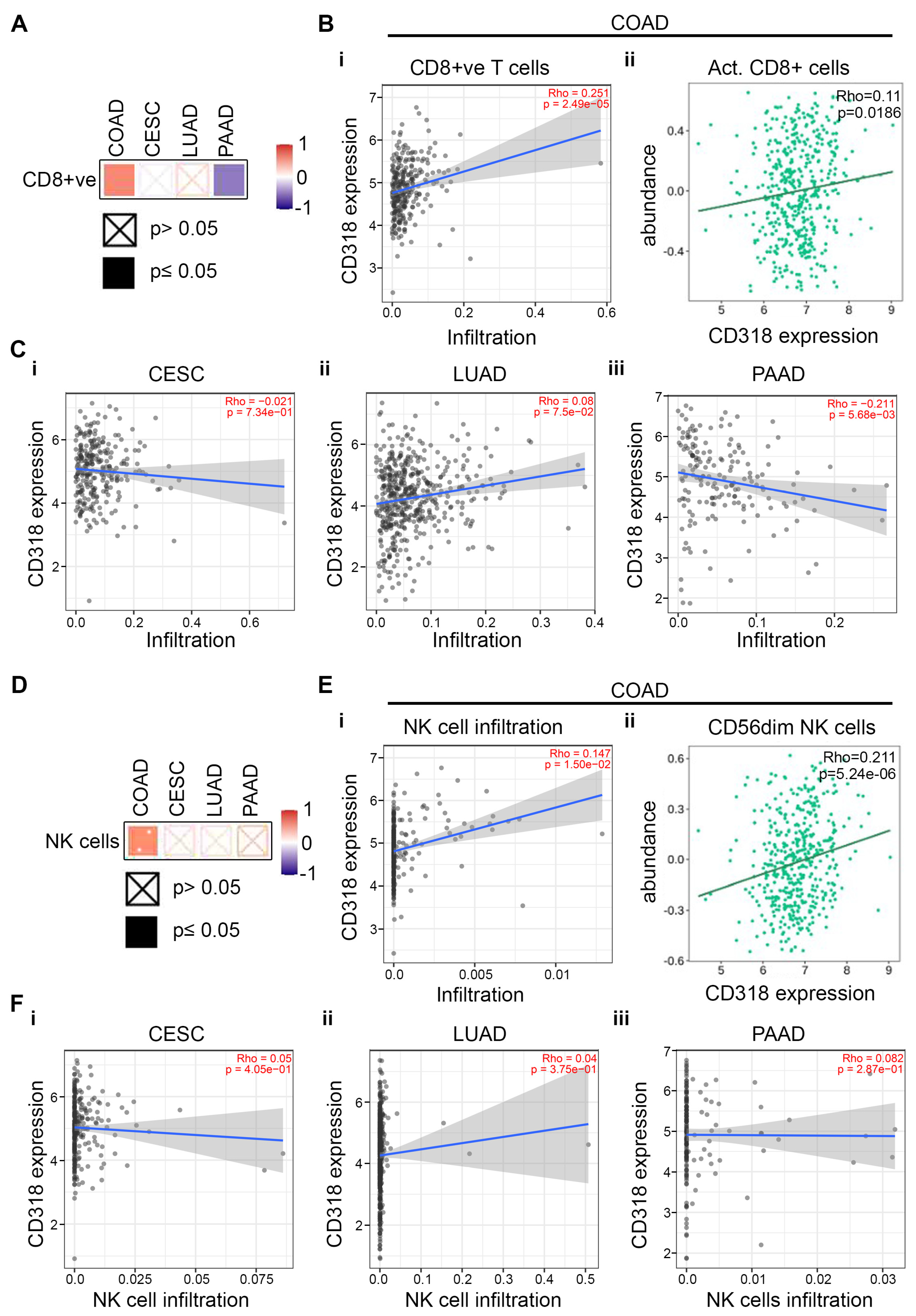
3.5. T and NK Cell-Mediated Cytotoxicity Through CD160 and CD96 Association and Interaction of CD318 in COAD Cancer
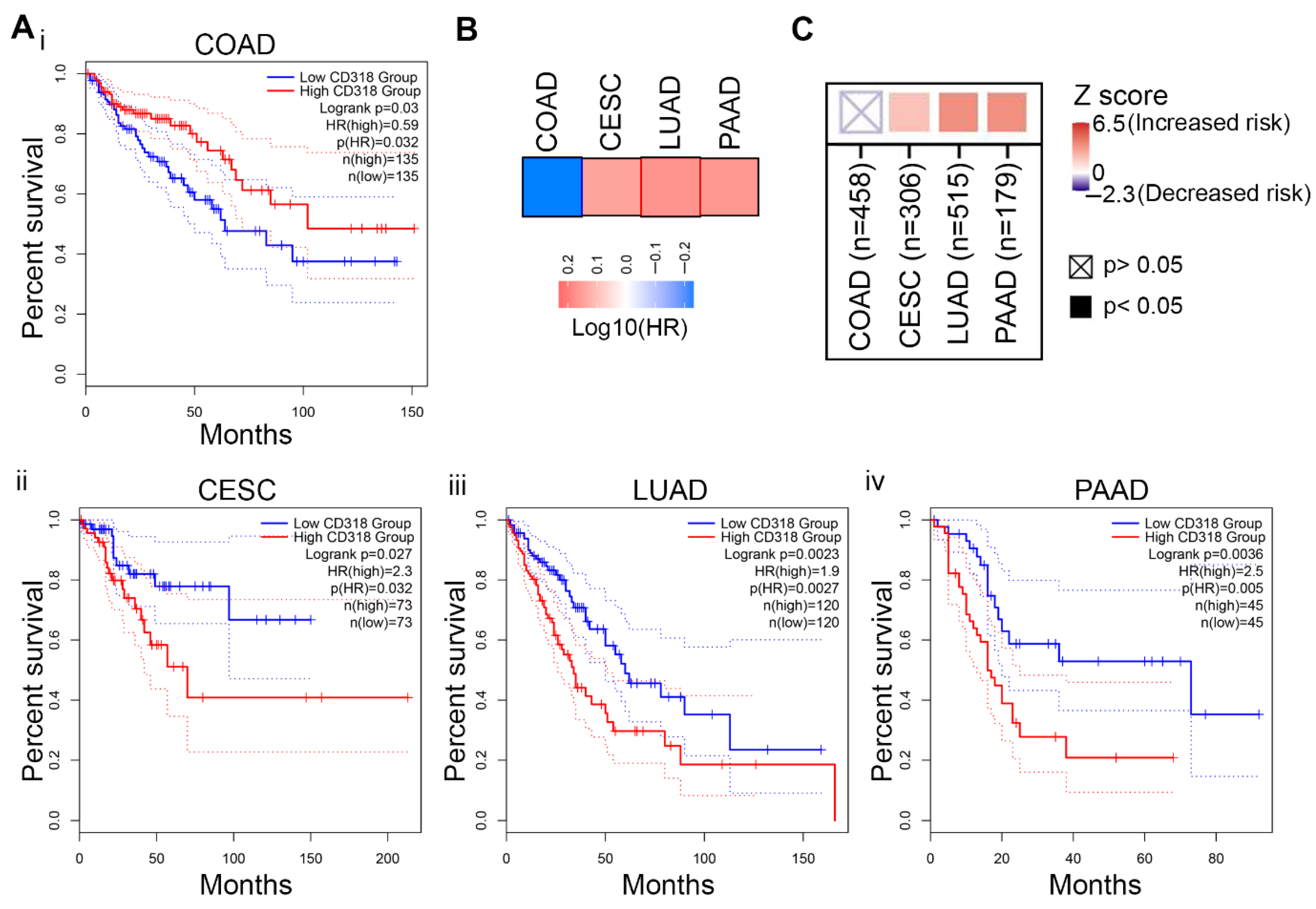
3.6. Difference in Immune Response Affects the Prognostic Significance of CD318 in COAD Compared to CESC, LUAD, and PAAD
4. Discussion
5. Conclusions
Future Directions and Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scherl-Mostageer, M.; Sommergruber, W.; Abseher, R.; Hauptmann, R.; Ambros, P.; Schweifer, N. Identification of a novel gene, CD318, overexpressed in human colorectal cancer. Oncogene 2001, 20, 4402–4408. [Google Scholar] [CrossRef]
- Liu, H.; Ong, S.E.; Badu-Nkansah, K.; Schindler, J.; White, F.M.; Hynes, R.O. CUB-domain-containing protein 1 (CD318) activates Src to promote melanoma metastasis. Proc. Natl. Acad. Sci. USA 2011, 108, 1379–1384. [Google Scholar] [CrossRef]
- Casar, B.; Rimann, I.; Kato, H.; Shattil, S.J.; Quigley, J.P.; Deryugina, E.I. In vivo cleaved CD318 promotes early tumor dissemination via complexing with activated β1 integrin and induction of FAK/PI3K/Akt motility signaling. Oncogene 2014, 33, 255–268. [Google Scholar] [CrossRef]
- Spassov, D.S.; Baehner, F.L.; Wong, C.H.; McDonough, S.; Moasser, M.M. The transmembrane src substrate Trask is an epithelial protein that signals during anchorage deprivation. Am. J. Pathol. 2009, 174, 1756–1765. [Google Scholar] [CrossRef][Green Version]
- Conze, T.; Lammers, R.; Kuci, S.; Scherl-Mostageer, M.; Schweifer, N.; Kanz, L.; Buhring, H.J. CD318 is a novel marker for hematopoietic stem cells. Ann. N. Y. Acad. Sci. 2003, 996, 222–226. [Google Scholar] [CrossRef]
- Buhring, H.J.; Kuci, S.; Conze, T.; Rathke, G.; Bartolovic, K.; Grunebach, F.; Scherl-Mostageer, M.; Brummendorf, T.H.; Schweifer, N.; Lammers, R. CD318 identifies a broad spectrum of normal and malignant stem/progenitor cell subsets of hematopoietic and nonhematopoietic origin. Stem Cells 2004, 22, 334–343. [Google Scholar] [CrossRef]
- Uekita, T.; Sakai, R. Roles of CUB domain-containing protein 1 signaling in cancer invasion and metastasis. Cancer Sci. 2011, 102, 1943–1948. [Google Scholar] [CrossRef]
- Wong, C.H.; Baehner, F.L.; Spassov, D.S.; Ahuja, D.; Wang, D.; Hann, B.; Blair, J.; Shokat, K.; Welm, A.L.; Moasser, M.M. Phosphorylation of the SRC epithelial substrate Trask is tightly regulated in normal epithelia but widespread in many human epithelial cancers. Clin. Cancer Res. 2009, 15, 2311–2322. [Google Scholar] [CrossRef]
- Ruth, J.H.; Gurrea-Rubio, M.; Athukorala, K.S.; Rasmussen, S.M.; Weber, D.P.; Randon, P.M.; Gedert, R.J.; Lind, M.E.; Amin, M.A.; Campbell, P.L.; et al. CD6 is a target for cancer immunotherapy. JCI Insight 2021, 6, e145662. [Google Scholar] [CrossRef]
- Enyindah-Asonye, G.; Li, Y.; Ruth, J.H.; Spassov, D.S.; Hebron, K.E.; Zijlstra, A.; Moasser, M.M.; Wang, B.; Singer, N.G.; Cui, H.; et al. CD318 is a ligand for CD6. Proc. Natl. Acad. Sci. USA 2017, 114, E6912–E6921. [Google Scholar] [CrossRef]
- Do, J.S.; Arribas-Layton, D.; Juan, J.; Garcia, I.; Saraswathy, S.; Qi, M.; Montero, E.; Reijonen, H. The CD318/CD6 axis limits type 1 diabetes islet autoantigen-specific human T cell activation. J. Autoimmun. 2024, 146, 103228. [Google Scholar] [CrossRef]
- Gonzalez Munoz, C.; Alvarez Arzola, R.; Calvo Perez, A.; Frometa Campanon, M.C.; Hernandez Casana, P.; Fernandez-Calienes Valdes, A.; Lorenzo-Luaces, P.; Mazorra Herrera, Z.; Crombet Ramos, T.; Labrada Mon, M. Itolizumab regulates activating and inhibitory signals on effector cells, improving their cytotoxicity against CD318+ tumor cell lines. Front. Immunol. 2025, 16, 1585597. [Google Scholar] [CrossRef]
- Uhlen, M.; Zhang, C.; Lee, S.; Sjostedt, E.; Fagerberg, L.; Bidkhori, G.; Benfeitas, R.; Arif, M.; Liu, Z.; Edfors, F.; et al. A pathology atlas of the human cancer transcriptome. Science 2017, 357, eaan2507. [Google Scholar] [CrossRef]
- Kawase, N.; Sugihara, A.; Kajiwara, K.; Hiroshima, M.; Akamatsu, K.; Nada, S.; Matsumoto, K.; Ueda, M.; Okada, M. SRC kinase activator CD318 promotes hepatocyte growth factor-induced cell migration/invasion of a subset of breast cancer cells. J. Biol. Chem. 2022, 298, 101630. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Kusakabe, D.; Watari, K.; Kawahara, A.; Azuma, K.; Akiba, J.; Taniguchi, M.; Kuwano, M.; Ono, M. AXL/CD318/SRC axis confers acquired resistance to osimertinib in lung cancer. Sci. Rep. 2022, 12, 8983. [Google Scholar] [CrossRef] [PubMed]
- Bolos, V.; Gasent, J.M.; Lopez-Tarruella, S.; Grande, E. The dual kinase complex FAK-Src as a promising therapeutic target in cancer. OncoTargets Ther. 2010, 3, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Leroy, C.; Shen, Q.; Strande, V.; Meyer, R.; McLaughlin, M.E.; Lezan, E.; Bentires-Alj, M.; Voshol, H.; Bonenfant, D.; Alex Gaither, L. CUB-domain-containing protein 1 overexpression in solid cancers promotes cancer cell growth by activating Src family kinases. Oncogene 2015, 34, 5593–5598. [Google Scholar] [CrossRef][Green Version]
- Miyazawa, Y.; Uekita, T.; Hiraoka, N.; Fujii, S.; Kosuge, T.; Kanai, Y.; Nojima, Y.; Sakai, R. CUB domain-containing protein 1, a prognostic factor for human pancreatic cancers, promotes cell migration and extracellular matrix degradation. Cancer Res. 2010, 70, 5136–5146. [Google Scholar] [CrossRef]
- Miura, S.; Hamada, S.; Masamune, A.; Satoh, K.; Shimosegawa, T. CUB-domain containing protein 1 represses the epithelial phenotype of pancreatic cancer cells. Exp. Cell Res. 2014, 321, 209–218. [Google Scholar] [CrossRef]
- Qi, X.; Li, Z.; Zhang, J.; Li, H.; Zhang, G.; Li, M.; Li, B.; Fu, Y.; Cai, M.; Wang, H.; et al. Mechanistic insights into CD318 clustering on non-small-cell lung cancer membranes revealed by super-resolution fluorescent imaging. iScience 2023, 26, 106103. [Google Scholar] [CrossRef]
- Dagnino, S.; Bodinier, B.; Guida, F.; Smith-Byrne, K.; Petrovic, D.; Whitaker, M.D.; Haugdahl Nost, T.; Agnoli, C.; Palli, D.; Sacerdote, C.; et al. Prospective Identification of Elevated Circulating CD318 in Patients Years before Onset of Lung Cancer. Cancer Res. 2021, 81, 3738–3748. [Google Scholar] [CrossRef]
- Nakashima, K.; Uekita, T.; Yano, S.; Kikuchi, J.I.; Nakanishi, R.; Sakamoto, N.; Fukumoto, K.; Nomoto, A.; Kawamoto, K.; Shibahara, T.; et al. Novel small molecule inhibiting CD318-PKCdelta pathway reduces tumor metastasis and proliferation. Cancer Sci. 2017, 108, 1049–1057. [Google Scholar] [CrossRef]
- Xia, J.; Ozaki, I.; Matsuhashi, S.; Kuwashiro, T.; Takahashi, H.; Anzai, K.; Mizuta, T. Mechanisms of PKC-Mediated Enhancement of HIF-1α Activity and its Inhibition by Vitamin K2 in Hepatocellular Carcinoma Cells. Int. J. Mol. Sci. 2019, 20, 1022. [Google Scholar] [CrossRef]
- Selno, A.T.H.; Schlichtner, S.; Yasinska, I.M.; Sakhnevych, S.S.; Fiedler, W.; Wellbrock, J.; Klenova, E.; Pavlova, L.; Gibbs, B.F.; Degen, M.; et al. Transforming growth factor beta type 1 (TGF-beta) and hypoxia-inducible factor 1 (HIF-1) transcription complex as master regulators of the immunosuppressive protein galectin-9 expression in human cancer and embryonic cells. Aging 2020, 12, 23478–23496. [Google Scholar] [CrossRef]
- Ueno, M.; Maeno, T.; Nomura, M.; Aoyagi-Ikeda, K.; Matsui, H.; Hara, K.; Tanaka, T.; Iso, T.; Suga, T.; Kurabayashi, M. Hypoxia-inducible factor-1alpha mediates TGF-beta-induced PAI-1 production in alveolar macrophages in pulmonary fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 2011, 300, L740–L752. [Google Scholar] [CrossRef]
- Mingyuan, X.; Qianqian, P.; Shengquan, X.; Chenyi, Y.; Rui, L.; Yichen, S.; Jinghong, X. Hypoxia-inducible factor-1alpha activates transforming growth factor-beta1/Smad signaling and increases collagen deposition in dermal fibroblasts. Oncotarget 2018, 9, 3188–3197. [Google Scholar] [CrossRef]
- Kim, B.G.; Malek, E.; Choi, S.H.; Ignatz-Hoover, J.J.; Driscoll, J.J. Novel therapies emerging in oncology to target the TGF-beta pathway. J. Hematol. Oncol. 2021, 14, 55. [Google Scholar] [CrossRef]
- Patel, B.; Silwal, A.; Eltokhy, M.A.; Gaikwad, S.; Curcic, M.; Patel, J.; Prasad, S. Deciphering CD59: Unveiling Its Role in Immune Microenvironment and Prognostic Significance. Cancers 2024, 16, 3699. [Google Scholar] [CrossRef]
- de Folmont, A.; Bourhis, J.-H.; Chouaib, S.; Terry, S. Multifaceted Role of the Transforming Growth Factor ? on Effector T Cells and the Implication for CAR-T Cell Therapy. Immuno 2021, 1, 160–173. [Google Scholar] [CrossRef]
- Marie, J.C.; Letterio, J.J.; Gavin, M.; Rudensky, A.Y. TGF-β1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J. Exp. Med. 2005, 201, 1061–1067. [Google Scholar] [CrossRef]
- Chiang, E.Y.; de Almeida, P.E.; de Almeida Nagata, D.E.; Bowles, K.H.; Du, X.; Chitre, A.S.; Banta, K.L.; Kwon, Y.; McKenzie, B.; Mittman, S.; et al. CD96 functions as a co-stimulatory receptor to enhance CD8(+) T cell activation and effector responses. Eur. J. Immunol. 2020, 50, 891–902. [Google Scholar] [CrossRef]
- Liu, F.; Huang, J.; He, F.; Ma, X.; Fan, F.; Meng, M.; Zhuo, Y.; Zhang, L. CD96, a new immune checkpoint, correlates with immune profile and clinical outcome of glioma. Sci. Rep. 2020, 10, 10768. [Google Scholar] [CrossRef]
- Lepletier, A.; Lutzky, V.P.; Mittal, D.; Stannard, K.; Watkins, T.S.; Ratnatunga, C.N.; Smith, C.; McGuire, H.M.; Kemp, R.A.; Mukhopadhyay, P.; et al. The immune checkpoint CD96 defines a distinct lymphocyte phenotype and is highly expressed on tumor-infiltrating T cells. Immunol. Cell Biol. 2019, 97, 152–164. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, A.; Xu, J.; Qiu, C.; Zhu, L.; Qiu, C.; Fu, W.; Wang, Y.; Ye, L.; Fu, Y.X.; et al. CD160 Plays a Protective Role During Chronic Infection by Enhancing Both Functionalities and Proliferative Capacity of CD8+ T Cells. Front. Immunol. 2020, 11, 2188. [Google Scholar] [CrossRef]
- Sun, H.; Xu, J.; Huang, Q.; Huang, M.; Li, K.; Qu, K.; Wen, H.; Lin, R.; Zheng, M.; Wei, H.; et al. Reduced CD160 Expression Contributes to Impaired NK-cell Function and Poor Clinical Outcomes in Patients with HCC. Cancer Res. 2018, 78, 6581–6593. [Google Scholar] [CrossRef]
- Poli, A.; Michel, T.; Theresine, M.; Andres, E.; Hentges, F.; Zimmer, J. CD56bright natural killer (NK) cells: An important NK cell subset. Immunology 2009, 126, 458–465. [Google Scholar] [CrossRef]
- Rodriguez-Mogeda, C.; van Ansenwoude, C.M.J.; van der Molen, L.; Strijbis, E.M.M.; Mebius, R.E.; de Vries, H.E. The role of CD56(bright) NK cells in neurodegenerative disorders. J. Neuroinflammation 2024, 21, 48. [Google Scholar] [CrossRef]
- Uekita, T.; Fujii, S.; Miyazawa, Y.; Iwakawa, R.; Narisawa-Saito, M.; Nakashima, K.; Tsuta, K.; Tsuda, H.; Kiyono, T.; Yokota, J.; et al. Oncogenic Ras/ERK signaling activates CD318 to promote tumor invasion and metastasis. Mol. Cancer Res. 2014, 12, 1449–1459. [Google Scholar] [CrossRef]
- He, Y.; Davies, C.M.; Harrington, B.S.; Hellmers, L.; Sheng, Y.; Broomfield, A.; McGann, T.; Bastick, K.; Zhong, L.; Wu, A.; et al. CD318 enhances Wnt signaling in colorectal cancer promoting nuclear localization of beta-catenin and E-cadherin. Oncogene 2020, 39, 219–233. [Google Scholar] [CrossRef]
- Salzer, E.; Santos-Valente, E.; Keller, B.; Warnatz, K.; Boztug, K. Protein Kinase C delta: A Gatekeeper of Immune Homeostasis. J. Clin. Immunol. 2016, 36, 631–640. [Google Scholar] [CrossRef]
- Hao, Y.; Baker, D.; Ten Dijke, P. TGF-beta-Mediated Epithelial-Mesenchymal Transition and Cancer Metastasis. Int. J. Mol. Sci. 2019, 20, 2767. [Google Scholar] [CrossRef]
- Oh, S.A.; Li, M.O. TGF-beta: Guardian of T cell function. J. Immunol. 2013, 191, 3973–3979. [Google Scholar] [CrossRef]
- Gurrea-Rubio, M.; Wu, Q.; Amin, M.A.; Tsou, P.S.; Campbell, P.L.; Amarista, C.E.; Ikari, Y.; Brodie, W.D.; Mattichak, M.N.; Muraoka, S.; et al. Activation of Cytotoxic Lymphocytes Through CD6 Enhances Killing of Cancer Cells. Cancer Immunol. Immunother. 2024, 73, 34. [Google Scholar] [CrossRef]
- Huang, H.; Pan, Y.; Mai, Q.; Zhang, C.; Du, Q.; Liao, Y.; Qin, S.; Chen, Y.; Huang, J.; Li, J.; et al. Targeting CD318 boost CD8+ T cells-mediated cytotoxicity in cervical cancer via the JAK/STAT signaling pathway. J. Immunother. Cancer 2024, 12, e009416. [Google Scholar] [CrossRef]
- Wong, S.C.; Yeh, C.C.; Zhang, X.Y.; Hsieh, C.Y.; Lo, C.C.; Kuo, T.T.; Lin, C.C.; Chao, C.H.; Liu, J.P.; Chang, L.C.; et al. Inhibition of CD318 by 8-isopentenylnaringenin synergizes with EGFR inhibitors in lung cancer treatment. Mol. Oncol. 2023, 17, 1648–1665. [Google Scholar] [CrossRef]
- Fukuchi, K.; Steiniger, S.C.; Deryugina, E.; Liu, Y.; Lowery, C.A.; Gloeckner, C.; Zhou, B.; Kaufmann, G.F.; Quigley, J.P.; Janda, K.D. Inhibition of tumor metastasis: Functional immune modulation of the CUB domain containing protein 1. Mol. Pharm. 2010, 7, 245–253. [Google Scholar] [CrossRef]
- Ji, D.; Shang, G.; Wei, E.; Jia, Y.; Wang, C.; Zhang, Q.; Zeng, L. Targeting CD318 gene transcription coactivated by BRD4 and CBP/p300 in castration-resistant prostate cancer. Oncogene 2022, 41, 3251–3262. [Google Scholar] [CrossRef]
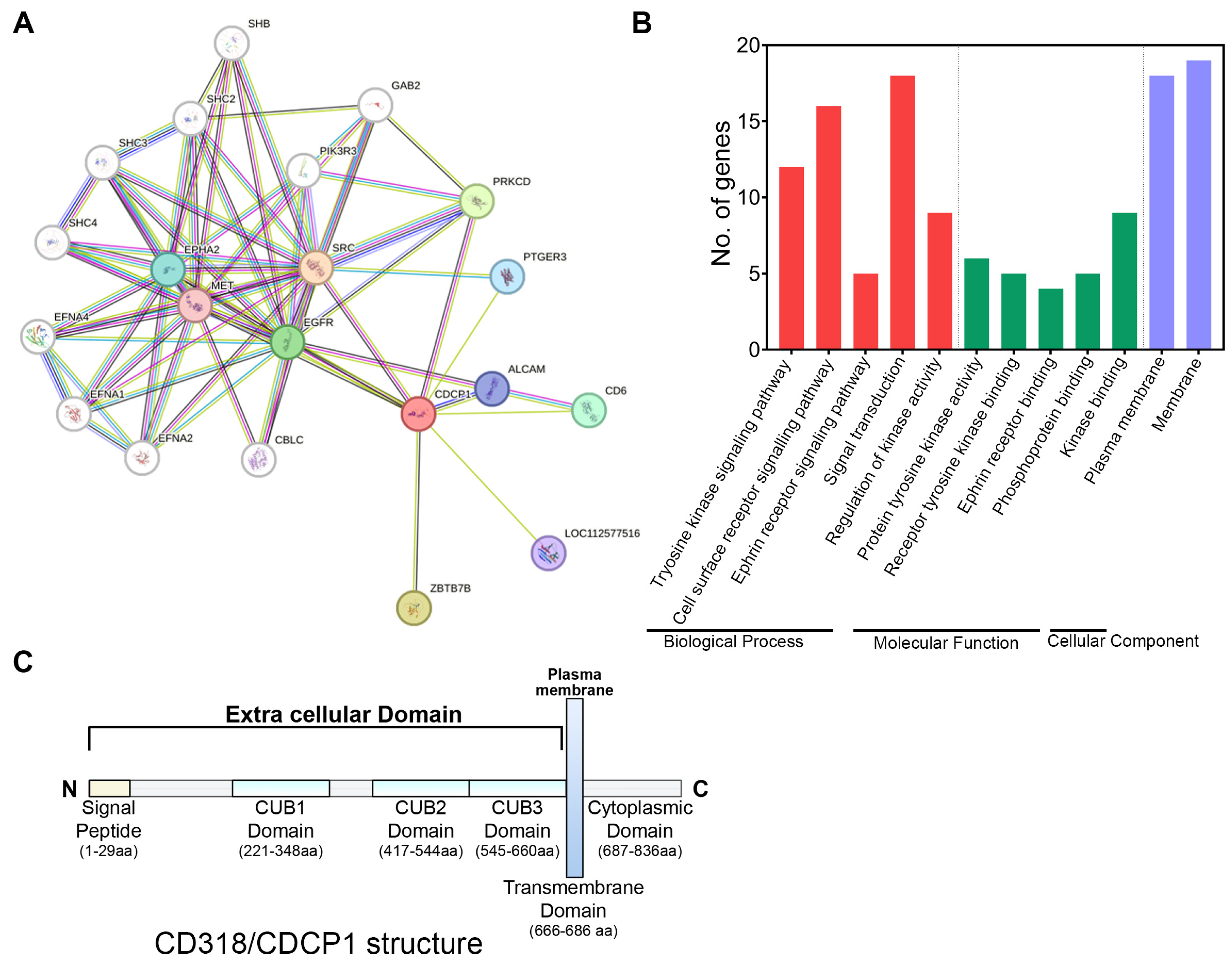
| Pathway | Description | Matching Proteins |
|---|---|---|
| hsa01521 | EGFR tyrosine kinase inhibitor resistance | EGFR, MET, SRC |
| hsa04015 | Rap1 signaling pathway | EGFR, MET, EPHA2, SRC |
| hsa04520 | Adherens junction | EGFR, MET, SRC |
| hsa05120 | Epithelial cell signaling in Helicobacter pylori infection | EGFR, MET, SRC |
| hsa04915 | Estrogen signaling pathway | EGFR, PRKCD, SRC |
| hsa04360 | Axon guidance | MET, EPHA2, SRC |
| hsa04510 | Focal adhesion | EGFR, MET, SRC |
| hsa05205 | Proteoglycans in cancer | EGFR, MET, SRC |
| hsa04014 | Ras signaling pathway | EGFR, MET, EPHA2 |
| Cancer Type | TPM Median Expression | p Value | % of Five-Year Survival with High Expression | % of Five-Year Survival with Low Expression | Protein Expression | Significance | Condition |
|---|---|---|---|---|---|---|---|
| Breast cancer | 11.75 | 0.049 | 81 | 93 | Low/medium | Not prognostic | |
| Cervical cancer | 24.23 | 0.011 | 52 | 75 | Medium | Potential prognostic | Unfavorable |
| Colorectal cancer | 19.72 | 0.0052 | 79 | 52 | High/medium | Potential prognostic | Favorable |
| Endometrial cancer | 13.47 | 0.47 | 68 | 56 | Low/medium | Not prognostic | |
| Glioma | 2.02 | 0.98 | 0 | 13 | Low | Not prognostic | |
| Head and neck cancer | 27.76 | 0.008 | 42 | 52 | Low/medium | Not prognostic | |
| Liver cancer | 0.23 | 0.014 | 44 | 63 | Low | Not prognostic | |
| Lung adenocarcinoma | 12.31 | 9.2 × 10−6 | 27 | 46 | Medium | Validated prognostic | Unfavorable |
| Lung squamous cell carcinoma | 18.51 | 0.027 | 39 | 52 | Medium | Not prognostic | |
| Melanoma cancer | 1.75 | 0.058 | 29(3 yr) | 57(3 yr) | Low | Not prognostic | |
| Ovarian cancer | 9.87 | 0.005 | 24 | 37 | Low/medium | Not prognostic | |
| Pancreatic cancer | 21.32 | 0.00028 | 16 | 53 | Medium | Potential prognostic | Unfavorable |
| Prostate cancer | 6.38 | 0.069 | 100 | 97 | Low/medium | Not prognostic | |
| Renal cancer | 4.59 | 0.001 | 49 | 67 | Low | Not prognostic | |
| Stomach cancer | 21.29 | 0.021 | 38 | 34 | Low/medium | Not prognostic | |
| Bladder Urothelial Carcinoma | 15.46 | 0.15 | 5 | 4 | Low/medium | Not prognostic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, B.; Curcic, M.; Eltokhy, M.A.; Prasad, S. Differential Role of CD318 in Tumor Immunity Affecting Prognosis in Colorectal Cancer Compared to Other Adenocarcinomas. J. Clin. Med. 2025, 14, 5139. https://doi.org/10.3390/jcm14145139
Patel B, Curcic M, Eltokhy MA, Prasad S. Differential Role of CD318 in Tumor Immunity Affecting Prognosis in Colorectal Cancer Compared to Other Adenocarcinomas. Journal of Clinical Medicine. 2025; 14(14):5139. https://doi.org/10.3390/jcm14145139
Chicago/Turabian StylePatel, Bhaumik, Marina Curcic, Mohamed Ashraf Eltokhy, and Sahdeo Prasad. 2025. "Differential Role of CD318 in Tumor Immunity Affecting Prognosis in Colorectal Cancer Compared to Other Adenocarcinomas" Journal of Clinical Medicine 14, no. 14: 5139. https://doi.org/10.3390/jcm14145139
APA StylePatel, B., Curcic, M., Eltokhy, M. A., & Prasad, S. (2025). Differential Role of CD318 in Tumor Immunity Affecting Prognosis in Colorectal Cancer Compared to Other Adenocarcinomas. Journal of Clinical Medicine, 14(14), 5139. https://doi.org/10.3390/jcm14145139






